Abstract
Obstructive Sleep Apnea Syndrome (OSAS) may contribute to the increasing frequency of metabolic disorders. Intermittent hypoxia (IH) is a major characteristic of the syndrome. However, the existing indices of hypoxia in sleep cannot express accurately the effect of the mild desaturations. In this study, a total of 51 patients without other comorbidities were examined by polysomnography (PSG). Hypoxia parameters were analyzed, in the intervals with low values of SpO2 signal. More specifically, the thresholds were set at 94 and 92% and the average value (M) of the SpO2 signal, in areas below thresholds, were calculated. Moreover, the desaturations were analyzed, together with their duration within the recording in terms of SpO2 signal parameters. The patients’ blood sample was analyzed for metabolic parameters. In total, 28 individuals were diagnosed with severe OSAS, (Apnea Hypopnea index (AHI) 59.11 ± 26.10/h, averSpO2 91.64 ± 4.50%, minSpO2 78.18 ± 10.26%, t < 90 21.42 ± 28.64 and ODI 35.48 ± 33.79/h). A statistically significant correlation between the average M92 value with insulin levels (r = 0.401, p < 0.03) and homeostasis model assessment (HOMA) (r = 0.431, p < 0.022) was displayed. Likewise, a correlation between the amount of desaturations and fasting glucose (r = 0.400, p < 0.035) was observed. Moreover, a statistically significant correlation between the desaturations’ average value with insulin (r = 0.378, p < 0.047) and CRP (r = 0.400, p < 0.035) levels was also revealed. A strong correlation also emerged from the cumulative desaturations’ duration as recorded by the SpO2 signal with fasting glucose levels (r = 0.964 p < 0.001), glycosylated hemoglobin (r = 0.860, p < 0.000) and HOMA index (r = 0.580, p < 0.001). The results suggest that the Hypoxia factors derived from SpO2 signal analysis, are strongly correlated with the insulin resistance and with fasting glucose levels. The correlations of the proposed hypoxia parameters were found to be stronger than the already known hypoxia indices, deriving from the PSG, however a more extended analysis is necessary in order to consolidate the findings of this study.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
It has already been reported that Obstructive Sleep Apnea Syndrome (OSAS) is characterized by recurrent episodes completely of partial obstruction of the upper airways. It is characterized by hypoxic episodes alternating with normal oxygenation and by arousals aiming at restoring airflow which result in fragmentation of sleep. Obesity is considered as a major risk factor for the development and progression of OSAS. The incidence of the syndrome in obese or severely obese patients is almost twice than adults with normal weight [1]. The deposition of fat around the chest reduces chest compliance and functional residual capacity, conditions that can lead to increased oxygen demand [2]. The visceral obesity is considered a common risk factor for the onset of diabetes and OSAS.
There is a rapid increase in recent years of both obesity and diabetes, which has been attributed to environmental, socioeconomic, demographic factors as well as lifestyle changes [3]. Many clinical studies suggest that up to 50% of patients with type 2 diabetes have moderate to severe OSAS. Most patients with diabetes report insufficient sleep, both in duration and quality. The prevalence of sleep apnea in patients with type 2 diabetes is estimated at 2–70%. A remarkable finding of the study by Foster et al. was the high prevalence of undiagnosed OSAS (86.6%) among obese patients with type 2 diabetes [4]. This report requires certainly further study on the link between diabetes and sleep apnea.
Intermittent hypoxia (IH), characterized by small repetitive desaturation cycles followed by rapid re-oxygenation, is a feature of the OSAS. Many researchers have developed experimental models in animals and at cellular level in order to study the role of IH. Studies in normal and obese mice, has shown that chronic exposure to IH contributes to decreased insulin sensitivity, a result that is partially reversible after stimulus discontinuation [5].
Nocturnal oxyhemoglobin desaturation and OSAS severity has been associated with metabolic parameters including higher fasting insulin, glucose levels and impaired insulin sensitivity [6].
The present study aims to explore whether parameters derived from the SpO2 signal during sleep can be related to insulin resistance, with the perspective to generate hypoxia markers that can predict diabetes mellitus in the future.
Materials and Methods
Data Description
The data for this work were collected in one year period, following all ethical procedures. Totally, 51 patients without known comorbidities, who had been referred to the sleep lab of the G.H. “G. Papanikolaou”, Thessaloniki, Greece with symptoms suggesting sleep-related breathing disorders were included in the study. All patients had undergone clinical and laboratory testing. A full polysomnography (PSG) was performed for the assessment of their sleep. Finally, 28 patients were diagnosed with OSAS and 23 were found to have normal breathing function in sleep. A blood sample was collected after at least 8 h of overnight fasting. The following parameters were examined: high sensitivity C-reactive protein, fibrinogen, homocysteine, complete lipid profile, blood glucose as well as glycosylated hemoglobin, insulin levels and HOMA (Homeostasis model assessment) which is an insulin resistance marker. The characteristics of the subjects are displayed in Table 1.
The SpO2 signals were extracted from the night PSG recording, in standard EDF format. All signals had a sampling frequency of 3 Hz. Figure 1 depicts an example SpO2 signal of a PSG study of an OSAS patient with AHI 42/h. In this figure, many mild and some serious desaturations occur throughout the night.
SpO2 Signal Analysis
The SpO2 signal was processed in Matlab following the procedure described below:
-
1.
Signal preprocessing to remove artifacts and improve signal.
-
2.
Calculation of hypoxia parameters: setting the threshold to 92 and 94%, and considering the signal values below the thresholds, statistical parameters were calculated for this set up of values. The parameters M92 and M94 refer to the calculated average values, with the respective thresholds employed.
-
3.
Detection of desaturation events (Dev), and related parameters. Desaturation events refer to areas with continuous values below threshold, characterized by the start, end and duration of the event. The number of Dev and their total duration at the time of recording, the Dev duration statistics (mean, standard deviation, min and max), and the percentage of Dev that were long than 6 points (2 s) were calculated as candidate markers.
Statistical Analysis
A statistical analysis of the calculated parameters was performed in order to investigate potential correlation between the characteristics of the signal and the insulin resistance.
Pearson correlation coefficient was used, between the proposed newly introduced hypoxia markers and metabolic markers, and considering the already used hypoxia markers as measured by PSG.
Results
As previously reported, a total of 51 patients with an average age of 46.6 ± 8.84 years were studied. The patients were in general obese with a body mass index (BMI) 31.45 ± 5.81.
During the first interview they reported sleepiness during the day, restless sleep, intense snoring and episodes of breathing during their sleep, recorded by relatives who accompanied them on the day of the interview. They all went through a full PSG study, and on average had AHI 34.02 ± 33.93/h. Blood sampling was performed after overnight fasting of at least 8 h as reported. 28 patients were diagnosed with AHI 59.11 ± 26.10/h, while the other 22 patients had normal sleep with AHI/h of 3.47 ± 0.6.
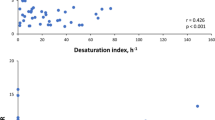
The blood sample values are depicted in Table 2. There was a statistically significant correlation between mean M92 with insulin levels (r = 0.401, p < 0.035) and HOMA (r = 0.431, p < 0.022), as well as the amount of desaturations with fasting glucose (r = 0.400, p < 0.035). In addition, there was a statistically significant correlation between mean duration of desaturations with insulin (r = 0.378, p < 0.047) and CRP (r = 0.400, p < 0.035). A strong correlation was also revealed between the total duration of desaturation of SpO2 with fasting glucose (r = 0.964, p < 0.000), glycosylated hemoglobin (r = 0.860, p < 0.000) and HOMA (r = 0.580, p < 0.001) (Table 3).
In addition, a statistical analysis was performed between metabolic indices and markers of hypoxia deriving from the PSG. A statistically significant positive correlation between t < 90 and insulin levels (r = 0. 496, p < 0.007), HOMA (r = 0.432, p = 0.022) was found, while a negative correlation between insulin and AverSpO2 (r = −0.552, p = 0.022) and the minSpO2 (r = −0.486, p < 0.009) was observed. A negative correlation was also found between AverSpO2 and HOMA (r = −0.379, p < 0.047).
An additional statistically significant correlation was found between minSpO2 and CRP (r = 0.400, p < 0.035).
Discussion and Conclusion
There is lack of studies on the effect of IH on insulin resistance and on the development of diabetes mellitus. Most researchers in their attempt to correlate IH with insulin resistance have used hypoxia markers from PSG, such as the average saturation of oxyhemoglobin- AverSpO2, the minimum SpO2-minSpO2, time below 90% −t < 90 and the ODI-Oxygen Desaturation Index.
ODI is the hourly average number of desaturation episodes, which are defined as at least 4% decrease in saturation from the average saturation in the preceding 120 s, and lasting 10 s.
The purpose of the present study is to analyze the SpO2 signal from the PSG, in order to investigate the correlation between nocturnal hypoxia and metabolic disorders in otherwise patients. There is lack of studies in literature on the analysis of PSG signals (except EEG) and even less is the data regarding the SpO2 signal. All patients in this study were obese with BMI 31.45 ± 5.81 and at a pre-diabetic stage. 28 of them were diagnosed with OSAS with an AHI 47.14 ± 11.54/h.
Hypoxia markers derived from SpO2 signal analysis that are used in this study, are in agreement with the existing evidence that supports the effect of IH on insulin resistance and on glucose metabolism. The IH index currently employed is ODI. In the present study no statistically significant correlation was found between ODI and the HOMA index, insulin and fasting glucose, but significant correlation was found between AverSpO2, t < 90 and minSpO2 with the same metabolic markers [7].
Muraki et al. [8] studied the effect of IH in patients with OSAS in the highly sensitive C-reactive protein. The index that was used as a marker to observe the intermittent hypoxia was the ODI. The severity of nocturnal IH was determined by 3% ODI levels. ODI scores of 5–15 episodes/h, corresponding to mild, moderate to severe intermittent hypoxia, were found to be associated with elevated levels of CRP. The results of the study are in agreement with the results of our study. An important correlation was found between CRP and the mean SpO2 signal desaturations.
As regards analysis of the SpO2 signal, in a recent study of Moret-Bonillo et al. [9] analysis of SpO2 and respiratory (airflow, abdominal and thoracic signals) signals was performed, for the detection and quantification of respiratory pauses in the patient’s respiratory activity and the generation of diagnostic patterns of sleep apnea syndrome. In a pilot study of Renata Trimer et al., the SpO2 signal variability (SpO2V) derived from SpO2 intervals (SpO2i) between successive SpO2 values of the pulse wave was studied. The aim was to use the signal variability SpO2V as a predictor marker of sleep apnea syndrome [10]. Alvarez et al. analyzed the SpO2 signal using central tendency measure (CTM), measures indicating the point to which values of a data/observations group tend to accumulate, and is considered the “center” of the distribution of observations. The result of their study was that the CTM can be used as a reliable diagnostic tool for the sleep apnea syndrome [11].
General research on the analysis of signals from PSG and specific SpO2 signal, revolves around finding the most reliable indicators for diagnosis of OSAS [12, 13].
This study proposed new markers of IH. The results of this study are in agreement with the data from the international literature about the role of IH in glucose metabolism and insulin resistance. In addition, it is important to mention that the new hypoxic indicators employed in this work, presented a stronger correlation to metabolic indices, than the hypoxic indicators so far used by PSG. These findings can lead to better utilization of SpO2 data and help in the prognosis and treatment of metabolic syndromes by addressing their causes.
References
Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK (2010) Interactions between obesity and obstructive sleep apnea implications for treatment. Chest 137(3):711–719. https://doi.org/10.1378/chest.09-0360
Malhotra A, White DP (2002) Obstructive sleep apnoea. Lancet 360(9328):237–245. https://doi.org/10.1016/S0140-6736(13)60734-5
Touma C, Pannain S Does lack of sleep cause diabetes? Cleve Clin J Med 78:549–58
Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB et al (2009) Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 32:1017–1019. https://doi.org/10.2337/dc08-1776
Drager LF, Li J, Reinke C et al (2011) Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 19:2167–2174. https://doi.org/10.1038/oby.2011.240
Moon K, Naresh M, Punjabi, NM, Rashmi N, Aurora RN (2015) Obstructive sleep apnea and type 2 diabetes in older adults. Clin Geriatr Med 31(1):139–ix. https://doi.org/10.1016/j.cger.2014.08.023
Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE (2004) Sleep-disordered breathing, glucose intolerance, and insulin resistance: the sleep heart health study. Sleep heart health study investigators. Am J Epidemiol 15, 160(6):521–530. https://doi.org/10.1093/aje/kwh261
Muraki I, Tanigawa T, Yamagishi K, Sakurai S, Ohira T, Imano H, Kitamura A, Kiyama M, Sato S, Shimamoto T, Konishi M, Iso H (2010) Nocturnal intermittent hypoxia and C reactive protein among middle-aged community residents: a cross-sectional survey. CIRCS Invest Thorax 65(6):523–527. https://doi.org/10.1136/thx.2009.128744
Moret-Bonillo V, Alvarez-Estévez D, Fernández-Leal A, Hernández-Pereira E (2014) Intelligent approach for analysis of respiratory signals and oxygen saturation in the sleep apnea/hypopnea syndrome. Open Med Inform J 8:1–19. https://doi.org/10.2174/1874431101408010001
Mendes RG, Delfino A, Oliveira JR, Arena R, Cabiddu R, Borchi-Silva A (2016) Correlation between desaturation indices of oxygen saturation variability in severe obstructive sleep apnea :a pilot study. J Resp Cardiov Phy Ther 4(1):3–11
Alvarez D, Hornero R, García M, del Campo F, Zamarrón C (2007) Improving diagnostic ability of blood oxygen saturation from overnight pulse oximetry in obstructive sleep apnea detection by means of central tendency measure. Artif Intell Med 41(1):13–24. (Epub 2007). https://doi.org/10.1016/j.artmed.2007.06.002
Koley BL, Dey D (2014) On-line detection of apnea/hypopnea events using SpO2 signal: a rule-based approach employing binary classifier models. IEEE J Biomed Health Inform. 18(1):231–239. https://doi.org/10.1109/JBHI.2013.2266279
Garde A, Dehkordi P, Wensley D, Ansermino JM, Dumont GA (2015) Pulse oximetry recorded from the phone oximeter for detection of obstructive sleep apnea events with and without oxygen desaturation in children. Conf Proc IEEE Eng Med Biol Soc 7692–7695. https://doi.org/10.1109/EMBC.2015.7320174
Acknowledgements
We want to thank the sleep lab of the G.H. “G. Papanikolaou”, Thessaloniki, Greece, for the data collection.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Perantoni, E., Steiropoulos, P., Filos, D., Maglaveras, N., Nikolaou, K., Chouvarda, I. (2018). Association Between SpO2 Signal Characteristics and Sleep Architecture with Insulin Resistance in Patients with Obstructive Sleep Apnea Syndrome. In: Maglaveras, N., Chouvarda, I., de Carvalho, P. (eds) Precision Medicine Powered by pHealth and Connected Health. ICBHI 2017. IFMBE Proceedings, vol 66. Springer, Singapore. https://doi.org/10.1007/978-981-10-7419-6_21
Download citation
DOI: https://doi.org/10.1007/978-981-10-7419-6_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7418-9
Online ISBN: 978-981-10-7419-6
eBook Packages: EngineeringEngineering (R0)