Abstract
Three fern species, namely, Pteris vittata L, Ampelopteris prolifera (Retz.) Copel., and Diplazium esculentum (Retz.) Sw., were grown on three different amendments of fly ash (FA) with garden soil (GS), viz., 100% GS as control, 50% FA+50% GS, and 100% FA. Their growth, metal accumulation, and response to antioxidants were evaluated. It was observed that all of these species accumulated significant amount of metals in their fronds and rhizomes (including rhizoids), while the amount of metal being accumulated by each fern varied. Results revealed that there was a significant increase in their biomass and photosynthetic pigments, for all the test species grown on 50% FA-amended GS in comparison to control; however, it further decreased in ferns grown on 100% FA, indicating that 50% FA amendment did not generate oxidative stress in ferns as well as it seems favorable substratum for fern growth.
Furthermore, while the activity of antioxidant enzymes such as melanoaldehydes (MDA), superoxide dismutase (SOD), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX) increased to a considerable extent in 50% FA amendment, it was found to be maximum in the case of 100% FA amendment. In all the species, the fronds accumulated more metals than rhizomes; they also experienced more oxidative stress as the activities of antioxidant enzymes were observed to be higher in frond’s biomass. Overall, the results of the experiment showed fly ash-induced metabolic adaptation in these ferns and further utility of these species in phytoremediation of toxic metals from fly ash as well as ecorestoration of fly ash landfills with the same species.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Coal is one of the chief sources of electricity generation in India. A large number of power-generating stations in India are coal based (Khan and Khan 1996), wherein a huge quantity of fly ash (FA) is generated in the form of coal combustion residue. The global generation of FA is estimated to be above 600 million tons per year (Singh and Siddiqui 2003), and India contributes to the number by generating 120 million tons FA every year from its 82 power plants; this amount is likely to exceed to 150 million tons by 2020. This huge quantity of FA requires enough space and money for storage/dumping. Physicochemical studies on FA have been revealed that it contains significant amount of noxious metals and metalloids, which percolated from FA and resulted a hazard to receiving ecosystem. Management of such contaminated sites becomes imperative in view of ecological health and hygiene (Kumari et al. 2013; Pandey and Singh 2010). Although a big proportion of the FA has been utilized in building materials and pit filling in mines as well as other landfilling purposes, even then a huge quantity of the FA need environmental friendly uses. Its utilization for agriculture and cultivation of nonagricultural crops like ferns would be an alternative for its disposal/utilization.
Consequently, the revegetation of the FA landfill area by the FA-tolerant plants is the only lucrative and eco-friendly solution/alternative for the management of FA as well as to maintain an aesthetic and pollution-free pleasant landscape (Pandey 2012; Rai et al. 2004; Vajpayee et al. 2000; Wong and Wong 1990). A number of leguminous plant species have been proven suitable in ecorestoration of FA dikes (Gupta et al. 2004; Jambhulkar and Juwarkar 2009; Rai et al. 2004; Ram et al. 2008), and amalgamation of multiple chemical constituents of FA as well as combination of N2-fixing microbes has been found useful in enhancing growth of the plants. Some rhizospheric bacteria also enhance metal mobilization in FA and consequently uptake in plants (Tiwari et al. 2008, 2010). Besides, some fern species have also been reported as hyperaccumulator of metals including arsenic (Ma et al. 2001a, b; Mehrag 2002; Singh et al. 2006), which could be also used for phytoremediation of FA (Kumari et al. 2011, 2013).
Plants growing on FA dikes experience a variety of physiological and biochemical stresses due to the presence of toxic metals. Due to the presence of high pH; low levels of N, P, and K; and high concentration of many toxic elements like Cu, Pb, Hg, Cd, Ni, Al, and As in FA (Haynes 2009; Mehra et al. 1998; Pandey and Singh 2010), some plants showed visual toxicity symptoms, but the common fern species, studied during present work, namely, Pteris vittata, Ampelopteris prolifera, and Diplazium esculentum, did not show any visible toxicity symptoms. It could be due to some internal defense mechanism in these plants against toxicity of heavy metals.
It is known that plants developed internal defense mechanism in the presence of antioxidative enzymes to protect the damage caused by free radicals. Metal compounds, upon entry into the plant cells, produce reactive oxygen species either by direct electron transfer involving metal cations or as a consequence of metal-mediated metabolic reactions. However, to cope up with reactive oxygen species, plant has scavenging system mediated by nonenzymatic antioxidant and enzymatic antioxidant systems like melanoaldehyde (MDA), superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and glutathione (GSH) (Sinha and Gupta 2005; Sinha et al. 2005). Such plants under stress condition also synthesize metal-binding peptides (phytochelatins) showing high level of nonprotein thiols (NPSH) and cysteine (Grill et al. 1987; Zenk 1996). Further, arsenic-induced biochemical changes in P. vittata have been reported (Cao et al. 2004; Srivastava et al. 2005), whereas FA-induced biochemical studies are reported in both non-nodulated species Cassia siamea Lamk. (Kumar et al. 2002) and nodulated species chickpea of leguminous family (Pandey et al. 2010). FA-induced biochemical changes in Prosopis juliflora L. have been done by Sinha et al. (2005).
However, such studies have been conducted on many crop plants and vegetation with varying degree of success, but such studies on the ferns experiencing FA stress are limited, and a single report has been made by Kumari et al. (2013) to explain antioxidant systems in the fern Thelypteris dentata growing on varied level of FA amendments. Therefore, the present study was planned to pursue the objectives on how these species could establish themselves in the vicinity of FA, even in the presence of toxic metals and lack of N, P, and K. In this context, the present study aimed to elucidate the internal defense mechanism by analyzing antioxidative enzymes in target ferns generated to combat with metal stress of FA. Thus, the present study is also an attempt to eco-friendly management of FA landfills with nonedible, ornamental, and potential metal accumulator ferns which may prove a useful alternative for FA utilization.
7.2 Material and Methods
7.2.1 Sample Collection and Preparation
FA used in this experiment was collected randomly from dumping sites of National Thermal Power Corporation, Kanti, Muzaffarpur (Bihar), India in large plastic bags and brought to the garden field near experimental fern house. The garden soil (GS) was collected from CSIR-Institute of Himalayan Bioresource Technology, Palampur, India. Before preparation of various amendments, both FA and GS were air dried for 7 days and sieved through 2 mm mesh. These amendments were denoted as 100% FA, 50% FA (50% FA+50% GS), and 100% GS alone served as control. Sporophylls of P. vittata, A. prolifera, and D. esculentum were collected from the plants growing naturally in FA dikes of NTPC Kanti, Muzaffarpur (Bihar), India, in spore packets made by brown papers.
7.2.2 Experimental Setup
Collected sporophylls of all the three species with mature spores were kept in desiccators for 4 weeks. Spores from sporophyll of each fern were released on the tissue paper automatically and by destructing the sporangia with the help of needle. Spores were surface sterilized by 2% sodium hypochlorite for 2 min and rinsed four times with sterilized deionized water. Spores were sprinkled on petri plates containing 25 ml P&T medium solidified with addition of 1% agar as stock culture. Cultures were placed in culture room (temperature, 22 ± 2°; photoperiod, 16:8 h; a light intensity of 350 μ mol m−2 s−1). After 1 week of sowing, germination of spores started which was observed at weekly interval under microscopes till complete gametophyte formation. Nine earthen pots were taken of 2 kg air-dried FA capacity. Three sets of pot were prepared by filling FA with GS: first set containing only 100% FA, second set containing 50:50 ratio of FA and GS, and third set containing only 100% GS which was treated as control. Each set of experiment was performed in triplicates.
Similarly, nine earthen pots (1 kg capacity) were prepared for each species, and experiments were set up in randomized block design. Each pot was transplanted with three healthy gametophytes (5 weeks old) of P. vittata, A. prolifera, and D. esculentum, from stock culture, respectively. Pots were arranged in three plastic trays, one tray for each species, and covered by acclimatization hood and put randomly inside experimental fern house of IHBT, Palampur. A dish was placed under each pot to avoid leaching during the experiments. For healthy growth of gametophyte, moisture was maintained by spraying tap water daily or as and when necessary, and it is allowed to grow till sporophyte forms five to six fronds. The plants were grown for approximately 90 days after inoculation in pots and then harvested and washed in tap water. Harvested plants were washed repeatedly, blotted dry, and separated into the aboveground (fronds) and belowground (rhizomes including roots) portions weighed (fresh weight (FW) basis). The uptakes of metals as well as various growth, biochemical parameters, and antioxidative enzymes were studied in harvested samples.
7.2.3 Physicochemical Characterization of Different Amendments of FA and GS
FA and GS prepared for pot experiment were analyzed for their physicochemical characterization. The pH and electrical conductivity were determined through pH meter and conductivity meter by adding distilled water in FA in 1:5 ratio (Piper 1966), total organic carbon (Walkely and Black 1934), total nitrogen (micro-Kjeldahl digestion: Nelson and Sommer 1982), available phosphorus (Oleson and Sommers 1982), and cation exchange capacity (CEC) extracted with ammonium acetate (Allen et al. 1974).
7.2.4 Plant Growth Parameters (Biomass and Photosynthetic Pigments)
Plants were harvested after 90 days of growth in different amendments and repeatedly washed with double-distilled water. Plants were blotted dry, and the biomass (fresh weight) of belowground part and aboveground parts was measured on fresh weight basis. Biomass of plants growing on various amendments of FA was observed by recording the fresh weight of both parts of the plant (data not shown separately) and was expressed as total biomass of each fern on various amendments on fresh weight (FW) basis. For estimation of photosynthetic pigments, leaf frond (300 mg) was ground in chilled 80% acetone in dark. After centrifugation at 10,000×g for 10 min. at 4 °C, absorbance of the supernatants was taken at 480, 510, 645, and 663 nm. Chlorophyll and carotenoids contents were calculated using the formula given by Arnon (1949) and Dexbury and Yentch (1956), respectively.
7.2.5 Metal Estimation in Fronds and Root Parts
The harvested fern sample was washed and separated in aboveground (fronds) and belowground parts (Rhizome with roots) and then oven-dried at 80 °C for 24 h. The oven-dried plant tissue (frond and root) of treated and control plants was grinded at room temperature and digested with 0.1 g of sample in glass digestion tube of 250 mL along with mixture of concentrated nitric acid (HNO3) and perchloric acid (HClO4) (V/V 3:1) at 140 °C. After digestion the solution was cooled, filtered, and made up to 50 mL with distilled water for heavy metal analysis. The heavy metal measurements in the foliar and root samples were performed with Flame Atomic Absorption Spectrophotometer (Perkin Elmer, Model A Analyst 300) following Tripathi et al. (2008).
7.2.6 Lipid Peroxidation
The level of lipid peroxidation in roots and leaves was measured in terms of melanoaldehyde (MDA) content as estimated by thiobarbituric acid reaction following the method of Heath and Packer (1968) with slight modification. Plant tissues (500 mg) were homogenized in 5 ml of 0.1% TCA. The homogenate was centrifuged at 10,000× g for 15 min. For every 1 ml of aliquot, 4 ml of 20% TCA containing 0.5% thiobarbituric acid was added. Mixture was heated at 95 °C for 30 min and then cooled quickly on ice bath. The resulting mixture was centrifuged at 10,000-×g for 15 min, and the absorbance of the supernatants was taken at 532 and 600 nm. The non-specific absorbance at 600 nm was subtracted from the absorbance at 532 nm. The concentration of MDA was calculated and expressed by total thiobarbituric acid reaction substrates (TBARS) in term of μmole g−1 by using an extinction coefficient of 155 mM−1 cm−1.
7.2.7 Analysis of Antioxidative Enzymes
Plant material (500 mg, leaves or roots) was homogenized in 100 mM potassium phosphate buffer (pH 7.0) containing 0.1 mm EDTA and 1% polyvinylchloride (w/v) at 4 °C. Homogenate was filtered through four layers of cheesecloth and centrifuged at 15,000×g for 15 min at 4 °C. Supernatant was used to measure the activities of enzymes.
7.2.7.1 Superoxide Dismutase (SOD)
The activity of SOD was assayed by measuring its ability to inhibit the photochemical reduction of nitrobluetetrazolium according to the method of Beauchamp and Fridovich (1971). The 3 ml reaction mixture contained 40 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 mM nitrobluetetrazolium, 2 μm riboflavin, and 0.1 μm EDTA, and a suitable aliquot of enzyme extract riboflavin was added; at the end the test tubes were shaken and placed 30 cm below the light source consisting of 15 watt fluorescent lamp. Switching on the light started the reaction, and after 30 min, switching off the light stopped the reaction. A tube containing a protein kept in the dark served as blank, while the control tube was without the enzyme and kept in the light. The absorbance of the solution was taken at 560 nm. Activity of SOD is the measure of NBT reduction in light without protein minus NBT reduction with protein. One unit of activity is the amount of protein required to inhibit 50% initial reduction of NBT under light.
7.2.7.2 Ascorbate Peroxidase (APX)
The activity of APX was measured according to the method of Nakano and Asada (1981) by estimating the rate of ascorbate oxidation (extinction coefficient 2.8 mM−1 cm−1). The 3 ml reaction mixture contains 50 mM phosphate buffer (pH, 7.0), 0.1 mM H202, 0.5 mM sodium ascorbate, 0.1 mM EDTA, and a suitable aliquot of enzyme extract. The change in absorbance was monitored at 290 nm, and enzyme activity was expressed as μmole of ascorbate oxidized min−1 g−1 fw.
7.2.7.3 Guaiacol Peroxidase (GPX)
GPX activity was assayed according to the method of Hemeda and Klein (1990). A 100 ml of reaction mixture was prepared by adding 10 ml of 1% guaiacol (v/v), 10 ml of 0.3% H2O2, and 80 ml of 50 mM phosphate buffer (pH, 6.6). 75 μl of enzyme extract was added to reaction mixture with a final volume of 3 ml. The increase in absorbance due to oxidation of guaiacol (extinction coefficient 26.6 mM−1 cm−1) was monitored at 470 nm. Enzyme activity was expressed as μmole of guaiacol oxidized min−1 g−1 fw.
7.2.8 Statistical Analysis
All the values are presented as mean ± SD (n = 3) in figures and tables. The data was subjected to analysis of variance (ANOVA) to test the significant differences by using software package SYSTAT-9.0.
7.3 Results and Discussion
All the three fern species were grown on three different amendments of FA and GS, viz., 100% GS, 50% FA: 50% GS, 100% FA) for 90 days. Physicochemical studies were carried out to assess the physical status and metal toxicity present in the above amendments used during pot experiment. Oxidative stress created by the metals accumulated by plants was measured by changes in the biomass, photosynthetic pigments, TBARS, SOD, and antioxidant enzymes. Further, we tested the hypothesis that antioxidant molecules APX and GPX were involved in the detoxification of FA-induced stress in ferns. Since aboveground part (especially frond) contains more metal contents in comparison to belowground parts, aboveground part experienced more oxidative stress. Present study evaluated the extent of FA-induced oxidative stress and associated metal detoxification systems in three target fern species.
7.3.1 Physicochemical Characterization of Different Substratum
Analysis of physicochemical properties of different substratum used during present study is depicted in Table 7.1. Result reveals that FA was alkaline but GS used during experiment was acidic in nature. The pH and electrical conductivity (EC) of FA were 9.3 ± 0.03 and 7.51 ± 0.52 dS m−1, while the pH and EC of GS were 6.4 ± 0.02 and 1.61 ± 0.03 dS m−1, respectively. Cation exchange capacity (CEC) of GS was significantly higher in comparison to 100% FA, but it was found in moderate content at 50:50 ratios of FA and GS. However, the presence of total N and P in FA was almost negligible (0.01 and 0.02, respectively) in comparison to GS but having better water holding capacity, which retains moisture inside. The concentration of trace elements in different substrates such as GS-, FA-, and FA-treated soils is also given in Table 7.1. The level of trace elements (Fe, Si, Ni, Al, Mg, Cu Cd, Pb, and As) in 100% FA was significantly high (student’s t-test significant at p < 0.05) as compared to GS (control), while the concentration of As, Cd, and Pb in GS was below detectable limit (BDL). Results of the present study reveal that FA contains many plant growth essential micronutrient elements that are used in normal plant metabolism because they are essential constituents of various coenzymes and food substances like carbohydrate, fat, and protein, but the role of heavy metals is not known in plant metabolism, and they pose significant health risks to the receiving environment. The results of the present study are in support of earlier studies (Haynes 2009; Mehra et al. 1998; Pandey and Singh 2010). Chemically, all naturally existing elements can be found in FA (Klien et al. 1995), which is also subsequently enriched in trace elements compared with the parent coal. Among the elements enriched in ashes were Zn, Fe, Ni, Mn, Cu, Cd, Pb, Cr, B, Al, Si, and As (Mehra et al. 1998).
7.3.2 Metal Accumulation in Ferns Growing on FA Amendments
All the three fern species grown on different amendments of FA accumulated significant amount of Fe, Cu, Al, Ni, Si, Pb, Cr, and Cd in their fronds and rhizomes (Fig. 7.1). However, the concentration of metals in different plant parts (fronds and rhizome including roots) and order of preference for uptake and accumulation of metals in the plant tissues varied in each fern species. In the present study, P. vittata accumulated maximum iron followed by Al, Ni, Si, Cu, Cr, Pb, and Cd in frond part, but in rhizome metals accumulated in the order of Al>Fe>Cu>Si>Cr>Pb>Ni>Cd in 100% as well as 50% FA, but in 100% GS the patterns changed slightly as Fe>Al>Cu>Ni>Si>Pb>Cr>Cd. In each amendment, A. prolifera accumulated metals in Fe>Al>Si>Cu>Ni>Cr>Pb>Cd pattern. In fronds of D. esculantum, the metal was accumulated in the order of Fe>Al>Si>Ni>Cu>Cr>Pb>Cd in 100% FA and in 50% FA but negligible silicon in fronds grown on 100% GS. In D. esculantum, accumulation of metals in rhizome was found in the order of Fe>Al>Si>Ni>Cu>Cr>Pb>Cd at various amendments. Although metal accumulation was almost equal in other two species A. prolifera and D. esculentum, P. vittata accumulated about two times more heavy metals (Fe, Al, Cr, Pb, and Cd) than other two species in both plant parts. Further, all the three species accumulated significant amount of all the metals tested in this study in fronds than rhizomes including roots (Fig. 7.1a–c). Results showed potential of all the three fern species to grow on FA without any visible phytotoxic symptoms.
Many plants are known to accumulate high quantity of toxic metals in their tissues, and hence they are being used for phytoremediation of contaminated sites (Baker and Brooks 1989; Wenzel and Jockwer 1999). Among such plants, ferns have been demonstrated to accumulate and resist high metal toxicity growing on contaminated soil (Honjo et al. 1980; Ma et al. 2001a, b). Despite of this fact, these species have never been utilized for removal of metals from contaminated wastes like FA. Results obtained during the present study clearly demonstrated a very high potential of P. vittata, A. prolifera, and D. esculentum in removal of metals from FA-amended soil. Further, it was also observed that fronds contain higher amount of heavy metals than rhizomes of the plants in all the three test species. It indicates that accumulated metal content was translocated and deposited inside frond which is reported as a good symbol of efficient hyperaccumulator, because suitability of a plant for phytoremediation could be determined by its ability to produce high aboveground biomass and high bioconcentration and transfer factors of metals (Feyiga et al. 2004). Several studies reported the suitability of P. vittata for phytoremediation of As-contaminated lands (Feyiga et al. 2004; Singh et al. 2006) and also recently reported in phytoremediation of FA-contaminated lands (Kumari et al. 2011). Application of A. prolifera and D. esculentum for phytoremediation of FA has first time reported in this paper. Our results showed that iron accumulation was maximum in all the three species which could be attributed to the high requirements of iron for plants for various metabolic activities as constituent of many enzymes and cytochromes of certain porphyrins in plant growth (Hewitt 1998). These findings confirm earlier reports on terrestrial plants growing on FA-amended soil (Gupta et al. 2007; Kumari et al. 2011; Rai et al. 2004).
7.3.3 Effect of FA Amendments on Biomass and Photosynthetic Pigments of Ferns
The comparative growth parameters of all the three species were observed weekly, and their rate of germination and growth rate were studied on P&T medium solidified with 1% agar under laboratory conditions (Table 7.2). The percentage rate of germination and growth was maximum in P. vittata L. in comparison to other two species, i.e., A. prolifera and D. esculentum. Both species took approximately equal time for germination and possessed almost equal growth rate. Sex ontogeny and differential growth stages were also appeared delayed in comparison to P. vittata.
Ferns grown on various amendments showed variation in their growth patterns; hence, they possessed varied biomass. Most significant increase in biomass was observed on 50% concentration of FA in all the three test species. P. vittata performed maximum increase by 40% in total biomass growing on 50% FA, and it decreases on 100% FA by denoting only 20% increase in total biomass. A. prolifera showed 20% increase in total biomass on 50% amendments, but there was no significant increase found on 100% FA as compared to control. Similarly D. esculentum performed 15% increase in total biomass on 50% amendments but decreased biomass on 100% FA amendments in comparison to control. At 100% FA, all the test species experienced toxicity in some extent, and consequently biomass decreases in comparison to 50% FA (Fig. 7.2). However, there were no visible toxicity symptoms observed in all the three species.
As exposure of 100% FA possessed metal toxicity in ferns, the photosynthetic pigments also exerted by the same and consequently decreased chlorophyll a and b and total chlorophyll (Fig. 7.2), but ferns growing on 50% FA enhanced chlorophyll a and b and total chlorophyll by 20–25% in P. vittata. Ferns grown on 50% FA amendments showed always enhanced photosynthetic pigments in comparison to control in all species. However, chlorophyll content was decreased at 100% FA, but carotenoid contents were enhanced in all the three species. It showed young sporophytes raised on maximum dose of FA loss in photosynthetic pigments due to metal toxicity present in FA. Thus, the result obtained by study of biomass and photosynthetic pigments suggests that 50% FA amendment do not generate oxidative stress in ferns and seem to be suitable substratum for healthy fern growth (Fig. 7.2).
Carotenoid is a class of natural fat-soluble pigments found in plants and plays an important role in photosynthetic process as well as membrane-associated antioxidant activity. The decline in chlorophyll concentration implied heavy metal-induced stress due to FA in all the three species. Earlier Pteris vittata has been shown to have reduced chlorophyll content in higher-dose exposure of arsenic, but in lesser doses it showed enhancement in chlorophyll content (Singh et al. 2006). Similar result was obtained during present study on various FA amendments. The same trend was followed in other two species D. esculantum and A. prolifera. The chlorophyll data suggest that a combination of antioxidant compounds and enzymes resulted in a greater protection of the photosynthetic system of metal-tolerant Pteris vittata and other hyperaccumulator ferns against ROS (Singh et al. 2006).
7.3.4 Effect of FA Amendments on Lipid Peroxidation and Antioxidant Enzymes
7.3.4.1 Lipid Peroxidation (MDA)
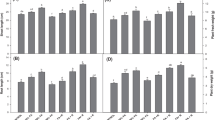
The effect of FA toxicity on lipid peroxidation was determined by evaluating MDA content in the form of TBARS content of the fern tissues. It was found maximum in more toxic site, i.e., in 100% FA and minimum in control (100% GS) site. A significant increase by 47%, 31%, and 27% in TBARS was observed in frond tissues of P. vittata, A. prolifera, and D. esculentum, respectively, as compared to ferns grown on control. Similarly, MDA content was enhanced in rhizome tissues of P. vittata, A. prolifera, and D. esculentum by 41%, 25%, and 28%, respectively. As metal content in fronds was found higher in comparison to rhizome tissues, the level of MDA in frond tissues was also found higher. MDA level increased by increasing ratio of FA amendments in GS. Lipid peroxidation in terms of MDA was found maximum in the case of sporophytes of P. vittata, grown in 100% FA as compared to other treatments showing its lower scavenging ability. Therefore, high levels of lipid peroxidation in fern tissues are also indirect indicator of metal stress provided by FA (Fig. 7.3).
Effect of different amendments of fly ash on SOD (μ mg−1 fw), APX (min−1 g−1fw), MDA content (μ mol g−1 fw), and GPX (μ mol g−1 fw) of three fern species (a) P. vittata, (b) A. prolifera, and (c) D. esculentum on fronds and rhizomes including roots after 90 days. Similar letters are not significant (P < 0.5 level) a/c to Duncan’s multiple range test
The formation of TBARS in plants exposed to adverse environmental conditions is an indicator of free-radical formation in the tissues, and it may be used as an index of lipid peroxidation in biological systems (Heath and Packer 1968). There is considerable evidence that inorganic exposure results in the generation of ROS in plants (Hartley-Whitaker et al. 2001). ROS induced by the ferns growing in FA-amended soil showed an enhanced level of melanoaldehyde compounds, which is degree-dative product of a variety of biologically important compounds like amino acid, protein, and carbohydrate; therefore, increased concentration of MDA in these ferns following FA stress is an indicator of metal-induced oxidative damage as reported in earlier studies (Kumari et al. 2013). This indicated that all these target species possessed a defense system to protect themselves against oxidative damages as evidenced by increased activity of antioxidant enzymes.
7.3.4.2 Superoxide Dismutase (SOD)
SOD is an essential component of plant’s antioxidative defense system. The SOD activities of the frond tissue in all the three species were found significantly higher in 100% FA-grown ferns. It was found enhanced by 57%, 27%, and 34% in frond part of P. vittata, A. prolifera, and D. esculentum, respectively, on ferns grown on 100% FA. Similarly, in belowground part, the level of SOD was found higher in FA-grown ferns by 42%, 24%, and 26%. It was further observed that fronds maintained higher SOD activity than roots and rhizomes, and it increased by increasing level of FA stress (Fig. 7.3).
Antioxidant enzymes are considered to be an important defense system of plants against oxidative stress caused by metals (Weckx and Clijsters 1997). The activities of SOD, APX, and GPX in general showed simultaneous induction and decline, which may be due to their co-regulation (Shigeoka et al. 2002). Significant increase in the level of SOD in all three ferns was found in this study. However, the highest level of SOD was found in P. vittata fronds grown on 100% FA indicating its greater scavenging or acclimatization capacity to FA stress by producing rapidly antioxidative defense system. SOD plays an important role in dismutation of free hydroxyl radicals by the formation of hydrogen peroxides. However, during well-organized functioning of SOD, which prevents oxygen-driven cell damage and converting it to H2O2 which is then reduced to water and molecular oxygen by the action of various enzymes like APX and GPX working at different location of cell.
7.3.4.3 Ascorbate Peroxidase (APX)
In comparison to fronds, the root tissues contained higher APX concentration in all the three test species. In 100% FA, fronds of P. vittata showed 18% increase in APX activity, but it increased by 22% in belowground part. A. prolifera and D. esculentum showed approximately similar APX activity. But both species showed maximum APX content in belowground part (rhizomes and roots) by 28% and 24% increase than control. In frond part of both these species, APX activity increased when treated with maximum FA toxicity by 18% and 14%, respectively (Fig. 7.3).
Ascorbate is an essential compound in plant tissues and has a major role in relation to enzymatic and nonenzymatic oxidation reactions in the biological systems (Gupta et al. 1999; Smirnoff 1996). It can react directly by reducing superoxide, hydrogen peroxide, and hydroxyl radical or quenching singlet oxygen and functions as co-substrate of plant oxidases, such as the ascorbate peroxidase system, which produces dehydroascorbate (Halliwell 1982). Though APX play key role in functioning of chloroplast in ascorbate glutathione cycle, but in present study the role of APX during metal detoxification seems not much significant, as it shown slight increase in ferns growing on 100% FA in comparison to GPX, which is basically a cell-bound enzyme found in cytoplasm play better response to combat metal toxicity in ferns.
7.3.4.4 Guaiacol Peroxidase (GPX)
GPX activities perform remarkably higher in FA-grown ferns than control, but it also showed similarity with APX and was found higher in root tissues than fronds. When treated with 100% FA, it showed maximum increase by 71% in P. vittata fronds and 60% in rhizome parts. Other two species A. prolifera and D. esculentum showed approximately similar increase by 52% and 50% in fronds and by 50% and 55% in rhizomes, respectively. Almost similar GPX activity was performed by both species, and no significant changes in GPX activity of both plant parts were observed (Fig. 7.3).
In present study, GPX activity in both fronds and rhizomes showed remarkable better response as compared to APX. GPX thus seemed to play a lead role in metal detoxification. GPX is thought to be stress marker enzymes (Castillo 1986), and its higher induction may indicate stress exerted by heavy metals, which can be correlated with amount of accumulated metal.
7.4 Conclusions
The present study concludes feasibility of using P. vittata, D. esculantum, and A. prolifera in phytoremediation of metals from FA. Metal-accumulating ferns, despite showing slight reduction in the biomass and photosynthetic pigments, showed enhanced level of antioxidative enzymes; thus, these plants could scavenge oxygen radicals due to stimulated activities of SOD and GPX. However, contribution of APX found is not much significant, and other mechanisms of thio-metabolism and phytochelatin induction could be ruled out. The result of present study also suggests that 50% amendment ratio is most suitable substratum for healthy and luxuriant growth of ferns, which address a major problem of FA management in agriculture of ornamental ferns. Further studies were aimed to analyze both GSH and PC biosynthesis in these potential ferns under FA stress. Besides these, molecular mechanism underlying hyperaccumulation in ferns is also prerequisite for better understanding of the complete mechanism of metal detoxification.
References
Allen SE, Grimsha WHM, Parkinson JA, Quarnby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publisher, Oxford
Arnon DI (1949) Copper enzymes in isolated chloroplast, polyphenol/oxidase in Beta vulgaris. Plant Physiol 24:1–15
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements – a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44 (1):276–287
Cao X, Ma LQ, Tu C (2004) Antioxidant responses to arsenic in the arsenic hyperaccumulator Chinese brake fern (Pteris vittata L.) Environ Pollut 128:463–468
Castillo FJ (1986) Extracellular peroxidases as markers of stress? In: Grepin H, Penel C, Gaspar T (eds) Molecular and physiological aspects of plant peroxidases. University of Geneva Press, Geneva, pp 419–426
Dexbury AC, Yentch CS (1956) Plankton pigment monograph. J Mar Res 5:93–101
Feyiga OA, Ma LQ, Xinde C, Rathinasabapathi B (2004) Effects of heavy metals on growth and arsenic accumulation in the arsenic hyperaccumulator Pteris vittata L. Environ Pollut 132:289–296
Grill E, Winnacker EL, Zenk MH (1987) Phytochelatins, a class of heavy metal binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A 84:439–443
Gupta M, Cuypers H, Vangronsveld H, Clijsters (1999) Copper effects the enzymes of the ascorbate-glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiologiqua Plant 106:262–267
Gupta DK, Rai UN, Sinha S, Tripathi RD, Nautiyal BD, Rai P, Inouhe M (2004) Role of rhizobium (CA-1) inoculation in increasing growth and metal accumulation in Cicer arietinum L. growing under fly ash stress condition. Bull Environ Contam Toxicol 73:424–431
Gupta AK, Dwivedi S et al (2007) Metal accumulation and growth performance of Phaseolus vulgaris grown in fly ash amended soil. Bioresour Technol 98:3404–3407
Halliwell H (1982) Ascorbic acid and the illuminated chloroplast. In: Seib PA, Tolbert BM (eds) Ascorbic acid: chemistry, metabolism and uses. American Chemical Society, Washington, DC, pp 263–274
Hartley-Whitakar J, Ainsworth G, Vooijs R, Ten WB, Schat H, Mehrag AA (2001) Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol 126:299–306
Haynes RJ (2009) Reclamation and revegetation of fly ash disposal sites – challenges and research needs. J Environ Manag 90:43–53
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast 1 kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hemeda HM, Klein BP (1990) Effects of naturally occurring anti-oxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hewitt EJ (1998) The role of mineral elements in the activity of plant enzymes. In: Ruhl W (ed) Hand buch der pfflanzen physiologie, vol IV. Springer, Berlin, p 427
Honjo T, Suganuma H, Satomi N (1980) The vegetation of the pollution areas caused by the lead title in Kanazawa Castle. J Phytogeogr Taxon 27:70–73
Jambhulkar HP, Juwarkar AA (2009) Assessment of bioaccumulation of heavy metals by different plant species grown on fly ash dump. Ecotoxicol Environ Saf 72:1122–1128
Khan MR, Khan MW (1996) The effect of fly ash on plant growth and yield of tomato. Environ Pollut 92:105–111
Klien DH, Andew AW et al (1995) Pathways of thirty seven trace elements through coal fired power plants. Environ Sci Technol 9:973–979
Kumar A, Vajpayee P, Ali MB, Tripathi RD, Singh N, Rai UN, Singh SN (2002) Biochemical responses of Cassia siamea Lamk. grown on coal combustion residue (fly ash). Bull Environ Contam Toxicol 68:675–683
Kumari A, Lal B, Pakade YB, Chand P (2011) Assessment of bioaccumulation of heavy metals by Pteris vittata L. growing in the vicinity of fly ash. Int J Phytoremediation 13:779–787
Kumari A, Pandey VC, Rai UN (2013) Feasibility of fern Thelypteris dentata for revegetation of coal fly ash landfills. J Geochem Explor 128:147–152
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y (2001a) A fern that hyperaccumulates arsenic. Nature 409:579
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y (2001b) A fern that hyperaccumulates arsenic-addendum. Nature 410:411–438
Mehra A, Farago ME, Banerjee DK (1998) Impact of fly ash from coal fired station in Delhi, with particular reference to metal contamination. Environ Monit Assess 50:15–35
Mehrag AA (2002) Variation in arsenic accumulation/hyperaccumulation in ferns and their allies. New Phytol 157:25–31
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Nelson DW, Sommer LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL (ed) Methods of soil analysis, ASA Monogr 9 (2), 2nd edn. American Society of Agronomy, Madison, pp 539–579
Oleson SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keene DR (eds) Methods of soil analysis, Part 2 Chemical and Microbiological. Properties ASA, Madison, pp 403–427
Pandey VC (2012) Invasive species based efficient green technology for phytoremediation of fly ash deposits. J Geochem Explor 123:13–18
Pandey VC, Singh N (2010) Impact of fly ash incorporation in soil systems. Agric Ecosyst Environ 136:16–27
Pandey VC, Singh JS, Kumar A, Tewari DD (2010) Accumulation of heavy metals by chickpea grown in FA treated soil: effect on antioxidants. Clean (Weinh) 38:1116–1123
Piper CS (1966) Soil and plant analysis. Inter Science, New York
Rai UN, Pandey K, Sinha S, Singh A, Saxena R, Gupta DK (2004) Revegetating fly-ash landfills with Prosopis juliflora L. impact of different amendments and rhizobium inoculation. Environ Int 30:293–300
Ram LC, Jha SK, Tripathi RC, Masto RE, Selvi VA (2008) Remediation of fly ash landfills through plantation. Remediation 18:71–90
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase iso-enzyme. J Exp Bot 53:1305–1319
Singh IP, Siddiqui ZA (2003) Effects of fly ash and Helminthosporium oryzae on growth and yield of three cultivars of rice. Bioresour Technol 86:73–78
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in P vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Sinha S, Gupta AK (2005) Translocation of metals from fly ash amended soil in the plant of Sesbania cannabina L Ritz: effect on antioxidants. Chemosphere 61:1204–1214
Sinha S, Rai UN, Bhatt K, Pandey K, Gupta AK (2005) Fly ash induced oxidative stress and tolerance in Prosopis juliflora L. grown on different amended substrates. Environ Monit Assess 102:447–457
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Srivastava M, Ma LQ et al (2005) Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot 56:1335–1342
Tiwari S, Kumari B, Singh SN (2008) Microbe-induced changes in metal extractability from fly ash. Chemosphere 71:1284–1294
Tiwari S, Kumari B, Singh SN (2010) Evaluation of metal mobility/immobility in fly ash induced by bacterial strains isolated from the rhizospheric zone of Typha latifolia growing on fly ash dumps. Bioresour Technol 99:1305–1310
Tripathi RD, Dwivedi S et al (2008) Role of blue green algae biofertilizer in ameliorating the nitrogen demand and fly-ash stress to the growth and yield of rice (Oryza sativa L.) plants. Chemosphere 70:1919–1929
Vajpayee P, Rai UN et al (2000) Management of fly ash landfills with Cassia surattensis Burm. – a case study. Bull Environ Contam Toxicol 65:675–682
Walkely YA, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Weckx JEJ, Chlisters HMM (1997) Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 35:405–410
Wenzel WW, Jockwer F (1999) Accumulation of heavy metals in plants grown on mineralized soils of the Austrian Alps. Environ Pollut 104:145–155
Wong JWC, Wong MH (1990) Effects of fly ash on yields and elemental composition of two vegetables, Brassica parachinensis and Brassica chinensis. Agric Ecosyst Environ 30:254–264
Zenk MH (1996) Heavy metals detoxification in higher plants – a review. Gene 179:21–30
Acknowledgment
We thank Directors, CSIR-Institute of Himalayan Bioresource Technology, Palampur, H.P., and CSIR-National Botanical Research Institute, Lucknow, UP, India, for motivation of collaborating research to exchange required research facilities. Alka Kumari is grateful to DST for providing financial support under WOS-A scheme (SR/WOS-A/LS-117/2008).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumari, A. (2017). Fly Ash-Induced Metabolic Adaptations in Three Ferns. In: Shukla, V., Kumar, S., Kumar, N. (eds) Plant Adaptation Strategies in Changing Environment. Springer, Singapore. https://doi.org/10.1007/978-981-10-6744-0_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-6744-0_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6743-3
Online ISBN: 978-981-10-6744-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)