Abstract
Glow discharge mass spectrometry (GDMS) is a very sensitive technique to analyze elements in solid samples using glow discharge plasma. Samples serve as the cathode, and discharge cells serve as the anode.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Mass spectrometry
- Glow discharge
- Ionization
- Wide dynamic range
- Quantitative analysis
- Relative sensitivity factor
- Metal
- Trace element
36.1 Principle
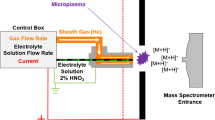
Glow discharge mass spectrometry (GDMS) is a very sensitive technique to analyze elements in solid samples using glow discharge plasma. Samples serve as the cathode and discharge cells serve as the anode. A glow discharge can easily be obtained by applying a direct potential to a sample surrounded by an inert gas, which acts as the plasma support gas. The sample surface is irradiated with ions of the plasma support gas accelerated by an electric field in the cathode dark region, and neutral atoms are sputtered. These atoms diffuse into the plasma and are ionized mainly by the electron impact process \( \left( {M + e^{ - } \to M^{ + } + 2e^{ - } } \right) \) and Penning ionization process \( \left( {{\text{Ar}}^{ *} + M \to M^{ + } + {\text{Ar}} + e^{ - } } \right) \), as shown in Fig. 36.1. Then, these ions are attracted to the anode and transported through a defining slit to a mass analyzer. Ions with only a given mass-to-charge ratio are transmitted through the mass analyzer, and the ion beam intensity is measured. The ion beam intensity of an analyzed element is normalized by that of the matrix element (or sometimes another reference element), and the elemental mass fraction is determined. As glow discharge plasmas are quite stable, a high reproducibility is expected. It is possible to analyze most elements in the periodic table, irrespectively of their fractions, from trace elements (~sub ppb level) to matrix elements, simultaneously. GDMS is usually applied to conductive materials such as metals and semiconductors in bulk. However, GDMS is also applicable to non-conductive materials by placing a metal mask containing an aperture on the surface of a non-conductive sample. The mask serves as the cathode. Conductive powder samples can also be measured by pressing the powder into an appropriate shape, as described later. In the case of non-conductive powders, the powders are first mixed with a conductive medium and then pressed into an appropriate shape prior to measurement. The technical specifications for GDMS operation have been reported as an ISO/TS and are thus available as a reference [1].
36.2 Features
-
Direct analysis of solid sample is feasible without any special pre-treatment before measurement.
-
High stability and reproducibility are attained.
-
As the dynamic range is quite wide, both trace elements and matrix elements can be analyzed simultaneously in a single specimen.
-
Quantitative analyzes within approximately one order of magnitude are possible without any standard samples.
-
Isotope analysis is possible.
-
Elements are ionized with electrons or meta-stable gases, as a result of which the appearance of few multivalent ions can occur, and the effects due to interference are reduced.
36.3 Instrumentation
A glow discharge mass spectrometer consists of a glow discharge cell and a mass analyzer. The sample, which serves as the cathode, is placed in the cell, and an inert gas plasma is maintained. For the mass analyzer, a double-focusing magnetic-sector instrument is conventionally employed to allow for high-resolution analysis. By increasing the magnitude of the magnetic field, ions with a larger mass-to-charge ratio can be transmitted through the analyzer, providing better mass separation. An electrostatic analyzer acts as an energy filter and transmits only ions with appropriate energies. A high resolving power of more than 4000 is commonly realized and is sufficient to overcome most interference effects.
The glow discharge plasma is also used for glow discharge optical emission spectrometry (GD-OES). Another type of mass spectrometry that employs a plasma source is inductively coupled plasma mass spectrometry (ICP-MS). In ICP-MS, samples are dissolved and introduced into the plasma with the solvent. Therefore, it is impossible to analyze solids directly by ICP-MS. By contrast, GDMS does not allow the analysis of liquid samples. Double-focusing magnetic-sector instruments are also used in secondary ion mass spectrometry (SIMS). These plasmas and mass spectrometry techniques have been reported elsewhere.
36.4 Measurements
36.4.1 Discharge and Ionization
The ion beam intensities observed strongly depend on both the plasma support gas and discharge conditions, which are determined by the current, potential, and gas pressure. Typically, instruments are operated under conditions of direct potential and direct current at 0.5–2 kV and 1–300 mA, respectively. The direct-current source may be operated under the constant current mode or constant potential mode. In both cases, the designed constant current or potential is achieved by adjusting the plasma support gas pressure. Ar is commonly used as the plasma support gas. However, helium is also used. The first ionization potential and potential energy in the meta-stable state of helium are high, which is conducive to measuring elements with high ionization energies such as N and O.
36.4.2 Samples
Samples should preferably be prepared in the form of pins or disks. Holders suitable for these shapes are available. The use of disk-shaped samples is expected to yield higher ion beam intensities. In the case of pin-shaped samples, the dependence of ion beam intensities on sample geometry may be high, as shown in the next section. Thus, disk-shaped samples are much more desirable.
36.4.3 Mass Analyzes
The mass fractions are determined by multiplying the ion beam ratios (IBRs) by relative sensitivity factors (RSFs). The RSFs are defined as follows:
where \( C \) is the mass fraction, \( I \) is the ion beam intensity, \( {\text{Abd}} \) is the abundance of the isotope, and suffices \( X \) and \( R \) indicate that the respective quantities are attributed to the analyzed and reference (usually, the matrix) elements, respectively. On the other hand, sputtering phenomena involve individual atoms, and thus, it is sometimes convenient to use atomic fractions. For this purpose, relative ion yields (RIYs) are also used, defined as follows:
where \( M \) is relative atomic weight and \( C^{{\prime }} \) is atomic fraction, and all other notation is the same as in the equation for RSF. The mass fraction of an element X in a single-element matrix sample can be obtained as follows, by setting \( C_{\text{R}} \) to unity:
where the reference element for RSFx is the matrix element. In cases where the RSFs for the matrix element are not available, it is possible to correct the IBRs using RSFs of a non-matrix element. GDMS instrument manufacturers usually provide RSF values, commonly in reference to Fe, but in principle, these values can be used for any matrix. The mass fraction of element X in a multi-element matrix sample can be described as follows:
where \( m \) is an abundant element (typically with a mass fraction of more than 1%).
The ion beam intensities and RSFs are affected by not only the discharge conditions, but also the sample geometry. Furthermore, RSFs may depend on the matrix elements and the fractions of the analyzed elements. Therefore, precise analyzes require the use of RSF values obtained using compositions similar to those of the analyzed samples. As an example of the dependence of RSFs on sample shape, the comparison of RSFs obtained using pin-shaped and disk-shaped samples prepared from the same bulk sample is shown in Fig. 36.2, the matrix element being Fe. It should be noticed that there exists a nearly linear relationship. Another example is shown in Fig. 36.3, where the dependence of the RSFs of pin-shaped samples on the sample length exposed to the plasma is plotted.
The ionization probabilities of the elements in the plasma strongly depend on their first ionization potentials. In general, for elements with smaller first ionization potentials, the ionization probabilities are expected to be higher and the RSFs tend to be smaller. The relationship between the first ionization potential and RSF is shown in Fig. 36.4 for an Fe matrix and Ar plasma support gas. As shown in Figs. 36.2, 36.3, and 36.4, the RSFs for most of the elements fall within approximately one order of magnitude, and thus, quantitative GDMS analysis in this range is possible without standard samples for calibration. This feature is quite convenient for surveying impurities in samples.
When two ions have the nearly same mass-to-charge ratio, interference will occur, which seriously affects the analysis results. However, this problem can be resolved in most cases using a magnetic-sector instrument with a resolving power of more than 4000. The resolving power is defined as \( \frac{M}{\Delta \,M} \), where \( \Delta M \) is the mass difference between two adjacent peaks and \( M \) is the mean mass of the two peaks or the mass of either peak. It is recommended to choose the most appropriate isotopes free of interferences and with high abundances. When Ar is used for the plasma support gas, an interference between Ca (atomic weight: 39.9625907) and Ar (atomic weight: 39.9623831) is expected, where \( {M \mathord{\left/ {\vphantom {M {\Delta M}}} \right. \kern-0pt} {\Delta M}} \) ≈ 193,000. In the case of Ca, no abundant isotopes exist (the abundance of the second abundant isotope of 44Ca is 2%). Therefore, for the analysis of trace Ca, it is recommended to employ a plasma support gas other than Ar. As an example, the interference spectrum between 28Si+ (atomic weight: 27.976927, mass fraction: 400 ppm) and 56Fe2+ (atomic weight: 55.93494, mass fraction: 70%) for a Nd magnet is shown in Fig. 36.5. Figure 36.5 also reveals that the appearance of multivalent ions is less probable, as the ionizations in GDMS consist of two independent processes, namely, sputtering and ionization, as shown in Fig. 36.1.
36.5 Applications
As GDMS is a relatively simple and stable method, high reproducibility can be expected, and the technique is widely applicable to trace element analysis of metals and semiconductors. It is indispensable for the detection of impurities in pure metals and alloys. However, a large area of the sample is exposed to the plasma, and atoms are sputtered from the whole exposed area. Therefore, it is difficult to measure impurity distribution, e.g., segregation on a flat surface.
The depth profiles of the sample composition can be determined through repeated measurements. Sputtering rates will be relatively large and magnetic scanning will be necessary. Thus, the high depth resolutions achievable through SIMS are not feasible in GDMS. However, in SIMS, only a target element can be measured, while in GDMS, all elements can be measured simultaneously. Figure 36.6a shows an example of a depth profile of multiple-Al-implanted SiC. Depth profiles for all atoms included are measured by a single operation. Figure 36.6b compares the depth profiles of Al obtained by GDMS and SIMS. Owing to the high sputtering rate, GDMS cannot be used for the analysis of surfaces and thin films.
(a) Depth profile for SiC and (b) comparison between GDMS and SIMS. Reproduced from Seiji Akahori, The TRC News, 201607-04 [2]. (Courtesy of Toray Reseach Center)
References
ISO/TS 15338:2009 Surface chemical analysis-GD-MS-Introduction to use
Akahori, S.: The TRC News, 201607-04 (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Saka, T. (2018). Glow Discharge Mass Spectrometry. In: The Surface Science Society of Japan (eds) Compendium of Surface and Interface Analysis. Springer, Singapore. https://doi.org/10.1007/978-981-10-6156-1_36
Download citation
DOI: https://doi.org/10.1007/978-981-10-6156-1_36
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6155-4
Online ISBN: 978-981-10-6156-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)






 : 2.0 mm,
: 2.0 mm,
 : 2.5 mm,
: 2.5 mm,
 : 3.0 mm). (Courtesy of Daido Bunseki Research)
: 3.0 mm). (Courtesy of Daido Bunseki Research)

