Abstract
Molecular motor proteins are amazing biological units that are responsible for the transformation of the chemical or biological components into mechanical works. These molecular machines express stronger energy conversion than man-made systems, which inspired scientists to pursue the target of improved performance of current synthetic devices. Thus, it is significant to explore interesting features of biomolecular motors, and design the novel intelligent platforms that mixed motor proteins with synthetic materials. Biomimetic molecular assembly enables the possibility for the in vitro reconstruction of biomolecular motors, further provides a variety of functionalized strategies. In this chapter, we gave a detailed introduction for one of the most familiar biomolecular motors, adenosine triphosphatase (ATPase), and deepen the understanding of their working mechanism and clarified how to conjugate ATPase with the artificially synthetic materials. In addition, some promising examples and significant comments were highlighted to display reconstructed performance of ATPase during this exploring voyage.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
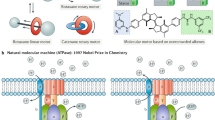
Biomolecular machines such as myosin, kinesin, dynein, and adenosine triphosphate synthase (ATPase) attract great research interests since they were initially discovered and observed [1,2,3]. ATPase is a class of biomolecular motor protein that is extensively present in mitochondria, chloroplast, prokaryotic algae, and photosynthetic bacterium [4, 5]. ATPase plays a range of fascinating roles in the energy metabolism, and is involved in physiological activity, catalytically synthesize basic energy unit, adenosine triphosphate (ATP). Various types of energy can be further generated from the hydrolysis of ATP molecules, and power most cellular motility, intracellular transportation, and physiological metabolic activity in the living organisms. ATPase has more than 100 types according to their distributed location and function, and mainly include Ca2+-ATPase, H+/K+-ATPase, H+-ATPase Na+/K+-ATPase, Mg2+-ATPase, Zn2+-ATPase, and so on [6,7,8]. These types of ATPases display great contribution in the exchange and transportation of various ions in biological entities, importantly balance the ion homeostasis in the intracellular organelles.
Proton-pumping ATPase is an energy-linked enzyme and can be responsible for the regulation of proton concentration, and play important role in phosphorylation. As the transmembrane protein, ATPase is served as a smart channel in biological membrane that the proton can go through [9]. The proton stream can trigger the conformational change of ATPase to spin, achieving energy conversion from the chemical energy into mechanical work [10]. Many researches demonstrate that energy-linked ATPase enzyme can be magically separated from biological entities and be incorporated into the synthetic systems for the improvement of their biomimetic properties [11]. Integration of photoactive components into ATPase-based system could endow the biohybrid materials with the function of light harvesting property and further energy conversion, resulting in the fabrication of light-responsive devices [12]. The ATPase incorporated synthetic materials own several inherent properties like the biological unit. Compare with the traditional materials, these novel biohybrid systems display amazing high-efficiency because of the introduction of natural components.
Miraculous structures in nature show their great interesting points, and exhibit much smarter than man-made materials. Actually, bionics not only promotes people to study the nature, but also enable the natural components to participate in the construction of hybrid materials [13]. In the microworld, these structures have their own advanced functions and elegant properties. Over past twenty years, scientists have devoted many efforts to develop novel biomimetic systems [14]. Several hybrid platforms made of synthetic materials and bioactive units have been presented, and they have significantly achieved the breakthrough on the modification and functionalization of traditional materials. In this chapter, we mainly focus on how to reconstitute F-type ATPase-based structures and explore their biomimetic performance in vitro. Molecular assembly of bionics has been proved quite useful in the design and preparation of functional bioengineered materials. This approach has great potential for the assembly of structured materials, and develops several assembled mechanism based on electrostatic interaction, hydrogen bond, covalent bond, and metal coordination [15]. The natural biomacromolecules extracted from biological entities significantly inspire the design of biohybrid materials and optimally integrate the biological properties with the synthetic materials. The appearance of ATPase enzyme-based hybrid materials meets the current energy topics to design the novel energy-linked materials and devices, significantly open a new perspective to perform the energy capture and further conversion.
2 ATPase Family: Types, Structure, and Components
ATPase is a class of transmembrane proteins made of several structured subunits, and widely exists in the living organisms [16]. According to the distribution, these types of ATPase are given peculiar properties and specific functions, and are responsible for the various ions transport and exchange, biological signal transmission, and energy transduction [17]. In cellular physiological process, ATPase enables many necessary metabolites for cellular metabolism to import, simultaneously take charge of the export of toxins and wastes. Such a kind of protein-based biomolecular machines are of vital importance in the biological entities. ATPase is a big family and has its own category, and can be systematically divided into three types: P-type ATPase, V-type ATPase, and F-type ATPase (Table 10.1).
P-type ATPases are a large group of evolutionarily related pumps to transport ions that widespread across many biological entities from bacteria to human, mainly reside in bacteria, fungi, archaea, and eukaryotic plasma membrane and organelles [18]. At present, P-type ATPases including more than 20 different types of structures have been discovered [19]. All P-type ATPases have a central domain with α-helical structure and perform the transportation of many metal ions (Mg2+, Ca2+, Na+, K+, Zn2+, Cd2+, Cu+, Hg2+) [20,21,22,23,24,25]. During the process of cations across the entire membrane, P-type ATPase uses its own two conformational regiments, called E1 and E2, to maintain the electrochemical gradient [26]. The generated conformational change effectively regulates the ions stream, significantly prevents the formation of back-flow state when the membrane opens the channel for ions to go through. Actually, the ions transportation process is accompanied with the energy consumption due to the change of conformational regiments that are triggered by the ATP hydrolysis. The P-type ATPase acts a smart housekeeper in the regulation of ions transportation during transmembrane behavior.
As one of the membrane-associated enzymes, vacuolar-type H+-ATPase (V-ATPase) plays an important role in a series of vesicles-mediated physiological activities [27, 28]. V-type ATP is a multimeric complex-like enzyme with remarkably diverse features and functions that resides in eukaryotic organisms and their organelles [29]. In the eukaryotic cells, V-type ATPases are energy-consumed proton pumps and are responsible for the acidification of cellular interior and the proton across the plasma membrane. During the proton delivery, ATP hydrolysis miraculously triggers the rotation of V-type ATPase to form a proton gradient, effectively regulates the pH value in the intracellular compartment and vesicular cell organelles [30]. V-ATPases are mainly composed of two structured parts, V1 domain and V2 domain [31, 32]. The V1 domain contains eight different subunits that are responsible to propel the rotation of V-type ATPase via ATP hydrolysis. The V0 domain has six different subunits that can transport protons to pass the membrane. Moreover, plasma membrane-associated V-type ATPase can be employed to participate in the type identification for various cells [33]. The subunits of the V-ATPase reside in certain cells that have a tissue-specific manner to express, resulting in the given information for the detection of cell types. Several exampled cells such as macrophages, tumor cells, osteoclasts, and insect goblet cells can be exactly distinguished because of that they have specific functions and process in the renal acidification, pH homeostasis, and tumor invasion.
F-type ATPases are usually known as the F0F1 ATPase, and also called ATP synthase (ATPase for short in the following text) or proton-translocating ATPase [34, 35]. ATPases are extensively found in chloroplast thylakoid membrane, mitochondrial inner membrane, and bacterial plasma membrane [36]. F-type ATPase is an energy-linked enzyme coupled with oxidative phosphorylation that perform fantastic task to produce the energy molecule, ATP, for cellular consumption [37]. Compared with P-type ATPase and V-type ATPase, F-type ATPase transports proton through the generated proton gradient across the biological membrane. More importantly, there is no energy consumption during transport process. Upon the electrochemical gradient, the rotation of ATPase is immediately triggered as the form of mechanical work, meanwhile starts the proton flux delivery. In exceptional circumstances, ATPase also can reversely rotate when the ATP concentration outside of membrane is pretty high [38]. In some bacteria, sodium ions can be instead of proton as the “fuel” to drive the rotation of ATPase [39].
Similar to V-type ATPase, F0F1 ATPase is also a transmembrane protein composed of two separate domains, extrinsic portion F1 and transmembrane portion F0 (Fig. 10.1a) [40]. F1 portion is a stator constructed by five subunits, α, β, γ, δ, and ε, which have many catalytic sites that are responsible for the ATP synthesis or ATP hydrolysis. F0 portion is a hydrophobic membrane-bond part across the biological membrane that contains three subunits named a, b, and c, and regulate the proton flow to start the rotation. The two portions are connected together by the bridge of central stalk (γ and ε). During the ATP synthesis, this rotary protein motor effectively transfers adenosine diphosphate (ADP) and phosphate into ATP molecules, exhibits marvelous conversion efficiency compare to the current man-made machines. The proton flows generate an electrochemical gradient through F0 portion that drives rotation of the F0 portion and the connected stalk (Fig. 10.1b), inducing the conformational changes of F1 portion that drives the catalytic synthesis of ATP [41]. Remarkably, this complicated enzyme can also perform ATP hydrolysis to drive the reverse rotation that pumps proton against the electrochemical gradient.
There are a lot of ATPases enriched in the thylakoid membrane of chloroplast and inner membrane of mitochondria. As energy-linked protein, ATPase not only participate the oxidative phosphorylation, but also carry out the conversion between chemical energy and mechanical works. In the plant cells, ATPase mainly couple with photosynthesis proteins to achieve various tasks or assistant process, such as ATPase synthesis, water photolysis, proton delivery, and electron transfer [42]. Mitochondria are the energy factory that possess high density of ATPase, and conduct the continuous ATP synthesis for the energy supply in cells [43]. Some prokaryotic algal and photosynthetic bacteria also can use their own ATPase to acquire the energy and further conversion [44].
3 In Vitro Assembly of ATPase Enzyme Mediated Devices
The bionics has been proved helpful since it was used to manufacture nature-designed structures. The biomimetic concept is not only learning from nature, but also inspire scientists employ the natural components to modify and improve the current assembled systems [45,46,47]. Recently, many reports talk about how to fabricate the biohybrid materials and devices composed of the naturally biological units and artificially synthetic systems. These introduced biological units have eye-catching work efficiency and favorable biocompatibility, which can significantly enhance the performance of traditional biomimetic materials and devices. Inspired from this, ATPase enzymes possess great potential to participate in the fabrication of rotation-based nanomachines. The scientists significantly introduce ATPase enzyme as the rotary motor to couple with several synthetic structures and achieve the biological components triggered rotation.
3.1 The Rotation of ATPase
ATPase is a motor protein with great investigation in detail, which has well definition in structure information and rotary mechanism. In 1997, Noji et al. [48] split F1F0-ATPase into two parts, and retained the F1 portion composed of γ subunit and αβ-hexamer. Subsequently, the bare γ subunit is coupled with streptavidin-conjugated actin filament, and the opposite side of αβ-hexamer is immobilized on the substrate by His-Tag (Fig. 10.2a). In the presence of ATP molecules, F1-ATPase starts to hydrolyze ATP due to no proton channel (F0 portion) and no proton concentration gradient (Fig. 10.2b). Thus, the fluorescent actin filament can spin because of that generated energy of ATP hydrolysis drives the rotation of γ subunit.
a The schematic diagram of F1-ATPase powered rotation of actin filament. b The crystal structure of F1-ATPase. c The time-lapse images of the rotating actin filament. Reproduced with permission from Ref. [48]. Copyright 1997, Nature Publishing Group
View from the membrane side, the filament is rotated in an anticlockwise manner more than 100 revolutions (Fig. 10.2c). This is the first time to use F1-ATPase motor for powering the rotation of employed structure, and also achieve the direct observation of single ATPase rotation. This approach opens a bright research aspect for biomolecular motor, and inspires the scientists devote many efforts to deeply investigate the rotary behavior of ATPase-based synthetic devices.
To use the rotation torque generated by ATPase, many synthetic nanoengineered structures have been introduced in this rotary platform for the further investigation. Several biohybrid nanodevices based on F1F0-ATPase which are sequentially developed and fabricated, and significantly promote the progress of molecular machines. Emergent fabrication techniques enable the desired structure feasible to acquire, for example, Soong et al. [49] prepare F1-ATPase-based rotator (Fig. 10.3a). This rotary nanodevice is immobilized on the Ni post. The Ni rod as the nanopropeller is assembled on the γ subunit side of ATPase via specific conjugation of biotin-streptavidin. Subsequently, ATP generated assay is immediately added to power the rotation of this nanodevice. To simplify the modified process of ATPase motor, the attached nanopropeller is directly conjugated on the bottom of F0 portion without dismantling any accessories of ATPase motor (Fig. 10.3b) [50]. The αβ-hexamer side is still connected with the substrate by His-Tag. Accompany with ATP molecules, this F1F0 ATPase exhibits graceful rotary performance in powering inorganic propeller.
In the previous study, the rod-like structures play important role on the act of attached propeller. Meanwhile, some other types of fantastically employed structures have been used to couple F1-ATPase, such as gold nanoparticle, dimeric particles and DNA double-strand linked gold nanorod [51,52,53,54,55,56]. These featured propellers equipped ATPases can be performed for further mechanical exploration and special tasks. As Fig. 10.3c shown, this rotary nanodevice performs the maximum work per 120° step, which is approximately equal to the thermodynamical maximum work that can be extracted from a single ATP hydrolysis under a broad range of conditions [55]. During the reversible rotation, the external torque and the chemical potential of ATP hydrolysis are both precisely controlled by the discrete 120° steps. Furthermore, the ATPase motor can also couple with double-strand DNA to develop the rotary biosensor. DNA double stranded is connected with the F1-ATPase to form a biosensor for the detection of the specific bridged gold nanorods (Fig. 10.3d), which enable the sensitive discrimination depend on the rotation, resulting in the sensitive limitation of one zeptomole [56]. Thus, these rotary nanodevices based on the manipulation of propeller components and conjugated technology can help to develop novel features of rotary nanodevices and enhance their complexity and functions, as well as inspire many biological applications.
3.2 ATP Synthesis in ATPase Incorporated Liposomes
Since the investigation of protein motors, F1F0-ATPase is the most familiar motor because of specific feature and structure. Due to proton gradient-responsive rotation, the ATPase motors have been employed to perform miraculous rotation through conjugating with the biomacromolecules. Liposome is a classical vesicle that can be composed of several polymers and lipids [57], and have well biocompatibility for the hybridization with the ATPase. Usually, an enclosed space is formed by liposomes to generate the proton gradient for the activation of ATPase rotation. Like the biological membrane in cells, the naturally isolated space is formed by lipid membranes, and effectively provides the proper condition for the generation of proton gradient. To construct biomimetic membrane, an artificial vesicles made of lipid mixture, porphyrin-naphthoquinone molecular triad (1, C-P-Q), and lipophilic quinone (Qs) is prepared and used for the incorporation of ATPase (Fig. 10.4a) [58]. This proton-pumping photocycle system can perform the photon-induced electron transfer and proton delivery in liposomes in the presence of visible light, resulting in the proton gradient for the ATP synthesis.
All F-types ATPase have the specific properties in transmembrane function and proton delivery, generating the proton gradient to produce ATP molecules. A designed 120 nm liposome is composed of phosphatidylcholine and 5 mol% phosphatidic acid. Each liposome contain 1.3 × 105 lipid molecules, valinomycin (~800 molecules), and one F1F0-ATPase from chloroplast (Fig. 10.4b) [59]. This chemiosmotic model system significantly demonstrates the scientific relationship between H+/ATP ratio and Gibbs free energy of ATP synthesis as a function of the changed pH and transmembrane electrochemical potential, and calculates the standard Gibbs free energies of ATP synthesis is 37 ± 2 kJ/mol (pH 8.45) and 36 ± 3 kJ/mol (pH 8.05), respectively.
Liposome-based sealed-cavity provides ideal research model to form the proton gradient, and play important role in the reconstitution of ATPase enzyme due to well biocompatibility. These amphipathic molecules not only form the hermetic structure for themselves, but also can be employed to encapsulate other materials for the regulation of proton concentration. With the development of biotechnology, several bioactive molecules could be introduced to enhance the diversity of ATPase mediated liposomes, such as functional proteins, polysaccharide, polymers, and DNA molecule. Many controllable mechanisms could be used to adjust permeability of biomembrane, and control the transmembrane behavior of the protons.
3.3 ATPase-Linked Polymeric Structures
Due to rapid development of modern biotechnology and nanoscience, it is possible to design and fabricate biohybrid functional materials. Layer-by-layer (LbL) assembled capsules are promising candidates and draw much attention in constructing hybrid systems [60,61,62,63,64]. LbL assembled capsules with adjusted size, shape, wall thickness, and permeability were prepared by alternative assembly of multilayer materials on particle templates and subsequently dissolving core templates [65, 66]. The hollow structure endows the assembled capsules to be an ideal transporter for diverse cargos, such as DNA, proteins, and drug molecules [67]. Because of the above remarkable advantages, it is expected that the assembled capsules can be considered as functional container to reconstitute rotary motor-based biomimetic systems.
In 2007, Li et al. firstly report the LbL assembled polyelectrolyte microcapsules coated by ATPase incorporated lipid bilayers that is used for ATP synthesis [68]. Compared to the previously reported strategy, this lipid membrane modified microcapsules provide an approach to simulate the real cell membrane, and also make the design and application of new biomimetic structural materials to be possible. As a membrane protein, F0F1-ATP synthase has been successfully reconstituted in the liposomes to build biomimetic membrane systems (Fig. 10.5a) [68]. The F0F1-ATP synthase hybrid LbL assembled poly(allylamine hydrochloride) (PAH)/poly(acrylic acid) (PAA) microcapsules are employed to simulate ATP biosynthesis process in the living cells. The proton gradient can be generated by the change of pH values, which plays a key role to generate ATP molecules and also decide the rate of ATP synthesis. ATP generating behavior in real cells can be simulated and reproduced by combining ATPase with the proton gradient. Consequently, F0F1-ATP synthase hybrid microcapsules can retain the biological activity of F0F1-ATPase, indicating that many other bioactive membrane-bound proteins can also be reconstituted using this method.
The reconstitution of ATPase in the LbL assembled polymeric structures. a The F0F1 ATPase in lipid-coated PAH/PAA microcapsules for ATP synthesis. b The reconstitution of F0F1-ATPase in lipid-coated Hb microcapsules. c GOD loaded polymeric microcapsule as the bioreactor for ATP biosynthesis. Reprinted with the permission from Ref. [72]. Copyright 2013, American Chemical Society; d ATPase incorporated polymeric nanoporous films for pH-responsive ATP synthesis. Reprinted with the permission from Ref. [73]. Copyright 2011, Royal Society of Chemistry
Glucose is always used in F0F1-ATPase microcapsules to obtain continuous proton gradient for ATP generation by the enzymatic catalysis of glucose oxidase (GOD) [69, 70]. The oxidation of glucose can produce gluconic acid and generate a proton gradient. Thus, the proton gradient can be maintained for a longer time by adding glucose and sustaining drives the biological synthesis of ATP molecules from the microcapsules. The microcapsules with ATP generation that are assembled by glutaraldehyde (GA) cross-linked hemoglobin (Hb) and F0F1-ATPase (Fig. 10.5b) [71]. Proton gradient between the interior and exterior of the F0F1-ATPase-microcapsule is generated by adding glucose and GOD solution, and it promotes the rotation of ATPase and induces the synthesis of ATP. To acquire the continuous proton gradient across the microcapsules, GOD as building block is directly used to fabricate F0F1-ATPase incorporated microcapsules. Once the glucose is injected, a proton gradient between the inside and outside of microcapsules is continuously generated by the catalytic hydrolysis of glucoses. This approach reveals that the amount of ATP production was continuously increased along with the continuous proton gradient. In other case, polymeric microcapsules can contribute their hollow structure to load GOD molecules that instead of being as the assembled materials (Fig. 10.5c) [72]. In addition, the two-dimensional polymeric film can also be incorporated with F1F0-ATPase to perform ATP synthesis (Fig. 10.5d) [73]. This smart film can effectively generate the proton gradient according to the manipulated pH value of two separated sides, resulting in the activation of ATP synthesis.
Hence, these rotating biological molecular motors, F0F1-ATPase, which can be assembled in the lipid membrane-coated microcapsules or two-dimensional film. Due to a proton gradient generation, the process of ATP production can be successfully performed. It is possible to use F0F1-ATPase-based systems for the storage of ATP and provide biological energy on demand.
4 External Field Modulated ATP Synthesis
ATP molecule is the basic energy unit in the biological entities, which enables physiological activity work on the regular running mode. The ATPases are a type of smart system in the biological entities and know how and when to start ATP synthesis or ATP hydrolysis on their own benefit. However, a critical issue gets great concern is how to control the in vitro performance of ATPase on demand. Rapid development of nanotechnology provides it possible to achieve the fabrication and manipulation in micro/nanoscale, and highly meet the requirement of ATPase modification and functionalization. Unlike in vivo, in vitro assembly of ATPase is not smart enough, resulting in that many driven mechanisms are used to regulate work performance of ATP synthesis. Currently, several external fields such as the magnetic field, light, and electric field, that promise an untouching approach to trigger on/off the ATP synthesis. This is really favorable to preserve the bioactivity of ATPase proteins. More importantly, it is a simple and valid method to achieve precisely remote manipulation.
4.1 Magnetic Field-Driven ATP Synthesis
Magnetic field is a most common phenomenon generated by the permanent magnet or electromagnetic effect. This inspires the fabrication and development of several magnetic materials composed of elements Fe, Co, and Ni, further develops the magnetism-responsive telecontrolled manner. Thus, scientists use magnetic field to propel the rotation of ATPase and produce ATP molecules [74,75,76,77,78]. During whole process, there is no proton gradient across the ATPase motor, and directly achieve the transfer from mechanical rotation into chemical energy.
Similar to the up-mentioned ATPases-powered rotator and propeller, ATPases are upside-down immobilized again on the substrate by His-Tag conjugation. Subsequently, many structured magnetic materials such as particle, dimeric particle, and rod, were conjugated on the side of γ subunit via biotin-streptavidin-biotin approach. The magnetic particle modified F1-ATPase can be rotated by using six steerable electromagnets, achieving a modulated ATP synthesis (Fig. 10.6a) [79]. In physiological conditions, ATP synthesis is an energy-consumed reaction that requires 80–100 pN nm energy. Using external magnetic field trigger ATPase for ATP synthesis that needs only about 30 pN nm of free energy to produce one molecule ATP because of no external energy consumption in local medium. Compared with the magnetic field manipulated by magnet, magnetic tweezers have better performance in flexible operation and high precision. The single-molecule manipulation and micromanufacture are integrated together to study the mechanochemical transformation of this system. The magnetic bead modified F1-ATPase is immobilized in femtoliter-sized hermetic chamber to synthesize ATP by using the rotating magnetic field (Fig. 10.6b) [80]. A clockwise rotating magnetic tweezers can trigger the ATP synthesis as usual. Interestingly, the “switch off” magnetic tweezers can immediately end the rotation, and induce ATPase rotate in anticlockwise to perform ATP hydrolysis due to high concentration of ATP molecules. It demonstrates that the “switching on/off” ATP synthesis/hydrolysis of ATPase motors can be flexibly regulated by the manipulation of magnetic tweezers.
The magnetic field triggered “switch on/off” ATP synthesis. a Magnetically driven rotation of F1-ATPase for ATP synthesis. Reprinted with the permission from Ref. [79] Copyright 2004, Nature Publishing Group; b Rotary magnetic tweezers manipulated ATP production. Reprinted with the permission from Ref. [80]. Copyright 2005, Nature Publishing Group; c Rotary magnetic field mediated approach for the behavior research of ATP hydrolysis. d The torque composed of plastic bead-connected Ni rod for the magnetically manipulated rotation. Reprinted with the permission from Ref. [82]. Copyright 2010, American Chemical Society
Magnetic field manipulated rotation also provides a strong tool to study biological behaviors or processes of ATPase during ATP hydrolysis. Inorganic phosphate (Pi) release, ADP release, and ATP binding are the important status and independent processes, respectively. The single-molecule imaging technology can directly observe the conformational change of ATPase to elucidate the abovementioned status. Scientists use the fluorescence-marked ATP molecule to research what happen in ATP hydrolysis process (Fig. 10.6c) [81]. To develop and improve this rotary nanosystem, many efforts have been devoted to fabricate series of magnetism-responsive propellers for the rotatory motors. A designated propeller is assembled by plastic bead and Au capped Ni rod which is connected on the side of γ subunit (Fig. 10.6d) [82]. Compared with other conjugated structures, this specific propeller can significantly enhance the resolution of rotary ATPase motor, and provide a strategy for the research of torque behavior during ATP synthesis.
The magnetic field propelled rotation of ATPase enzyme significantly achieves the regulation of ATP synthesis. More importantly, rotary process of ATPase enzyme can be successfully operated in clockwise or anticlockwise without proton gradient, resulting in a controlled transform between ATP synthesis and ATP hydrolysis. With the development of biotechnology and nanotechnology, high precise manipulation in single molecule gives the design sparks of diverse nanostructures for conjugation of ATPase rotary motor, and enables the energy conversion from mechanical rotation into chemical power.
4.2 Light Triggered ATP Synthesis
Sunlight plays an irreplaceable role in the living organism [83]. In the living plant cells or photosynthetic bacterium, ATP synthesis can be intelligently regulated by sunlight. Inspired by chloroplasts, several light-responsive proteins and enzymes are introduced to couple with ATPase for the controlled performance of ATP synthesis. Currently, many novel materials exhibit light-responsive behavior upon given wavelength, such as ultraviolet light, visible light, and near-infrared light [84,85,86]. Reconstituting smart platforms in vitro to simulate the natural biological process is very important to deeply understand the mechanisms of photosynthesis, and develop biomimetic materials.
To achieve the “on-demand” ATP synthesis and rotation, many efforts have been devoted to design the “open/close” feature of ATP synthesis for ATPase assembled architectures [87,88,89,90]. Photosynthesis in green plants can regulate ATP synthesis in the presence of light. Inspired by this, a light-induced ATP synthesis process is reconstructed by using a multiprotein inlaid polymersome system (Fig. 10.7a) [91]. ATP molecule is generated by coupled reactions between light-responsive bacteriorhodopsin (BR), transmembrane proton pump, and F0F1-ATPase, reconstituted in polymersomes. These inserted BR protein provide a light sensitive channel for the regulation of proton concentration in polymerliposome, resulting in the rotation of ATPase to produce ATP. This artificially hybrid proteopolymersome have great potential application in several fields ranging from the investigation of cellular physiology to the synthesis and assembly of bioinspired materials.
Light triggered the rotation of ATPase enzyme for ATP synthesis. a Bacteriothodopsin (BR) mediated ATP synthesis in the presence of light. Reprinted with the permission from Ref. [91]. Copyright 2005, American Chemical Society; b Ranaspumin-2 protein-based light-to-bioenergy converted platform. Reprinted with the permission from Ref. [92]. Copyright 2010 American Chemical Society; c Lipids-photosystem II-CaCO3 particle for the light-responsive ATPase synthesis. Reprinted with the permission from Ref. [12]. Copyright 2016, American Chemical Society
To acquire the enhanced ATPase synthesis in vitro, Montemagno and coworkers present an in vitro artificial photosynthesis platform that conjugates the necessary enzymes of the Calvin cycle with a nanosize photophosphorylation system engineered into a foam architecture by using the Túngara frog surfactant protein Ranaspumin-2 (Fig. 10.7b) [92]. This unique protein allows lipid vesicles and the coupled enzyme to concentrate in the microscale interlaced foam nets, which transforming the photon-derived chemical energy into carbon fixation and sugar production. Light-induced ATP synthetic process usually occurs in the thylakoid membrane of green plants. A light sensitive protein, photosystem II (PSII), is employed to construct F0F1-ATPase inserted proteoliposome-coated PSII-based microspheres by using molecular assembly approach (Fig. 10.7c) [12]. Upon light illumination, PSII can split water into protons, oxygen, and electrons and can generate a proton gradient for ATPase to produce ATP. This biomimetic platform gives a strategy to simulate the photophosphorylation process, and may facilitate the development of ATP-driven devices by remote light control. In addition, ATPase-based rotary system can be used in the biomedical aspect. This assembled rotary platform can be introduced to enhance thrombolysis along with the urokinase against the thrombus [93]. The δ-subunit-free F0F1-ATPase motor is obtained by reconstructing an original chromatophore, which is extracted from Rhodospirillum rubrum. The removal of δ-subunit-free aims to enhance the rotation of F0F1-ATPase motor. Upon the light illumination, the photosynthesis center triggers the generation of proton that results in the rotation of ATPase motor protein. After the targeting modification of anti-fibrinogen antibody, this ATPase-based nanodevice can act as a drill to mechanically destroy the thrombosis. Along with the urokinase, the introduced ATPase significantly accelerates the dissolution of thrombus in the presence of light.
Like the manipulation of magnetic field, light-induced ATP synthesis is a biological friendly approach that has the flexible controllability and high precision in manipulation. This strategy uses remote control to trigger on/off the rotation of ATPase enzyme without needing close contact. Significantly, it could remain the bioactivity of ATPase enzyme and enhance their work performance in vitro. The light triggered rotation of ATPase opens a door to regulate molecular machine for ATP synthesis, and devotes great contribution to develop the light-responsive materials and nanodevices.
4.3 Electric Field-Driven ATP Synthesis
In 1976, the first demonstration reports that an external electric field is used to impact the thylakoid membranes in the presence of ADP and 32Pi and results in the formation of 32P labeled ATP molecules [94]. Usually, the reconstituted ATPase structures for ATP synthesis are reported by the assembled approach. External electric field-driven ATP synthesis has both high time resolution and a flexible control across the membrane potential [95, 96]. Thus, it is a valid strategy for ATPase to investigate the kinetic behavior and energy conversion in the presence of external electric field.
Proton-driven rotary molecular motors can use potential energy generated by proton flux as the driving force. The transmembrane behavior of protons could produce electric potential gradient on both sides of the membrane. Proton delivery through the membrane is dependent on the F0 portion of F1F0-ATPase, inducing the rotation of ATPase motor. Usually, F0 portion contains a-subunit, b-subunit, and c-subunit. The ring-like c-subunit is responsible for the channel of proton transportation across the membrane that bridges the high potential and low potential (Fig. 10.8a) [97]. The generated potential gradient can generate a motive force to start the rotary transportation of proton. During cross membrane process of proton, a-subunit of ATPase plays an important role to generate an electric field. Like the particle in the electric field, proton can be manipulated by electric field in c-subunit to move from an electrode to another (Fig. 10.8b).
The mechanism of electric field-driven ATP synthesis. a Lateral view of F1F0-ATPase. b The distribution of electric field in a-subunit and c-subunit. Reprinted with the permission from Ref. [97]. Copyright 2013, Miller et al.
In the living organisms, membrane-bond ATPase always couples with several external fields to achieve the mechanical rotation and ATP synthesis or hydrolysis. Electric field is a better approach to simulate special environment and check the in vitro performance of ATPase-based complex. A rotary electric field is introduced to trigger the rotation of ATPase conjugated dimeric polystyrene beads with the diameter of 460 nm (Fig. 10.9) [98]. This specially designed propeller is a dielectric and biologically attached to the side of γ-subunit. The F1 portion is fixed on the Ni-NTA-coated coverslip substrate and accompanied with a rotary electric field. The rotating electric field is composed of four electrodes that own a frequency of 10 MHz is generated by using sinusoidal voltages. Due to the dielectric feature, the dimeric polystyrene beads can be rotated by the electrical rotary field. Then, the peak-to-peak voltage of the applied sinusoidal voltage is increased (Fig. 10.9 upper left) that results in dimeric polystyrene beads attached F1-ATPase rotate faster. Moreover, the rotary direction can be controlled by the reversing rotation of external electric field. The above strategy is the first electric field-driven ATPase-based rotary device.
At present, electric field-driven ATPase rotation is still in challenge so that many requisite efforts have been attributed to develop the optimized devices and deeply study the rotary mechanism. It is important to effectively use the generated potential energy for the further energy conversion, and reveal the detail of rotary molecular machine. To enhance the stability in the presence of electric field, many realistic models and auxiliary theories should be further developed. Also, diverse of attached structures can be designed and matched for ATPase to perform electric field-driven rotation. Electric field for the manipulation of ATPase rotation has smart features and great potential to achieve advanced transform from the electric energy into chemical energy or mechanical work.
5 Conclusions and Perspectives
Biomimetic materials-based assembly has attracted great concerns because of great biocompatibility and programmable feature on benefit. ATPase motor is a regulatory protein distributed in biological membrane that is responsible for the proton delivery across membrane. The molecular assembly of ATPase reconstitution employs bioactive enzyme and synthetic material to achieve construction of biohybrid materials. Based on the rotation of ATPase, many rotary nanodevices are miraculously designed to perform energy transformation between chemical energy and mechanical rotation, further develop external field manipulated approaches to regulate the rotation. In this chapter, detail introduction and promising examples are mainly discussed, and it strengthens the understanding of the biomolecular rotary motor.
In vitro reconstitution of ATPase inspires research sparks of bioactive matters and ultimately develops diverse assembled materials with fascinating functions. Scientists have successfully introduced biological macromolecules and synthetic structures as the attached propellers for ATPase. Consider the regulation of rotation, several strategies such as the magnetic field, light, and electric field that have been used to switch on/off the rotation of ATPase. Due to the own properties of propellers, manipulation of external fields possesses giant priority in controlling the rotation direction of ATPase with clockwise and anticlockwise, and then perform the conversion between ATP synthesis and ATP hydrolysis. Thus, this is significant to enhance the operated precision and enables the design of nanosize smart structures to make it possible to carry out various biological tasks. However, ATPase-based rotary systems suffer from technological barriers such as biological stability and bioactivity so that long-time work in vitro is expectant performance. These present limitations as breakthrough points can be deeply studied in the future research, and provide basic supports to achieve the construction of biomimetic devices in diverse applications.
References
van den Heuvel MGL, Dekker C (2007) Motor proteins at work for nanotechnology. Science 317:333–336
Hess H, Bachand GD, Vogel V (2004) Powering nanodevices with biomolecular motors. Chem Eur J 10:2110–2116
Hess H, Bachand GD (2005) Biomolecular motors. Nano Today 8:22–29
Junge W, Müller DJ (2011) Seeing a molecular motor at work. Science 333:704–705
Schliwa M, Woehlke G (2003) Molecular motors. Nature 422:759–765
Rensing C, Mitra B, Rosen BP (1997) The zntA gene of Escherichia coli encodes a Zn (II)-translocating P-type ATPase. Proc Natl Acad Sci USA 94:14326–14331
MacLennan DH, Brandl CJ, Korczak B, Green NM (1985) Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 316:696–700
Murata T, Yamato I, Kakinuma Y, Leslie AGW, Walker JE (2005) Structure of the rotor of the V-type Na+-ATPase from Enterococcus hirae. Science 308:654–659
Stock D, Leslie AGW, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286:1700–1705
Wang H, Oster G (1998) Energy transduction in the F1 motor of ATP synthase. Nature 396:279–282
Yasuda R, Noji H, Yoshida M Jr, Kinosita K, Itoh H (2001) Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature 410:898–904
Feng X, Jia Y, Cai P, Fei J, Li J (2016) Coassembly of photosystem II and ATPase as artificial chloroplast for light-driven ATP synthesis. ACS Nano 10:556–561
Pfeifer R, Lungarella M, Iida F (2007) Self-organization, embodiment, and biologically inspired robotics. Science 318:1088–1093
Dreyfus R, Baudry J, Roper ML, Fermigier M, Stone HA, Bibette J (2005) Microscopic artificial swimmers. Nature 437:862–865
Jia Y, Li J (2015) Molecular assembly of schiff base interactions: construction and application. Chem Rev 115:1597–1621
Junge W, Sielaff H, Engelbrecht S (2009) Torque generation and elastic power transmission in the rotary F0F1-ATPase. Nature 459:364–370
Møller JV, Juul B, le Maire M (1996) Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta 1286:1–51
Morsomme P, Chami M, Marco S, Nader J, Ketchum KA, Goffeau A, Rigaud A (2002) Characterization of a hyperthermophilic P-type ATPase from Methanococcus jannaschii expressed in yeast. J Biol Chem 277:29608–29616
Chan H, Babayan V, Blyumin E, Gandhi C, Hak K, Harake D, Kumar K, Lee P, Li T, Liu H, Lo T, Meyer CJ, Stanford S, Zamora KS (2010) The P-types ATPase superfamily. J Mol Microbiol Biotechnol 19:5–104
von Ballmoos C, Wiedenmann A, Dimroth P (2009) Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem 78:649–672
Veshaguri S, Christensen SM, Kemmer GC, Ghale G, Møller MP, Lohr C, Christensen AL, Justesen BH, Jørgensen IL, Schiller J, Hatzakis NS, Grabe M, Pomorski TG, Stamou D (2016) Direct observation of proton pumping by a eukaryotic P-type ATPase. Science 351:1469–1473
Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat A, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 38:1184–1191
Patrick ML, Aimanova K, Sanders HR, Gill SS (2006) P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol 209:4638–4651
Rensing C, Fan B, Sharma R, Mitra B, Rosen BP (2000) Cop A: an Escherichia coli Cu (I)-translocating P-type ATPase. Proc Natl Acad Sci USA 97:652–656
Axelsen KB, Palmgren MG (1998) Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 46:84–101
Bublitz M, Morth JP, Nissen P (2012) P-type ATPase at a glance. J Cell Sci 124:2515–2519
Weng X, Huss M, Wieczorek H, Beyenbach KW (2003) The V-type H+-ATPase in malpighian tubules of Aedes aegypti: localization and activity. J Exp Biol 206:2211–2219
Beyenbach KW, Wieczorek H (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209:577–589
Graham LA, Flannery AR, Stevens TH (2003) Structure and assembly of the yeast V-ATPase. J Bioenerg Biomembr 35:301–312
Recchi C, Chavrier P (2006) V-ATPase: a potential pH sensor. Nat Cell Biol 8:107–109
Sagermann M, Stevens TH, Matthews BW (2001) Crystal structure of the regulatory subunit H of the V-type ATPase of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 98:7134–7139
Hinton A, Bond S, Forgac M (2009) V-ATPase functions in normal and disease processes. Pflugers Arch-Eur J Physiol 457:589–598
Marshansky V, Futai M (2008) The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20:415–426
Lipfert J, van Oene MM, Lee M, Pedaci F, Dekker NH (2015) Torque spectroscopy for the study of rotary motion in biological systems. Chem Rev 115:1449–1474
Buchachenko AL, Kuznetsov DA, Breslavskaya NN (2012) Chemistry of enzymatic ATP synthesis: an insight through the isotope window. Chem Rev 112:2042–2058
Okuno D, Iino R, Noji H (2011) Rotation and structure of F0F1-ATP synthase. J Biochem 149:655–664
Abrahams JP, Leslie AGW, Lutter R, Walker JE (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370:621–628
Jr Kinosita K, Adachi K, Itoh H (2004) Rotation of F1-ATPase: how an ATP-driven molecular machine may work. Annu Rev Biophys Biomol Struct 33:245–268
Mesbah NM, Wiegel J (2011) The Na+-translocating F1F0-ATPase from the halophilic, alkalithermophile Natranaerobius thermophilus. Biochim Biophys Acta 1807:1133–1142
Schalley CA, Beizai K, Vögtle F (2001) On the way to rotaxane-based molecular motors: studies in molecular mobility and topological chirality. Acc Chem Res 34:465–476
Uchihashi T, Iino R, Ando T, Noji H (2011) High-speed atomic force microscopy reveals rotary catalysis of rotorless F1-ATPase. Science 333:755–758
Pain D, Blobel G (1987) Protein import into chloroplasts requires a chloroplast ATPase. Proc Natl Acad Sci USA 84:3288–3292
Senior AE (1973) The structure of mitochondrial ATPase. Biochim Biophys Acta 301:249–277
Sowa Y, Rowe AD, Leake MC, Yakushi T, Homma M, Ishijima A, Berry RM (2005) Direct observation of steps in rotation of the bacterial flagellar motor. Nature 437:916–919
Shu Y, Yue J, Ou-Yang Z (2010) F0F1-ATPase, rotary motor and biosensor. Nanoscale 2:1284–1293
Omote H, Sambonmatsu N, Saito K, Sambongi Y, Iwamoto-Kihara A, Yanagida T, Wada Y, Futai M (1999) The γ-subunit rotation and torque generation in F1-ATPase from wide-type or uncoupled mutant Escherichia coli. Proc Natl Acad Sci USA 96:7780–7784
Yasuda R, Noji H Jr, Kinosita K, Yoshida M (1998) F1-ATPase is a highly efficient molecular motor that rotates with discrete 120o steps. Cell 93:1117–1124
Noji H, Yasuda R, Yoshida M Jr, Kinosita K (1997) Direct observation of the rotation of F1-ATPase. Nature 386:299–302
Soong RK, Bachand GD, Neves HP, Olkhovets AG, Craighead HG, Montemagno CD (2000) Powering an inorganic nanodevice with a biomolecular motor. Science 290:1555–1558
Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto-Kihara A, Ueda I, Yanagida T, Wada Y, Futai M (1999) Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 26:1722–1724
Suzuki T, Tanaka K, Wakabayashi C, Saita E, Yoshida M (2014) Chemomechanical coupling of human mitochondrial F1-ATPase motor. Nat Chem Biol 10:930–936
Hayashi S, Ueno H, Shaikh AR, Umemura M, Kamiya M, Ito Y, Ikeguchi M, Komoriya Y, Iino R, Noji H (2012) Molecular mechanism of ATP hydrolysis in F1-ATPase revealed by molecular simulations and single-molecule observations. J Am Chem Soc 134:8447–8454
Bachand GD, Soong PK, Neves HP, Olkhovets A, Craighead HG, Montemagno CD (2001) Precision attachment of individual F1-ATPase biomolecular motors on nanofabricate substrates. Nano Lett 1:42–44
Furuike S, Nakano M, Adachi K, Noji H Jr, Kinosita K, Yokoyama K (2011) Resolving stepping rotation in thermus thermophilus H+-ATPase/synthase with an essentially drag-free probe. Nat Commun 2:233
Toyabe S, Watanabe-Nakayama T, Okamoto T, Kudo S, Muneyuki E (2011) Thermodynamic efficiency and mechanochemical coupling of F1-ATPase. Proc Natl Acad Sci USA 108:17951–17956
York J, Spetzler D, Xiong F, Frasch WD (2008) Single-molecule detection of DNA via sequence-specific links between F1-ATPase motors and gold nanorod sensors. Lab Chip 8:415–419
Safinya CR, Ewert KK (2012) Liposomes derived from molecular vases. Nature 489:372–374
Steinberg-Yfrach G, Rigaud JL, Durantini EN, Moore AL, Gust D, Moore TA (1998) Light-driven production of ATP catalysed by F0F1-ATP synthase in an artificial photosynthetic membrane. Nature 392:479–482
Turina P, Samoray D, Gräber P (2003) H+/ATP ratio of proton transport-coupled ATP synthesis and hydrolysis catalysed by CF0F1-liposomes. EMBO J 22:418–426
De Koker S, Hoogenboom R, De Geest BG (2012) Polymeric multilayer capsules for drug delivery. Chem Soc Rev 41:2867–2884
Donath E, Sukhorukov GB, Caruso F, Davis SA, Möhwald H (1998) Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew Chem Int Ed 37:2202–2205
De Cock LJ, De Koker S, De Geest BG, Grooten J, Vervaet C, Remon JP, Sukhorukov GB, Antipina MN (2010) Polymeric multilayer capsules in drug delivery. Angew Chem Int Ed 49:6954–6973
Peyratout CS, Dähne L (2004) Tailor-made polyelectrolyte microcapsules: from multilayers to smart containers. Angew Chem Int Ed 43:3762–3783
Tong W, Song X, Gao C (2012) Layer-by-layer assembly of microcapsules and their biomedical applications. Chem Soc Rev 41:6103–6124
Shao J, Xuan M, Dai L, Si T, Li J, He Q (2015) Near-infrared activated nanocalorifiers in microcapsules: vapor bubble generation for in vivo enhanced cancer therapy. Angew Chem Int Ed 54:12782–12787
Shao J, Xuan M, Si T, Dai L, He Q (2015) Biointerfacing polymeric microcapsules for in vivo near-infrared light-triggered drug release. Nanoscale 7:19092–19098
Such GK, Johnston APR, Caruso F (2011) Engineered hydrogen-bonded polymer multilayers: from assembly to biomedical applications. Chem Soc Rev 40:19–29
Duan L, He Q, Wang K, Yan X, Cui Y, Möhwald H, Li J (2007) Adenosine triphosphate biosynthesis catalyzed by F0F1 ATP synthase assembled in polymer microcapsules. Angew Chem Int Ed 46:6996–7000
He Q, Duan L, Qi W, Wang K, Cui Y, Yan X, Li J (2008) Microcapsules containing a biomolecular motor for ATP biosynthesis. Adv Mater 20:2933–2937
Duan L, Qi W, Yan X, He Q, Cui Y, Wang K, Li D, Li J (2009) Proton gradients produced by glucose oxidase microcapsules containing motor F0F1-ATPase for continuous ATP biosynthesis. J Phys Chem B 113:395–399
Qi W, Duan L, Wang K, Yan X, Cui Y, He Q, Li J (2008) Motor protein CF0F1 reconstituted in lipid-coated hemoglobin microcapsules for ATP synthesis. Adv Mater 20:601–605
Li J, Wang Y, Ha W, Liu Y, Ding L, Li B, Zhang S (2013) Cyclodextrin-based microcapsules as bioreactors for ATP biosynthesis. Biomacromol 14:2984–2988
Dong H, Nie R, Hou X, Wang P, Yue J, Jiang L (2011) Assembly of F0F1-ATPase into solid state nanoporous membrane. Chem Commun 47:3102–3104
Hirono-Hara Y, Ishizuka K Jr, Kinosita K, Yoshida M, Noji H (2005) Activation of pausing F1 motor by external force. Proc Natl Acad Sci USA 102:4288–4293
Saita E, Suzuki T Jr, Kinosita K, Yoshida M (2015) Simple mechanism whereby the F1-ATPase motor rotates with near-perfect chemomechanical energy conversion. Proc Natl Acad Sci USA 112:9626–9631
Adachi K, Oiwa K, Yoshida M, Nishizaka T Jr, Kinosita K (2012) Controlled rotation of the F1-ATPase reveals differential and continuous binding changes for ATP synthesis. Nat Commun 3:1022
Gaspard P, Gerritsma E (2007) The stochastic chemomechanics of the F1-ATPase molecular motor. J Theor Biol 247:672–686
Craighead H (2006) Future lab-on-a-chip technologies for interrogating individual molecules. Nature 442:387–393
Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M Jr, Kinosita K (2004) Mechanically driven ATP synthesis by F1-ATPase. Nature 427:465–468
Rondelez Y, Tresset G, Nakashima T, Kato-Yamada Y, Fujita H, Takeuchi S, Noji H (2005) Highly coupled ATP synthesis by F1-ATPase single molecules. Nature 433:773–777
Adachi K, Oiwa K, Nishizaka T, Furuike S, Noji H, Itoh H, Yoshida M Jr, Kinosita K (2007) Coupling of rotation and catalysis in F1-ATPase revealed by single-molecule imaging and manipulation. Cell 130:309–321
Palanisami A, Okamoto T (2010) Torque-induced slip of the rotary motor F1-ATPase. Nano Lett 10:4146–4149
Bang M, Chisholm P (2009) Living sunlight: how plants bring the earth to life. Science 326:1483
Danielson E, Golden JH, McFarland EW, Reaves CM, Weinberg WH, Wu X (1997) A combinatorial approach to the discovery and optimization of luminescent materials. Nature 389:944–948
Grinberg I, West DV, Torres M, Gou G, Stein DM, Wu L, Chen G, Gallo EM, Akbashev AR, Davies PK, Spanier JE, Rappe AM (2013) Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 503:509–512
Xuan M, Wu Z, Shao J, Dai L, Si T, He Q (2016) Near infrared light-powered Janus mesoporous silica nanoparticle motors. J Am Chem Soc 138:6492–6497
Inoue K, Ono H, Abe-Yoshizumi R, Yoshizawa S, Ito H, Kogure K, Kandori H (2013) A light-driven sodium ion pump in marine bacteria. Nat Commun 4:1678
Hoffmann A, Hildebrandt V, Heberle J, Büldt G (1994) Photoactive mitochondria: in vivo transfer of a light-driven proton pump into the inner mitochondrial membrane of Schizosaccharomyces pombe. Proc Natl Acad Sci USA 91:9367–9371
Luo T, Soong R, Lan E, Dunn B, Montemagno C (2005) Photo-induced proton gradients and ATP biosynthesis produced by vesicles encapsulated in a silica matrix. Nature Mater 4:220–224
Diez M, Zimmermann B, Börsch M, König M, Schweinberger E, Steigmiller S, Reuter R, Felekyan S, Kudryavtsev V, Seidel CAM, Gräber P (2004) Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nat Struct Mol Biol 11:135–141
Choi H, Montemagno CD (2005) Artificial organelle: ATP synthesis from cellular mimetic polymersomes. Nano Lett 5:2538–2542
Wendell D, Todd J, Montemagno C (2010) Artificial photosynthesis in ranaspumin-2 based foam. Nano Lett 10:3231–3236
Duan X, Liu L, Jiang W, Yue J (2015) Visible thrombolysis acceleration of a nanomachine powered by light-driving F0F1-ATPase motor. Nanoscale Res Lett 10:1–10
Vinkler C, Korenstein R (1982) Characterization of external electric field-driven ATP synthesis in chloroplasts. Proc Natl Acad Sci USA 79:3183–3187
Bauermeister H, Schlodder E, Gräber P (1988) Electric field-driven ATP synthesis catalyzed by the membrane-bound ATP-synthase from chloroplasts. Ber Bunsenges Phys Chem 92:1036–1039
Kaim G, Dimroth P (1998) Voltage-generated torque drives the motor of the ATP synthase. EMBO J 17:5887–5895
Jr Miller JH, Rajapakshe KI, Infante HL, Claycomb JR (2013) Electric field driven torque in ATP synthase. PLoS ONE 8:e74978
Watanabe-Nakayama T, Toyabe S, Kudo S, Sugiyama S, Yoshida M, Muneyuki E (2008) Effect of external torque on the ATP-driven rotation of F1-ATPase. Biochem Bioph Res Co 366:951–957
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Xuan, M., Jia, Y., Li, J. (2017). Reconstitution of Motor Protein ATPase. In: Li, J. (eds) Supramolecular Chemistry of Biomimetic Systems. Springer, Singapore. https://doi.org/10.1007/978-981-10-6059-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-6059-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6058-8
Online ISBN: 978-981-10-6059-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)