Abstract
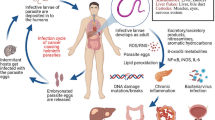
Parasitic infection remains as a persistent public health problem and can be carcinogenic. Three helminth parasites, namely, Clonorchis sinensis (liver fluke) and Opisthorchis viverrini as well as Schistosoma haematobium (blood fluke), are classified as Group 1 carcinogens by the World Health Organization’s International Agency for Research on Cancer (IARC Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis), World Health Organization, International Agency for Research on Cancer, 2011). Infection by these parasites is frequently asymptomatic and is thus rarely diagnosed at early exposure. Persistent infection can cause severe cancer complications. Until now, the cellular and molecular mechanisms linking fluke infections to cancer formation have yet to be defined, although many studies have focused on these mechanisms in recent years, and numerous findings were made in various aspects of parasite-associated cancers. Herein, we only introduce the fluke-induced cholangiocarcinoma (CCA) and bladder carcinoma and mainly focus on key findings in the last 5 years.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Cholangiocarcinoma and Liver Flukes
The small liver flukes O. viverrini and C. sinensis are helminths that can affect humans. These flukes are particularly prevalent in the Southeast and East Asia, particularly in countries such as Thailand, Lao PDR, Vietnam, and Cambodia [1,4,4]. These countries have a strikingly high incidence of CCA (hepatic cancer of the bile duct epithelium) and approximately 700 million people at risk of infection. Moreover, 10 and 35 million people are infected by O. viverrini and C. sinensis, respectively [2]. Thus, liver fluke infections are considered a serious global public health concern.
O. viverrini is represented in literature as a cause of hepatobiliary disease often referred to clinically as “opisthorchiasis,” particularly CCA [5, 6]. The disease is acquired by ingesting contaminated raw or undercooked freshwater fish or fish products, such as fish sauces and fermented fishes. The flukes reside in the small intrahepatic bile ducts and cause chronic inflammation and, eventually, CCA. Similarly, C. sinensis is considered as an agent of CCA. However, the detailed mechanisms in the pathogenesis of CCA caused by C. sinensis are less clear than those caused by O. viverrini. Meanwhile, infections caused by Opisthorchis felineus, another kind of liver fluke, have no observed association with CCA so far [7]. O. felineus holds a highly close phylogenetic relationship with O. viverrini. Moreover, the biology of the former and the medical and epidemiological implications of the related infection are suggested to be essentially identical to those of O. viverrini and C. sinensis. Thus, further studies are warranted to assess the contribution of opisthorchiasis to CCA.
O. viverrini is endemic in the northeastern region of Thailand, which has the highest worldwide incidence of CCA. This geographical coincidence typically supports the liver fluke–CCA causal relationship. Pathophysiological research demonstrated the capability of O. viverrini to induce cancer in artificially infected laboratory animals (hamsters) [8]. The main liver fluke–CCA causal mechanisms were observed to be involved in the mechanical damage caused by parasite activities and immunopathology due to infection-related inflammation. Each of these effects has several minor counterparts. In brief, liver flukes generate tumor microenvironments that result in CCA.
12.1.1 Risk Factors for Liver Fluke-Associated Cholangiocarcinoma
To date, studies that were able to attain the valuable risk factors of liver fluke-associated CCA are few, indicating that the disease factors are exceedingly complex. A meta-analysis found that liver fluke infection is significantly associated with cholangitis, cholecystitis, cholelithiasis, hepatocellular carcinoma, and CCA [9]. Moreover, heavy infection was observed to be significantly associated with high incidence of hepatobiliary pathological changes.
Apart from heavy infection, risk factors were reported to affect the liver fluke–CCA relation. Liver fluke infections may exhibit hepatobiliary abnormalities and chronic infection that may lead to CCA development. A study on a population in the northeast of Thailand found that the prevalence of O. viverrini infection has decreased because of a liver fluke control program that has been implemented over the decades [10]. However, the prevalence of PDF remained high. In 55,246 subjects, the overall prevalence of PDF was 33.0 %, and males were at higher risk in developing PDF than females. The study also showed that old age (≥70 years) and hepatitis B are associated with increased PDF risk. By contrast, the number of praziquantel treatments and diabetes mellitus cases were significantly associated with decreased PDF risk. Another report suggested that fermented food consumption can exacerbate cholangitis and cholangiofibrosis, which are risk factors for CCA-associated opisthorchiasis [11].
12.1.2 Cholangiocarcinoma-Associated Gene Variation
Genetic changes have been widely reported to be associated with liver fluke-related CCA. Ong et al. reported that whole-exome and targeted sequencing not only can verify frequent mutations in known CCA-related genes, including TP53 (44 %), KRAS (16.7 %), and SMAD4 (16.7 %), but also can identify somatic mutations in 10 newly implicated genes, including MLL3 (14.8 %), ROBO2 (9.3 %), RNF43 (9.3 %), PEG3 (5.6 %), and GNAS oncogene (9.3 %) [12]. Gene functions can be grouped according to the deactivation of histone modifiers, activation of G protein signaling, and genome stability loss. Another report compared CAA associated with O. viverrini with those not associated with O. viverrini. The study revealed mutations in novel CCA-related genes associated with chromatin remodeling (BAP1, ARID1A, MLL3, and IDH1/2), WNT signaling (RNF43 and PEG3), and KRAS/G protein signaling (GNAS and ROBO2) [13].
Genetic variations are frequently reported in liver fluke-related CCA. In addition, when the epigenetic changes and miRNAs were characterized in liver fluke-related CCA, they were found to hold potential as diagnostic or prognostic biomarkers. Sriraksa et al. reported that aberrant hypermethylation of a certain loci is a common event in liver fluke-related CCA and thus may potentially contribute to cholangiocarcinogenesis. In their results, a number of CpG islands (OPCML, SFRP1, HIC1, PTEN, and DcR1) showed frequent hypermethylation, and 91 % of CCA were methylated in at least one CpG island [14]. Furthermore, patients with methylated DcR1 exhibited significantly longer overall survival than those without. Runglawan et al. revealed that the levels of urinary miR-192 and miR-21 are higher in the risk group of subjects than those in healthy individuals. This result suggests that increased miR-192 and miR-21 levels in host urine may provide better predictive values in areas endemic for O. viverrini than they do in nonendemic regions [11].
Liver fluke-induced chronic inflammation plays a crucial role in cholangiocarcinogenesis through distinct signatures of genetic, epigenetic, and transcriptional alterations. These alterations indicate a unique pathogenic process in liver fluke-related CCA and thus may hold potential clinical implications in CAA diagnostics, therapeutics, and prevention.
12.1.3 Cholangiocarcinoma-Associated Parasite Proteins
Liver fluke-secreted proteins were previously shown to accelerate human cholangiocytes. The endocytosis of liver fluke proteins by host epithelial cells affects the pathways and induces the parasites to cause a highly devastating form of cancer, that is, CCA. Although the detailed mechanisms by which cells internalize liver fluke-secreted proteins remains unclear, recent studies implied that liver fluke proteins have a role in pathogenesis and highlighted an approach for vaccine development against this infectious cancer.
O. viverrini excretory/secretory products (OvESs) have been a focus of study. These products are especially internalized by biliary cells and postulated to be responsible for the chronic inflammation and proliferation of cholangiocytes. The physical attachment of O. viverrini to the biliary epithelium causes damage by releasing highly immunogenic OvES. When internalized preferentially by liver cell lines, OvESs induce liver cell proliferation and promote IL6 secretion [15].
Proteins secreted by O. viverrini accelerate wound resolution in human cholangiocytes. Smout et al. demonstrated that a gene encoding granulin-like growth factor (Ov-GRN-1), which was derived from recombinant O. viverrini, induces angiogenesis, an essential mechanism for malignant tumor development. In addition, Monica et al. showed that Ov-GRN-1 induces angiogenesis and accelerates wound healing in mice. In fact, wound healing and cancer progression have remarkable similarities, such as new blood vessel growth in a process called angiogenesis. Thus, determining the effect of Ov-GRN-1 in CCA progression is valuable [16].
Recent reports have highlighted the presence of secreted extracellular vesicles (EVs) in helminths. In particular, O. viverrini secretes EVs that induce a proinflammatory or tumorigenic phenotype in human cholangiocytes. Chaiyadet et al. demonstrated that O. viverrini EVs are released in the secreted products of carcinogenic liver flukes. These EVs are then internalized by cholangiocytes, subsequently driving cell proliferation and IL-6 secretion and promoting an inflammatory but simultaneously modulatory environment. These processes ultimately facilitate the CCA development [17].
Thioredoxin from O. viverrini was reported to inhibit the oxidative stress-induced apoptosis of bile duct epithelial cells and cholangiocytes. Immunolocalization revealed the presence of liver fluke thioredoxin within the cholangiocytes. The cells exposed to thioredoxin were observed to downregulate the apoptotic genes in mitogen-activated protein kinase pathway and upregulate antiapoptosis-related genes, including apoptosis signaling kinase 1, caspase 9, caspase 8, caspase 3, and survivin. These results suggest that O. viverrini thioredoxin can inhibit apoptosis and facilitate carcinogenesis. As such, thioredoxin may play an important role for liver fluke oxidoreductase in O. viverrini-induced CCA [18].
12.1.4 Immunopathology, Tissue Damage, and Cholangiocarcinoma
Cholangiocarcinoma (CCA) incidence related to chronic O. viverrini infection is a multifactorial process encompassing immunopathological mechanism, tissue damage, and signal pathway activation. Immune-mediated pathogenesis in response to liver fluke infection is a major driving force of CCA onset. Liver fluke-associated CCA involves different pro- and anti-inflammatory cytokines that may instigate cancer development. In O. viverrini infection, elevated total serum IL-6, and IL-6 production stimulated by O. viverrini in PBMC have been reported in infected individuals with advanced periductal fibrosis. Surapaitoon et al. reported 11 cytokine profiles, including those of IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, TNF-α, and LT-α, which were increased in CCA incidence associated with O. viverrini infection relative to the same profiles in uninfected normal controls. The results suggest the dysregulation of immune response in liver fluke-associated CCA [19].
Oxidative tissue damage caused by the free radicals released from effector cells has been observed to surround infected bile ducts. Thanee et al. suggested that the accumulation of CD44v suppresses p38MAPK expression in transforming bile duct cells and is linked to poor prognosis in CCA patients [20]. CD44 is a single transmembrane protein involved in cancer development and expressed in a wide variety of isoforms. Moreover, the expression of CD44v on a cell surface stabilizes xCT and promotes glutathione synthesis as defense against reactive oxygen species (ROS). This process enhances cancer development and increases chemotherapy resistance. Apinya et al. identified the oxysterols Triol and 3K4 as potential mediators of cholangiocarcinogenesis. Triol and 3K4 induce DNA damage and cell apoptosis via mitochondrial-dependent mechanisms. Chronic liver fluke infection increases the production of ROS/RNS when chronic inflammation occurs in the biliary system. Oxysterols and free radicals can induce biliary epithelial cell apoptosis. Ineffective DNA repair and persistent exposure to DNA damaging agents select for resistant cells that clonally expand to become malignant.
Signaling pathways involved in chronic inflammation regulate the progression of liver fluke-associated CCA. Yothaisong et al. reported a significant activation of the PI3K/AKT signaling pathway with PTEN suppression in human CCA tissues. Watcharin et al. suggested that the PKA signaling pathway and the switching of PKA regulatory subunits from Prkar2b/PKAII to Prkar1a/PKAI are involved in the development of CCA via altered cell transformation and proliferation [13]. Furthermore, the overexpression of Prkar1a can lead to the secretion of extracellular PKA (ECPKA) outside the CCA cells. Thus, the signal pathway activation may be responsible for the CCA development induced by O. viverrini. Targeting the components of these particular pathways may prove beneficial for the development of effective treatment, early diagnostics, and prevention strategies for CCA.
Inflammation is a well-known key component of tumor microenvironments. CCA is induced by chronic inflammation caused by the combination of several mechanical damage types. Immunosuppressive prednisolone was found to enhance early CCA in Syrian hamsters with liver fluke infection. This result suggests that host immune responses occur to ameliorate pathology and are also crucially associated with the pathogenesis of O. viverrini infection. Thus, imbalanced host immunity may enhance cancer-related inflammation.
12.1.5 Liver Fluke-Associated Microbiome and Cholangiocarcinoma
Microbial interaction with host cells can influence the health of the host considerably and has been implicated in liver fluke-associated CCA. The bacterial families Dietziaceae, Pseudomonadaceae, and Oxalobacteraceae are distinct microbiomes that dominate bile duct tissues. Chng et al. compared groups associated with those not associated with O. viverrini and then identified the enrichment for specific enteric bacteria (Bifidobacteriaceae, Enterobacteriaceae, and Enterococcaceae). They found that Bifidobacteriaceae was enriched and dominant in the O. viverrini microbiome and thus provided a mechanistic link to the parasite. Functional analysis revealed that altered microbiota increases the production of bile acids and ammonia in O. viverrini tissues and thus is linked to carcinogenesis [21]. These findings denote that parasitic infections and tissue microenvironments can influence each other and both can contribute to cancer.
12.2 Bladder Carcinoma and Blood Flukes
Schistosomiasis is a neglected tropical disease transmitted to humans from freshwater snails [22]. This disease is caused by a blood fluke of the genus Schistosoma. Schistosomiasis is considered as the most important helminthiasis that causes high rates of morbidity and mortality. Schistosomes affect at least 76 countries and 200–400 million people worldwide. S. haematobium is the causative agent of urogenital schistosomiasis and is responsible for two-thirds of the 200–400 million cases of human schistosomiasis worldwide [22, 23]. This infection is also associated with a high incidence of squamous cell carcinoma of the bladder, which is prevalent in the developing world [24, 25].
S. haematobium cercariae penetrate the skin and then transform into schistosomula. After infecting the subcutaneous tissue, the schistosomula enter the circulation and travel to the lungs and liver, where they achieve sexual maturity before entering into the vesical venous plexus. The eggs released from paired adults travel to the bladder, become trapped in the bladder wall, and release antigens and other metabolites. The eggs then lodge into the tissues and produce granulomatous inflammation that can lead to fibrosis [26, 27].
Long-term urinary schistosomiasis has been associated with the development of bladder cancer [28]. This disease is the leading cause of bladder cancer and occurs following years of chronic inflammation, fibrosis, and hyperproliferation. As a result, bladder cancer has become a significant health problem in the rural areas of Africa and the Middle East, where S. haematobium is prevalent [29, 30]. This information supports the association between malignant transformation and infection caused by this blood fluke. A study on an adult rural population in a Ghana region with endemic urinary schistosomiasis revealed the potential schistosome-associated bladder cancer problem and increasing association among age, severe bladder abnormalities, and occurrence of cancer biomarkers. Data on the epidemiological extent in different geographical areas estimate a schistosome-associated bladder cancer incidence of 3–4 cases per 100,000 [31]. This value suggests that schistosome-associated bladder cancer is an important public health concern in areas where S. haematobium is prevalent.
12.2.1 Bladder Carcinoma-Associated Parasite Genes
Bladder cancer is often difficult to diagnose without invasive measures, such as cystoscopy. However, benefitting from the development of molecular diagnostic techniques, some biomarkers were recognized as candidates for diagnosis and prognosis of this neglected tropical disease-linked cancer. In a recent study, liquid chromatography–mass spectrometry analysis was performed on urine from Angolans diagnosed with urogenital schistosomiasis and schistosome-associated bladder cancer. The metabolites were analyzed and expected to provide deep insight into the schistosome-associated bladder cancer. The analysis revealed numerous estrogen-like metabolites, including catechol estrogen quinones, CEQ-DNA-adducts, and novel metabolites derived directly from 8-oxo-7,8-dihydro-2′-deoxyguanosine, which were identified in urine of all patients [32].
A correlation was observed among the frequency of the biomarkers of bladder cancer associated with S. haematobium, those with p53, and sialylated glycans. The correlation highlights a missing link between infection and cancer development. The eggs of S. haematobium express sLea and sLex antigens in mimicry of human leukocyte glycosylation and thus may play a role in colonization and disease dissemination [33]. These results may facilitate early identification of infected patients at a high risk of developing bladder cancer and guide the future development of noninvasive diagnostic tests.
12.2.2 Bladder Carcinoma-Associated Parasite Proteins
Chronic infection with S. haematobium is associated with squamous cell carcinoma of the bladder. However, the molecular mechanisms underlying this association are poorly understood. Previous data revealed that the soluble extracts of mixed-sex adult S. haematobium worms (SWAP) are tumorigenic [22]. Moreover, the estrogen-related molecules in SWAP downregulate the estrogen receptors (ERs) in estrogen-responsive cells [22, 34]. Schistosome estrogens present in the sera of schistosomiasis patients repress the transcription of ERs in urothelial cells [34].
The soluble egg antigens of S. haematobium (Sh-SEA) exhibit potent tumorigenic capacity, because the S. haematobium eggs are in the developmental stage wherein they can directly cause urogenital disease during schistosomiasis haematobia. The findings confirmed that Sh-SEA can stimulate cell proliferation, reduce apoptosis, and increase the oxidative stress of urothelial cells. Furthermore, the presence of catechol estrogens in Sh-SEA might induce bladder cancer. These catechol estrogens are formed by a prospective estrogen–DNA adduct-mediated pathway in the S. haematobium eggs [22].
12.2.3 Immunopathology, Tissue Damage, and Bladder Carcinoma
Schistosomes elicit chronic inflammatory responses in both humans and mice. In S. haematobium infection, the prolonged inflammatory response is thought to contribute to the development of squamous cell carcinoma. Furthermore, the dependence of schistosomes on host factors for successful infection is evolutionarily conserved in S. haematobium. When infecting its host, schistosomes use common host mechanisms. In addition, schistosomes use immune signals for its development. The contributions of the host genes, which are discrete from immune system genes, must be understood, because these contributions are necessary for parasite establishment and development. Previous studies addressing the host–parasite interactions during schistosomiasis focused on a subset of immune response genes used to mount a Th1/Th2 response during infection. These genes include critical immune response genes, such as IL-4, IL-6, and IL-10, which control the Th1/Th2 response. In brief, regulatory pathways accommodate host permissiveness to schistosome establishment and productive schistosome infection and parasitism [35].
Schistosomiasis haematobia is a chronic infection. The adult and egg-producing schistosomes can live for many years. Thus, reinfections frequently occur and thus can lead to bladder cancer. To discuss the basic mechanisms that are potentially common in cancers, many studies focused on the role of both estrogens and ERs on the carcinogenesis associated with urogenital schistosomiasis. Botelho et al. observed a noteworthy elevation in estradiol serum levels [34]. They also observed that the serum levels of the luteinizing and follicle-stimulating hormones remained normal. Thus, they hypothesized that excess estradiol is external to the host. The molecule responsible for the effect may be an estradiol-like molecule derived from S. haematobium. This molecule is an antagonist of estradiol and thus repressed the transcriptional activity of the ERs. ER transcriptional activity was suppressed in urothelial cells, and ER expression was also inhibited in the mouse bladders in response to S. haematobium infection. These findings revealed the estrogen metabolism and ER signaling pathways associated with cancer induction in the context of S. haematobium infection [36, 37].
Another kind of hormonal and estrogenic molecule was identified in the Sh-SEA. The majority of these compounds are catechol estrogens, which are formed through the hydroxylation of steroid aromatic ring A. The hydroxylation of both C-2 and C-3 on a steroid ring is apparent and is subjected to further oxidation into an estradiol-2,3-quinone [38, 39]. The genotoxic effects of estrogen metabolites may be attributed to the oxidation of catechol estrogens to quinones, followed by redox cycling and formation of reactive oxygen species, which react with DNA. The metabolism of estrogens and production of depurinating estrogen–DNA adducts can be implicated in a pathway underlying host cell DNA damage promoted by S. haematobium and eventually lead to cell transformation. The carcinogenic effect of this estrogen–DNA adduct-mediated pathway may explain the link between chronic schistosomiasis haematobia and squamous cell carcinoma of the bladder [22].
At present, many mechanisms remain unclear, and further studies are necessary to understand how schistosomiasis haematobia leads to the squamous cell carcinoma of the bladder.
12.3 Other Parasite-Associated Cancers
Although certain cancer-related parasites belong to helminths, recent works have reported the association between unicellular protozoa and tumor. Despite its small size, protozoa can cause a series of diseases and public health problems worldwide. Their ability to induce tumor must be given sufficient attention. Herein, we list two kinds of tumors potentially caused by unicellular protozoa.
12.3.1 Toxoplasma and Brain Tumors
Toxoplasmosis is caused by Toxoplasma gondii and is a highly prevalent parasitic disease. This condition is estimated to affect a third of the world’s human population [40, 41]. Two recent studies highlighted a positive correlation between the prevalence of brain tumors and T. gondii at the national and international scales [42, 43]. Unfortunately, these studies are correlative, and the links between T. gondii and cancer are complex. Thus, a causality between T. gondii and brain tumors was not attained. Even so, further research could shed light on the possible mechanisms underlying this association.
12.3.2 Trichomonas and Prostate Cancer (PCA)
Trichomonas vaginalis is an extracellular flagellated parasitic protozoan that causes a relatively common parasitic sexually transmitted infection. The disease holds an annual incidence of over 3 million. The majority of infections is asymptomatic in men and thus is often undiagnosed and untreated. These untreated cases are hypothesized to promote chronic persistent prostatic infection and resultant urethritis and prostatitis [44, 45].
T. vaginalis was previously suspected to be associated with the development of PCA [46]. For decades, a couple of clinical studies seemed to support this correlation. However, two recent studies gave negative results. A study that included 146 men with advanced prostate cancer demonstrated that T. vaginalis serostatus is not associated with increased risk of metastatic or fatal prostate cancer (odds ratio <1) [47]. This result does not support the increased risk of advanced or fatal prostate cancer in men infected with T. vaginalis. In another study, Zhu et al. suggested that the culture supernatant of T. vaginalis inhibits prostate cancer growth by disrupting the proliferation and promotion of apoptosis [48].
Epidemiologic evidence for the association of T. vaginalis with prostate cancer is inconsistent, and the role of T. vaginalis in PCA development remains controversial. Thus, further study may help elucidate the association between PCA and T. vaginalis infection.
References
IARC. (2011) Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis), World Health Organization, International Agency for Research on Cancer
Petney TN, Andrews RH, Saijuntha W, Wenz-Mucke A, Sithithaworn P (2013) The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol 43(12–13):1031–1046
Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P et al (2012) The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int 61(1):10–16
Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A et al (2011) Opisthorchiasis and opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop 120(Suppl 1):S158–S168
Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C (2014) Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21(5):301–308
Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E et al (2012) The tumorigenic liver fluke Opisthorchis viverrini – multiple pathways to cancer. Trends Parasitol 28(10):395–407
Fedorova OS, Kovshirina YV, Kovshirina AE, Fedotova MM, Deev IA, Petrovskiy FI, et al. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: a review of medical statistics. Parasitol Int. 2016;S1383–5769(16):30236–30237
Wilcox BA, Echaubard P. Balancing biomedical and ecological perspectives in research framing of liver fluke and cholangiocarcinoma in NE Thailand. Parasitol Int. 2016;S1383–5769(16):30258–30256
Xia J, Jiang SC, Peng HJ (2015) Association between liver fluke infection and hepatobiliary pathological changes: a systematic review and meta-analysis. PLoS One 10(7):e0132673
Intajarurnsan S, Khuntikeo N, Chamadol N, Thinkhamrop B, Promthet S (2016) Factors associated with periductal fibrosis diagnosed by ultrasonography screening among a high risk population for cholangiocarcinoma in Northeast Thailand. Asian Pac J Cancer Prev 17(8):4131–4136
Silakit R, Loilome W, Yongvanit P, Thongchot S, Sithithaworn P, Boonmars T, et al. Urinary microRNA-192 and microRNA-21 as potential indicators for liver fluke-associated cholangiocarcinoma risk group. Parasitol Int. 2015;S1383–5769(15):00165–00168
Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W et al (2012) Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 44(6):690–693
Yothaisong S, Thanee M, Namwat N, Yongvanit P, Boonmars T, Puapairoj A et al (2014) Opisthorchis Viverrini infection activates the PI3K/ AKT/PTEN and Wnt/β-catenin signaling pathways in a Cholangiocarcinogenesis model. Asian Pac J Cancer Prev 15(23):10463–10468
Sriraksa R, Zeller C, El-Bahrawy MA, Dai W, Daduang J, Jearanaikoon P et al (2011) CpG-island methylation study of liver fluke-related cholangiocarcinoma. Br J Cancer 104(8):1313–1318
Chaiyadet S, Smout M, Johnson M, Whitchurch C, Turnbull L, Kaewkes S et al (2015) Excretory/secretory products of the carcinogenic liver fluke are endocytosed by human cholangiocytes and drive cell proliferation and IL6 production. Int J Parasitol 45(12):773–781
Botelho MC, Alves H, Richter J (2016) Wound healing and cancer progression in Opisthorchis viverrini associated cholangiocarcinoma. Parasitol Res 115(7):2913–2914
Chaiyadet S, Sotillo J, Smout M, Cantacessi C, Jones MK, Johnson MS et al (2015) Carcinogenic liver fluke secretes extracellular vesicles that promote cholangiocytes to adopt a tumorigenic phenotype. J Infect Dis 212(10):1636–1645
Matchimakul P, Rinaldi G, Suttiprapa S, Mann VH, Popratiloff A, Laha T et al (2015) Apoptosis of cholangiocytes modulated by thioredoxin of carcinogenic liver fluke. Int J Biochem Cell Biol 65:72–80
Surapaitoon A, Suttiprapa S, Khuntikeo N, Pairojkul C, Sripa B (2017) Cytokine profiles in Opisthorchis viverrini stimulated peripheral blood mononuclear cells from cholangiocarcinoma patients. Parasitol Int 66(1):889–892
Thanee M, Loilome W, Techasen A, Sugihara E, Okazaki S, Abe S et al (2016) CD44 variant-dependent redox status regulation in liver fluke-associated cholangiocarcinoma: a target for cholangiocarcinoma treatment. Cancer Sci 107(7):991–1000
Chng KR, Chan SH, Ng AH, Li C, Jusakul A, Bertrand D et al (2016) Tissue microbiome profiling identifies an enrichment of specific enteric bacteria in Opisthorchis Viverrini associated cholangiocarcinoma. EBioMedicine 8:195–202
Botelho MC, Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL et al (2013) Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: an oestrogen-DNA adducts mediated pathway? Int J Parasitol 43(1):17–26
van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD et al (2003) Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 86(2–3):125–139
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118(12):3030–3044
Hodder SL, Mahmoud AA, Sorenson K, Weinert DM, Stein RL, Ouma JH et al (2000) Predisposition to urinary tract epithelial metaplasia in Schistosoma haematobium infection. Am J Trop Med Hyg 63(3–4):133–138
Stavitsky AB (2004) Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun 72(1):1–12
Pearce EJ, MacDonald AS (2002) The immunobiology of schistosomiasis. Nat Rev Immunol 2(7):499–511
Mostafa MH, Sheweita SA, O'Connor PJ (1999) Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev 12(1):97–111
Zheng YL, Amr S, Saleh DA, Dash C, Ezzat S, Mikhail NN et al (2012) Urinary bladder cancer risk factors in Egypt: a multicenter case-control study. Cancer Epidemiol Biomark Prev 21(3):537–546
Salem S, Mitchell RE, El-Alim El-Dorey A, Smith JA, Barocas DA (2011) Successful control of schistosomiasis and the changing epidemiology of bladder cancer in Egypt. BJU Int 107(2):206–211
Shiff C, Veltri R, Naples J, Quartey J, Otchere J, Anyan W et al (2006) Ultrasound verification of bladder damage is associated with known biomarkers of bladder cancer in adults chronically infected with Schistosoma haematobium in Ghana. Trans R Soc Trop Med Hyg 100(9):847–854
Gouveia MJ, Santos J, Brindley PJ, Rinaldi G, Lopes C, Santos LL et al (2015) Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett 359(2):226–232
Santos J, Fernandes E, Ferreira JA, Lima L, Tavares A, Peixoto A et al (2014) P53 and cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with Schistosoma haematobium infection. PLoS Negl Trop Dis 8(12):e3329
Botelho MC, Alves H, Barros A, Rinaldi G, Brindley PJ, Sousa M (2015) The role of estrogens and estrogen receptor signaling pathways in cancer and infertility: the case of schistosomes. Trends Parasitol 31(6):246–250
Nair SS, Bommana A, Bethony JM, Lyon AJ, Ohshiro K, Pakala SB et al (2011) The metastasis-associated protein-1 gene encodes a host permissive factor for schistosomiasis, a leading global cause of inflammation and cancer. Hepatology 54(1):285–295
Botelho MC, Soares R, Vale N, Ribeiro R, Camilo V, Almeida R et al (2010) Schistosoma haematobium: identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Exp Parasitol 126(4):526–535
Botelho MC, Ribeiro R, Vale N, Oliveira P, Medeiros R, Lopes C et al (2012) Inactivation of estrogen receptor by Schistosoma haematobium total antigen in bladder urothelial cells. Oncol Rep 27(2):356–362
Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S et al (1997) Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci U S A 94(20):10937–10942
Cavalieri EL, Rogan EG (2011) Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol 125(3–5):169–180
Ewald PW (2009) An evolutionary perspective on parasitism as a cause of cancer. Adv Parasitol 68:21–43
Vittecoq M, Elguero E, Lafferty KD, Roche B, Brodeur J, Gauthier-Clerc M et al (2012) Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect Genet Evol 12(2):496–498
Thirugnanam S, Rout N, Gnanasekar M (2013) Possible role of Toxoplasma gondii in brain cancer through modulation of host microRNAs. Infect Agent Cancer 8(1):8
Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, Missé D (2012) Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol Lett 8(1):101–103
Dirkx M, Boyer MP, Pradhan P, Brittingham A, Wilson WA (2014) Expression and characterization of a β-fructofuranosidase from the parasitic protist Trichomonas vaginalis. BMC Biochem 15:12
Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC et al (2013) Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 40(3):187–193
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Shui IM, Kolb S, Hanson C, Sutcliffe S, Rider JR, Stanford JL (2016) Trichomonas vaginalis infection and risk of advanced prostate cancer. Prostate 76(7):620–623
Zhu Z, Davidson KT, Brittingham A, Wakefield MR, Bai Q, Xiao H et al (2016) Trichomonas vaginalis: a possible foe to prostate cancer. Med Oncol 33(10):115
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Feng, M., Cheng, X. (2017). Parasite-Associated Cancers (Blood Flukes/Liver Flukes). In: Cai, Q., Yuan, Z., Lan, K. (eds) Infectious Agents Associated Cancers: Epidemiology and Molecular Biology. Advances in Experimental Medicine and Biology, vol 1018. Springer, Singapore. https://doi.org/10.1007/978-981-10-5765-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-5765-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5764-9
Online ISBN: 978-981-10-5765-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)