Abstract
The mainstream of microfluidic chip research has transformed from the platform construction and method development to a wide range of applications. As a typical multiphase micro-functional unit, the droplet can be used as an independent microreactor with a volume ranging from pL to nL and formation rate up to thousands of droplets per second. It has the advantages of restricted diffusion, accelerated mixing, high heat transfer, effective mass transfer and so on. Droplet-based microfluidic technology has been emerged as a powerful tool to carry out high throughput screening and droplet manipulation research. Thus cell analysis, especially single cell analysis, and cell manipulation in droplets are easy and effective to be implemented with the assistance of a series of analytical methods. In this Chapter, we have briefly introduced an overview of droplet generation and corresponding principle by microfluidic chip. Besides, some usual methods coupled with droplet analysis have also been presented such as fluorescence analysis, mass spectrometry, capillary electrophoresis and others. Then we have mainly discussed the progress of droplets in cell analysis of recent decades. Finally, a summary and possible predication of droplet-based microfluidic chip are made at the end of this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The microfluidic technology, also known as Lab Chip, or Micro-Total-Analysis Systems (μTAS), has developed rapidly in last decades and became core tools of research in a broad range of fields, such as biology, medicine, pharmacy, tissue engineering , material science and so on [1,2,3]. Especially in cell biological studies, microfluidic technology can revolutionize and replace the traditional methods of utilizing multi-well plates and microscopy slides for the observation and analysis of cell cultures in vitro. The main reasons of such dramatical superiority are from giant advantages of microfluidic technology in cell research [4,5,6,7], including: (1) the sizes of microfluidic channels are usually range from 10 to 100 μm, which is almost commensurate with cell size and shape, making it benefit for the accurate manipulation and analysis; (2) the multidimensional mesh structure of microfluidic chips has formed sealed micro-environment, similar with cellular environment in vivo; (3) the requirement of cells and other reagents can be significantly reduced as the tiny space in microfluidic chips, thus saving much costs and sample consumption; (4) microfluidic chip can integrate diverse technologies and laborious research operations, such as sample preparation, introduction, cell culture, reaction, sorting, separation and detection, into a piece of intact chip, contributing to integration and automation of practical application in the future; (5) it can get abundant biological information via high-throughput assay; (6) the primary materials constructing microfluidic chips, such as PDMS (Polydimethylsiloxane), have good biocompatibility, good permeability and transparence, convenient for cell culture, observation and study; (7) high surface to volume ratios can accelerate energy transfer and often reduce reaction time, providing effective cell research environment. All of these inherent and extrinsic superiority make microfluidic technology expand fast and widely applied.
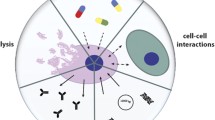
Droplet-based microfluidics , as one subcategory of microfluidics, is a newly developed technology for precise and accurate manipulation of many or single individual small volume droplet [8]. It not only possesses the advantages of microfluidic technology above mentioned, but also overcomes some shortcomings of conventional limitation, such as the restricted liquid mixing in the channel due to laminar flow driven pressure, cross contamination of samples easily happened in narrow space and so on [9]. At the same time, this technology also has a higher specific surface area, higher mass and heat transfer efficiency, shorter diffusion distance, less reagent consumption and other merits. Two immiscible phases, such as water and oil, are utilized to produce discrete tiny droplets of femoliter to microliter volumes, with a high speed up to 20,000 per second [10]. One phase is named as continuous phase (also mobile phase), and the other is dispersed phase. Their roles and compositions can be exchanged depending on purpose of study. Unlike other microfluidic continuous-flow systems, droplet-based microfluidic technology creates a clear separation space between droplets, thus to isolate and obtain smaller, easier to control, independent but intact rooms for cell research. Since a large number of micro-chambers can be produced in a very short time, parallel processing and experiments can be easily achieved, which provides the basis for obtaining large amounts of data. Hence, droplet-based microfluidic technology is also more superior than the common microfluidic technology in high-throughput study and analysis. As mentioned reasons, droplet-based microfluidic technology is widely used in enzyme kinetics research and analysis, protein crystallization, nanoparticle and molecular synthesis, single cell analysis and so on (Fig. 7.1). In the whole process of droplet-based microfluidic technology, a series of functions such as micro-droplet generation, transport, split, fusion, sorting , positioning, capture and control of two-phase behavior, can be realized easily and combined with other analytical detection techniques for further study (Fig. 7.2) [13,14,15]. Therefore, it has been developed rapidly in recent years and become one potential trend of microfluidic technology.
Some main applications of droplet-based microfluidic technology (Reprinted with permission from Ref. [11])
A whole of unit operations for droplet microfluidics. a–j droplet generation; mixing and generation; fusion; short-term incubation; stationary storage; detection; sorting; re-injection; splitting and off-chip incubation (Reprinted with permission from Ref. [12])
Of course, some shortcomings not beneficial for cell culture and research also exist in droplet-based microfluidic technology. For example, the interior of a microdroplet can provide a really suitable physical and chemical environment, but an interfacial tension is created at its surface due to direct contacting with the continuous phase, which may have an adverse effect on cell growth [16]. Thus, Baroud et al. separated the continuous phase from the microchannel to reduce the side effect of interfacial tension on the droplet interior [17]. Another aspect is the small amount of consumption and use of the reagents and cells in microfluidic droplet technology, which reversely bothers the detection of certain trace substances and needs high technical requirements compared with traditional methods. It desires for appropriate technology of analysis and detection to make further progress and development to satisfy the demands. So far, droplet-based microfluidic technology has already been combined with a variety of technologies, such as electric field, surface acoustic wave, for cells sorting and screening, as well as some detection technologies such as fluorescence , mass spectrometry, raman spectroscopy, electrophoresis , PCR (polymerase chain reaction), and electrical detection methods, for the detection and analysis of cells and related substances. Schmid et al. combined surface acoustic wave with micro-droplet technology to achieve sequential droplet sorting at high speeds up to 3000 per second without large shear forces production [18]. Mazutis et al. severally injected cells and green fluorescent protein-conjugated antibodies from two wells of a Y-shaped structure to produce droplets of packaging cells and antibodies. As a process of culture and fluorescent screening, they have detailed a binding assay for detecting antibodies secreted from single mouse hybridoma cell [19]. Hatch et al. utilized the combination of droplet microfluidics with PCR technology to manufacture a continuous, high throughput, automated portable devices for realizing multichannel and simultaneous fluorescence imaging [20]. With the constant development and progress of these means and the emergence of new technologies, droplet-based microfluidic technology will play a greater role in cell analysis and achieve more effective analysis through mutual combination.
Taking into account of above favorable and unfavorable factors, droplet-based microfluidic technology have mainly focused on cell manipulation , metabolite analysis and single cell analysis in cell research at present. The ability to generate discrete droplets via droplet-based microfluidic technology provides a new strategy for the studies of cell-cell interactions as employment of different designs in microfluidic chip, or formation of three-dimensional tumor spheroids by using some special materials such as gel. Sabhachandani et al. have reported a method to obtain three-dimensional spherical tumor of MCF-7 (human breast cancer cell) and co-cultured with HS-5 lactococcus lactis by individual injecting cells contained alginate solution and Ca2+ solution into the channel from both sides of the T-shaped structure chip, thus to produce the cell-loaded monodispersed calcium alginate droplets [21]. It’s capable to produce single cell droplets quickly and efficiently by proper adjustment of flow rate and concentration as the high flux and small volume in droplets generation. Brouzes et al. conducted droplets fused via utilizing multiple droplets generated microfluidic structures, allowing single cells to be cultivated for several days in different drug environments, then to implement the cytotoxicity assays and analysis using the same method [22]. Droplet-based microfluidic technology has special and dramatical advantages of simple, fast, cheap, efficient and no aids of aptamers and antibodies in single cell generation when compared with other methods, so most of the studies have focused on single cell analysis in the field of cell research.
Hence, we will mainly review the development and progress of droplet-based microfluidic technology in cell analysis over the years, to summarize its advantages and shortcomings, and to look forward to its potentials and prospects in future. This chapter is mainly introduced in the following sections: principles of droplet generation, methods for droplet generation, analytical methods in droplet microfluidic, single cell analysis and cell manipulation .
7.2 Principles of Droplet Generation
It’s very simple to generate continuous droplets via use of microfluidic chips today after years of study and development. The earliest droplet generation dates back to 1950s, when de Fonbrune sprayed water droplets into mineral oil to produce stable droplets in 1947, and some similar approaches are still in use nowadays [23]. Subsequent Lederberg and Nossal have successfully encapsulated single cells with droplets through modified methods and proved the rule that a cell would only produce an antibody [24]. The most common methods for generating continuous droplets today are based on the use of geometrical structures such as V-shaped crossings or T-shaped crossings, to severally inject two mutually immiscible liquids at high speed, producing droplets of high flux. The basic principles of these methods are almost similar. As soon as the two immiscible liquids come into contact, the interface are formed. One of the liquid phases would be self-dispersal into discrete droplets and surrounded by the other liquid phase due to the role of surface tension [25]. It mainly depends on the liquid phases, flow velocity and the surface energy of the channel to decide the affiliation of monodisperse droplet. For example, the aqueous phase become dispersed phase when oil and water are used for two liquid phases and typically hydrophobic PDMS as channel. Whereas, the oil phase may change into dispersed phase if the hydrophobic channel is hydrophilic modification [26].
The droplets also need to meet some specific characters in practical applications although easy formation, just as droplets for cell encapsulation, including whether the size of the droplet is appropriate and uniform, the shape meets the demand, the composition and distribution are the identical, and the monodispersity reaches the standard [27]. Some droplets, also named smart droplets, can specifically receive external stimulation, such as temperature, pH, UV irradiation, magnetic field and electric field, and respond in an active manner, by combining smart or intelligent materials (Fig. 7.3). The formation and stability of droplets are often the reflection of the balance among inertia force, viscous resistance, shear force, buoyancy force and interfacial tension. In addition, particularly high surface area to volume ratios caused by the limitation of small sizes of the microchannel and droplet, and the boundary effect in the channel make the behavior of the droplet peculiarly complicated. Some parameters are commonly used for describing the microfluidic kinetic of droplet formation, such as surface tension , Reynolds number, Weber number , Bond number , capillary number , surfactant effect and so on [25].
Explanation and examples of five smart droplets. 1 droplet size decreases with increasing temperature; 2 droplets are positively charged at pH 4 and becoming neutral at pH 9; 3 two droplets are fused together under the exposure to UV light; 4 and 5 are magnetically induced particles and electrically induced particles (Reprinted with permission from Ref. [30])
7.2.1 Surface Tension σ
The surface tension σ is a kind of tension acting on the surface due to the imbalance of molecular attraction and quantized in units of newton per meter (N/m) or joules per square meter (J/m2). The surface tension can greatly influence the droplet behavior because the interface area is totally surrounded by the continuous phase, leading to large interfacial area and dramatically strong force. So the droplets will decrease the interface area as much as possible to reduce the surface energy in the presence of surface tension. In theory, the droplet should be the size of circular when its surface energy performs minimal. But the droplets in the microfluidic chips have been found to be tend towards a certain oval shape rather than totally circular in the actual process, possible the cause of small size of the channel and the boundary effects [28].The curvature of the interface can lead to pressure jump, named Laplace pressure (ΔP), between the outside and inside of the interface as the deformation of the droplet interface curvature. One formula is used to describe the Laplace pressure, as \(\Delta {\text{P}} =\upsigma\left( {\frac{1}{{{\text{R}}_1}} + \frac{1}{\text{R}_2}} \right)\), where R1 and R2 are the radius of curvature at different locations in droplet interface, and have inverse proportion to Laplace pressure. The interfaces of droplets have elasticity under the effect of ΔP. This property is used to carry out the droplet control and manipulation in recent years, such as droplet capture, merger and dilution [29].
7.2.2 Dimensionless Numbers
The dimensionless number is an important parameter for discrimination of different fluids, main including Reynolds number, Weber number, Bond number and capillary number .
-
(1)
Reynolds number (Re), is a dimensionless number to characterize fluid flow through a chip channel. Re = ρUl/μ, where ρ, U, μ and l represent mass density, characteristic velocity, dynamic viscosity and characteristic length, respectively. Re is the ratio of the inertia force to the viscous force, and a value of Re ≤ 2300 indicates that the liquid is a laminar flow under selected conditions. In general, the Re of fluid in the microfluidic channel is small, which means negligible inertial force and the regular fluid condition [31].
-
(2)
Weber number (We), is used to describe the movement of the fluid. We = ρU2 l/σ, where ρ, U and σ represent the fluid density, the characteristic velocity of the flow and surface tension. Weber number represents the ratio of the inertial force to the surface tension . The droplet is likely to be disintegrated if the Weber number is larger than a certain critical value when droplets are formed by droplet-based microfluidic technology, and the droplet will be deformed but not broken if the Weber number is at a moderate value [32].
-
(3)
Bond number (Bo) , is the ratio of gravity to surface tension . Bo = ∆ρgl2/σ, g and ∆ρ represent acceleration of gravity and density difference between the two liquids, respectively. Bo is usually much smaller than 1 at the microscale, meaning that the effect of gravity can be ignored.
-
(4)
Capillary number (Ca), also named as the interfacial tension number , is defined as the ratio of viscous stress to surface tension. Ca = μcU/σ, where μc is the fluid viscosity, and U is the velocity, and σ also represents the surface tension. A low value of Ca means that the surface tension are dominant over viscous stress, and the droplets perform spherical to reduce the surface area and energy. While a high value of Ca appears opposite meaning that the viscous stress plays a dominant role, and the droplets will become deformation by the flow and present asymmetric structure [33].
From the definition of above, we can discover that the Reynolds number, Weber number and Bond number have small effect on droplet generation in most cases, while the surface tension and capillary number own a dominate role on the micrometer scale of microfluidic technology. Furthermore, other factors have also contribute their efforts, such as the hydrophilic of channel, boundary effects and so on. It is worth noting that the design of the microchannel still plays an important role in the generation of droplets.
7.3 Methods for Droplet Generation
There are many methods have been developed for droplet formation, the core of droplet-based microfluidic technology, after extensive and years of study. In general, these methods can be categorized into two groups, passive and active approaches. The passive methods are mainly based on different microfluidic geometries, such as T-junction, flow focussing structure and co-flowing structure, utilizing the principle of hydrodynamics for droplet formation [34, 35]. This method is simple, efficient and fast, so it is widely studied and used, but the flexibility of droplet is relatively poor as limited by channel geometry [36]. The active approaches are primarily driven through the control of external field to generate droplets, such as pneumatic drive, optical drive, thermal drive, electric field drive and other methods [30]. People can control the size and frequency of the generated droplets well by this method without totally dependence of chip structure, but the velocity of droplet production is very slow. We will mainly focus on the passive approach since it’s the most common configuration of droplets formation.
7.3.1 T-junction
T-junction are the most commonly used microfluidic geometry for droplet generation (Fig. 7.4a). Thorsen firstly used T-junction to generate droplets in 2001 [37]. In this configuration, the main channel, injected with the mobile phase, and the side channel, introduced with the dispersed phase, are intersected at focused zone in a perpendicular way to create droplets, under the effect of surface tension and shear stress. At the same time, two asymmetric forces, upstream water pressure of the side channel and the pressure of the mobile phase in the main channel, together with the surface tension, can cut off continuous dispersed phase to single and independent droplets [38]. A brief summary of the droplet formation process is dropping, spraying, and deformation. The frequency and size of generated droplet can be effectively regulated and controlled by adjustment of the flow rate, channel size and liquid viscosity. For example, increasing channel width of the downstream can effectively accelerate the rate of droplets formation and improve the stability of droplets. And multi-wrapped droplets can be achieved through reasonable use of multiple T-junction (Fig. 7.4b) [39]. However, cells encapsulated in droplets will be greater effected due to the asymmetry force in droplet generation process, so T-junction is most commonly used in other aspects but not cell analysis.
Common methods of generating droplet. a The two most commonly used methods of passive generation droplets are the T-junction and the flow focussing, and the less commonly used co-flowing method (Reprinted with permission from Ref. [40]). b A double T-junction cross structure to obtain droplets of oil/water/oil structure (Reprinted with permission from Ref. [39]). c Pulsed laser-generated holes promote droplets on demand (Reprinted with permission from Ref. [41])
7.3.2 Flow Focusing Structure
In flow focussing structures , three channels, one main channel and two symmetric side channels, are focused on a narrow region connecting the downstream channel (Fig. 7.4a). In brief, the dispersed phase is injected into the side channels, while the continuous phase is introduced into main channel, which approach the confluence at the straight line in the downstream channel. The advantage of this design is that the dispersed phase in focused zone will only bear the driving force on the main channel and force from other directions would be counteracted as the symmetrical structure of side channels, leading to less affected and more stable droplets. It can be seen that the size of the droplet is related to the velocity ratio of the two phases. The larger the flow rate of the mobile phase is when compared with the dispersed phase, the smaller and faster the generated droplet is. In addition, the size of the focusing region, the viscosity of the liquid can also influence the formation and size of droplets. Similarly, the application of multiple flow focussing structures is designed to obtain multilayered droplets, which are often used for cell co-culture in cell analysis [42]. However, some small satellites droplets are often produced accompanied with droplets generation in this process, which greatly reduces the monodispersity of the whole system. So some control methods, such as screening systems, adjusting the flow rate, selecting the appropriate two phases and designing appropriate focused zone, have been studied to control the non-uniformity [43].
T-junction and flow focussing structure are the basic structures for droplet formation in droplet-based microfluidic technology. Different designs are usually implemented in the actual process to meet the needs of different research. Song et al. vertically connected the downstream channel in flow focussing structures with another main channel to form a second T-junction, thereby achieving the purpose of mixing three reagents in one droplet [44]. Adamson et al. integrated the T-junction with the dendritic bifurcation channels to generate multiple droplet arrays [45]. Both methods are simple, fast and easy to be achieved, so they have been widely used in droplet based microfluidic technology.
7.3.3 Co-flowing Structure
The co-flowing structure has utilized a drive pipe to create interface between two co-flowing immiscible fluids (Fig. 7.4a). That is to say, the dispersed phase flows into the inner channel while the outer channel is mobile phase. One serious disadvantage of this structure is that the dispersed phase has suffered strong viscous shear force of continuous phase for its roomy diameter. As the continuous phase-touched droplets march forward, their surface tension force have counteracted with drag force, thus to pinch the droplet off from the continuous phase. Compared with other two methods, this approach is complex and difficult to a certain extent, and strong uniform forces in the process of generation make it unusually applied for cell research.
7.3.4 Other Methods
Though some methods to generate droplets, such as air pressure driving, light driving, heat driving and electric field driving, are not convenient, fast and high flux as the former methods, they also attract a lot of research due to the stable size and controllable frequency of the droplets [46]. The basic principle of these methods is that the dispersed phase is pinched into the mobile phase to form droplets by the power of regular, controlled, continuous pulse pressure, which is applied to the dispersed phase near the mobile phase. Park et al. irradiated pulsed laser light into the dispersed phase to produce small voids, which can extrude the dispersed phase into the mobile phase, and then form droplets of small volumes (Fig. 7.4c) [41]. Collins et al. imposed the surface acoustic waves to the dispersed phase and utilized the generated sound to force the dispersed phase into the mobile phase, forming droplets under the action of shear stress and surface tension . The size and frequency of droplets could be adjusted and controlled by the frequency and amplitude of the surface acoustic waves [47]. He et al. applied a high-voltage pulse to the dispersed phase so as to move the two-phase interface towards the direction of potential. The dispersed phase could be incised into single droplet with a minimal volume of fL or pL in this process [48]. The droplets formed by these methods have been widely used in mass spectrometry analysis, protein detection, DNA analysis, particle encapsulation and other fields, but not in cell analysis, especially cell manipulation . One reason is that some extra field driving, such as electric field driving, maybe have bad impact on cell or biological sample by produced high voltage. Besides, the frequency of droplet generation is low, and the homogeneity of the wrapped cells is poor by using such methods, which is far inferior to T-junction and flow focussing structure . Therefore, these technologies need to be further improved and optimized.
7.4 Analytical Methods in Droplet Microfluidic
The analytical methods in droplet microfluidic, especially cell analysis, have a higher standards whether off-line analysis or online analysis, due to the high flux of generated droplets, the small size of the droplets, a small amount of cells or even single cell usually contained in one droplet, together with the different size and frequency of the droplets in different experiments. Now the commonly used methods are fluorescence, mass spectrometry, electrochemical detection , capillary electrophoresis and so on.
7.4.1 Fluorescence
Fluorescence detection is the most common and effective method in microfluidic technology. This method usually requires an assistance of fluorescence reagents and fluorescence microscopy. It has the characteristics of high specificity, sensitivity and accuracy, and can be used for analysis of the enzyme activity, proteins , DNA, small molecules, even single molecules and individual cells (Fig. 7.5a) [19, 49,50,51]. For example, Chen et al. were able to detect enzyme activity for a long time by encapsulating enzyme proteins in fluorescent substrates contained droplets [52]. Harris et al. utilized microfluidic and fluorescence techniques to successfully detect single-molecule nucleotide sequences without amplification of the sequence [53]. Gu et al. have constructed a multifunctional droplet manipulation platform, which could achieve many functions of efficient single cell encapsulation, single cell enzyme activity analysis, and single cell DNA purification with the aid of fluorescence microscopy (Fig. 7.5c) [55]. In these analysis, quantitative analysis of fluorescence intensities and high-resolution images via the use of high-resolution microscopy are often required to obtain more complete and meaningful data. However, the effective analysis of the droplets need to be conducted in the condition of slow generation velocity or idle state, or after being collected and captured since the droplets are generated very quickly, because production frequency of droplets are usually much faster than frame number of a charge-coupled camera (Fig. 7.5b) [54, 56]. Shi et al. attached a droplet trapping array behind the T-junction, capturing the formed droplets in a specific small space and then analyzing them [57]. Fidalgo et al. reduced the flow rates of generated droplets and left the fluorescent droplets and water droplets alternately flowing past the fluorescence detection region, allowing for selective separation of the fluorescent droplets [58]. Thousands of droplets can be monitored simultaneously for long periods of time using fluorescence detection because of the small size of droplets, so a large amount of statistical data and parallel results can be obtained in an experiment. Baret et al. could directly detect the bacteria of overexpressing β-galactosidase in a large number of Escherichia coli encapsulated droplets, and compare their enzyme activity using fluorescence detection [59].
Several common methods for combining droplets with fluorescence detection. a Direct mixing with a Y-shaped channel (Reprinted with permission from Ref. [19]). b Addition of target reagents by droplets fusion (Reprinted with permission from Ref. [54]). c The different reagents were gradually added to obtain blending droplets (Reprinted with permission from Ref. [55])
Fluorescence detection method can also provide the information of spatial distribution of the material in the droplet, such as protein secretion, cell migration , droplet breakage, and connection between droplets [60]. For example, Khorshidi et al. continuously observed and analyzed the dynamic behavior of a large number of droplets containing HEK293T single cells up to 11 h using automated microscopy and image analysis [61]. Chokkalingam et al. encapsulated microspheres of capturing secretions on T-cell membranes, and then incubated, cooled, gelled the single-cell droplets, finally detecting the secreted cytokines by flow cytometry after adding fluorescent antibodies [62]. Courtois et al. have studied the time-dependent release of molecules from droplets by observing the fluorescence of the fluorescent derivatives in the droplets [63]. The emergence of fluorescence lifetime imaging microscopy, has achieved observation in spatial distribution and ultra-short time interval imaging as low as 1 microsecond, providing possible for ultra-sensitive and ultra-fast detection [64]. Solvas has observed the mixing behavior of two droplets in microsecond time resolution by using a fluorescence lifetime imaging microscope [65]. Laser-induced fluorescence microscopy can be realized for analysis and detection of highly sensitive and high-throughput, making it possible for a large number of droplets detection, such as single-cell analysis, PCR detection, cell screening and so on [51, 66]. For example, Agrest et al. have implemented ultra-high throughput cell sorting to study biochemical reactions and protein evolution with the assistance of laser-induced fluorescence microscopy [67]. Therefore, the visualization, localization and quantification of fluorescence detection make it well suited for droplet analysis and cell analysis.
7.4.2 Mass Spectrometry (MS)
Many study have investigated the association of microfluidic chip with mass spectrometry as it is a label-free method and can provide the structure information of substance compared with fluorescence detection methods [68, 69]. Lin’s group has carried out a series of study about combination of microfluidic chip with mass spectrometry and made great progress by developing micro-solid phase extraction cartridge for sample pretreatment and constructing MS connector. This combination of microfluidic chip with MS has been widely used for cell analysis, cell co-culture , drug metabolism and cancer therapy research. Chen et al. directly linked microfluidic chip to the mass spectrometry via attaching a micro-solid phase extraction cartridge to the end of the microfluidic structure for removal of salt in the cell culture medium [70]. However, combination of the droplet microfluidic with mass spectrometry is more difficult, mainly as the followings: (1) The mobile phase surrounding the droplets tends to interfere the detection of mass spectrometry and dilute the target content in the droplet; (2) The cells and target molecules are very rare as small volume of droplets, thus requiring high sensitivity of the mass spectrometer; (3) The cell culture medium can’t be directly accessed into mass spectrometry for containing much salts when cells are contained in the droplets; (4) High flux of the droplets generation also limits its combination with mass spectrometry [71,72,73].
The first thought of researchers is to separate the droplet from the mobile phase, in order to reduce the interference of mobile phase when facing these difficulties [77]. Smith et al. collected and stored the surfactant-stabilized droplets, and then re-injected into the microfluidic chip attached to the mass spectrometer to perform high-flux droplet analysis [78]. Fidalgo et al. have constructed a two-phase interface of water-oil in a chip, each leading to different channels, and then pushed the generated droplets into the water phase from the oil phase by using the electric field (Fig. 7.6a) [68]. The droplets were sprayed into mass spectrometry after moving into the fused silica nozzle. Su et al. drove the generated droplets onto the glass to form arrays, and then pipetted them into the capillary that connecting with the mass spectrometer for detection [79]. Similarly, Küster et al. formed droplets microarray in an indium tin oxide-coated glass, which were then detected by matrix assisted laser desorption ionization (MALDI) [80]. Of course, some people also used clever designs to omit these steps [79, 81]. Gasilova et al. punched an observation hole in the top of the main channel and connected with the mass spectrometer inlet, and then sprayed the droplets of flowing through the observation port by using the high pressure, thus accompanying little of mobile phase and minimum effect on the MS analysis (Fig. 7.6b). In addition, the droplets have been generated without mobile phase or just chosen as sampling device due to the existence of the above difficulties (Fig. 7.6c, d). For example, Chen et al. generated single-cell droplets without mobile phase by utilizing piezoelectric ceramics to squeeze cell-containing organic solvents. So the droplets could be directly dropped onto a high-pressure needle and sprayed into mass spectrometry for obtaining information [75]. Lee and Zhang et al. used droplets as agent to extract individual cell and then for mass spectrometry analysis by laser or electric spraying [82, 83]. Liu et al. employed a small diameter capillary to inhale the cell culture medium from multi-channel microfluidic chip by capillary action, and then got stable droplets with height difference into the paper spray mass spectrometry to obtain metabolite signal [76]. Even so, many difficulties and problems still remain unresolved. Therefore, the combination of droplet microfluidic technology and mass spectrometry also need more in-depth research and more technical support.
Some usually ways of combination droplet microfluidics with mass spectrometry. a The droplets were separated from the mobile phase by an electric field (Reprinted with permission from Ref. [68]). b The droplets are directly sprayed into the mass spectrum from the channel using high voltage pulses (Reprinted with permission from Ref. [74]). c Single cell droplets were directly produced by piezoelectric ceramics to drip onto the high pressure needle (Reprinted with permission from Ref. [75]). d Capillary sampling and generation of droplets combined with multi-channel microfluidic chip and paper spray mass spectrometry (Reprinted with permission from Ref. [76])
7.4.3 Electrochemical Detection and Capillary Electrophoresis
Electrochemical detection is a simple, rapid, effective, high sensitivity and low cost detection method, and has been mainly used to measure the electrical signal changes, for detection of the droplet size, frequency, speed and conductivity [84,85,86]. Luo et al. have firstly measured droplet size and ion concentration with a detection limit of 20 μM by utilizing different electrochemical signals as the different impedance in water and oil [87]. Moiseeva et al. also successfully detect the droplet chains based on thin-film electrodes and the difference of two-phase impedance [85]. In addition, the droplet size, speed and frequency can be well analyzed since changes in capacitance when droplets flow into the specific detection area. Liu and Elbuken have successfully measured the capacitance change of the droplet stream by different arrangement of the electrodes, and then analyzed these properties of the droplets [88, 89].
Capillary electrophoresis is a simple, fast and efficient separation technique, which is often used in conjunction with other detection methods. If the components of the droplet are complex or easily interfered with each other, capillary electrophoresis can be used for separation to obtain a good analytical result before entering the detector. Edgar et al. were able to simultaneously analyze the various substances contained in the droplets via droplets separation of capillary electrophoresis and detection by using laser-induced fluorescence [90]. As the low efficiency of capillary electrophoresis in microfluidic chip, Wang et al. modified the surface of the chip to get high efficiency of capillary electrophoresis, and successfully detect the neurotransmitters in the brain of rats by such method [91].
7.4.4 Other Methods
Except for the above methods, other usually methods are Raman spectroscopy, chemiluminescence, absorption spectrophotometry, nuclear magnetic resonance and so on. Cecchini et al. have constructed an ultrafast surface-enhanced Raman scattering system for the detection and analysis of droplets, with highest rate up to 32 droplets per second. [92]. Trivedi et al. allowed quick and efficient screening of droplet libraries by combining droplet generation with absorbance detection [49]. Shen et al. integrated the three reagents required in chemiluminescence into one droplet to complete luminescence and detection by means of liquid-liquid extraction after trapping droplets on both sides of the main channel [93].
There are still many problems in the analysis of droplets when they are applied to cell analysis although many detection methods have been carried out. Fluorescence, capillary electrophoresis , and chemiluminescence need assistant of additional reagents to complete the detection. Mass spectrometry, Raman spectroscopy, nuclear magnetic resonance, and absorption spectrophotometry, require complex pre-treatment, or still seriously interfere the detection owing to matrix effect, or have relatively low detection limit. Therefore, further technical progress, improvement and the establishment of new methods have broad prospects and requirement in droplet microfluidic technology.
7.5 Single Cell Analysis
Tumor is a disease that seriously threatens the health of human beings nowadays, and the heterogeneity of tumor cells is one of the important reasons for its easy relapse and difficult treatment [94]. As the traditional analysis is often the average results of abundant cells, some key information is likely to be hidden. So the analysis of individual tumor cells shows great meaning and has been widely concerned and explored for better mastering and understanding the occurrence, development, transferring and treatment of tumors [95, 96]. Droplet microfluidic technology has an intrinsic advantage in single cell analysis because droplets can provide a limited environment with easy accessibility by regulation of cell loading patterns. Besides, it’s also convenient to carry out analysis and detection of cellular secretion and factors because of long survival of cells in droplets and metabolism in a narrow space [97]. Meanwhile, the droplet also is a moving chamber that can be high-throughput screening according to the property of the droplets and cells, which is contributing to observation and analysis of follow-up [98].
7.5.1 Encapsulation of Single Cells
One of the key points in single cell analysis is the separation of single cells from a large population of cells. There are two main ways for single cells encapsulation by using droplet microfluidic technology: passive and active methods [10]. Passive encapsulation is a way that actuates cells into droplets by adjusting the flow rate, cell concentration and channel width (Fig. 7.7a). This method is convenient, simple and high throughput, but the number of cells in each droplet is variable and presents a pattern of Poisson distribution, even no cells in considerable number of droplets [99, 100]. The active encapsulation is the utilization of external force such as sound, electricity, light, magnetic to wrap cells into droplets based on microfluidic chip (Fig. 7.7b). This approach is more accurate and produces higher proportion of single cell droplets than passive method, but with shortcomings of low throughput and efficiency [101, 102].
Common methods of encapsulating single cell into one droplet. a Basic T-junction and flow focussing structures were used to encapsulate single cells (Reprinted with permission from Ref. [103]). b Surface acoustic wave was used to actively wrap a single cell (Reprinted with permission from Ref. [104]). c Regulating cells for stable spacing by high aspect ratio channels to obtain high occupancy rates for single cell droplets (Reprinted with permission from Ref. [108])
Passive encapsulation is the most common method of generating single-cell droplets today, and a large number of studies have used this method. The main process is that the cells are suspended to a certain concentration in the dispersed phase and incised into single droplet to complete single cell separation in the confluence zone by flowing phase on the basis of typical T-junction or flow focussing structure [105]. The droplets produced in this way don’t have uniform number of cells in each droplet with a presentation of Poisson distribution as the spatial distribution of the cells and the moment of reaching the focusing region are random, thus greatly reducing its research significance [106]. The most common approach to solve this problem is the sorting of droplets, which will be described in detail later. Others have come up with some sensible methods, such as regulation the rates of cell arriving at the focal region and droplet production to the same, making one cell in one droplet [107]. On that account, Edd et al. engendered a more stable spacing between cells by passing through a high-aspect-ratio channel, and kept the frequency of arriving at the focusing region with the same as droplets, resulting in a significant increase in the ratio of single cell droplets (Fig. 7.7c) [108]. The realization of this method is based on a series of hydrodynamics, such as hydrodynamic repulsive effect, repellent effect and parabolic shear gradient, which make the cells arranged orderly in long, wide and high, respectively, and easily controlled by reasonable regulation. Kemna et al. used a helical channel to align the cells in good order of horizontal and vertical, and most of the droplets only contained a single cell by a straightforward confluence with flowing phase in flow focussing structure. [102]. This method needs to arrange appropriate conditions of the cells in advance, so its application is limited as the heterogeneity of different cells during the process and it’s difficult to balance the flow rate.
Active encapsulation refers to droplets generation with the assistance of external force, for the main advantage of accurate single-cell droplets production. Such as sound, electricity, light, magnetic force can be applied near the interface of the two phases and drive the cells into the focus area to control the generation of single cell droplets [104, 109,110,111]. He et al. immediately applied a high voltage pulse to produce a single cell droplet once the cells reached the two-phase interface with optical capture of the cell site [112]. Schoendube et al. used two electrodes to precisely determine the position and velocity of the droplets and installed a piezoelectric actuator in the focal region. After the electrode-measured signal was transferred to the piezoelectric actuator in real time, the force released by the piezoelectric actuator perfectly contacted with cells, generating single-cell droplets [113]. Another method was that the surface acoustic wave was used to act on the cell-containing dispersed phase to generate a thin liquid bridge, and then the mobile phase divided the bridge into droplets, but the number of cells is also uncertain by this method [101]. It is evident that the generation of single-cell droplets by active encapsulation results in very low rates due to the need of precise control of the cell’s location for exerting external forces, even in comparison with passive encapsulation. However, single-cell analysis often require a large number of samples for statistics and comparison. Um et al. constructed a microwell array containing mesh-grid for simultaneous merging and storage of single-cell droplets in a high-throughput manner [114]. This way is still not fast as passive method and also an off-line method, so how to effectively improve the rate of single cell droplets is an important problem to be faced in active methods.
7.5.2 Droplet Sorting
As mentioned above, the number of cells in a droplet is often not deterministic, thus making subsequent experiments much more difficult. The main purpose of sorting is to select high-purity single cell droplets or other required droplets. For example, Wu et al. screened fluorescent-labeled lymphoma cells at high-speed by using laser pulse excitation, up to 45,000 cells per second (Fig. 7.8c) [115]. Another goal of sorting is to reduce the work of analysis in follow-up. A large number of cell-free or multiple cells-contained droplets can be filtered out by sorting, greatly reducing the subsequent experimental workload. Sorting is mainly based on the different characters among the target droplets with other droplets, such as physical size, magnetic, mechanical properties or optical properties and so on [109, 111, 116]. Jing et al. instituted a small column array after flow focussing structure and sorted cells-contained droplets by adjusting the spacing of the column, due to different sizes of the cell droplet and empty droplets (Fig. 7.8b) [116]. Chen et al. covered cells with magnetic beads to obtain magnetic droplets after cells encapsulation, which were then separated from all the droplets depended on the attraction of an electromagnet. Nam et al. used a standing surface acoustic wave to encapsulate cells into droplets and separate single cell droplet based on density (Fig. 7.8a) [117]. There are two ways of sorting: active and passive sorting, according to the different modes of action [118].
Active cell droplet sorting based on microfluidic chip. a Using surface acoustic wave to sort out the cells without cells, containing a small number of cells and many cells of the droplets and collecting them (Reprinted with permission from Ref. [117]). b Size screening by micro-column arrays (Reprinted with permission from Ref. [116]). c Fluorescence detection and high purity pulsed laser screening (Reprinted with permission from Ref. [115])
Passive sorting is a way that makes full use of microfluidic chip structure and the physical properties of droplets to sort, without external field or force [119]. Size screening of micro-column arrays in passive method is a common approach as the presence of cells in the droplets changes the size of the droplets [120]. Joensson et al. set up a micro-column array to let droplets move towards different directions according to their size in order to allow droplets for determinate lateral movement. Some sorting methods are also on the basis of the dynamic effect of liquid [121]. Chabert et al. rendered the droplets lateral movement and spatial dispersion by using shear flow and compression flow of liquid, respectively, to achieve the purpose of self-sorting [122]. In addition, methods making use of channels with selective size and different fluid forces are also commonly used [123, 124]. Passive sorting doesn’t need much equipment and is relatively simple to operate. Nevertheless, it is less used in practical application because it owns low efficiency and large error, and requires a complete understanding of every parameters in droplets for being likely to redesign the chip once any changed.
Active sorting, based on the droplet itself or the external cause of the differences in the physical or chemical properties, utilizes technology to find these difference and screens droplets by exerting external force [125]. Active methods provide the most flexible method for droplet sorting, and have employed most measurable parameters of droplets. The most common, efficient and accurate method is depended on the optical properties of the droplets, especially the fluorescence intensity [126]. Cell expression proteins , secretory proteins, surface proteins, and other molecules can be fluorescent labeled for sorting with a property of the high specificity, sensitivity, and easy detection [59, 127]. For example, Wu et al. sorted generated single-cell droplets at 1000 Hz by using fluorescent-labeled cells and laser sorting, obtaining droplets containing single cells up to 98% [128]. Joensson et al. used this principle and the enzyme signal amplification technique to analyze low-level surface markers in individual cells and to sort cells with higher marker content [129]. Fluorescence-activated dielectrophoresis force, as one of the commonly used driving forces, can be combined with fluorescence detection, and move the charged droplets in the electric field to achieve the purpose of droplet sorting. Agresti et al. sorted droplets based on fluorescence-induced electrophoretic equipment, realizing to screen 108 droplets in 10 h [67]. In addition, the electric field force, magnetic force, surface acoustic wave and other forces are also often used as the driving force for active sorting [103, 111, 130,131,132]. Chen et al. labeled cells with magnetic particles and then sorted out droplets with cells with the assist of magnetic fields [126]. The more the cells are contained in the droplet, the farer the droplets will move to both sides of the channel by surface acoustic wave. Franke et al. sorted the droplets in a wide channel to select out droplets containing no cells, a few cells and multiple cells by using this principle without prior labelling and cell encapsulation into liquid droplet [131]. Active sorting can greatly increase sorting efficiency and speed with high accuracy, based on not only whether the droplets contain cells, but also the characters of the cells in the droplet [22, 62]. At the same time, active sorting is less dependent on the structure of microfluidic chip than passive sorting, and still works on a wide range of droplet sizes, but usually requires additional reagents or equipment, which is one of the reasons for its higher sensitivity [18, 133].
7.5.3 Protein Analysis
The heterogeneity of cancer cells has reflected in different proteins expression of different cells, but single-cell protein analysis has been always a major challenge in cell research because of the small amount of protein in a single cell [134, 135]. While conventional methods such as flow cytometry can carry out single-cell protein analysis to a certain extent, but its practical significance is greatly weakened because of its damage to cells, and analysis without real-time [136, 137]. The single cell in the droplet is not only high-throughput, long-term survival, easy to detect, but also limited to a tiny space and not easily damaged, so it’s considered as an ideal place for single cell protein analysis in recent years [10, 138].
The protein of cells mainly includes three types: surface protein, secretory protein and internal protein [139]. Because proteins can’t amplify as DNA by polymerase chain reaction (PCR) , the basic principle for trace protein detection is to amplify signals like enzyme-linked immunosorbent assays (ELISA) [60, 140]. Surface proteins and secretory proteins are easily captured by the addition of antibodies, so the basic idea is to collect protein by the antibody or aptamer, and then enhance signal through the other end of the antibody (Fig. 7.9a). Kony et al. connected antibodies with avidin bridge, which was linked with special DNA chain after capturing the cell surface protein . The DNA chain can conduct amplification of the signal through the DNA rolling chain amplification for protein detection [141]. In the detection of secreted proteins, nanoparticles modified with specific antibody were usually wrapped in the cell surface to perform enrichment and signal amplification in order to get better results as the protein will disperse into whole parts of the droplet [142]. Konry et al. used the microfluidic droplet system again to analyze the amount of surface protein CD86 and secreted protein IL-6 in single dendritic cells stimulated with drugs and co-cultured with T cells, demonstrating the feasibility of this method [145]. In addition, the feature that protease can specificly decompose corresponding substrate has also been used to detetct secreted protease by analysis of production (Fig. 7.9b). Antibodies are hard to enter the internal of cells due to the presence of cell membranes, so the detection of internal proteins is more difficult [146]. Now it’s mainly implemented by extracting proteins from the cell internal after cell lysis or allowing cells to express fluorescent protein by transfection, and then to detect, except for some specific dyes that can entering the interior of the cell to label protein (Fig. 7.9c) [144]. For example, Huebner et al. have successfully detected droplet size, cell occupancy and fluorescence expression of a single cell through combination of T-junction, bending channel, laser induced fluorescence microscopy and transfected cells [51].
Analysis of single cell proteins in microdroplets. a Surface proteins of single cell were analyzed by enzyme—amplified signal method (Reprinted with permission from Ref. [143]). b Simultaneous determination of a variety of cell secretory protein activity analysis (Reprinted with permission from Ref. [60]). c Cell surface proteins and secretory proteins were simultaneously monitored in single cell droplets (Reprinted with permission from Ref. [144])
7.5.4 Single Cell PCR
PCR, the abbreviation of polymerase chain reaction, is one of the most commonly used nucleic acid amplification means. High-throughput DNA sequencing method has been applied by combination droplet microfluidic technology with PCR in recent years [11]. Droplets provide a small, independent space, and a good environment for controllable and efficient replication reaction, reducing pollution of moving process in the traditional method [140]. In single-cell PCR, diluted cells or samples are dispersed into a large number of droplets, each with only a single or even no target DNA sequence, so a large number of parallel experiments can be carried out simultaneously, thus greatly increasing the throughout [150]. Moreover, template nucleic acid molecules are only located in droplets without fusion into the mobile phase in droplet microfluidic PCR, avoiding nucleic acid recombination with multiple cells in a conventional method, and not generating short, chimeric and other artificial products [151, 152]. These factors make droplet PCR very suitable for single-cell and single-molecule nucleic acid amplification. For example, Zhong et al. used multiple real-time droplet PCR to break the barrier of the traditional PCR, in which a color can only indicate one target [153].
Currently, there are two main approaches available to perform microfluidic PCR in droplet: digital microfluidic PCR and continuous-flow microfluidic PCR. Digital microfluidic PCR is performed by placing reagents and amplification reactions in fixed or semi-fixed droplets for accurate flow control, droplet manipulation, and rapid detection [154, 155]. Pekin et al. screened the mutated genes in a 20,000-fold normal gene and accurate quantified by using digital microfluidic droplet PCR [156]. While the continuous-flow microfluidic PCR is different as droplets are often in continual motion in microfluidic devices (Fig. 7.10c), which makes the operation great challenge and is less use but higher throughout and suitable for continuous amplification and detection [149, 157]. Mohr et al. integrated cell lysis, complementary DNA synthesis and gene amplification into the chip by manipulating the droplets using the magnetic field dynamics after adding cells and magnetic beads into the droplets [158]. Microfluidic PCR in droplet can orderly process a large number of droplets in a short time with a higher throughout. Difficulties in single-cell PCR mainly exist in three processes: cell lysis , DNA extraction, and PCR. The lysate after cell lysis can strongly inhibit the PCR reaction reversely, and it will also interfere with the PCR results if the DNA extraction is incomplete or contains impurities. To solve these problems, Novak et al. used agarose as the dispersed phase to encapsulate single cells and released the DNA from the cells with sodium dodecyl sulfate and proteinase K, in which impurities could be washed away, and finally reacted with the PCR reagents to proceed amplification and analysis (Fig. 7.10a) [147]. Some by-products or undesired reagents can be removed in this way, but it is not suitable for simultaneous cell staining and gene analysis due to the presence of the gel. Eastburn et al. have constructed a microfluidic platform to perform the whole PCR process by diluting the droplets containing the cell lysate with a large water droplet to reduce the inhibitory effect, and then separating parts of the droplets for amplification and analysis after adding the PCR reagents (Fig. 7.10b) [148]. In the PCR process, two types of thermal cycling are often used: on-chip and off-chip. The off-chip thermal cycling is simple, convenient, efficient, and capable of handling a large number of droplets simultaneously, but the off-chip thermal cycling is more convenient for automating [159]. Kim et al. performed droplets PCR reaction with the aid of laser for heating, obtaining good results because the laser can be well controlled to heat the droplets only and produce thermal reactions very quickly [160].
Droplet microfluidic chip for single-cell PCR. a Single cell PCR analysis system based on gel droplets (Reprinted with permission from Ref. [147]). b A common water-in-oil droplet platform was used for single-cell PCR analysis (Reprinted with permission from Ref. [148]). c Architecture of a complete real-time PCR device (Reprinted with permission from Ref. [149])
7.6 Cell Manipulation
Though the size of droplet is small, it can be much larger than a single cell, so droplets can be used not only for single-cell analysis, but also for manipulation of multiple cells [161]. When many cells are encapsulated in droplets, the number of cells in the droplet obeys no longer a Poisson distribution, but rather a Gaussian distribution [39]. For droplet manipulation of cells, it is important not just by droplet generation and sorting, but by a series of other operations: fusion, splitting, and mixing [27, 162]. Some special cell manipulations can be achieved through droplets operations, such as cell three-dimensional (3D) culture and cell co-culture .
Compared with conventional 2D culture system, 3D cell culture more accords with the condition of cells in the organism, which makes people understand the cell differentiation, tissue production and function more deeply and is meaningful to study cells and tissues as important components of body [163]. Cells culture in gel is considered to be the most commonly used method for 3D cell culture as the gel has good biocompatibility, provides good support for cells growth and is convenient for the addition of cytokines [164]. Meanwhile, gel can perform the conversion between the liquid and semi-solid state under certain conditions, which is exploited to carry out micro-droplet 3D culture. Headen and colleagues wrapped human bone marrow mesenchymal stem cells with RGD (Arg-Gly-Asp peptide) functionally modified liquid polyethylene glycol gel, which would become coagulation state under the action of triethanolamine, and then cells microspheres were successfully formed after cultivation (Fig. 7.11a) [165]. The formation of gel droplets by droplet microfluidic technology can not only accurately control the size, shape and dispersibility of the gel, but also precisely regulate the chemical composition of the gel. Now there are two ways for formation of microgel droplets. One is to firstly generate droplets with small molecule monomers, and then polymerize these monomers to form micro-gel (Fig. 7.11b) [166]. Such way is convenient to operate and easy to implement with closely linked two-step operation, but difficult to control the degree of reaction and morphology of droplets. The other way serves prepolymer as disperse phase to form droplets, and then to develop micro-gel via cross-linking reaction by adding cross-linking agent. The difficulties in this approach are that the prepolymer is a non-Newtonian fluid and owns different fluid properties contrast with the mobile phase, while its advantage is getting more controllable microgels [167]. The latter method is more suitable for cell culture, such as commonly used alginate, agarose, carrageenan, gelatin, and so on [168, 169]. For example, Siltanen et al. formed stem cells encapsulated hydrogels in a way of pre-polymerisation and then cross-linking, and have found that the formed stem cell spheres expressed higher content of endoderm markers [170]. Now three kinds of cross-linking methods are commonly used: chemical crosslinking, temperature crosslinking and ion crosslinking. Kumacheva et al. encapsulated cells and formed microgels with an agarose precursor capable of gelatinization by thermal inversion [171]. Liu et al. formed calcium ion droplets and alginate droplets, respectively, and then fused two droplets to produce microgels to achieve cell encapsulation and 3D culture as the property that alginate turns into gelation in presence of calcium ions [166]. The 3D cell culture in droplet provides an effective to study drug screening, cell differentiation and phenotypic change. Du and colleges accomplished drug screening of a large number of cell microspheres formed by microfluidic droplet array just with a small amount of drugs [172].
Cells were wrapped in biocompatible gel for 3D cell culture. a The microfluidic device was used to generate droplets with macromonomers encapsulating the cells and then were polymerized to form gel [165]. b The droplets containing two components of synthetizing gel are formed and the gel was formed by droplet fusion [166]. (Reprinted with permissions from Refs. [165] and [166])
Except for 3D culture, cell co-culture is also an important direction for biological research as the cells in vivo are not isolated, but closely contacts with other cells through directly or indirectly communication. Since the droplets are born in sequence and can be operated with fusion and separation between each other, Tumarkin et al. mixed two cell lines contained in agarose with a Y-channel, and formed droplets through a T-junction, and finally developed into micro-gel by the temperature cross-linking, in order to get the purpose of two cells co-culture [175]. Yu et al. fused two types of droplets pathway containing E. coli and phage, respectively, by applying the same method for the detection of phage content in water after culture for a certain time (Fig. 7.12a). Chen et al. have developed a new core-shell structure consisting of human hepatocellular carcinoma cells and NIH-3T3 cells, respectively, by wrapping the water droplets with gels and analyzed the secretion of albumin and urea in single culture and co-culture (Fig. 7.12b). In addition, Zhang et al. printed arrays of two cell with accurate and controllable interval by using Inkjet to study intercellular drug metabolism (Fig. 7.12c). Liu and colleges applied high-voltage pulse into three metal nozzles which were next to each other, so that the generated droplets got fused together in the process of spray for cell co-culture [176]. In contrast with other methods, cells co-culture in droplet enables co-culture between a few cells, or even single cells and high-throughput study, and provides a more precise control of droplet behavior. Furthermore, cell patterning [177], the study of cell growth [178], and live cell monitoring [179], through union of droplets and nanoparticles are also widely used in droplet microfluidic technology.
Some common methods for cell co-culture using droplet microfluidic technology. A Cell co-culture was performed using droplet fusion. a droplet generation; b droplet fusion; c culture in droplets; d optical detection (Reprinted with permission from Ref. [173]). B core-shell structure for co-culture. a calcium ion-induced alginate crosslinked network; b microfluidic chip and partial enlarged view of core-shell structure; c hepatocytes located in the nucleus and fibroblasts located in shell (Reprinted with permission from Ref. [42]). C Inkjet printing was used to perform cell co-culture in droplet. a schematic of complete system; b an inkjet print, integrated chip, and drug metabolism process (Reprinted with permission from Ref. [174])
7.7 Summary and Prospect
The droplet-based microfluidic technology inherits the advantages of microfluidic technology and further performs some characters of high-throughput, low consumption, and higher specific surface area, so it has been developed rapidly in recent years and has become a hotspot and powerful tool in the fields of chemical and biological research. It mainly depends on T-junction, flow focussing structures or external forces, to produce monodisperse and high-flux droplets with a minimal volume of fL or pL. This process is easy to operate and provides tiny and independent space for single or small number of cells. The generated cell droplets are charactered with a variety of analytical methods, such as fluorescence, mass spectrometry, electrical detection and chemiluminescence, to fulfill aim of study the cell states, phenotypes, metabolism , genes and so on. The single-cell analysis, 3D cell culture and cell co-culture are well controlled by the design of the microfluidic chip structure and some pluripotent operations of fusion, splitting, sorting and mixing, which offers a possible direction in single cell level research and analysis.
For future droplet-based microfluidic technology, we expect that it will allow better integration with various detection methods for real-time assay of cell metabolism and other markers, or incorporation with new biocompatible materials to establish new methods and systems for cell research. Since droplets are high-throughput, some unexpected conclusion may be obtained by combination experimental results with big data analysis. One possible field of future droplet microfluidics may be the development of portable devices that apply some simple and sensitive detection techniques such as electrical or chemiluminescent detection for diagnosis of diseases. Even with few applications, active methods of generating droplets can overcome many disadvantages of passive methods in encapsulating cells and will promote progress in study of single cell and cell manipulation if further developed. In addition, efficient and extensive drug screening, development of new isothermal amplification methods and other applications also have broad prospects. We believe that droplet-based microfluidic technology will play a more important role in cell research and analysis, tumor therapy, disease diagnosis in the near future.
References
Woodruff K, Maerkl SJ (2016) A high-throughput microfluidic platform for mammalian cell transfection and culturing. Sci Rep 6:23937. doi:10.1038/srep23937
Vidi PA, Maleki T, Ochoa M, Wang L, Clark SM, Leary JF, Lelievre SA (2014) Disease-on-a-chip: mimicry of tumor growth in mammary ducts. Lab Chip 14(1):172–177. doi:10.1039/c3lc50819f
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328(5986):1662–1668. doi:10.1126/science.1188302
Chen Q, Wu J, Zhuang Q, Lin X, Zhang J, Lin JM (2013) Microfluidic isolation of highly pure embryonic stem cells using feeder-separated co-culture system. Sci Rep 3:2433. doi:10.1038/srep02433
Bergstrom G, Nilsson K, Mandenius CF, Robinson ND (2014) Macroporous microcarriers for introducing cells into a microfluidic chip. Lab Chip 14(18):3502–3504. doi:10.1039/c4lc00693c
Deng Y, Zhang Y, Sun S, Wang Z, Wang M, Yu B, Czajkowsky DM, Liu B, Li Y, Wei W, Shi Q (2014) An integrated microfluidic chip system for single-cell secretion profiling of rare circulating tumor cells. Sci Rep 4:7499. doi:10.1038/srep07499
Shapiro OH, Kramarsky-Winter E, Gavish AR, Stocker R, Vardi A (2016) A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nat Commun 7:10860. doi:10.1038/ncomms10860
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442(7101):368–373. doi:10.1038/nature05058
Niu XZ, Zhang B, Marszalek RT, Ces O, Edel JB, Klug DR, deMello AJ (2009) Droplet-based compartmentalization of chemically separated components in two-dimensional separations. Chem Commun 41:6159–6161. doi:10.1039/b918100h
Niu X, deMello AJ (2012) Building droplet-based microfluidic systems for biological analysis. Biochem Soc Trans 40(4):615–623. doi:10.1042/BST20120005
Song H, Chen DL, Ismagilov RF (2006) Reactions in droplets in microfluidic channels. Angew Chem Int Edit 45(44):7336–7356. doi:10.1002/anie.200601554
Kintses B, van Vliet LD, Devenish SR, Hollfelder F (2010) Microfluidic droplets: new integrated workflows for biological experiments. Curr Opin Chem Biol 14(5):548–555. doi:10.1016/j.cbpa.2010.08.013
Boybay MS, Jiao A, Glawdel T, Ren CL (2013) Microwave sensing and heating of individual droplets in microfluidic devices. Lab Chip 13(19):3840–3846. doi:10.1039/c3lc50418b
Srisa-Art M, Bonzani IC, Williams A, Stevens MM, deMello AJ, Edel JB (2009) Identification of rare progenitor cells from human periosteal tissue using droplet microfluidics. Analyst 134(11):2239–2245. doi:10.1039/b910472k
Wang T, Zhu H, Zhuo J, Zhu Z, Papakonstantinou P, Lubarsky G, Lin J, Li M (2013) Biosensor based on ultrasmall MoS2 nanoparticles for electrochemical detection of H2O2 released by cells at the nanomolar level. Anal Chem 85(21):10289–10295. doi:10.1021/ac402114c
Schaerli Y, Hollfelder F (2009) The potential of microfluidic water-in-oil droplets in experimental biology. Mol BioSyst 5(12):1392–1404. doi:10.1039/b907578j
Baroud CN, de Saint Vincent MR, Delville JP (2007) An optical toolbox for total control of droplet microfluidics. Lab Chip 7(8):1029–1033. doi:10.1039/b702472j
Schmid L, Weitz DA, Franke T (2014) Sorting drops and cells with acoustics: acoustic microfluidic fluorescence-activated cell sorter. Lab Chip 14(19):3710–3718. doi:10.1039/c4lc00588k
Mazutis L, Gilbert J, Ung WL, Weitz DA, Griffiths AD, Heyman JA (2013) Single-cell analysis and sorting using droplet-based microfluidics. Nat Protoc 8(5):870–891. doi:10.1038/nprot.2013.046
Hatch AC, Fisher JS, Tovar AR, Hsieh AT, Lin R, Pentoney SL, Yang DL, Lee AP (2011) 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip 11(22):3838–3845. doi:10.1039/c1lc20561g
Sabhachandani P, Motwani V, Cohen N, Sarkar S, Torchilin V, Konry T (2016) Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 16(3):497–505. doi:10.1039/c5lc01139f
Brouzes E, Medkova M, Savenelli N, Marran D, Twardowski M, Hutchison JB, Rothberg JM, Link DR, Perrimon N, Samuels ML (2009) Droplet microfluidic technology for single-cell high-throughput screening. Proc Natl Acad Sci U S A 106(34):14195–14200. doi:10.1073/pnas.0903542106
Theberge AB, Courtois F, Schaerli Y, Fischlechner M, Abell C, Hollfelder F, Huck WT (2010) Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew Chem Int Edit 49(34):5846–5868. doi:10.1002/anie.200906653
Nossal GJ, Lederberg J (1958) Antibody production by single cells. Nature 181(4620):1419–1420
Baroud CN, Gallaire F, Dangla R (2010) Dynamics of microfluidic droplets. Lab Chip 10(16):2032. doi:10.1039/c001191f
Shen F, Li Y, Liu Z-M, Cao R-T, Wang G-R (2015) Advances in micro-droplets coalescence using microfluidics. Chin J Anal Chem 43(12):1942–1954. doi:10.1016/s1872-2040(15)60886-6
Yang C-G, Xu Z-R, Wang J-H (2010) Manipulation of droplets in microfluidic systems. TrAC Trends Anal Chem 29(2):141–157. doi:10.1016/j.trac.2009.11.002
Niu X, Gulati S, Edel JB, deMello AJ (2008) Pillar-induced droplet merging in microfluidic circuits. Lab Chip 8(11):1837–1841. doi:10.1039/b813325e
Bayley H, Cronin B, Heron A, Holden MA, Hwang WL, Syeda R, Thompson J, Wallace M (2008) Droplet interface bilayers. Mol BioSyst 4(12):1191–1208. doi:10.1039/b808893d
Wu J, Wen W, Sheng P (2012) Smart electroresponsive droplets in microfluidics. Soft Matter 8(46):11589. doi:10.1039/c2sm26286j
Movahednejad E, Ommi F, Hosseinalipour SM (2010) Prediction of droplet size and velocity distribution in droplet formation region of liquid spray. Entropy 12(6):1484–1498. doi:10.3390/e12061484
Gu H, Duits MH, Mugele F (2011) Droplets formation and merging in two-phase flow microfluidics. Int J Mol Sci 12(4):2572–2597. doi:10.3390/ijms12042572
Zhao C-X, Middelberg APJ (2011) Two-phase microfluidic flows. Chem Eng Sci 66(7):1394–1411. doi:10.1016/j.ces.2010.08.038
Cao J, Köhler JM (2015) Droplet-based microfluidics for microtoxicological studies. Eng Life Sci 15(3):306–317. doi:10.1002/elsc.201400074
Zec H, Shin DJ, Wang TH (2014) Novel droplet platforms for the detection of disease biomarkers. Expert Rev Mol Diag 14(7):787–801. doi:10.1586/14737159.2014.945437
Fleury J-B, Schiller UD, Thutupalli S, Gompper G, Seemann R (2014) Mode coupling of phonons in a dense one-dimensional microfluidic crystal. New J Phys 16(6):063029. doi:10.1088/1367-2630/16/6/063029
Thorsen T, Roberts RW, Arnold FH, Quake SR (2001) Dynamic pattern formation in a vesicle-generating microfluidic device. Phys Rev Lett 86(18):4163–4166. doi:10.1103/PhysRevLett.86.4163
Basova EY, Foret F (2015) Droplet microfluidics in (bio)chemical analysis. Analyst 140(1):22–38. doi:10.1039/c4an01209g
Okushima S, Nisisako T, Torii T, Higuchi T (2004) Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir 20(23):9905–9908. doi:10.1021/la0480336
Collins DJ, Neild A, deMello A, Liu AQ, Ai Y (2015) The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation. Lab Chip 15(17):3439–3459. doi:10.1039/c5lc00614g
Park SY, Wu TH, Chen Y, Teitell MA, Chiou PY (2011) High-speed droplet generation on demand driven by pulse laser-induced cavitation. Lab Chip 11(6):1010–1012. doi:10.1039/c0lc00555j
Chen Q, Utech S, Chen D, Prodanovic R, Lin JM, Weitz DA (2016) Controlled assembly of heterotypic cells in a core-shell scaffold: organ in a droplet. Lab Chip 16(8):1346–1349. doi:10.1039/c6lc00231e
Tan YC, Lee AP (2005) Microfluidic separation of satellite droplets as the basis of a monodispersed micron and submicron emulsification system. Lab Chip 5(10):1178–1183. doi:10.1039/b504497a
Song H, Tice JD, Ismagilov RF (2003) A microfluidic system for controlling reaction networks in time. Angew Chem Int Edit 115(7):792–796. doi:10.1002/anie.200390203
Adamson DN, Mustafi D, Zhang JX, Zheng B, Ismagilov RF (2006) Production of arrays of chemically distinct nanolitre plugs via repeated splitting in microfluidic devices. Lab Chip 6(9):1178–1186. doi:10.1039/b604993a
Xiao Z, Niu M, Zhang B (2012) Droplet microfluidics based microseparation systems. J Sep Sci 35(10–11):1284–1293. doi:10.1002/jssc.201200115
Collins DJ, Alan T, Helmerson K, Neild A (2013) Surface acoustic waves for on-demand production of picoliter droplets and particle encapsulation. Lab Chip 13(16):3225–3231. doi:10.1039/c3lc50372k
He M, Kuo JS, Chiu DT (2005) Electro-generation of single femtoliter- and picoliter-volume aqueous droplets in microfluidic systems. Appl Phys Lett 87(3):031916. doi:10.1063/1.1997280
Huebner A, Srisa-Art M, Holt D, Abell C, Hollfelder F, deMello AJ, Edel JB (2007) Quantitative detection of protein expression in single cells using droplet microfluidics. Chem Commun 12:1218–1220. doi:10.1039/b618570c
Kapanidis AN, Strick T (2009) Biology, one molecule at a time. Trends Biochem Sci 34(5):234–243. doi:10.1016/j.tibs.2009.01.008
Casadevall iSX, Niu X, Leeper K, Cho S, Chang SI, Edel JB, deMello AJ (2011) Fluorescence detection methods for microfluidic droplet platforms. JoVE-J Vis Exp 58. doi:10.3791/3437
Chen CH, Sarkar A, Song YA, Miller MA, Kim SJ, Griffith LG, Lauffenburger DA, Han J (2011) Enhancing protease activity assay in droplet-based microfluidics using a biomolecule concentrator. J Am Chem Soc 133(27):10368–10371. doi:10.1021/ja2036628
Harris TD, Buzby PR, Babcock H, Beer E, Bowers J, Braslavsky I, Causey M, Colonell J, Dimeo J, Efcavitch JW, Giladi E, Gill J, Healy J, Jarosz M, Lapen D, Moulton K, Quake SR, Steinmann K, Thayer E, Tyurina A, Ward R, Weiss H, Xie Z (2008) Single-molecule DNA sequencing of a viral genome. Science 320(5872):106–109. doi:10.1126/science.1150427
Trivedi V, Doshi A, Kurup GK, Ereifej E, Vandevord PJ, Basu AS (2010) A modular approach for the generation, storage, mixing, and detection of droplet libraries for high throughput screening. Lab Chip 10(18):2433–2442. doi:10.1039/c004768f
Gu SQ, Zhang YX, Zhu Y, Du WB, Yao B, Fang Q (2011) Multifunctional picoliter droplet manipulation platform and its application in single cell analysis. Anal Chem 83(19):7570–7576. doi:10.1021/ac201678g
Huebner A, Bratton D, Whyte G, Yang M, Demello AJ, Abell C, Hollfelder F (2009) Static microdroplet arrays: a microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip 9(5):692–698. doi:10.1039/b813709a
Shi W, Qin J, Ye N, Lin B (2008) Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip 8(9):1432–1435. doi:10.1039/b808753a
Fidalgo LM, Whyte G, Bratton D, Kaminski CF, Abell C, Huck WT (2008) From microdroplets to microfluidics: selective emulsion separation in microfluidic devices. Angew Chem Int Edit 47(11):2042–2045. doi:10.1002/anie.200704903
Baret JC, Miller OJ, Taly V, Ryckelynck M, El-Harrak A, Frenz L, Rick C, Samuels ML, Hutchison JB, Agresti JJ, Link DR, Weitz DA, Griffiths AD (2009) Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 9(13):1850–1858. doi:10.1039/b902504a
Chen CH, Miller MA, Sarkar A, Beste MT, Isaacson KB, Lauffenburger DA, Griffith LG, Han J (2013) Multiplexed protease activity assay for low-volume clinical samples using droplet-based microfluidics and its application to endometriosis. J Am Chem Soc 135(5):1645–1648. doi:10.1021/ja307866z
Khorshidi MA, Rajeswari PK, Wahlby C, Joensson HN, Andersson Svahn H (2014) Automated analysis of dynamic behavior of single cells in picoliter droplets. Lab Chip 14(5):931–937. doi:10.1039/c3lc51136g
Chokkalingam V, Tel J, Wimmers F, Liu X, Semenov S, Thiele J, Figdor CG, Huck WT (2013) Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics. Lab Chip 13(24):4740–4744. doi:10.1039/c3lc50945a
Courtois F, Olguin LF, Whyte G et al (2009) Controlling the retention of small molecules in emulsion microdroplets for use in cell-based assays. Anal Chem 81(8):3008–3016. doi:10.1021/ac802658n
Farkas DL, Nicolau DV, Leif RC, Song YS, Won YJ, Lee S-H, Kim DY (2014) Fluorescence lifetime imaging of lipids during 3T3-L1 cell differentiation. Langmuir 8947:89471W. doi:10.1117/12.2036160
Casadevall iSX, Srisaart M, Demello AJ, Edel JB (2010) Mapping of Fluidic Mixing in Microdroplets with 1 μs Time Resolution Using Fluorescence Lifetime Imaging. Anal Chem 82(9):3950–3956. doi:10.1021/ac100055g
Mazutis L, Araghi AF, Miller OJ, Baret JC, Frenz L, Janoshazi A, Taly V, Miller BJ, Hutchison JB, Link D, Griffiths AD, Ryckelynck M (2009) Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis. Anal Chem 81(12):4813
Agresti JJ, Antipov E, Abate AR, Ahn K, Rowat AC, Baret JC, Marquez M, Klibanov AM, Griffiths AD, Weitz DA (2010) Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc Natl Acad Sci U S A 107(9):4004–4009. doi:10.1073/pnas.0910781107
Fidalgo LM, Whyte G, Ruotolo BT, Benesch JL, Stengel F, Abell C, Robinson CV, Huck WT (2009) Coupling microdroplet microreactors with mass spectrometry: reading the contents of single droplets online. Angew Chem Int Edit 48(20):3665–3668. doi:10.1002/anie.200806103
Wang XL, Zhu Y, Fang Q (2014) Coupling liquid chromatography/mass spectrometry detection with microfluidic droplet array for label-free enzyme inhibition assay. Analyst 139(1):191–197. doi:10.1039/c3an01917a
Chen Q, Wu J, Zhang Y, Lin JM (2012) Qualitative and quantitative analysis of tumor cell metabolism via stable isotope labeling assisted microfluidic chip electrospray ionization mass spectrometry. Anal Chem 84(3):1695–1701. doi:10.1021/ac300003k
Sun S, Slaney TR, Kennedy RT (2012) Label free screening of enzyme inhibitors at femtomole scale using segmented flow electrospray ionization mass spectrometry. Anal Chem 84(13):5794–5800. doi:10.1021/ac3011389
Kuster SK, Pabst M, Jefimovs K, Zenobi R, Dittrich PS (2014) High-resolution droplet-based fractionation of nano-LC separations onto microarrays for MALDI-MS analysis. Anal Chem 86(10):4848–4855. doi:10.1021/ac4041982
Luo C, Ma Y, Li H, Chen F, Uchiyama K, Lin JM (2013) Generation of picoliter droplets of liquid for electrospray ionization with piezoelectric inkjet. J Mass Spectrom 48(3):321–328. doi:10.1002/jms.3159
Gasilova N, Yu Q, Qiao L, Girault HH (2014) On-chip spyhole mass spectrometry for droplet-based microfluidics. Angew Chem Int Edit 53(17):4408–4412. doi:10.1002/anie.201310795
Chen F, Lin L, Zhang J, He Z, Uchiyama K, Lin JM (2016) Single-cell analysis using drop-on-demand inkjet printing and probe electrospray ionization mass spectrometry. Anal Chem 88(8):4354–4360. doi:10.1021/acs.analchem.5b04749
Liu W, Lin JM (2016) Online monitoring of lactate efflux by multi-channel microfluidic chip-mass spectrometry for rapid drug evaluation. ACS Sens 1:344–347. doi:10.1021/acssensors.5b00221
Kelly RT, Page JS, Marginean I, Tang K, Smith RD (2009) Dilution-free analysis from picoliter droplets by nano-electrospray ionization mass spectrometry. Angew Chem Int Edit 48(37):6832–6835. doi:10.1002/anie.200902501
Smith CA, Li X, Mize TH, Sharpe TD, Graziani EI, Abell C, Huck WT (2013) Sensitive, high throughput detection of proteins in individual, surfactant-stabilized picoliter droplets using nanoelectrospray ionization mass spectrometry. Anal Chem 85(8):3812–3816. doi:10.1021/ac400453t
Su Y, Zhu Y, Fang Q (2013) A multifunctional microfluidic droplet-array chip for analysis by electrospray ionization mass spectrometry. Lab Chip 13(10):1876–1882. doi:10.1039/c3lc00063j
Kuster SK, Fagerer SR, Verboket PE, Eyer K, Jefimovs K, Zenobi R, Dittrich PS (2013) Interfacing droplet microfluidics with matrix-assisted laser desorption/ionization mass spectrometry: label-free content analysis of single droplets. Anal Chem 85(3):1285–1289. doi:10.1021/ac3033189
Verboket PE, Borovinskaya O, Meyer N, Gunther D, Dittrich PS (2014) A new microfluidics-based droplet dispenser for ICPMS. Anal Chem 86(12):6012–6018. doi:10.1021/ac501149a
Lee JK, Jansson ET, Nam HG, Zare RN (2016) High-resolution live-cell imaging and analysis by laser desorption/ionization droplet delivery mass spectrometry. Anal Chem 88(10):5453–5461. doi:10.1021/acs.analchem.6b00881
Zhang XC, Wei ZW, Gong XY, Si XY, Zhao YY, Yang CD, Zhang SC, Zhang XR (2016) Integrated droplet-based microextraction with ESI-MS for removal of matrix interference in single-cell analysis. Sci Rep 6:24730. doi:10.1038/srep24730
Zhu Y, Fang Q (2013) Analytical detection techniques for droplet microfluidics—a review. Anal Chim Acta 787:24–35. doi:10.1016/j.aca.2013.04.064
Moiseeva EV, Fletcher AA, Harnett CK (2011) Thin-film electrode based droplet detection for microfluidic systems. Sens Actuat B-Ch 155(1):408–414. doi:10.1016/j.snb.2010.11.028
Cahill BP, Land R, Nacke T, Min M, Beckmann D (2011) Contactless sensing of the conductivity of aqueous droplets in segmented flow. Sens Actuat B-Ch 159(1):286–293. doi:10.1016/j.snb.2011.07.006
Luo C, Yang X, Fu Q, Sun M, Ouyang Q, Chen Y, Ji H (2006) Picoliter-volume aqueous droplets in oil: electrochemical detection and yeast cell electroporation. Electrophoresis 27(10):1977–1983. doi:10.1002/elps.200500665
Liu S, Gu Y, Le Roux RB, Matthews SM, Bratton D, Yunus K, Fisher AC, Huck WT (2008) The electrochemical detection of droplets in microfluidic devices. Lab Chip 8(11):1937–1942. doi:10.1039/b809744e
Elbuken C, Glawdel T, Chan D, Ren CL (2011) Detection of microdroplet size and speed using capacitive sensors. Sens Actuat A-Ph 171(2):55–62. doi:10.1016/j.sna.2011.07.007
Edgar JS, Pabbati CP, Lorenz RM et al (2006) Capillary electrophoresis separation in the presence of an immiscible boundary for droplet analysis. Anal Chem 78(19):6948. doi:10.1021/ac0613131
Wang M, Roman GT, Perry ML et al (2009) Microfluidic chip for high efficiency electrophoretic analysis of segmented flow from a microdialysis probe and in vivo chemical monitoring. Anal Chem 81(21):9072. doi:10.1021/ac901731v
Cecchini MP, Hong J, Lim C, Choo J, Albrecht T, Demello AJ, Edel JB (2011) Ultrafast surface enhanced resonance Raman scattering detection in droplet-based microfluidic systems. Anal Chem 83(8):3076–3081. doi:10.1021/ac103329b
Shen H, Fang Q, Fang ZL (2006) A microfluidic chip based sequential injection system with trapped droplet liquid-liquid extraction and chemiluminescence detection. Lab Chip 6(10):1387–1389. doi:10.1039/b605332g
Buettner F, Natarajan KN, Casale FP, Proserpio V, Scialdone A, Theis FJ, Teichmann SA, Marioni JC, Stegle O (2015) Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat Biotechnol 33(2):155–160. doi:10.1038/nbt.3102
Schubert C (2011) Single-cell analysis: The deepest differences. Nature 480(7375):133. doi:10.1038/480133a
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134(5):703–707. doi:10.1016/j.cell.2008.08.021
Lim B, Reddy V, Hu X, Kim K, Jadhav M, Abedini-Nassab R, Noh YW, Lim YT, Yellen BB, Kim C (2014) Magnetophoretic circuits for digital control of single particles and cells. Nat Commun 5:3846. doi:10.1038/ncomms4846
Spiller DG, Wood CD, Rand DA, White MR (2010) Measurement of single-cell dynamics. Nature 465(7299):736–745. doi:10.1038/nature09232
Kang A, Park J, Ju J, Jeong GS, Lee SH (2014) Cell encapsulation via microtechnologies. Biomaterials 35(9):2651–2663. doi:10.1016/j.biomaterials.2013.12.073
Li JL, Day D, Gu M (2010) Design of a compact microfludic device for controllable cell distribution. Lab Chip 10(22):3054–3057. doi:10.1039/c0lc00090f
Moon D, Im DJ, Lee S, Kang IS (2014) A novel approach for drop-on-demand and particle encapsulation based on liquid bridge breakup. Exp Therm Fluid Sci 53:251–258. doi:10.1016/j.expthermflusci.2013.12.016
Kemna EW, Schoeman RM, Wolbers F, Vermes I, Weitz DA, van den Berg A (2012) High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip 12(16):2881–2887. doi:10.1039/c2lc00013j
Hong J, deMello AJ, Jayasinghe SN (2010) Bio-electrospraying and droplet-based microfluidics: control of cell numbers within living residues. Biomed Mater 5(2):21001. doi:10.1088/1748-6041/5/2/021001
Collins DJ, Alan T, Neild A (2014) The particle valve: on-demand particle trapping, filtering, and release from a microfabricated polydimethylsiloxane membrane using surface acoustic waves. Appl Phys Lett 105(3):033509. doi:10.1063/1.4891424
Amini H, Lee W, Di Carlo D (2014) Inertial microfluidic physics. Lab Chip 14(15):2739–2761. doi:10.1039/c4lc00128a
Lagus TP, Edd JF (2013) A review of the theory, methods and recent applications of high-throughput single-cell droplet microfluidics. J Phys D Appl Phys 46(11):114005. doi:10.1088/0022-3727/46/11/114005
Morimoto Y, Tan WH, Tsuda Y, Takeuchi S (2009) Monodisperse semi-permeable microcapsules for continuous observation of cells. Lab Chip 9(15):2217–2223. doi:10.1039/b900035f
Edd JF, Di CD, Humphry KJ et al (2008) Controlled encapsulation of single-cells into monodisperse picolitre drops. Lab Chip 8(8):1262. doi:10.1039/B805456H
Brouzes E, Kruse T, Kimmerling R, Strey HH (2015) Rapid and continuous magnetic separation in droplet microfluidic devices. Lab Chip 15(3):908–919. doi:10.1039/c4lc01327a
Morimoto Y, Tan WH, Takeuchi S (2009) Three-dimensional axisymmetric flow-focusing device using stereolithography. Biomed Microdevic 11(2):369–377. doi:10.1007/s10544-008-9243-y
Verbruggen B, Tóth T, Cornaglia M, Puers R, Gijs MAM, Lammertyn J (2014) Separation of magnetic microparticles in segmented flow using asymmetric splitting regimes. Microfluid Nanofluid 18(1):91–102. doi:10.1007/s10404-014-1409-8
He M, Edgar JS, Jeffries GDM et al (2009) Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets. Anal Chem 77(6):1539. doi:10.1021/ac0480850
Schoendube J, Wright D, Zengerle R, Koltay P (2015) Single-cell printing based on impedance detection. Biomicrofluidics 9(1):014117. doi:10.1063/1.4907896
Um E, Rha E, Choi SL, Lee SG, Park JK (2012) Mesh-integrated microdroplet array for simultaneous merging and storage of single-cell droplets. Lab Chip 12(9):1594. doi:10.1039/C2LC21266H
Wu TH, Chen Y, Park SY, Hong J, Teslaa T, Zhong JF, Di Carlo D, Teitell MA, Chiou PY (2012) Pulsed laser triggered high speed microfluidic fluorescence activated cell sorter. Lab Chip 12(7):1378–1383. doi:10.1039/c2lc21084c
Jing T, Ramji R, Warkiani ME, Han J, Lim CT, Chen CH (2015) Jetting microfluidics with size-sorting capability for single-cell protease detection. Biosens Bioelectron 66:19–23. doi:10.1016/j.bios.2014.11.001
Nam J, Lim H, Kim C, Yoon Kang J, Shin S (2012) Density-dependent separation of encapsulated cells in a microfluidic channel by using a standing surface acoustic wave. Biomicrofluidics 6(2):24120–2412010. doi:10.1063/1.4718719
Chen A, Byvank T, Chang WJ, Bharde A, Vieira G, Miller BL, Chalmers JJ, Bashir R, Sooryakumar R (2013) On-chip magnetic separation and encapsulation of cells in droplets. Lab Chip 13(6):1172–1181. doi:10.1039/c2lc41201b
McGrath J, Jimenez M, Bridle H (2014) Deterministic lateral displacement for particle separation: a review. Lab Chip 14(21):4139–4158. doi:10.1039/c4lc00939h
Tan Y-C, Ho YL, Lee AP (2007) Microfluidic sorting of droplets by size. Microfluid Nanofluid 4(4):343–348. doi:10.1007/s10404-007-0184-1
Joensson HN, Uhlen M, Svahn HA (2011) Droplet size based separation by deterministic lateral displacement-separating droplets by cell–induced shrinking. Lab Chip 11(7):1305–1310. doi:10.1039/c0lc00688b
Chabert M, Viovy JL (2008) Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc Natl Acad Sci U S A 105(9):3191–3196. doi:10.1073/pnas.0708321105
Landenberger B, Hofemann H, Wadle S, Rohrbach A (2012) Microfluidic sorting of arbitrary cells with dynamic optical tweezers. Lab Chip 12(17):3177–3183. doi:10.1039/c2lc21099a
Shim JU, Ranasinghe RT, Smith CA et al (2013) Ultrarapid generation of femtoliter microfluidic droplets for single-molecule-counting immunoassays. ACS Nano 7(7):5955–5964. doi:10.1021/nn401661d
Levison HF, Morbidelli A (2003) The formation of the Kuiper belt by the outward transport of bodies during Neptune’s migration. Nature 426(6965):419–421. doi:10.1038/nature02120
Chen Y, Wu TH, Kung YC, Teitell MA, Chiou PY (2013) 3D pulsed laser-triggered high-speed microfluidic fluorescence-activated cell sorter. Analyst 138(24):7308–7315. doi:10.1039/c3an01266b
Huang LR, Edward CC, Robert HA, James CS (2004) Continuous particle separation through deterministic lateral displacement. Science 14(304):987–990. doi:10.1126/science.1094567
Wu L, Chen P, Dong Y, Feng X, Liu BF (2013) Encapsulation of single cells on a microfluidic device integrating droplet generation with fluorescence-activated droplet sorting. Biomed Microdevic 15(3):553–560. doi:10.1007/s10544-013-9754-z
Joensson HN, Samuels ML, Brouzes ER et al (2009) Detection and analysis of low-abundance cell-surface biomarkers using enzymatic amplification in microfluidic droplets. Angew Chem Int Edit 48(14):2518–2521. doi:10.1002/anie.200804326
Link DR, Grasland-Mongrain E, Duri A, Sarrazin F, Cheng Z, Cristobal G, Marquez M, Weitz DA (2006) Electric control of droplets in microfluidic devices. Angew Chem Int Edit 45(16):2556–2560. doi:10.1002/anie.200503540
Franke T, Braunmuller S, Schmid L, Wixforth A, Weitz DA (2010) Surface acoustic wave actuated cell sorting (SAWACS). Lab Chip 10(6):789–794. doi:10.1039/b915522h
Eastburn DJ, Sciambi A, Abate AR (2014) Identification and genetic analysis of cancer cells with PCR-activated cell sorting. Nucleic Acids Res 42(16):e128. doi:10.1093/nar/gku606
Kemna EW, Segerink LI, Wolbers F et al (2013) Label-free, high-throughput, electrical detection of cells in droplets. Analyst 138(16):4585–4592. doi:10.1039/C3AN00569K
Yu D, Gustafson WC, Han C, Lafaye C, Noirclerc-Savoye M, Ge WP, Thayer DA, Huang H, Kornberg TB, Royant A, Jan LY, Jan YN, Weiss WA, Shu X (2014) An improved monomeric infrared fluorescent protein for neuronal and tumour brain imaging. Nat Commun 5:3626. doi:10.1038/ncomms4626
Cesinaro AM, Natoli C, Grassadonia A, Tinari N, Iacobelli S, Trentini GP (2002) Expression of the 90 K tumor-associated protein in benign and malignant melanocytic lesions. J Invest Dermatol NLM 119(1):187–190. doi:10.1046/j.1523-1747.2002.17642.x
Ramdzan YM, Polling S, Chia CP, Ng IH, Ormsby AR, Croft NP, Purcell AW, Bogoyevitch MA, Ng DC, Gleeson PA, Hatters DM (2012) Tracking protein aggregation and mislocalization in cells with flow cytometry. Nat Methods 9(5):467–470. doi:10.1038/nmeth.1930
Franci C, Takkunen M, Dave N, Alameda F, Gomez S, Rodriguez R, Escriva M, Montserrat-Sentis B, Baro T, Garrido M, Bonilla F, Virtanen I, Garcia de Herreros A (2006) Expression of Snail protein in tumor-stroma interface. Oncogene 25(37):5134–5144. doi:10.1038/sj.onc.1209519
Guo MT, Rotem A, Heyman JA, Weitz DA (2012) Droplet microfluidics for high-throughput biological assays. Lab Chip 12(12):2146–2155. doi:10.1039/c2lc21147e
Kang D-K, Monsur Ali M, Zhang K, Pone EJ, Zhao W (2014) Droplet microfluidics for single-molecule and single-cell analysis in cancer research, diagnosis and therapy. TrAC-Trend Anal Chem 58:145–153. doi:10.1016/j.trac.2014.03.006
Hummer D, Kurth F, Naredi-Rainer N, Dittrich PS (2016) Single cells in confined volumes: microchambers and microdroplets. Lab Chip 16(3):447–458. doi:10.1039/c5lc01314c
Konry T, Smolina I, Yarmush JM, Irimia D, Yarmush ML (2011) Ultrasensitive detection of low-abundance surface-marker protein using isothermal rolling circle amplification in a microfluidic nanoliter platform. Small 7(3):395–400. doi:10.1002/smll.201001620
Easley CJ, Rocheleau JV, Head WS, Piston DW (2009) Quantitative measurement of zinc secretion from pancreatic islets with high temporal resolution using droplet-based microfluidics. Anal Chem 81(21):9086–9095. doi:10.1021/ac9017692
Joensson HN, Samuels ML, Brouzes ER, Medkova M, Uhlen M, Link DR, Andersson-Svahn H (2009) Detection and analysis of low-abundance cell-surface biomarkers using enzymatic amplification in microfluidic droplets. Angew Chem Int Edit 48(14):2518–2521. doi:10.1002/anie.200804326
Konry T, Golberg A, Yarmush M (2013) Live single cell functional phenotyping in droplet nano-liter reactors. Sci Rep 3:3179. doi:10.1038/srep03179
Konry T, Dominguez-Villar M, Baecher-Allan C, Hafler DA, Yarmush ML (2011) Droplet-based microfluidic platforms for single T cell secretion analysis of IL-10 cytokine. Biosens Bioelectron 26(5):2707–2710. doi:10.1016/j.bios.2010.09.006
Wu N, Zhu Y, Brown S, Oakeshott J, Peat TS, Surjadi R, Easton C, Leech PW, Sexton BA (2009) A PMMA microfluidic droplet platform for in vitro protein expression using crude E. coli S30 extract. Lab Chip 9(23):3391–3398. doi:10.1039/b911581a
Novak R, Zeng Y, Shuga J, Venugopalan G, Fletcher DA, Smith MT, Mathies RA (2011) Single-cell multiplex gene detection and sequencing with microfluidically generated agarose emulsions. Angew Chem Int Edit 50(2):390–395. doi:10.1002/anie.201006089
Eastburn DJ, Sciambi A, Abate AR (2013) Ultrahigh-throughput mammalian single-cell reverse-transcriptase polymerase chain reaction in microfluidic drops. Anal Chem 85(16):8016–8021. doi:10.1021/ac402057q
Hatch AC, Ray T, Lintecum K, Youngbull C (2014) Continuous flow real-time PCR device using multi-channel fluorescence excitation and detection. Lab Chip 14(3):562–568. doi:10.1039/c3lc51236c
Zhang Y, Ozdemir P (2009) Microfluidic DNA amplification—a review. Anal Chim Acta 638(2):115–125. doi:10.1016/j.aca.2009.02.038
Zhang Y, Jiang HR (2016) A review on continuous-flow microfluidic PCR in droplets: advances, challenges and future. Anal Chim Acta 914:7–16. doi:10.1016/j.aca.2016.02.006
Curcio M, Roeraade J (2003) Continuous segmented-flow polymerase chain reaction for high-throughput miniaturized DNA amplification. Anal Chem 75(1):1–7. doi:10.1021/ac0204146
Zhong Q, Bhattacharya S, Kotsopoulos S, Olson J, Taly V, Griffiths AD, Link DR, Larson JW (2011) Multiplex digital PCR: breaking the one target per color barrier of quantitative PCR. Lab Chip 11(13):2167–2174. doi:10.1039/c1lc20126c
Wang P, Jing F, Li G, Wu Z, Cheng Z, Zhang J, Zhang H, Jia C, Jin Q, Mao H, Zhao J (2015) Absolute quantification of lung cancer related microRNA by droplet digital PCR. Biosens Bioelectron 74:836–842. doi:10.1016/j.bios.2015.07.048
Leman M, Abouakil F, Griffiths AD, Tabeling P (2015) Droplet-based microfluidics at the femtolitre scale. Lab Chip 15(3):753–765. doi:10.1039/c4lc01122h
Pekin D, Skhiri Y, Baret JC, Le Corre D, Mazutis L, Salem CB, Millot F, El Harrak A, Hutchison JB, Larson JW, Link DR, Laurent-Puig P, Griffiths AD, Taly V (2011) Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 11(13):2156–2166. doi:10.1039/c1lc20128j
Hartung R, Brosing A, Sczcepankiewicz G, Liebert U, Hafner N, Durst M, Felbel J, Lassner D, Kohler JM (2009) Application of an asymmetric helical tube reactor for fast identification of gene transcripts of pathogenic viruses by micro flow-through PCR. Biomed Microdevic 11(3):685–692. doi:10.1007/s10544-008-9280-6
Mohr S, Zhang YH, Macaskill A, Day PJR, Barber RW, Goddard NJ, Emerson DR, Fielden PR (2007) Numerical and experimental study of a droplet-based PCR chip. Microfluid Nanofluid 3(5):611–621. doi:10.1007/s10404-007-0153-8
Leng X, Zhang W, Wang C, Cui L, Yang CJ (2010) Agarose droplet microfluidics for highly parallel and efficient single molecule emulsion PCR. Lab Chip 10(21):2841–2843. doi:10.1039/c0lc00145g
Kim H, Dixit S, Green CJ et al (2009) Nanodroplet real-time PCR system with laser assisted heating. Opt Express 17(1):218–227. doi:10.1364/OE.17.000218
Yun H, Kim K, Lee WG (2013) Cell manipulation in microfluidics. Biofabrication 5(2):022001. doi:10.1088/1758-5082/5/2/022001
Ding Y, Qiu F, Casadevall iSX, Chiu F, Nelson B, deMello A (2016) Microfluidic-based droplet and cell manipulations using artificial bacterial flagella. Micromachines 7(2):25. doi:10.3390/mi7020025
Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21(12):745–754. doi:10.1016/j.tcb.2011.09.005
Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E (2013) Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500(7461):217–221. doi:10.1038/nature12298
Headen DM, Aubry G, Lu H, Garcia AJ (2014) Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv Mater 26(19):3003–3008. doi:10.1002/adma.201304880
Liu K, Deng Y, Zhang N, Li S, Ding H, Guo F, Liu W, Guo S, Zhao X-Z (2012) Generation of disk-like hydrogel beads for cell encapsulation and manipulation using a droplet-based microfluidic device. Microfluid Nanofluid 13(5):761–767. doi:10.1007/s10404-012-0998-3
Seiffert S (2011) Functional microgels tailored by droplet-based microfluidics. Macromol Rapid Commun 32(20):1600–1609. doi:10.1002/marc.201100342
Sakakihara S, Araki S, Iino R, Noji H (2010) A single-molecule enzymatic assay in a directly accessible femtoliter droplet array. Lab Chip 10(24):3355–3362. doi:10.1039/c0lc00062k
Tumarkin E, Kumacheva E (2009) Microfluidic generation of microgels from synthetic and natural polymers. Chem Soc Rev 38(8):2161–2168. doi:10.1039/b809915b
Siltanen C, Yaghoobi M, Haque A, You J, Lowen J, Soleimani M, Revzin A (2016) Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids. Acta Biomater 34:125–132. doi:10.1016/j.actbio.2016.01.012
Kumachev A, Greener J, Tumarkin E, Eiser E, Zandstra PW, Kumacheva E (2011) High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation. Biomaterials 32(6):1477–1483. doi:10.1016/j.biomaterials.2010.10.033
Du GS, Pan JZ, Zhao SP, Zhu Y, den Toonder JM, Fang Q (2013) Cell-based drug combination screening with a microfluidic droplet array system. Anal Chem 85(14):6740–6747. doi:10.1021/ac400688f
Yu JQ, Huang W, Chin LK, Lei L, Lin ZP, Ser W, Chen H, Ayi TC, Yap PH, Chen CH, Liu AQ (2014) Droplet optofluidic imaging for lambda-bacteriophage detection via co-culture with host cell Escherichia coli. Lab Chip 14(18):3519–3524. doi:10.1039/c4lc00042k
Zhang J, Chen F, He Z, Ma Y, Uchiyama K, Lin JM (2016) A novel approach for precisely controlled multiple cell patterning in microfluidic chips by inkjet printing and the detection of drug metabolism and diffusion. Analyst 141(10):2940–2947. doi:10.1039/c6an00395h
Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, Peerani R, Purpura K, Zandstra PW, Kumacheva E (2011) High-throughput combinatorial cell co-culture using microfluidics. Integr Biol 3(6):653–662. doi:10.1039/c1ib00002k
Liu Z, Shum HC (2013) Fabrication of uniform multi-compartment particles using microfludic electrospray technology for cell co-culture studies. Biomicrofluidics 7(4):44117. doi:10.1063/1.4817769
Peng Lee C, Hsin Chen Y, Hang Wei Z (2013) Fabrication of hexagonally packed cell culture substrates using droplet formation in a T-shaped microfluidic junction. Biomicrofluidics 7(1):14101. doi:10.1063/1.4774315
Chen H, Sun J, Wolvetang E, Cooper-White J (2015) High-throughput, deterministic single cell trapping and long-term clonal cell culture in microfluidic devices. Lab Chip 15(4):1072–1083. doi:10.1039/c4lc01176g
Taylor RJ, Falconnet D, Niemisto A, Ramsey SA, Prinz S, Shmulevich I, Galitski T, Hansen CL (2009) Dynamic analysis of MAPK signaling using a high-throughput microfluidic single-cell imaging platform. Proc Natl Acad Sci U S A 106(10):3758–3763. doi:10.1073/pnas.0813416106
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wang, J., Lin, JM. (2018). Droplet-Based Microfluidic Technology for Cell Analysis. In: Lin, JM. (eds) Cell Analysis on Microfluidics. Integrated Analytical Systems. Springer, Singapore. https://doi.org/10.1007/978-981-10-5394-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-5394-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5393-1
Online ISBN: 978-981-10-5394-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)