Abstract
Human milk is a vital source of nutrient as well as a continuous source of bacteria to newborn. Microbes are present in milk aid to initiation and development of infant gut microflora. These bacteria play a vital role in lessening of incidences and severity of infection to the child. Breast milk protects the newborn against infectious diseases, as it consists of different antimicrobial compounds, immunoglobulin, immune component cells, and bacteriocins secreted by probiotic bacteria, which all together provoke the growth of the helpful bacteria in neonate gut. However, breastfeeding mothers may also experience a condition called mastitis. Mastitis, one of the most common conditions experienced by breastfeeding mother, is an inflammation of connective tissue within the mammary gland. It is caused by a mixture of pathogenic bacteria and often treated with antimicrobials. The recent advances in metagenomic sequencing and amplicon sequencing technologies, which try to capture all the DNA information from the biological sample, have been widely used for the characterization of microbial community present within a sample and identification of unknown etiological agents involved in diseased condition. In the present review, effort has been made to understand the development of milk microflora and also the microbial diversity in healthy and infected breast. The present article reveals that breast milk is a source of more life than we envision.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The human microbiome is defined as collection of microbial species that colonize many body sites, including human milk. The human microbiome project was undertaken by the National Institutes of Health with a goal to conduct survey of microbes present within the body and those resting on human body and the potential impact these communities have on health. However, one of the key systems was ignored, human milk. Human milk was conventionally considered as sterile; however, recent examination discovered a constant foundation of commensal, mutualistic, and probiotic bacteria in human milk.
2 Human Milk
Human milk is an intricate biological fluid which fulfills the nutritional supplies of newborn baby, helps in the development of infant immune system, and provides defense against pathogens (Morrow and Rangel 2004). Bioactive molecules like polyamines, oligosaccharides, fatty acids, lactoferrin, lysozyme, immunoglobulin, immune-competent cells, and antimicrobial peptides present in colostrum and milk (Newburg 2005) are the main constituents involved in providing defense. Recent studies articulated the presence of not only environmental bacteria but also the symbiotic and probiotic bacteria in the milk which are transmitted through milk to the infant and hence contribute in initial colonization of gut microflora of the infant (Martín et al. 2009). Daily consumption of human milk by an infant is 800 ml/day; this in fact contributes to transport of 1 × 105 to 1 × 107 bacteria each day leading to their colonization in gut microflora (Heikkilä and Saris 2003). Human milk protects against gastrointestinal infections (Duijts et al. 2010), respiratory infections (Nishimura et al. 2009), and allergic diseases (Greer et al. 2008; Ip et al. 2008). According to the American Academy of Pediatrics (AAP), it also trims down the possibility of diseases like inflammatory bowel disease (IBD), obesity, or diabetes.
As neonates are born with immature immune system, they are more prone to get infected. At that time breastfeeding helps in building up the immune system by providing fatty acids, α-lactalbumin, sIgA, oligosaccharides, lactoferrin, lysozyme, antioxidants, and cytokine molecules bearing immune-protective role (Chirico et al. 2008; Goldman 2007). Human milk proteome consists of 976 proteins, out of which plentiful possess immunogenic property (Molinari et al. 2013; Gao et al. 2012). In addition to immune molecules, human milk also consists of blood-derived leukocytes which get transported to milk via the paracellular pathway. Bacteria present in human milk play numerous roles in the infant gut; they reduce the occurrence and severity of infections, produce antimicrobial compounds, or improve intestinal barrier function by enhancing mucin production and dropping intestinal permeability (Olivares et al. 2014). Studies have shown that accumulation of Lactobacillus strain, isolated from human milk, reduces the incidence of gastrointestinal infection, upper respiratory tract infections, and total number of infections to 46%, 27%, and 30% (Maldonado et al. 2012). These microorganisms also contribute in digestion by breaking down sugars and proteins and also participate in the right maturation of the infant immune system.
3 Origin of Microflora in the Human Milk
Physiological and hormonal alteration occurring during and after pregnancy increased gut permeability which in turn helps in the transfer of gut microflora to the mammary gland. Dendritic cells and macrophages also play an important role in the migration of microbes to the mammary gland (Fernández et al. 2013). These bacteria are transferred from maternal community to breast milk via the entero-mammary pathway (Fig. 7.1). Along with above apparent mechanisms, the retrograde flux between the mother’s skin microbes and infant’s oral microbes may also help in the development of the human milk microbiome (Makino et al. 2011; Albesharat et al. 2011).
Origin of microflora in human breast milk. Source: Fernández et al. (2013)
4 Mechanism of Health-Promoting Probiotic Bacteria
The milk microbiota plays a significant role in decreasing the frequency of infection to the newborn babies due to their probiotic properties (Fig. 7.2). Probiotics have a potential to produce antimicrobial substance like bacteriocins which work as antagonists to the pathogenic bacteria and their efficient antagonistic activity is by alone or synergistically. These antimicrobial substances can be protein and bioactive peptides. Bacteriocins are important antimicrobial peptides which have therapeutic activity against intestinal pathogenic microbes (Thirabunyanon et al. 2009; Verdenelli et al. 2009; Gaudana et al. 2010). They also produce metabolites, i.e., acetic and lactic acids, which reduce the pH in the intestine and generate adverse environment for pathogen to survive (Ridwan et al. 2008). Probiotics can remove pathogens using competitive exclusion and/or blocking their attachment at the intestinal epithelium cells by competing for the glycoconjugate receptors (Vanderpool et al. 2008).
5 Cell of Human Milk
Human milk alters in composition since colostrum to late lactation and varies within feeds and between mothers. Human milk consists of 75% leukocytes, i.e., neutrophils, erythrocytes, macrophages, and lymphocytes, and 25% epithelial cells (Paape and Weinland 1988). The epithelial cells of the glands are normally shed and get renewed, but at the time of infection, the number increases. The white blood cells work as a defense mechanism which fight against the infection and help in the repair of damaged tissue. During inflammation, it was observed that the level of neutrophils increases by 90% in human milk to fight against infection (Miller et al. 1985; Cooey and Harmon 1994). Moreover, composition of somatic cells in human milk changes with respect to lactation cycle and type of secretion (Table 7.1). Generally, the number of SCC in human milk from healthy mammary gland is approximately 1 × 105 cells/ml, while challenge with bacterial infection causes it to increase above 1 × 106 cells/ml (Bytyqi et al. 2010). Of the somatic cells, leukocyte is the most studied cell type in human milk, and depending on stage of lactation and health status of breastfeeding dyad, it may account for considerable portion of human milk (Boutinaud and Jammes 2002; Hassiotou et al. 2012; Cregan 2002; Ho et al. 1979). Many of these leukocytes are activated, motile, and interactive (Smith and Goldman 1970). This suggests that they confer active immunity to the infant (Wirt et al. 1991). This was further supported by in vivo studies in animal models showing active transfer of milk leukocyte through the intestinal epithelium into the blood circulation, and movement to and engraftment in different organs, including the mesenteric nodes, liver, and spleen (Weiler et al. 1983; Zhou et al. 2000; Michie et al. 1998; Schnorr and Pearson 1984).
6 Microbial Profiling of Human Milk
During the last decades, microbiological studies that focused on human milk were restricted to the identification of potential pathogenic bacteria in stored milk or milk retrieved from maternal infected human milk, but microbes present in healthy human milk are unexplored (El-Mohandes et al. 1993; Wright et al. 1998). Standard microbiological based culturing methods can only detect small proportion of bacteria because the great majority of bacteria on earth are not culturable in laboratory condition. To identify these unculturables and estimate real bacterial diversity, culture-independent method is required. Sequence-based identification of microbial species through sequencing has overcome the limitation. The nine hypervariable regions of 16S rRNA can be used for identification of bacterial species. Amplification of 16S rRNA region using universal primer is useful for estimation of bacterial diversity.
7 Culture-Dependent Assessment of Human Milk Microbial Diversity
Initial report of culture-dependent methods for studying human milk microbial diversity came in 2003 by Dr. Juan Rodriguez with his associate researcher R. Martin. They isolated a total of 178 isolates from each mother and infant pair (human milk, nipple areola, infant’s mouth and feces) and subjected it to randomly amplified polymorphic DNA (RAPD) analysis and identified by 16S rDNA sequencing. Bacteria having identical profiles in mother and child pair were identified as Lactobacillus gasseri and Enterococcus faecium. Surprisingly, none of the lactic acid bacteria isolated from breast skin shared RAPD profiles into other sources (Martín et al. 2003).
After that Grönlund et al. (2007) studied the association of maternal fecal and breast milk bifidobacteria and infant fecal bifidobacteria using real-time PCR from 61 mother-infant pairs. They found that Bifidobacterium longum was the most abundant species isolated from breast milk. Moreover, they concluded that Bifidobacterium adolescentis and Bifidobacterium bifidum colonization frequency and count correlated significantly among mother and infant pairs (Grönlund et al. 2007).
Collado et al. (2009) in their study examined 50 breast milk samples for the presence of differential bacterial genera by using qPCR technique. They found that Staphylococcus, Streptococcus, Bifidobacterium, and Lactobacillus were the most abundant genera in all the samples. In addition, Collado et al. (2012) studied the effect of maternal weight and weight gain during pregnancy on milk microbiota (56 mothers, 22 overweight and 34 normal weight) using qPCR. Staphylococcus group bacteria were observed in higher number, whereas Bifidobacterium group was in lower level, in overweight mother compared to normal-weight mother. Moreover, they found higher prevalence of Akkermansia muciniphila in higher number in breast milk of overweight mothers (Collado et al. 2009).
Solís et al. (2010) studied the development of lactic acid bacteria and bifidobacteria during the first 3 months of life in 20 vaginally delivered breastfed infants and mothers. Streptococcus, Lactobacillus, and Bifidobacterium were the most dominant genera in breast milk contributing to the initial establishment of microbiota in newborn (Solís et al. 2010).
Albesharat et al. (2011) isolated a total 700 isolates of LAB from fecal sample of breastfeeding mother, feces of their infant, from breast milk, and from fermented food that is normally consumed in Syria, and characterized it by RAPD and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Their study demonstrates occurrence of 36 different species of Lactobacillus, Enterococcus, Streptococcus, Weissella, and Pediococcus. Interestingly, they found identical RAPD genotype of L. plantarum, L. fermentum, L. brevis, Enterococcus faecium, Enterococcus faecalis, and P. pentosaceus in feces of mother, in breast milk of mother, and in feces of her babies (Albesharat et al. 2011).
In 2014, Khodayar-Pardo et al. studied the bacterial population present in 32 Spanish breastfeeding women using quantitative PCR and determine the influence of lactational stage, gestational age, and delivery mode on milk microbiota. They identified Enterococcus, Lactobacillus, and Streptococcus spp. as the dominant bacterial group. They also concluded that Bifidobacterium is found more commonly in vaginal than cesarean deliveries (Khodayar-Pardo et al. 2014).
Afterward, Soto et al. (2014) isolated Bifidobacterium, Lactobacillus, Enterococcus, and Staphylococcus species from breast milk of 47 Slovenian lactating mother. Moreover, Gonzalez et al. (2013) also found Staphylococcus, Streptococcus, and Lactobacillus genera in breast milk collected from 121 Mozambique women (Albesharat et al. 2011).
8 Culture-Independent Assessment of Human Milk Microbial Diversity
In 2011, Hunt et al. used a new approach (454 pyrosequencing), which utilized specific primer targeting the V1–V2 hypervariable region of 16S rRNA gene of bacteria. They characterized microbial diversity and temporal stability of bacterial profiles in healthy human milk collected from 16 US women over a 4-week period (Hunt et al. 2011). Half of the bacterial sequences were contributed by nine “core” OTUs which include Pseudomonas, Staphylococcus, Serratia, Corynebacterium, Ralstonia, Streptococcus, Sphingomonas, Bradyrhizobium, and Propionibacterium. Moreover, the proportion of these core OTUs varied greatly between subjects.
Similarly, Cabrera-Rubio et al. (2012) studied bacterial diversity in human milk over three different time points (colostrum 1 and 6 months postpartum) in 18 Finnish women (Cabrera-Rubio et al. 2012). They found that human milk microbiome changes over lactation period. Bacteria belonging to Weissella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus were more abundant in colostrum. While in 1- and 6-month milk samples, Veillonella, Leptotrichia, and Prevotella, typical inhabitants of oral cavity, increased significantly. Moreover, they concluded that milk from obese mother tends to be altered and less diverse than normal-weight mothers.
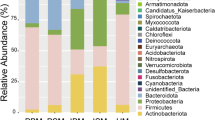
Jost et al. (2013) examined bacterial diversity in breast milk of seven mothers at three different sampling points (days 3–6, 9–14, and 25–30 postpartum) using culture-dependent and culture-independent techniques. They found that Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria were the most abundant phyla, including representatives from the genera Pseudomonas, Staphylococcus, Ralstonia, Streptococcus, Bacteroides, Blautia, and Bifidobacterium. Moreover, they also found, for the first time, bacteria belonging to Faecalibacterium and Roseburia, which are butyrate producers and important for colonic health (Jost et al. 2013).
After that, Ward et al. (2013) performed metagenomic functional analysis of a pooled milk samples from ten donor mothers using Illumina sequencing. Over 360 bacterial genera were identified with predominance of sequences belonging to Proteobacteria and Firmicutes. In addition, they also concluded that human milk is less diverse than the feces of infant and mother at the phylum level. Human milk contained prominent amounts of genetic component which link with nitrogen membrane transport, stress response, metabolism, and immunomodulatory functions (Ward et al. 2013).
In addition, Urbaniak et al. (2014) studied bacterial diversity in human milk collected every 2 weeks over a 4-month period from lactating mothers undergoing chemotherapy of Hodgkin’s lymphoma. They found that chemotherapy causes substantial alteration in microbiome from healthy controls, with reduction in genera such as Bifidobacterium, Eubacterium, Staphylococcus, and Cloacibacterium (Urbaniak et al. 2014).
Two recent independent studies by Cabrera-Rubio et al. (2015) and Urbaniak et al. (2016) studied the milk microbiota composition of healthy women and correlated it to birthing method. In addition to birthing method, Urbaniak et al. (2016) also studied alteration of milk microbiota with gestation time and infant gender. Urbaniak et al. (2016) in their study collected human milk from 39 Canadian mothers and analyzed microbial profiles by 16S rRNA sequencing using Illumina platform. They found Proteobacteria and Firmicutes as most dominant phyla and Staphylococcus, Pseudomonas, Streptococcus, and Lactobacillus as most abundant genera. However, comparison of bacterial profile between term and preterm infants, vaginal and C-section deliveries, and male and female showed no statistical significant difference (Urbaniak et al. 2016). In contrast, Cabrera-Rubio et al. (2015), in their study, compared milk microbiome of six vaginally delivered mothers and four cesarean delivered mothers and found significant separation of milk microbiome based on mode of delivery (Cabrera-Rubio et al. 2015).
The microbiota of breast milk from 90 Chinese lactating women was analyzed with two different collection procedures (without aseptic cleansing and after aseptic cleaning) by Olga Sakwinska et al. (2016). They found that Streptococci and Staphylococci were the most abundant in both the group and results were consistent with that of previous study. However, they revealed that breast milk collected without aseptic cleansing and rejection of foremilk had higher abundance of Acinetobacter sp. Moreover, bifidobacteria and Lactobacilli were present in few samples but with low abundance (Sakwinska et al. 2016).
9 Overview of Mastitis
Mastitis is an inflammation of connective tissue within the mammary gland (Gianneechini et al. 2002; Zhao and Lacasse 2008). The term comes from the Greek word masto-referring to the mammary gland and its meaning inflammation. It is characterized by physical, chemical, and bacteriological changes in the breast milk. It is the most common condition experienced by lactating mothers. Incidence of occurrence of mastitis varies extensively because of difference in breastfeeding method. As per the data of WHO (World Health Organization), overall 2–33% of breastfeeding mothers are thought to be infected with mastitis (WHO 2000). Studies conducted in the USA, New Zealand, Finland, and Australia suggest that 20–25% of breastfeeding women have chances of developing mastitis (Kinlay et al. 1998; Fetherston 1997; Foxman et al. 2002). Although mastitis is a very common condition, very few studies are conducted on it till date (Foxman et al. 2002). Mastitis usually affects lactating women; hence, it is known as lactational mastitis.
Mastitis is a deliberately painful condition experienced by breastfeeding mothers. It is mainly found to be prevalent during second and third week postpartum. Mastitis can be caused by an infection or excess of milk remaining in the milk tissue (milk stasis). Mastitis is usually the result of a blocked milk duct that hasn’t cleared. Milk banked up behind the blocked duct can be forced into nearby breast tissue, causing the tissue to become inflamed. Sometimes it may occur due to sudden stop of breastfeeding. Infectious mastitis develops when bacteria commonly found on skin enter the mammary gland through cracked nipples and multiply in the fatty tissue of mammary gland resulting in infection.
9.1 Mastitis: A Dysbiosis of Breast Milk Bacteria
Breast milk has got vibrant bacterial diversity mainly that related with skin and non-skin. These bacteria are transported from maternal community to breast milk via the entero-mammary pathway. Pathogenesis of mastitis could have resulted from enrichment of pathogenic bacteria in milk and mutual healthy milk microflora killed due to toxins released by pathogenic bacteria.
9.1.1 Types of Mastitis
Scandinavian researchers suggested in the 1980s that mastitis should be classified into two classes: clinical mastitis and subclinical mastitis.
Clinical mastitis: It is characterized by the presence of gross inflammatory signs and symptoms.
Clinical mastitis can be divided into three types:
-
1.
Preacute mastitis: Inflammation and changes in milk composition. Systemic signs like fever, depression, shivering, loss of appetite, and loss of weight.
-
2.
Acute mastitis: Similar to preacute mastitis, but with mild signs like fever and mild depression.
-
3.
Subacute mastitis: In this type of mastitis, signs of inflammation are minimal and no visible systemic signs.
Subclinical mastitis: This form of mastitis is characterized by change in milk composition with no signs of gross inflammation or milk abnormalities.
Mastitis is associated with increased somatic cells, free fatty acids, and interleukin-8 concentrations (Hunt et al. 2013). However, fresh milk produced by a mastitis gland has free fatty acids (FFAs) and when stored at 4 °C exhibits greater rates if lipolysis occurs (Randolph and Erwin 1974; Murphy et al. 1989).
9.2 Mastitis and Somatic Cell Count
Somatic cells are white blood cells; their number increases during bacterial infection in order to fight against pathogenic bacteria (Sharma et al. 2011). Thus somatic cells can be a better indicator of infectious condition in mammary gland. Somatic cell count in women with mastitis usually has an elevated count compared to healthy women (Hunt et al. 2013; Hassiotou et al. 2013). Intramammary infection results in a significant increase in the somatic cell count level in the breast milk. In response to invasion of mammary gland by bacteria, leukocytes are released into the milk to kill the bacteria, which results in increases in somatic cell numbers and ultimately leads to inflammation and blocked milk ducts. Moreover somatic cells contain lipolytic and proteolytic enzymes, which degrade fats and proteins, respectively. Upon challenge by bacterial infection, the amount of destructive enzymes carried out by increased somatic cells results into deterioration of milk fat and protein. Somatic cell count is often used for diagnosis of mastitis in case of bovine animals.
9.3 Etiology of Mastitis
Etiological agents of mastitis can be infectious or noninfectious. Organisms which may cause mastitis are bacteria, viruses, mycoplasma, yeasts, and algae. Gram-positive, catalase-positive bacteria are mostly isolated from mastitis-infected milk. It can be caused by microbes, such as Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus uberis, Streptococcus agalactiae, Staphylococcus epidermidis, Corynebacterium bovis, Corynebacterium pyogenes, Klebsiella sp., and Candida albicans. Among all of these microorganisms, the most important are Staphylococcus aureus and Staphylococcus epidermidis, which is a common cause of mastitis, and it is commonly isolated from mastitis-infected milk. In Brazil, studies reported the predominance of Staphylococcus aureus over other disease-causing agents in all regions of the country (Rodrigues et al. 2015). Other than this, coagulase-negative Staphylococci are considered as minor mastitis-causing pathogens. Nineteen distinct species of coagulase-negative Staphylococci have been revealed to date. Members of the Staphylococcus epidermidis subgroup include S. hominis, S. warneri, S. capitis, and S. haemolyticus. Variety of pathogenic organism causing mastitis can be divided into two groups: contagious mastitis pathogens and environmental mastitis pathogens.
Contagious mastitis pathogens: These are commonly found on the skin and enter into the mammary gland through cracked or sore nipples. The major contagious pathogens are Staphylococcus aureus and Streptococcus agalactiae.
Environmental mastitis pathogens: Environmental mastitis pathogens are also known as opportunistic mastitis pathogens because they will take the opportunity to cause mastitis and cause intramammary infections sporadically. The most common environmental mastitis pathogens are Staphylococcus chromogenes, Staphylococcus simulans, Staphylococcus xylosus, Staphylococcus epidermidis, Staphylococcus hyicus, and Staphylococcus haemolyticus.
9.3.1 Staphylococcus aureus and Its Virulence Gene
Staphylococcus aureus is a gram-positive bacteria associated with many serious diseases in humans as well as animals, and it is found to be the most predominant bacteria causing human mastitis with relevant losses in the dairy industry (Bjork et al. 2014; Li et al. 2009). S. aureus is the most common species of Staphylococci to cause Staphylococcus infections. It is frequently found in the human respiratory tract and on the skin. Although S. aureus is not always pathogenic, it is a common cause of skin infections (e.g., boils), respiratory disease (e.g., sinusitis), and food poisoning. Other than this S. aureus can cause a range of illnesses, from minor skin infections, such as pimples, impetigo, boils (furuncles), cellulitis folliculitis, carbuncles, scalded skin syndrome, and abscesses, to life-threatening disease such as pneumonia, meningitis, osteomyelitis, endocarditis, toxic shock syndrome, bacteremia, and sepsis. Virulence factors, such as surface proteins that promote colonization of host tissues, surface factors that inhibit phagocytic engulfment (protein A), biochemical properties that enhance their survival in phagocytes (catalase production), immunological disguises (protein A, coagulase clotting factor), and acquired resistance to microbial agents, are often expressed by S. aureus. Clumping factor is the surface agent that acts as adhesions. Coagulase is tightly bound to the surface of S. aureus and coats its surface with fibrin upon contact with blood. This fibrin-coated S. aureus resists phagocytosis. Protein A binds to IgG in wrong orientation in serum, thus preventing opsonization and phagocytosis.
The role of bacteria in lactational mastitis is still questionable. Although it is most common among lactating women, there is lack of scientific analysis on it. Culture-dependent and culture-independent assessment of mastitis-infected breast milk can provide in-depth analysis of microflora involved in diseased condition. Culture-dependent studies involve classical microbiological techniques and it has several drawbacks, while culture-independent studies involve assessment of microflora by metagenomic approach with the recent next-generation sequencing technology.
9.4 Culture-Dependent Assessment of Mastitis
There are many conventional techniques used for isolation and identification of pathogenic bacteria. Isolation of pathogenic bacteria on the sheep blood agar is widely used in many laboratories because pathogenic bacteria grow via engulfing the red blood cell and appear as greenish colony. Otherwise if pathogens are not present in breast milk, they cannot grow on blood agar. Molecular typing (molecular markers) techniques such as polymerase chain reaction (PCR) technology provided additional approaches that have been reported and is considered as the most powerful technique for the control and investigation of pathogens. But culture-based approach to isolate microorganism from any environment does not provide comprehensive information on composition of bacterial communities. This technique also failed to determine microorganism which cannot grow in laboratory condition. Most of the studies performed till date on mastitis involve classical microbiological techniques to identify etiology of mastitis.
Kvist et al. (2008) compare bacterial composition in milk samples collected from 192 women with a clinical mastitis and 466 healthy donors. They found that S. aureus was present in 45% of women with mastitis and 31% of healthy donors. In both the group, mean colony counts were identical and no correlation was observed between colony counts and symptom severity. Finding hints that the presence of S. aureus in breast milk does not always result in clinical mastitis and it is always present in healthy human milk.
Delgado et al. (2008) recognized the role of coagulase-negative Staphylococcus spp. in human mastitis. Employing pulsed-field gel electrophoresis, they found that S. epidermidis was present in 85% (17/20) of samples collected, while S. aureus in 40% (8/20) of samples. After that they compared strains of S. epidermidis present in women with mastitis and women with healthy human milk. They found that women with clinical signs of infection were more likely to harbor strains of S. epidermidis with the icaD (33 vs. 11%, p ¼ 0.03), which was correlated with biofilm production. Thus, virulence factors of S. epidermidis strains found in breast milk may play a vital role in pathogenesis.
Using 16S-specific PCR primers, Shriram et al. (2015) identified bacteria belonging to Staphylococcus and Pseudomonas genera from human milk of 32 mastitis women. Moreover, the authors found 17 genera and 30 different species from mastitis milk suggesting diverse community in diseased condition (Patel et al. 2016).
9.5 Culture-Independent Assessment of Mastitis
Traditionally microbial genome sequencing has been restricted to only a small number of organisms which can be grown in pure culture in laboratory. Progressive development of culture-independent methods has allowed researchers to sequence microbial communities directly from environmental samples. Culture-independent techniques deal with the isolation of total DNA from the environmental sample. Culture-independent approach is commonly referred to as “metagenomic” or “community genomics.” Metagenomics is applied literally to describe any culture-independent analysis of microbial communities. With the recent development in more advances sequencing techniques, which try to capture all the DNA information from the biological sample have been widely used for the characterization of microbial community present within a sample and identification of unknown etiological agents involved in diseased condition. Moreover, this type of technology provides identification of thousands of sequences per sample, which increases the possibility to observe less frequent phylotypes that may have significant importance in disease condition. Metagenomics can also be applied to solve practical challenges in the field of medicine, agriculture, sustainability, and ecology. Numerous microbiome studies have been carried out to assess the composition of the bacterial communities inhabiting a variety of human body locations, including the gut (Zhao and Lacasse 2008), oral cavity (Nasidze et al. 2009; Belda-Ferre et al. 2012), vagina (Ravel et al. 2011), skin (Costello et al. 2009), and human milk (Jost et al. 2013; Belda-Ferre et al. 2012; Ward et al. 2013). All of these studies were focused on the bacterial component of the microbiome.
So far only one study has been reported discussing metagenome of breast milk from mastitis-infected women. Jimenez et al. (2015) performed shotgun sequencing of ten healthy and ten mastitis-infected breast milk samples. They found that Staphylococcus aureus clearly dominated the microbiome in the samples from the women with acute mastitis, whereas high abundance of Staphylococcus epidermidis-related reads was observed in the milk of those suffering from subacute mastitis (Jimenez et al. 2015).
9.6 Prevention and Control
Antibiotics are regularly used to treat mastitis. But nowadays development of multiple resistances by different bacteria has led to failure of treatment. It is due to indiscriminate use of antimicrobials without checking its in vitro sensitivity to the causing bacteria (Oliver and Murinda 2012). In addition to antibiotic resistance, formation of biofilm is also an important virulence factor implicated by mastitis-causing pathogens, which allow survival of bacteria at high antimicrobial concentration (Hoiby et al. 2010). Alternative treatment for the antibiotics can be probiotic therapy and herbal therapy.
9.7 Probiotic Therapy
Development of new strategies based on probiotics is an alternative or complement to antibiotic therapy for the management of mastitis and is particularly appealing. Use of lactic acid bacteria as oral administration of lactobacilli isolated from breast milk for the treatment of mastitis has been used by researchers (Jimenez et al. 2008). Human milk consists of bacterial species like Lactobacillus gasseri, Lactobacillus reuteri, Lactobacillus salivarius, Lactobacillus fermentum, or Bifidobacterium breve with probiotic properties. These bacteria have shown promising results as probiotic agents that might be useful in treating mastitis.
9.8 Herbal Therapy
There has been not a single study published till date indicating use of herbal therapy on human mastitis. But in veterinary field, there are some studies focused on the use of natural herbal plant as a remedy for mastitis. It has been reported that garlic tincture or aloe gel can be used as a fast remedy from mastitis (Pol and Ruegg 2007). In literature antimicrobial properties of garlic extracts and Aloe vera gels have already been reported (Ross et al. 2001; Agarry et al. 2005). But the use of these compounds to successfully treat mastitis has not been described. In one clinical trial, they have specifically evaluated the clinical efficacy of a botanical treatment to treat subclinical mastitis (Abaineh and Sintayehu 2001). Two different doses of a dried leaf powder of an African perennial herb (Persicaria senegalense) were fed for 3–5 days to cows infected with subclinical mastitis. Results of this trial indicated positive effect of herbal medicines in eradication of mastitis but conceded that more research is necessary.
10 Conclusion and Future Aspects
In conclusion, there are now convincing proofs that human milk consists of diverse and feasible microbial population, which initially colonize the infant gut. Somehow, variations in microbial profiling in different studies were due to behavioral, environmental, and genetic differences or a consequence of methodological variation. As such, the era has moved away from the past belief that breast milk is sterile and acknowledged the rich microbial community present in human milk.
However, dysbiosis of breast milk microbial community results in a development of mastitis. Monitoring changes in mastitis-causing microflora with metagenomic platforms might be helpful in building a strategy to overcome this problem.
References
Abaineh D, Sintayehu A (2001) Treatment trial of subclinical mastitis with the herb Persicaria senegalense (Polygonaceae). Trop Anim Health Prod 33(6):511–519
Agarry OO, Olaleye MT, Bello-Michael CO (2005) Comparative antimicrobial activities of aloe vera gel and leaf. Afr J Biotechnol 4(12):1413–1414
Albesharat R, Ehrmann MA, Korakli M, Yazaji S, Vogel RF (2011) Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol 34(2):148–155
Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, Romero H, Simon-Soro A, Pignatelli M, Mira A (2012) The oral metagenome in health and disease. ISME J 6(1):46–56. doi:10.1038/ismej.2011.85
Bhatt VD, Vaidya YH, Kunjadia PD, Kunjadia AP, Patel R (2012) Isolation and characterization of probiotic bacteria from human milk. Int J Pharm Sci Health Care 3(2):62–70
Bjork S, Bage R, Kanyima BM, Andre S, Nassuna-Musoke MG, Owiny DO, Persson Y (2014) Characterization of coagulase negative staphylococci from cases of subclinical mastitis in dairy cattle in Kampala, Uganda. Ir Vet J 67(1):12. doi:10.1186/2046-0481-67-12
Boutinaud M, Jammes H (2002) Potential uses of milk epithelial cells: a review. Reprod Nutr Dev 42(2):133–147
Bytyqi H, Rrustemi M, Mehmeti H, Kryeziu A, Gjinovci V, Gjonbalaj M (2010) Milk production in commercial cattle dairy farms in Kosova. Stočarstvo 63(4):275–285
Cabrera-Rubio R, Carmen Collado M, Laitinen K, Salminen S, Isolauri E, Mira A (2012) The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 96(3):544–551
Cabrera-Rubio R, Mira-Pascual L, Mira A, Collado MC (2015) Impact of mode of delivery on the milk microbiota composition of healthy women. J Dev Orig Health Dis 7(1):54–60. doi:10.1017/S2040174415001397
Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A (2008) Antiinfective properties of human milk. J Nutr 138(9):1801S–1806S
Collado MC, Delgado S, Maldonado A, Rodríguez JM (2009) Assessment of the bacterial diversity of breast milk of healthy women by quantitative real‐time PCR. Lett Appl Microbiol 48(5):523–528
Collado MC, Laitinen K, Salminen S, Isolauri E (2012) Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr Res 72(1):77–85
Cooey PM, Harmon MW (1994) Religious imagination and the body: a feminist analysis: a feminist analysis. Oxford University Press, Oxford
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R (2009) Bacterial community variation in human body habitats across space and time. Science 326(5960):1694–1697
Cregan MD (2002) The paracellular pathway and the lactating human breast. University of Western Australia, Australia
Delgado S, Arroyo R, Martin R, Rodriguez JM (2008) PCR-DGGE assessment of the bacterial diversity of breast milk in women with lactational infectious mastitis. BMC Infect Dis 8:51. doi:10.1186/1471-2334-8-51
Duijts L, Jaddoe VWV, Hofman A, Moll HA (2010) Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 126(1):e18–e25
El-Mohandes AE, Schatz V, Keiser JF, Jackson BJ (1993) Bacterial contaminants of collected and frozen human milk used in an intensive care nursery. Am J Infect Control 21(5):226–230
Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM (2013) The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 69(1):1–10
Fetherston C (1997) Characteristics of lactation mastitis in a Western Australian cohort. Breastfeed Rev 5(2):5–11
Foxman B, D'Arcy H, Gillespie B, Bobo JK, Schwartz K (2002) Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. Am J Epidemiol 155(2):103–114
Gao X, Zhang Q, McMahon RJ, Woo JG, Davidson BS, Morrow AL (2012) Semi-quantitative analysis of milk proteomes reveals new evolving activities in carbohydrate metabolism in breastfeeding women. FASEB J 26(1_MeetingAbstracts):lb287
Gaudana SB, Dhanani AS, Bagchi T (2010) Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br J Nutr 103(11):1620–1628
Gianneechini R, Concha C, Rivero R, Delucci I, Moreno Lopez J (2002) Occurrence of clinical and sub-clinical mastitis in dairy herds in the West Littoral Region in Uruguay. Acta Vet Scand 43(4):221–230
Goldman AS (2007) The immune system in human milk and the developing infant. Breastfeed Med 2(4):195–204
Gonzalez R, Maldonado A, Martin V, Mandomando I, Fumado V, Metzner KJ, Sacoor C et al (2013) Breast milk and gut microbiota in African mothers and infants from an area of high HIV prevalence. PLoS One 8(11):e80299. doi:10.1371/journal.Pone.0080299
Greer FR, Sicherer SH, Wesley Burks A (2008) Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121(1):183–191
Grönlund M‐M, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S, Isolauri E (2007) Maternal breast‐milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy 37(12):1764–1772
Hassiotou F, Trengove N, Tat Lai C, Filgueira L, Blancafort P, Hartmann PE (2012) Breastmilk stem cells: an overview of the current knowledge. In: Breastfeeding and lactation symposium, Vienna, Austria
Hassiotou F, Hepworth AR, Metzger P, Tat Lai C, Trengove N, Hartmann PE, Filgueira L (2013) Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin Transl Immunol 2(4):e3. doi:10.1038/cti.2013.1
Heikkilä MP, Saris PEJ (2003) Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol 95(3):471–478
Ho FC, Wong RL, Lawton JW (1979) Human colostral and breast milk cells: a light and electron microscopic study. Acta Paediatr 68(4):389–396
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35(4):322–332. doi:10.1016/j.Ijantimicag.2009.12.011
Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA (2011) Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 6(6):e21313
Hunt KM, Williams JE, Shafii B, Hunt MK, Behre R, Ting R, McGuire MK, McGuire MA (2013) Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeed Med 8(1):105–110. doi:10.1089/bfm.2011.0141
Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J (2008) Breastfeeding and maternal and infant health outcomes in developed countries. US Department of Health and Human Services, Agency for Healthcare Research and Quality, Rockville, MD. Evidence Report/Technology Assessment (153)
Jimenez E, Delgado S, Maldonado A, Arroyo R, Albujar M, Garcia N, Jariod M, Fernandez L, Gomez A, Rodriguez JM (2008) Staphylococcus epidermidis: a differential trait of the fecal microbiota of breast-fed infants. BMC Microbiol 8:143. doi:10.1186/1471-2180-8-143
Jimenez E, de Andres J, Manrique M, Pareja-Tobes P, Tobes R, Martinez-Blanch JF, Codoner FM, Ramon D, Fernandez L, Rodriguez JM (2015) Metagenomic analysis of milk of healthy and mastitis-suffering women. J Hum Lact. doi:10.1177/0890334415585078
Jost T, Lacroix C, Braegger C, Chassard C (2013) Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr 110(7):1253–1262. doi:10.1017/S0007114513000597
Khodayar-Pardo P, Mira-Pascual L, Collado MC, Martinez-Costa C (2014) Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol 34(8):599–605
Kinlay JR, O'Connell DL, Kinlay S (1998) Incidence of mastitis in breastfeeding women during the six months after delivery: a prospective cohort study. Med J Aust 169(6):310–312
Kvist LJ, Larsson BW, Hall-Lord ML, Steen A, Schalen C (2008) The role of bacteria in lactational mastitis and some considerations of the use of antibiotic treatment. Int Breastfeed J 3:6. doi:10.1186/1746-4358-3-6
Li JP, Zhou HJ, Yuan L, He T, Hu SH (2009) Prevalence, genetic diversity, and antimicrobial susceptibility profiles of Staphylococcus aureus isolated from bovine mastitis in Zhejiang Province, China. J Zhejiang Univ Sci B 10(10):753–760. doi:10.1631/jzus.B0920072
Makino H, Kushiro A, Ishikawa E, Muylaert D, Kubota H, Sakai T, Oishi K, Martin R, Ben Amor K, Oozeer R (2011) Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl Environ Microbiol 77(19):6788–6793
Maldonado J, Cañabate F, Sempere L, Vela F, Sanchez AR, Narbona E, López-Huertas E, Geerlings A, Valero AD, Olivares M (2012) Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr 54(1):55–61
Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J, Fernández L, Rodríguez JM (2003) Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 143(6):754–758
Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, Rodríguez JM (2009) Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol 75(4):965–969
Michie CA, Tantscher E, Rot A (1998) The long term effects of breastfeeding: a role for the cells in breast milk? [Editorial]. J Trop Pediatr 44(1):2–3
Miller WR, Scott WN, Morris R, Fraser HM, Sharpe RM (1985) Growth of human breast cancer cells inhibited by a luteinizing hormone-releasing hormone agonist. Nature 313(5999):231–233
Molinari CE, Casadio YS, Hartmann BT, Arthur PG, Hartmann PE (2013) Longitudinal analysis of protein glycosylation and β-casein phosphorylation in term and preterm human milk during the first 2 months of lactation. Br J Nutr 110(01):105–115
Morrow AL, Rangel JM (2004) Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis 15(4):221–228
Murphy SC, Cranker K, Senyk GF, Barbano DM, Saeman AI, Galton DM (1989) Influence of bovine mastitis on lipolysis and proteolysis in milk. J Dairy Sci 72(3):620–626
Nasidze I, Li J, Quinque D, Tang K, Stoneking M (2009) Global diversity in the human salivary microbiome. Genome Res 19(4):636–643. doi:10.1101/gr.084616.108
Newburg DS (2005) Innate immunity and human milk. J Nutr 135(5):1308–1312
Nishimura T, Suzue J, Kaji H (2009) Breastfeeding reduces the severity of respiratory syncytial virus infection among young infants: a multi‐center prospective study. Pediatr Int 51(6):812–816
Olivares M, Albrecht S, De Palma G, Ferrer MD, Castillejo G, Schols HA, Sanz Y (2014) Human milk composition differs in healthy mothers and mothers with celiac disease. Eur J Nutr 54(1):119–128
Oliver SP, Murinda SE (2012) Antimicrobial resistance of mastitis pathogens. Vet Clin North Am Food Anim Pract 28(2):165–185. doi:10.1016/j.Cvfa.2012.03.005
Paape MJ, Weinland BT (1988) Effect of abraded intramammary device on milk yield, tissue damage, and cellular composition. J Dairy Sci 71(1):250–256
Patel SH, Vaidya YH, Joshi CG, Kunjadia AP (2016) Culture-dependent assessment of bacterial diversity from human milk with lactational mastitis. Comp Clin Pathol 25(2):437–443
Pol M, Ruegg PL (2007) Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin. J Dairy Sci 90(1):249–261. doi:10.3168/jds.S0022-0302(07)72626-7
Randolph HE, Erwin RE (1974) Influence of mastitis on properties of milk: X. Fatty acid composition. J Dairy Sci 57(8):865–868. doi:10.3168/jds.S0022-0302(74)84978-7
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S et al (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):4680–4687. doi:10.1073/pnas.1002611107
Ridwan BU, Koning CJM, Besselink MGH, Timmerman HM, Brouwer EC, Verhoef J, Gooszen HG, Akkermans LMA (2008) Antimicrobial activity of a multispecies probiotic (Ecologic 641) against pathogens isolated from infected pancreatic necrosis. Lett Appl Microbiol 46(1):61–67
Rodrigues MDA, Gindri L, Silva ADD, Guex CG, Santos SOD, Hörner R (2015) Prevalence of methicillin-resistant Staphylococcus aureus in a University Hospital in the South of Brazil.Braz J Pharm Sci 51(1):35–41
Ross ZM, O'Gara EA, Hill DJ, Sleightholme HV, Maslin DJ (2001) Antimicrobial properties of garlic oil against human enteric bacteria: evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl Environ Microbiol 67(1):475–480. doi:10.1128/AEM.67.1.475-480.2001
Sakwinska O, Moine D, Delley M, Combremont S, Rezzonico E, Descombes P, Vinyes-Pares G, Zhang Y, Wang P, Thakkar SK (2016) Microbiota in breast milk of Chinese lactating mothers. PLoS One 11(8):e0160856. doi:10.1371/journal.Pone.0160856
Schnorr KL, Pearson LD (1984) Intestinal absorption of maternal leucocytes by newborn lambs. J Reprod Immunol 6(5):329–337
Sharma N, Singh NK, Bhadwal MS (2011) Relationship of somatic cell count and mastitis: an overview. Asian Australas J Anim Sci 24(3):429–438
Shriram HP, Vaidya YH, Joshi CG, Kunjadia AP (2015) Culture-dependent assessment of bacterial diversity from human milk with lactational mastitis. Comparative Clinical Pathology 25(2);437–443
Smith CW, Goldman AS (1970) Interactions of lymphocytes and macrophages from human colostrum: characteristics of the interacting lymphocyte. J Reticuloendothel Soc 8(1):91–104
Solís G, de Los Reyes-Gavilan CG, Fernández N, Margolles A, Gueimonde M (2010) Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 16(3):307–310
Soto A, Martín V, Jiménez E, Mader I, Rodríguez JM, Fernández L (2014) Lactobacilli and bifidobacteria in human breast milk: influence of antibiotherapy and other host and clinical factors. J Pediatr Gastroenterol Nutr 59(1):78
Thirabunyanon M, Boonprasom P, Niamsup P (2009) Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol Lett 31(4):571–576
Urbaniak C, McMillan A, Angelini M, Gloor GB, Sumarah M, Burton JP, Reid G (2014) Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome 2:24. doi:10.1186/2049-2618-2-24
Urbaniak C, Angelini M, Gloor GB, Reid G (2016) Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 4(1):1. doi:10.1186/s40168-015-0145-y
Vaidya Y, Patel S, Patel R, Joshi C, Kunjadia A (2015) Exploring the microbiota of human milk using the culture-dependent method. Int J 3(5):462–471
Vanderpool C, Yan F, Brent Polk D (2008) Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis 14(11):1585–1596
Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, Cresci A (2009) Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr 48(6):355–363
Ward TL, Hosid S, Ioshikhes I, Altosaar I (2013) Human milk metagenome: a functional capacity analysis. BMC Microbiol 13:116. doi:10.1186/1471-2180-13-116
Weiler IJ, Hickler W, Sprenger R (1983) Demonstration that milk cells invade the suckling neonatal mouse. Am J Reprod Immunol 4(2):95–98
WHO (2000) Mastitis causes and management
Wirt DP, Adakins LT, Palkowetz KH, Schmalsteig FC, Goldman AS (1991) Activated-memory T-cells in human-milk (HM). In: Pediatric research. Williams & Wilkins, Baltimore, MD
Wright KC, Feeney AM (1998) The bacteriological screening of donated human milk: laboratory experience of British Paediatric Association’s published guidelines. J Infect 36(1):23–27
Zhao X, Lacasse P (2008) Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci 86(13 Suppl):57–65. doi:10.2527/jas.2007-0302
Zhou L, Yoshimura Y, Huang Y‐Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M (2000) Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast‐feeding after birth. Immunology 101(4):570–580
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Joshi, C., Kunjadiya, A. (2017). Human Milk Microbiome: A Perspective to Healthy and Infected Individuals. In: Singh, R., Kothari, R., Koringa, P., Singh, S. (eds) Understanding Host-Microbiome Interactions - An Omics Approach. Springer, Singapore. https://doi.org/10.1007/978-981-10-5050-3_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-5050-3_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5049-7
Online ISBN: 978-981-10-5050-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)