Abstract
The textile industry sees currently a fast development of legal and voluntary restrictions of chemicals content in textile products. However, the on-going phase-out work focuses on evaluating the environmental and health aspects of chemicals. The technical performance in the end application for the chemical does not receive the same attention. In addition, many research projects committed to evaluating hazardous substances and their possible alternatives also neglects the technical performance. The technical performance is left to the companies to evaluate. This may lead to inefficiency in the substitution process and also have the consequence that companies never dare to take the step to practical substitution, at least not in a proactive way. This chapter presents a model for practical substitution, developed and evaluated in several case studies, whereof two in the textile field: water and soil repellent textile coating materials and flame retarded textiles. From the general lessons learnt, an improved substitution methodology with widespread applicability has been defined.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Chemicals
- Toxicity
- Practical substitution
- Perfluorinated substances
- Flame retardants
- Functional properties
1 Introduction
Chemicals are used among other things to provide function in materials and products. Some chemicals do show hazard characteristics that are of great concern. Thus, there is a clear need for phase out actions of such hazardous chemicals that today are used in materials and products. But, we cannot phase out chemicals without replacing their functionality.
In the manufacturing of textiles large amounts of chemicals are used. A quantitative study of the consumption of chemicals during the life cycle of textiles showed that between 1 and 5 kg of chemicals are used per kg textiles (Olsson et al. 2009). Some of the substances are harmful to health and/or the environment, with properties such as sensitizing, human toxic, eco-toxic, persistent or bio-accumulative (Munn 2011).
This chapter presents a model for practical substitution including also the technical and economical performance of alternative chemistries. The first section gives a background to why hazardous chemicals are used in textile production and occur in the ready-made textile product. Furthermore it describes the state-of-the-art regarding the legal and voluntary initiatives to phase-out hazardous chemicals in the textile industry. Finally the conditions required for a viable substitution are explained. Two specific examples are addressed where the authors have in practice applied, and iteratively developed, the suggested substitution model. The latter example involves textile chemistry and is a further development of a model used for phase out of hazardous flame retardants in plastic components in electronics as well as textiles.
1.1 Use of Chemicals in Textile Production
A wide variety of chemical substances with various functionalities, applications and properties are used in textile manufacturing. Chemical substances can be grouped in several different ways (Swedish Chemicals Agency 2004a), based on:
-
chemical structure (phthalates, polychlorinated biphenyls etc.),
-
functional properties (plasticizers, flame retardants etc.), or
-
toxicological properties (endocrine disrupters, carcinogens, etc.).
While the chemical structure is a singular property, both the functional and toxicological properties are not; one substance may have one or many functional properties (e.g. both be a plasticizer and a flame retardant) and also one of many toxicological properties (e.g. both be endocrine disruptive and carcinogenic).
Figure 1 shows the long sequence of process steps in textile production and the type of chemicals that are used in each step. In this overview, the chemicals have been described after their functional properties. The grouping of chemicals after their functional properties is a key factor in the model for practical substitution. The functional properties of chemicals can be further divided into:
Examples of commonly occurring chemicals (in red) in the textile life cycle. The chemicals are described after which function they deliver in each process step. Figure from Roos (2016)
-
Effect chemicals, which provide function to the final textile product (softeners, plasticizers etc.). These functions are usually selected by the product designer and/or the procurer. Sometimes this group of chemicals is addressed as “functional chemicals”, hence giving function to the final product.
-
Processing chemicals, which are used in the processing of textiles in the production (antifoaming agents, catalysts etc.). These functions are selected by the process engineer or sometimes specified by the chemical company to achieve compatibility with chemicals added to provide final effect.
This way of grouping the chemicals in two sets (process chemicals vs. effect chemicals) will facilitate for companies to organize and target their chemical management including the efforts to substitute hazardous chemicals (Roos 2016).
1.1.1 Effect Chemicals
Effect chemicals (or functional chemicals) are added to give an article a specific function. Effect chemicals contribute to design or any feasible technical function in the final product, e.g. colorants. Flame retardants in clothing and furniture save a large number of lives each year. Clothing with a high degree of water and dirt repellence is necessary in many workplaces but also in high demand in sports and for the comfort in everyday life. Further, we need to use biocides for a safe and reliable healthcare situation. However, these chemicals bearing toxic properties contribute to the environmental burden of this class of products. For effect chemicals there is a need for certain concentration in the final product in order to achieve the desirable function (Swedish Chemicals Agency 2014).
Some examples of functional (or effect) chemicals are:
-
Colorants (dyestuffs and pigments)
-
Oil, soil and water repellents
-
Plasticizers
-
Flame retardants
-
Fragrances
-
Alloys
-
Biocides for defined functionalities in the article e.g. disinfectants
-
Stabilizers e.g. antioxidants, UV/light stabilizers and anti-degradants.
The effect chemicals that are used should have good compatibility, such as good solubility in the materials (Posner 2009). Some effect chemicals require good affinity to the fibers, for example as dyes in cellulose. In order to sustain the desired function in the final textile product during the usage phase the “function” should have the most favorable ageing characteristics possible. In other words, there is no point in the functional chemistry to last twice as long as the rest of the garment or vice versa the for instance color to fade after the first washes for a textile product meant to last several years. The effect chemicals are not only relevant to high degree in the use phase and the possible exposure, but also to the end-of-life scenario for a textile product. Especially chemicals threatening the vision of non-toxic circular materials or disturbing a recycling process are of importance.
1.1.2 Processing Chemicals
The other category are called processing chemicals, also called auxiliary chemicals, that are necessary to make processes work, but they do not provide any desired properties to the final product and are therefore not meant to remain in the finished product.
Some examples of process chemicals are:
-
Organic solvents
-
Surfactants e.g. wetting and dispersing agents
-
Softeners
-
Curative agents
-
Accelerators
-
Chain extenders
-
Lubricants
-
Defoaming agent
-
Catalysts
-
Hardening agents
-
Vulcanizing agent (rubber)
-
Retarder (rubber)
-
Complexing agent
-
Salts
-
Acids and bases
-
Reactive resins (e.g. binders and adhesives) for various finishing treatments
-
Biocides as preservatives in the process or during storage and transport e.g. fungicides and preservatives.
-
Tanning agents (leather)
-
Drying agents
-
Intermediates, precursors and monomers.
Remains of the process chemicals may be found in the finished product and cause health and/or environmental problems. A process chemical which remains as impurities in the final product often has a relatively low concentration, compared with the concentration of an effect/functional chemical in the final product.
1.2 Development of Legal and Voluntary Restrictions of Chemicals Content in Textile Products
In 2002, when the global leaders gathered at the Johannesburg World Summit on Sustainable Development, detrimental impacts from chemicals was one of the highlighted sustainability challenges. The participating countries agreed to the following goal:
by the year 2020, chemicals are produced and used in ways that minimize significant adverse impacts on the environment and human health
(United Nations 2002, paragraph 23)
This goal was then specified further at the International Conference on Chemicals Management (ICCM) in Dubai four years later, where the Strategic Approach to International Chemicals Management (SAICM) was adopted (UNEP 2006). This policy framework for sound management of chemicals has since then affected chemicals management in several sectors including textile and fashion.
1.2.1 Development of Legal Restrictions Addressing Chemicals in Textiles
In 2007, the European chemicals legislation REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) (European Commission 2006) entered into force. The REACH legislation was a harmonization of the chemicals legislation in the European Union and the European Economic Area (EEA) countries (Norway, Iceland and Lichtenstein) (hereinafter called the EU). REACH also implied that chemicals became regulated in many product groups where chemicals were not previously regulated in the EU. Another effect that REACH brought about was that several other countries followed the REACH example and developed similar regulations, in popular terms often called “China REACH” (China Ministry of Environmental Protection (MEP) 2010), “India REACH” (Government of India 2012) or “K-REACH” (South Korean Ministry of the Environment 2011).
However, the possibilities to use legislative measures as a tool to counteract problems with hazardous chemicals in textiles is impaired by a major challenge: the fact that any national (or federal) regulation of chemicals is limited to actions inside their area of jurisdiction (Roos 2015). This limitation means that, for example, the European legislation can only regulate the chemical content of products produced in, imported to or used in the EU. The textile supply chain is on the contrary global to its nature; textile products and semi-finished products are constantly exported and imported across country borders. In the absence of a legislative framework that covers the entire textile supply chain, the industry has instead acted via voluntary initiatives to secure a responsible chemicals management. Legislation can thus be identified as one measure to achieve the SAICM objectives, where industry voluntary action is another of at least equal importance (Roos 2015).
1.2.2 Development of Voluntary Restrictions Addressing Chemicals in Textiles
Today, there are many on-going international activities for development of voluntary schemes addressing hazardous chemicals in textiles. For example, a broad range of textile labels exist; currently 108 textile ecolabels are listed in the Ecolabel Index (Ecolabel Index 2016). The most common environmental textile label globally is the Oeko-Tex® 100 certification (OEKO-TEX® Association 2017), followed by BlueSign (BLUESIGN® 2017) and Global Organic Textile Standard (GOTS 2017). One important recent initiative is the Outdoor Industry Association (OIA) and the Sustainable Apparel Coalition (SAC) who jointly developed the Chemicals Management Framework, also called the Chemicals Management Module (CMM) (OIA 2014) which has also been integrated into SAC’s Higg Index 2.0 (SAC 2017). Several other management tools addressing specific chemicals are available through the Substitution and Alternatives Assessment Toolbox (SAAT), recently developed by the Organisation for Economic Co-operation and Development (OECD) (OECD 2015).
In order to show compliance with existing legislation and to fulfill customer demands, some kind of chemical management is utilized by all actors on the textile market. Current company chemical management systems are designed and intended to handle the obstacles. For textile brands many companies use so called Restricted Substance List (RSL) as their core tool in the chemical management system. The RSL normally consists of chemical names as well as CAS numbersFootnote 1 of the specific substances (AAFA 2015). In some cases the lists also give guidance to where the chemicals may be found in the production or with information of the function provided (AFIRM 2015; Swedish Chemicals Group/Swedish Textile Importer’s Association 2016). Substances included in such lists mainly consist of restricted substances often but not always related to the textile materials (Roos et al. 2017). Some front runners occasionally and additionally include substances that may be, but not yet are, regulated in their RSLs together with already regulated and often textile-relevant substances such as carcinogens, endocrine disruptors and skin sensitizers (ChemSec 2017).
Most of the above mentioned tools has in common that they provide “negative lists”, that is lists of unwanted substances, and little guidance on how to perform the substitution. The exception is BlueSign and GOTS that also provide “positive lists” of chemical products that have been evaluated and identified by the schemes as more environmentally friendly alternatives.
1.3 Substitution of Substances, Materials or Products
Most of the work with substitution of hazardous chemicals aims at finding a substitute substance with less harmful properties than the original substance (Swedish Chemicals Agency 2007). In practice, substitution can refer either to chemical substances, to chemical products (i.e. commercial mixtures), to materials and even to products (Leonards 2011). Furthermore, the approach for substitution can be based on either hazard or risk. Inherent safety, also called primary prevention, consists in the elimination of a hazard. It is contrasted with secondary prevention that consists in reducing the risk associated with a hazard (Swedish Chemicals Agency 2007). Regardless of approach and type of substitution, the substitute product system (where the substitute can be a chemical substance, chemical product, material, technical solution or textile product) need to provide the same function as was provided in the original product system.
In the textile industry, a lot of the focus has been put on effect chemicals with hazardous properties, such as halogenated flame retardants and perfluorinated durable water repellent (DWR) agents. The requirements on alternative effect chemicals are therefore described below.
1.3.1 Requirements on Alternative Effect Chemicals
Substitute effect chemicals can appreciably impair the properties of the textile material. The basic problem in substitution is the trade-off between the decrease in performance of the textile material caused by the substitution and the (perceived) customer requirements and the foreseen lowered hazard. In addition to fulfilling the appropriate function (color, fire retardants, water repellency etc.), a viable alternative effect chemical shall, at most, fulfill all of the below qualities (Norwegian Pollution Control Authority (SFT) 2009):
-
Functional properties
-
Provide the same function as was provided by the original effect chemical
-
Not alter electrical properties
-
-
Mechanical properties
-
Not significantly alter the mechanical properties of the textile material
-
Be easy to incorporate into the host textile material
-
Be compatible with the host textile material
-
Be easy to extract/remove for recyclability of the textile material
-
-
Physical properties
-
Be colorless or at least non-discoloring (not applicable for dyestuffs and pigments)
-
Have good light stability
-
Be resistant towards ageing and hydrolysis
-
Not cause corrosion
-
-
Health and environmental properties
-
Not have harmful health effects
-
Not have harmful environmental properties
-
-
Commercial viability
-
Be commercially available and cost-effective.
-
1.3.2 State-of-the-Art Substitution Work
Among the on-going initiatives with phasing-out hazardous chemicals in the textile industry, the focus is mainly on evaluating the environmental and health aspects of chemicals, and describing the current emission levels. The technical performance in the end application for the possible substitute chemicals does not receive the same attention (AAFA 2015; ChemSec 2017; UNEP/POPS/POPRC.8/INF/17 2012; ZDHC 2014). In addition, many research projects committed to evaluating hazardous substances and their possible alternatives also neglects the technical performance (Howard and Muir 2010; Quinete et al. 2010; Wang et al. 2013a). The technical performance is left to the companies to evaluate. This may lead to inefficiency in the substitution process and also have the consequence that companies never dare to take the step to practical substitution, at least not in a proactive way.
Further, focus is sometimes put on equal technical performance but also the performance level can be questioned. That in turn requires performance criteria to match the new performance level. But also the “culture” and the way things have previously been working or performed need to be questioned. In other words the substitution work needs much more attention on the performance side.
Many substitution processes result in solutions that are rather specific for specific applications compared to some of the former solutions. Some examples are halogenated flame retardants used in a variety of applications that needs a several substitutes to cover the same number of applications.
1.4 Life Cycle Perspective
Life cycle assessment (LCA) is an ISO-standardized method for quantitative evaluation of the environmental performance of products and services throughout the life cycle (Baumann and Tillman 2004). The life cycle of a product is generally divided into four main phases: raw material extraction, production, use and end-of-life, see Fig. 2. Environmental performance is measured in the form of environmental impacts of emissions to air, water and soil as well as consumption of resources (energy, water, land and material), in the different stages of the life cycle.
The life cycle perspective of the LCA is essential in order to avoid sub-optimization, i.e. improving just a part of a system in a manner that negatively affects other parts of the system. Sub-optimization can occur when only parts of the life cycle are studied and the overall performance is not evaluated. The life cycle perspective is thus important also for substitution work, to assure that the substitution of a substance in one sub-part of the life cycle (e.g. application of a durable water repellent coating) does not lead to increased toxicity in another sub-part (e.g. increased toxic emissions from dyehouses due to a shorter life length of the product).
2 Model Creation
This section gives the background to the creation of the model and the context in which it was developed. Experts in the fields of environmental, analytical, organic and physical chemistry, physics, ecotoxicology, economics, risk assessment and life cycle assessment (LCA) were all involved and engaged in order to take a holistic view on substitution of substances. Further, the requirements for a viable substitution in practice have been identified in dialogue with industry partners, which has been crucial for understanding the implications of the real-life changes that need to be made in the supply chains.
The development of the model for practical substitution has been based on empirical experiences from case studies. In systems analysis, case studies play an important role for method development where, according to Dubois and Gadde (2002), using a logic-based systematic combining approach “has been showed to be particularly useful for the development of new theories”, letting methodological framework, empirical fieldwork, and case analysis evolve simultaneously.
2.1 First Development Steps
The creation of the model suggested here began in the project ENFIRO (Life Cycle Assessment of Environment-Compatible Flame Retardants).Footnote 2 ENFIRO followed a prototypical case study approach in which existing and alternative flame retardants were evaluated regarding their flame retardant properties, their influence on the function of products once incorporated, and their environmental and toxicological properties.
There were at the time several non-brominated flame retardants existing on the market. However, there was limited information available about the environmental and toxicological impact of these alternatives. Furthermore, the alternatives were difficult to apply before tests had shown that they did not adversely affect the quality of consumer products. ENFIRO evaluated viable substitution options for a number of brominated flame retardants. These flame retardants were studied in five applications: textile coatings, intumescent paint, electronic components, printed circuit boards and injection moulded products. In Sect. 3.2, the results from the case study with textile coatings are provided.
The ENFIRO approach developed followed a chemical substitution cycle anchored in four major elements (Fig. 3). In the first element the alternative halogen-free flame retardants were prioritized and the most viable alternatives selected. The second major element focussed on the technical performance (fire and application), hazard and exposure assessment of the selected halogen-free flame retardants. The collected information was analyzed in a comparative hazard and risk assessments (third element). Finally, information on production costs and socio-economics of the halogen-free flame retardants and related products, was added to give a holistic picture together with impact assessment studies using life-cycle assessments (LCA) (fourth element). This finally resulted in a recommendation of certain halogen-free flame retardants and their related product combinations.
2.2 Generalization of the Model
The ENFIRO model was built to meet the needs of the specific needs of the ENFIRO project. In order to develop procedures that can be used both as a basis for legislation and for substitution in practical product development, in textiles as well as other consumer products, a generalization of the model was needed.
The generalized model is intended to be able to be applied on any substance group and to any type of substitution (the substitute can be a chemical substance, chemical product, material or product). In addition, the model should be able to be used by any consortia covering the fields of environmental, analytical, organic and physical chemistry, physics, ecotoxicology, economics, risk assessment and life cycle assessment (LCA) that is needed for the specific case in order to achieve robust results. Which these fields are for a specific case need to be identified in dialogue with academic and industry partners.
To the model was added a first step where mapping and characterization of substances of high concern are related to other substances of concern as a way to prioritize chemicals and products to be included in substitution processes (Fig. 4). The next steps are based on the ENFIRO approach for substitution of hazardous substances, including testing technical and economical viability.
3 Evaluation via Case Studies
The model for practical substitution of hazardous chemicals has been evaluated in several case studies, whereof two in the textile field: water and soil repellent textile coating materials (the SUPFES projectFootnote 3) and flame retarded textiles (the ENFIRO projectFootnote 4).
3.1 The SUPFES Project
The project SUPFES (Substitution in practice of prioritised fluorinated compounds for textile applications),Footnote 5 2014–2017, has taken a holistic view on the use of per- and polyfluoroalkyl substances (PFAS) in the textile industry studying emissions, life cycles as well as human and aquatic toxicity. To create possibilities for real change the SUPFES project also looks at practical substitution of fluorinated compounds in textile applications. This means that substitutes need to be evaluated not only for their health and environmental properties but they need also to be evaluated for their technical properties (function, durability and compatibility with textile processing). Within this project the level of performance have also been thoroughly discussed for different textile applications since the performance level provided by perfluorinated compounds are high but rather many alternatives exist for lower levels of performance.
3.1.1 Current Use of Per- and Polyfluoroalkyl Substances (PFAS)
Per- and polyfluorinated chemical products are extremely versatile and are used in a variety of industrial and consumer applications and products. Some of these chemical products contain or release per- and polyfluoroalkyl substances (PFAS) that are documented to be persistent as well as accumulating in the human body as well as in the environment. The most common textile applications for PFAS-based chemicals are as water- and oil-repellent agents (for so called durable water repellent (DWR) treatments). Other applications include use in digital printing processes to prevent bleed-through of the fabric.
The PFAS substance group is known to be (or transform into substances that are) persistent, i.e. does not degrade in the environment. PFAS are in addition bioaccumulative, i.e. their concentration in organisms can become higher than that of the surrounding environment. PFAS have been detected in the ground and water in remote areas, such as the Arctic as well as in the blood of small children, adults and other mammals (Posner et al. 2013). The rising levels of PFAS found in the environment are of high concern because these substances have been linked to adverse health effects, such as delayed puberty onset, elevated cholesterol levels, reduced immunologic responses to vaccination and over-representation of attention-deficit/hyperactivity disorder (ADHD) in children (Bergman et al. 2013). Two PFAS are currently subject to legal restriction: perfluorooctane sulfonic acid (PFOS) with CAS RN 1763-23-1 is restricted under the global Stockholm Convention and perfluorooctanoic acid (PFOA) with CAS RN 335-67-1 is restricted in Norway. Other PFAS are proposed for regulations.
The fluorochemistry has been in production and on the global market since the early 1950th where the so called long-chain fluorochemistryFootnote 6 has dominated the market for decades. There are two main chemistries that used to dominate this market namely the so called PFOS-related chemistry and the PFOA-related chemistry. In 2003 there was a voluntarily phase out in 2003 of the production of PFOS by the most important global producer 3 M, which marked a major decrease in global production and use. Production before 2003 was mostly for surface treatment such as textile and for paper protection (UNECE 2006). Subsequently several countries and regions worldwide have introduced phase out programs and legislation to limit the use of the PFOS-related chemistry.
In 2009 PFOS and related substances where declared Persistent Organic Pollutants (POP) by the Stockholm Convention that is one of the UNEP activities under the SAICM strategy. For a long period PFOA-related chemistry was considered an alternative to PFOS-related chemistry, but this too has demonstrated severe health and environmental properties and is currently in several global phase out programs to be replaced by the so called short-chain fluorochemistry and non-fluorinated chemistries such as polysiloxanes, waxes and paraffins.
3.1.2 Characterization of Original Substance in Use
The situation with PFOS and PFOA being restricted in several applications at the same time as the market is searching intensively after alternative chemistries has led to that manufacturers of DWR agents are very secretive with the content of their products. The first step of the SUPFES project was therefore to characterize the PFAS currently in use.
-
Characterization of PFAS in use
Textile materials on the market were screened for the traditional long-chain PFAS (hereafter called old PFAS), alternative PFAS, and novel fluorinated compounds. The main aims were to characterize the diffuse sources and identify the PFAS used in materials and goods. A literature search and the SUPFES stakeholders were used to associate different types of PFAS with different chemical products. This information was used to collect materials and goods, followed by an analytical screening of PFAS.
Textile samples from outdoor clothing like trousers, jackets, gloves etc., were provided by different suppliers from the outdoor industry. A first screening of the materials for the content of the traditional long-chain PFASs and alternative short chain PFASs (Table 1) was performed by a simple and at the time non-validated extraction method with methanol as extraction solvent and quantification by LC-MS/MS. Results of the screening showed that a variety of PFASs were present in the samples, with some PFOS and PFOA concentrations exceeding the norm set by the European Union for PFOS (1 µg/m2) (EU 2006) and the Norwegian government for PFOA (1 µg/m2) (Lovdata 2014). PFASs profiles showed that for some samples comparability patterns were found. No comparability was detected between the type of chemistry used by the textile industry (C6 or C8) and the profiles detected by the screening (Andersson et al. 2014).
-
Development of an extraction method of PFCAs, PFSAs and FOSA in textile samples
Although some analyses on PFASs in textiles have already been performed by others (Herzke et al. 2012; Knepper et al. 2014), no peer reviewed validated extraction methods were published. For the screening of PFASs in the 34 textile samples methanol was used, because it had already successfully been used for extracting PFASs from several matrixes (van Leeuwen et al. 2009; Backe et al. 2013; Weiss et al. 2013; Wang et al. 2013b). However, because Knepper et al. (2014) used acetone/ acetonitrile (80:20, v/v) to extract PFASs from textile samples, both the acetone/acetonitrile mixture as well as methanol were optimized as extraction solvent. The number of sequential extractions and extraction time on a shaking device were optimized by performing sequential extractions of five textile samples with either acetone/acetonitrile (80:20, v/v) or methanol and with different extraction times.
The developed extraction method (Van Der Veen et al. 2016) for PFASs analysis in textile samples is validated by a recovery assessment, a repeatability assessment, and a reproducibility assessment. For the recovery assessment two textile samples were spiked in triplicate on two different concentration levels. For the repeatability assessment three samples were extracted in triplicate in the same series. For the reproducibility assessment the three samples were extracted in triplicate on three different days.
The 34 textile samples have been reanalyzed for, together with 11 additional textile samples, with the developed and validated method. Five of the 11 additional samples consisted of two different colors. Those different colors were analyzed as separate samples, which resulted in a total of 50 textile samples. Although 4:2 FTSA, 6:2 FTSA and 8:2 FTSA were not included in the validation, those PFASs were quantified with the developed method as well. PFASs profiles were calculated for the textile samples based on the results.
-
Environmental and exposure assessment of PFAS
For the environmental and exposure assessment, analytical methods for the outdoor environment were developed. Physical-chemical property data for the identified PFAS were taken from the literature or generated using existing structure-property relationship models (EPIsuite, SPARC). The property data were used to predict the environmental fate of the chemicals. Key matrices for environmental and human exposure (sewage sludge, effluent, food, air) were sampled and analyzed for the chemical(s). Waste water and sewage sludge were sampled in a nested design to identify variability in contaminant flows in collaboration with a wastewater treatment plant in Stockholm, Sweden. Additionally, key performance data for sludge management at the wastewater treatment plant were collected to facilitate system analysis of the consequences of increased and decreased sludge quality on the environmental performance of sludge management. To assess indoor contamination, dust and indoor air samples were analyzed from microenvironments where textile consumer products are used (homes, offices, clothes shop). The analytical data were used to calculate environmental and human exposure to the identified PFAS.
-
Selection of prioritized substances
The prioritization was made using a scheme developed for the purpose, where all elements of the analyses above were set in preference order. The scheme had five elements: (1) sources, (2) product/PFAS combinations, (3) leaching and emission, (4) environmental levels, and (5) toxicological and ecotoxicological properties of the PFAS. The prioritized results (Table 2) served as the basis for the selection of alternative compounds.
3.1.3 Alternative Selection
A list of potential and already implemented alternatives was first assembled. Both PFASs with shorter chain lengths than the old PFASs (e.g. based on perfluorobutanesulfonic acid (PFBS) or 6:2 instead of 8:2 fluorotelomer chemistry) as well as non-fluorinated alternatives (siloxanes/polysiloxanes, dendrimers and waxes). The selection of potential alternatives was based on the “Technical paper on the identification and assessment of alternatives to the use of perfluorooctane sulfonic acid in open applications” (UNEP/POPS/POPRC.8/INF/17 2012) and on information from the associated partners. A literature review was prepared in which a detailed summary of the different chemistries in durable water repellency (DWR) products was provided (Holmquist et al. 2016). A range of common brands providing alternatives to long chain PFAS are listed in Table 3 (UNEP/POPS/POPRC.8/INF/17 2012).
Figure 5 shows a summary of the groups of chemicals that were selected as alternatives, namely hydrocarbons (including waxes) (HC-DWR), silicon based chemistry (Si-DWR) and short chain fluorocarbons (FC-DWR). In addition information about the repellency performance in relation to the end groups of each chemical structure is presented.
Groups of chemicals: hydrocarbons (including waxes) (HC-DWR), silicon based chemistry (Si-DWR) and short chain fluorocarbons (FC-DWR) including their respectively repellency performance. Figure modified from Holmquist et al. (2016)
3.1.4 Toxicity and Exposure Assessment
The same procedure as described in Sect. 3.1.2 was applied to assess the identified alternatives that were already in use (e.g. PFBS-based chemicals). For the potential alternatives that were still in the development phase and not in commerce yet, a modeling approach was taken. A preliminary hazard assessment was undertaken of different DWR chemistries (Holmquist et al. 2016). This was then further refined as new information was created in experimental testing made in SUPFES and outside the project.
Physical-chemical properties were estimated using structure-property relationship models (EPIsuite, SPARC). Different emission scenarios were used in the model and the alternatives as well as the old PFASs (benchmarks) were computed in the estimation model. Conclusions could then be drawn in terms of the probable behavior, transport and fate of the alternatives relative to the old PFASs (i.e. are the alternatives “better” or “worse”).
A literature search for toxicological and ecotoxicological properties of selected alternatives was carried out and the data was compared to corresponding data from the old PFASs. In vitro toxicity testing was performed both for the alternatives as well as for the old PFASs. The established in vitro assays included testing for induction of endocrine disruption, dioxin-like toxicity, genotoxicity, cytotoxicity, and thryroid hormone binding. The in vitro toxicity was further tested after a biotransformation step using different species’ microsomes. Additionally, two standard subchronic ecotoxicity tests were performed with brackish/marine species, i.e. a macroalga (Ceramium Tenuicorne) and a crustacean (Nitocra Spinipes) representative of the Baltic Sea. In all toxicity and ecotoxicity testing, the old PFASs were used as benchmark chemicals, with the aim to characterize the relative toxicity of the alternatives (“better” or “worse” approach).
3.1.5 Case Studies
The overall aim of SUPFES is to demonstrate practical substitution in consumer products. The textile industry sector had clearly expressed needs of meeting both legal and customer demands for surface treatments aiming at water and dirt repellence. To ensure the success of the proposed substitution model, case studies using alternatives in prototypes were performed using a life cycle perspective. Different textiles were selected to represent important sources studied in Step 1 of the substitution model. The case studies included both prototype manufacturing as well as technical performance testing.
The Swedish outdoor brand Haglöfs and the research institute Swerea IVF commissioned water and dirt repellent fabrics for outdoor jacket using proposed alternatives (fluorinated and non-fluorinated alternatives) using conventional solvent phase chemistry and gas phase chemistry (plasma), for input to the technical testing and the emission studies.
3.1.6 Impact and Risk Assessment
Risk estimates for prototypes were based on scenarios describing
-
1.
the degree of exposure of humans and the environment under normal conditions,
-
2.
the frequency of these exposures,
-
3.
the probability for the occurrence of these exposure scenarios, and
-
4.
the hazard characteristics of the substances in combination.
A newly developed methodology by one of the project partners, Vrije Universiteit (VU) in Amsterdam, for measuring emissions of chemicals from materials to air under controlled conditions was used for non-targeted as well as targeted analysis. This methodology enables analysis of chemical compounds in materials; test for emissions to air, analyze in air, dust, wastewater streams, sewage sludge, and recipient water as well as take water samples from a washing machine.
Life cycle assessment (LCA) was used to evaluate the balance of inputs and outputs of systems in different categories related to resource use, human health and ecological areas in different steps. Information on potential toxic effects on human health and the environment, along with physical and chemical properties of the alternatives of interest, was used as input to the LCA. The scenarios in the LCA examined a base case (current products), an improved case (with substitutions) and a zero option (where such substances are not used and thus the technical performance is not provided). The impacts on downstream processes (e.g. wastewater treatment and sludge management) were considered for these scenarios.
To verify the economic viability of suggested designs, life cycle cost (LCC) calculations were performed. The economic viability of the developed technology will be analyzed and interpreted with the chosen software.
3.1.7 Technical Performance
Products with PFASs are often made for outdoor use due to their water and oil repelling and release properties. In order to validate the performance of the prototype(s) developed in the project and to compare the prototype(s) with the existing fluoro-based technology, the following properties were taken into account:
-
General properties; washability, compatibility with dyestuffs
-
Mechanical properties; resistance to abrasion and tearing
-
Physical properties; water vapor resistance (“Skin Model”), water and oil repellency, overall comfort
Durable water repellence (DWR) and related performance attributes include not just water repellency, but normally performance for below attributes is required:
-
Water repellency: various static and dynamic tests are used to measure water repellency. Since there is no set definition of water repellency, the conditions of the test must be stated when specifying water repellency. Water repellent fabrics are generally defined as fabrics which resist being wetted by water; water drops will roll off the fabric. Water repellency depends on the nature of the fiber surface, the porosity of the fabric and the dynamic force behind the impacting water spray.
-
Water proof: this concept is in itself an overstatement; a more descriptive term is “impermeable to water”.
-
Oil repellency: tested by placing a drop of oil on the fabric and observing whether the drop resides on top the fabric or whether it penetrates. A homologous series of hydrocarbons decreasing in surface tension is used to rate the fabric’s oil repellency. The hydrocarbon with the lowest surface tension to remain on top and not penetrate is indicative of the fabric’s repellency. The lower the surface tension of the liquid, the better is the fabric’s resistance to oily stains.
-
Stain repellency: the ability of a treated fabric to withstand penetration of liquid soils under static conditions involving only the weight of the drop and capillary forces.
-
Durability (e.g., to laundering, light exposure, abrasion, dry cleaning, etc.).
-
Other (Soil repellency, stain release, soil release etc.).
It is important to distinguish between water repellent and water proof fabrics. A fabric is made water repellent by depositing a hydrophobic material on the fiber’s surface; however, waterproofing requires filling the pores as well. Water repellent fabrics have open pores and are permeable to air and water vapor, hence they provide the function of “breathability”, i.e. moisture can be transferred through the material. Water repellent fabrics will always permit the passage of liquid water once hydrostatic pressure is high. Water proof fabrics on the other hand are resistant to the penetration of water under much higher hydrostatic pressure than water repellent fabrics, but do not provide the breathability function.
3.1.8 Finalizing the SUPFES Project
The SUPFES project will be finalized during 2017.
3.2 The ENFIRO Project
The project ENFIRO (Life Cycle Assessment of Environment-Compatible Flame Retardants),Footnote 7 2009–2012, investigated the substitution options for some brominated flame retardants and compared the hazard, exposure, fire, and application performances.
Many brominated flame retardants are known to have unintended detrimental effects on the environment and human health. The situation before ENFIRO started was that less toxic alternatives appeared to already be available on the market, however, comprehensive information on their possible toxicological effects was lacking. ENFIRO investigated the substitution options for some brominated flame retardants and compared the hazard, exposure, fire, and application performances. Based on these results, risk and impact assessments were carried out. In total 14 halogen-free flame retardants as alternatives for decabromodiphenyl ether (decaBDE), tetrabromobisphenol A (TBBPA), and brominated polystyrenes (BPS) were selected. These flame retardants were studied in five applications: textile coatings, intumescent paint, electronic components, printed circuit boards and injection moulded products.
ENFIRO followed a prototypical case study approach in which new alternative flame retardants were evaluated. The evaluation included flame retardant properties, their influence on the function of products once incorporated, and their environmental and toxicological properties. The main objectives were:
-
To deliver a comprehensive dataset on viability of production and application, environmental safety, and a life cycle assessment of the alternative flame retardants.
-
To recommend certain flame retardant/product combinations for future study based on risk and impact assessment studies.
3.2.1 Current Use of Flame Retardants
Fires are among the most common causes of harm to people and property around the world. In the last ten to fifteen years, a number of risk-benefit analyses have been performed based on cases with actual fires. The conclusion from these risk-benefit analyses is that measures taken to improve fire safety lead to a clear reduction in the number of deaths and severe injuries. Such measures include increased and improved applications of various forms of flame-retardants. The need for improved fire safety has stimulated the development of better and more effective flame retardants. Also the development of legislation and extensive safety requirements for protection against fire has given rise to tough fire standards for a number of materials handled in situations where there is a risk of fire (Swedish Chemicals Agency 2006, 2004b).
Some halogenated flame retardants have unintended detrimental effects on the environment and human health, and are subject to legal restrictions. The European directive 2011/65/EU on the restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS) (European Commission 2011), addresses for example several halogenated flame retardants while for textile applications, flame retardants are regulated by the European Regulation (EC) No. 1907/2006 REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) (European Commission 2006).
Today, flame retardants are mostly used in the area of electronics, for example in the manufacturing of printed circuit boards, plastic casings for electronics, including mobile phone equipment. Polymeric materials are by far the most common material type containing flame retardants; the largest quantities of flame retardants (around 90%) are supplied to raw-material manufacturers in the plastics industry. A smaller proportion of world production of flame retardants (around 10%) is supplied to the textile and paper industries. The aromatic polybrominated diphenyl ethers (PBDE) have been one of the most commonly used flame retardants. The compound containing ten bromine atoms under the name decabromodiphenyl ether (decaBDE), with CAS RN 1163-19-5 is used in textile applications. DecaBDE is the most commonly occuring flame retardant among the organic aromatic bromine compounds. DecaBDE, which belongs to the category of additiveFootnote 8 flame retardants, is produced in quantities of tonnes per year around the world. Furthermore, DecaBDE is always used in conjunction with antimony trioxide (ATO), which has a harmonized classification under the European Union CLP regulation as suspected of causing cancer (H351) (European Commission 2008).The EU Risk Assessment Report (RAR) of 2002 concluded that further information was required about the persistent, bioaccumulative and toxic (PBT) properties, and that DecaBDE is likely to be very persistent (vP). The substance has some similarities in its behavior to a very persistent and very bioaccumulative (vPvB) substance and its breakdown to substances with PBT or vPvB properties could occur in the environment (for example by metabolism in fish). The RAR further concluded that there are uncertainties regarding possible neurotoxic effects by mammals in laboratory studies as well as secondary poisoning. Possible formation of more toxic and accumulative products such as lower BDE congeners and brominated dibenzofurans in the environment should also be investigated.
The current knowledge about DecaBDE shows that the available assessment methodology might not be applicable to this substance, and in general also not to other brominated flame retardant’s. It can be concluded that there is a continued need to monitor environmental contamination for both the substance in itself and also its more toxic and bioaccumulative degradation products. This uncertainty surrounding DecaBDE is expected to be clarified through further testing and long term biomonitoring (European Chemicals Bureau 2007).
3.2.2 Prioritization and Selection
In the first phase of ENFIRO a prioritization and selection of alternative flame retardants was carried out. The main objective was to identify a range of non-brominated flame retardants that were considered viable alternatives to specific commercial brominated flame retardants on the market. The identification was carried out using the scientific literature and other reliable scientific sources based on how they affect the material’s characteristics of the polymers that are flame retarded. Such characteristics included compatibility, electrical properties, and various ageing properties and was based on already available data on toxicity, exposure risks and environmental fate. This resulted in the assessment of viability criteria for specific flame retardant applications that consisted of flame retarded marketable polymers.
At the start of the project an overview of existing data on alternative flame retardants was made. One of the most important findings was that large data gaps and contradicting information still existed for alternative flame retardants, which also showed the need for ENFIRO. The combination of polymers with the halogen-free flame retardants that were identified as commercially viable alternatives to specific commercial brominated flame retardants [TBBP-A, decaBDE, brominated polystyrene (BPS)] are presented in Table 4. The selection criteria were that the flame retardants should be halogen-free, commercially available, and that some information on the compatibility behavior in polymer materials should be available. The list of halogen-free flame retardants was further updated after consultation with the ENFIRO Stakeholder Forum consisting of flame retardant producers, formulators, end-users, environmental organisations, and others, and after initial screening tests. The list contains phosphorus flame retardants, inorganic tin-based flame retardants, nanoclays and combination of nanoclays with phosphinates. Based on the selected halogen-free flame retardants a literature survey of fire behavior including general characteristics of flame retardant chemicals, thermal degradation properties of the selected flame retardants, and a literature survey on the flammability and toxicity of the selected prototype base polymers and flame retardants was made. Literature data on the flame retardancy of selected systems using halogen-free flame retardants with comparison to brominated flame retardants were also presented.
One of the objectives of ENFIRO was to perform an ecotoxicological and health hazard characterisation of the selected halogen-free flame retardants. Literature data on acute toxicity and ecotoxicity tests of the selected halogen-free flame retardants was collected. The ecotoxicity data showed that a lack of data or contradictory data existed for halogen-free flame retardants that made it difficult to assess the alternative flame retardants and points to the need for reliable experimental data. This was further confirmed for data on specific end points based on a molecular and cellular level, with emphasis on geno-, endocrine-, and neuro-toxicity. Some of these toxicity end points were studied in ENFIRO to fill the data gaps on Ah-receptor, mutagenicity, thyroid hormone binding, endocrine disruption, and neurotoxicity.
Available data for physical-chemical properties for the selected non-halogenated flame retardants was reviewed. The physical-chemical data was used to assess environmental fate and behavior. It was found that estimation tools for organic substances exist but no reliable estimation tools were at the time available for inorganic substances, which means that the assessment of environmental occurrence of inorganic flame retardants was a major challenge. A review of the physical-chemical properties and the (eco)toxicity data for the alternative flame retardants was published (Waaijers et al. 2013).
Information on the economic aspects of the prioritized halogen-free flame retardants was collected as well. The focus was to give an overview about the flame retardants market and about the related industry which is highly influenced by recent trends. Pinpointed were those economic data that were collected through the ENFIRO project with the help of the project partners and the ENFIRO Stakeholder Forum members, in order to complete the Life Cycle Costing.
A schematic presentation of the most viable flame retardants was made per technical area, application, polymer, brominated flame retardants and alternative flame retardants, but a ranking was not possible as too many data gaps existed.
3.2.3 Hazard Exposure
Of the 14 alternative flame retardants that had initially been selected, seven were found to be less toxic than some of the brominated flame retardants and also that they accumulated less in the food chain. The environmental fate models that were used predicted that the organic halogen-free flame retardants would be found primarily in soils, sediments and dust and to a lesser extent in water and air. Controlled air emission experiments showed that all organic halogen-free flame retardants emitted from polymers at elevated temperature but not at lower temperatures. Leaching experiments showed that both halogen-free flame retardants and brominated flame retardants can leach to water. For some polymers no differences in leaching behavior were found between brominated flame retardants and halogen-free flame retardants, but some halogen-free flame retardant systems had higher leaching properties than polymeric based brominated flame retardants. The type of polymer is the main parameter determining the leaching behavior. Analysis of organic halogen-free flame retardants in dust from micro-environments and environmental samples showed highest concentrations on and around electronic equipment, in sediment and in sewage sludge.
3.2.4 Fire and Application Performance
All the selected alternative flame retardants fulfilled the regulatory fire test. A method was developed using intrinsic flammability properties as well as a simple method for characterizing the fire performance and fire toxicity of polymers using three parameters (fire spread, smoke/carbon monoxide, inefficiency of combustion). With this model a comparative fire performance assessment of halogen-free flame retardants versus brominated flame retardants could be made. An important finding was that halogen-free systems demonstrated clear benefits, for example less visible smoke, less toxic components in smoke and in some cases lower peak heat release rate. Regarding mechanical properties, the polymers with brominated and halogen-free flame retardant showed similar loss compared to the polymer alone. All formulations (halogen-free flame retardant and brominated flame retardant) showed equal or better performance regarding processability for injection moulding. For all polymer systems investigated a halogen-free flame retardant option was found. The results for the printed circuit boards showed that the halogen-free flame retardants were as good as or better compared to the reference printed circuit boards produced using brominated flame retardants. A novel intumescent coating system was developed for pure high impact polystyrene (HIPS), showing good fire performance results and excellent results were obtained for the industry fire standards relevant to the electronics industry as well.
3.2.5 Risk Assessment
The environmental and human risk assessments carried out during the ENFIRO project showed that the predicted environmental and human exposure concentrations were below the toxicity thresholds for the selected halogen-free flame retardants. However, the lower risk of halogen-free flame retardants compared to brominated flame retardants is mainly due to the lower hazards of the halogen-free flame retardants, and not due to a lower exposure. Reducing the leaching of halogen-free flame retardants from polymer materials is a next challenge for the development of new flame retardants.
3.2.6 Impact Assessment
The comparative life cycle assessment (LCA) of brominated flame retardant vs halogen-free flame retardants, using a laptop as case study showed that the waste phase was the most relevant phase. In particular, improper electronics waste treatment, leading to the formation of brominated dioxins from brominated flame retardants, had a strong negative impact on the LCA-scores. Overall the LCA performance of the halogen-free flame retardant scenario was better than for the brominated flame retardant scenario. The same life cycles were also evaluated on social criteria using a Social Life Cycle Assessment (S-LCA). Several hotspots are found in the raw material mining phase. In conclusion, ENFIRO showed that viable alternative flame retardants are available. Some halogen-free flame retardants showed less risk for the environment and human health, and show similar fire performance and technical application capabilities as brominated flame retardant
3.2.7 Conclusions from the ENFIRO Project
During the ENFIRO project, a unique approach to assess the data at three different levels was developed: the chemical (flame retardant), material and the product (Fig. 6).
ENFIRO’s three levels of comparative assessment. Modified from Leonards (2011)
The project followed a tiered approach (Fig. 7), starting in the first tier with a prioritization and selection of alternative flame retardants taking into account the viability of flame retardant production, application, flammability in product system, hazards, and exposure of the flame retardants. This generated a list of viable alternatives and identified knowledge gaps. To fill some of the data gaps, screening studies of the selected flame retardants were performed. The screening studies focused on relatively rapid hazard characterization tests, exposure assessment modeling and fire performance tests.
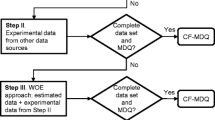
ENFIRO tiered approach of screening and case studies. Modified from Leonards (2011)
Based on the evaluation of the screening results and literature information a further selection of viable flame retardants was narrowed down to be able to carry out in-depth studies on a selection (Tier 2). These studies covered chronic toxicity tests, neurotoxicity, a battery of in vitro tests, persistency, and monitoring of the behavior of the alternative flame retardants in the outdoor and indoor environments. In parallel, elaborated fire performance (realistic fire smoldering and flaming incidents) tests and technical assessments of the flame retardants in various applications were compared with traditional brominated flame retardant systems.
4 Evaluation of Interventions
The conclusions from the two case studies are presented below. In the ENFIRO case, the foreseen intervention has been to substitute one chemical (the brominated flame retardant decaBDE) with another (ammonium polyphosphate (APP) in combination with pentaerythritol (PER), melamine polyphosphate (MPP) and zinc borate (ZB)). In the SUPFES case, the one-to-one substitution of a hazardous chemical with a less toxic alternative has been one identified route. However, the possibility that the perceived requirements from customers turn out to be negotiable has also been considered, in a scenario where the customer is willing to make a compromise between environmental impact and technical functionality.
4.1 SUPFES Evaluation
In the case of DWR treatment of textiles the textile sector has since the beginning of the SUPFES project moved towards alternatives. The importance of SUPFES is therefore high and the results are being communicated along the study rather than after finalizing the project. The characterization of original substances in use was used to associate different types of PFAS with different chemical products. Figure 8 shows that the commercial chemical products labeled as “C6” chemistry also to a large extent contained “C8” chemistry and vice versa, as well as other fluorinated chain lengths.
Characterization of the commercial C6 and C8 products and association of different types of PFAS with different chemical products. Figure based on Van der Veen et al. (2016)
The project has so far been able to conclude that when oil repellence is needed there are no viable alternatives to traditional long chain PFAS. At the same time the hazard assessment have shown both for long chain and to some extent short chain PFAS that they are rather alarming, especially due to their persistence. Regarding emission studies they do not under normal condition give rise to leakage during use. However, emission of stressed materials such as aging in UV and humidity may contribute to emissions.
Alternatives assessed do not offer the same level of technical performance. In addition some of them do emit during use and therefore lose their performance rapidly. They may also have to be added in higher amounts/concentration during application process. But indications are in most cases that non-fluorinated alternatives all in all give less environmental impact or health impact than fluorinated alternatives in general. Figure 9 shows the results from a GreenScreen (Clean Production Action 2014) chemical hazard assessment (Holmquist et al. 2016).
Hazard assessment for selected water repellent agent related substances that reach the environment via diffuse emissions, figure modified from Holmquist et al. (2016). Hazard classification abbreviations: vL = very low, L = low, M = moderate, H = high, vH = very high, PEA = potentially endocrine active, DG = data gap. Classifications in italics are of low confidence and in bold of high confidence. Classifications based on estimated data are marked with an asterisk (*). The endpoints are in order: Carcinogenicity (C), Mutagenicity and Genotoxicity (M), Reproductive Toxicity (R), Developmental Toxicity (incl. Developmental Neurotoxicity) (D), Endocrine Activity (E), Acute Mammalian Toxicity (AT), Systemic Toxicity and Organ Effects (incl. Immunotoxicity) (ST), Neurotoxicity (N), Acute Aquatic Toxicity (AA), Chronic Aquatic Toxicity (CA), Persistence (P) and Bioaccumulation (B)
However, many of the alternatives, including short chain perfluorinated compounds, have not been thoroughly assessed to the same extent as long chain perfluorinated compounds. Therefore, a few alternatives have shown hazardous properties in the same level as PFAS. For example, in the case of silicon-based water and dirt-repellent agents, such agents have recently caused concern due to the fact that both precursors and breakdown products have documented toxic, persistent and bioaccumulative properties.
The materials chosen in the study do not generate fiber loss which can otherwise be an important source of emission. Thus, ecodesign of the combination of alternative and material as well as intended use and performance levels should therefore be considered.
4.2 ENFIRO Evaluation
The efficiency of flame retardants is dependent on the textile polymer systems. Therefore, two different fiber types were investigated with two different coating polymers frequently used on the market. Polyamide (PA) weave and polyether terephtalate (PET) weave (also referred to as polyester) are used as filament plain weaves. The coating polymers were water based emulsion systems without cross linkers. The polymers in the two emulsions are acrylic respectively polyurethane (PUR). A reference was also made for comparing the studied systems with best practice. This best practice was composed with decaBDE/antimony trioxide system. Dispersions with alternative flame retardants (ammonium polyphosphate (APP), melamine polyphosphate (MPP), pentaerythritol (PER)), coating of substrates, and fire testing of the coatings were performed. Test vehicles were tested for fire retardant behavior, peel adhesion strength between weave and coating, tensile properties of pure coating and friction. Representative textiles were used in this study and test vehicles were fire tested according to appropriate fire standards, required for the specific use. Dispersions of acrylic and polyurethanes were used for coatings on PET and PA weave.
Results showed that for suitable flame retardancy for PUR on PET weave 30% of MPP is needed. The combination with APP and PER is not more effective. A formulation of three halogen-free flame retardants (MPP, APP, PER) gives improved extinguishing compared to decaBDE. This halogen-free flame retardant combination is suitable for PUR on PA weave. The minimum amount of halogen-free flame retardant needed is 20% in solid coating, and the effectiveness is similar to decaBDE. Acrylics on PET weave can be flame proofed with 30% APP, but the combination with MPP and PER is not more effective. In this case also the combination of MPP, APP and PER gives similar extinguishing compared to decaBDE systems. For acrylics on PA weave none of the tested halogen-free flame retardants seem to be effective.
Tensile tests were performed on the coating according to modified SS-EN ISO 13934-1:1999. Bromine containing formulations show high tensile strength and maintained or improved elongation at break for both PUR and acrylic. The halogen-free flame retardant formulations are good for acrylic but poor elongation at break for PUR. The test also showed that the bromine formulations make a more flexible coating which is an advantage in many cases. The peel tests showed that optimized halogen-free flame retardant formulations (MPP, APP, PER) with PUR on PET weave had a 57% drop compared to decaBDE systems. The PUR on PA weave system performed better with the halogen-free flame retardants and showed only a 17% drop of peeling compared to the decaBDE. Interestingly, the acrylic on PET and PA weave textile system gave no coating with the decaBDE system but the MPP, APP, PER system gave adhesion.
Based on literature information, databases, and the ENFIRO hazard assessment, seven of the selected halogen-free flame retardants showed to have less issues of toxicity concern (listed as “no immediate concern” in Table 5) than some brominated flame retardants. For two of the halogen-free flame retardants, the results varied between aquatic toxicity studies in the literature (moderate-low and high-low toxicity, respectively). This variation may be due to the amount of triphenyl phosphate (TPP) present in the technical products; TPP is a by-product and know to be very toxic for aquatic organisms. The indication of high toxicity impact does cause concern for environment and humans for these two substances.
Also the results for TPP by itself cause concern: TPP is classified according to REACH as very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment, and needs to avoid release to the environment. Further, bisphenol-A bisphosphate (BDP) is a persistent compound. Finally, a third compound that is of concern and needs further study is the nanoclay (nano-MMT), which showed a strong in vitro neurotoxicity effect. Also the fate (leaching) of this compound from polymers needs further study. For an overview of the hazards see Table 5.
5 Conclusions
This chapter has presented a model for practical substitution where in addition to evaluating the environmental and health performance of alternatives, the technical and economical performance of alternative chemistries are also included.
This chapter also provided two examples of practical substitution using the model, referring to the EU FP7 program ENFIRO and the FORMAS funded SUPFES. The main conclusion is to work across different disciplines to generate knowledge about technical performance in parallel to a thorough evaluation of the environmental performance and health aspects. The technical performance evaluation should also include a discussion about acceptable performance level and different relevant or newly developed performance criteria.
In general, a substitution model including evaluation for both technical as well as environmental and health performance requires an interdisciplinary approach to create and/or suggest feasible alternative solutions. From a sustainability perspective it is vital that the substitution has a net positive effect when considering the entire life cycle. Thus, all relevant impact categories thus have to be considered to avoid new solutions that have less impact from a toxicity perspective but, for instance, have detrimental climate impact. But it is also important to avoid substituting an old and well-known toxic chemistry with a new, less known chemistry that may still be equally toxic.
Notes
- 1.
Chemical Abstracts Service Registry Numbers.
- 2.
- 3.
- 4.
- 5.
- 6.
The term “long-chain PFAS” has been defined by OECD (2013) as:
-
i.
PFCAs with 7 and more perfluoroalkyl carbons, such as PFOA (with 8 carbons or C8 PFCA) and PFNA (with 9 carbons or C9 PFCA);
-
ii.
PFSAs with 6 and more perfluoroalkyl carbons, such as PFHxS (with 6 perfluoroalkyl carbons, or C6 PFSA) and PFOS (with 8 perfluoroalkyl carbons or C8 PFSA); and
-
iii.
Substances that have the potential to degrade to long-chain PFCAs or PFSAs, i.e. precursors such as PASF- and fluorotelomer-based compounds.
-
i.
- 7.
- 8.
Additive means that the flame retardant is only physically bound to the flame retardant material, unlike the reactive flame retardants that are chemically bound.
References
AAFA (2015) AAFA restricted substance list (WWW Document). https://www.wewear.org/rsl/. Accessed 26 Oct 2015
AFIRM (2015) AFIRM supplier toolkit
Andersson H, Harder R, Peters G, Cousins I (2014) SUPFES: environmental risk assessment on short-chain per- and polyfluoroalkyl substances applied to land in municipal sewage sludge. In: 6th international workshop on per- and polyfluorinated alkyl substances—PFASs. Idstein, Germany
Backe WJ, Day TC, Field JA (2013) Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. Military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ Sci Technol 47(10):5226–5234
Baumann H, Tillman A-M (2004) The Hitchhiker’s guide to LCA. Studentlitteratur, Lund, Sweden
Bergman Å, Heindel J, Jobling S, Kidd K, Zoeller RT (2013) State of the science of endocrine disrupting chemicals, 2012. Toxicol Lett. United Nations environment programme and the World Health Organization, New York and Geneva. doi:10.1016/j.toxlet.2012.03.020
BLUESIGN® (2017) BLUESIGN ® (WWW Document). http://www.bluesign.com/. Accessed 24 Aug 2013
ChemSec (2017) SIN list (WWW Document). URL Canadian Hazardous Products Act (Phthalates regulation SOR/ 2010-298). Accessed 20 Mar 2016
China Ministry of Environmental Protection (MEP) (2010) Provisions on the environmental administration of new chemical substances in China. Order No. 7 of the Ministry of Environmental Protection (MEP). China
Clean Production Action (2014) GreenScreen® hazard criteria (WWW Document). http://www.greenscreenchemicals.org/info/guidance-and-method-documents-downloads:GreenScreen®. Accessed 31 Jan 2017
Dubois A, Gadde L-E (2002) Systematic combining: an abductive approach to case research. J Bus Res 55:553–560
Ecolabel Index (2016) Ecolabel index (WWW Document). http://www.ecolabelindex.com/ecolabels/?st=category,textiles. Accessed 24 Aug 2013
European Chemicals Bureau (2007) Review on production processes of decabromodiphenyl ether (decaBDE) used in polymeric applications in electrical and electronic equipment, and assessment of the availability of potential alternatives to decaBDE. Brussels, Belgium
Commission European (2008) Regulation (EC) No 1272/2008 of the European parliament and of the council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending regulation (EC). Off J Eur, Union 353
Commission European (2006) Regulation (EC) No 1907/2006 of the European parliament and the council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European chemicals agency, amending directive 1999/45/E. Off J Eur Union L396:0001–0851
Commission European (2011) Directive 2011/65/EU of the European parliament and of the council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off J Eur Union L174:88–110
GOTS, 2017. Global Organic Textile Standard (GOTS)
Government of India (2012) Draft national chemicals policy (Draft NCP-2012)
Holmquist H, Schellenberger S, van der Veen I, Peters GM, Leonards PEG, Cousins I (2016) Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing. Environ Int 91:251–264
Herzke D, Olsson E, Posner S (2012) Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway—A pilot study. Chemosphere 88(8):980–987. https://doi.org/10.1016/j.chemosphere.2012.03.035
Howard P, Muir DCG (2010) Identifying new persistent and bioaccumulative organics among chemicals in commerce. Environ Sci Technol 2277
Knepper TP, Frömel T, Gremmel C, van Driezum I, Weil H, Vestergren R, Cousins IT (2014) Understanding the exposure pathways of per- and polyfluoralkyl substances (PFASs) via use of PFASs-containing products—risk estimation for man and environment. Texte 47/2014. Dessau-Roßlau, Germany
Leonards P (2011) Life cycle assessment of environment-compatible flame retardants (Prototypical Case Study), ENFIRO. NORMAN Network of Reference Laboratories for Monitoring Emerging Environmental Pollutants New Bulletin 1–20
Munn K (2011) The chemicals in products project: case study of the textiles sector. Switzerland, Geneva
Norwegian Pollution Control Authority (SFT) (2009) Guidance on alternative flame retardants to the use of commercial pentabromodiphenylether. Oslo, Norway
OECD (2013). OECD/UNEP Global PFC Group. Synthesis paper on per- and polyfluoronated chemicals (PFCs)
OECD (2015) Substitution and alternatives assessment toolbox (SAAT) (WWW Document). www.oecdsaatoolbox.org/. Accessed 26 Oct 2015)
OEKO-TEX® Association (2017) OEKO-TEX® standard 100 (WWW Document). https://www.oeko-tex.com. Accessed 24 Aug 2013)
Olsson E, Posner S, Roos S, Wilson K (2009) Kartläggning av kemikalieanvändning i kläder. Mölndal, Sweden
Outdoor Industry Association (2014) Chemicals Management Module (CMM) (WWW Document). https://outdoorindustry.org/advocacy/corporate-responsibility/chemicals-management-module/. Accessed 1 June 2016
Posner S (2009) ENFIRO. Life cycle assessment of environment-compatible flame retardants. WP2 prioritization and selection
Posner S, Roos S, Brunn Poulsen P, Jörundsdottir H, Gunnlaugsdottir H, Trier X, Astrup Jensen A, Katsogiannis A, Herzke D, Bonefeld-Jörgensen E, Jönsson C, Pedersen G, Ghisari M, Jensen S (2013) Per- and polyfluorinated substances in the Nordic Countries. TemaNord, Nordic Council of Ministers. doi:10.6027/TN2013-542
Quinete N, Orata F, Maes A, Gehron M, Bauer K, Moreira I, Wilken RD (2010) Degradation studies of new substitutes for perfluorinated surfactants. Arch Environ Contam Toxicol 59:20–30
Roos S (2016) Advancing life cycle assessment of textile products to include textile chemicals. Chalmers University of Technology, Inventory data and toxicity impact assessment
Roos S (2015) Towards sustainable use of chemicals in the textile industry: how life cycle assessment can contribute. Chalmers University of Technology, Gothenburg
Roos S, Jönsson C, Posner S (2017) Nordic textile initiative—report on labelling of chemicals in textiles. Denmark, Copenhagen
SAC (2017) Sustainable Apparel Coalition (SAC) (WWW Document). http://apparelcoalition.org/the-higg-index/. Accessed 24 Aug 2013
South Korean Ministry of the Environment (2011) Act on the registration and evaluation of chemicals (K-REACH). South Korea
Swedish Chemicals Agency (2014) Chemicals in textiles—risks to human health and the environment. KemI Report 6/14. Stockholm, Sweden
Swedish Chemicals Agency (2007) The substitution principle. Stockholm, Sweden
Swedish Chemicals Agency (2006) Hexabromocyclododecane (HBCDD) and tetrabromobisphenol-A (TBBPA). Government Commission Report, Stockholm, Sweden
Swedish Chemicals Agency (2004a) KemI Rapport 6/04—Information om varors innehåll av farliga kemiska ämnen. Sundbyberg, Sweden
Swedish Chemicals Agency (2004b) Survey and technical assessment of alternatives to decabromodiphenyl ether (decaBDE) in textile applications. Stockholm, Sweden
Swedish Chemicals Group/Swedish Textile Importer’s Association (2016) Chemicals guidance. Mölndal, Sweden
UNECE (2006) Overview of existing information on PFOS production, use, emissions and pathways to the environment and cost/benefits with alternatives/substitutes. Bern, Switzerland
UNEP (2006) Strategic approach to international chemicals management (SAICM). Switzerland, Geneva
UNEP, POPS, POPRC.8, INF, 17 (2012) Technical paper on the identification and assessment of alternatives to the use of perfluorooctane sulfonic acid in open applications. Switzerland, Geneva
United Nations (2002) Report of the world summit on sustainable development, Reissued edn. United Nations publications, New York
van Leeuwen SPJ, van Velzen MJM, Swart CP, van der Veenm I, Traag WA, de Boer J (2009) Halogenated contaminants in farmed salmon, trout, tilapia, pan- gasius, and shrimp. Environ Sci Technol 43:4009–4015
Van Der Veen I, Weiss JM, Hanning AC, De Boer J, Leonards PEG (2016) Development and validation of a method for the quantification of extractable perfluoroalkyl acids (PFAAs) and perfluorooctane sulfonamide (FOSA) in textiles. Talanta 147:8–15. doi:10.1016/j.talanta.2015.09.021
Waaijers SL, Kong D, Hendriks HS, de Wit CA, Cousins IT, Westerink RHS, Leonards PEG, Kraak MHS, Admiraal W, de Voogt P, Parsons JR (2013) Persistence, bioaccumulation, and toxicity of halogen-free flame retardants. Rev Environ Contam Toxicol 222:1–71
Wang D-G, Norwood W, Alaee M, Byer JD, Brimble S (2013a) Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment. Chemosphere 93:711–725
Wang Z, Cousins IT, Scheringer M, Hungerbühler K (2013b) Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60:242–248
Weiss JM, Van der Veen I, De Boer J, Van Leeuwen SPJ, Cofino W, Crum S (2013) Analytical improvements shown over four interlaboratory studies of perfluoroalkyl substances in environmental and food samples. TrAC—Trends in Analytical Chemistry 43:204–216. https://doi.org/10.1016/j.trac.2012.10.005
ZDHC (2014) Roadmap to Zero Discharge of Hazardous Chemicals (ZDHC) (WWW Document). http://www.roadmaptozero.com/. Accessed 1 June 2014
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jönsson, C., Posner, S., Roos, S. (2018). Sustainable Chemicals: A Model for Practical Substitution. In: Muthu, S. (eds) Detox Fashion. Textile Science and Clothing Technology. Springer, Singapore. https://doi.org/10.1007/978-981-10-4876-0_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-4876-0_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4875-3
Online ISBN: 978-981-10-4876-0
eBook Packages: EngineeringEngineering (R0)