Abstract
A new Escherichia coli-Bacillus subtilis shuttle vector pBE2R was constructed to provide a convenient tool for the construction of the mutant library in the directed evolution of the alkaline protease. pBE2R was constructed by fusing P43 promoter with signal peptide as well as propeptide sequence of the apr E gene to the pBE2 vector. Alkaline protease can be expressed successfully in Bacillus subtilis WB600 by introducing the mature peptide encoding gene into the pBE2R vector with the ligation of BamH I which located at the downstream of the first amino acid of the alkaline protease mature peptide. Five insertional positions around the junction between propeptide and mature peptide were tested to detect the critical residues involved in the autoprocessing of the propeptide. Finally, it was deduced that the four critical amino acids “TTMA” which located around the 3′ terminal of the propeptide, as well as the maintenance of its integrity were necessary for the effective autoprocessing of the propeptide. Furthermore, the effect of autoprocessing of the propeptide on the formation of an active protease suggested that the propeptide was a potent inhibitor to the mature domain although it was needed in the folding of the protein before secreting.
Run Wei and Xiao-mei Liang contributed equally to this work.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The serine alkaline protease, secreted from a wide variety of Bacillus species, is an important industrial enzyme and a model system for protein engineering. The alkaline protease can be widely used in washing powders and dehairing hides for its high activity and stability. The alkaline protease generally secreted extracellular for the purpose of scavenging nutrients is specific for aromatic or hydrophobic residues, such as tyrosine, phenylalanine and leucine. However, they are highly sensitive to phenyl methyl sulphonyl fluoride and diisopropyl-fluorophosphate. The Bacillus-originated alkaline proteases are mesophilic enzymes with a molecular weight range of 15–30 kDa and an isoelectric point near pI 9. They reflect high activity at 50–70 °C, however, the activity were reduced significantly at low temperature, like 20 °C [1].
Directed evolution has rapidly emerged as a powerful strategy for improving the characteristics of various enzymes in a targeted manner. To generate large gene variant libraries, it is possible to optimize an enzyme for its specific applications through combining error-prone PCR or DNA shuffling with the high-throughput screening which is used to select the specific properities of an enzyme, such as thermostability, catalytic activity and substrate specificity [2]. Therefore, it is available to improve the activity of the alkaline protease at low temperature through the directed evolution. The construction of the mutant library was a key step in the process of the alkaline protease directed evolution. In this report, a new Escherichia coli-Bacillus subtilis shuttle vector pBE2R was constructed to establish a platform for the high-throughput screening of the alkaline protease in the process of the alkaline protease directed evolution.
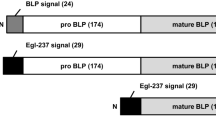
The alkaline protease derived from Bacillus alcalophilus TCCC11263 can be expressed successfully in B. subtilis WB600 by using the pBE2R vector [3]. Apart from this, in the process of construction of the pBE2R vector we found that the correct cleavage of the propeptide after secretion was significant for the formation of the functional protein. As other subtilisins, the B. alcalophilus TCCC11263 alkaline protease of 380 residues is synthesized as an inactive precursor that is composed of a 27 residues signal peptide for protein secretion, a 84 residues propeptide for folding and formation of the active protease and a 269 residues mature peptide for the catalytic protein. The signal peptide is cleaved as the protein crosses the inner membrane, and the propeptide remains covalently attached until the protein is secreted from the cell [4]. The propeptides are relatively common in Bacillus secretory proteins, and they are divided into two different kinds, long and short [5]. The propeptide region generally functions as a facilitator of folding, stability, and even secretion of the protein. In most cases, the propeptide domain is cleaved from the enzymatic domain autocatalytically to release an active protease. Removal of the propeptide by autocleavage during processing is crucial for the secretion and production of subtilisins [6]. Takahashi et al. [7] showed that the autoprocessing efficiency of a subtilisin E mutant with altered specificity for acid residues was improved by substituting the autoprocessing site Tyr-1 with Asp or Glu. Grande et al. [8] reported that insertion within a 9-amino-acid region in the propeptide caused dramatic reduction in LasA enzymatic activity. All mutant proLasA proteins were still secreted, but extracellular stability was low due to clustered insertions within the propeptide. However, little is known about the critical residues involved in the autoprocessing of the B. alcalophilus alkaline protease. Therefore, another primary goal of this study was to identify the critical residues correlated to the autocleavage of the propeptide of the B. alcalophilus alkaline protease.
2 Materials and Methods
2.1 Strains and Plasmids
E. coli DH5α, B. subtilis WB600, B. alcalophilus TCCC11263 were stored by Tianjin university of science and technology laboratory. The plasmid pWB980 was kindly provided by Sui-Lam Wong (University of Calgary, Canada). The plasmid pBE2 was kindly provided by NanKai University.
2.2 Growth Medium and Conditions
E. coli DH5α, B. subtilis WB600 were cultured aerobically in LB medium at 37 °C. For recombinant selection, the kanamycin and ampicillin were applied to the above media. The final concentration of Kanamycin and Ampicillin was 30 and 100 µg/mL respectively. The skim milk plate containing Kanamycin (30 µg/mL) was used for the preliminary screening of the mutants.
2.3 Related Enzymes and Reagents
GeneRuler, restriction endonuclease and T4 DNA ligase were purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. Taq DNA polymerases and Unstained Protein Molecular Weight Marker were purchased from Fermentas. PCR primers were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. All other reagents were analytically pure.
2.4 Construction of the pBE2R
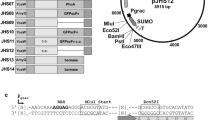
P43 promoter is a strong promoter containing two overlapping promoters which are recognized in vitro by σ55- and σ37-containing RNA polymerase holoenzymes from B. subtilis [9]. The 317 base pair length fragment P43 promoter was released from the plasmid pWB980 by digestion with EcoR I and KpnI. The fragment was isolated by gel purification and inserted into the MCS of the pBE2 vector directionally. Cohesive ends ligation of both fragments resulted in the plasmid pBE2a. Then the DNA fragment containing signal peptide and pro-peptide of alkaline protease derived from B. alcalophilus TCCC11263 was amplified by the Polymerase Chain Reaction (PCR). Based on the DNA sequence of the alkaline protease (aprE) of B. alcalophilus reported on NCBI, a primer pair (Psp1 and Psp2) (Table 1) with indicated engineered restriction sites were designed to amplify the signal peptide and pro-peptide gene (sp).
The KpnI-BamH I fragment of the PCR product was inserted into pBE2a directionaly, forming plasmid pBE2R. Then the pBE2R was transformed into E. coli DH5α. The pBE2R was sent to Shanghai Sangon Biological Engineering Technology & Services Co., Ltd for DNA sequencing.
The mature peptide encoding gene of alkaline protease was introduced into the pBE2R to detect the function of the new shuttle vector. A primer pair (Pmp1and Pmp2) (Table 1) were designed to amplify the mature peptide encoding gene. The BamH I-SalI fragment of the PCR product of the mature peptide (mp) was introduced into pBE2R directionaly to generate pBE2R-mp. Then the recombinant plasmid was transformed into B. subtilis WB600 to express the alkaline protease.
2.5 Construction of the Mutants with Different Insertional Positions of the Restriction Sites Around the Region Between Propeptide and Mature Peptide
Four pairs primers were designed to amplify the fragment of signal and pro-sequences of alkaline protease with the different insertional positions of restriction site around the region between propeptide and mature peptide. The BamH I-SalI fragments of PCR products were inserted into the pBE2a to construct plasmids containing different insertional positions. According to the insertional position, The recombinant plasmids were named pBE2a∷Rs109, pBE2a∷Rs110, pBE2a∷Rs111, pBE2a∷Rs112, respectively. The mature peptide encoding gene was amplified by PCR. Then the PCR products were introduced into the recombinant plasmids described above, generating plasmids pBE2a∷Rs109-mp′, pBE2a∷Rs110-mp′, pBE2a∷Rs111-mp′ and pBE2a∷Rs112-mp′. Meanwhile, a recombinant plasmid pBE2a-apr containing the signal and pro-sequences, as well as the mature sequence of the alkaline protease was constructed without the insertion of restriction site at the junction between propeptide and mature peptide. Then these recombinant plasmids were transformed into the B. subtilis WB600 to express the alkaline protease. The skim milk plate containing Kanamycin (30 µg/mL) was used to screen the mutants preliminarily.
2.6 Assay of the AprE Activity
The B. subtilis WB600 carrying recombinant plasmids were cultured in 100 mL LB medium containing Kanamycin (30 µg/mL) at 37 °C for 48 h. The culture supernatants were collected by centrifugation at 4 °C and 12,000 r/min for 10 min. The supernatants were used as crude enzyme solutions for the activity determination of the AprE protease. The general methods of determination for industrial enzymes was employed to assay the activity of the alkaline protease quantitatively.
2.7 SDS-PAGE Analysis
The SDS-PAGE was employed to detect the relative content of the extracellular proteins. The hosts were cultured in 100 mL LB medium containing Kanamycin (30 µg/mL) at 37 ℃ for 48 h. Supernatants and the cells were harvested by centrifugation at 4 ℃ and 5000×g for 10 min, respectively. Supernatants were precipitated with 100% TCA at −20 ℃ for 5–10 min and then 4 ℃ for 12 h. Centrifugating at 4 ℃ and 5000×g for 10 min to collect the precipitate. The precipitate was washed by acetone for three times and volatiled thoroughly. Then the precipitates were dissolved with the 1× loading buffer before they were boiled. The cells collected by centrifugation described above were suspended with 40 µL of distilled water and 10 µL of 1× loading buffer. Then the solutions were boiled at 100 ℃ for 20 min before loading. SDS-PAGE was performed in a 30% polyacrylamide gel.
3 Results
3.1 Expression of the Alkaline Protease (AprE)
The Escherichia coli-Bacillus subtilis shuttle vector pBE2R was constructed by fusing P43 promoter and signal as well as pro-sequence of the aprE gene to the pBE2 vector (Figs. 1 and 2). The plasmid was verified by double digestion (KpnI/BamH I). The results demonstrated that the sequences inserted into pBE2.
The mature peptide encoding gene of alkaline protease was introduced into pBE2R to generate pBE2R-mp, which was transformed into B. subtilis WB600 to detect the function of the new shuttle vector. The transformants were cultured on the skim milk plate containing Kanamycin (30 µg/mL) at 37 ℃ for 16 h. It was observed from the production of the proteolytic ring that the shuttle vector pBE2R can express the alkaline protease successfully in B. subtilis WB600 (Fig. 3).
3.2 Deduction of the Critical Residues Involved in the Autoprocessing of the Propeptide
The new constructed shuttle vector pBE2R can be used in the directed evolution of the alkaline protease. The mutational gene encoding mature peptide of the alkaline protease produced by error-prone PCR and DNA shuffling can be inserted into the MCS of pBE2R with the connection of BamH I. Therefore, the pBE2R provided a convenient tool for the high-throughput screening of the forward mutation in the directed evolution of the alkaline protease.
In the process of pBE2R construction, we found that the insertional position of BamH I which was located at the junction between the propeptide and mature peptide had a significant effect on the activity of the alkaline protease. It was indicated that the inactive protease was due to the aborting autoprocessing of the propeptide after secretion. Five recombinant plasmids pBE2a∷Rs109~pBE2a∷Rs112 and pBE2a-apr were constructed to detect the critical residues involved in the autoprocessing of the propeptide. Then the mature peptide encoding gene amplified by PCR was ligated to the pBE2a∷Rs109~pBE2a∷Rs112 to generate pBE2a∷Rs-mp′ respectively. Finally, the plasmids pBE2a∷Rs-mp′ and pBE2a-apr were transformed into B. subtilis WB600 to express the alkaline protease.
It was observed that the proteolytic ring could be produced by the hosts carrying pBE2a-apr and pBE2a∷Rs112 but could not by the others carrying pBE2a∷Rs109~pBE2a∷Rs111. The results showed that the activity of the alkaline protease produced by the hosts carrying pBE2a-apr and pBE2a∷Rs112-mp′ could be detected and were parallel. While the activity of the protease produced by the hosts carrying pBE2a∷Rs109-mp′~pBE2a∷Rs111-mp′ could not be detected (Table 2). The determination of the activity of AprE was consistent with the results of the proteolytic ring.
To determine if there are mutations in the coding domain of the alkaline protease which couldn’t produce the proteolytic ring. DNA sequencing was used to detect the mutation in the mature peptide encoding gene which was probably produced by PCR. However, the results of the DNA sequencing revealed that there was no mutation in the coding region of the mature peptide. The protein electrophoresis was employed to determine if the insertions adversely affected the secretion of the alkaline protease. The electrophoresis result of the intracellular protein showed that no increased accumulation of 41-kDa proAprE protein was observed in cell (Fig. 4a). The results of the extracellular protein electrophoresis showed that there was no obvious difference in the secretion level between the two active proteases which were produced by the hosts carrying pBE2a-apr and pBE2a∷Rs112-mp′, respectively, while there was a great difference between the active protease and the inactive protease. The active proteases, a 38-kDa protein bond could be detected in the hosts producing inactive proteases, while the mature peptide which is 29-kDa in molecular mass was not detected (Fig. 4b). The results described above implied that the mutant proAprE protein resulted from the different insertions of BamH I appeared to be secreted normally but abnormally in autoprocessing of the propeptide outside of the cell.
The SDS-PAGE results of the intracellular and extracellular protein from different Samples. a The SDS-PAGE result of the intracellular protein. The strains examined were as follows 1–5 hosts carrying pBE2a∷Rs109-mp′~pBE2a∷Rs112-mp′ and pBE2a-apr, respectively; 6 protein marker. b The SDS-PAGE result of the extracellular protein. 1 Protein marker; the strains examined were as follows: 2–3 hosts carrying pBE2a-apr and pBE2a∷Rs112-mp′; 4–6 hosts carrying pBE2a∷Rs109-mp′~pBE2a∷Rs111-mp′; 7 host of carrying pBE2 (negative control)
In our experiments, the insertion occured at the residues 109, 110, 111 and 112, respectively (Table 3). The insertion of the restriction sites between propeptide and mature peptide might interfere with the recognition of the autoprocessing site so that the pro-peptide couldn’t be cleaved from preproenzyme correctly. It was deduced from the experimental results described above that the amino acids “TTMA” which located at the junction between propeptide and mature peptide, as well as its arranging sequence were critical in autoprocessing of the propeptide. The autoprocessing would be disturbed when this amino acid sequence was destroyed.
4 Discussion
This report described the construction of the Escherichia coli-Bacillus subtilis shuttle vector pBE2R and the identification of the critical residues involved in the autoprocessing of the propeptide in alkaline protease. The new shuttle vector contained P43 promoter and the signal as well as pro-peptide encoding gene of alkaline protease. It was demonstrated that the signal peptide of the alkaline protease could guide the foreign protein to secrete effectively. The propeptide acting as an intramolecular chaperone had a significant effects on the folding and stability of the protease, as well as the formation of the active protease [10,11,12,13,14,15]. In our experiment, It was shown that the deletion of the propeptide resulted in the acute instability of the alkaline protease. The alkaline protease could not fold itself into a suitable state without the assistance of the propeptide so that could be recognized and digested by the proteases produced by the host (not data shown). On the other hand, the correct cleavage of the propeptide after the secretion of the protein was also indispensable to an active protease. It was revealed from the mutant experimental results that several crucial amino acids “TTMA” locating at the 3′ terminal of the propeptide, as well as the maintenance of its integrated sequence played a key role in the autoprocessing of the propeptide. The autoprocessing might be disturbed when this amino acid sequence was destroyed. In addition, the inactive protease caused by the aborted autoprocessing of the propeptide suggested that the propeptide was a potent inhibitor of the mature domain although it was needed in the folding of the protein before secreting.
The pBE2R could be used to express the alkaline protease in B. subtilis WB600 successfully. The shuttle vector pBE2R not only provided a convenient tool for the expression of the foreign protein in the B. subtilis, but also established a platform for the high-throughput screening of the alkaline protease in the process of the directed evolution of the alkaline protease. The mutated encoding genes of the mature peptide which were produced by error-prone PCR and DNA shuffling were introduced into the pBE2R to express the alkaline protease with the different mutation in B. subtilis WB600. The desired mutant containing different positive characters was obtained after multiple rounds of error-prone PCR and DNA shuffling as well as the high-throughput screening.
The test for the expression of mesophilic alpha-amylase gene in B. subtilis WB600 by using pBE2R revealed that the long propeptide of the alkaline protease could not guide the alpha-amylase which belongs to the short propeptide protein to produce the functional protein.
References
Gupta R, Beg Q, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Bryan PN (2001) Protein engineering of subtilisin. Biochim Biophys Acta 1543:203–222
Siezen RJ, Leunissen JAM (1997) Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci 6:501–523
Kessler E, Safrin M, Gustin JD, Ohman DE (1998) Elastase and LasA protease of Pseudomonas aeruginosa are secreted with their propeptides. J Biol Chem 273:30225–30231
Simonen M, Palva I (1993) Protein secretion in Bacillus species. Microbiol Rev 57(1):109–137
Hsu CC, Tsai YC, Chang YS, Lisw SH, Mei HC (2002) Mutational analysis of the autoprocessing site of subtilisin YaB-G124A. Biochem Biophys Res Commun 291:165–169
Takahashi M, Hasuura Y, Nakamori S et al (2001) Improved autoprocessing efficiency of mutant subtilisins E with altered specificity by engineering of the pro-region. J Biochem 130:99–106
Grande KK, Gustin JK, Kessler E et al (2007) Identification of critical residues in the propeptide of LasA protease of Pseudomonas aeruginsa involved in the formation of a stable mature protease. J Bacteriol 189(11):3960–3968
Wang PZ (1984) Doi RH Overlapping promoters transcribed by Bacillus subtilis σ55- and σ37-RNA polymerase holoenzymes during growth and stationary phases. J Biol Chem 259(13):8619–8625
Barequet IS, Ben SGM, Kessler E et al (2004) Pseudomonas aeruginosa LasA protease in treatment of experimental staphylococcal keratitis. Antimicrob Agents Chemother 48:1681–1687
Cunningham EL, Mau T, Truhlar SME et al (2002) The proregion N-terminal domain provides specific interactions required for catalysis of alpha-lytic protease folding. Biochemistry 41:8860–8867
Chang AK, Park JW, Lee EH et al (2007) The N-terminal propeptide of Vibrio vulnificus extracellular metalloprotease is both an inhibitor of and a substrate for the enzyme. J Bacteriol 189(19):6832–6838
Falzon L, Patel S, Chen YJ et al (2007) Autotomic behavior of the propeptide in propeptide-mediated folding of prosubtilisin E. J Mol Biol 366:494–503
Shinde U, Inouye M (2000) Intramolecular chaperones: polypeptide extensions that modulate protein folding. Semin Cell Dev Biol 11:35–44
Pulido M, Saito K, Tanaka S et al (2006) Ca2+-dependent maturation of subtilisin from a hyperthermophilic archaeon, Thermococcus kodakaraensis: the propeptide is a potent inhibitor of the mature domain but is not required for its folding. Appl Environ Microbiol 72(6):4154–4162
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21176190). We thank professor Sui-Lam Wong (University of Calgary, Canada) for providing the pWB980 plasmid kindly. We also thank NanKai University for providing the pBE2 plasmid kindly.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Wei, R., Liang, Xm., Xuan, Mj., Yuan, Hy., Lu, Fp., Li, M. (2018). Construction of the Escherichia coli-Bacillus subtilis Shuttle Vector pBE2R and Identification of the Critical Residues Involved in the Autoprocessing of the Propeptide of the Alkaline Protease. In: Liu, H., Song, C., Ram, A. (eds) Advances in Applied Biotechnology. ICAB 2016. Lecture Notes in Electrical Engineering, vol 444. Springer, Singapore. https://doi.org/10.1007/978-981-10-4801-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-4801-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4800-5
Online ISBN: 978-981-10-4801-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)