Abstract
Primary immune thrombocytopenic purpura (ITP), the most common isolated thrombocytopenia of childhood, is involved in immunological mechanisms. ITP has shown heterogeneous pathophysiology, clinical features, and response to treatment. Most children with primary ITP recover within 6–12 months, but some patients who develop refractory or chronic ITP require especially careful medical management. To date, most conventional treatments consist of immunosuppressive or immune-modulating drugs. The International Consensus Report on the management of primary ITP has stated the goal for ITP management as achieving a safe level of platelet counts to avoid severe bleeding and minimizing therapy-related adverse effects. More recently, a new class of drugs, rituximab and thrombopoietin receptor (TPO-R) agonists, have been developed for the use in treating patients with ITP including children. The increasing clinical usage of these agents might improve therapeutic approaches and managements for children with ITP.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Immune Thrombocytopenic Purpura
- Thrombopoietin Receptor
- Immune Thrombocytopenic Purpura Patient
- Chronic Immune Thrombocytopenic Purpura
- International Consensus Report
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Primary immune thrombocytopenic purpura (ITP), the most common isolated thrombocytopenia in children, is characterized by increased platelet destruction in the spleen and by impaired platelet production in the bone marrow [1, 2]. Today, ITP is regarded not as a single disease but collectively as various thrombocytopenic diseases that are commonly involved by immunological mechanisms [1, 3], although its diagnosis yet needs exclusion of any definite disease.
Since the publication of the International Consensus Report [3] and revised American Society of Hematology guidelines [4], the attitude in which pediatrician manage ITP has begun to change. Patients are treated based on symptoms and signs rather than a mere platelet count. The goal of this management is to maintain a safe platelet count to a level that will minimize or stop bleeding and to lessen the therapy-related adverse effects of importance for children [5]. More recently, in addition to conventional drugs for ITP, the clinical application of newly developed agents, such as rituximab and thrombopoietin receptor (TPO-R) agonists, has demonstrated the efficacy and safety of these agents for children with refractory or chronic ITP.
This review provides a brief summary of treatment for childhood ITP in the first half and, in the second half, emphasizes the clinical studies of rituximab and TPO-R agonists for primary ITP in children.
2 Management of Children with ITP
In 2009, new terminology for ITP was adopted based on the duration: “newly diagnosed” from diagnosis until 3 months, “persistent” from 3 to 12 months, and “chronic ITP” lasting for longer than12 months [6].
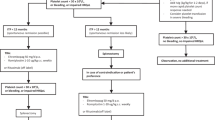
The International Consensus Report [3] has stated on the management of patients with primary ITP. Bleeding of children with newly diagnosed ITP most commonly shows mucocutaneous symptom (petechiae, ecchymosis, or epistaxis), but only 3% of cases have significant bleeding such as severe epistaxis or gastrointestinal bleeding. In particular, the incidence of intracranial hemorrhage (ICH) is rare (0.1–0.5%), but its prediction is difficult in a practical sense [7]. Severe bleeding is more likely with platelet counts of less than 10 × 109/L, whereas major bleeding is rarely seen with platelet counts of more than 30 × 109/L. Most cases of children with newly diagnosed ITP without severe bleeding can be managed using a “watch-and-wait” policy under close observation and with consent of parents. Children with ITP showing significant bleeding must consider hospital admission and treatment if accompanied by marked thrombocytopenia (lower than 20 × 109/L). The management of children with persistent ITP is fundamentally the same as that for those with newly diagnosed ITP.
The goal of treatment for children with persistent/refractory or chronic ITP is to maintain platelet counts at higher than safety levels and to minimize the therapy-related side effects, especially those associated with long-term administration of corticosteroids. The main first-line treatments include corticosteroids and intravenous immunoglobulin (IVIG) as described below.
In Japan, the guideline previously proposed by the Japanese Society of Pediatric Hematology/Oncology had been used widely for management of childhood ITP [8]. Recently, however, the new consensus of terminology and standardization as well as clinical application of new drugs has engendered an increased need for a revised guideline for children with ITP in Japan.
3 Conventional Treatments [4, 8]
Conventional treatments for ITP include immunosuppressive therapies that primarily aim to reduce platelet destruction (e.g., corticosteroids, azathioprine, cyclosporine, cyclophosphamide, mycophenolate mofetil, rituximab, and vinca alkaloids), immune-modulating agents that prevent macrophage destruction of antibody-coated platelets (e.g., intravenous immunoglobulin and intravenous anti-D), and surgical therapy that prevents platelet sequestration (i.e., splenectomy). However, patients with ITP do not always respond to conventional “immunosuppressive” treatments.
3.1 First-Line Treatments
Corticosteroids and intravenous immunoglobulin (IVIG) are two major conventional drugs used in the first-line therapy for children with ITP in Japan. Children with newly diagnosed ITP presenting with bleeding symptom and <20 × 109/L of platelet counts are treated with prednisolone or prednisone (1–2 mg/kg), which may be effective to induce a response in children within 2–7 days until response. Because of side effects associated with prolonged administration of corticosteroids, they should be used for a maximum of 2 weeks. In a standard way, IVIG is administered at doses of 0.4 g/kg/day (2–5 days) or 0.8–1 g/kg (single day). A rapid increase of platelet is induced in more than 80% of patients usually with a shorter durable response than corticosteroids. With these first-line treatments, approximately 70–80% of children with newly diagnosed ITP are likely to have a complete response within 6–12 months after diagnosis.
However, patients who are severe/refractory or chronic ITP with insufficient response to first-line treatments need careful medical care, in addition to second-line treatments if necessary. Conventional second-line treatments include immunosuppressive agents such as cyclosporine, azathioprine, or mycophenolate. Although splenectomy is another reliable second-line therapy with 70–80% of efficacy at initial response, its application to children has become less frequent because of postoperative risk of infection and, more recently, because of the introduction of new agents such as rituximab and TPO-R agonists in clinical areas.
3.2 Choice of the First-Line Therapy and Chronicity of ITP
When physicians start to treat children with newly diagnosed ITP, the choice of corticosteroids or IVIG might be made dependent on clinical expertise, patient preference, or urgent need to increase platelet counts (generally more rapid response to IVIG than to corticosteroids), rather than on the predictive property for preventing chronic ITP development.
Although the individual course of a child with ITP is difficult to predict, Heitink-Polle et al. assessed therapeutic predictors of chronic ITP using a meta-analysis that revealed significantly fewer chronic ITP patients treated with IVIG (odd ratio: 0.71) than those with other treatments [9]. Moreover, a significantly higher risk for chronic ITP was found for patients treated with a combination therapy of IVIG and standard-dose methylprednisolone (SDMP) than with other treatments (odd ratio: 2.67). Although this meta-analysis might be useful in clinical practice, these data must be verified with more precise evaluation using with multivariate, not univariate, analysis or prospective clinical trials.
4 Rituximab
Rituximab is a chimeric monoclonal antibody that targets CD20 antigen on the B-cell surface. Rituximab was used at first for treatment of B-cell lymphoma. Then, its target diseases were expanded to autoimmune diseases. Previous reports of rituximab used for children with ITP are shown in Table 1 [10,11,12,13,14,15,16], in which only few data are available with regard to the long-term efficacy of rituximab for childhood ITP. Recently, a retrospective study in Japan reported the long-term effects of rituximab for 22 children with refractory ITP, for whom the initial CR rate was as high as 41% (9/22), decreased gradually to a 14% (3/22) relapse-free CR rate at 5 years after the first rituximab therapy [16]. Results of this study also suggest that repeated rituximab administration is a promising therapy because patients who had received multiple courses of rituximab after relapse responded each time without adverse effects. They achieved remission during long-term observation.
As shown in Fig. 1, the downward tendency of relapse-free survival (RFS) after initial response is one of major concerns to therapeutic effectiveness of rituximab [15, 16]. From a long-term follow-up conducted for more than 5 years, Patel et al. showed that 52% (34/66) of initial responders subsequently relapsed. Actually, 28 relapsed within 1 year and 6 relapsed during 1–2 years, but none relapsed after 2 years, suggesting that observation for at least 2 years is necessary to assess the long-term efficacy of rituximab for children with chronic ITP [17].
Relapse-free survival rate of subjects who keep continuing first response to the first course of rituximab during long-term follow-up. The gray-shaded area of the initial 4 weeks indicates the evaluation period of the initial response to rituximab [16]
A recent meta-analysis including 324 pediatric patients showed that a pooled CR (platelet count >100 × 109/L) rate and an overall response (platelet count >30 × 109/L) rate were 39 and 68%, respectively [18]. Rituximab therapy might be promising for refractory ITP patients, and, therefore, it might offer relief from bleeding symptoms and allow for avoidance of splenectomy. However, infection is a major concern, and there have been reports in children of pneumonia, varicella, and reactivation of hepatitis C [4, 18,19,20].
The relationship of clinical variables with response of rituximab and relapse-free factors has been studied [15, 17, 21]. Patel et al. [17] reported that patients showing a higher degree of response continued to remain in remission for a long-term period compared to those with a lesser degree of response (relapse rate within 1 year; 7% (2/28) in CR vs. 40% (4/10) in PR). Nor were significant predictors of splenectomy, gender, or age for response of rituximab and relapse-free factors. Although the efficacy of rituximab was not high, Matsubara et al. indicated that the initial responders to rituximab achieved remission at significantly higher rate, even after relapse than nonresponders did. Consequently, in clinical practice, the initial response is a useful indicator of the subsequent remission rate.
Moreover, the rituximab treatment regimen is not necessarily consistent among ITP patients. A recent systematic review pointed that there is no “standard” dose for rituximab treatment in children [18].
4.1 Development of TPO-R Agents
For refractory or chronic children with ITP whose risk of severe bleeding would grow even with the second-line treatments, a strong desire has persisted for therapeutic agents with distinct action mechanisms. The discovery and development of a human recombinant TPO (rh-TPO, the first generation of TPO) proved that the activation of TPO receptor can increase thrombopoiesis of megakaryocytes, facilitating TPO-based therapies as treatment for ITP [22,23,24].
However, the initial clinical trials demonstrated that healthy volunteers who received rh-TPO became severely thrombocytopenic because of cross-reactivity between autoantibodies to rh-TPO and endogenous TPO. This result led to the development of new agents that stimulate TPO receptor but led to little immunogenic adverse effect. The newly developed TPO-R agonists (the second generation of TPO) belong to either TPO non-peptide mimetics (eltrombopag) or TPO peptide mimetics (romiplostim) that increase platelet production by promoting the maturation of BM megakaryocytes through activation of TPO receptor signaling.
The properties of eltrombopag (daily p.o. medicine) and romiplostim (weekly SC injection) are presented in Table 2. The approved administration of eltrombopag is 50 mg/day initial dose and 75 mg/day maximum in Europe and the United States, but for the East Asian patients, the applied dosage was reduced to 12.5 mg/day initial and 50 mg/day maximum because of ethnic difference of drug responsiveness [25].
In the prospective and randomized studies for adult with chronic ITP, 109 of 135 patients (79%) showed significant increases of platelet counts, decreases of concurrent drugs, and lower demand of rescue therapies [26]. Therapeutic effects were not significantly influenced by prior treatments, previous splenectomy, or pretreatment platelet counts (Fig. 2).
For romiplostim, the initial dose is 5 μg/kg/week SC and adjusted up to 10 μg/kg/week at maximum with no marked racial difference of drug responsiveness. Similarly to eltrombopag, the efficacy of romiplostim was approximately 80% for adult with chronic ITP. In a randomized and double-blinded clinical trial for 125 patients [27], the percentage of patients who attained reduction or discontinuation of concurrent drugs was 87% for the romiplostim cohort and 38% for the placebo cohort. Moreover, a randomized clinical trial for patients who were refractory ITP without splenectomy, the subsequent execution rate of splenectomy was 9% for the romiplostim cohort and 36% for the standard therapy cohort, suggesting the possible avoidance of splenectomy by incorporating TPO-R agonists to treatment for refractory ITP [28].
The most common adverse effects of TPO-R agonists were headache, although nasopharyngitis and liver dysfunction were also observed for eltrombopag [26, 27, 29,30,31]. Thrombosis was reported to have a low rate of incidence, but its causal relation remains unclear [28, 31]. As long-term adverse effects, major concerns are the development of myelofibrosis, depletion of hematopoietic stem cells, and induction of other bone marrow abnormalities including malignant diseases. Further investigation of those areas of concern is expected to lead to better understanding.
4.2 Clinical Studies of TPO-R Agonists for Children
Recently, many reports have described the efficacy and safety of TPO-R agonists for children with chronic ITP who had been treated unsuccessfully with the first-line treatments (Table 3) [32,33,34,35,36,37,38]. Patients included children (1–17 years old) with chronic/refractory ITP who received romiplostim or eltrombopag at doses adjusted to less than the maximum dose to maintain platelet counts at least >50 × 109/L. In 2011, the first randomized clinical trials with romiplostim were reported: short-term observation (12 weeks) for 18 and 22 patients for whom the respective pretreatment duration was 2.4 and 2.5 years [32, 33]. These studies showed that the efficacy to maintain >50 × 109/L of platelet counts without rescue medication was 83.5–88% for patients with romiplostim, as compared to 0% for those with placebo. Nevertheless, the small number of patients and the short-term duration of treatment of these studies might limit the generalization of their conclusions. More recently, a larger study of 62 children (romiplostim, 42; placebo, 20) during 24 weeks of treatment duration revealed that the efficacy of romiplostim was 52% for the romiplostim group as compared to 10% for the placebo group [38]. Another randomized double-blinded and subsequent open-labeled study with eltrombopag has demonstrated its efficacy as 40% for the eltrombopag group, compared to 3% for those of placebo [37]. The efficacy of eltrombopag increased further to 80% (70/87 patients) of efficacy during the following 24 weeks of the open-labeled period. These two recent studies confirmed that both romiplostim and eltrombopag TPO-R agonists are effective for chronic/refractory ITP in children. For these periods, no drug resistance or autoantibody against intrinsic TPO was detected.
Few therapy-associated severe adverse effects leading to forced discontinuation or lethalness have been observed, including headache, epistaxis, local pain, cough and vomiting commonly for both drugs, and liver dysfunction for eltrombopag. Although TPO-R agonists are effective and safe for short-term treatment, a retrospective study of children receiving TPO-R agonists for up to 53 months showed that one of 24 patients developed myelofibrosis in grade 2 [35]. The guideline of the American Society of Hematology continues to advise a cautious attitude related to TPO-R agonists for children with ITP [3].
4.3 Long-Term Safety and Discontinuation of TPO-R Agonists
It has been shown that some adult patients remain CR without treatment after discontinuation [39]. Approximately, 30% of adult patients who received these drugs might be able to maintain a safe or normal platelet count after stopping them [5]. Therefore, it remains to clarify whether TPO-R agonists could shorten the period until recovery or facilitate spontaneous cure in a subset of patients with refractory or chronic ITP.
5 Conclusion
In these years, with advances in understanding of pathophysiology of ITP and development of new therapeutic agents for ITP, there have been dynamic changes in the management and therapeutic approaches for children with ITP. However, from the viewpoint of children in the process of hematological and immunological development, further investigation needs to clarify their therapeutic role and long-term safety of those new agents for children with primary ITP.
References
Nugent DJ. Immune thrombocytopenic purpura of childhood. Hematology Am Soc Hematol Educ Program. 2006:97–103.
Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–21.
Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–86.
Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207.
Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32:875–87.
Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93.
Imbach P, Kuhne T, Muller D, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr Blood Cancer. 2006;46:351–6.
Shirahata A, Ishii E, Eguchi H, et al. Consensus guideline for diagnosis and treatment of childhood idiopathic thrombocytopenic purpura. Int J Hematol. 2006;83:29–38.
Heitink-Poll K, Nijsten J, Boonacker C, et al. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood. 2014;124:3295–307.
Wang J, Wiley JM, Luddy R, et al. Chronic immune thrombocytopenic purpura in children: assessment of rituximab treatment. J Pediatr. 2005;146:217–21.
Taube T, Schmid H, Reinhard H, et al. Effect of a single dose of rituximab in chronic immune thrombocytopenic purpura in childhood. Haematologica. 2005;90:281–3.
Bennett CM, Rogers ZR, Kinnamon DD, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107:2639–42.
Rao A, Kelly M, Musselman M, et al. Safety, efficacy, and immune reconstitution after rituximab therapy in pediatric patients with chronic or refractory hematologic autoimmune cytopenias. Pediatr Blood Cancer. 2008;50:822–5.
Mueller BU, Bennett CM, Feldman HA, et al., Pediatric Rituximab/ITP Study Group; Glaser Pediatric Research Network. One year follow-up of children and adolescents with chronic immune thrombocytopenic purpura (ITP) treated with rituximab. Pediatr Blood Cancer. 2009;52:259–62.
Parodi E, Rivetti E, Amendola G, et al. Long-term follow-up analysis after rituximab therapy in children with refractory symptomatic ITP: identification of factors predictive of a sustained response. Br J Haematol. 2009;144:552–8.
Matsubara K, Takahashi Y, Hayakawa A, et al. Long-term follow-up of children with refractory immune thrombocytopenia treated with rituximab. Int J Hematol. 2014;99:429–36.
Patel VL, Mahévas M, Lee SY, et al. Outcome at 5 years following response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–95.
Ling Y, Zhang L, Gao J, et al. Rituximab for children with immune thrombocytopenia: a systematic review. PLoS One. 2012;7:e36698.
Ng PC, Lee KK, Lo AF, et al. Anti B cell targeted immunotherapy for treatment of refractory autoimmune haemolytic anaemia in a young infant. Arch Dis Child. 2003;88:337–9.
Goto S, Goto H, Tanoshima R, et al. Serum sickness with an elevated level of human anti-chimeric antibody following treatment with rituximab in a child with chronic immune thrombocytopenic purpura. Int J Hematol. 2009;89:305–9.
Grace RF, Bennett CM, Ritchey AK, et al. Response to steroids predicts responses to rituximab in pediatric chronic immune thrombocytopenia. Pediatr Blood Cancer. 2012;58:221–5.
Fox JE. Platelet activation: new aspects. Haemostasis. 1996;26(Suppl 4):102–31.
Machlus KR, Italiano Jr JE. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–96.
Kaushansky K. Thrombopoietin: the primary regulator of platelet production. Blood. 1995;86:419–31.
Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10:799–806.
Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomized, phase 3 study. Lancet. 2011;377:393–402.
Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403.
Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–99.
Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–8.
Bussel JB, Kuter DJ, George JN, et al. AMG531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–81.
Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–71.
Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118:28–36.
Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011;90:1341–4.
Pasquet M, Aladjidi N, Guiton C, et al. Romiplostim in children with chronic immune thrombocytopenia (ITP): the French experience. Br J Haematol. 2014;164:266–71.
Ramaswamy K, Hsieh L, Leven E, et al. Thrombopoietic agents for the treatment of persistent and chronic immune thrombocytopenia in children. J Pediatr. 2014;165:600–5.
Bussel JB, Hsieh L, Buchanan GR, et al. Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP). Pediatr Blood Cancer. 2015;62:208–13.
Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386:1649–358.
Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:45–54.
Katsutani S, Tomiyama Y, Kimura A, et al. Oral eltrombopag for up to three years is safe and well-tolerated in Japanese patients with previously treated chronic immune thrombocytopenia: an open-label, extension study. Int J Hematol. 2013;98:323–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Imaizumi, M. (2017). Management and Treatment of Primary Immune Thrombocytopenia in Children. In: Ishida, Y., Tomiyama, Y. (eds) Autoimmune Thrombocytopenia . Springer, Singapore. https://doi.org/10.1007/978-981-10-4142-6_22
Download citation
DOI: https://doi.org/10.1007/978-981-10-4142-6_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4141-9
Online ISBN: 978-981-10-4142-6
eBook Packages: MedicineMedicine (R0)