Abstract
This chapter introduces the concept of patient research partners (PRPs) in clinical research and presents recommendations to support PRPs that have been developed in the field of rheumatology. PRPs are encouraged to participate throughout the research process because their experiential knowledge is valued. For this collaboration to be fruitful, all participants in the research process should agree to principles of trust, respect, transparency, partnerships, communication, diversity, confidentiality and co-learning to support patient involvement in research. On this basis, recommendations are presented that relate to the role, research phases, number, recruitment, selection, support, training, acknowledgement and reporting of PRPs. This provides guidance that can help researchers and PRPs in a variety of clinical research settings such as grant assessment, agenda setting, designing and conducting a clinical study of a health technology, development of a disease-specific core outcome set including endpoints relevant to patients, patient-reported outcome measures and dissemination of findings, all of which are highly relevant to HTA processes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
This chapter introduces the concept of patient research partners (PRPs) in clinical research and presents recommendations to support PRPs that have been developed in the field of rheumatology. PRPs are encouraged to participate throughout the research process because their experiential knowledge is valued. For this collaboration to be fruitful, all participants in the research process should agree to principles of trust, respect, transparency, partnerships, communication, diversity, confidentiality and co-learning to support patient involvement in research. On this basis, recommendations are presented that relate to the role, research phases, number, recruitment, selection, support, training, acknowledgement and reporting of PRPs. This provides guidance that can help researchers and PRPs in a variety of clinical research settings such as grant assessment, agenda setting, designing and conducting a clinical study of a health technology, development of a disease-specific core outcome set including endpoints relevant to patients, patient-reported outcome measures and dissemination of findings, all of which are highly relevant to HTA processes.
National initiatives, such as INVOLVE in the UK (INVOLVE 2016), the Strategy for Patient-Oriented Research in Canada (ISPOR 2016) and the work of the Patient-Centered Outcomes Research Institute (PCORI, Chap. 30) in the USA, demonstrate how public funders of clinical research have been developing their processes to ensure that researchers work with patients in the design and conduct of a clinical trial. More recently, health technology manufacturers have started to explore how they can work with patients to improve the relevance and efficiency of clinical trials within the legal constraints placed upon them about interactions with patients (Chap. 33). Alongside this, there has been increasing recognition that patients must influence the design of clinical studies to ensure they meet the needs of HTA.
Case studies and systematic reviews indicate that, in the past, there has been some consultation with patients to provide input to clinical research (Shippee et al. 2015; Boote et al. 2012). Examples of more extensive collaboration with patients in particular phases of a study have also been reported, for instance, developing research agendas (Abma et al. 2014), developing research protocols (Wilson et al. 2015) or dissemination (Gagnon et al. 2009). But what do we know about patient participation throughout the research cycle? How can researchers enable patients to provide meaningful contributions to each phase of research? The recommendations presented in this chapter provide practical guidance on how patients can be included as research partners in clinical research and have relevance for both health technology developers and HTA bodies.
2 Patient Research Partners
Since the beginning of the century, the role of patients in clinical research has gradually become more influential. The role of passive research subject has evolved into that of patient reviewer, patient advisor and PRP. The latter role should be clearly distinguished from that of study participant who gives informed consent and enters a clinical trial to donate blood or tissue or fill out a questionnaire. Collaboration as a PRP implies equal partnership and a direct dialogue between the patient and the researcher. Here, PRPs are expected to also perform managerial and oversight roles (Dudley et al. 2015). The distinction between both roles demarcates the difference between doing research to, about or for patients and doing research with patients (Staniszewska et al. 2012).
In rheumatology, the concept of PRP was introduced by Outcome Measures in Rheumatology (OMERACT) (Hewlett et al. 2006), an international organisation that develops core outcome sets and core measurement instrument sets for clinical trials. Since 2002, PRPs have been involved in identifying new domains that are important for patients and assessing measurement instruments for content validity and feasibility (e.g. burden for patients). In 2007 the European League Against Rheumatism (EULAR) adopted the concept of PRPs to support patient-researcher partnerships in the development of disease management recommendations. At that time, patients and researchers expressed a lack of knowledge and skills on building such partnerships. This prompted EULAR to formulate a set of practical guidelines that could direct and support participants to collaborate in the context of management recommendation development (de Wit et al. 2011). More recently, also OMERACT formalised PRP involvement by publishing practical recommendations, including a set of three overarching principles for patient involvement (Cheung et al. 2016).
OMERACT Overarching Principles for Participation of PRP
-
OMERACT values the experiential knowledge of PRPs as critical to outcome research.
-
Engaging PRPs as integral participants throughout the research process is a fundamental OMERACT principle.
-
All OMERACT participants subscribe to the principles of trust, respect, transparency, partnerships, communication, diversity, confidentiality and co-learning with respect to patient involvement in research.
The purpose of PRPs is to provide the experiential knowledge of the impact of an illness and use of the health technology on daily life and to ensure that the perspectives of patients are preserved throughout the research process. A PRP operates as an active and equal member of the research team. A PRP can be called an expert patient when representing a patient organisation or when they are able to present a wider perspective on the disease, going beyond their individual experience. For some conditions, parents or caregivers can take on the role of a PRP. PRPs can contribute perspectives about their illness in different ways, being on a patient panel, patient reference group or guideline working group (Pittens et al. 2013) or as a member of a research steering group or Scientific Advisory Board (Teunissen et al. 2013). They can take responsibility for providing patients’ perspectives in setting research priorities, research design, reviewing literature, recruitment methods, collecting data, analysis and interpretation of findings and dissemination. In addition, it is their duty to ensure that patients’ perspectives are not lost at any stage of the research by providing these perspectives whenever appropriate or suggesting methods to capture these perspectives, for example, by suggesting consultation of a wider group of patients through a survey, interview, focus group or mixed method study. We believe that the added value of PRP participation outweighs potential risks and disadvantages but recognise that patients must be supported to contribute fully as partners in research.
Potential Tasks of PRPs in HTA
-
Identifying questions and unmet needs that are important for patients to inform HTA agenda setting
-
Promoting incorporation of patients’ perspectives through existing literature or initiating new qualitative studies
-
Considering ethical issues in HTA
-
Critically reviewing of evidence
-
Identifying and prioritising outcomes that are important for patients
-
Identifying eligible target groups or subgroups
-
Advocating the interests of minorities and difficult to reach groups and encouraging researchers to make additional efforts to incorporate their perspectives (e.g. through home visits)
-
Demonstrating the short- and long-term real-world implications of an intervention
-
Assessing the burden of treatment options in daily life
-
Providing the local context of health delivery
-
Advocating access to appropriate interventions
-
Supporting dissemination of findings to lay audiences (for instance, by writing lay summaries or giving presentations)
3 EULAR Recommendations for Collaboration
The EULAR recommendations for PRP collaboration address some of the challenges of patient participation identified in Chap. 5 and provide support to researchers as well as PRPs to help avoid risks of bias and other pitfalls.
3.1 Role
Participation of patient research partners is strongly recommended for clinical research projects and for the development of recommendations and guidelines and should be considered for all other research projects.
How patient involvement is implemented in research depends on the objectives of a study and the health system in a particular country. Ideally, meaningful patient involvement implies combining patient contributions through various consultation methods and direct participation in the research team (such as in the example in Sect. 8.3.3). Creating opportunities for an open dialogue between patients and researchers and building sustainable relationships can be time-consuming and demanding for both PRPs and researchers. Therefore, depending on the intensity of the agreed tasks and responsibilities, a watchful eye should be kept to balance what is desirable and what is feasible.
3.2 Research Phases
Participation of patient research partners should be considered in all phases of the project to provide experiential knowledge, with the aim of improving the relevance, quality and validity of the research process.
In the past 10 years, PRPs have been involved in many research phases (Shippee et al. 2015). They have enriched research agendas with themes that are relevant to patients (Abma et al. 2014) and contributed to drafting research calls, formulating research questions, developing treatment recommendations, reviewing grant applications and supporting dissemination and implementation.
Although the form and timing of involvement may be adapted to the scope of the project, it is recommended that involvement of PRPs should start as early as possible. Studies have demonstrated that involvement of PRPs in trial design frequently leads to choosing endpoints more relevant to patients, more user-friendly instruments and procedures and valuable suggestions for improving recruitment rates (Haywood et al. 2014; de Wit et al. 2013).
3.3 Number of PRPs
A minimum of two patient research partners should be involved in each project.
In general, it is strongly recommended to involve more than one PRP in a research project (de Wit et al. 2011; Cheung et al. 2016). This ensures multiple views from patients during meetings and continuity in the event of a relapse in illness or drop-out of one of the PRPs. It also creates opportunities for prior consultation or preparation (de Wit et al. 2011). In OMERACT it has been agreed that the research leadership takes responsibility for appropriate representation of patients’ perspectives in the research project.
The primary task and responsibility of the PRPs is to help a research team think through the design and conduct of a study. Based on their personal experience with the disease and what they know about fellow patients, they may suggest phases where the perspectives of patients are relevant and advise on ways to obtain those perspectives. It is not the responsibility of the PRPs to guarantee representativeness. One or two PRPs on a research team or steering committee cannot represent all perspectives of the entire target population. The perspective of patients is heterogeneous as a result of age, gender, social-economic status, cultural background, disease duration and severity and other factors. Therefore the participation of PRPs should be complemented by other forms of patient involvement to enhance diversity and validity of the patients’ perspectives (Legare et al. 2011). In the example below, the research team took responsibility for the integration of different forms of patient involvement in the development of the EULAR Psoriatic Arthritis Impact of Disease score (PsAID) (Gossec et al. 2013; De Wit et al. 2015a; Kirwan et al. 2016).
Example of Full Patient Participation in Clinical Research: The Development of a Patient-Reported Outcome in Psoriatic Arthritis
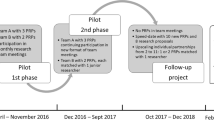
In the development of the PsAID, a disease-specific patient-derived quality of life instrument for psoriatic arthritis, patients were involved in various steps of the participation ladder. The involvement of patients as study participants in a domain prioritisation exercise and in the validation phase was complemented by involvement as advisors in a series of cognitive interviews in ten countries and as collaborative partners in the overall research team. In the latter role, ten PRPs participated in two international face-to-face meetings and contributed to the:
-
Identification of domains of impact important to people with psoriatic arthritis
-
Definition and phrasing of items
-
Translation of the draft questionnaire into the national language
-
Interpretation of findings from the validation study
-
Choice of recall period
-
Number of the items
-
Format and anchors of the instrument
All patient research partners who agreed became co-authors of the final PsAID publication.
Finally, two expert patients were member of the steering group of the project.
3.4 Recruitment
Identification of potential PRPs should be supported by a clear description of expected contributions.
The initiative for PRP recruitment lies with the investigator, who preferably contacts the appropriate patient organisation and provides a clear job description clarifying expectations and benefits of involvement. Not all diseases have patient organisations and only a few have established networks of trained PRPs. For this reason, some research teams have to find alternative recruitment strategies, for instance, through patient magazines, health professionals, national patient umbrella organisations or established virtual networks such as ‘Patients in Research’ (UK) or ‘Patients Like Me’ (USA). EULAR recruits PRPs through national patient member organisations, while OMERACT prefers to identify PRPs through the clinics of physician-researchers. The latter are often best situated to assess the competences of potential patients for taking on the PRP role.
All strategies involve risk of bias and have pros and cons that depend on the research context and objectives of the patient involvement. For instance, in the phase of fund-raising, the formal endorsement of a research study by a well-known patient organisation is important, while in the elaboration of a disease-specific quality of life instrument, the contributions of individual PRPs are important.
Studies have demonstrated that it is effective to discuss mutual expectations on contributions and the level of participation prior to a research project (Abma et al. 2009). This will help the researcher to create a realistic picture of the required time investment, frequency of meetings and tasks of the PRP. Conversely, the same is true for patient representatives’ expectations in terms of research outcomes and collaboration with the investigator which should be shared with the investigator. Importantly, both parties should participate in this discussion on the basis of equality and clearly specify their limits and possibilities.
As investigators and PRPs develop their thinking as a project proceeds, and needs and expectations may evolve as well, it is wise to evaluate the collaboration at regular intervals. Does the investigator provide sufficient information and support? Is the PRP not over- or underburdened and will there be a follow-up to this research project? It can also be useful for patient organisations and investigators to use the evaluation outcomes internally to adjust and improve their procedures, support and policies.
3.5 Selection
The selection process of patient research partners should take into account communication skills, motivation and constructive assertiveness in a team setting.
Over the years, we have learned that PRPs should not only be selected for their experiential knowledge or membership of a patient organisation but also for their competences to collaborate in a team setting. Some researchers may argue that selection for language skills, affinity or knowledge of clinical research and the ability to travel and communicate with professionals will only attract highly educated patients that are not representative for the patient group under study (van de Bovenkamp 2010). Although it is true that PRPs are not representative for the entire patient population, the fact that strict selection may indeed constitute a risk of bias is no argument to relinquish PRP involvement. Diversity of patients’ perspectives should be captured through the use of appropriate research methods, and educated PRPs form an additional source for the research team (Mayer 2012).
Researchers should know that various forms of participation require various competencies and skills and should select PRPs in accordance with their expected role and tasks. Generally, the advice for researchers who start with PRP involvement for the first time is to start small and to identify two or three patients from the own institution that might be interested in clinical research. During an introduction meeting, a draft research proposal can be presented followed by an exploration among the patients for their potential interest in the study and level or intensity of involvement.
3.6 Support
The principal investigator must facilitate and encourage the contribution of patient research partners and consider their specific needs.
The responsibility of the investigator to enable PRPs to contribute to research in a meaningful way is crucial (Nierse et al. 2012; Hewlett et al. 2006). The investigator should provide timely and individualised information and ensure an open and safe atmosphere during meetings (Elberse et al. 2010). The type of support may vary from using understandable language and explaining difficult terms or concepts, asking open questions and inviting patients to share their perspectives to writing lay summaries, arranging access to libraries or medical databases or taking care of logistics. Depending on the role and project, the PRP may be offered a job description or formal contract, outlining issues such as responsibilities, confidentiality, conflict of interest, available support and education. Sometimes the principal investigator may appoint a designated PRP supporter in the research team. To support clinical trial teams with limited resources, the clinical trial research centre of the University of Liverpool has started to develop a toolkit that provides resources for planning, supporting, recording and evaluating patient involvement along the research pathway (Bagley et al. 2016).
A realistic budget for patient involvement and prompt reimbursement of expenses can also be regarded as support. PRP involvement requires time and money; costs for travel, accommodation, attendance fees, out-of-pocket expenses or even compensation for worked hours should be considered and subsequently realistically budgeted. Compensation for the patient association should, on occasion, be included to cover costs for recruitment, training and support. There is currently no standard for what can be considered a reasonable compensation for the work of a PRP or patient association. It depends on factors such as the PRP’s preferences and the expected time investment, and it should be customised for each individual project (De Wit et al. 2016).
Network of PRPs
EULAR has established a network of over 40 educated PRPs coming from all parts of Europe and representing ten rheumatic conditions. They are involved in a broad range of research activities varying from developing disease management recommendations and reviewing grant application to participating in clinical research studies, committees of the European Medicines Agency (EMA) and Innovative Medicines Initiative (IMI) consortia and dissemination of findings. A designated coordinator at the EULAR secretariat ensures appropriate matching of research projects and PRPs and organises support and training if needed. Researchers and PRPs are provided with reference cards for collaboration and a background brochure. PRPs are invited for biannual training and evaluation meetings and alerted on training opportunities. Seven members have participated in a medicine development training course organised by the European Patients’ Academy (EUPATI). In 2016 two members followed the first EULAR course on health economics in rheumatology.
In some countries, national organisations take on the task of supporting PRPs. For instance, in the UK the National Institute for Health Research (NIHR) runs induction meetings for public members who join advisory committees to help them integrate, understand their role and meet professional members. The NIHR also provides a buddy system and organises networking events for public members to meet fellow members to share experiences and provide support.
3.7 Training
The principal investigator must ensure that patient research partners receive information and training appropriate to their roles.
Optimising PRP participation requires adequate capacity building. PRPs’ ability to provide constructive and competent collaboration cannot always be assumed. For this reason, some institutes that foster patient participation in research offer training. Also, EUPATI offers an intensive training programme for patient representatives to understand the medicine development process (see Chap. 36). EULAR provides annual evaluation and training days to the members of the EULAR PRP network. Researchers are invited to present best practices of patient involvement or to provide additional education on, for example, critical appraisal of scientific articles or basic statistics. PRPs are encouraged to share experiences, train communication skills and learn to deal with power imbalances within a research team.
An important aim of the education is to make participants aware of the potential strengths and limitations of the role of a PRP. They learn not only to appreciate the value of personal illness and experiences of healthcare use, they also have to acquire the competence to balance their personal preferences or personal interests against other issues affecting research.
Researchers should also be taught the conditions for effective patient participation. It is often wrongly assumed that investigators have the required knowledge and competences for PRP’s participation (de Wit et al. 2015b). In some countries master classes or coaching programmes are offered to familiarise investigators with the added value of patients’ perspectives and methods of participatory research (de Wit et al. 2015b).
3.8 Acknowledgement
The contribution of patient research partners to projects should be appropriately recognised, including co-authorship when eligible.
There are many ways, both material and immaterial, to acknowledge the contribution of patients (see Table 8.1). Becoming co-authors or timely reimbursement can be regarded as examples of nonmonetary methods of acknowledgement. PRPs regularly express the lack of feedback from researchers on the value of their input and how the researcher has incorporated their comments and suggestions in the project. PRPs appreciate confirmation that their participation matters.
Although many patients are content with their voluntary role as PRP, the investment in terms of time and energy might become substantial and justify payment, in particular for patients who have a job and have to take days off from work to participate in a research meeting. Some organisations have rules for financial compensation for PRPs who do committee work or research projects. The resource section on the INVOLVE website (www.invo.org.uk) contains guidance and practical advice on payment and other methods for recognising the time, skills and expertise provided by PRPs. EMA has developed rules for a daily allowance, and pharmaceutical companies sometimes agree on a formal contract with PRPs paying a fixed rate per hour.
3.9 Reporting
The nature of PRP involvement should be reported throughout the research process, at least in the initial research proposal and final reports.
OMERACT encourages researchers to be explicit about the strategy of patient involvement. The publication policy, including peer-reviewed scientific publications and lay summaries and articles targeting the general public, should be discussed with PRPs during team meetings.
In accordance with the Guidance for Reporting Involvement of Patients and Public (Staniszewska et al. 2011), investigators are expected to report the intended or implemented patient involvement in research proposals and scientific publications, both positive outcomes and any negative consequences. Requiring reporting of patient involvement may avoid tokenism and enhance transparency of the research. It will also stimulate mutual sharing of lessons learned, challenges and pitfalls and, by doing so, improve the systematic evaluation of patient involvement in practice. Finally, funders of clinical research want to be informed about the added value of patient and PRP involvement to legitimise their investments in this field.
4 Challenges
4.1 PRP Training and the Risk of Pseudo-professionalisation
Chapter 3 raises concerns about the professionalism of patients and their role in research. However, with the training opportunities offered by public research networks for patients seeking to become involved in research, it looks like the debate about the potential risk of patients losing their experiential knowledge as a consequence of being educated has come to an end in favour of the benefits of proper education of PRPs. However, there is still the risk that patients become professional researchers aligning easily with ‘real’ researchers (Dudley et al. 2015). More robust knowledge is needed to examine what kind of attitude, knowledge and skills training is needed to become an effective PRP with maintenance of the unique value of the authentic patient’s experiences.
Not only PRPs need training but also researchers have to learn the basic principles and accept the practical implications of PRP involvement in clinical research. This is necessary because researchers are still reluctant to involve patients as collaborating partners. Partnership implies that control of some parts of the study should be shared with PRPs and that flexibility is required, for instance, to include new outcome measures that are important to patients although less frequently used in clinical research. It might also mean that research questions need reformulation, that inclusion or exclusion criteria need to be changed or that ways of administration or burdensome research protocols need to be adjusted to make them more patient friendly. In this regard, the King’s Fund initiative has started a shared leadership programme that explores training and development interventions to establish collaborative relationships among professionals, patients and caregivers (Seale 2016).
Researchers may experience a lack of know-how or feel insecure about the amount of freedom that payers or regulators allow them to address issues important to patients. It is true that involving PRPs in the research process is time-consuming and may cause new dilemmas. Both researchers and PRPs should become aware of the practical, moral and legal implications of participatory research. Participants should also be taught the difference between the patient-health professional relationship in the clinic and that of collaborating colleagues in the context of research (Hewlett et al. 2006). This equal relationship is an essential condition for the establishment of a genuine dialogue free of power or status differences.
4.2 Ethical Considerations
With regard to the legal framework of PRP involvement, we do not have much experience in the context of clinical research. Because PRPs are not approached as study participants or respondents, uncertainty exists whether a researcher needs ethical approval to include a PRP in the research team. Similarly, although the PRP will not be exposed to any intervention, does the PRP have to sign a consent form? INVOLVE, in collaboration with the National Research Ethics Services, developed a document, stating that people do not need ethical approval when they act as specialist advisers, meaning actively involved in planning or advising on research. However, when a patient’s involvement results in direct contact with study participants, the ethics committee should be consulted (www.nres.npsa.nhs.uk). This could be the case when PRPs take part in the conduct of research, for instance, by co-moderating a focus group, coding interview transcripts or assisting in recruiting by providing information to patients as a contact person. The principal investigator should ensure that PRPs are formally certified or receive appropriate support and supervision. Finally, does the PRP have to fulfil all the ICMJEFootnote 1 requirements (ICMJE 2015) to qualify for authorship of a peer-reviewed manuscript? In the absence of a standardised approach, researchers are expected to make a fair choice between an acknowledgement box or offering co-authorship.
We do not have experience of privately funded research undertaken by health technology developers, but we feel that the recommendations presented here to support inclusion of patients as research partners would inform the growing interest in ‘patient-focused drug development’ as outlined in Chap. 33.
5 Conclusion
The EULAR recommendations can help researchers to involve patients in the conduct of research, including development of successful recruitment strategies, identification of patient relevant outcomes and dissemination of results. PRP involvement requires an investment in time and commitment from the researcher. Regular exchange of mutual expectations between PRPs and researchers is beneficial and will prevent tokenism. Ensuring the representativeness of patients’ perspectives and in particular the role and added value of PRPs is still challenging. It is one of the responsibilities of PRPs to help the research team to preserve patients’ perspectives throughout the different stages of the research process. They are not on the team to guarantee the representativeness of the study in person because two or three PRPs can never represent the perspectives of the entire patient population. That perspective should be obtained through appropriate methods such as literature reviews, patient surveys or qualitative research, for instance, narrative research, focus group or interview studies. PRPs can help researchers to improve their recruitment strategy, explore the best endpoints for a trial or ask the right questions in a focus group or survey in the right order. Future experiences will teach us how collaborations between researchers and PRPs can be fruitful in the research considered in HTA.
Notes
- 1.
International Committee of Medical Journal Editors
References
Abma TA, Nierse C, Widdershoven G. Patients as partners in responsive research: methodological notions for collaborations in mixed research teams. Qual Health Res. 2009;19:401–15. doi:10.1177/1049732309331869.
Abma TA, Pittens CA, Visse M, Elberse JE, Broerse JE. Patient involvement in research programming and implementation: a responsive evaluation of the dialogue model for research agenda setting. Health Expect. 2014; doi:10.1111/hex.12213.
Bagley H, Short H, Larman NL, Hickey HR, Gamble CL, Woolfall K, Young B, Williamson PR. A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials – a work in progress. Res Involv Engag. 2016;2:1–14. doi:10.1186/s40900-016-0029-8.
Boote J, Baird W, Sutton A. Involving the public in systematic reviews: a narrative review of organizational approaches and eight case examples. J Comp Eff Res. 2012;1:409–20. doi:10.2217/cer.12.46.
Cheung PP, de Wit M, Bingham 3rd CO, Kirwan JR, Leong A, March LM, et al. Recommendations for the involvement of patient research partners (PRP) in OMERACT working groups. A report from the OMERACT 2014 working group on PRP. J Rheumatol. 2016;43:187–93. doi:10.3899/jrheum.141011.
de Wit M, Abma T, Koelewijn-van Loon M, Collins S, Kirwan J. Involving patient research partners has a significant impact on outcomes research: a responsive evaluation of the international OMERACT conferences. BMJ Open. 2013;3 doi:10.1136/bmjopen-2012-002241.
de Wit MP, Berlo SE, Aanerud GJ, Aletaha D, Bijlsma JW, Croucher L, Da Silva JA, Glusing B, Gossec L, Hewlett S, Jongkees M, Magnusson D, Scholte-Voshaar M, Richards P, Ziegler C, Abma TA. European league against rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis. 2011;70:722–6. doi:10.1136/ard.2010.135129.
de Wit M, Bloemkolk D, Teunissen T, Van Rensen A. Voorwaarden voor succesvolle betrokkenheid van patiënten bij medisch wetenschappelijk onderzoek. Tijdschr Gezondheidsr. 2016;94:92–102.
de Wit MP, Elberse JE, Broerse JE, Abma TA. Do not forget the professional - the value of the FIRST model for guiding the structural involvement of patients in rheumatology research. Health Expect. 2015b;18:489–503. doi:10.1111/hex.12048.
de Wit M, Kvien T, Gossec L. Patient participation as an integral part of patient reported outcomes development guarantees the representativeness of the patient voice – a case-study from the field of rheumatology. RMD Open. 2015a;1:e000129. doi:10.1136/rmdopen-2015-000129.
Dudley L, Gamble C, Allam A, Bell P, Buck D, Goodare H, et al. A little more conversation please? Qualitative study of researchers' and patients' interview accounts of training for patient and public involvement in clinical trials. Trials. 2015;16:190. doi:10.1186/s13063-015-0667-4.
Elberse JE, Caron-Flinterman JF, Broerse JE. Patient-expert partnerships in research: how to stimulate inclusion of patient perspectives. Health Expect. 2010;14(3):225–39. doi:10.1111/j.1369-7625.2010.00647.x.
Gagnon MP, Lepage-Savary D, Gagnon J, St-Pierre M, Simard C, Rhainds M, et al. Introducing patient perspective in health technology assessment at the local level. BMC Health Serv Res. 2009;9:54. doi:10.1186/1472-6963-9-54.
Gossec L, de Wit MPT, Heiberg T, Maccarone M, Balanescu A, Balint P et al. Elaboration and preliminary validation of the psoriatic arthritis impact of disease (psaid) questionnaire. A 13-country eular initiative with involvement of patient research partners from each country. Paper presented at the EULAR congress, Berlin, 2013.
Haywood K, Brett J, Salek S, Marlett N, Penman C, Shklarov S, et al. Patient and public engagement in health-related quality of life and patient-reported outcomes research: what is important and why should we care? Findings from the first ISOQOL patient engagement symposium. Qual Life Res. 2014;24(5):1069–76. doi:10.1007/s11136-014-0796-3.
Hewlett S, De Wit M, Richards P, Quest E, Hughes R, Heiberg T, et al. Patients and professionals as research partners: challenges, practicalities, and benefits. Arthritis Rheum. 2006;55:676–80. doi:10.1002/art.22091.
ICMJE Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. 2015. http://www.icmje.org/icmje-recommendations.pdf. Accessed 12 July 2015.
INVOLVE. Website supporting public involvement in NHS, public health and social care research. 2016. http://www.invo.org.uk/. Accessed 10 Feb 2016.
ISPOR Strategy for patient-oriented research. Canadian Institutes of Health Research. 2016. http://www.cihr-irsc.gc.ca/e/41204.html. Accessed 18 Oct 2016.
Kirwan J, de Wit M, Frank L, Haywood K, Salek S, Brace-McDonnell S et al. Patient as partners in health outcomes research: learnings from the field. Value Health. 2016;20(3):481–86.
Legare F, Boivin A, van der Weijden T, Pakenham C, Burgers J, Legare J, et al. Patient and public involvement in clinical practice guidelines: a knowledge synthesis of existing programs. Med Decis Mak. 2011;31:E45–74. doi:10.1177/0272989X11424401.
Mayer M. Seeking what matters: patients as research partners. Patient. 2012;5:71–4. doi:10.2165/11632370-000000000-00000.
Nierse CJ, Schipper K, van Zadelhoff E, van de Griendt J, Abma TA. Collaboration and co-ownership in research: dynamics and dialogues between patient research partners and professional researchers in a research team. Health Expect. 2012;15:242–54. doi:10.1111/j.1369-7625.2011.00661.x.
Pittens CA, Vonk Noordegraaf A, van Veen SC, Anema JR, Huirne JA, Broerse JE. The involvement of gynaecological patients in the development of a clinical guideline for resumption of (work) activities in the Netherlands. Health Expect. 2013; doi:10.1111/hex.12121.
Seale B. Patients as partners. Building collaborative relationships among professionals, patients, carers and communities. London: The King’s Fund; 2016.
Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18:1151–66. doi:10.1111/hex.12090.
Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care. 2011;27:391–9. doi:10.1017/S0266462311000481.
Staniszewska S, Haywood KL, Brett J, Tutton L. Patient and public involvement in patient-reported outcome measures: evolution not revolution. Patient. 2012;5:79–87. doi:10.2165/11597150-000000000-00000.
Teunissen GJ, Visse MA, Laan D, de Boer WI, Rutgers M, Abma TA. Patient involvement in lung foundation research: a seven year longitudinal case study. Health. 2013;5:320–30. doi:10.4236/health.2013.52A043.
van de Bovenkamp HM. The limits of patient power. Examining active citizenship in Dutch health care [dissertation]. Rotterdam: Erasmus University; 2010.
Wilson P, Mathie E, Keenan J, McNeilly E, Goodman C, Howe A, et al. ReseArch with patient and public invOlvement: a RealisT evaluation – the RAPPORT study. Health Serv Deliv Res. 2015; doi:10.3310/hsdr03380.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
de Wit, M., Gossec, L. (2017). Patients as Collaborative Partners in Clinical Research to Inform HTA. In: Facey, K., Ploug Hansen, H., Single, A. (eds) Patient Involvement in Health Technology Assessment. Adis, Singapore. https://doi.org/10.1007/978-981-10-4068-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-4068-9_8
Published:
Publisher Name: Adis, Singapore
Print ISBN: 978-981-10-4067-2
Online ISBN: 978-981-10-4068-9
eBook Packages: MedicineMedicine (R0)