Abstract
The soil microbiome is a diverse system composed of microorganisms with different functions. Microorganisms known as plant growth-promoting microorganisms (PGPMs) can help plants with nutrient uptake and consequently with crop yields. From this class of microorganisms, we can isolate nitrogen-fixing bacteria (NFB), phosphorus-solubilizing microorganisms (PSMs), and the microbes that are able to produce phytohormones. The use of these microorganisms in improving nutrient uptake by plants has been acceptable because of reduced costs and the safety of application for humans and the environment. It is for this reason that inoculant products have been developed. During the process of inoculant development, it is possible to use molecular biology techniques, such as 16S rRNA gene sequencing. This technique helps with the identification of potential microorganisms adapted for different conditions and crops. Moreover, these microorganisms can be used in degradable areas or as pathogen controls. It is also important to consider the siderophore, which is a biological molecule produced by various bacteria, and which has an immense application in agriculture. Another important symbiosis that occurs is realized by mycorrhizas, which are essential for transferring nutrients and water from the soil to plants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Soil Microbiome
- Plant Growth-promoting Microorganisms (PGPMs)
- Phosphorus-solubilizing Microorganisms (PSMs)
- Siderophores
- Indole-3-pyruvic Acid
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
The association between plants, soil, and soil microbes is like a system, which influences plant health and productivity. To illustrate this, recent advances in “omics” research can provide a common understanding and management of these interactions (Chaparro et al. 2012).
The phytomicrobiome is characterized by microbial communities related to plants (Smith and Zhou 2014; Smith et al. 2015). This phytomicrobiome can be divided into the rhizomicrobiome, which is located in and around the roots or the rhizosphere (Lundberg et al. 2012), the phyllomicrobiome, which refers to the microbiome present in aerial parts of the plant (Rastogi et al. 2012; Kembel et al. 2014), and the endosphere, which is inside the plant (Berg et al. 2014). The structure of these microbial communities can vary according to the interactions between plant – microorganism and/or microorganism-microorganism. These connections are mediated by compounds that are released by plants or microorganisms as exudates (East 2013). Understanding the form and function of these compounds is essential for the possibility of using these microbes to develop new technologies for crop growth promotion, industrial process optimization (e.g., fermentation), and biocontrol mechanism development (East 2013).
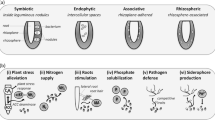
Of all the microbial communities, the rhizomicrobiome shows most relevance for field crops. This group of microorganisms is very diverse and dynamic in response to environmental conditions and the interactions between plants and microbes, which in some cases are specific. Plant growth–promoting microorganisms (PGPMs) are one group from the rhizomicrobiome which live in soil or close to plant roots (Gray and Smith 2005; Mabood et al. 2014). These groups of bacteria are distinguished by some inherent characters: (i) able to be established at the root surface; (ii) remain and compete with other microbes; (iii) promote plant growth (Kloepper 1994).
PGPMs have acquired relevance in agriculture because they are considered an alternative to the traditional management of crops (Bhattacharyya and Jha 2012), and an environmentally friendly practice. A huge miscellanea of microbes have been used as PGPMs (Ahemad and Kibret 2014); one of the most used is Rhizobia, due to the high yield increases that result when inoculated in plants (Rathore 2014). Moreover, PGPMs are classified according to their functionality (Gray and Smith 2005; Mabood et al. 2014). As biofertilizers, they can intensify the acquisition of nutrients by plants, through nitrogen fixation (Vessey 2003; Bhattacharyya and Jha 2012) and phosphate solubilization (Inui-Kishi et al. 2012; Trabelsi and Mhamdi 2013). Other uses of PGPMs are as phytostimulators (inducing plant growth through phytohormes), rhizomediators (used for restoration of degraded environments) (Antoun and Prévost 2005), and for the production of metal chelators and siderophores (Vessey 2003; Bhattacharyya and Jha 2012).
Other mechanisms of PGPMs include the production of 1-aminocycloropropane-1-carboxyla deaminase (maintaining ethylene levels in plant tissues under stress situations) (Penrose and Glick 2003), the induction of innate resistance or suppression of disease by antibiotics produced by fungi or bacteria (Antoun and Prévost 2005; Bhattacharyya and Jha 2012), the production of cell wall lytic enzymes (Haas and Defago 2005; Rathore 2014), and quorum sensing and interference on biofilm formation (Bhattacharyya and Jha 2012).
PGPMs can be divided into two groups according to the proximity of bacteria to the root (Gray and Smith 2005). One group is called ePGPM (extracellular) and is present in the rhizosphere or on the rhizoplane. Agrobacterium, Arthobacter, Azospirillum, Pseudomonas, and Serratia are just some examples of ePGPMs (Bhattacharyya and Jha 2012). Another group is iPGRM (intracellular), which are present inside roots cells and are represented by Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, and Rhizobium (Gray and Smith 2005).
In summary, microorganisms can act in coordination with the soil microbiome for the purpose of benefiting plant health and development. Much evidence shows that plants are able to determine microbial communities through root exudates. These exudates maintain a molecular conversation according to the plants’ phenological stage, interaction with others species, and management techniques (Chaparro et al. 2012). This chapter discusses the detail and actual knowledge about the importance of the soil microbiome on nutrient acquisition together with other applications of these microorganisms.
6.2 Soil Microbiome Diversity and Function
The rhizosphere of plants is a huge source of soil microbes (Köberl et al. 2013), which are captivated by root secretions and/or rhizodeposits (Compant et al. 2010). Plant species can co-ordinate the rhizosphere microbiome, which is dependent on the soil microbial community (Smalla et al. 2001). Furthermore, microbial communities are contingent on soil type, pedoclimatic conditions, plant health, phenological stage, and edaphoclimatic factors (Singh and Mukerji 2006).
The higher activity observed by microbes in the rhizosphere brings several biological and ecological benefits to the environment and improves plant yield. The rhizosphere contains a large number of microorganisms with the ability to fix nitrogen, solubilize phosphorus (P), enhance plant pathogen resistance (Arjun and Harikrishnan 2011), and help recover degraded environments (Antoun and Prévost 2005). These microorganisms assume an important aspect from an agronomic point of view, as we can outline below.
Knowledge of the diversity of the rhizosphere is very limited. It has been estimated that less than 1% of the soil microbiome has been isolated in pure culture. In order to understand the soil microbiome, metagenomics can help in analyzing complex genomes of microbial communities through culture-independent molecular approaches (Peix et al. 2007). Moreover, with molecular approaches it is possible to verify the existence and determine the quantity of microorganisms (Oliveira et al. 2009). For bacterial diversity analysis, the molecular marker 16S rRNA gene is utilized (Richardson et al. 2011). Arjun and Harikrishnan (2011) investigated the microbial diversity present in the rice rhizosphere from a paddy field ecosystem in Kerala, India. They used culture-independent molecular techniques, 16S rRNA clone library generation obtained by RFLP, sequencing, and phylogenetic analysis. Through sequence analysis of 16S rRNA genes, they observed major diversity in the bacterial community, with the majority of microbes being related to Proteobacteria. Just a small portion of the 16S rRNA sequences were highly similar to rRNA from the Acidobacteria, Firmicutes, and Bacterioides groups. Knowledge of the less known microbial community is very useful for the comprehension of their individual roles as related to plant health, yield, and metabolic capabilities. Moreover, metagenomics promises to bring many more questions regarding the uncultivable fraction of the rhizosphere community.
Köberl et al. (2013) present a study performed with the purpose of analyzing the microbiome of medicinal plants (Matricaria chamomilla L., Calendula officinalis L., and Solanum distichum Schumach. and Thonn.) planted in an organic desert farm in Egypt. These plants have a distinguished microbiome due to their particular and structurally divergent active secondary metabolites. These secondary metabolites present the major reason for their high specificity for related microorganisms (Qi et al. 2012). Soil microbiomes of desert environments are more abundant in Gram-positive bacteria related to pathogen suppression. These authors observed an evident selection of specific microbes by plants, as well as highly specific diazotrophic communities that demonstrated the importance of plant species on microbial diversity. Moreover, they found Bacillus spec. div. strains were able to promote plant growth and improve flavonoid production. These results emphasize the numerous connections between the plant microbiome and the plant metabolome.
Several surveys have demonstrated that the soil microbiome diversity has been reduced due to the intensification of land use in in the agriculture (Maeder et al. 2002; de Vries et al. 2013), This demonstrates some of the negative effects of agriculture on the environment and the unsustainability of agricultural production (Sala et al. 2000). The decline in soil biodiversity is sometimes discussed in terms of functional redundancy. Functional redundancy suggests that different species can have the same function in an ecosystem, and therefore declines in species diversity do not necessarily affect ecosystem functioning. Research by Philippot et al. (2013) counters this viewpoint, suggesting that microbial diversity loss can affect ecosystem processes.
Mendes et al. (2015) hypothesize that the microbial community diversity and functional diversity are much lower in undisturbed than disturbed soils, with consequences for functional redundancy in the soil microbiome. To explain this hypothesis in detail, they used soil DNA shotgun metagenomics to assess the soil microbiome in a chronological sequence of land use with native forest, followed by deforestation and cultivation of soybean and pasture in different seasons. The results obtained by these authors demonstrated that an agriculture and pasture soil shows the most diversity and higher functional redundancy. Conversely, the equilibrium in forest ecosystems was maintained with a lower diversity and higher abundance of microorganisms. These results indicate that land use is an important factor in the composition of the soil microbiome. Knowledge of the diversity of the soil microbial community could help in the identification of microbial candidates to act as PGPMs and for development of inoculant products.
6.3 Biological Nitrogen Fixation
Nitrogen (N) is one of the most important elements for plant development because it is an essential part of nucleic acids, enzymes, and proteins. Seventy-eight percent of N is in gaseous form. Despite this, N is unavailable to plants and is thus considered one of the most growth-limiting nutrients (Dalton and Krammer 2006). To become available to plants, atmospheric nitrogen (N2) needs to be modified or fixed to ammonia (NH3) by nitrogen-fixing microorganisms (Kim and Rees 1994). Biological nitrogen fixation (BNF) contributes to two-thirds of the nitrogen fixed worldwide. Mild temperature is generally one of the conditions that promotes BNF by diazotrophic microorganisms, which are dispersed in nature (Raymond et al. 2004).
Microorganisms with BNF activity are separated into groups as being (a) symbiotic N2-fixing bacteria, including the rhizobiaceae family (Ahemad and Khan, 2012b); (b) non-leguminous trees (e.g. Frankia); and (c) non-symbiotic (free living and endophytes) nitrogen-fixing forms like cyanobacteria Anabaena, Nostoc, Azospirrilum, Azoarcus, and others (Battacharrya and Jha 2012). Non-symbiotic microorganisms provide a small amount of the fixed nitrogen that is required by plants (Glick 2012) due to the limitation of carbon and the inhibition of nitrogenase by oxygen, which is the enzyme responsible by nitrogen fixation (Oldroyd and Downie 2004).
For the purpose of acquiring nutrients, plants have formed symbiotic interactions with microorganisms such as legumes and rhizobia. In this symbiosis, the bacteria penetrate the plant root and remain restricted in intracellular space, such as nodules, where N2 is transformed into ammonia, which is then absorbed by plants (Oldroyd and Downie 2008).
6.3.1 Molecular Signaling in Nitrogen-Fixing Bacteria
The molecular communication between plant and bacteria occurs due to the detection of flavonoids and related molecules that are secreted from legume roots by rhizobia (Perret et al. 2000). Flavonoids are transcriptional regulators recognized by NodD proteins that bind to a signaling molecule and have the possibility to activate gene expression (Long 1996).
The formation of nodules in legume roots is activated by nodulation (Nod) components (NC), which are signaling molecules that induce developmental changes in plants. Nod components have in their structure a chitin backbone with an N-linked fatty acid moiety connected with a non-reducing terminal sugar. Some modifications can occur in NC structure among species of rhizobia. These modifications can define the specificity between the rhizobia and the host plant (Oldroyd and Downie 2008).
Bacterial invasion normally happens by root hair cells or disruption of root cells. The entry via root hair cells begins by bacterial attachment to root hairs (Oldroyd and Downie 2004). Concurrently, cortical cells activate cell partitioning to establish the nodule meristem. The epidermal response to the entry of roots is correlated with the recognition of nodulation components that govern calcium spiking-subordinate signaling pathway, and with root hair distortion through a signaling pathway autonomous to calcium spiking. Cortical cell division is correlated with an increase in the concentration of cytokinin and auxin. Transcription factors (TFs) from nodulating signaling pathway, NSP1 and NSP2, are essential for nodulation and are obligatory in the epidermis with induction of initial nodulation genes (INODs), cortical cell division, and nodule inception (NIN). These TFs are responsible for activating NC gene expression. In disruption invasion, violation occurs through the epidermis and the microbes receive access to cortical cells. The relation with Nod components is considered important in some species, which endure disruption entry, but NC-independent of disruption invasion also exists and may be associated with rhizobial modifications of cytokinins (Fig. 6.1) (Oldroyd and Downie 2008; Oldroyd et al. 2011).
Changes in the roots during the nodulation process. NC nodulation components, INODs initial nodulation genes, NS nodule start, LysM-RLK lysin motif receptor kinase, LHK1 Lothushistidine kinase (Adapted from Oldroyd and Downie 2008)
After the nodule formation occurs, the bacteria enlarge and differentiate into nitrogen-fixing forms, and are denominated as bacteroids. These bacteroids are surrounded by plant membrane, known as a symbiosome that is a kind of organelle, which has the function of reducing nitrogen (Oldroyd and Downie 2004).
The enzyme responsible for nitrogen fixation in diazotrophs is generally molybdenum nitrogenase. As an alternative to molybdenum nitrogenase, some diazotrophic microorganims have vanadium and/or iron nitrogenases. The structure of the molibidenum nitrogenase enzyme is composed of nifDK and nifH genes (Rubbio and Ludden 2008).
6.3.2 Optimization of Elements of Biological Nitrogen-Fixing System
One of the main topics discussed in the Edinburg Declaration on Reactive Nitrogen (2011) was related to improving nitrogen availability to plants (Gutierrez 2012). Here we can consider biological nitrogen fixation (BNF) (Galloway et al. 2004).
Due to the importance of BNF in decreasing the necessity for chemical nitrogen fertilizers, it will be interesting to understand how to improve the biological process. For this, we can verify what happens during the optimal use of a nitrogen-fixing system, search for new plant-microorganism fixing associations, and transfer the ability of BNF to non-fixing microbes (Olivares et al. 2013).
There are many ways to improve the BNF through legumes, like legume adoption by farmers during combined cultivation and rotations between crops (Sessitsch et al. 2002). With the purpose of maximizing BNF by legumes, nodulation with the appropriate rhizobia should be evaluated. However, there are soils with low numbers of compatible rhizobia, thus it is necessary to carry out inoculation and to select an inoculant strain. For the selection of this strain it is necessary to verify the bacterial compatibility and nitrogen-fixing efficiency with the plant. Moreover, environmental conditions need to be analyzed because they may limit BNF activity and periodical inoculation must also be adopted (Hungria et al. 2005).
The election of inoculants is usually based on existing microbe diversity. Therefore, there are some genetic modifications that can be done with the purpose of enhancing the BNF of a given strain, by reiteration or overexpression of genes related to nitrogenase enzyme activity. Peralta et al. (2004) investigated improving the symbiotic efficiency in Rhizobium etli – Phaseolus vulagris. With the purpose of improving nitrogenase production, these authors built a chimeric nifHDK operon regulated by a strong nifHc promoter and verifed it in symbiosis with P. vulgaris. Bacterial strains with overexpression of nitrogenase had increased nitrogenase activity, plant weight (improved around 32%), concentration of nitrogen in plants and seed, and seed yield. Moreover, the overexpression of the chimeric nifHDK operon contributed to increased symbiosis. In another study, Wang et al. (2013) recognized a cluster consisting of nine nif genes in the genome of Paenibacillus sp. WLY78. With the purpose of analyzing the genetic requirements for fixing nitrogen, they inserted a Paenibacillus nif gene cluster in Escherichia coli. A minimum nif gene cluster allows the production of active nitrogenase in E. coli. However, on deletion analysis it was verified that in addition to the core nif genes, hesA (one of the genes of nif clusters) participates in an important aspect on nitrogen fixation and is sensitive to molybdenum. Wang et al. (2013) wanted to demonstrate the possibility of transferring the BNF activity with a short set of nif gene cluster. Breeding for enhanced nitrogen fixation is not an easy task, but you can analyze characteristics such as plant and seed yield, and others, to quantify the efficiency of BNF (Olivares et al. 2013).
6.4 Phosphorus in Soil
Phosphorus and nitrogen are important to keeping a healthy nutritional life for plants, but, unlike nitrogen, phosphorus is not present in large amounts that can become available to plants.
Many peculiarities are associated with phosphorus nutrition and this element shows an important role in metabolic processes (Khan et al. 2010). Microbial associations are associated with P-fixation as with N-fixation in legumes. Phenological development, crop yield, and resistance to plant diseases are also described in relation to P nutrition (Hao et al. 2002).
A considerable amount of inorganic P is quickly retained in insoluble mineral complexes after frequent application of phosphate fertilizers (Rodriguez and Fraga 1999; Igual et al. 2001). The retention of P is around 75% of the total amount applied. Organic forms (20–80% of P in soils) are the other important storage of immobilized P (Richardson et al. 1994). Phosphorus is present in soil on average at around 0.05% (w/w); however, only 0.1% of it is usable by plants (Zhu et al. 2011). Physicochemical (adsorption – desorption) and biological (immobilization-mineralization) means are responsible for soil P dynamics and consequently for P fixation. Most P that is administrated as fertilizer becomes static through a condensation reaction with Al3+, Fe+3, and Ca+2 (Hao et al. 2002).
The application of chemical fertilizers represents an extra cost to agricultural production and moreover causes negative impacts on different environments (Tilman et al. 2001). Reduction of fertility by lost microbial diversity, which consequently reduces the crop yields, is also observed (Gyaneshwar et al. 2002). Beyond that, P has limited sources in rock phosphate and there are estimations that the reserve of P will be exhausted in the current century (Cordell et al. 2009). The high cost of P chemical fertilizers and problems with P availability make it necessary to search for an environmental alternative and a production strategy for improving crop yields without environmental problems. One option available to achieve this purpose is the use of microbe inoculated fertilizer with P-solubilizing properties in agriculture (Sharma et al. 2013).
6.4.1 Phosphorus-Solubilizing Microorganisms
In 1903 the natural occurrence of phosphorus-solubilizing microbes (PSMs) was observed. These have the ability to use diverse means to solubilize and mineralize P, with the purpose of converting inorganic and organic soil P into available forms for plants (Khan et al. 2007).
Bacteria represent the majority of the microbial community with 1–50%, with fungi constituting only 0.1–0.5% of the total respective population. Several species of bacteria and fungi have been tested in relation to their potential as a PSM. Generally, these microbes are found in the phytomicrobiome and soil areas with P deposits. The isolation can be done by serial dilution methods or through enhancement culture techniques (Zaidi et al. 2009). Another area that a PSM could be isolated in is in stressful environments, such as the moderately halophic bacterium, Kushneria sp., found by Zhu et al. (2011), in the solid residue of Daqiao saltern on the east coast of China, YCWA18.
Many factors could affect the potential of these PSMs, such as the amount of iron ore, temperature, carbon, and nitrogen origin. One of the biggest problems that generates controversy is the source of insoluble P used to isolate PSMs: tricalcium phosphate (TCP). The TCP is able to select a huge number of isolates. However, when these isolates are evaluated in relation to the available P provided to plants, many of them fail as PSMs because there are often other sources of P that are less soluble than TCP such as iron/aluminum/calcium phosphate (Bashan et al. 2013).
Soil is complex and shows a lot variation depending on pH and chemical properties; in this way no P source can be used as a universal selection factor. Thus, the best way to assess how these microbes are evaluated is in which type of soil (alkaline, acid, or rich in organic matter) the PSMs will be applied. Bashan et al. (2013) suggested the use of specific compounds such as calcium phosphate (alkaline soils), iron/aluminum phosphate (acidic soils), and phytases (organic soils). The PSMs that show greater solubilization in vitro are selected for field trials before the production of biofertilizer (Sharma et al. 2013).
Excessive numbers of microorganisms show PSM capacity, and include bacteria, fungi, and actinomycetes. Among the bacterial communities, Pseudomonas and Bacillus have been described as PSMs. Some of the bacteria with PSM skills are Rhodococus, Arthrobacter, Serratia, Chryseobacterium, Gordonia, Phyllobacterium, Delftia sp. (Wani et al. 2005), Enterobacter sp., Pantoea, Klebisiella (Chung et al. 2005), Vibrio proteolyticus, Xanthobacter agilis (Vazquez et al. 2000) (Sharma et al. 2013), and Burkholderia (Inui-Kishi et al. 2012).
There are some nitrogen-fixing microorganisms such as rhizobia that have also shown PSM activity (Ahemad and Kibret 2014; Zaidi et al. 2009). Among fungi Penicillium and Aspergillus represent the most effective solubilizers (Reyes et al. 2002).
6.4.2 Mechanisms of P-Solubilization by Soil Microbial Communities
Microorganisms are able to increase the ability of plants to obtain P from soil through different mechanisms, such as increased root extension by mycorrhizal associations, or by hormonal stimulation of root hair development and root growth (Hayat et al. 2010; Richardson and Simpson 2011). Another method is through metabolic mechanisms that are efficient in providing unavailable P present in soil as organic and inorganic forms (Fig. 6.2). Moreover, the existence of labile C in these microorganisms appears as a reservoir of P, through immobilization. Thus, the dispensation of P retained by microbes occurs when the cell dies (Richardson and Simpson 2011).
Diagram of phosphorus cycle (Adapted from Richardson and Simpson 2011)
6.4.3 Inorganic P-Solubilization
The majority of available inorganic phosphate is H2PO4 −, which normally occurs at a lower pH. Nevertheless, with the increase in pH, other phosphate forms such as HPO4 2− and HPO4 3− become more predominant.
The availability of inorganic P through PSMs occurs mainly by organic acid production (Zaidi et al. 2009). Other ways include: lowering pH by H+ extrusion (Parks et al. 1990), improved capitation of cations ligated with P, or establishment of a soluble compound with metal ions (calcium, aluminum, and iron) that releases P (Maliha and Samina 2004) and the release of protons accompanying respiration or NH4+ assimilation when the solublization occurs without acid production (Illmer and Schimer 1995). The effect on pH is due the liberation of organic acid, originated from microbial metabolism, mostly by oxidative respiration or fermentation processes (Trolove et al. 2003). The predominant acids that are related to PSM activity are gluconic, oxalic, citric, succinic (Khan et al. 2007), lactic, tartaric (Venkateswarlu et al. 1984; Khan et al. 2007), and aspartic (Venkateswarlu et al. 1984) acids. There are also inorganic acids (sulphur and nitric acid) (Siqueira and Franco 1988). One example of higher potential in solubilizing P is caused by gluconic and 2-keto gluconic acids synthetized by direct oxidation in Erwinia herbicola and Pseudonomas cepacea (Goldstein et al. 1993; Goldstein 1994; Goldstein 1995). The capacity of PSMs to utilize inorganic phosphate is regulated by specific genes. Understanding these genes is very important because it is possible to use them in biotechnology applications. Genes correlated with PSMs are glucose dehydrogenase (GDH), gluconate dehydrogenase (GADH), and pirroloquinoline–quinone (PQQ), which are already identified and cloned in different bacteria (Sashidhar and Podile 2010). However, the recombinant bacteria that express these genes need to be studied in more depth and approved by regulatory laws, because the use of these PSMs can enhance the eutrophication of rivers (Siqueira et al. 2004). Fraga et al. (2001) observed accumulation of extracellular phosphatase when the napA phosphatase gene from Morganella morganii was cloned in Burkholderia cepacia IS-16 (strain used for inoculant). There may be more advantages in developing genetically modified microorganisms (PSMs) over transgenic plants for improved plant development. It is possible to combine more than one plant growth–promoting characteristic in a unique organism, and develop an inoculant that can be used for different cultivated plants (Ahemad and Kibret 2014; Rodriguez et al. 2006). Molecular genetics yield information that elucidates the mechanisms associated with PSMs. Comparative genomic and transcriptomic sequencing of the microbiome, and differential gene expression analyses have found potential targets such as enzymes, metabolites, and transport proteins that are related to the PSM process that leads to the enhancement of P availability and use by plants (Krishnaraj and Dahale 2014).
6.4.4 Organic P-Solubilization
While inorganic P becomes unavailable by precipitation and chemical adsorption, the organic P is retained in the organic matter of soil (Sharma et al. 2013).
Organic P represents 30–80% of the total soil (Menezes-Blackburn et al. 2013). It is found as inositol phosphate (30–50% of total amount of organic P), nucleic acids, and nucleotides (3–5%), phospholipids, and others (low quantities) (Siqueira et al. 2004). The use of organic P by plants and microbes requires hydrolysis of organic P from soil (Richardson and Simpson 2011; Sharma et al. 2013). Hydrolysis is known as the mineralization of organic P in soil and occurs mainly by the action of phosphatase enzymes (Fig. 6.3).
Mineralization reaction of organic phosphorus by (1) phytases, (2) nucleases, (3) nucleotidases, (4) phospholipases (Adapted from Siqueira et al. 2004)
In general, fungi have more hydrolytic activity on phytate than bacteria; however, bacteria and plants can produce phytate. The nucleases are mainly produced by rhizospheric microorganisms and phospholipases by actinomycetes (Siqueira et al. 2004).
Phytate is the main stock form (60–80%) of organic P in several soils and plant tissues (1–5% of weight) (Singh and Satyanarayana 2011). In plants, phytases cause the liberation of P from phytate degradation. However, the capacity of plants to obtain P immediately from phytate is very limited. Arabidopisis plants supplemented with phytate were considerably benefited with P when they were engineered with the phytase gene (phyA) originated from Aspergillus niger (Richardson et al. 2005).
Phosphatases are a class of abundant enzymes that are used by PSMs and are widely present in studies. These enzymes can be set into acid and alkaline phosphatase, according to soil characteristic (acid or alkaline) (Jorquera et al. 2008). In plants the production of acid enzymes is more predominant than alkaline phosphatase, suggesting that this is a specific characteristic of PSMs (Criquet et al. 2004).
Microorganisms are the main source of phosphorus mineralization enzymes (Richardson 1994). The activity and synthesis process of these enzymes depends on environmental conditions that are suppressed with high contents of phosphorus or stimulated in limited conditions. In conditions with low availability of P, bacteria have the ability to acquire P in their biomass, which at the final cycle will be mineralized and become available to plants and other organisms (Gyaneshwar et al. 2002).
6.5 Phytohormone Production
A phytohormone is defined as an organic signal molecule synthesized in plant organs or tissues that can be translocated to other regions and presents a specific response. However, phytohormones can also be active in tissues where they are created (Baca and Elmerich 2007). Their production has been studied as one of the main instruments by which PGPMs may increase plant growth (Iqbal and Hasnain 2013).
Auxins, cytokinins, gibberellins, ethylene, and abscisic acid are classes of widely recognized phytohormones (Zahir et al. 2004).
In nature, two modes of phytohormones are accessible to plants. They are endogenous production by the plant tissues, and phytohormones that are made available by associated microbes (Patten and Glick 1996).
Fungi inoculants are advantageous compared with bacterial ones because of their efficiency at spreading over the rhizosphere. Trichoderma species represents a class of fungi found in the rhizosphere. Trichoderma strains can colonize plant roots and improve plant progress in growth and development. These effects are influenced by microbial production of indole-3-acetic acid (IAA) and indolic compounds (Ortíz-Castro et al. 2009).
During plant growth and development, signaling molecules intermediate the contact with microorganisms, performing a relevant communication. Microbes have the means to recognize a plant host and start colonizing the rhizosphere by production of plant growth–regulating substances like phytohormones. Furthermore, these microbial-produced compounds are perceived by plants, which respond and further influence the type of microorganisms found. This represents a molecular conversation that determines the relationship between plants and microbes from pathogenesis to symbiosis (Bais et al. 2004).
Many bacteria and fungi can produce auxins using different pathways, which increases the potential to form associations with plants. Moreover, epiphytic and rhizospheric microfloras of plants are of utmost relevance in the conversion of tryptophan (which is present in plant exudates) into IAA (Tsavkelova et al. 2006).
The main known phytohormone is IAA. At least 80% of the rhizospherical bacteria synthetize IAA (Patten and Glick 1996). In addition to IAA, other indolic compounds that are physiologically active for plants are also produced by rhizospheric microbes (Lebuhn et al. 1997; El-Khawas and Adachi 1999).
On the other hand, IAA and cytokinins derived from bacteria are also related to the virulence of several interactions between microorganisms like genus Agrobacterium, Pseudomonas, and pathogenic Erwinia (Lichter et al. 1995; Morris 1986; Spaepen et al. 2007).
Cytokinins are other relevant phytohormones. The physiological effect of cytokinin is the enhancement of cell division (Frankenberger and Arshad 1995). Although it is difficult to identify these molecules, they can be detected using bioassays (Nieto and Frankenberger 1990).
Microorganisms have the ability to synthetize kinetin, zeatin, isopentenyladenine, and other cytokinin derivatives. Rhizobacteria of the genera Azotobacter, Azospirillum, Arthrobacter, Bacillus, Rhizobium, Pseudomonas, and also streptomycetes are capable of synthetizing cytokinins (Tsavkelova et al. 2006).
PGPMs also produce gibberellins (GAs). There are over 89 known GAs (Dobbelaere et al. 2003), which are numbered GA1 through GA89 in approximate order of their discovery (Frankenberger and Arshad 1995; Arshad and Frankenberger 1998). The most accepted gibberellin is GA3, while the most active is GA1. The main physiological role of gibberellins is stem elongation and increased internode length (Davies 1995).
The fourth phytohormone to be discovered is abscisic acid (ABA). This was detected by radioimmunoassay or thin-layer chromatography in supernatant cultures of plant-associative bacteria Azospirillium and Rhizobium sp. (Dangar and Basu 1987; Dobbelaere et al. 2003). ABA responses are related to stomatal closure and root proliferation. Therefore, its presence in the rhizosphere is of paramount relevance for plant growth in water-deficient environments (Frankenberger and Arshad 1995).
Ethylene is another important phytohormone. Its role is related to root elongation inhibition, auxin transport, senescence, and abscission promotion of various organs, and fruit ripening (Bleecker and Kende 2000; Glick et al. 2007). Reducing levels of ethylene in plants may be one of the growth-promoting activities of PGPMs. These reductions are due to the activity of enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which reduces ACC, the precursor of ethylene (Yang and Hoffman 1984).
Cadaverine, a poliamine that enhances root growth and helps to reduce osmotic stress, was reported in rice inoculated with Azospirillim brasilense Az39 (Cassán et al. 2009).
6.5.1 Applications
Associative rhizobacteria may provide favorable progress in plant development due to production of phytohormones, nitrogen fixation, biosynthesis of antimicrobial substances, and enhanced water or mineral nutrition uptake (Saharan and Nehra 2011).
There are many reports presenting the beneficial impact of bacteria inoculation on orchids. An early paper was published by Knudson (1922), who inoculated the seeds of Epidendrum and Laeliocattleya with a diazotrophic strain, Rhizobium leguminosarum, to stimulate germination. Wilkinson et al. (1989, 1994) described the propagation of Pterostylis vittata seeds co-inoculated with a mycorrhizal fungus and strains of orchid-associated bacteria belonging to Pseudomonas putida, Bacillus sphaericus, B. cereus, and Arthrobacter sp.
The in vitro germination of mature seeds of the species Dendrobium moschatum was enhanced by inoculation of bacterial cultures (Tsavkelova et al. 2007). Seed germination was enhanced by Mycobacterium sp. and Sphingomonas sp. in in vitro inoculations.
Strains of Bacillus sp. and Enterobacter sp. isolated as endophytic bacteria were used to stimulate Cattleya walkeriana seedlings. This was done by increasing such characteristics as fresh weight, dry weight, and plant survival during ex vitro acclimatization, which is considered the bottleneck stage for orchid seedling propagation (Galdiano Júnior et al. 2011). One of these isolates, Enterobacter asburiae, produces an acid ectophosphatase, which can be a mechanism for the solubilization of mineral phosphates (Sato et al. 2016), configuring a PGPM with two growth-promoting activities (IAA production and solubilization of mineral phosphate).
Growth promotion was also observed when seedlings of Cattleya loddigesii generated in vitro were inoculated with a bacterial suspension of Paenibacillus lentimorbus and P. macerans strains (Faria et al. 2013). Sphingomonas paucimobilis ZJSH1 significantly promoted the growth of D. officinale seedlings, enhancing fresh weight and stem length (Yang et al. 2014).
6.6 The Importance of Siderophores
The siderophore is a biological molecule produced by various bacteria and has wide applications in various fields, such as improving soil fertility in agriculture. Bacterial strains that do not use any other means of biocontrol can act as biocontrol agents by using the siderophores that they produce. Therefore, siderophores from PGPMs prevent phytopathogens from acquiring iron and can be a limiting factor for their proliferation (Kloepper et al. 1980).
Available results indicate PGPM siderophores have a much better affinity for iron than to pathogens (Schippers et al. 1987). As a result, lack of iron in the rhizosphere incapacitates proliferation of fungal pathogens. By reason of biocontrol, PGPMs out-compete fungal pathogens for accessible iron.
However, plant growth is not altered by the iron reduction in the rhizosphere caused by the siderophores, because most plants use less iron than microorganisms (O’Sullivan and O’Gara 1992). Also, some plants can bind and consequently make up the biocontrol PGPM iron-siderophore complex (Bar-Ness et al. 1991; Wang et al. 1993).
6.7 Mycorrhizae
Mycorrhizal symbioses are widespread and common in terrestrial ecosystems around the globe, occurring in nearly all soils (Smith and Read 2008). The symbiotic fungi are often crucial for absorbing water and nutrients from the soil and transferring them to plants (Orwin et al. 2011).
Mycorrhizae are grouped into two types: ectomycorrhizae and endomycorrhizae. Endomycorrhizae are defined by dense mycelial sheaths near the roots with intercellular hyphal invasions of the root cortex. They are limited to forest trees in temperate regions. All other plants represent endomycorrhizae, characterized by fungi forming external hyphal networks in the soil and penetrating the cortical cells of roots (Bolan 1991).
Mycorrhizal fungi are known to secrete phytohormones such as GA3, IAA, ABA, zeatin, and zeatin riboside (Wu et al. 2002; Liu et al. 2010). The mycorrhizal fungus Trichoderma sp., isolated from Pleione bulbocodioides, increased seed germination up to 84.6%, while the control presented lower germination (77.6%) on OMA medium (Yang et al. 2008).
Pathogenic and growth-promoting fungal species are IAA yielders (Tsavkelova et al. 2006). Four different Fusarium species isolated as endophytic fungi from Dendrobium moschatum also produced IAA (Tsavkelova et al. 2003, 2012), while mycorrhizal fungus in association with Dendrobium densiflorum produced vitamins and GA (Wu et al. 2002).
6.8 Alternative Use of Plant Growth–Promoting Microorganisms
Several bacterial genera are necessary elements in maintaining the balance of soils. They are implicated in distinct biotic activities of soil ecosystems, making them a functional for nutrient turnover and enhancing maintenance for agricultural production (Ahemad et al. 2009; Chandler et al. 2008). The association of plants with bacteria can be considered good, pernicious, or neutral depending on the motif of their action on plant growth (Dobbelaere et al. 2003).
The use of PGPMs has become a habitual practice in various regions of the world. PGPMs are native to the soil and plant rhizosphere, and play an important function in biological control of plant pathogens, suppressing a broad spectrum of bacteria, fungi, and nematodes. PGPMs can also give protection against viral diseases (Sivasakthivelan et al. 2013).
Bacteria in the genera Agrobacterium, Bacillus, Burkholderia, Pseudomonas, and Streptomyces are frequently studied biocontrol agents. They eliminate plant disease through at least one mechanism: induction of systemic resistance and production of siderophores or antibiotics (Tenuta 2003). Among others, actinobacteria commonly inhabit the rhizosphere making it possible to characterize them as PGPMs (Franco-Correa and Chavarro-Anzola 2016).
These microorganisms are found very close to the roots epidermis, which secretes signal molecules for defense versus invasion of distinct microorganisms in the root area. At this stage, the distinction takes place between symbiotic, associative, pathogenic, or neutralistic association of the microorganisms with the plant (Hayat et al. 2010).
PGPM strains occur in many taxonomic groups and are present in the rhizosphere of plants in any given soil (Kyselková et al. 2009; Almario et al. 2013). This suggests that PGPMs colonize and coexist in the same rhizosphere soil as non-PGPM groups of the bacterial community. Studies have shown the existence of a particular gene (Table 6.1) and of relevant properties of PGPMs, which provide positive effects on plant growth, health, and their ability to inhibit phytopathogens (Bertrand et al. 2001; Barriuso et al. 2005; Upadhyay et al. 2009).
The biological control lineage Pseudomonas fluorescens Psd was analyzed for IAA organic synthesis and the logical preparation on it as a PGPM. While the indole pyruvic acid (IPyA) route usually connected with PGPMs was absent, the indole acetamide (IAM) pathway is commonly noticed in plant pathogens and was expressed in the Psd strain. Overexpression of IAM pathway genes iaaM-iaaH, from P. syringae subsp. savastanoi radically increased IAA levels and demonstrated a prejudicial effect on sorghum root development (Saranraj et al. 2013; Sivasakthivelan and Saranraj 2013).
PGPMs are efficient in secreting molecules as antibiotics into the rhizosphere to control pathogenic microbes, producing iron-chelating molecules (Raaijmakers et al. 2002). They also induce phytoalexin production in association with plants, and have broad acceptance as providing an agricultural advantage (Lifshitz et al. 1986; Halverson and Handelsman 1991). Phytoalexins are antimicrobial compounds with low molecular weights that are both synthesized by and accumulated in plants after exposure to microorganisms (Dakora 1985; Dakora et al. 1993; Van Peer et al. 1990, 1991).
In the rhizosphere of various leguminous and non-leguminous crops, species of Pseudomonas and Bacillus have been identified (Table 6.2) that help in plant colonization and suppression of phytopathogens (Parmar and Dufresne 2011).
PGPMs produce beneficial effects on plant health by accelerating nutrient availability, assimilation, and growth by suppressing diseases caused by phytopathogens (Franco-Correa and Chavarro-Anzola 2016). In the quest to improve soil fertility and crop yield while reducing the negative impact of chemical fertilizers on the environment, there is a need to exploit PGPMs for beneficial agriculture. Moreover, PGPMs can also prevent the deleterious effects of stresses from the environment (Paul and Nair 2008). They are also able to increase the capacity of plants to sequester heavy metals and can help plants withstand abiotic stresses (Jing et al. 2007; Saharan and Nehra 2011; Tak et al. 2013).
Bio-inoculants with diverse symbiotic (Bradyrhizobium, Mesorhizobium, Rhizobium) and non-symbiotic (Azomonas, Azospirillum, Azotobacter, Bacillus, Klebsiella, Pseudomonas) microbes are used to promote plant growth and development under various stresses like heavy metals (Ma et al. 2009a, b; Wani and Khan 2010), herbicides (Ahemad and Khan 2010), insecticides (Ahemad and Khan 2009), fungicides (Ahemad and Khan 2012a), and salinization (Mayak et al. 2004). Diverse varieties of rhizobacteria help to improve plant nutrition thus increasing plant health or stress tolerance (Vacheron et al. 2013).
Ferreira et al. (2010) showed similarities between cepacian exopolysaccharide (EPS) produced by members of the Burkholderia cepacia complex (BCC complex) and the EPS synthesized by B. graminis, B. phytofirmans, B. phymatum, and B. xenovorans (Estrada-De Los Santos et al. 2001). According to Ferreira et al. (2010), EPS may have a role in the tolerance of Burkholderia species to ion stress and desiccation.
Bloemberg and Lugtenberg (2001) showed the expression of some genes involved in the defensive response as well as genes expressed under conditions of drought, salt, and stress. The experiments with isolates of B. graminis, a species that has been isolated from the rhizosphere of pasture, corn, and wheat, resulted in an improvement in shoot height and neck diameter, as well as inducing a protective response to salt and drought stress in tomato plants (Barriuso et al. 2005, 2008).
PGPMs are able to produce gibberellic acid or ABA, or to control the level of these hormones in plants (Richardson et al. 2009; Dodd et al. 2010). The ABA is well known for its involvement in drought stress. Bauer et al. (2013) showed that during water stress, increases in ABA levels cause closing of stomata, thereby limiting water loss.
Habib et al. (2016), studying the ACC deaminase-containing PGPM isolate Enterobacter sp. UPMR18, concluded that it could be an effective bio-resource for enhancing salt tolerance and growth of okra plants under salinity stress. Microorganisms synthesizing the ACC deaminase enzyme can cleave ACC to 𝛼-ketobutyrate and ammonia, thus decreasing ethylene stress in plants (Rashid et al. 2012).
Ethylene is a simple gaseous hormone critical for many plant developmental stages (Abeles et al. 1992). The ethylene-mediated stress response can be activated by many environmental factors such as heavy metal contamination, high salinity, flooding, drought, and phytopathogens. Ethylene can also inhibit stimulation of cell proliferation and elongation by repressing auxin response factor synthesis (Dugardeyn and Van Der Straeten 2008). PGPMs, besides promoting plant growth by employing certain mechanisms and protecting the plant from salinity, can also increase plant tolerance against stress conditions (Shrivastava and Kumar 2015).
Conclusion
Understanding the mechanisms used by PGPMs enables the elucidation of their impact on nutrient cycling and on the protection of crops against disease. Moreover, functioning analysis, diversity, and gene expression patterns of PGPM populations in soil will be a precondition to developing a management action plan for sustainable agriculture. Future research and understanding of the mechanisms of PGPMs will pave the way to finding more competent rhizobacterial strains. These yet-to-be-found strains may work under diverse agro-ecological conditions to protect the environment, and produce enough food for an increasing world population.
References
Abeles FB, Morgan PW, Salveit MEJ (1992) Ethylene in plant biology. Academic Press, New York
Ahemad M, Khan MS (2009) Effect of insecticide-tolerant and plant growth promoting Mesorhizobium on the performance of chickpea grown in insecticide stressed alluvial soils. J Crop Sci Biotechnol 12:213–222
Ahemad M, Khan MS (2010) Growth promotion and protection of lentil (Lens esculenta) against herbicide stress by Rhizobium species. Ann Microbiol 60:735–745
Ahemad M, Khan MS (2012a) Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek] using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi J Biol Sci 19:451–459
Ahemad M, Khan MS (2012b) Effects of pesticides on plant growth promoting traits of Mesorhizobium strain MRC4. J Saudi Soc Agric Sci 11:63–71
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26:1–20
Ahemad M, Khan MS, Zaidi A, Wani PA (2009) Remediation of herbicides contaminated soil using microbes. In: Khan MS, Zaidi A, Musarrat J (eds) Microbes in sustainable agriculture. Nova Science Publishers, New York, pp 261–284
Almario J, Kyselková M, Kopecký J, Ságová-Marecková M, Muller D, Grundmann GL et al (2013) Assessment of the relationship between geologic origin of soil, rhizobacterial communityco mposition and soil receptivity to tobacco black root rot in Savoie region (France). Plant Soil. doi:10.1007/s11104-013-1677-1
Antoun H, Prévost D (2005) Ecology of plant growth promoting rhizobacteria. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 1–38
Arjun JK, Harikrishnan K (2011) Metagenomic analysis of bacterial diversity in the rice rhizosphere soil microbiome. Biotechnol Bioinf Bioeng 1(3):361–367
Arshad M, Frankenberger WT Jr (1998) Plant growth regulating substances in the rhizosphere. Microbial production and function. Adv Agron 62:46–51
Baca BE, Elmerich C (2007) Microbial production of plant hormones. In: Elmerich C, Newton WE (eds) Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Springer, Dordrecht, pp 113–143
Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9:26–32
Bar-Ness E, Chen Y, Hadar Y et al (1991) Siderophores of Pseudomonas putida as an iron source for dicot and monocot plants. In: Chen Y, Hadar Y (eds) Iron nutrition and interactions in plants. Kluwer Academic, Dordrecht, pp 271–281
Barriuso J, Pereyra MT, Lucas Garcia JA, Megias M, Gutierrez Manero FJ, Ramos B (2005) Screening for putative PGPR to improve establishment of the symbiosis Lactarius deliciosus-Pinus sp. Microb Ecol 50:82–89
Barriuso J, Ramos Solano B, Fray RG, Camara M, Hartmann A, Gutierrez Manero FJ (2008) Transgenic tomato plants alter quorum sensing in plant growth-promoting rhizobacteria. Plant Biotechnol J 6:442–452
Bashan Y, Kammev AA, de Bashan LE (2013) Tricalcium phosphate is innappopriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49:465–479
Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, Mendel RR, Bittner F, Hetherington AM, Hedrich R (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 1:53–57
Belimov AA, Dodd IC, Safronova VI, Hontzeas N, Davies WJ (2007) Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. J Exp Bot 58:1485–1495
Berg G, Grube M, Schloter M, Smalla K (2014) Unravelling the plant microbe: looking back and future perspectives. Front Microbiol 5:148
Bertrand H, Nalin R, Bally R, Cleyet-Marel JC (2001) Isolation and identification of the most efficient plant growth-promoting bacteria associated with canola (Brassica napus). Biol Fertil Soils 33:152–156
Bhattacharyya PN, Jha DK (2012) Plant growth-promotig rhixobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1250
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bolan NS (1991) A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134:189–207
Bruto M, Prigent-Combaret C, Muller D, Moënne-Loccoz Y (2014) Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci Rep 4:6261
Cassán F, Maiale S, Masciarellia O, Vidal A, Luna V, Ruiz O (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Cazorla FM, Duckett SB, Bergstrom FT, Noreen S, Odik R et al (2006) Biocontrol of avocado Dematophora root rot by the antagonistic Pseudomonas fluorescens PCL 1606 correlates with the production 2-hexyl-5-propyl resorcinol. Mol Plant-Microbe Interact 19:418–428
Chandler D, Davidson G, Grant WP, Greaves J, Tatchell GM (2008) Microbial biopesticides for integrated crop management: an assessment of environmental and regulatory sustainability. Trends Food Sci Tech 1(9):275–283
Chaparro J, Sheflin A, Manter D, Vivanco J (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499
Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, Sa T (2005) Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 37(10):1970–1974
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and flood thought. Glob Environ Chang 19:292–305
Criquet S, Ferre E, Farner EM, Le Petit J (2004) Annual dynamics of phosphatase activities in na evergreen oak litter- influence of biotic and abiotic factors. Soil Biol Biochem 36:1111–1118
Dakora FD (1985) Use of intrinsic antibiotic resistance for characterisation and identification of rhizobia from nodules of Vigna unguiculata (L) Walp. and Phaseolus vulgaris (L). Acta Microbiol Pol 34:187–194
Dakora FD, Joseph CM, Phillips DA (1993) Alfalfa (Medicago sativa L.) root exudates contain isoflavonoids in the presence of Rhizobium meliloti. Plant Physiol 101:819–824
Dalton DA, Kramer S (2006) Nitrogen-fixing bacteria in non-legumes. Springer, Dordrecht, pp 105–113
Dangar TK, Basu PS (1987) Studies on plant growth substances, IAA metabolism and nitrogenase activity in root nodules of Phaseolus aureus Roxb. var. mungo. Biol Plant 29:350–354
Davies PJ (1995) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant hormones: physiology, biochemistry, and molecular biology, 2nd edn. Kluwer, Dordrecht, pp 1–12
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149
Dodd IC, Zinovkina NY, Safronova VI, Belimov AA (2010) Rhizobacterial mediation of plant hormone status. Ann Appl Biol 157:361–379
de Vries FT, Thébault E, Liiri M et al (2013) Soil food web properties explain ecosystem services across European land use systems. Proc Natl Acad Sci U S A 110:14296–14301
Dugardeyn J, Van Der Straeten D (2008) Ethylene: fine-tuning plant growth and development by stimulation and inhibition of elongation. Plant Sci 175:59–70
East R (2013) Soil science comes to life: plants may be getting a little help with their tolerance of drought and heat. Nature 501:18–19
El-Khawas H, Adachi K (1999) Identification and quantification of auxins in culture media of Azospirillum and Klebsiella and their effect on rice roots. Biol Fertil Soils 28:377–381
Erturk Y, Ercisli S, Haznedar A, Cakmakci R (2010) Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol Res 43:91–98
Estrada-De Los Santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant associated nitrogen fixers with wide environmental and geographic distribution. Appl Environ Microbiol 67:2790–2798
Faria DC, Dias AC, Melo IS, de Carvalho Costa FE (2013) Endophytic bacteria isolated from orchid and their potential to promote plant growth. World J Microbiol Biotechnol 29:217–221
Fedorov D, Ivanova E, Doronina N, Trotsenko Y (2008) A new system of degenerate oligonucleotide primers for detection and amplification of nifHD genes. Microbiologiia 77:247–249
Ferreira AS, Leitao JH, Silva IN, Pinheiro PF, Sousa SA, Ramos CG, Moreira LM (2010) Distribution of cepacian biosynthesis genes among environmental and clinical Burkholderia strains and role of cepacian exopolysaccharide in resistance to stress conditions. Appl Environ Microbiol 76:441–450
Fraga R, Rodriguez H, Gonzalez T (2001) Transfer of the gene encoding the Nap A acid phosphatase from Morganella morganni to Burkholderia cepacia strain. Acta Biotechnol 21:359–369
Franco-Correa M, Chavarro-Anzola V (2016) Actinobacteria as plant growth-promoting rhizobacteria. doi:10.5772/61291
Frankenberger WTJ, Arshad M (1995) Phitohormones in soil: microbial production and function. Dekker, New York
Galdiano Júnior RF, Pedrinho EAN, Castellane TCL, Lemos EGM (2011) Auxin-producing bacteria isolated from the roots of Cattleya walkeriana, an endangered Brazilian orchid, and their role in acclimatization. Rev Bras Ciênc Solo 35:729–737
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present and future. Biogeochemistry 70:153–226
Gauthier D, Diem HG, Dommergues Y (1981) In Vitro nitrogen fixation by two actinomycete strains isolated from Casuarina nodules. Appl Environ Microbiol 41(1):306–308
Glick BR (2012) Plant growth promoting bacteria: mechanisms and application. Scientifica 2012:963401
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339
Goldstein AH (1995) Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by Gram negative bacteria. Biol Agric Hortic 12(2):185–193
Goldstein AH (1994) Involvement of quinoprotein glucose dehydrogenase in the solubilization of exogeneous phosphates by Gram-negative bacteria. In: Torriani-Gorini A, Yagliard E, Silver S (eds) Phosphate in microorganisms: cellular and molecular biology. ASM Press, Washington, DC, pp 197–203
Goldstein AH, Rogers RD, Mead G (1993) Mining by microbe. Bio/Technology 11:125–1254
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412
Gutierrez RA (2012) Systems biology for enhanced plant nitrogen nutrition. Science 336:1673–1675
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res Int. doi:10.1155/2016/6284547
Hafeez FY, Yasmin S, Ariani D, Mehboob-ur-Rahman Z, Malik KA (2006) Plant growth-promoting bacteria as biofertilizer. Agron Sustain Dev 26:143–150
Halverson LJ, Handelsman J (1991) Enhancement of soybean nodulation by Bacillus cereus UW85 in the field and in a growth chamber. Appl Environ Microbiol 57:2767–2770
Han H, Supanjani S, Lee KD (2006) Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ 52(3):130–136
Hao X, Cho CM, Racz GJ, Chang C (2002) Chemical retardation of phosphate diffusion in an acid soil as affected by liming. Nutr Cycl Agroecosyst 64:213–224
Hayat R, Safdar Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Hungria M, Campo RJ, Mendes IC (2005) Reinoculation increasing soybean grain yield in Brazil. In: Proceedings of the 14th international nitrogen fixation congress, Springer, Dordrecht, p 315
Iavicoli A, Boutet E, Buchala A, Metraux JP (2003) Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol Plant-Microbe Interact 16:851–858
Igual M, Valverde EA, Cervantes E, Velásquez E (2001) Phosphate-solubilizing bactéria as inoculants for agriculture: use of update molecular techniques in their study. Agronomie 21:561–568
Illmer P, Schimer F (1995) Solubilization of inorganic calcium phosphates solubilization mechanisms. Soil Biol Biochem 27:257–263
Inui-Kishi RN, Kishi LT, Picchi SC, Barbosa JC, Lemos MTO, Marcondes J, Lemos EG d M (2012) Phosphorus solubilizing and IAA production activities in plant growth promoting rhizobacteria from Brazilian soils under sugarcane cultivation. ARPN J Eng Appl Sci 7:1446–1454
Iqbal I, Hasnain S (2013) Auxin producing pseudomonas strains: biological candidates to modulate the growth of Triticum aestivum beneficially. Am J Plant Sci 4:1693–1700
Jaizme-Vega MC, Rodriguez-Romero AS, Guerra MSP (2004) Potential se of rhizobacteria from the Bacillus genus to stimulate the plant growth of micropropagated bananas. Fruits 59(2):83–90
Jing YD, He ZL, Yang XE (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8:192–207
Jorquera MA, Hernandez MT, Rengel Z, Marschner P, Mora MD (2008) Isolation of culturable phosphor bacteria with both phytate mineralization and phosphate solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils 44:1025–1034
Kembel SW, O’Connor TK, Arnold HK, Hubbell SP, Wright SJ, Green JL (2014) Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc Natl Acad Sci U S A 38:13715–13720
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 27:29–43
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010) Plant growth promotion by phosphate solubilizing fungi—current perspective. Arch Agron Soil Sci 56:73–98
Kim J, Rees DC (1994) Nitrogenase and biological nitrogen fixation. Biochemist 33:389–397
Kloepper JW (1994) Plant growth-promoting rhizobacteria (other systems). In: Okon Y (ed) Azospirillum/plant associations. CRC Press, Boca Raton, pp 111–118
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:5776
Knudson L (1922) Nonsymbiotic germination of orchid seeds. Bot Gaz 73:1–25
Köberl M, Schimdt R, Ramadan EM, Bauer R, Berg G (2013) The microbiome of medicinal plants: diversity and importance for plant growth, quality, and health. Front Microbiol 4:1–9
Krishnaraj PU, Dahale SK (2014) Mineral phosphate solubilization: concepts and prospects in sustainable agriculture. Proc Indian Natl Sci Acad 80:389–405
Kyselková M, Kopecký J, Frapolli M et al (2009) Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. ISME J 3:1127–1138
Lebuhn M, Heulin T, Hartmann A (1997) Production of auxin and other indolic and phenolic compounds by Paenibacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol Ecol 22:325–334
Lichter A, Barash I, Valinsky L, Manulis S (1995) The genes involved in cytokinin biosynthesis in Erwinia herbicola pv. gypsophilae, characterization and role in gall formation. J Bacteriol 177:4457–4465
Lifshitz R, Kloepper JW, Scher FM et al (1986) Nitrogen-fixing pseudomonads isolated from roots of plants grown in the Canadian High Arctic. Appl Environ Microbiol 51:251–255
Liu HX, Luo YB, Liu H (2010) Studies of mycorrhizal fungi of Chinese orchids and their role in orchid conservation in China – a review. Bot Rev 76:241–262
Long SR (1996) Rhizobium symbiosis: nod factors in perspective. Plant Cell 8:1885–1898
Lundberg DS, Lebeis SL, Paredes SH et al (2012) Defining the core Arabdopsis thaliana root. Nature 488:86–90
Ma Y, Rajkumar M, Freitas H (2009a) Isolation and characterization of Ni mobilizing PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere 75:719–725
Ma Y, Rajkumar M, Freitas H (2009b) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater 166:1154–1161
Mabood F, Zhou X, Smith DL (2014) Microbial signaling and plant growth promotion. Can J Plant Sci 94:1051–1063
Maeder P, Fliessbach A, Dubois D et al (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Maliha R, Samina K, Najma A et al (2004) Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms under in vitro consitions. Pak J Biol Sci 7:187–196
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Mendes LW, Tsai SM, Navarrete AA et al (2015) Soil-borne microbiome: linking diversity to function. Microb Ecol 70:255–265
Menezes-Blackburn D, Jorqueira MA, Greiner R et al (2013) Phytases and phytase-labile organic phosphorus in manures and soils. Crit Rev Environ Sci Technol 43:916–954
Morris RO (1986) Genes specifying auxin and cytokinin biosynthesis in pathogens. Annu Rev Plant Physiol 37:509–538
Nieto KF, Frankenberger WT Jr (1990) Microbial production of cytokinins. In: Bollag JM, Stotzky G (eds) Soil biochemestry, vol 6. Dekker, New York, pp 191–248
O’Sullivan DJ, O’Gara F (1992) Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Oldroyd GED, Downie JA (2004) Calcium, kinases and nodulation signaling in legumes. Nature 5:566–576
Oldroyd GED, Downie JA (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59:519–546
Oldroyd GED, Murray JD, Poole PS, Downie JA (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 45:119–144
Olivares J, Bedmar EJ, Sanjuán J (2013) Biological nitrogen fixation in the context of global change. MPMI 26:486–494
Oliveira CA, Sa NMH, Gomes EA et al (2009) Assessment of the mycorrhizal community in the rhizosphere of maize (Zea mays L.) genotypes contrasting for phosphorus efficiency in the acid savannas of Brazil using denaturing gradient gel electrophoresis (DGGE). Appl Soil Ecol 41:249–258
Orhan E, Esitken A, Ercisli S, et al (2006) Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci Hortic 111(1):38–43
Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4:701–712
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA (2011) Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol Lett 14:493–502
Parks EJ, Olson GJ, Brinckman FE, Baldi F (1990) Characterization by high performance liquid chromatography (HPLC) of the solubilization of phosphorus in iron ore by fungus. J Ind Microbiol Biotechnol 5:183–189
Parmar N, Dufresne J (2011) Beneficial interactions of plant growth promoting rhizosphere microorganisms. In: Singh A, Parmar N, Kuhad RC (eds) Bioaugmentation, biostimulation and biocontrol. Springer-Verlag, Berlin Heidelberg, pp 27–42
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Paul D, Nair S (2008) Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol 48:378–384
Peix A, Velazquez E, Martýnez-Molina E (2007) Molecular methods for biodiversity analysis of phosphate solubilizing microorganisms (PSM). In: Velazquez E, Rodriguez-Barrueco C (eds) First international meeting on microbial phosphate solubilization. Springer, Berlin, p 97–100
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Plant 118:10–15
Peralta H, Mora Y, Salazar E, Encarnacion S, Palacios R, Mora J (2004) Engineering the nifH promoter region and abolishing poly-Â - hydroxybutyrate accumulation in Rhizobium etli enhance nitrogen fixation in symbiosis with Phaseolus vulgaris. Appl Environ Microbiol 70(6):3272–3281
Perret X, Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev 64:180–201
Philippot L, Spor A, Hénault C et al (2013) Loss in microbial diversity affects nitrogen cycling in soil. ISME J 7:1609–1619
Pirlak M, Kose M (2009) Effects of plant growth promoting rhizobacteria on yield and some fruit properties of strawberry. J Plant Nutr 32:1173–1184
Qi X, Wang E, Xing M, Zhao W, Chen X (2012) Rhizosphere and nonrhizosphere bacterial community composition of the wild medicinal plant Rumex patientia. World J Microbiol Biotechnol 28:2257–2265
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81:537–547
Rashid S, Charles TC, Glick BR (2012) Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol 61:217–224
Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JHJ (2012) Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822
Rathore P (2014) A review on approaches to develop plant growth promoting rhizobacteria. Intl J Recent Sci Res 5:403–407
Raymond J, Siefert JL, Cr S, Blankendhip RE (2004) The natural history of nitrogen fixation. Mol Biol Evol 21:541–554
Reyes I, Bernier L, Antoun H (2002) Rock phosphate solubilization and colobization of maize rizosphere by wild and genetically modified strains of Pennicilium rugulosum. Microb Ecol 44:39–48
Rezzonoco F, Binder C, Defago G, Moenne-Loccoz Y (2005) The type III secretion system of biocontrol Pseudomonas fluorescencs KD targets the phytopathogenic chromista Pythium ultimum and promotes cucmuber protection. Mol Plant-Microbe Interact 9:991–1001
Richardson AE (1994) Soil microorganisms and phosphorus availability. In: Pankhurst CE, Doubeand BM, Gupta WSR (eds) Soil biot: management in sustainable farming systems. CSIRO, Victoria, pp 50–62
Richardson AE, Simpson RJ (2011) Soil microorganism mediating phosphorus availability. Plant Physiol 156:989–996
Richardson AE, George TS, Hens M, Simpson RJ (2005) Utilization of soil organic phosphorus by higher plants. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment. CABI, Wallingford, pp 165–184
Richardson AE, Baréa JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Rodrigues EP, Rodrigues CS, de Oliveira ALM, Baldani VL, Teixeira da Silva JA (2008) Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.) Plant Soil 302:249–261
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rodriguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21
Rubio LM, Ludden PW (2008) Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu Rev Microbiol 62:93–111
Saharan BS, Nehan V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Sala OE, Chapin FS, Armesto JJ et al (2000) Biodiversity—global biodiversity scenarios for the year 2100. Science 287:1770–1774
Saranraj P, Sivasakthivelan P, Siva SS (2013) Prevalence and production of plant growth promoting substance by Pseudomonas fluorescens isolated from paddy rhizosphere soil of Cuddalore district, Tamil Nadu, India. Afr J Basic Appl Sci 5(2):95–101
Sashidhar B, Podile AR (2010) Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol 109:1–12
Sato VS, Galdiano RF, Rodrigues GR, Lemos EGM, Pizauro JM (2016) Kinetic characterization of a novel acid ectophosphatase from Enterobacter asburiae. J Microbiol 54:106–113
Schippers B, Bakker AW, Bakker AHM (1987) Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practice. Annu Rev Phytopathol 25:339–358
Sessitsch A, Howieson JG, Perret X, Antoun H, Martínez Romero E (2002) Advances in rhizobium research. Crit Rev Plant Sci 21:323–378
Sharma SK, Johri BN, Ramesh A, Joshi OP, Prasad SVS (2011) Selection of plant growth-promoting Pseudomonas spp. that enhanced productivity of soybean-wheat cropping system in central India. J Microbiol Biotechnol 21:1127–1142
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Singh G, Mukerji KG (2006) Root exudates as determinant of rhizospheric microbial biodiversity. In: Mukerji KG, Manoharachary C, Singh J (eds) Microbial activity in the rhizosphere. Spinger, Berlin, pp 39–53
Singh B, Satyanarayana T (2011) Microbial phytases in phosphorus acquisition and plant growth promotion. Physiol Mol Biol Plants 17:93–103
Siqueira JO, Franco AA (1988) Biotecnologia do solo: fundamentos e perspectivas. ESAL/FAEPE, Lavras. (in Portuguese)
Siqueira JO, Andrade AT, Faquin V (2004) O papel dos microrganismos na disponibilização e aquisição de fósforo pelas plantas. In: Yamada T, Stipp e Abdalla SR (eds) Fósforo na agricultura brasileira. Potafos publishers, Piracicaba, pp 117–156
Sivasakthivelan P, Saranraj P (2013) Azospirillum and its formulations: a review. Intl J Microbiol Res 4(3):275–287
Smalla K, Wieland G, Buchner A et al (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67:4742–4751
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, Amsterdam
Smith DL, Zhou X (2014) An effective integrated research approach to study climate change in Canada. Can J Plant Sci 94:995–1008
Smith DL, Subramanian S, Lamont JR, Bywater-Ekegärd M (2015) Signalling in the phytomicrobiome: breath and potential. Front Plant Sci 6:1–8
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Supanjani HS, Han JS, Jung KD, Lee KD (2006) Rock phosphate potassium and rock solubilizing bacteria as alternative, sustainable fertilizers. Agron Sustain Dev 26(4):233–240
Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol 223. Springer Science Bussiness Media, New York, pp 33–52
Tenuta M (2003) Plant growth promoting rhizobacteria: prospects for increasing nutrient acquisition and disease control. http://www.umanitoba.ca/faculties/afs/MAC_proceedings/2003/pdf/tenuta_rhizobacteria.pdf. Accessed 24 Feb 2016
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schesinger WH, Simberloff D, Wackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284
Trabelsi D, Mhamdi R (2013) Microbial inoculants and their impact on soil microbial communities: a review. Biomed Res 1:13
Trolove SN, Hedley MJ, Kirk GJD, Bolan NS, Loganathan P (2003) Progress in selected areas of rhizosphere research on P acquisition. Aust J Soil Res 41(3):471
Tsavkelova EA, Cherdyntseva TA, Netrusov AI (2003) Phytohormones production by the fungi associated with orchids. Mycol Phytopathol 37:75–83
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol 42:117–126
Tsavkelova EA, Cherdyntseva TA, Klimova SY, Shestakov AI, Botina SG, Netrusov AI (2007) Orchid-associated bacteria produce indole-3-acetic acid, promote seed germination, and increase their microbial yield in response to exogenous auxin. Arch Microbiol 188:655–664
Tsavkelova E, Oeser B, Oren-Young L, Israeli M, Sasson Y, Tudzynski B, Sharon A (2012) Identification and functional characterization of the genes for indole-3-acetamide-mediated IAA biosynthesis in plant-associated Fusarium species. Fungal Genet Biol 49:48–57
Upadhyay SK, Singh DP, Saikia R (2009) Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr Microbiol 59:489–496
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356
Valdés M, Pérez N, Estrada P, Caballero J, Peña J, Normand P, Hirsch A (2005) Non-Frankia actinomycetes isolated from surface-sterilized roots of Casuarina equisetifolia fix nitrogen. Appl Environ Microbiol 71(1):460–466
Van Peer R, Punte HL, de Weger LA, Schippers B (1990) Characterization of root surface and endorhizosphere pseudomonads in relation to their colonization of roots. Appl Environ Microbiol 56:2462–2470
Van Peer R, Nieman GJ, Schippers B (1991) Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 81:728–734
Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Fertil Soils 30(5-6):460–468
Venkateswarlu B, Rao AV, Raina P, Ahmad N (1984) Evaluation of phosphorus solubilization by microorganisms isolated from arid soil. J Ind Soc Soil Sci 32:273–277
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vessey JK, Buss TJ (2002) Bacillus cereus UW85 inoculation effects on growth, nodulation, and N accumulation in grain legumes. Controlled environmental studies. Can J Plant Sci 82:282–290
Wang Y, Brown HN, Crowley DE, Szaniszlo PJ (1993) Evidence for direct utilization of a siderophore, ferrioxamine B, in axenically grown cucumber. Plant Cell Environ 16:579–585
Wang L, Zhang L, Liu Z, Zhao D, Liu X, Zhang B, Xie J, Hong Y, Li P, Chen S, Dixon R, Li J (2013) A minimal nitrogen fixation gene cluster from Paenibacillus sp. WLY78 enables expression of active nitrogenase in Escherichia coli. PLoS Genet 3:1–11
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48:3262–3267
Wani PA, Zaidi A, Khan AA, Khan MS (2005) Effect of phorate on phosphate solubilization and indole acetic acid (IAA) releasing potentials of rhizospheric microorganisms. Ann Plant Protect Sci 13:139–144
Wilkinson KG, Dixon KW, Sivasithamparam K (1989) Interaction of soil bacteria, mycorrhizal fungi and orchid seed in relation to germination of Australian orchids. New Phytol 112:429–435
Wilkinson KG, Dixon KW, Sivasithamparam K, Ghisalberti EL (1994) Effect of IAA on symbiotic germination of an Australian orchid and its production by orchid-associated bacteria. Plant Soil 159:291–295
Wu JP, Qian J, Zheng SZ (2002) A preliminary study on ingredient of secretion from fungi of orchid mycorrhizal. Chin J Appl Ecol 13:845–848
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yang YL, Liu ZY, Zhu GS (2008) Study on symbiotic seed germination of Pleione bulbocodioides (Franch) Rolfe. Microbiology 35:909–912. (in Chinese with English abstract)
Yang S, Zhang X, Cao Z, Zhao K, Wang S, Chen M, Hu X (2014) Growth-promoting Sphingomonas paucimobilis ZJSH1 associated with Dendrobium officinale through phytohormone production and nitrogen fixation. Microb Biotechnol 7:611–620
Zahir AZ, Arshad M, Frankenberger WT Jr (2004) Plant growth promoting rhizobacteria: application and perspectives in agriculture. Adv Agron 81:97–168
Zahir ZA, Shah MK, Naveed M, Akhter MJ (2010) Substrate dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. J Microbiol Biotechnol 20:1288–1294
Zaidi A, Khan MS, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung 56:263–284
Zhu F, Qu L, Hong X, Sun X (2011) Isolation and characterization of phosphate solubilizing Halophilic Bacterium Kushneria sp. YCWA 18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid Based Complement Alternat Med 2011:1–6
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Inui Kishi, R.N., Galdiano Júnior, R.F., Val-Moraes, S.P., Kishi, L.T. (2017). Soil Microbiome and Their Effects on Nutrient Management for Plants. In: Kumar, V., Kumar, M., Sharma, S., Prasad, R. (eds) Probiotics in Agroecosystem. Springer, Singapore. https://doi.org/10.1007/978-981-10-4059-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-4059-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4058-0
Online ISBN: 978-981-10-4059-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)