Abstract
Capacitation is defined as an ensemble of several physiological, molecular and cellular changes in the spermatozoa, making them fertilization competent. It is considered as an obligate requirement for sperm fertility, since failures in sperm capacitation affect the fertilization potential. This chapter discusses the hallmarks of capacitation, including molecular changes involved in this phenomenon. Laboratory-based studies on human spermatozoa (molecular studies and sperm function tests based on capacitation and its associated events: hyperactivation, acrosome reaction and tyrosine phosphorylation) have been discussed with a view to highlight the pressing need for translating this information into the clinical practice. Additionally, a requirement to develop molecular markers/sperm function tests based on protein tyrosine phosphorylation has been emphasized. The latter have come to the fore with increasing incidence of infertility and frequent use (and need) of assisted reproductive technologies like IVF and ICSI.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Sperm capacitation
- Male infertility
- Molecular marker
- Sperm function tests
- Hyperactivation
- Acrosome reaction
- Tyrosine phosphorylation
- ARTs
Key Points
-
Sperm capacitation, discovered in 1951, independently by CR Austin and MC Chang, is considered as an obligate requirement for sperm fertility.
-
The last six decades have seen a considerable rise in laboratory-based studies on human sperm capacitation and its associated phenomena: hyperactivation, acrosome reaction and protein tyrosine phosphorylation.
-
Clinical tests based on the identified molecular markers are rather scarce, with one test, viz. Androvia Cap-Score™ showing promising results in being able to discriminate fertile from infertile men.
-
In the present era of assisted reproductive techniques (ARTs), especially ICSI, it is mandatory to develop reliable sperm function tests based on capacitation and other related phenomena to ensure the selection of the “healthiest” spermatozoa.
1 Introduction
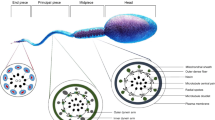
In mammals, after having gone through the journey of formation in testis and maturation in epididymis; spermatozoa, the male gamete, isn’t quite ready yet to marry the female gamete, the oocyte. It still has to undergo a whole battery of changes—this time—in the female reproductive tract, to fertilize the oocyte (Fig. 5.1). This ensemble of post-ejaculation changes in the spermatozoa has been collectively called as sperm “capacitation”. Capacitation renders the spermatozoa functionally mature.
Life cycle of sperm: After production in testis in sufficient numbers with normal shape, the spermatozoa undergo maturation in epididymis, gain motility and undergo capacitation in the female reproductive tract, then acrosome react after oocyte binding and penetrate and activate the egg, resulting in successful fertilization. The blue arrows indicate the site of event
Origin of spermatozoa in the testis is followed by its capacitation (after ejaculation) in the female reproductive tract and ultimately fertilization with oocyte in the fallopian tube. Sperm contribution to fertilization to assess the “male factors” is usually estimated through evaluation of semen parameters, namely, sperm count, morphology and motility (World Health Organization 2010). Quite often, in spite of these parameters being normal and these males being termed as normozoospermic (normal count, motility and morphology); the infertility still exists in the male partner. Such cases of idiopathic (unknown etiology) infertility have been attributed substantially to the problems in sperm capacitation (Tucker et al. 1987; Matzuk and Lamb 2002; Esposito et al. 2004; Hildebrand et al. 2010; Nandi and Homburg 2016).
2 What Is Sperm Capacitation?
Sperm capacitation has been defined as the “ensemble of all the physiological, molecular and cellular changes in the spermatozoa, which are necessary to make it fertilization competent”. It was independently discovered by Austin and Chang in 1951 (Austin 1951; Chang 1951). Although discovered more than half a century ago, capacitation is still regarded as a “poorly understood” phenomenon, owing to the fact that each mammalian species has its unique features at the physical (time of capacitation) and molecular level (Chang 1984) that are difficult to monitor, since it takes place in the female reproductive tract (either in the oviduct or in the vicinity of the egg).
Sperm capacitation is a prerequisite for successful fertilization as evidenced from the observations that a block in capacitation causes male infertility (Tucker et al. 1987; Matzuk and Lamb 2002; Esposito et al. 2004; Hildebrand et al. 2010). Therefore, there has been a pressing need to understand sperm capacitation in all the individual species making it a focus of investigations of many gamete biologists worldwide. Most progress in understanding the phenomenon of capacitation has been because of in vitro methods for capacitation (Yanagimachi 1969). In the procedure, freshly ejaculated or epididymal spermatozoa are washed and incubated at physiological conditions in a defined medium that mimics the female oviductal fluid (Dow and Bavister 1989). The medium normally has the following composition: electrolytes, metabolic energy source and a macromolecule to allow for cholesterol efflux like serum albumin (Yanagimachi 1969, 1994). Several in vitro studies have revealed that during capacitation, spermatozoa undergo a number of biochemical and biophysical changes (Fig. 5.2), such as increase in membrane fluidity (Davis et al. 1980; Cross 1998; Buffone et al. 2009; Salvolini et al. 2013), activation of trans-bilayer signalling events (Go and Wolf 1985; Visconti et al. 1998; Gadella and Harrison 2000; Flesch et al. 2001; Sheriff and Ali 2010; Ickowicz et al. 2012), changes in redox status of spermatozoa leading to generation of reactive oxygen species (ROS) (de Lamirande and Gagnon 1992; Aitken 1995; O’Flaherty et al. 2006; Musset et al. 2012), removal of stabilizing proteins (Shivaji et al. 1990; Villemure et al. 2003; Leahy and Gadella 2011) and phosphorylation of proteins (Leyton and Saling 1989; Visconti et al. 1995; Mitra and Shivaji 2004; Arcelay et al. 2008; Mitchell et al. 2008; Kota et al. 2009; Katoh et al. 2014).
3 Hallmarks of Capacitation
Capacitation is generally monitored by recording protein tyrosine phosphorylation (pY), hyperactivation (Yanagimachi 1994; Kulanand and Shivaji 2001; Baker et al. 2006) and acrosome reaction (Ward and Storey 1984; Meizel and Turner 1991; Aitken 1995; Curry and Watson 1995; Mitra and Shivaji 2004; Varano et al. 2008; Bragado et al. 2012; Jaldety and Breitbart 2015), which are also considered as the “hallmarks of capacitation” (Fig. 5.2). Capacitation changes lead to the transformation in the motility pattern of spermatozoa from a progressively motile cell to a more vigorous, but less progressive, motile cell (Yanagimachi 1969; Suarez and Dai 1992; Mortimer and Swan 1995; Ho and Suarez 2001). This type of motility is termed as “hyperactivation”, and subsequent to this, capacitation ends with the ability of spermatozoa to undergo “acrosome reaction”, during which the spermatozoa releases the hydrolytic enzymes to facilitate its penetration and fusion with the oocyte—finally leading to fertilization. The increase in pY is another distinctive feature of the mammalian spermatozoa associated with capacitation. This molecular change is considered as an important characteristic of mammalian capacitation and has been addressed by various groups worldwide in varied animal models (Visconti and Kopf 1998; Visconti et al. 1999; Kulanand and Shivaji 2001; Lefièvre et al. 2002; Jha et al. 2003; Shivaji et al. 2007, 2009; Arcelay et al. 2008; Mitchell et al. 2008; Kota et al. 2009).
3.1 Hyperactivation
Hyperactivation, which is defined as “a distinct change in the sperm motility from a symmetrical to an asymmetrical pattern, is crucial for fertilization” (Yanagimachi 1969; Suarez 2008). The mammalian spermatozoa, while in the epididymis are immotile. But when released in the female reproductive tract/culture media, they quickly begin to swim and get hyperactivated (Morton et al. 1974), which imparts sperm the ability to traverse through the mucus-filled, labyrinthine lumen of the oviduct to reach the female gamete. Hyperactivation also helps the spermatozoa in penetrating the cumulus oophorus and the zona pellucida (Suarez et al. 1991; Suarez 2008). This activated spermatozoon generates a near symmetrical flagellar beat, which is called as a “planar motility” pattern. This planar motility propels the spermatozoa in an almost linear trajectory (Suarez and Dai 1992; Mortimer and Swan 1995; Ho et al. 2002). The amplitude of the flagellar bend is usually increased only on one side of the hyperactivated spermatozoa. This increased uneven amplitude leads to a circular, wriggling and whiplash type of motility pattern of the spermatozoa as shown in Fig. 5.3, and these movements are assessed objectively by using the computer-assisted sperm analysis (CASA) system (Shivaji et al. 1995; Panneerdoss et al. 2012). Hyperactivation is initiated and maintained by the involvement of a number of physiological factors like calcium, bicarbonate, cAMP and metabolic substrates (Visconti et al. 1999).
3.2 Acrosome Reaction
Acrosome reaction is an absolute crucial step for successful fertilization, as it is due to acrosomal secretions alone that the sperm makes its progress through the investments surrounding the egg. In fact, males with spermatozoa lacking the acrosome are infertile (Baccetti et al. 1991). During the acrosome reaction, multiple fusions occur between the plasma membrane and the outer acrosomal membrane in the anterior region of the head. These multiple fusions lead to the formation of extensive hybrid membrane vesicles and subsequent exposure of the inner acrosomal membrane and acrosomal contents (Cardullo and Florman 1993). These stages of acrosome reaction have been depicted in Fig. 5.4.
Schematic representation of various stages in the progression of the sperm acrosome reaction (adapted from Curry and Watson 1995)
3.3 Protein Tyrosine Phosphorylation
Protein tyrosine phosphorylation (pY), a post-translational event, is also considered as hallmark of capacitation. pY is a regulatory mechanism which controls many processes, such as cell cycle control, cytoskeleton assembly, cellular growth, receptor regulation and ionic current modulation (Hunter 2000; Pawson 2004; Vizel et al. 2015). The first evidence of protein tyrosine phosphorylation in spermatozoa was provided by Leyton and Saling (1989) in mouse. Later, Visconti et al. (1995) showed a correlation between sperm capacitation and protein tyrosine phosphorylation in mouse spermatozoa, and soon this increase was demonstrated in spermatozoa of various other species during capacitation, including human (Leclerc et al. 1996; Osheroff et al. 1999), hamster (Kulanand and Shivaji 2001), cat (Pukazhenthi et al. 1998), pig (Tardif et al. 2001), boar (Kalab et al. 1998), bovine (Galantino-Homer et al. 1997, 2004), equine (Pommer et al. 2003), cynomolgus monkey (Mahony and Gwathmey 1999), tammar wallaby and brushtail possum (Sidhu et al. 2004), guinea pig (Kong et al. 2008) and ram (Grasa et al. 2006).
Naz and Rajesh (2004) proposed a model for tyrosine phosphorylation pathways during sperm capacitation. The model suggests that sperm capacitation involves three main signalling pathways, namely, a cAMP/PKA-dependent pathway (pathway I) [unique to spermatozoa], a receptor tyrosine kinase pathway (pathway II) and a non-receptor protein tyrosine kinase pathway (pathway III). A crosstalk between tyrosine kinase and cAMP-dependent kinase signalling pathways in human sperm motility regulation is a unique feature in spermatozoa (Bajpai and Doncel 2003). SRC family kinases (SFKs) known to play an important role in this capacitation-associated increase in protein tyrosine phosphorylation (Battistone et al. 2013) are shown to be downstream of PKA. The target proteins for PKA could be protein tyrosine kinase(s) or protein tyrosine phosphatase(s) or both. These kinase(s) and phosphatase(s) then regulate the downstream phosphorylation of their substrate proteins at their tyrosine residues leading to a cascade of signalling events. Till date, a number of kinases have been identified (Table 5.1), which are involved in the process of capacitation, and the list is still expanding (Lawson et al. 2008; Mitchell et al. 2008; Varano et al. 2008; Goupil et al. 2011; Battistone et al. 2013; Wang et al. 2015). Although several kinases have been identified in the spermatozoa (Table 5.1), their functional relevance is seen only in vitro and mostly in animal models. The importance of the identified kinases and thus the regulation of tyrosine phosphorylation in male fertility/infertility has not yet been explored much.
4 Diagnosis and Prognosis of Male Infertility/Fertility: Importance of Capacitation-Based Sperm Function Tests
In humans, the prognosis and diagnosis of male fertility has been a subject of research worldwide. As mentioned earlier, a good percentage of human pregnancy failures can be attributed to decreased male fertility or male factor infertility (Thonneau et al. 1991; Sharlip et al. 2002; Lee and Foo 2014). To evaluate human sperm fertility, there has always been a consistent effort to get in place sperm function tests, owing to low predictive power of standard seminal parameters (motility, concentration and morphology) (Oehninger 1995; Carrell 2000; Muller 2000; Aitken 2006; Lefièvre et al. 2007; Vasan 2011; De Jonge and Barratt 2013; Esteves et al. 2014; Oehninger et al. 2014). Attempts have been made in laboratories for decades to design sperm function tests based on capacitation and its associated events/parameters for predicting male fertility.
Sperm penetration tests, including the sperm mucus penetration test and sperm penetration assay, are being routinely used in fertility centres. In addition, various biochemical and biophysical changes during capacitation (Zaneveld et al. 1991; Benoff 1993; Martínez and Morros 1996; Cross 1998; Travis and Kopf 2002; Visconti et al. 2002, 2011; Mitra and Shivaji 2005; Signorelli et al. 2012; Aitken and Nixon 2013) also are being utilized for designing sperm-function tests, for instance, determining the cholesterol efflux, examining activation of ion channels, evaluating protein phosphorylation changes, measuring intracellular calcium and pH and reactive oxygen species, monitoring hyperactivation and acrosome reaction, etc. Three of these events/changes are discussed in the following sections.
4.1 Monitoring Hyperactivation (HA)
One of the indicators of capacitation is the display of HA by spermatozoa (Burkman 1984). Sperm motility, hyperactivation and related motility kinematic parameters like average path velocity (VAP), curvilinear velocity (VCL), straight line velocity (VSL), linearity (LIN), amplitude of lateral head displacement (ALH), straightness (STR) and beat cross frequency (BCF) are assessed using CASA (Larsen et al. 2000; Freour et al. 2009). Based on the aforesaid kinematic parameters, namely, VCL, LIN and ALH, the non-hyperactivated spermatozoa (exhibiting planar motility pattern) can be differentiated from the hyperactivated spermatozoa (exhibiting either circular or helical motility patterns) using the SORT facility of the CASA (Youn et al. 2011).
Impaired sperm hyperactivation (HA) has been observed in human patients with infertility (Wong et al. 1993; Munier et al. 2004; Wiser et al. 2014). Wiser et al. evaluated spermatozoa from the normal patients who were to undergo IVF. They found that patients with increased hyperactivated motility had significantly higher fertilization rate compared to the group with no increased hyperactivated motility. Several groups have also found a good correlation between sperm hyperactivation, zona-induced acrosome reaction and zona binding (Liu et al. 2007); sperm motility, capacitation and tyrosine phosphorylation (Yunes et al. 2003; Buffone et al. 2005); and oocyte penetration (Wang et al. 1991), thus presenting HA as a good prognostic parameter for sperm fertility.
4.2 Monitoring Acrosome Reaction (AR)
Only capacitated spermatozoa are known to undergo acrosome reaction, underscoring its importance in predicting sperm capacitation and fertility potential of spermatozoa (Bielfeld et al. 1994). Acrosomal status in human spermatozoa is monitored with the fluorescent conjugated lectins (PNA, peanut agglutinin, and PSA, Pisum sativum agglutinin) (Cross and Meizel 1989). Additionally, several methods of assessing induced AR in vitro have been designed, where the ability of spermatozoa to acrosome react in the presence of calcium-mobilizing agents, such as calcium ionophore (A23187) or the physiological inducers like progesterone and zona pellucida proteins, is assessed (Brucker and Lipford 1995 ; Bastiaan et al. 2002). There are other fluorescent tests to evaluate the acrosome, like chlortetracyclin (CTC) staining, in which staining can differentiate three different sperm populations: the uncapacitated and acrosome intact (F pattern), the capacitated and acrosome intact (B pattern) and the capacitated and acrosome reacted (AR pattern) (Kholkute et al. 1992; Dasgupta et al. 1994).
The fact that in vivo, acrosome reaction is induced by progesterone and zona proteins, evaluation of induced acrosome reaction is routinely used as a predictor of sperm quality for utilization in clinics for assisted reproductive technologies (ARTs) (Shimizu et al. 1993; Coetzee et al. 1994; Fusi et al. 1994; Yovich et al. 1994; Glazier et al. 2000; Makkar et al. 2003). Quite often, spontaneous acrosome reaction is also evaluated and correlated with sperm fertility (Bielsa et al. 1994; Parinaud et al. 1995; Tavalaee et al. 2014; Wiser et al. 2014).
4.3 Monitoring Tyrosine Phosphorylation (pY)
In human spermatozoa, increase in global protein tyrosine phosphorylation occurs during capacitation and is correlated with the fertilizing ability of the spermatozoa (Yunes et al. 2003; Liu et al. 2006; Barbonetti et al. 2008, 2010; Mendeluk et al. 2010; Kwon et al. 2014; Sati et al. 2014).
In spite of the importance of pY in human sperm capacitation, laboratory studies and clinic-based sperm-function tests on pY are very scarce. Such studies have to be in place to determine the predictive capability of pY of sperm fertility. As discussed for the kinases as well earlier, profiling of infertile patients’ samples with appropriate controls is essential to develop sperm function tests based on this important molecular event during sperm capacitation.
5 From Bench to Clinics: Male Fertility Biomarkers and ARTs
There has been a steady rise in the molecular studies on the role of capacitation and its associated events (hyperactivation, acrosome reaction and tyrosine phosphorylation) in male fertility, in vitro (Fig. 5.5a, b). In spite of such extensive work being carried out at the laboratory level, these studies do not seem to have found application in the clinics yet. There are only a handful of clinics globally which seem to offer basic sperm capacitation/acrosome reaction tests as a part of routine sperm analysis, e.g. FIVMadrid; Poma Fertility; Androvia Life Sciences; University of Utah Hospitals and Clinics; the Male Fertility Lab, University of Washington; and Genetics & IVF Institute (references for website information). This data/information presented and discussed here is based on literature survey and searches on the World Wide Web, and real picture regarding the clinical usage of sperm capacitation tests might differ and remains to be determined.
(a) Relative percentages of studies in different categories (as shown) conducted over the last four decades worldwide (total number of studies =363). (b) Threefold increase in molecular studies has taken place in the last decade (2007–2016) as compared to the previous one (1996–2006). The publications were taken from PubMed (http://www.ncbi.nlm.nih.gov/pubmed/). The search was done using the following terms: [sperm capacitation, fertilization, infertility, human(s), infertility, infertile], [sperm, sperm capacitation, human(s), infertility, infertile, fertilization, hyperactivation] and [sperm, sperm capacitation, human(s), infertility, infertile, fertilization, acrosome reaction]. Based on their content, the publications (related to humans) were assigned to six categories and then percentages calculated, as shown in the pie chart
There is a pressing need to evaluate the potential of the capacitation-associated sperm molecules/events and sperm function tests as biomarkers (or predictors) of sperm fertility/infertility. One promising sperm function/molecular test in this direction has been the “Androvia Cap-Score™ test”—a clinical test based on sperm surface ganglioside, GM1 (http://www.androvialifesciences.com/cap-score-sperm-function-test/). This test is based on the work of Dr. Alex Travis and is based on the localization of GM1 on sperm head (Buttke et al. 2006; Selvaraj et al. 2007). GM1 is a sperm membrane component that regulates the opening and closing of specific calcium ion channels on the surface of sperm head. Androvia uses technology that identifies the ability of sperm to undergo capacitation. Since capacitation, hyperactivation and the acrosome reaction require an influx of calcium ions, by identifying the presence and location of GM1 in the sperm membrane across a number of sperm and identifying how many sperm are undergoing capacitation, a “Cap-Score™” can be generated that is predictive of the fertilizing ability of sperm in the ejaculate. The company Androvia claims that their preliminary research has already validated the ability of the test to discriminate between fertile and infertile populations of men, thus gaining clinical significance as a molecular marker/sperm function test.
Sperm capacitation and its associated events are the very basis of intrauterine insemination (IUI) and in vitro fertilization (IVF), the first line of ART management for couples with unexplained infertility/subfertility (Muratori et al. 2011; Wiser et al. 2014; Tosti and Ménézo 2016). In the cases of IUI and IVF, where cryopreserved spermatozoa are used, knowledge of sperm capacitation is especially useful for extending the health and life span of the sperm (and thus success of the ART), since it is known that freeze-thawed spermatozoa exhibit a precocious acrosome reaction-like phenotype, suggesting capacitation-like event during the process of cryopreservation (Gomez et al. 1997). Though extensively used in domestic species (such as bovine, pigs and dogs), it is well known and accepted that cryopreservation damages sperm, with a large number of cells losing their fertility potential after freezing/thawing (Cormier and Bailey 2003). Knowledge about mechanisms involved in capacitation/acrosome reaction would help in efforts towards minimizing the cryo-damage to spermatozoa and improve the success rate in ARTs, as being used in the livestock industry (Singh et al. 2014; Layek et al. 2016).
The life cycle of sperm is complex and involves a series of events, which have to be perfect for successful fertilization—viz. production in testis in sufficient numbers with normal shape, maturation in epididymis, gain of motility, successful capacitation, hyperactivation and acrosome reaction, oocyte binding and penetration, activation of the ovum and ultimately successful fertilization. All these parameters ought to be looked at in defining a “healthy” spermatozoon, and defects in any of these complex events can cause male infertility. The use of ICSI (intracytoplasmic sperm injection) bypasses many of these events, increasing the risk of choosing the “compromised spermatozoa”. To avoid this, as already emphasized, it is imperative to develop new pre-ART molecular markers/sperm-function tests, for use in the clinics (Muratori et al. 2011; Natali and Turek 2011). Although few efforts to define predictive tests for ICSI success have already begun with limited success (Vural et al. 2005; Setti et al. 2012; Brown et al. 2013; Breznik et al. 2013; Meerschaut et al. 2013), further research in this direction is much needed.
Concluding Remarks
It is well accepted now that conventional semen analysis is unable to precisely predict sperm fertility potential, thus warranting search of biomarkers for fertility/infertility based on newer research (Weber et al. 2005; Lewis 2007; Lamb 2010). Attempts to translate the molecular information about capacitation—from laboratories to clinic—and to develop capacitation-based molecular markers/sperm function assays (besides other tests) is the need of the hour, especially in the era of assisted reproductive technologies like ICSI. The pre-ART tests would permit the clinicians and the infertile couples to make a more informed decision about the treatment/procedure and be assured of its success. It, thus, becomes necessary to continue improving our understanding of sperm capacitation, not only for the basic understanding of sperm physiology but also to understand its functionality, both in vivo and in vitro, ultimately translating into higher success rate in assisted reproductive technologies.
References
Aitken RJ (1995) Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev 7(4):659–668
Aitken RJ (2006) Sperm function tests and fertility. Int J Androl 29(1):69–75
Aitken RJ, Nixon B (2013) Sperm capacitation: a distant landscape glimpsed but unexplored| NOVA. The University of Newcastle’s Digital Repository
Almog T, Lazar S, Reiss N, Etkovitz N, Milch E, Rahamim N, Dobkin-Bekman M, Rotem R, Kalina M, Ramon J, Raziel A (2008) Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality. J Biol Chem 283(21):14479–14489
Alvau A, Battistone MA, Gervasi MG, Navarrete FA, Xu X, Sánchez-Cárdenas C, De la Vega-Beltran JL, Da Ros VG, Greer P, Darszon A, Krapf D (2016) The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 143(13):2325–2333
Androvia LifeSciences (2016). http://www.androvialifesciences.com/cap-score-sperm-function-test/. Accessed 11 Sept 2016
Aparicio IM, Bragado MJ, Gil MC, Garcia-Herreros M, Gonzalez-Fernandez L, Tapia JA, Garcia-Marin LJ (2007) Porcine sperm motility is regulated by serine phosphorylation of the glycogen synthase kinase-3α. Reproduction 134(3):435–444
Aquila S, Gentile M, Middea E, Catalano S, Andò S (2005) Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinology 146(2):552–557
Arcelay E, Salicioni AM, Wertheimer E, Visconti PE (2008) Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int J Dev Biol 52(5–6):463–472
Austin CR (1951) Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 4(4):581–596
Awda BJ, Buhr MM (2010) Extracellular signal-regulated kinases (ERKs) pathway and reactive oxygen species regulate tyrosine phosphorylation in capacitating boar spermatozoa. Biol Reprod 83(5):750–758
Baccetti B, Burrini AG, Collodel G, Piomboni P, Renieri T (1991) A “miniacrosome” sperm defect causing infertility in two brothers. J Androl 12(2):104–111
Bajpai M, Doncel GF (2003) Involvement of tyrosine kinase and cAMP-dependent kinase cross-talk in the regulation of human sperm motility. Reproduction 126(2):183–195
Baker MA, Hetherington L, Aitken RJ (2006) Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci 119(15):3182–3192
Barbonetti A, Vassallo MRC, Cinque B, Antonangelo C, Sciarretta F, Santucci R, D’Angeli A, Francavilla S, Francavilla F (2008) Dynamics of the global tyrosine phosphorylation during capacitation and acquisition of the ability to fuse with oocytes in human spermatozoa. Biol Reprod 79(4):649–656
Barbonetti A, Vassallo MRC, Cordeschi G, Venetis D, Carboni A, Sperandio A, Felzani G, Francavilla S, Francavilla F (2010) Protein tyrosine phosphorylation of the human sperm head during capacitation: immunolocalization and relationship with acquisition of sperm-fertilizing ability. Asian J Androl 12(6):853–861
Bastiaan HS, Menkveld R, Oehninger S, Franken DR (2002) Zona pellucida induced acrosome reaction, sperm morphology, and sperm–zona binding assessments among subfertile men. J Assist Reprod Genet 19(7):329–334
Battistone MA, Da Ros VG, Salicioni AM, Navarrete FA, Krapf D, Visconti PE, Cuasnicu PS (2013) Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol Hum Reprod 19(9):570–580
Battistone MA, Alvau A, Salicioni AM, Visconti PE, Da Ros VG, Cuasnicú PS (2014) Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Mol Hum Reprod 20(11):1054–1066
Benoff S (1993) Preliminaries to fertilization: the role of cholesterol during capacitation of human spermatozoa. Hum Reprod 8(12):2001–2006
Bielfeld P, Anderson RA, Mack SR, De Jonge CJ, Zaneveld LJ (1994) Are capacitation or calcium ion influx required for the human sperm acrosome reaction? Fertil Steril 62(6):1255–1261
Bielsa MA, Andolz P, Gris JM, Martinez P, Egozcue J (1994) Andrology: which semen parameters have a predictive value for pregnancy in infertile couples? Hum Reprod 9(10):1887–1890
Bordeleau LJ, Leclerc P (2008) Expression of hck-tr, a truncated form of the src-related tyrosine kinase hck, in bovine spermatozoa and testis. Mol Reprod Dev 75:828–837
Bragado MJ, Gil MC, Martin-Hidalgo D, de Llera AH, Bravo N, Moreno AD, Garcia-Marin LJ (2012) Src family tyrosine kinase regulates acrosome reaction but not motility in porcine spermatozoa. Reproduction 144(1):67–75
Breitbart H, Etkovitz N (2011) Role and regulation of EGFR in actin remodeling in sperm capacitation and the acrosome reaction. Asian J Androl 13(1):106–110
Breznik BP, Kovačič B, Vlaisavljević V (2013) Are sperm DNA fragmentation, hyperactivation, and hyaluronan-binding ability predictive for fertilization and embryo development in in vitro fertilization and intracytoplasmic sperm injection? Fertil Steril 99(5):1233–1241
Brown DB, Merryman DC, Rivnay B, Houserman VL, Long CA, Honea KL (2013) Evaluating a novel panel of sperm function tests for utility in predicting intracytoplasmic sperm injection (ICSI) outcome. J Assist Reprod Genet 30(4):461–477
Brucker C, Lipford GB (1995) The human sperm acrosome reaction: physiology and regulatory mechanisms. An update. Hum Reprod Update 1(1):51–62
Buffone MG, Calamera JC, Verstraeten SV, Doncel GF (2005) Capacitation-associated protein tyrosine phosphorylation and membrane fluidity changes are impaired in the spermatozoa of asthenozoospermic patients. Reproduction 129(6):697–705
Buffone MG, Doncel GF, Calamera JC, Verstraeten SV (2009) Capacitation‐associated changes in membrane fluidity in asthenozoospermic human spermatozoa. Int J Androl 32(4):360–375
Burkman LJ (1984) Characterization of hyperactivated motility by human spermatozoa during capacitation: comparison of fertile and oligozoospermic sperm populations. Arch Androl 13(2–3):153–165
Buttke DE, Nelson JL, Schlegel PN, Hunnicutt GR, Travis AJ (2006) Visualization of GM1 with cholera toxin B in live epididymal versus ejaculated bull, mouse, and human spermatozoa. Biol Reprod 74(5):889–895
Cardullo RA, Florman HM (1993) Strategies and methods for evaluating acrosome reaction. Methods Enzymol 225:136
Carrell DT (2000) Semen analysis at the turn of the century: an evaluation of potential uses of new sperm function assays. Arch Androl 44(1):65–75
Chang MC (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168:697–698
Chang MC (1984) The meaning of sperm capacitation a historical perspective. J Androl 5(2):45–50
Chen WY, Ni Y, Pan YM, Shi QX, Yuan YY, Chen AJ, Mao LZ, Yu SQ, Roldan ER (2005) GABA, progesterone and zona pellucida activation of PLA2 and regulation by MEK‐ERK1/2 during acrosomal exocytosis in Guinea pig spermatozoa. FEBS Lett 579(21):4692–4700
Cheng CY and Mruk DD (2012) The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol Rev 64:16–64. doi: 10.1124/pr.110.002790
Chieffi P, Barchi M, Di Agostino S, Rossi P, Tramontano D, Geremia R (2003) Prolin-rich tyrosine kinase 2 (PYK2) expression and localization in mouse testis. Mol Reprod Dev 65(3):330–335
Coetzee K, Olmedo J, Lombard CJ (1994) Induced acrosome reactions as fertility predictor. J Assist Reprod Genet 11(9):470–473
Cormier N, Bailey JL (2003) A differential mechanism is involved during heparin-and cryopreservation-induced capacitation of bovine spermatozoa. Biol Reprod 69(1):177–185
Cotton L, Gibbs GM, Sanchez-Partida LG, Morrison JR, de Kretser DM, O'Bryan MK (2006) FGFR-1 signaling is involved in spermiogenesis and sperm capacitation. J Cell Sci 119(1):75–84
Cross NL (1998) Role of cholesterol in sperm capacitation. Biol Reprod 59(1):7–11
Cross NL, Meizel S (1989) Methods for evaluating the acrosomal status of mammalian sperm. Biol Reprod 41(4):635–641
Curry MR, Watson PF (1995) Sperm structure and function. Gametes—the spermatozoon. Cambridge University Press, Cambridge, pp 45–69
DasGupta S, O’Toole C, Mills CL, Fraser LR (1994) Fertilization and early embryology: effect of pentoxifylline and progesterone on human sperm capacitation and acrosomal exocytosis. Hum Reprod 9(11):2103–2109
Davis BK, Byrne R, Bedigian K (1980) Studies on the mechanism of capacitation: albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc Natl Acad Sci 77(3):1546–1550
De Jonge CJ, Barratt CL (2013) Methods for the assessment of sperm capacitation and acrosome reaction excluding the sperm penetration assay. Spermatogenesis: Methods and Protocols, 113–118.
Dow MP, Bavister BD (1989) Direct contact is required between serum albumin and hamster spermatozoa for capacitation in vitro. Gamete Res 23(2):171–180
Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA (2004) Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A 101(9):2993–2998
Esteves SC, Sharma RK, Gosálvez J, Agarwal A (2014) A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol 46(6):1037–1052
Etkovitz N, Tirosh Y, Chazan R, Jaldety Y, Daniel L, Rubinstein S, Breitbart H (2009) Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev Biol 334:447–457
Feng HL, Sandlow JI, Zheng LJ (2005) C‐kit receptor and its possible function in human spermatozoa. Mol Reprod Dev 70(1):103–110
FIVMadrid, http://fivmadrid.es/en/treatments-fivmadrid/semen-analysis-sperm-capacitation-test/, accessed on 11th September 2016
Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, Colenbrander B, Gadella BM (2001) Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci 114(19):3543–3555
Freour T, Jean M, Mirallie S, Langlois ML, Dubourdieu S, Barriere P (2009) Predictive value of CASA parameters in IUI with frozen donor sperm. Int J Androl 32(5):498–504
Fusi FM, Viganò P, Daverio R, Busacca M, Vignali M (1994) Effects of the coculture with human endometrial cells on the function of spermatozoa from subfertile men. Fertil Steril 61(1):160–167
Gadella BM, Harrison RA (2000) The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 127(11):2407–2420
Galantino-Homer HL, Visconti PE, Kopf GS (1997) Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3'5'-monophosphate-dependent pathway. Biol Reprod 56(3):707–719
Galantino‐Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS (2004) Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation‐associated protein tyrosine phosphorylation. Mol Reprod Dev 67(4):487–500
Gallardo Bolaños JM, Balao da Silva C, Martin Munoz P, Plaza Davila M, Ezquerra J, Aparicio IM, Tapia JA, Ortega Ferrusola C, Peña FJ (2014) Caspase activation, hydrogen peroxide production and Akt dephosphorylation occur during stallion sperm senescence. Reprod Domest Anim 49(4):657–664
Genetics & IVF Institute (2016). http://www.givf.com/fertility/maleinfertility.shtml. Accessed 11 Sept 2016
Glazier DB, Diamond SM, Marmar JL, Gibbs M, Corson SL (2000) A modified acrosome induction test. Arch Androl 44(1):59–64
Go KJ, Wolf DP (1985) Albumin-mediated changes in sperm sterol content during capacitation. Biol Reprod 32(1):145–153
Gomez MC, Catt JW, Gillan L, Evans G, Maxwell WM (1997) Effect of culture, incubation and acrosome reaction of fresh and frozen-thawed ram spermatozoa for in vitro fertilization and intracytoplasmic sperm injection. Reprod Fertil Dev 9(7):665–673
González-Fernández L, Macías-García B, Loux SC, Varner DD, Hinrichs K (2013) Focal adhesion kinases and calcium/calmodulin-dependent protein kinases regulate protein tyrosine phosphorylation in stallion sperm. Biol Reprod 88(6):138
Goupil S, La Salle S, Trasler JM, Bordeleau LJ and Leclerc P. (2011) Developmental expression of SRC-related tyrosine kinases in the mouse testis. J Androl 2011;32:95–110
Grasa P, Cebrián-Pérez JÁ, Muiño-Blanco T (2006) Signal transduction mechanisms involved in in vitro ram sperm capacitation. Reproduction 132(5):721–732
Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, Auer J, Serres C, Kahrizi K, Najmabadi H, Beckmann JS, Smith RJ (2010) Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet 18(11):1178–1184
Ho HC, Suarez SS (2001) Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 122:519–526
Ho HC, Granish KA, Suarez SS (2002) Hyperactivated motility of bull sperm is triggered at the axoneme by Ca 2+ and not cAMP. Dev Biol 250(1):208–217
Hunter T (2000) Signaling—2000 and beyond. Cell 100(1):113–127
Ickowicz D, Finkelstein M, Breitbart H (2012) Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl 14(6):816–821
Jaldety Y, Breitbart H (2015) ERK1/2 mediates sperm acrosome reaction through elevation of intracellular calcium concentration. Zygote 23(05):652–661
Jha KN, Kameshwari DB, Shivaji S (2003) Role of signaling pathways in regulating the capacitation of mammalian spermatozoa. Cell Mol Biol (Noisy-le-Grand) 49(3):329–340
Kalab P, Pěknicová J, Geussova G, Moos J (1998) Regulation of protein tyrosine phosphorylation in boar sperm through a cAMP‐dependent pathway. Mol Reprod Dev 51(3):304–314
Katoh Y, Takebayashi K, Kikuchi A, Iki A, Kikuchi K, Tamba M, Kawashima A, Matsuda M, Okamura N (2014) Porcine sperm capacitation involves tyrosine phosphorylation and activation of aldose reductase. Reproduction 148(4):389–401
Kholkute SD, Meherji P, Puri CP (1992) Capacitation and the acrosome reaction in sperm from men with various semen profiles monitored by a chlortetracycline fluorescence assay. Int J Androl 15(1):43–53
Kierszenbaum AL, Rivkin E, Talmor-Cohen A, Shalgi R, Tres LL (2009) Expression of full-length and truncated Fyn tyrosine kinase transcripts and encoded proteins during spermatogenesis and localization during acrosome biogenesis and fertilization. Mol Reprod Dev 76:832–843. doi:10.1002/mrd.21049
Kong LJ, Shao B, Wang GL, Dai TT, Xu L, Huang JY (2008) Capacitation-associated changes in protein-tyrosine-phosphorylation, hyperactivation and acrosome reaction in Guinea pig sperm. Asian Australas J Anim Sci 21(2):181
Kota V, Dhople VM, Shivaji S (2009) Tyrosine phosphoproteome of hamster spermatozoa: role of glycerol‐3‐phosphate dehydrogenase 2 in sperm capacitation. Proteomics 9(7):1809–1826
Krapf D, Ruan YC, Wertheimer EV, Battistone MA, Pawlak JB, Sanjay A, Pilder SH, Cuasnicu P, Breton S, Visconti PE (2012). cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev Biol 369:43–53. doi: 10.1016/j.ydbio.2012.06.017
Kulanand J, Shivaji S (2001) Capacitation associated changes in protein tyrosine phosphorylation, hyperactivation and acrosome reaction in hamster spermatozoa. Andrologia 33:95–104
Kumar P, Meizel S (2005) Nicotinic acetylcholine receptor subunits and associated proteins in human sperm. J Biol Chem 280:25928–25935
Kwon WS, Rahman MS, Pang MG (2014) Diagnosis and prognosis of male infertility in mammal: the focusing of tyrosine phosphorylation and phosphotyrosine proteins. J Proteome Res 13(11):4505–4517
Lalancette C, Faure RL, Leclerc P (2006) Identification of the proteins present in the bull sperm cytosolic fraction enriched in tyrosine kinase activity: a proteomic approach. Proteomics 6:4523–4540
Lamb DJ (2010) Semen analysis in 21st century medicine: the need for sperm function testing. Asian J Androl 12(1):64–70
Lamirande E, Gagnon C (1992) Reactive oxygen species and human spermatozoa. J Androl 13(5):379–386
Larsen L, Scheike T, Jensen TK, Bonde JP, Ernst E, Hjollund NH, Zhou Y, Skakkebæk NE, Giwercman A, Danish First Pregnancy Planner Study Team (2000) Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. Hum Reprod 15(7):1562–1567
Lawson C, Goupil S, Leclerc P (2008) Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod 79:657–666. doi: 10.1095/biolreprod.108.070367
Layek SS, Mohanty TK, Kumaresan A, Parks JE (2016) Cryopreservation of bull semen: evolution from egg yolk based to soybean based extenders. Anim Reprod Sci 172:1–9
Leahy T, Gadella BM (2011) Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 142(6):759–778
Leclerc P, de Lamirande E, Gagnon C (1996) Cyclic adenosine 3', 5'monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod 55(3):684–692
Lee LK, Foo KY (2014) Recent insights on the significance of transcriptomic and metabolomic analysis of male factor infertility. Clin Biochem 47(10):973–982
Lefièvre L, Jha KN, Lamirande E, Visconti PE, Gagnon C (2002) Activation of protein kinase a during human sperm capacitation and acrosome reaction. J Androl 23(5):709–716
Lefièvre L, Bedu-Addo K, Conner SJ, Machado-Oliveira GS, Chen Y, Kirkman-Brown JC, Afnan MA, Publicover SJ, Ford WCL, Barratt CL (2007) Counting sperm does not add up any more: time for a new equation? Reproduction 133(4):675–684
Lewis SE (2007) Is sperm evaluation useful in predicting human fertility? Reproduction 134(1):31–40
Leyton L, Saling P (1989) 95 kd sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell 57(7):1123–1130
Liu DY, Clarke GN, Baker HWG (2006) Tyrosine phosphorylation on capacitated human sperm tail detected by immunofluorescence correlates strongly with sperm–zona pellucida (ZP) binding but not with the ZP-induced acrosome reaction. Hum Reprod 21(4):1002–1008
Liu DY, Liu ML, Clarke GN, Baker HWG (2007) Hyperactivation of capacitated human sperm correlates with the zona pellucida-induced acrosome reaction of zona pellucida-bound sperm. Hum Reprod 22(10):2632–2638
Luna C, Colás C, Pérez-Pé R, Cebrián-Pérez JA, Muiño-Blanco T (2012) A novel epidermal growth factor-dependent extracellular signal-regulated MAP kinase cascade involved in sperm functionality in sheep. Biol Reprod 87(4):93
Luo J, Gupta V, Kern B, Tash JS, Sanchez G, Blanco G, Kinsey WH (2012) Role of FYN kinase in spermatogenesis: defects characteristic of Fynnull sperm in mice. Biol Reprod 86:1–8. doi: 10.1095/biolreprod.111.093864
Mahony MC, Gwathmey T (1999) Protein tyrosine phosphorylation during hyperactivated motility of cynomolgus monkey (Macaca fascicularis) spermatozoa. Biol Reprod 60:1239–1243
Makkar G, Ng EHY, Yeung WSB, Ho PC (2003) The significance of the ionophore‐challenged acrosome reaction in the prediction of successful outcome of controlled ovarian stimulation and intrauterine insemination. Hum Reprod 18(3):534–539
Martínez P, Morros A (1996) Membrane lipid dynamics during human sperm capacitation. Front Biosci 1:d103–d117
Matzuk MM, Lamb DJ (2002) Genetic dissection of mammalian fertility pathways. Translocations 45:46XY
Meerschaut FV, Leybaert L, Nikiforaki D, Qian C, Heindryckx B, De Sutter P (2013) Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum Reprod 28(1):87–98
Meizel S, Turner KO (1991) Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol 77(1–3):R1–R5
Mendeluk GR, Sardi-Segovia LM, Chenlo PH, Pugliese MN, Repetto H, Curi S, Ariagno J, Prentki Santos E, Paez P, Passanante EG, Palaoro LA (2010) Assessment of human sperm protein tyrosine phosphorylation by immunocytochemistry in a clinical andrology laboratory. Preliminary data. Biotech Histochem 84(6):321–328
Mitchell LA, Nixon B, Baker MA, Aitken RJ (2008) Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod 14(4):235–243
Mitra K, Shivaji S (2004) Novel tyrosine-phosphorylated post-pyruvate metabolic enzyme, dihydrolipoamide dehydrogenase, involved in capacitation of hamster spermatozoa. Biol Reprod 70(4):887–899
Mitra K, Shivaji S (2005) Proteins implicated in sperm capacitation. Indian J Exp Biol 43(11):1001
Mortimer ST, Swan MA (1995) Variable kinematics of capacitating human spermatozoa. Hum Reprod 10:3178–3182
Morton B, Harrigan-Lum J, Albagli L, Jooss T (1974) The activation of motility in quiescent hamster sperm from the epididymis by calcium and cyclic nucleotides. Biochem Biophys Res Commun 56(2):372–379
Muller CH (2000) Rationale, interpretation, andrology lab corner validation, and uses of sperm function tests. J Androl 21(1):10–30
Munire M, Shimizu Y, Sakata Y, Minaguchi R, Aso T (2004) Impaired hyperactivation of human sperm in patients with infertility. J Med Dent Sci 51(1):99–104
Muratori M, Marchiani S, Tamburrino L, Forti G, Luconi M, Baldi E (2011) Markers of human sperm functions in the ICSI era. Front Biosci 16:1344–1363
Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, El Jamali A (2012) NOX5 in human spermatozoa expression, function, and regulation. J Biol Chem 287(12):9376–9388
Nandi A, Homburg R (2016) Unexplained subfertility: diagnosis and management. Obstetrician Gynaecologist 18(2):107–115
Natali A, Turek PJ (2011) An assessment of new sperm tests for male infertility. Urology 77(5):1027–1034
Naz RK, Rajesh PB (2004). Role of tyrosine phosphorylation in sperm capacitation / acrosome reaction. Reprod Biol Endocrinol 2:75
Nixon B, Bielanowicz A, Anderson AL, Walsh A, Hall T, Mccloghry A, Aitken RJ (2010) Elucidation of the signaling pathways that underpin capacitation‐associated surface phosphotyrosine expression in mouse spermatozoa. J Cell Physiol 224(1):71–83
O’Flaherty C, de Lamirande E, Gagnon C (2006) Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med 40(6):1045–1055
Oehninger S (1995) An update on the laboratory assessment of male fertility. Hum Reprod 10(suppl 1):38–45
Oehninger S, Franken DR, Ombelet W (2014) Sperm functional tests. Fertil Steril 102(6):1528–1533
Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS (1999) Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod 5(11):1017–1026
Panneerdoss S, Siva AB, Kameshwari DB, Rangaraj N, Shivaji S (2012) Association of lactate, intracellular pH, and intracellular calcium during capacitation and acrosome reaction: contribution of hamster sperm dihydrolipoamide dehydrogenase, the E3 subunit of pyruvate dehydrogenase complex. J Androl 33(4):699–710
Parinaud J, Vieitez G, Moutaffian H, Richoilley G, Labal B (1995) Andrology: variations in spontaneous and induced acrosome reaction: correlations with semen parameters and in-vitro fertilization results. Hum Reprod 10(8):2085–2089
Pawson T (2004) Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116(2):191–203
Poma Fertility (2016). www.pomafertility.com/patients/infertility/acrosome-reaction-test-ar/. Accessed 11 Sept 2016
Pommer AC, Rutllant J, Meyers SA (2003) Phosphorylation of protein tyrosine residues in fresh and cryopreserved stallion spermatozoa under capacitating conditions. Biol Reprod 68(4):1208–1214
Pukazhenthi BS, Long JA, Wildt DE, Ottinger MA, Armstrong DL, Howard J. (1998) Regulation of sperm function by protein tyrosine phosphorylation in diverse wild felid species. J Androl 19:675–685
Roa-Espitia AL, Hernández-Rendón ER, Baltiérrez-Hoyos R, Muñoz-Gotera RJ, Cote-Vélez A, Jiménez I, González-Márquez H, Hernández-González EO (2016) Focal adhesion kinase is required for actin polymerization and remodeling of the cytoskeleton during sperm capacitation. Biol Open 5(9):1189–1199
Rotfeld H, Hillman P, Ickowicz D, Breitbart H (2012) PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction 147(3):347–356
Sagare-Patil V, Vernekar M, Galvankar M, Modi D (2013) Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol Cell Endocrinol 374:82–91
Salvolini E, Buldreghini E, Lucarini G, Vignini A, Lenzi A, Di Primio R, Balercia G (2013) Involvement of sperm plasma membrane and cytoskeletal proteins in human male infertility. Fertil Steril 99(3):697–704
Sati L, Cayli S, Delpiano E, Sakkas D, Huszar G (2014) The pattern of tyrosine phosphorylation in human sperm in response to binding to zona pellucida or hyaluronic acid. Reprod Sci 21(5):573–581
Selvaraj V, Buttke DE, Asano A, McElwee JL, Wolff CA, Nelson JL, Klaus AV, Hunnicutt GR, Travis AJ (2007) GM1 dynamics as a marker for membrane changes associated with the process of capacitation in murine and bovine spermatozoa. J Androl 28(4):588–599
Setti AS, Braga DPAF, Figueira RCS, Iaconelli A Jr, Borges E Jr (2012) The predictive value of high-magnification sperm morphology examination on ICSI outcomes in the presence of oocyte dysmorphisms. J Assist Reprod Genet 29(11):1241–1247
Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD, Overstreet JW (2002) Best practice policies for male infertility. Fertil Steril 77(5):873–882
Sheriff DS, Ali EF (2010) Perspective on plasma membrane cholesterol efflux and spermatozoal function. J Hum Reprod Sci 3(2):68
Shimizu Y, Nord EP, Bronson RA (1993) Progesterone-evoked increases in sperm [Ca 2+] i correlate with the egg penetrating ability of sperm from fertile but not infertile men. Fertil Steril 60(3):526–532
Shivaji S, Scheit KH, Bhargava PM (1990) Proteins of seminal plasma. Wiley
Shivaji S, Peedicayil J, Devi LG (1995) Analysis of the motility parameters of in vitro hyperactivated hamster spermatozoa. Mol Reprod Dev 42(2):233–247
Shivaji S, Kumar V, Mitra K, Jha KN (2007) Mammalian sperm capacitation: role of phosphotyrosine proteins. Soc Reprod Fertil Suppl 63:295–312
Shivaji S, Kota V, Siva AB (2009) The role of mitochondrial proteins in sperm capacitation. J Reprod Immunol 83(1):14–18
Sidhu KS, Mate KE, Gunasekera T, Veal D, Hetherington L, Baker MA, Aitken RJ, Rodger JC (2004) A flow cytometric assay for global estimation of tyrosine phosphorylation associated with capacitation of spermatozoa from two marsupial species, the tammar wallaby (Macropus eugenii) and the brushtail possum (Trichosurus vulpecula). Reproduction 127(1):95–103
Signorelli J, Diaz ES, Morales P (2012) Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res 349(3):765–782
Singh DK, Deshmukh RK, Narayanan PK, Shivaji S, Siva AB (2017) SRC family kinases in hamster spermatozoa: Evidence for the presence of LCK. Reproduction. Accepted
Singh VK, Kumar R, Atreja SK (2014) Cryo-survival, cryo-capacitation and oxidative stress assessment of buffalo spermatozoa cryopreserved in new soya milk extender. Livest Sci 160:214–218
Suarez SS (2008) Control of hyperactivation in sperm. Hum Reprod Update 14(6):647–657
Suarez SS, Dai X (1992) Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol Reprod 46(4):686–691
Suarez SS, Katz DF, Owen DH, Andrew JB, Powell RL (1991) Evidence for the function of hyperactivated motility in sperm. Biol Reprod 44(2):375–381
Tardif S, Dubé C, Chevalier S, Bailey JL (2001) Capacitation is associated with tyrosine phosphorylation and tyrosine kinase-like activity of pig sperm proteins. Biol Reprod 65(3):784–792
Tardif S, Dubé C, Bailey JL (2003) Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol Reprod 68(1):207–213
Tavalaee M, Deemeh MR, Arbabian M, Kiyani A, Nasr‐Esfahani MH (2014) Relationship between fertilization rate and early apoptosis in sperm population of infertile individuals. Andrologia 46(1):36–41
The Male Fertility Lab University of Washington (2016). http://faculty.washington.edu/cmuller/MFL/Sperm_Function_Tests.html. Accessed 11 Sept 2016
Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A (1991) Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989). Hum Reprod 6(6):811–816
Tosti E, Ménézo Y (2016) Gamete activation: basic knowledge and clinical applications. Hum Reprod Update 22(4):420–439
Travis AJ, Kopf GS (2002) The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest 110(6):731–736
Tucker MJ, Leong MKH, Leung CKM, Wong CJY, Chan HHY (1987) Is delayed capacitation a complicating factor in the treatment of idiopathic infertility by intrauterine insemination? J Assist Reprod Genet 4(4):245–247
University of Utah Hospitals & Clinics (2016). http://healthcare.utah.edu/andrology/tests.php. Accessed 11 Sept 2016
Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M (2008) Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod 23(12):2652–2662
Vasan SS (2011) Semen analysis and sperm function tests: how much to test? Indian J Urol (IJU) J Urol Soc India 27(1):41
Villemure M, Lazure C, Manjunath P (2003) Isolation and characterization of gelatin-binding proteins from goat seminal plasma. Reprod Biol Endocrinol 1(1):1
Visconti PE, Kopf GS (1998) Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 59(1):1–6
Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS (1995) Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121(4):1129–1137
Visconti PE, Galantino‐Homer HANNAH, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS (1998) The molecular basis of sperm capacitation. J Androl 19(2):242–248
Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf GS, Tezón JG (1999) Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod 61(1):76–84
Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB (2002) Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 53(1):133–150
Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A (2011) Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 13(3):395–405
Vizel R, Hillman P, Ickowicz D, Breitbart H (2015) AKAP3 degradation in sperm capacitation is regulated by its tyrosine phosphorylation. Biochimica et Biophysica Acta (BBA)-General Subjects 1850(9):1912–1920
Vural B, Sofuoglu K, Caliskan E, Delikara N, Aksoy E, Uslu H, Karan A (2005) Predictors of intracytoplasmic sperm injection (ICSI) outcome in couples with and without male factor infertility. Clin Exp Obstet Gynecol 32(3):158–162
Wang C, Leung A, Tsoi WL, Leung J, Ng V, Lee KF, Chan SY (1991) Evaluation of human sperm hyperactivated motility and its relationship with the zona‐free hamster oocyte sperm penetration assay. J Androl 12(4):253–257
Wang J, Qi L, Huang S, Zhou T, Guo Y, Wang G, Guo X, Zhou Z, Sha J (2015) Quantitative phosphoproteomics analysis reveals a key role of insulin growth factor 1 receptor (IGF1R) tyrosine kinase in human sperm capacitation. Mol Cell Proteomics 14(4):1104–1112
Ward CR, Storey BT (1984) Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol 104(2):287–296
Weber RF, Dohle GR, Romijn JC (2005) Clinical laboratory evaluation of male subfertility. Adv Clin Chem 40:317–364
Wiser A, Sachar S, Ghetler Y, Shulman A, Breitbart H (2014) Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: preliminary results. Andrologia 46(3):313–315
World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen. World Health Organization, Geneva
Yanagimachi R (1969) In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil 18(2):275–286
Yanagimachi R (1994) Mammalian fertilization. Physiol Reprod 1:189–317
Youn JS, Cha SH, Park CW, Yang KM, Kim JY, Koong MK, Kang IS, Song IO, Han SC (2011) Predictive value of sperm motility characteristics assessed by computer-assisted sperm analysis in intrauterine insemination with superovulation in couples with unexplained infertility. Clin Exp Reprod Med 38(1):47–52
Yovich JM, Edirisinghe WR, Yovich JL (1994) Use of the acrosome reaction to ionophore challenge test in managing patients in an assisted reproduction program: a prospective, double-blind, randomized controlled study. Fertil Steril 61(5):902–910
Yunes R, Doncel GF, Acosta AA (2003) Incidence of sperm-tail tyrosine phosphorylation and hyperactivated motility in normozoospermic and asthenozoospermic human sperm samples. Biocell-Mendoza 27(1):29–36
Zaneveld LJD, De Jonge CJ, Anderson RA, Mack SR (1991) Human sperm capacitation and the acrosome reaction. Hum Reprod 6(9):1265–1274
Acknowledgements
The author is in receipt of grant from CSIR (XIIth FYP grant “PROGRAM”) for research on molecular basis of sperm capacitation and gratefully acknowledges the support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Deshmukh, R.K., Siva, A.B. (2017). Sperm Capacitation: The Obligate Requirement for Male Fertility. In: SINGH, R., Singh, K. (eds) Male Infertility: Understanding, Causes and Treatment. Springer, Singapore. https://doi.org/10.1007/978-981-10-4017-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-4017-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4016-0
Online ISBN: 978-981-10-4017-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)