Abstract
Neutropenia is defined as a decrease in the number of circulating neutrophils in the peripheral blood with absolute neutrophil count less than 1000–1500/μL. Chronic neutropenia in pediatric patients is divided into three groups. Extrinsic factors, such as antibodies, some drugs, and nutritional deficiencies, lead to excessive destruction of neutrophils. Autoimmune neutropenia is a benign form of neutropenia shown in infancy to early childhood. Spontaneous recovery of neutropenia usually occurs within a few months to a few years. Acquired disorders of myeloid and stem cells present hypoplasia of myeloid cells. Congenital neutropenia is intrinsic defects in granulocytes or their progenitors and includes a heterogenous group of disorders. More than ten responsible gene mutations have been identified in congenital neutropenia. Most common congenital neutropenia is due to the gene mutation of neutrophil elastase. The hallmark of profound neutropenia is increased susceptibility to bacterial infections, cutaneous cellulitis, deep tissue abscesses, pneumonia, and septicemia. Almost patients with congenital neutropenia have been responded to administration of G-CSF. However, long-term use of G-CSF has the risk of the development of MDS/AML, suggesting the necessity of the careful follow-up. Hematopoietic stem cell transplantation should be considered for the curable treatment in severe congenital neutropenia.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Hematopoietic Stem Cell Transplantation
- Absolute Neutrophil Count
- Neutrophil Elastase
- Paroxysmal Nocturnal Hemoglobinuria
- Intrinsic Disorder
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Definition and Classification
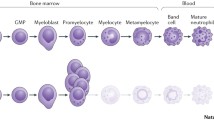
Neutropenia is defined as a decrease in the number of circulating neutrophils in the peripheral blood. Normal neutrophil levels should be varied for age, race, and other factors. Neutrophils predominate at birth, but gradually decrease in the first few days of life. During infancy, neutrophils constitute of 20–30% of circulating white blood cells (WBC). Near same level of neutrophils and lymphocyte is found in the peripheral blood at the age of 5–6 years. Approximately 60–70% of neutrophils are observed in the circulating WBC during adolescent. The lower limit for normal absolute neutrophil counts (ANC) is 1000/μL in infants between 2 weeks and 1 year of age and that is 1500/μL for children older than 1 year of age. Neutropenia can be transient or chronic that persists beyond 6 months.
Neutropenia in pediatric patients is classified into three causes, neutropenia caused by extrinsic to marrow myeloid cells, acquired disorders of myeloid and stem cells, and intrinsic disorders of proliferation and differentiation of myeloid and stem cells. The former causes include infections; drug-induced, immune neutron, and reticuloendothelial sequestration; bone marrow replacement; cancer chemotherapy; or radiation therapy to bone marrow.
The second causes consist of aplastic anemia, vitamin B12 or folate deficiency, acute and chronic leukemia, myelodysplasia, paroxysmal nocturnal hemoglobinuria, and chronic idiopathic neutropenia. The third causes are intrinsic disorders presented in Table 5.1 [1, 2].
2 Clinical Manifestations of Neutropenia
The hallmark of neutropenia is increased susceptibility to bacterial infections. The most types of pyogenic infections observed in patients with neutropenia are cutaneous cellulitis, superficial or deep cutaneous abscesses, lymphadenopathy, upper and/or lower respiratory tract infections, and septicemia. Stomatitis, gingivitis, and periodontitis are often chronic problems, resulting in the loss of teeth. Perianal and perirectal inflammation and otitis media occur as well. Susceptibility to bacterial infection even in patients with severe neutropenia can be quite variable, depending on the underlying pathophysiology.
The representative disorders presenting neutropenia in infancy and childhood are described as follows.
3 Neutropenia Caused by Intrinsic Defects of Neutrophils or Their Progenitors (Congenital Neutropenia)
The current classification of congenital neutropenia is listed in Table 5.1 with the modification of the proposal of International Union of Immunological Societies Expert Committee presented in 2015 [2]. Severe congenital neutropenia (SCN) includes a heterogenous group of disorders with different responsible gene mutations and is divided into five disorders, SCN1–5. SCN1 is autosomal dominant or sporadic patterns of inheritance, and in this group of patients, most of them (60–80%) have diverse mutations in the neutrophil elastase gene (ELANE) [3, 4]. SCN3 is known as Kostmann disease following recessive pattern of inheritance. Its underlying genetic defect is due to homozygous or compound heterozygous mutations in the HAX1 gene. HAX1 mutations may be associated with neurologic deficits [5, 6]. Most patients with SCN have experienced frequent episodes of bacterial infections. Prior to the development of granulocyte colony-stimulating factor (G-CSF), patients died of infections and/or their complications. The administration of G-CSF succeeds in the increase of ANC and the decrease of infectious episodes in more than 95% of SCN patients. However, patients with SCN requiring the long-term use of high-dose G-CSF are at risk for developing myelodysplasia/acute myelogenous leukemia [7]. Hematopoietic stem cell transplantation should be considered for the curable treatment in SCN patients.
Cyclic neutropenia is a rare autosomal dominant disorder and presents in infancy or childhood. The disorder is characterized by regular, periodic oscillation, with the ANC ranging from normal to less than 200/μL, mirrored by reciprocal cycling of monocytes. The mean oscillatory period of the cycle is 21 days. During the neutropenic period, almost all patients suffer from malaise, fever, stomatitis, gingivitis, periodontitis, or pharyngitis, lymph node enlargement, and occasionally pneumonia. Gene sequencing showed mutations in the gene for ELANE, similar to SCN1 [3, 4]. It has been speculated that neutropenia results from activation of apoptotic pathway by mutant forms of ELANE. Approximately 5–10% of patients developed fatal overwhelming infection before the availability of G-CSF.
4 Increased Destruction of Neutrophils (Acquired Neutropenia)
Immune-mediated neutropenia is usually associated with the presence of antineutrophil antibodies, resulting in the excessive destruction of neutrophils.
Alloimmune neonatal neutropenia occurs after transplacental transfer of maternal alloantibodies. Maternal antibodies are produced by the incompatibility of neutrophil antigens between mother and babies, similar mechanism of Rh hemolytic disease. Infants may be asymptomatic or they may have infections, such as pyoderma, omphalitis, and pneumonia. Neutropenia usually resolves by 3 months of age with the disappearance of circulating antibodies. Treatment consists of supportive care and the administration of appropriate antibiotics with or without G-CSF.
Primary autoimmune neutropenia (AIN) is a benign form of neutropenia shown in infancy to early childhood. Patients usually have moderate to severe neutropenia with ANC < 500/μL. The median age of presentation is 8 to 10 months ranging 3–30 months. Approximately 90% of children show benign infections, such as pyoderma, otitis media, lymphadenopathy, and upper and lower respiratory tract infections with no life-threatening and responsive-to-standard antibiotics. The ANC varies from 0 to 500/μL, and monocytosis is common. The bone marrow picture reveals the myeloid hyperplasia with marked reduction of segmented neutrons due to their destruction by antibodies. Antineutrophil antibodies are often detected in serum by immunofluorescence test using flow cytometry [8]. However, the test occasionally has false-negative or false-positive results. The careful diagnosis should be necessary with seeing patients’ clinical course. Spontaneous recovery of neutron usually occurs within a few months to a few years. The median age at recovery is 30 months ranging 7–72 months. The specific treatment is not generally necessary because severe infection is rare. The administration of appropriate antibiotics with or without low-dose G-CSF is recommended in severe infections. Prophylactic use of sulfamethoxazole-trimethoprim is useful for patients suffering from frequent infections and/or recurrence of otitis media [9].
References
Walkovich KJ, Newburger PE. Leukopenia. In: Kliegman RM, Stanton BF, Schor NF, St. Games III JW, Behrman RE, editors. Nelson textbook of pediatrics. 20th edn. Philadelphia: Elsevier; 2016. p. 1047–53.
Picard C, Al-Herz W, Bousfiha A, et al. Primary immunodeficiency diseases: an update on the classification from the international Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35:696–726.
Horwitz M, Benson KF, Person RE, et al. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Genet. 1999;23:433–6.
Dale DC, Person RE, Bolyard AA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96:2317–22.
Klein C, Grudzien M, Appaswamy G, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet. 2007;39:86–92.
Ishikawa N, Okada S, Miki M, et al. Neurodevelopmental abnormalities associated with severe congenital neutropenia due to the R86X mutation in the HA1 gene. J Med Genet. 2008;45:802–7.
Rosenberg PS, Alter BP, Bolyard AA, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628–35.
Kobayashi M, Nakamura K, Kawaguchi H, et al. Significance of the detection of antineutrophil antibodies in children with chronic neutropenia. Blood. 2002;99:4366–9.
Kobayashi M, Sato T, Kawaguchi H, et al. Efficacy of prophylactic use of trimethoprim-sulfamethoxazole in autoimmune neutron in infancy. J Pediatr Hematol Oncol. 2003;25:553–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kobayashi, M., Mizoguchi, Y., Karakawa, S., Okada, S., Kawaguchi, H. (2017). Neutropenia (In Infancy and Childhood). In: Ishii, E. (eds) Hematological Disorders in Children. Springer, Singapore. https://doi.org/10.1007/978-981-10-3886-0_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-3886-0_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3885-3
Online ISBN: 978-981-10-3886-0
eBook Packages: MedicineMedicine (R0)