Abstract
Over the past two decades, the stem cell field has developed methods to differentiate human pluripotent stem cells (hPSCs) into specific tissue types. These studies have been largely driven by developmental biologists who have identified pathways and tissue-specific markers that can be used to direct the differentiation of hPSCs. Furthermore, the identification of Lgr5+ adult stem cells in the mouse small intestine led to the development of protocols to grow these stem cells into self-organizing, self-renewing, multicellular “organoids.” PSC-derived and adult organoids derived from human samples now allow researchers to study cell lineage processes and model complex cell-cell interactions. Both PSC-derived gastrointestinal organoids and those generated from freshly excised tissue have many properties of gastrointestinal physiology. However, PSC-derived gastrointestinal organoids are generated through a stepwise differentiation that largely mimics gastrointestinal development and is therefore a good system to study congenital defects of the human GI tract. Moreover, PSC-derived organoids are complex and contain mesodermal cell types comprising smooth muscle and subepithelial myofibroblasts. Lastly, developmentally inspired approaches have been used to tissue engineer human PSC-derived organoids with a functional enteric nervous system. In this chapter we describe the development of both the intestine and stomach. We then describe how pathways identified by developmental studies can be used to direct the differentiation of pluripotent stem cells into human intestinal organoids (HIOs) and human gastric organoids (HGOs). In addition, we discuss potential applications of these systems for studying human gastrointestinal development and disease and in engineering GI tissues for eventual transplantation-based therapies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Definitive endoderm
- Foregut
- Midgut
- Hindgut
- Human pluripotent stem cells
- Gastric organoids
- Intestinal organoids
10.1 Establishment of Endoderm and Patterning into Foregut, Midgut, and Hindgut Domains
The definitive endoderm, one of the primary germ layers that are established during gastrulation, gives rise to the epithelium that lines the entire gastrointestinal tract (Spence et al. (2011a). Wnt and Nodal signaling are required at various stages of gastrulation. Wnt signaling is required for gastrulation, and loss of beta-catenin, a key downstream effector of canonical Wnt signaling, results in embryos that fail to gastrulate (Haegel et al. 1995). Wnt signaling is also required for the maintenance of definitive endoderm, and knocking out beta-catenin in embryonic endoderm (including DE) results in its conversion to pre-cardiac mesoderm (Lickert et al. 2002). Mouse Nodal mutants fail to form or maintain the primitive streak and fail to form the definitive endoderm (Brennan et al. 2001).
The definitive endoderm is first present as a flat sheet of cells, which through the process of gut tube morphogenesis transforms into a primitive gut tube (Fig. 10.1a). Formation of the gut tube is initiated at the anterior and posterior ends of the embryo, where the endoderm folds over forming the foregut and hindgut. The folding of the foregut and hindgut tube continues until they meet in the midgut, forming a primitive gut tube. Establishing the molecular patterns in the endoderm happens throughout these stages of development and is governed by four major signaling pathways, Wnt, Fgf, retinoic acid (RA), and Bmp. In Xenopus and mouse embryos, Wnt/β-catenin signaling promotes posterior gene expression in endoderm while at the same time inhibiting anterior gene expression in the posterior (McLin et al. 2007; Sherwood et al. 2011; Zorn and Wells 2009). Bmp, Fgf, and RA all have been reported to promote posterior pattern of endoderm between the late gastrula and early somite stages of embryonic development in several vertebrate species (Bayha et al. 2009; Dessimoz et al. 2006; Huang et al. 1998; Kumar et al. 2003; Niederreither et al. 2003; Rankin et al. 2011; Roberts et al. 1995; Stafford and Prince 2002; Tiso et al. 2002; Wang et al. 2006; Wells and Melton 2000). However, it has been difficult to identify how these pathways act in concert to pattern the endoderm in the context of the embryo. This requires temporal and spatial manipulation of multiple signaling pathways.
The foregut gives rise to the esophagus, trachea, stomach, lungs, thyroid, liver, biliary system, and pancreas. The midgut gives rise to the small intestine, and the hindgut gives rise to the large intestine. The early regionalization of the gastrointestinal tract into foregut and midgut/hindgut domains can be observed by the region-specific expression of transcription factors (San Roman and Shivdasani 2011). At early somite stages, Sox2 is expressed in the foregut, while Cdx2 is expressed in the midgut/hindgut. At slightly later stages, a third marker, Pdx1, further delineates presumptive organ boundaries into Sox2+ anterior foregut, Sox2+/Pdx1+ posterior foregut (antrum), Pdx1+/Cdx2+ anterior intestine (duodenum), and Cdx2+ small and large intestine. Although Sox2 and Cdx2 expression domains are thought to mark foregut and midgut/hindgut, respectively, it should be noted that (1) Sox2+/Cdx2+ cells are present in e8.75–9.25 embryos (Sherwood et al. 2009a).
10.2 Intestinal Development
The intestine is derived from the midgut/hindgut tissue which expresses the homeobox transcription factor Cdx2 as early as e7.5 (Beck et al. 1995; Sherwood et al. 2009b, 2011). Cdx2 is critical for intestinal development, and when Cdx2 is conditionally knocked out in the endoderm using Foxa3-Cre, animals fail to form a colon, and the posterior small intestine is converted into stratified squamous tissue which molecularly and morphologically resembles the esophagus (Gao et al. 2009). The pathways which specify the midgut and hindgut from the common Cdx2 intestinal domain remain to be determined although studies from model organisms suggest that Wnt and Bmp signaling are involved (Kumar et al. 2003; Roberts et al. 1995; Sherwood et al. 2011; Tiso et al. 2002; Wills et al. 2008). Following patterning into midgut and hindgut domains, from e9.5 to e13.5, the intestine increases in length and circumference and undergoes a series of morphological transitions (Fig. 10.1a). At e12.5 the intestine displays a pseudostratified morphology which transitions to a simple cuboidal epithelium (Spence et al. 2011a; Wells and Spence 2014). At e14.5 the intestinal tract undergoes cytodifferentiation and villus formation through coordinated formation of mesenchymal clusters marked by Pdgfrα (Shyer et al. 2015; Walton et al. 2012, 2016). The process of villi formation is dependent on BMP and Sonic Hedgehog signaling as clusters of Pdgfrα + mesenchymal cells form at sites of high activity of these pathways. Villus morphogenesis leads to the compartmentalization of progenitor domains to the intervillous regions, a process which requires Shh and Bmp signaling (Shyer et al. 2015; Walton et al. 2012, 2016). Following cytodifferentiation, goblet cells and enteroendocrine cells can be detected in the small intestine and colon, while Paneth cells are not detected in the small intestine until after birth (Fordham et al. 2013; Mustata et al. 2013). The progenitors of the intervillous regions are dependent on the Wnt effector TCF4, and mice deficient in this transcription factor display loss progenitors by birth (Korinek et al. 1998). Although most developmental studies have focused on small intestinal development, development of the colon involves similar transitions with the exception of villus formation. At birth, the small intestine is comprised of villi with immature crypts, while the colon is comprised of a flat epithelium with immature crypts.
During the first 2 weeks after birth in mice, the intervillous regions of the small intestine and the base of crypts in the colon begin to invade the submucosa to establish the adult stem cell domain. At 2 weeks Paneth cells, which support the intestinal stem cells that will sustain self-renewal of the small intestine throughout the lifetime of the animal, are present in the base of the crypts (Sato et al. 2011b). Unlike the small intestine which contains Paneth cells, the colon and its stem cell niche are dependent on other cell types including cKit + goblet cells and Reg4+ cells deep crypt secretory cells (Rothenberg et al. 2012; Sasaki et al. 2016). In contrast to mice, humans are born with crypts (Montgomery et al. 1999) and Paneth cells, although colonization by microbes helps to mature Paneth cells. In humans, intestinal maturation occurs in utero such that human enterocytes at mid-gestation resemble those of suckling rodents. At 22 weeks the human intestine resembles that of fully weaned rodents (Montgomery et al. 1999). The developmental processes outlined above culminate in the development of the most proliferative organ in the body which undergoes self-renewal every 4–5 days in mice and every week in humans.
10.3 Generation of Three-Dimensional Intestinal Organoids from Human Pluripotent Stem Cells
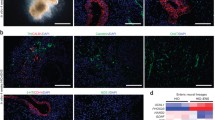
In the past few years, the identification of adult intestinal stem cell markers and the ability to isolate and culture these cells as organoids have led to significant advances in our understanding of intestinal regeneration and gastrointestinal cancer (Barker et al. 2007, 2009; Snippert et al. 2010) (Jung et al. 2011; Sato et al. 2009, 2011a, b), but they are not well suited to studies of developmental processes. However, a separate approach was developed that directed the differentiation of human pluripotent stem cells into human intestinal organoids (HIOs) using a process that recapitulates intestinal development. In this approach the Nodal mimetic Activin A is used to induce differentiation of pluripotent stem cells into definitive endoderm (D’Amour et al. 2005). Subsequent exposure of definitive endoderm to high levels of WNT3A and FGF4 induces patterning and morphogenesis into midgut/hindgut spheroids that express CDX2. Once formed, these midgut/hindgut spheroids can be grown in three-dimensional culture under conditions that favor intestinal growth giving rise to HIOs (Sato et al. 2009; Spence et al. 2011b). Moreover, growth of these spheroids closely recapitulates developmental events that occur in vivo. Midgut/hindgut spheroids transition from a simple cuboidal epithelium into a pseudostratified epithelium, which then undergoes cytodifferentiation and transitions into a polarized epithelium which contains zones of differentiation and proliferation. When compared to developing mouse intestine, these intestinal organoid cultures undergo strikingly similar transitions (Spence et al. 2011a) and contain all of the cell types found in the fetal gut (Fig. 10.1b). Furthermore, HIOs contain discreet epithelial domains that express the stem cell markers LGR5 and ASCL2, suggesting that early stages of intestinal stem cell development occur normally in organoid cultures.
Pluripotent stem cell-derived intestinal organoids also have another unique characteristic which is the presence of mesenchyme. Addition of FGF4 during the midgut/hindgut morphogenesis process allows the expansion of Brachyury-expressing Cdx2+ mesenchymal cells that codevelop with the intestinal epithelium. Follow-up studies also revealed that mesenchyme containing HIOs grow in the absence of Noggin and R-spondin, factors that are necessary for the maintenance of epithelial organoids (Watson et al. 2014). This suggests that the mesenchyme present in the HIOs provides endogenous factors that promote growth and maintenance of the epithelium. Conversely, the mesenchymal cells grow in the absence of exogenous factors which could promote the growth of these cells such as FGF9 and SHH. This suggests that the epithelium is capable of generating factors which support the growth of the mesenchyme. How this epithelial-mesenchymal cross talk drives growth and maturation of HIOs remains an open question.
It should be noted that human intestinal organoids can be generated through 3D cultures of aggregated 2D midgut/hindgut endoderm derived from PSCs (Fordham et al. 2013; Tamminen et al. 2015). Exposure of definitive endoderm to the GSK3B inhibitor CHIR99021, unlike WNT3A, allows generation of CDX2-positive endoderm in the absence of FGF4. These culture aggregates undergo self-organization and form organoids similar to those generated from induced morphogenesis. This suggests that once patterned, midgut/hindgut tissue retains the capacity to self-organize in the presence of correct environmental cues such as the extracellular matrix components and growth factors present in Matrigel.
10.4 Gastric Development
After patterning of the foregut and midgut/hindgut into distinct Sox2+ and Cdx2+ domains, respectively, the foregut domain is further patterned into anterior and posterior domains, the latter of which will give rise to the stomach (Zorn and Wells 2009). The stomach is then further subdivided into the proximal stomach that will give rise to the forestomach and corpus and the distal stomach which will give rise to the antrum which abuts the duodenum. Little is known about the specification and patterning of the stomach; however, as with other organs, this appears to require coordinated epithelial-mesenchymal signaling. In one example, the mesenchymal homeobox protein Barx1 is required for normal stomach development, and Barx1-null embryos have ectopic Cdx2 expression in the distal stomach remnant (Kim et al. 2005). Knockdown of Barx1 in gastric mesenchyme cultures results in reduced expression of secreted frizzled-related proteins (Sfrps), known inhibitors of Wnt signaling. This suggests that inhibition of Wnt in the distal stomach is required to prevent ectopic Cdx2 expression. The authors further demonstrate using Wnt reporter mice, that Wnt signaling is low in the distal stomach and high in the fundus and forestomach, suggesting that there is a Wnt-repressive mechanism patterning the antrum.
Several additional pathways have been implicated in anterior-posterior patterning of the stomach including retinoic acid, Indian hedgehog, and Sonic hedgehog (Ramalho-Santos et al. 2000; Wang et al. 2006). Mutants in the retinoic acid synthesizing enzyme Raldh2 display reduced expression of Pea3 and Nkx2–5 which are mesenchymal transcription factors expressed in the hindstomach. In addition mesenchymal transcription factors such as Bapx1 (Nkx3.2) and COUP-TFII are also required for proper stomach morphogenesis and patterning (Takamoto et al. 2005; Verzi et al. 2009). Bapx1 mutants’ stomach displays a truncated antral region, while COUP-TFII displays altered hindstomach epithelial patterning as well as perturbed smooth muscle differentiation. Furthermore several epithelial transcription factors regulate various aspects of stomach patterning including Hnf1β, Pdx1, and Gata4 (Haumaitre et al. 2005; Jacobsen et al. 2002; Larsson et al. 1996). Hnf1β and Pdx1 regulate patterning of the hindstomach, while Gata4 is required for proper cytodifferentiation of both the corpus and antrum. How these signaling pathways and transcription factors are integrated and coordinated remains an open question. For a more extensive review of stomach development, we refer the reader to this review (Kim and Shivdasani 2016).
Similar to the intestine, the stomach undergoes growth and morphogenesis and transitions (Fig. 10.1a) from a pseudostratified epithelium to a glandular epithelium comprised of simple columnar epithelial cells (McCracken et al. 2014). The corpus contains five distinct differentiated cell types, surface pit mucous cells which are located at the luminal surface and secrete mucus (predominantly Muc5AC), mucous neck cells (Muc6), chief cells which secrete pepsinogen, parietal cells which secrete HCL, tuft cells (unknown function), and endocrine cells which secrete hormones. In contrast, the antrum lacks chief cells and parietal cells but contains both types of mucous cells and G cells, which are endocrine cells that secrete the hormone gastrin and are restricted to the antrum. The epithelium of the corpus is maintained by a Mist1-expressing cell under homeostatic conditions, while the antrum is maintained by Lgr5+ stem cells located at the base of the glands (Barker et al. 2010; Hayakawa et al. 2015).
10.5 Generation of Three-Dimensional Gastric Tissue from Human Pluripotent Stem Cells
Exposure of definitive endoderm to high levels of Wnt and FGF promotes a posterior/Cdx2+ fate and induces gut tube morphogenesis, which results in the formation of midgut/hindgut tubelike structures. Evidence from model organisms suggests that BMP signaling is also required for posterior patterning; however, the relationship between WNT, BMP, and FGF in promoting a posterior fate was undetermined. To investigate the involvement of BMP in the WNT/FGF driven posteriorization of definitive endoderm, McCracken and colleagues determined the involvement of endogenous BMP signaling in promoting a posterior endoderm fate. Inhibition of endogenous BMP signaling through the addition of NOGGIN, while at the same time activating WNT and FGF, results in the formation of three-dimensional spheroids which lack CDX2 expression and instead express SOX2. This suggests that endoderm posteriorization is BMP dependent, but that morphogenesis is BMP independent. SOX2 expressing foregut spheroids could be further patterned into posterior foregut by the addition of retinoic acid. Once formed, these posterior foregut spheroids grow in three-dimensional culture and give rise to human gastric organoids (HGOs). HGOs express SOX2 and PDX1 suggesting that they are antral in nature (McCracken et al. 2014). Growth of spheroids into HGOs closely resembles developmental transitions that occur in vivo (Fig. 10.1b). Posterior foregut spheroids transition from a simple cuboidal epithelium into a pseudostratified epithelium and then into a glandular epithelium. This corresponds with cytodifferentiation and patterning of glands into luminal domains with surface mucous cells and glandular neck cells similar to the developing mouse stomach. In addition, HGOs have gastrin-expressing G cells, a hallmark of the antrum. Lastly, HGOs contain discreet epithelial domains that express the stem cell marker LGR5 and the progenitor marker SOX9, suggesting that early stages of gastric stem cell development occurred normally in these organoid cultures.
10.6 Applications of HIOs and HGOs
HIOs and HGOs offer an unprecedented opportunity for studying human gastrointestinal development and disease (Fig. 10.2). The utility of HIOs and HGOs is particularly important for studying aspects of human development and human disease which cannot be recapitulated in model organisms. The accessibility of HIOs and HGOs allows the interrogation of signaling pathways using recombinant proteins or small molecules. In addition, hPSCs are amenable to genetic manipulation including shRNA-mediated knockdown and lentiviral-mediated doxycycline-inducible gene expression, BAC transgenesis, and CRISPR-CAS9-mediated gene editing. This allows for generation of HIOs and HGOs with a vast array of tools. In this section we discuss how organoids can be used to model human gastrointestinal development and disease.
HIOs have been used to study the role of the transcription factors in the development of enteroendocrine cells (Du et al. 2012; Spence et al. 2011b). By overexpressing human Neurogenin-3 using adenovirus, Spence and colleagues were able to demonstrate that NEUROG3 was sufficient to induce the differentiation of intestinal enteroendocrine cells. This approach was improved by the incorporation of a lentiviral-mediated doxycycline-inducible system for gene overexpression (McCracken et al. 2014). This Dox-inducible system offers the ability to control the timing and dosage of transgene expression. Using such a system, HIOs have been used to demonstrate that overexpression of a dominant-negative form of FOXO1 is sufficient to convert a subset of enteroendocrine cells into insulin-expressing cells (Bouchi et al. 2014). There are now several approaches to study loss of function in HIOs. Lentiviral-mediated shRNA-mediated knockdown of NEUROG3 and ARX demonstrated the requirement of these factors for normal development of human intestinal enteroendocrine cells (Du et al. 2012; Spence et al. 2011b). CRISPR-CAS9-mediated knockout of NEUROG3 in human embryonic stem cells has been used to demonstrate the requirement of this transcription factor in endocrine pancreas development (McGrath et al. 2015). In addition, BAC-mediated transgenesis has been used to generate an LGR5-GFP and NEUROG3-GFP reporter lines that mark intestinal epithelial progenitors and endocrine cells in organoids (McCracken et al. 2014; Watson et al. 2014).
HIOs and HGOs have also been used for studying infectious disease and inflammatory responses. HIOs can be infected in order to grow and expand rotaviruses. HGOs have been used to model Helicobacter pylori infection (McCracken et al. 2014). HGOs infected with H. pylori display increased proliferation and CagA-dependent phosphorylation of C-met. Another study showed that HIOs express TNFα in response to hypoxia and that this effect is mediated by EPAS1 (Xue et al. 2013). HIOs have also been shown to maintain viable Clostridium difficile capable of generating the toxins TcdA and TcdB (Leslie et al. 2015). This allows modeling of epithelial barrier disruption caused by these bacterial toxins (Leslie et al. 2015). HIOs and HGOs could potentially be used to model other infectious diseases and study the impact of commensal bacteria on disease progression and epithelial function. Lastly, generation of HIOs and HGOs from patients can be used to study putative disease-causing mutations during GI development or the pathogenesis of GI diseases.
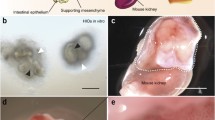
HIOs and HGOs lack an enteric nervous system which is responsible for peristalsis in vivo (Heanue and Pachnis 2007). The incorporation of enteric nerve cells is complicated since these cells are derived from neural crest cells, which originate at a distant site in the embryo and then migrate into the mesoderm of the gastrointestinal tract. However, incorporation of an ENS into HIOs has recently been achieved, resulting in intestinal tissues capable of peristalsis (Workman et al. 2017). By combining vagal neural crest cells with HIOs, neural crest cells were able to incorporate into the mesenchyme of organoids. Transplantation of these chimeric HIOs allowed maturation of the mesenchyme into smooth muscle which was innervated by neural crest-derived cells. To demonstrate the translational potential of this approach, this system was used to model a genetic form of Hirschsprung’s disease.
10.7 Limitations of HIOs and HGOs
Although HIOs and HGOs hold great promise as a tool for gastrointestinal research, these systems are not without their limitations. For example, HIOs are fetal in nature and lack expression of brush border enzymes like alkaline phosphatase, sucrase-isomaltase, and lactase, thus limiting their use for modeling absorptive diseases (Finkbeiner et al. 2015; Watson et al. 2014). However, following transplantation in vivo, HIOs undergo remarkable maturation and robustly express brush border and transport proteins. Moreover, in vivo-grown HIOs form villi, stem cell-containing crypts, and submucosal and myenteric muscle layers. These findings demonstrate that HIOs derived in vitro contain the progenitor cells capable of giving rise to more mature and functional intestinal tissue and that a close examination of pathways activated by in vivo transplantations may identify pathways which could be manipulated to improve maturation in vitro.
HGOs consistently form glandular units which appear to reflect early postnatal mouse development (McCracken et al. 2014). However, long-term growth of HGOs has remained a challenge, and growth in vivo has not yet been demonstrated. Identification of long-term culture conditions and in vivo growth will likely depend on expanding our knowledge base on the pathways that regulate embryonic growth and maturation of the stomach in model organisms. For example, there is little known about pathways that pattern the embryonic corpus/fundus or about what controls differentiation of fundic cell types such as the acid-producing parietal cells. Consequently, efforts to generate human fundic organoids that contain functional parietal cells have not yet been successful. Given the significant differences between the human and mouse stomach, developing human gastric organoid systems is critical for modeling human-specific processes.
HIOs and HGOs are also limited by the cell types that can be generated within the organoid. The blood vessels, immune cells, lymphatics, and an enteric nervous system are absent in HIOs. The lack of enteric nerves makes HIOs and HGOs unsuitable for gastrointestinal motility studies. In addition, these cells may contribute to maturation of intestinal tissue. There are several examples of successful efforts to incorporate additional cell types into PSC-derived organoids. Addition of human umbilical cord vascular endothelial cells (HUVECs) and mesenchymal stem cells to PSC-derived liver progenitors resulted in morphogenesis and development of embryonic liver tissue (Takebe et al. 2013, 2014). Similar successes have been achieved with other organ primordia including the kidney and intestine (Takebe et al. 2015) suggesting this may be possible to achieve with HIOs and HGOs. Finally, incorporation of immune cells should allow the development of complex models of inflammation in the gastrointestinal tract.
10.8 Future Directions
The development of methods to generate intestinal and gastric organoids, either through the directed differentiation of PSCs or from adult organ tissues, has been a seminal advance into human research. HIO and HGO technology will also benefit from new technologies such as high-throughput culture systems (for cell interaction and transcription analysis) and microfluidics platforms which can be used to mimic the flow of luminal contents through the intestine (Gracz et al. 2015; Ingber 2016). HIOs and HGOs have been used to model gastrointestinal pathogens, but these systems may also be applicable to other diseases. HIOs and HGOs derived from patients could be used to model congenital diseases which lead to malformations in the stomach or intestines. For example, HIOs could be derived from patients with congenital short bowel diseases in order to identify pathways which can be manipulated through drug treatment. TGFα mutations can be introduced into HGOs to study Ménétrier’s disease and the mechanism of disease progression. In vivo-transplanted HIOs could also be used to study rare malabsorption syndromes as well as to study mutations which affect intestinal barrier function.
HIOs and HGOs could also be used to study metaplasias of the stomach and intestine. Metaplasia, the conversion of one differentiated cell type into another, is considered a precancerous lesion in the stomach and intestine (Quinlan et al. 2007). Pyloric metaplasia of the ileum is common in patients suffering from ileocolonic Crohn’s disease, with one study reporting a 73% incidence (Soucy et al. 2012). Intestinal metaplasia of the stomach is common in patients with H. pylori-associated gastritis (Correa et al. 2010). Examination of pathways which regulate specification of HIOs versus HGOs could shed insight into how metaplasia occurs. In addition, HIOs and HGOs could be used to determine the role of inflammation in metaplasia by treating HIOs with inflammatory cytokines such as TNFα and IL-8. This could elucidate developmental mechanisms which are reactivated by prolonged chronic inflammation. The availability of tools and the accessibility of HIOs and HGOs will allow for countless studies on the development and pathogenesis of the gastrointestinal tract.
References
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457:608–611
Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M et al (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6:25–36
Bayha E, Jorgensen MC, Serup P, Grapin-Botton A (2009) Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One 4:e5845
Beck F, Erler T, Russell A, James R (1995) Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn Off Publ Am Assoc Anat 204:219–227
Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, Ratner LE, Egli D, Leibel RL, Accili D (2014) FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun 5:4242
Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RSP, Robertson EJ (2001) Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411:965–969
Correa P, Piazuelo MB, Wilson KT (2010) Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 105:493–498
D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541
Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM (2006) FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev 123:42–55
Du A, McCracken KW, Walp ER, Terry NA, Klein TJ, Han A, Wells JM, May CL (2012) Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol 365:175–188
Finkbeiner SR, Hill DR, Altheim CH, Dedhia PH, Taylor MJ, Tsai YH, Chin AM, Mahe MM, Watson CL, Freeman JJ et al (2015) Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation In Vitro and In Vivo. Stem Cell Rep 4(6):1140–1155
Fordham RP, Yui S, Hannan NR, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T et al (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13:734–744
Gao N, White P, Kaestner KH (2009) Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 16:588–599
Gracz AD, Williamson IA, Roche KC, Johnston MJ, Wang F, Wang Y, Attayek PJ, Balowski J, Liu XF, Laurenza RJ et al (2015) A high-throughput platform for stem cell niche co-cultures and downstream gene expression analysis. Nat Cell Biol 17:340–349
Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R (1995) Lack of beta-catenin affects mouse development at gastrulation. Development 121:3529–3537
Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S (2005) Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A 102:1490–1495
Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB et al (2015) Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28:800–814
Heanue TA, Pachnis V (2007) Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci 8:466–479
Huang D, Chen SW, Langston AW, Gudas LJ (1998) A conserved retinoic acid responsive element in the murine Hoxb-1 gene is required for expression in the developing gut. Development (Cambridge, England) 125:3235–3246
Ingber DE (2016) Reverse engineering human pathophysiology with organs-on-chips. Cell 164:1105–1109
Jacobsen CM, Narita N, Bielinska M, Syder AJ, Gordon JI, Wilson DB (2002) Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev Biol 241:34–46
Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E et al (2011) Isolation and in vitro expansion of human colonic stem cells. Nat Med 17:1225–1227
Kim TH, Shivdasani RA (2016) Stomach development, stem cells and disease. Development 143:554–565
Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA (2005) The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 8:611–622
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19:379–383
Kumar M, Jordan N, Melton D, Grapin-Botton A (2003) Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol 259:109–122
Larsson LI, Madsen OD, Serup P, Jonsson J, Edlund H (1996) Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev 60:175–184
Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR (2015) Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun 83:138–145
Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R (2002) Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell 3:171–181
McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404
McGrath PS, Watson CL, Ingram C, Helmrath MA, Wells JM (2015) The basic Helix-Loop-Helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes 64:2497–2505
McLin VA, Rankin SA, Zorn AM (2007) Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development (Cambridge, England) 134:2207–2217
Montgomery RK, Mulberg AE, Grand RJ (1999) Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 116:702–731
Mustata RC, Vasile G, Fernandez-Vallone V, Strollo S, Lefort A, Libert F, Monteyne D, Perez-Morga D, Vassart G, Garcia MI (2013) Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep 5:421–432
Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P (2003) The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development (Cambridge, England) 130:2525–2534
Quinlan JM, Colleypriest BJ, Farrant M, Tosh D (2007) Epithelial metaplasia and the development of cancer. Biochim Biophys Acta 1776:10–21
Ramalho-Santos M, Melton DA, McMahon AP (2000) Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127:2763–2772
Rankin SA, Kormish J, Kofron M, Jegga A, Zorn AM (2011) A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev Biol 351:297–310
Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Sonic hedgehog is an endodermal signal inducing Bmp-4 and hox genes during induction and regionalization of the chick hindgut. Development 121:3163–3174
Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F, Lobo N, Kulkarni S, Sim S, Qian D et al (2012) Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology 142(1195–1205):e1196
San Roman AK, Shivdasani RA (2011) Boundaries, junctions and transitions in the gastrointestinal tract. Exp Cell Res 317:2711–2718
Sasaki N, Sachs N, Wiebrands K, Ellenbroek SIJ, Fumagalli A, Lyubimova A, Begthel H, van den Born M, van Es JH, Karthaus WR et al (2016) Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A 113:E5399–E5407
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD et al (2011a) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011b) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418
Sherwood RI, Chen T-YA, Melton DA (2009a) Transcriptional dynamics of endodermal organ formation. Dev Dyn 238:29–42
Sherwood RI, Chen TY, Melton DA (2009b) Transcriptional dynamics of endodermal organ formation. Dev Dyn Off Publ Am Assoc Anat 238:29–42
Sherwood RI, Maehr R, Mazzoni EO, Melton DA (2011) Wnt signaling specifies and patterns intestinal endoderm. Mech Dev 128:387–400
Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ (2015) Bending gradients: how the intestinal stem cell gets its home. Cell 161:569–580
Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD et al (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143:134–144
Soucy G, Wang HH, Farraye FA, Schmidt JF, Farris AB, Lauwers GY, Cerda SR, Dendrinos KG, Odze RD (2012) Clinical and pathological analysis of colonic Crohn’s disease, including a subgroup with ulcerative colitis-like features. Mod Pathol 25:295–307
Spence JR, Lauf R, Shroyer NF (2011a) Vertebrate intestinal endoderm development. Dev Dyn Off Publ Am Assoc Anat 240:501–520
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM et al (2011b) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109
Stafford D, Prince VE (2002) Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol 12:1215–1220
Takamoto N, You L-R, Moses K, Chiang C, Zimmer WE, Schwartz RJ, DeMayo FJ, Tsai M-J, Tsai SY (2005) COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development (Cambridge, England) 132:2179–2189
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499:481–484
Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H (2014) Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc 9:396–409
Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K et al (2015) Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16:556–565
Tamminen K, Balboa D, Toivonen S, Pakarinen MP, Wiener Z, Alitalo K, Otonkoski T (2015) Intestinal commitment and maturation of human pluripotent stem cells is independent of exogenous FGF4 and R-spondin1. PLoS One 10:e0134551
Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F (2002) BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev 118:29–37
Verzi MP, Stanfel MN, Moses KA, Kim BM, Zhang Y, Schwartz RJ, Shivdasani RA, Zimmer WE (2009) Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology 136:1701–1710
Walton KD, Kolterud A, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL (2012) Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc Natl Acad Sci U S A 109:15817–15822
Walton KD, Whidden M, Kolterud A, Shoffner SK, Czerwinski MJ, Kushwaha J, Parmar N, Chandhrasekhar D, Freddo AM, Schnell S et al (2016) Villification in the mouse: Bmp signals control intestinal villus patterning. Development 143:427–436
Wang Z, Dolle P, Cardoso WV, Niederreither K (2006) Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol 297:433–445
Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y et al (2014) An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20:1310–1314
Wells JM, Melton DA (2000) Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development (Cambridge, England) 127:1563–1572
Wells JM, Spence JR (2014) How to make an intestine. Development (Cambridge, England) 141:752–760
Wills A, Dickinson K, Khokha M, Baker JC (2008) Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn Off Publ Am Assoc Anat 237:2177–2186
Workman MJ, Mahe MM, Trisno S. Poling HM (2017) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. http://www.nature.com/nm/journal/v23/n1/full/nm.4233.html
Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM (2013) Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145:831–841
Zorn AM, Wells JM (2009) Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25:221–251
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Múnera, J.O., Wells, J.M. (2017). Generation of Gastrointestinal Organoids Derived from Human Pluripotent Stem Cells. In: Tsuji, T. (eds) Organ Regeneration Based on Developmental Biology. Springer, Singapore. https://doi.org/10.1007/978-981-10-3768-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-3768-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3766-5
Online ISBN: 978-981-10-3768-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)