Abstract
The Runx family genes play important roles in development and cancer, largely via their regulation of tissue stem cell behavior. Their involvement in two organs, blood and skin, is well documented. This review summarizes currently known Runx functions in the stem cells of these tissues. The fundamental core mechanism(s) mediated by Runx proteins has been sought; however, it appears that there does not exist one single common machinery that governs both tissue stem cells. Instead, Runx family genes employ multiple spatiotemporal mechanisms in regulating individual tissue stem cell populations. Such specific Runx requirements have been unveiled by a series of cell type-, developmental stage- or age-specific gene targeting studies in mice. Observations from these experiments revealed that the regulation of stem cells by Runx family genes turned out to be far more complex than previously thought. For instance, although it has been reported that Runx1 is required for the endothelial-to-hematopoietic cell transition (EHT) but not thereafter, recent studies clearly demonstrated that Runx1 is also needed during the period subsequent to EHT, namely at perinatal stage. In addition, Runx1 ablation in the embryonic skin mesenchyme eventually leads to complete loss of hair follicle stem cells (HFSCs) in the adult epithelium, suggesting that Runx1 facilitates the specification of skin epithelial stem cells in a cell extrinsic manner. Further in-depth investigation into how Runx family genes are involved in stem cell regulation is warranted.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Runx
- Hmga2
- Hematopoietic stem cell
- Hair follicle stem cell
- Niche
- Fetal liver
- Leukemia
- Skin cancer
- Perinatal
- Aging
- DNA damage repair
- Conditional knockout mice
1 Introduction
In multicellular organisms, terminally differentiated cells of most tissues are short-lived and therefore require constant replenishment from stem cells for homeostasis and tissue repair. Stem cells are functionally defined as cells that can self-renew and are multipotent. When required, stem cells undergo cell division to self-renew and/or provide for downstream progenitors and differentiated cells. Stem cells can also reside in a dormant state. Importantly, deregulation of the stem cell compartment often leads to organ failure or tumorigenesis.
It is well documented that the Runx family genes (Runx1, Runx2, Runx3, and Cbfb) play a fundamental role in controlling the stem cell populations of various tissues (Table 9.1). They are central players in the fine-tuning of the balance among cell proliferation, differentiation, and cell cycle exit (summarized in Wang et al. 2010). In this review, the role of Runx and Cbfβ in two well characterized murine tissue stem cells, namely hematopoietic stem cells (HSCs) and hair follicle stem cells (HFSCs) , will be discussed.
2 Hematopoietic Stem Cells in the Blood
The hematopoietic system performs multiple functions, including oxygen transportation, blood clotting and providing immunity . Blood cells have a high turnover rate and are continuously replenished by HSCs and long term progenitors. HSCs, defined as cells that are multipotent and capable of long-term repopulating activity when transplanted into irradiated adult recipient mice (Cumano and Godin 2007), are generated during embryonic development in vertebrates (Fig. 9.1) (see chapter by Yzaguirre et al.). At E10.5-E11.5 of mouse development, the aorta-gonad-mesonephros (AGM) region is the primary intraembryonic hemogenic territory of HSC generation. Clusters of hematopoietic cells can be visualized emerging from the hemogenic endothelium into the lumen of the dorsal aorta—a phase known as endothelial-to-hematopoietic cell transition (EHT) (Jaffredo et al. 2005; Yokomizo and Dzierzak 2010; Kissa and Herbomel 2010). The HSC precursors, also known as pre-HSCs, that are generated from EHT must undergo maturation and acquire stem cell characteristics (Taoudi et al. 2008; Rybtsov et al. 2011) before becoming bona fide HSCs. The placenta is also capable of de novo HSC generation (Rhodes et al. 2008) and in fact harbors more HSCs than the AGM at its peak at E12.5–E13.5 (Gekas et al. 2005; Ottersbach and Dzierzak 2005).
Summary of the ontogeny of hematopoietic stem cells and requirement of Runx family genes. Timeline schematic diagram showing the key events which HSCs undergo and the main hematopoietic sites at which these events occur during ontogeny (top panel). Based on studies using knockout mouse models of Runx1, Runx3 and Cbfb, the time windows during which the Runx proteins are required have been established (bottom panel). Intensity of the blue boxes represent the mortality ratio observed. The HSC-associated phenotypes are also described in boxes. Note that the indicated information for the time period E12.5 to approximately 2 months and the period thereafter is derived from observations in Runx-deficient mice using the Vav1-iCre and Mx1-Cre system, respectively. Dotted lines delineate the times at which the Runx family gene is expressed. Abbreviations: HSC hematopoietic stem cell, EHT endothelial-to-hematopoietic transition, AGM aorta-gonad-mesonephros, FL fetal liver, BM bone marrow
Regardless of their sites of generation, the HSCs colonize the fetal liver (Dzierzak and Speck 2008; Cumano and Godin 2007), which not only provides a conducive environment for further maturation (Kieusseian et al. 2012), but which also serves as the predominant site for rapid HSC proliferation and differentiation to pools of various blood progenitors from E12 to E16 (Martinez-Agosto et al. 2007). Towards the end of the prenatal period, the HSCs proceed to colonize the bone marrow (Orkin and Zon 2008; Christensen et al. 2004), which will serve as the primary site of adult hematopoiesis throughout the postnatal life of the organism.
Interestingly, between 3 and 4 weeks after birth, most of the HSCs in the murine bone marrow abruptly transit from a predominantly proliferative state to a quiescent state, after which the HSC population size remains constant under steady state conditions (Bowie et al. 2006). Long-term progenitors, including classically defined short-term HSCs (ST-HSCs) and multipotent progenitors (MPPs), play a prime role in sustaining hematopoiesis under unperturbed conditions (Sun et al. 2014; Busch et al. 2015), while HSCs remain largely dormant in the lifetime of the organism and serves as a backup reservoir for the recovery from stressed hematopoiesis such as infection, cytotoxic drug treatment, transplantation or perhaps even aging.
As the organism ages, HSCs undergo a functional decline. Aged HSCs have a reduced self-renewal capability and a poorer ability to contribute to hematopoiesis compared to younger HSCs (Dykstra et al. 2011). As a compensatory mechanism to overcome their impaired function, their numbers are increased (Morrison et al. 1996). Aged HSCs also exhibit a biased differentiation potential towards the myeloid lineage (Dykstra et al. 2011). Previous studies have shown that accumulation of DNA damage , transcriptional changes, epigenetic modifications, and altered lineage contribution are factors which can contribute to the functional decline of HSCs (Geiger et al. 2013).
In addition to intrinsic changes through development and aging , HSCs are also subjected to external cues from the extracellular microenvironment. Although much still remains unknown about HSC niches during the developmental stage, two cell types that support HSC proliferation in the fetal liver are the Nestin+NG2+ pericytes located close to portal vessels (Khan et al. 2016) and lymphatic vessel endothelial receptor 1 (Lyve-1)+ sinusoidal endothelial cells (Iwasaki et al. 2010). The vascular network in the placental labyrinth may provide external cues for HSC proliferation (Rhodes et al. 2008). In the extensively studied bone marrow niche, HSCs receives appropriate signals for their maintenance and differentiation from multiple niche-constituting cells (summarized in Birbrair and Frenette 2016). Such cells include osteolineage cells, Nestin+ pericyte cells, CXCL12-abundant reticular (CAR) cells, leptin receptor (Lepr)+ perivascular stromal cells, endothelial cells, glial fibrillary acidic protein (GFAP)+ Schwann cells, sympathetic nerves, and even hematopoietic cells like CD169+ macrophages and megakaryocytes. CXCR4-CXCL12, Tie2-angiopoietin1 (Ang1), CD44-osteopontin (OPN), integrin α2 (or CD49b)-collagen, integrin α4β1-Vcam1, and Robo4-Slit2 have been found to be important for HSC-niche interactions. It is increasingly apparent that changes in the HSC niche affects the age-related changes in HSCs (Arora et al. 2014).
Differential control of HSC behavior by genes at different stages of development has been described. For example, Sox17 (Kim et al. 2007) and Scl (Lecuyer and Hoang 2004) are required for HSC generation or maintenance during early embryonic development but dispensable for adult HSCs, while Bmi1 (Park et al. 2003), Gfi1 (Hock et al. 2004), Tie2 (Puri and Bernstein 2003) and C/ebpα (Ye et al. 2013) are critical for self-renewal in adult HSCs but not in fetal HSCs. Knowledge about the intrinsic molecular mechanisms and important signals supplied by the niche to regulate HSC behavior may provide clues into establishing successful protocols for de novo HSC generation in vitro, ex vivo HSC expansion and HSC rejuvenation .
2.1 Runx During the Endothelial-to-Hematopoietic Cell Transition (EHT)
During early development of mouse embryos, Runx1 and Cbfb are indispensable for the generation of HSCs, specifically at EHT. While Runx1-positive cells are found in hemogenic endothelial cells and hematopoietic cell clusters in the dorsal aorta of the AGM region at E9.5–E11.5 in wild-type embryos (Yokomizo and Dzierzak 2010; Yokomizo et al. 2001; Ng et al. 2010; North et al. 1999), Runx1 −/− embryos showed lack of such hematopoietic cell clusters budding from the vessel wall (Yokomizo et al. 2001; North et al. 1999). As a consequence of the lack of EHT and thus the complete failure in generating HSCs and definitive hematopoiesis, both Runx1 −/− and Cbfb −/− mice die between E11.5 and E12.5 (Okada et al. 1998; Niki et al. 1997; Ng et al. 2010; Okuda et al. 1996; Wang et al. 1996a; b; Sasaki et al. 1996) (Fig. 9.1).
Employing different developmental stage- or tissue-specific Cre system to ablate Runx1 in mice, it was shown that the essential requirement for Runx1 function at this early developmental stage occurs within a specific time window. When Runx1 is abrogated in endothelial cells by vascular endothelial cadherin (VEC) promoter-driven Cre, the knockout mice showed embryonic lethality due to the lack of intra-aortic hematopoietic cell clusters and HSCs (Chen et al. 2009). However, embryonic lethality was not observed when Cre driven by the promoter of a pan-hematopoietic Vav1 gene, active starting from E10.5, was used to delete Runx1. Using temporal knockouts of Runx1 f/f ;Actb-Cre ERT and Runx1 f/f ;VEC-Cre ERT mice based on the tamoxifen-activated Cre recombinase system, it was established that during the early developmental stage, Runx1 is required at least up to E11.5, after which removal of both Runx1 alleles does not eliminate HSCs (Tober et al. 2013). These results appear to indicate that Runx1 is specifically required for HSC generation during EHT, but not thereafter.
In Runx2 −/− and Runx3 −/− mice, there are no inherent hematopoietic phenotypes at the early development stage including EHT. Yet, Runx2 was reported to be expressed in the AGM region, although Runx3 was not (Okada et al. 1998; Levanon et al. 2001). However, it is not clear whether the methods used for the examination of expression are sensitive and specific for individual Runx family genes. As such, the involvement of these genes during early development remains to be investigated. The roles of Runx1 in HSC generation during early development are described in detail elsewhere in this book (see chapter by Yzaguirre et al.).
2.2 Runx in Hematopoietic Stem Cells at the Peri-Natal Stage
Even after EHT is complete, it was reported that fetal livers from E14.5 Runx1 f/f ;Vav1-Cre embryos contained fourfold fewer functional HSCs than control fetal livers in a limiting dilution transplantation assay (Cai et al. 2011) and Cbfb f/f ;Vav1-Cre fetal livers were completely unable to reconstitute recipient mice (Tober et al. 2013). Therefore, it seems likely that the Runx-deficient fetal HSCs are compromised. Probably due to such defects, Cbfb f/f ;Vav1-iCre + mice were not born at Mendelian ratios (Wang et al. 2015), although Runx1 f/f ;Vav1-iCre + and Runx3 f/f ;Vav1-iCre + mice were (data not shown). Interestingly, Runx1 f/f ;Runx3 f/f ;Vav1-iCre + mice could not be obtained from crosses of Runx1 f/+ ;Runx3 f/f ;Vav1-iCre + with Runx1 f/f ;Runx3 f/f ;Vav1-iCre − mice (0 out of 73 mice genotyped) (Fig. 9.2). After birth, Runx1 f/f ;Vav1-iCre + and Cbfb f/f ;Vav1-iCre + mice exhibit poorer survival than Runx1 f/f ;Mx1-Cre + and Cbfb f/f ;Mx1-Cre + mice, respectively (Fig. 9.2a, d). Although the survival data do not directly translate to HSC defects, the most critical data supporting that the pronounced phenotypes in Runx-deficient Vav1-iCre + mice are due to functional HSC defects is derived from transplantation experiments. The ability of Cbfb f/f ;Vav1-iCre + adult HSCs to reconstitute recipient mice was severely impaired (Wang et al. 2015). Taken together, these results suggest that the perinatal absence of Runx family genes causes dysfunctional HSCs, even after EHT (Fig. 9.1).
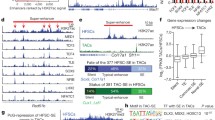
Comparison of phenotypes of Runx1, Runx3, Runx1 ; Runx3 and Cbfb conditional KO mice using different Cre-inducible systems. (a–d) Kaplan-Meier survival curves of (a) Runx1 f/f ;Mx1-Cre + (black line, n = 27) versus Runx1 f/f ;Vav1-iCre + (red line, n = 22), (b) Runx3 f/f ;Mx1-Cre + (black line, n = 11) versus Runx3 f/f ;Vav1-iCre + (red line, n = 12), (c) Runx1 f/f ;Runx3 f/f ;Mx1-Cre + (black line, n = 27) versus Runx1 f/f ;Runx3 f/f ;Vav1-iCre +, and (d) Cbfb f/f ;Mx1-Cre + (black line, n = 35) versus Cbfb f/f ;Vav1-iCre + (red line, n = 6) mice. Hundred percent and Fig. 9.2 (continued) forty percent embryonic/perinatal lethality observed in Runx1 f/f ;Runx3 f/f ;Vav1-iCre + and Cbfb f/f ;Vav1-iCre +, respectively, are marked in red crosses (†). The p-value from Mantel-Cox test is shown. Vertical ticks represent censored cases. Survival curves of wild-type controls are not shown for clarity purpose. (e) Frequency of hematopoietic stem/progenitor cell populations of Runx3 f/f ;Vav1-iCre − (n = 6) and Runx3 f/f ;Vav1-iCre + mice (n = 6) at 2 months old. Mean ± SEM of percentages in whole BM are shown. (f) White blood cell counts of Runx3 f/f ;Mx1-Cre − (n = 12) and Runx3 f/f ;Mx1-Cre + (n = 9) at 2 months post-induction, and Runx3 f/f ;Vav1-iCre − (n = 20) and Runx3 f/f ;Vav1-iCre + mice (n = 23) at 2 months old. Mean ± SEM of leukocyte counts are shown. (g) Diagram showing the time window of differential HSC properties (top) and the time when Vav1-iCre or Mx1-Cre are expressed in hematopoietic tissues (bottom). Abbreviations: KSL c-Kit+Sca-1+Lineage−, LT-HSC long-term hematopoietic stem cell (c-Kit+Sca-1+Lineage−CD34−Flt3−), ST-HSC short-term hematopoietic stem cell (c-Kit+Sca-1+Lineage−CD34+Flt3−), MPP multipotent progenitor (c-Kit+Sca-1+Lineage−CD34+Flt3+). Asterisk(s) represents significant difference (*p < 0.05, ***p < 0.001 and ****p < 0.0001, Student’s t-test)
Interestingly, a study involving reexpression of Runx1 in a reversible Runx1 knockout mouse model using Tie2-Cre supports such a notion (Liakhovitskaia et al. 2009). Although restoration of Runx1 expression rescues the ability of the mice to generate HSCs as fetal liver cells isolated from such mice were able to reconstitute irradiated recipient mice, the mice still exhibit lethality at birth. Possible causes of lethality was attributed to the abnormal development of sternum (Liakhovitskaia et al. 2010), but defects in HSC migration from fetal liver to the bone marrow cannot be ruled out. Furthermore, a recent study investigating the involvement of Runx1 during early development revealed that Runx1 is required for the maturation of pre-HSCs (Liakhovitskaia et al. 2014).
Notably, the reconstitution ability of E14.5 Cbfb f/f ;Vav1-Cre fetal livers (Tober et al. 2013) was much worse than Runx1 f/f ;Vav1-Cre fetal livers (Cai et al. 2011), indicating a possibility that Runx2 and Runx3 may be required after EHT . Supporting this possibility, it has been reported that there is a prominent increase in Runx2 levels when the intermediary pre-HSCs transit to become nascent HSCs (Zhou et al. 2016), suggesting that at least Runx2 may play a role at this transition stage. Furthermore, Runx3 f/f ;Vav1-iCre + mice exhibited increased HSC compartment accompanied by leukocytosis, which was only observed in aged Runx3 f/f ;Mx1-Cre + mice, but not the young ones (Fig. 9.2e, f). Surprisingly, Runx3 f/f ;Vav1-iCre + mice showed poorer survival (Fig. 9.2b), which was not observed in Runx3 f/f ;Mx1-Cre + mice (Wang et al. 2014).
The differences in phenotypes of Runx-deficient mice using the Vav1-iCre system versus Mx1-Cre system can be explained by the distinct requirement of a gene in developmental versus adult hematopoiesis (discussed in Koh et al. 2015): Vav1 promoter is activated at E10.5 onwards in all hematopoietic cells, including those during the maturation of pre-HSCs to nascent HSCs and the critical period of rapid HSC expansion, while Mx1 promoter is induced by polyinosinic–polycytidylic acid (pIpC) at the adult stage when HSCs are largely in quiescence (Fig. 9.2g). It is important to note that there are at least four distinct Vav1 promoter-driven Cre strains which could potentially delete in distinct but overlapping cell populations (Georgiades et al. 2002; Croker et al. 2004; Stadtfeld and Graf 2005; de Boer et al. 2003), and differences in phenotypes might be observed even amongst these four strains. Noteworthy is the enhanced expression of iCre (improved Cre) in the Vav1-iCre strain compared to the original Cre in the other Vav1 -Cre strains (Shimshek et al. 2002; de Boer et al. 2003), which may cause some level of toxicity (Loonstra et al. 2001; Silver and Livingston 2001) and poorer survival.
Taken together, Runx may be required for one or more of the following processes during the perinatal stage: (1) maturation of pre-HSCs to nascent HSCs, (2) HSC expansion in the placenta, fetal liver or bone marrow, (3) migration of HSCs from placenta to fetal liver and/or from fetal liver to bone marrow, and/or (4) the tightly controlled transition when the abrupt change from proliferative status to quiescence in HSC occurs .
2.3 Runx in Hematopoietic Stem Cells of Young Adults
The Runx family genes are also important for the maintenance of HSCs in adults. In contrast to the complete lack of HSCs in Runx1 −/− and Cbfb −/− mice, HSCs are still present in adult Runx1 or Cbfb conditional knockout mice using the Mx1-Cre system, but showed disrupted regulation (Ichikawa et al. 2004; Growney et al. 2005; Wang et al. 2015). Phenotypic HSCs in Runx1 f/f ;Mx1-Cre + mice exhibited a twofold expansion (Jacob et al. 2010). Recently, it has also been demonstrated that Cbfb f/f ;Mx1-Cre + show an even more pronounced expansion in the immunophenotypic HSC compartment (Wang et al. 2015), similar to the extent of expansion in Runx1 f/f ;Runx3 f/f ;Mx1-Cre + mice (Wang et al. 2014). These results suggest that both Runx1 and Runx3 serve to inhibit cell proliferation in HSCs, perhaps mediated by the following two molecular mechanisms.
First, Runx can directly inhibit cell cycle progression. Gene expression profiling of Runx1 f/f ; Runx3 f/f ;Mx1-Cre + HSC-enriched c-Kit+Sca-1+Lineage− (KSL) cells showed significantly elevated levels of high-mobility group AT -hook 2 (Hmga2) , a non-histone chromatin-interacting factor, which could have contributed to increased HSC self-renewal (Fig. 9.3). It has been shown that Runx1 plays a direct role in repressing the transcription of Hmga2 (Lam et al. 2014). The transcriptional regulation of cell cycle regulatory proteins (such as D-type cyclins (Strom et al. 2000; Bernardin-Fried et al. 2004) and p21 (Strom et al. 2000; Galindo et al. 2005) by Runx family genes may also affect the cell cycle status of HSCs. For example, downregulation of Cdkn1a (p21) in Runx1 f/f ;Runx3 f/f ;Mx1-Cre + KSL cells observed in gene expression profiling analysis may contribute to the expanded HSC population.
Schematic diagram depicting the Runx-Hmga2 axis in controlling HSC behavior during aging . Runx has been shown to inhibit transcription of Hmga2, which in turn regulates transcription of age-related players, p16INK4a and p19ARF indirectly, possibly via JunB. p16INK4a and p19ARF inhibits HSC self-renewal during aging. Hence, increased Runx1 expression in aged HSCs culminates in reduced self-renewal and the absence of Runx causes increased HSC self-renewal, albeit transient, via this Runx-Hmga2 axis
Additionally, Runx1 and Runx3 help to maintain stem cells in their quiescent state by regulation of the G0/G1 transition in a HSC niche -dependent manner, thereby controlling the HSC population size. Analysis of a panel of niche-related factors in Runx1 f/f ;Mx1-Cre + mice revealed that CXCR4 was downregulated . A luciferase reporter assay using the CXCR4 promoter, containing two Runx binding sites, showed that RUNX1 transactivates CXCR4 in a DNA-binding dependent manner, suggesting the direct transcriptional regulation of CXCR4 expression by RUNX1 (Jacob et al. 2010; Chin et al. 2016). Along with CXCR4 , another niche-interacting factor, integrin α2 (CD49b), was also downregulated in Runx1 f/f ;Mx1-Cre + HSCs. In the case of Runx1 f/f ;Runx3 f/f ;Mx1-Cre + mice, the KSL population showed downregulation of several critical niche-related factors: Vcam-1, Cxcr4 and Robo4 (Wang et al. 2014). Such inadequate expression of niche-related factors in Runx-deficient HSCs led to a weakened niche interaction and, as a result, less quiescence and expansion of HSCs could be induced.
Although the numbers of HSCs are increased in Runx1 and Cbfb conditional knockout mice , the HSCs are functionally impaired in the ability to repopulate hematopoiesis in recipient mice. While Runx1 f/f ;Mx1-Cre + HSCs can reconstitute recipient mice in a bone marrow transplantation experiment, the chimerism in Runx1 f/f ;Mx1-Cre + recipient mice decreased with time, unlike in control recipient mice (Growney et al. 2005). Furthermore, young adult Runx1 f/f ;Runx3 f/f ;Mx1-Cre +, Cbfb f/f ;Mx1-Cre + and surviving Cbfb f/f ;Vav1-iCre + mice exhibit a phenomenon called stem cell exhaustion (Wang et al. 2014). This is thought to be the result of the interplay of at least two mechanisms described below.
First, Runx proteins control DNA damage repair pathway(s) (Wang et al. 2014) (see chapter by Krishnan and Ito). In the absence of Runx1 and Runx3, KSL cells showed impairment in DNA damage repair, accentuated by an interstrand crosslinking agent. The increased DNA damage in Runx-deficient cells, coupled by the increased proliferation described earlier, renders high levels of replication stress to the cells, contributing to decreased HSC integrity and subsequent exhaustion of the stem cell pool. Second, Runx proteins regulate the transcription of several aforementioned niche -interacting factors. In Runx-deficient HSCs, there is compromised HSC-niche interactions important for maintaining HSC quiescence, which in turn causes the detachment of HSCs from the niche. Subsequently, the inability to maintain quiescence would result in the loss of long-term self-renewal capacity of Runx-deficient HSCs, eventually leading to stem cell exhaustion, despite the initial transient increase of HSCs.
The role of Runx2 in adult HSCs per se has yet to be studied, but Runx2 most probably affects HSC quiescence by generating the HSC niche components, namely the osteoblasts. Runx2 has been shown to be the principal transcriptional regulator of osteoblastic differentiation (Otto et al. 1997; Komori et al. 1997; Deguchi et al. 1999). This implies that the maintenance or replenishment of available HSC-niche sites during homeostatic bone turnover by Runx2 could potentially support stem cell quiescence, although more conclusive data is required. Additionally, Runx2 activates the transcription of OPN, an important factor for HSC quiescence, in osteoblasts (Sato et al. 1998). Contradictory, it has been recently demonstrated that Runx2 highly expressing immature osteoblasts were better than osteoblasts expressing lower levels of Runx2 for supporting proliferation and colony-forming capability of KSL population (Chitteti et al. 2010). Whether Runx2 is inhibitory or promoting for HSC quiescence remains to be further investigated.
2.4 Runx in Aging Hematopoietic Stem Cells
The phenotypes that distinguish aged HSCs from young HSCs include increased phenotypically defined HSC numbers, reduced self-renewal capacity, myeloid-biased differentiation, impaired homing to and enhanced mobilization from the bone marrow. Altered HSC-niche interactions can also play a role in HSC aging (Arora et al. 2014). Notably, the phenotypes commonly observed in various Runx-deficient mice seem to exhibit similarities to aging phenotypes (Fig. 9.1). When Runx family genes are ablated in the hematopoietic system, the HSC population as defined immunophenotypically is increased and there is a myeloid-biased differentiation. The motility of Runx-deficient HSCs is also increased. Hence, these features in Runx-deficient mice suggest a possibility that Runx family genes are essential to slow down the aging phenomenon.
Initial expansion and subsequent exhaustion of HSCs from Runx1 f/f ;Mx1-Cre + mice (Jacob et al. 2010) and earlier death of these mice than the control mice may be attributable to progeria, or premature aging. Similarly, expansion of HSCs in aged Runx3 f/f ;Mx1-Cre + mice may be caused by accelerated aging, although occurring to a lesser extent compared to Runx1 f/f ;Mx1-Cre + mice (Wang et al. 2013). Runx deficiency may thus promote aging of HSCs and plausible mechanisms are discussed below.
Hmga2 was identified to be the only gene whose expression not only progressively declines with age in HSCs, but is preferentially expressed in HSCs but not in differentiated cells (Nishino et al. 2008). Hmga2 regulates the Ink4a/Arf locus via JunB, mediating its effects on aging via p16Ink4a and p19Arf (Fig. 9.3). It is well documented that p16Ink4a induction contributes to the decline of HSC function in aged mice, whereas its absence in HSCs rescues their compromised self-renewal capacity in transplantation assays (Janzen et al. 2006). Lin28b-let-7 has been reported to regulate Hmga2 (Copley et al. 2013). As discussed earlier, Runx1 transcriptionally represses Hmga2 in HSCs and is thus another regulator of Hmga2. Alternatively, Runx1 can induce the expression of p19Arf directly (Linggi et al. 2002).
Notably, Ink4a/Arf deletion in Hmga2 mutant mice does not completely rescue the stem cell defects, suggesting that there are other mechanisms mediated by Hmga2, in addition to its effects on HSC self-renewal via p16Ink4a and p19Arf. Hmga2 is reported to be directly involved in DNA repair and aging -related DNA damage accumulation in HSCs might be due to the decrease of Hmga2 with age (Yu et al. 2014). Age-dependent accumulation of DNA damage in HSCs results in impaired self-renewal and thus decreased regenerative capacity of aged HSCs. As mentioned earlier, Runx proteins are also implicated in DNA repair and their roles in maintaining HSC genomic integrity could be via the regulation of Hmga2 (Wang et al. 2014). Taken together, it is imperative to determine if Runx family genes are indeed regulators of HSC ageing.
An alternative possibility for the ageing phenotype is that cytoplasmic sequestration of the IKK complex by Runx1 results in diminished NF-κB signaling (Nakagawa et al. 2011). In Runx-deficient cells, the derepression of NF-κB transcriptional targets can lead to increased senescence , and possibly the senescence-associated secretory phenotype (SASP) (Chien et al. 2011), resulting in a premature aging phenotype. Interestingly, the secretion of SASP factors can affect the cellular microenvironment, which may implicate Runx1 as having a role in influencing the HSCs and/or HSC niche via SASP.
FOXO3a is one of the few confirmed longevity genes (Flachsbart et al. 2009). SIRT1, a member of the sirtuin family of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases, is another anti-aging gene that triggers FOXO3a transcription via its deacetylation activity (Brunet et al. 2004). SIRT1 is thought to contribute to increased longevity by acting as a guardian against cellular oxidative stress and DNA damage (Haigis and Guarente 2006). Intriguingly, Runx2 physically interacts with Sirt1 (Shakibaei et al. 2012) and RUNX3 physically interacts with FOXO3a (Yamamura et al. 2006). In addition, the transcription of Runx2 was found to be co-mediated by SIRT1 and FOXO3a (Tseng et al. 2011). Interestingly, Runx2 is reported to be downregulated in aged HSCs (Chambers et al. 2007), and this decrease may be linked to the downregulation of SIRT1 and FOXO3a during aging. The close relationship between Runx family genes with longevity factors, FOXO3a and SIRT1, warrants further study into the role of Runx in HSC aging.
Based on the discussion above, one would then expect Runx1 to be expressed at low levels in aged HSCs which are increased in number and have a myeloid-biased differentiation phenotype. Paradoxically, Runx1, a myeloid-related gene, is upregulated in aged HSCs (Rossi et al. 2005; Chambers et al. 2007). Such discrepancy could be due to differential expression of the Runx1 isoforms in young versus aged mice. Runx1bEx6e, functionally similar to RUNX1a in human, enhances expansion of HSCs (Komeno et al. 2014; Osato 2014) and may be upregulated in aged HSCs, while Runx1bEx6+ inhibits HSC proliferation and could be downregulated in aged HSCs.
As the heterodimerization partner of the Runx proteins, the significance of Cbfβ upregulation (Rossi et al. 2005; Chambers et al. 2007) in aged HSCs remains to be further investigated. In general, the role of Runx family genes in aging is not well studied and stands as the important theme for future research.
2.5 Runx and Leukemia
In general, myeloid related hematopoietic malignancies are more predominant at old age, whereas lymphoid related ones occur at younger age. However, human RUNX-related myeloid leukemia such as inv(16) and t(8;21) are prevalent in childhood, as well as adolescents and younger adults (AYAs) (Mrozek et al. 2012). As mentioned above, results obtained from the mouse model studies clearly demonstrated that Runx family genes play a development- and age-dependent requirement in HSCs. Hence, the importance of Runx at the perinatal stage may underlie the aforementioned young onset of Runx leukemias.
RUNX1 point mutations are more frequently found in elderly acute myeloid leukemia (AML) patients. Recurrent mutations in genes such as DNMT3a, TET2 and ASXL1 are detected singly in aged individuals of the general population who do not exhibit hematological disorders and result in clonal expansion of HSCs harbouring these mutations (Jaiswal et al. 2014; Genovese et al. 2014). Based on the genetic landscape of mutations found in elderly healthy individuals and AML patients, it is thought that Runx1 point mutations occur secondary to such preceding mutations found in pre-leukemic HSCs to cause progression to leukemia (Xie et al. 2014). Runx deficiency leading to stem cell exhaustion is counterintuitive to leukemogenesis. Yet, RUNX1 mutations predispose cells to leukemogenesis by promoting a myeloid-biased cell status. This conundrum can be explained by the ability of the driver mutations to overcome the exhaustion conferred by RUNX1 mutations. For this reason, the advantageous state of pre-leukemic HSCs in aged individuals could explain why RUNX1 point mutations commonly occur in leukemias of older patients.
Notably, overexpression of Hmga2 , repeatedly discussed in this chapter (Fig. 9.3), has been reported in leukemias (Fusco and Fedele 2007; Tan et al. 2016). As such, the pronounced increased Hmga2 levels in the absence of Runx family genes may be relevant to leukemogenesis in Runx-deficient status, perhaps by increasing the stem cell pool in which cooperative mutations may occur.
3 Hair Follicle Stem Cells in the Skin
The skin epidermis is important for protection against external environmental insults and prevention against dehydration. The skin epidermis is constantly renewing and consists of different compartments, including the hair follicle , its surrounding interfollicular epidermis and sebaceous glands. At least three stem cell populations have been documented to maintain the epidermis, one of which is the HFSC located in the outermost layer of the follicle known as the outer root sheath. While the HFSCs contributes permanently only to the hair follicle, the HFSCs can temporarily regenerate all types of cells in the epidermal layer upon injury.
The hair follicle is an accessory structure of the epidermis embedded deep in the dermis of the mammalian skin. Of the four different types of hair present in the mouse pelage, the guard hair is the well studied and the timelines provided in this chapter are representative of this particular hair type. The formation of hair follicles require constant molecular interactions between the mesenchymal and epithelial cells. The morphogenesis, or development, of the hair follicle occurs beneath the skin surface and begins at approximately E14.5 in the mouse (Fig. 9.4a). The hair follicles develop through a series of intermediate structures that starts with budding from the epidermis and is fully mature when it extends into the dermis with an inner root sheath and hair shaft. The emergence and maturation of HFSCs takes place concurrent with the development of the hair follicle (reviewed in Sennett and Rendl 2012; Forni et al. 2012). Induction of early HFSC precursors begins at about E12.5, and HFSCs with slow cycling properties emerge in hair placodes, an intermediate hair follicle structure, several days later (Nowak et al. 2008). By the end of the hair follicle morphogenesis, the adult HFSCs reside in a niche known as the bulge region, where they are kept in a quiescent state. The bulge is a hair follicle structure that is part of the upper outer root sheath and located below the sebaceous gland. Adult HFSCs in the bulge can be identified by CD34 expression concurrent with either keratin 14 (K14)-expressing cells (Malanchi et al. 2008) or integrin α6 (Trempus et al. 2003).
Summary of the morphogenesis and cycling of hair follicle and requirement of Runx1 in hair follicle stem cells. (a) Timeline schematic diagram showing the key events of HFSCs /hair follicles during ontogeny. (b) Phenotypes exhibited by the Runx1 conditional knockout mouse models used to decipher the Runx1 role in HFSC are summarized. Colored boxes indicate the tissue type in which Runx1 is ablated (blue, epithelium; pink, mesenchyme). Note that the absence of “Runx1” in mesenchymal tissue represents undetectable expression of Runx1 in this tissue at the adult stage. Abbreviations: HFSC hair follicle stem cell, HF hair follicle, SG sebaceous gland
At about 17 days after birth, development of the hair follicle is complete and morphogenesis ends with the first catagen stage when destruction of the hair bulb occurs. The first adult hair cycle is initiated around postnatal day 21 by activation and proliferation of HFSCs in the bulge. Structurally, each hair follicle is composed of an upper non-cycling region and a cycling region (Zhang et al. 2009). The structure of the non-cycling region includes the stem cell-containing bulge (Morris et al. 2004), oil-producing sebaceous gland and the infundibulum through which the hair shaft passes. To continuously produce new hair shafts during homeostasis, the cycling portion of each hair follicle, the bulb, repeatedly undergoes a 3-week long hair cycle that comprises anagen (growth phase), catagen (degeneration phase) and telogen (quiescent phase) (Alonso and Fuchs 2006). During anagen, HFSCs undergo self-renewal and then some of these HFSCs leave the bulge only at the next telogen-anagen transition. Once outside the niche , these cells then undergo further differentiation into transit amplifying progenitor cells, known as the matrix, throughout anagen (Zhang et al. 2009). The matrix encloses a group of mesenchymal cells , called the dermal papillae, which integrates signals from the bulge and elicits instructions to the epidermis to activate HFSC proliferation and induce differentiation for hair follicle regeneration.
The skin undergoes significant changes in terms of structure and function with age. Aged HFSCs maintain their numbers and gene signatures. However, the period of quiescence becomes progressively longer with age, suggesting that quiescent HFSCs become increasingly resistant to activation (Keyes et al. 2013).
3.1 Runx in Hair Follicle Stem Cells at the Developmental Stage
Runx1 is expressed in both epithelial and mesenchymal regions in the area around the hair follicle during morphogenesis (Raveh et al. 2006; Osorio et al. 2011). At placode stage, Runx1 is expressed in cells of the single layer of ectoderm fated to become adult HFSCs , and the Runx1-expressing cells ultimately contribute to all layers of hair follicle during morphogenesis (Osorio et al. 2011). Such early specification of stem cells is important for hair follicle morphogenesis (Nowak et al. 2008). Close examination of epithelial-specific Runx1 knockout mice using Runx1 f/f ;K14-Cre + mice revealed that there was a delay in the formation of mature hair follicles due to delayed HFSC emergence (Fig. 9.4b), which could be explained by reduced Lef1 levels and reduced Wnt signaling in the epidermis and the adjacent dermis cells (Osorio et al. 2011). However, this defect was overcome with time, suggesting that Runx1 is crucial for the timely emergence of HFSCs during embryogenesis, but is dispensable for this process (Osorio et al. 2011).
Runx1-expressing cells are also detected in the dermal layer just beneath the epidermis at E14.5. This dermal population increases by E17.5 (Raveh et al. 2006; Osorio et al. 2011). Deletion of Runx1 by tamoxifen injections at E12.5, E13.5 and E14.5 in Runx1 CreER/f mice resulted in very efficient deletion in the dermis, but not epidermis, generating a knockout mouse model with Runx1 specifically ablated in mesenchymal tissue. In this mesenchymal knockout mouse model, the deletion of Runx1 led to formation of hair follicles throughout development, but they are converted into enormous sebaceous cysts that lacked the bulb and bulge regions at the first hair cycle (Osorio et al. 2011) (Fig. 9.4b). This phenotype is due to defective maturation of the early HFSC precursors, leading to preferential differentiation towards sebaceous glands over hair bulb lineages. Hence, it is evident that mesenchymal loss of Runx1 during embryogenesis affects hair follicle integrity much more than the epithelial loss of Runx1 does. Contrary to Runx1 deletion in the epithelium, Runx1 deficiency in the dermis resulted in upregulated Lef1 and Wnt signaling in both mesenchyme layer and epithelium, suggesting there are molecular interactions between the two compartments (Osorio et al. 2011).
Unlike Runx1 expression, the expression of Runx2 and Runx3 is much less pronounced in the epithelial layer of hair follicles. Runx2 is expressed in dermal papillae and bulb epithelium during hair follicle development, but is not expressed in other regions of the skin outside the hair follicles (Glotzer et al. 2008). Allografts of Runx2 null skin at E18.5, compared to those of wild-type controls, showed that Runx2 is dispensable for normal hair follicle morphogenesis and cycling (Glotzer et al. 2008). However, there was a delay in hair follicle development as E18.5 Runx2 null embryos had fewer follicles than the control embryos. This defect was shown to be due to decreased Sonic hedgehog expression.
Runx3 is expressed in the dermal layer just beneath the epidermal layer in placode and hair germ stages (Raveh et al. 2005). This is the region where the Runx3-expressing cells will form the dermal papillae. By birth, Runx3 is expressed in a cluster of cells that consisted of most, if not all, of the dermal papillae. The significance of this expression is worth further investigating as the involvement of Runx3 in HFSCs may act via its effects on the dermal papillae. Runx3 −/− mice bred on heterogeneous ICR, MF1 background are able to survive until adulthood and these mice did not show major changes in the overall morphology of the skin and its appendages. This may be due to compensation by Runx2 as Runx2 is also expressed in the dermal papillae (Glotzer et al. 2008). A population of stem cells termed skin-derived precursors (SKPs) has been discovered in the dermal papillae (Toma et al. 2001). Whether Runx2 and Runx3 plays a role in specification of these SKPs remains to be studied.
3.2 Runx in Hair Follicle Stem Cells of Young Adults
Runx1 has a dynamic expression in the murine hair follicle throughout the hair cycle and when expressed, it is found only in specific hair follicle compartments. Runx1 marks adult HFSCs in the lower bulge during telogen (Scheitz et al. 2012) and is detected throughout the bulge during anagen when HFSCs self-renew. Dermal expression of Runx1 is undetected at the onset of the first hair cycle (Raveh et al. 2006). Consistent with the absence of Runx1 expression in the adult mesenchyme, dermal excision of Runx1 by the induction of Runx1 CreER/f mice with tamoxifen at PD21 resulted in normal skin (Fig. 9.4b). The overall expression patterns of Runx1 in human and mouse skin are largely similar (Hoi et al. 2010), suggesting that the functions of Runx in both organisms are also conserved.
Interestingly, Runx1 is dispensable during hair follicle development but is crucial for normal regulation of the hair cycle at the transition into adult skin homeostasis. Gene targeting of Runx1 in the epithelial compartment in mice using K14-Cre system led to a delay in telogen-to-anagen transition of the first hair cycle (Osorio et al. 2008) (Fig. 9.4b). Immunofluorescence staining of epithelial Runx1-deficient hair follicles revealed the absence of specific hair lineage markers and progenitor matrix cell marker (Ephrin B1) characteristic of anagen phase at a time when wild-type hair follicles are in anagen. Using Runx1 f/f;β-actin-CreER mice injected with tamoxifen at various stages in the hair cycle, the requirement for Runx1 was found to be at the onset of anagen (Hoi et al. 2010). Clonogenic assays testing for skin stem cells resulted in cultured keratinocytes from Runx1 ∆4/∆4;K14-Cre mice having fewer and smaller colonies that are unable to survive long term (Osorio et al. 2008). BrdU labeling experiment demonstrated that the loss of Runx1 at anagen onset impaired the proliferation of CD34+ bulge cells in vivo (Hoi et al. 2010). Taken together, loss of Runx1 impairs proliferation of HFSCs and leads to delayed HFSC activation into cell cycle. Notably, the telogen block is spontaneously overcome with age (Hoi et al. 2010) or injury (Osorio et al. 2008), suggesting that Runx1 is not completely indispensable for HFSCs to exit quiescence and that Runx1 loss did not affect the differentiation potential and fate decision of HFSCs. Runx1 may work, at least partly, by downregulating Cdkn1a (p21) (Hoi et al. 2010) and keeping HFSCs poised for receiving proliferation signals (Lee et al. 2014). However, whereas deletion of Cdkn1a rescued the proliferation defects in Runx1 knockout keratinocytes in vitro, loss of Cdkn1a had the opposite effect in hair follicle cycling in vivo. Runx1;p21 double knockout mice showed an even more prolonged telogen phase, thought to be due to the upregulation of compensatory CDK inhibitor expression, such as p15 (Lee et al. 2013).
As in the case of HSCs, Hmga2 was found to be an important factor in the maintenance of long-term self-renewal capability of HFSCs (Chen et al. 2012). An in vitro RNA interference screen coupled with serial passages of HFSCs led to the identification of Hmga2, and this hit was validated by shRNA transduction experiment. Intriguingly, Runx1 was also identified as the top hit—absence of Runx1 reduces the self-renewal ability of HFSCs. As such, the Runx1-Hmga2 axis that is apparent in HSCs may operate in HFSCs as well. In this case, however, Runx1 may act as a positive regulator of Hmga2 expression. The exact mechanism remains to be further studied.
Runx3 expression in the dermal papillae persists throughout all stages of the hair cycle (Raveh et al. 2005). Durations of hair cycle stages were found to be unaffected in Runx3 −/− mice bred on heterogeneous ICR, MF1 background. As mesenchymal-epithelial interactions are important in the signals from dermal papillae to epidermis, it is possible that signals from Runx3-expressing cells in the dermal papillae may regulate HFSC function or numbers. Further investigation is warranted.
The expression of Runx2 during cycling is largely similar to that during development: Runx2 is expressed in dermal papillae and exhibits increasing asymmetric expression in bulb epithelium through anagen (Glotzer et al. 2008). Although Runx2 expression was detected at very low levels in freshly isolated CD34+integrin α6 + bulge cells (Hoi et al. 2010), its expression has not been detected in the bulge in vivo (Glotzer et al. 2008). Since there is some degree of overlap of expression of all three Runx genes, functional redundancy may operate in certain locations, such as the dermal papillae where both Runx2 and Runx3 are expressed. Hence, further in-depth analyses, such as analyses of Runx2;Runx3 double knockout mice are required to clarify the role of Runx in HFSCs.
In general, the expression of Runx1 seems to be non-overlapping with those of Runx2 and Runx3. Runx1 seems to play a role in both epithelial and mesenchymal compartments, thus regulating HFSC in both cell intrinsic and extrinsic manner , whereas the roles of Runx2 and Runx3 in potentially regulating HFSCs seem confined to cell extrinsic mechanisms.
3.3 Runx in Aging Hair Follicle Stem Cells
Even after repeated stimulation of skin wounding over a period of 1 year, Runx1 ∆4/∆4;K14-Cre hair follicles were able to continuously regenerate and result in new hair growth, suggesting that Runx1-deficient HFSCs maintained their long-term potential and did not result in the exhaustion of the stem cell pool (Osorio et al. 2008).
Although Runx proteins are not significantly differentially expressed with age in HFSCs , the transcription factor Nfatc1, implicated in hair follicle aging, was found to bind to the intronic regions of Runx1 (Keyes et al. 2013). In another study, the expression of type XVII collagen (COL17A1) decreased and/or its degradation or shedding by ELANE/elastase-2 (ELA2) increased with age in the HFSCs (Matsumura et al. 2016). As Runx1 has been demonstrated to transcriptionally activate ELA2 (Li et al. 2004; Lausen et al. 2006), Runx1 may function in aged HFSCs to promote aging . Since Runx1 poises HFSCs to respond rapidly to proliferation signals in young adult HFSCs, it will be interesting to find out if Runx is implicated in the prolonged telogen phase of aged HFSCs.
3.4 Runx and Skin Cancer
As HFSCs are a well appreciated source of skin appendage tumors such as basal cell carcinomas, Runx may be directly implicated in skin cancers (Lorz et al. 2009). Moreover, p21 encoded by Cdkn1a acts as a tumor suppressor in HFSCs (Topley et al. 1999) and Runx1 was found to negatively regulate Cdkn1a expression. As deletion of Cdkn1a only partially rescued the tumour impairment phenotype exhibited by Runx1 KO mice, other Runx1 targets are required for full tumour growth (Lee et al. 2013). Runx1 is also shown to be a downstream factor of p63 (Ortt et al. 2008), which is expressed at a high level in skin squamous cell carcinomas (Wrone et al. 2004). The p63 protein is a member of the p53 family and an essential factor for proper development of stratified epithelium (Mills et al. 1999; Yang et al. 1999). Unlike p53 (TP53) which acts as a tumor suppressor and is frequently mutated in cancers, p63 (TP63) is typically associated with gene amplification in cancers (Romano and Sinha 2014). It was shown that p63 binds to a Runx1 intronic enhancer and positively regulates its expression (Ortt et al. 2008). For the above reasons, it is plausible that Runx1 can act as an oncogene in skin tumorigenesis.
Indeed, Runx1 is highly expressed in mouse skin papilloma and squamous cell carcinomas (Hoi et al. 2010). The conditional loss of Runx1 in mouse epidermis impairs mouse skin tumorigenesis as mice in which Runx1 is deleted using K14-Cre showed drastically delayed and reduced skin papilloma and squamous cell carcinoma formation in response to skin DMBA/TPA carcinogenic treatment (Hoi et al. 2010). The Runx1 knockout bulge cells proliferated less than control cells even in response to stimulation by TPA, a strong proliferative-promoting agent (Hoi et al. 2010). Using timed deletion of Runx1 at different stages of tumorigenesis (initiation, promotion and maintenance), Scheitz et al. nicely demonstrated that Runx1 is specifically required at the initiation stage and its expression in HFSCs is responsible for initiating squamous cell carcinomas in mice (Scheitz et al. 2012). In the absence of proliferative agents, however, Runx1 is also crucial for the maintenance of CD34-expressing papilloma cancer stem cells (Scheitz et al. 2012). Absence of Runx1 under such circumstances causes tumour regression, leading to shrinkage of tumor size. In addition, knockdown of RUNX1 in the human skin cancer cell line, SCC13, renders the cells unable to grow (Scheitz et al. 2012). Further investigation revealed that RUNX1 acts to promote skin tumour growth by upregulating Stat3 activity via transcriptional repression of SOCS4 and potentially SOCS3 (Scheitz et al. 2012).
Overexpression of non-mutated RUNX3 has been reported in human basal cell carcinomas (Salto-Tellez et al. 2006). Whether the expression of RUNX3 is specifically required in the stem cell compartment for the development of this skin cancer subtype remains to be further investigated.
4 Future Perspectives
At least in HSCs and HFSCs , Runx seems to play converging roles, yet contrasting mechanisms operate. First, Runx1-expressing cells during early development mark stem cell precursors. Lack of Runx1 in the hematopoietic system led to complete failure in generating the blood system while lack of Runx1 in the skin led to impairment in generation of the mature hair follicle. Notably, the exact mechanism by which Runx1 affects blood and hair stem cells differs: Runx1 deficiency impairs stem cell emergence in the hematopoietic system in a cell intrinsic manner, whereas Runx1 ablation affects hair follicle development only via a cell extrinsic mechanism, indicating spatially different roles of Runx. Secondly, Runx1 works at the stem cell level to initiate adult stage of hematopoiesis and hair growth. Again, the mechanism differs: in young adults, Runx1 serves to promote proliferation in HFSCs, while it maintains quiescence in HSCs.
Such contrasting mechanisms in two different tissues, yet controlled by the same family of proteins, confound a proper understanding of fundamental core Runx functions. For example, while Hmga2 expression is upregulated in Runx-deficient HSCs, Runx seems to act in an opposite manner in HFSCs. In a similar scenario, p21 expression is positively regulated by Runx in HSCs but negatively in HFSCs. Possibly, intricate controls of the same pathway may be affected by other cell-type specific molecules, resulting in opposing effects.
In addition to HSCs and HFSCs, Runx function has also been detected in skeletal and mammary stem cells (Table 9.1). Our preliminary data suggest that Runx1 could be involved in stem cells of a wide spectrum of tissues, thus implying that Runx could potentially act as a global “master stem cell regulator”. Hence, it will be imperative to examine how Runx functions globally in the various tissue stem cells. By extending our knowledge of Runx roles in other tissue stem cells, we may be able to stratify their roles based on common machinery in regulating the stem cell compartment.
References
Alonso, L., & Fuchs, E. (2006). The hair cycle. Journal of Cell Science, 119(Pt 3), 391–393. doi:10.1242/jcs02793.
Arora, N., Wenzel, P. L., McKinney-Freeman, S. L., Ross, S. J., Kim, P. G., Chou, S. S., et al. (2014). Effect of developmental stage of HSC and recipient on transplant outcomes. Developmental Cell, 29(5), 621–628. doi:10.1016/j.devcel.2014.04.013.
Bernardin-Fried, F., Kummalue, T., Leijen, S., Collector, M. I., Ravid, K., & Friedman, A. D. (2004). AML1/RUNX1 increases during G1 to S cell cycle progression independent of cytokine-dependent phosphorylation and induces cyclin D3 gene expression. The Journal of Biological Chemistry, 279(15), 15678–15687. doi:10.1074/jbc.M310023200.
Birbrair, A., & Frenette, P. S. (2016). Niche heterogeneity in the bone marrow. Annals of the New York Academy of Sciences, 1370(1), 82–96. doi:10.1111/nyas.13016.
Bowie, M. B., McKnight, K. D., Kent, D. G., McCaffrey, L., Hoodless, P. A., & Eaves, C. J. (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. The Journal of Clinical Investigation, 116(10), 2808–2816. doi:10.1172/JCI28310.
Brunet, A., Sweeney, L. B., Sturgill, J. F., Chua, K. F., Greer, P. L., Lin, Y., et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science, 303(5666), 2011–2015. doi:10.1126/science.1094637.
Busch, K., Klapproth, K., Barile, M., Flossdorf, M., Holland-Letz, T., Schlenner, S. M., et al. (2015). Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature, 518(7540), 542–546. doi:10.1038/nature14242.
Cai, X., Gaudet, J. J., Mangan, J. K., Chen, M. J., De Obaldia, M. E., Oo, Z., et al. (2011). Runx1 loss minimally impacts long-term hematopoietic stem cells. PloS One, 6(12), e28430. doi:10.1371/journal.pone.0028430.
Chambers, S. M., Shaw, C. A., Gatza, C., Fisk, C. J., Donehower, L. A., & Goodell, M. A. (2007). Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biology, 5(8), e201. doi:10.1371/journal.pbio.0050201.
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E., & Speck, N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature, 457(7231), 887–891. doi:10.1038/nature07619.
Chen, T., Heller, E., Beronja, S., Oshimori, N., Stokes, N., & Fuchs, E. (2012). An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature, 485(7396), 104–108. doi:10.1038/nature10940.
Chien, Y., Scuoppo, C., Wang, X., Fang, X., Balgley, B., Bolden, J. E., et al. (2011). Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes & Development, 25(20), 2125–2136. doi:10.1101/gad.17276711.
Chin, D. W., Sakurai, M., Nah, G. S., Du, L., Jacob, B., Yokomizo, T., et al. (2016). RUNX1 haploinsufficiency results in granulocyte colony-stimulating factor hypersensitivity. Blood Cancer Journal, 6, e379. doi:10.1038/bcj.2015.105.
Chitteti, B. R., Cheng, Y. H., Streicher, D. A., Rodriguez-Rodriguez, S., Carlesso, N., Srour, E. F., & Kacena, M. A. (2010). Osteoblast lineage cells expressing high levels of Runx2 enhance hematopoietic progenitor cell proliferation and function. Journal of Cellular Biochemistry, 111(2), 284–294. doi:10.1002/jcb.22694.
Christensen, J. L., Wright, D. E., Wagers, A. J., & Weissman, I. L. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biology, 2(3), E75. doi:10.1371/journal.pbio.0020075.
Copley, M. R., Babovic, S., Benz, C., Knapp, D. J., Beer, P. A., Kent, D. G., et al. (2013). The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nature Cell Biology, 15(8), 916–925. doi:10.1038/ncb2783.
Croker, B. A., Metcalf, D., Robb, L., Wei, W., Mifsud, S., DiRago, L., et al. (2004). SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity, 20(2), 153–165.
Cumano, A., & Godin, I. (2007). Ontogeny of the hematopoietic system. Annual Review of Immunology, 25, 745–785. doi:10.1146/annurev.immunol.25.022106.141538.
de Boer, J., Williams, A., Skavdis, G., Harker, N., Coles, M., Tolaini, M., et al. (2003). Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European Journal of Immunology, 33(2), 314–325. doi:10.1002/immu.200310005.
Deguchi, K., Yagi, H., Inada, M., Yoshizaki, K., Kishimoto, T., & Komori, T. (1999). Excessive extramedullary hematopoiesis in Cbfa1-deficient mice with a congenital lack of bone marrow. Biochemical and Biophysical Research Communications, 255(2), 352–359. doi:10.1006/bbrc.1999.0163.
Dykstra, B., Olthof, S., Schreuder, J., Ritsema, M., & de Haan, G. (2011). Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. The Journal of Experimental Medicine, 208(13), 2691–2703. doi:10.1084/jem.20111490.
Dzierzak, E., & Speck, N. A. (2008). Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nature Immunology, 9(2), 129–136. doi:10.1038/ni1560.
Ferrari, N., Riggio, A. I., Mason, S., McDonald, L., King, A., Higgins, T., et al. (2015). Runx2 contributes to the regenerative potential of the mammary epithelium. Scientific Reports, 5, 15658. doi:10.1038/srep15658.
Flachsbart, F., Caliebe, A., Kleindorp, R., Blanche, H., von Eller-Eberstein, H., Nikolaus, S., et al. (2009). Association of FOXO3A variation with human longevity confirmed in German centenarians. Proceedings of the National Academy of Sciences of the United States of America, 106(8), 2700–2705. doi:10.1073/pnas.0809594106.
Forni, M. F., Trombetta-Lima, M., & Sogayar, M. C. (2012). Stem cells in embryonic skin development. Biological Research, 45(3), 215–222. doi:10.4067/S0716-97602012000300003.
Fusco, A., & Fedele, M. (2007). Roles of HMGA proteins in cancer. Nature Reviews. Cancer, 7(12), 899–910. doi:10.1038/nrc2271.
Galindo, M., Pratap, J., Young, D. W., Hovhannisyan, H., Im, H. J., Choi, J. Y., et al. (2005). The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. The Journal of Biological Chemistry, 280(21), 20274–20285. doi:10.1074/jbc.M413665200.
Geiger, H., de Haan, G., & Florian, M. C. (2013). The ageing haematopoietic stem cell compartment. Nature Reviews. Immunology, 13(5), 376–389. doi:10.1038/nri3433.
Gekas, C., Dieterlen-Lievre, F., Orkin, S. H., & Mikkola, H. K. (2005). The placenta is a niche for hematopoietic stem cells. Developmental Cell, 8(3), 365–375. doi:10.1016/j.devcel.2004.12.016.
Genovese, G., Kahler, A. K., Handsaker, R. E., Lindberg, J., Rose, S. A., Bakhoum, S. F., et al. (2014). Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England Journal of Medicine, 371(26), 2477–2487. doi:10.1056/NEJMoa1409405.
Georgiades, P., Ogilvy, S., Duval, H., Licence, D. R., Charnock-Jones, D. S., Smith, S. K., & Print, C. G. (2002). VavCre transgenic mice: A tool for mutagenesis in hematopoietic and endothelial lineages. Genesis, 34(4), 251–256. doi:10.1002/gene.10161.
Glotzer, D. J., Zelzer, E., & Olsen, B. R. (2008). Impaired skin and hair follicle development in Runx2 deficient mice. Developmental Biology, 315(2), 459–473. doi:10.1016/j.ydbio.2008.01.005.
Growney, J. D., Shigematsu, H., Li, Z., Lee, B. H., Adelsperger, J., Rowan, R., et al. (2005). Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood, 106(2), 494–504. doi:10.1182/blood-2004-08-3280.
Haigis, M. C., & Guarente, L. P. (2006). Mammalian sirtuins – emerging roles in physiology, aging, and calorie restriction. Genes & Development, 20(21), 2913–2921. doi:10.1101/gad.1467506.
Hock, H., Hamblen, M. J., Rooke, H. M., Schindler, J. W., Saleque, S., Fujiwara, Y., & Orkin, S. H. (2004). Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature, 431(7011), 1002–1007. doi:10.1038/nature02994.
Hoi, C. S., Lee, S. E., Lu, S. Y., McDermitt, D. J., Osorio, K. M., Piskun, C. M., et al. (2010). Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Molecular and Cellular Biology, 30(10), 2518–2536. doi:10.1128/MCB.01308-09.
Ichikawa, M., Asai, T., Saito, T., Seo, S., Yamazaki, I., Yamagata, T., et al. (2004). AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nature Medicine, 10(3), 299–304. doi:10.1038/nm997.
Iwasaki, H., Arai, F., Kubota, Y., Dahl, M., & Suda, T. (2010). Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood, 116(4), 544–553. doi:10.1182/blood-2009-08-240903.
Jacob, B., Osato, M., Yamashita, N., Wang, C. Q., Taniuchi, I., Littman, D. R., et al. (2010). Stem cell exhaustion due to Runx1 deficiency is prevented by Evi5 activation in leukemogenesis. Blood, 115(8), 1610–1620. doi:10.1182/blood-2009-07-232249.
Jaffredo, T., Nottingham, W., Liddiard, K., Bollerot, K., Pouget, C., & de Bruijn, M. (2005). From hemangioblast to hematopoietic stem cell: an endothelial connection? Experimental Hematology, 33(9), 1029–1040. doi:10.1016/j.exphem.2005.06.005.
Jaiswal, S., Fontanillas, P., Flannick, J., Manning, A., Grauman, P. V., Mar, B. G., et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. The New England Journal of Medicine, 371(26), 2488–2498. doi:10.1056/NEJMoa1408617.
Janzen, V., Forkert, R., Fleming, H. E., Saito, Y., Waring, M. T., Dombkowski, D. M., et al. (2006). Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature, 443(7110), 421–426. doi:10.1038/nature05159.
Kanaykina, N., Abelson, K., King, D., Liakhovitskaia, A., Schreiner, S., Wegner, M., & Kozlova, E. N. (2010). In vitro and in vivo effects on neural crest stem cell differentiation by conditional activation of Runx1 short isoform and its effect on neuropathic pain behavior. Upsala Journal of Medical Sciences, 115(1), 56–64. doi:10.3109/03009730903572065.
Keyes, B. E., Segal, J. P., Heller, E., Lien, W. H., Chang, C. Y., Guo, X., et al. (2013). Nfatc1 orchestrates aging in hair follicle stem cells. Proceedings of the National Academy of Sciences of the United States of America, 110(51), E4950–E4959. doi:10.1073/pnas.1320301110.
Khan, J. A., Mendelson, A., Kunisaki, Y., Birbrair, A., Kou, Y., Arnal-Estape, A., et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science, 351(6269), 176–180. doi:10.1126/science.aad0084.
Kieusseian, A., Brunet de la Grange, P., Burlen-Defranoux, O., Godin, I., & Cumano, A. (2012). Immature hematopoietic stem cells undergo maturation in the fetal liver. Development, 139(19), 3521–3530. doi:10.1242/dev.079210.
Kim, I., Saunders, T. L., & Morrison, S. J. (2007). Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell, 130(3), 470–483. doi:10.1016/j.cell.2007.06.011.
Kissa, K., & Herbomel, P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature, 464(7285), 112–115. doi:10.1038/nature08761.
Koh, C. P., Ng, C. E., Nah, G. S., Wang, C. Q., Tergaonkar, V., Matsumura, T., et al. (2015). Hematopoietic stem cell enhancer: a powerful tool in stem cell biology. Histology Histopathology, 30(6), 661–672. doi:10.14670/HH-30.661.
Komeno, Y., Yan, M., Matsuura, S., Lam, K., Lo, M. C., Huang, Y. J., et al. (2014). Runx1 exon 6-related alternative splicing isoforms differentially regulate hematopoiesis in mice. Blood, 123(24), 3760–3769. doi:10.1182/blood-2013-08-521252.
Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 89(5), 755–764.
Lam, K., Muselman, A., Du, R., Harada, Y., Scholl, A. G., Yan, M., et al. (2014). Hmga2 is a direct target gene of RUNX1 and regulates expansion of myeloid progenitors in mice. Blood, 124(14), 2203–2212. doi:10.1182/blood-2014-02-554543.
Lausen, J., Liu, S., Fliegauf, M., Lubbert, M., & Werner, M. H. (2006). ELA2 is regulated by hematopoietic transcription factors, but not repressed by AML1-ETO. Oncogene, 25(9), 1349–1357. doi:10.1038/sj.onc.1209181.
Lecuyer, E., & Hoang, T. (2004). SCL: From the origin of hematopoiesis to stem cells and leukemia. Experimental Hematology, 32(1), 11–24.
Lee, J., Hoi, C. S., Lilja, K. C., White, B. S., Lee, S. E., Shalloway, D., & Tumbar, T. (2013). Runx1 and p21 synergistically limit the extent of hair follicle stem cell quiescence in vivo. Proceedings of the National Academy of Sciences of the United States of America, 110(12), 4634–4639. doi:10.1073/pnas.1213015110.
Lee, S. E., Sada, A., Zhang, M., McDermitt, D. J., Lu, S. Y., Kemphues, K. J., & Tumbar, T. (2014). High Runx1 levels promote a reversible, more-differentiated cell state in hair-follicle stem cells during quiescence. Cell Reports, 6(3), 499–513. doi:10.1016/j.celrep.2013.12.039.
Levanon, D., Brenner, O., Negreanu, V., Bettoun, D., Woolf, E., Eilam, R., et al. (2001). Spatial and temporal expression pattern of Runx3 (Aml2) and Runx1 (Aml1) indicates non-redundant functions during mouse embryogenesis. Mechanisms of Development, 109(2), 413–417.
Li, F. Q., Person, R. E., Takemaru, K., Williams, K., Meade-White, K., Ozsahin, A. H., et al. (2004). Lymphoid enhancer factor-1 links two hereditary leukemia syndromes through core-binding factor alpha regulation of ELA2. The Journal of Biological Chemistry, 279(4), 2873–2884. doi:10.1074/jbc.M310759200.
Liakhovitskaia, A., Gribi, R., Stamateris, E., Villain, G., Jaffredo, T., Wilkie, R., et al. (2009). Restoration of Runx1 expression in the Tie2 cell compartment rescues definitive hematopoietic stem cells and extends life of Runx1 knockout animals until birth. Stem Cells, 27(7), 1616–1624. doi:10.1002/stem.71.
Liakhovitskaia, A., Lana-Elola, E., Stamateris, E., Rice, D. P., van't Hof, R. J., & Medvinsky, A. (2010). The essential requirement for Runx1 in the development of the sternum. Developmental Biology, 340(2), 539–546. doi:10.1016/j.ydbio.2010.02.005.
Liakhovitskaia, A., Rybtsov, S., Smith, T., Batsivari, A., Rybtsova, N., Rode, C., et al. (2014). Runx1 is required for progression of CD41+ embryonic precursors into HSCs but not prior to this. Development, 141(17), 3319–3323. doi:10.1242/dev.110841.
Linggi, B., Muller-Tidow, C., van de Locht, L., Hu, M., Nip, J., Serve, H., et al. (2002). The t(8;21) fusion protein, AML1 ETO, specifically represses the transcription of the p14(ARF) tumor suppressor in acute myeloid leukemia. Nature Medicine, 8(7), 743–750. doi:10.1038/nm726.
Loonstra, A., Vooijs, M., Beverloo, H. B., Allak, B. A., van Drunen, E., Kanaar, R., et al. (2001). Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America, 98(16), 9209–9214. doi:10.1073/pnas.161269798.
Lorz, C., Segrelles, C., & Paramio, J. M. (2009). On the origin of epidermal cancers. Current Molecular Medicine, 9(3), 353–364.
Malanchi, I., Peinado, H., Kassen, D., Hussenet, T., Metzger, D., Chambon, P., et al. (2008). Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature, 452(7187), 650–653. doi:10.1038/nature06835.
Martinez-Agosto, J. A., Mikkola, H. K., Hartenstein, V., & Banerjee, U. (2007). The hematopoietic stem cell and its niche: A comparative view. Genes & Development, 21(23), 3044–3060. doi:10.1101/gad.1602607.
Matsumura, H., Mohri, Y., Binh, N. T., Morinaga, H., Fukuda, M., Ito, M., et al. (2016). Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science, 351(6273), aad4395. doi:10.1126/science.aad4395.
Matsuo, J., Kimura, S., Yamamura, A., Koh, C., Hossain, M., Heng, D., Kohu, K., Voon, D., Hiai, H., Unno, M., So, J., Zhu, F., Srivastava, S., Meng, T., Yeoh, K., Osato, M., & Ito, Y. (2016) Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology, 152(1), 218–231. doi:10.1053/j.gastro.2016.09.018.
Mills, A. A., Zheng, B., Wang, X. J., Vogel, H., Roop, D. R., & Bradley, A. (1999). p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature, 398(6729), 708–713. doi:10.1038/19531.
Morris, R. J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., et al. (2004). Capturing and profiling adult hair follicle stem cells. Nature Biotechnology, 22(4), 411–417. doi:10.1038/nbt950.
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A., & Weissman, I. L. (1996). The aging of hematopoietic stem cells. Nature Medicine, 2(9), 1011–1016.
Mrozek, K., Marcucci, G., Nicolet, D., Maharry, K. S., Becker, H., Whitman, S. P., et al. (2012). Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. Journal of Clinical Oncology, 30(36), 4515–4523. doi:10.1200/JCO.2012.43.4738.
Nakagawa, M., Shimabe, M., Watanabe-Okochi, N., Arai, S., Yoshimi, A., Shinohara, A., et al. (2011). AML1/RUNX1 functions as a cytoplasmic attenuator of NF-kappaB signaling in the repression of myeloid tumors. Blood, 118(25), 6626–6637. doi:10.1182/blood-2010-12-326710.
Ng, C. E., Yokomizo, T., Yamashita, N., Cirovic, B., Jin, H., Wen, Z., et al. (2010). A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells, 28(10), 1869–1881. doi:10.1002/stem.507.
Niki, M., Okada, H., Takano, H., Kuno, J., Tani, K., Hibino, H., et al. (1997). Hematopoiesis in the fetal liver is impaired by targeted mutagenesis of a gene encoding a non-DNA binding subunit of the transcription factor, polyomavirus enhancer binding protein 2/core binding factor. Proceedings of the National Academy of Sciences of the United States of America, 94(11), 5697–5702.
Nishino, J., Kim, I., Chada, K., & Morrison, S. J. (2008). Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell, 135(2), 227–239. doi:10.1016/j.cell.2008.09.017.
North, T., Gu, T. L., Stacy, T., Wang, Q., Howard, L., Binder, M., et al. (1999). Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development, 126(11), 2563–2575.
Nowak, J. A., Polak, L., Pasolli, H. A., & Fuchs, E. (2008). Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell, 3(1), 33–43. doi:10.1016/j.stem.2008.05.009.
Okada, H., Watanabe, T., Niki, M., Takano, H., Chiba, N., Yanai, N., et al. (1998). AML1(−/−) embryos do not express certain hematopoiesis-related gene transcripts including those of the PU.1 gene. Oncogene, 17(18), 2287–2293. doi:10.1038/sj.onc.1202151.
Okuda, T., van Deursen, J., Hiebert, S. W., Grosveld, G., & Downing, J. R. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell, 84(2), 321–330.
Orkin, S. H., & Zon, L. I. (2008). Hematopoiesis: An evolving paradigm for stem cell biology. Cell, 132(4), 631–644. doi:10.1016/j.cell.2008.01.025.
Ortt, K., Raveh, E., Gat, U., & Sinha, S. (2008). A chromatin immunoprecipitation screen in mouse keratinocytes reveals Runx1 as a direct transcriptional target of DeltaNp63. Journal of Cellular Biochemistry, 104(4), 1204–1219. doi:10.1002/jcb.21700.
Osato, M. (2014). An unsung runt 6e isoform for HSC expansion. Blood, 123(24), 3684–3686. doi:10.1182/blood-2014-05-572891.
Osorio, K. M., Lee, S. E., McDermitt, D. J., Waghmare, S. K., Zhang, Y. V., Woo, H. N., & Tumbar, T. (2008). Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Development, 135(6), 1059–1068. doi:10.1242/dev.012799.
Osorio, K. M., Lilja, K. C., & Tumbar, T. (2011). Runx1 modulates adult hair follicle stem cell emergence and maintenance from distinct embryonic skin compartments. The Journal of Cell Biology, 193(1), 235–250. doi:10.1083/jcb.201006068.
Ottersbach, K., & Dzierzak, E. (2005). The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Developmental Cell, 8(3), 377–387. doi:10.1016/j.devcel.2005.02.001.
Otto, F., Thornell, A. P., Crompton, T., Denzel, A., Gilmour, K. C., Rosewell, I. R., et al. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 89(5), 765–771.
Park, I. K., Qian, D., Kiel, M., Becker, M. W., Pihalja, M., Weissman, I. L., et al. (2003). Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature, 423(6937), 302–305. doi:10.1038/nature01587.
Puri, M. C., & Bernstein, A. (2003). Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 100(22), 12753–12758. doi:10.1073/pnas.2133552100.
Raveh, E., Cohen, S., Levanon, D., Groner, Y., & Gat, U. (2005). Runx3 is involved in hair shape determination. Developmental Dynamics, 233(4), 1478–1487. doi:10.1002/dvdy.20453.
Raveh, E., Cohen, S., Levanon, D., Negreanu, V., Groner, Y., & Gat, U. (2006). Dynamic expression of Runx1 in skin affects hair structure. Mechanisms of Development, 123(11), 842–850. doi:10.1016/j.mod.2006.08.002.
Rhodes, K. E., Gekas, C., Wang, Y., Lux, C. T., Francis, C. S., Chan, D. N., et al. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell, 2(3), 252–263. doi:10.1016/j.stem.2008.01.001.
Romano, R. A., & Sinha, S. (2014). Family matters: Sibling rivalry and bonding between p53 and p63 in cancer. Experimental Dermatology, 23(4), 238–239. doi:10.1111/exd.12356.
Rossi, D. J., Bryder, D., Zahn, J. M., Ahlenius, H., Sonu, R., Wagers, A. J., & Weissman, I. L. (2005). Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9194–9199. doi:10.1073/pnas.0503280102.
Rybtsov, S., Sobiesiak, M., Taoudi, S., Souilhol, C., Senserrich, J., Liakhovitskaia, A., et al. (2011). Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. The Journal of Experimental Medicine, 208(6), 1305–1315. doi:10.1084/jem.20102419.
Salto-Tellez, M., Peh, B. K., Ito, K., Tan, S. H., Chong, P. Y., Han, H. C., et al. (2006). RUNX3 protein is overexpressed in human basal cell carcinomas. Oncogene, 25(58), 7646–7649. doi:10.1038/sj.onc.1209739.
Sasaki, K., Yagi, H., Bronson, R. T., Tominaga, K., Matsunashi, T., Deguchi, K., et al. (1996). Absence of fetal liver hematopoiesis in mice deficient in transcriptional coactivator core binding factor beta. Proceedings of the National Academy of Sciences of the United States of America, 93(22), 12359–12363.
Sato, M., Morii, E., Komori, T., Kawahata, H., Sugimoto, M., Terai, K., et al. (1998). Transcriptional regulation of osteopontin gene in vivo by PEBP2alphaA/CBFA1 and ETS1 in the skeletal tissues. Oncogene, 17(12), 1517–1525. doi:10.1038/sj.onc.1202064.
Scheitz, C. J., Lee, T. S., McDermitt, D. J., & Tumbar, T. (2012). Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. The EMBO Journal, 31(21), 4124–4139. doi:10.1038/emboj.2012.270.
Sennett, R., & Rendl, M. (2012). Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Seminars in Cell & Developmental Biology, 23(8), 917–927. doi:10.1016/j.semcdb.2012.08.011.
Shakibaei, M., Shayan, P., Busch, F., Aldinger, C., Buhrmann, C., Lueders, C., & Mobasheri, A. (2012). Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PloS One, 7(4), e35712. doi:10.1371/journal.pone.0035712.
Shimshek, D. R., Kim, J., Hubner, M. R., Spergel, D. J., Buchholz, F., Casanova, E., et al. (2002). Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis, 32(1), 19–26.
Silver, D. P., & Livingston, D. M. (2001). Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Molecular Cell, 8(1), 233–243.
Stadtfeld, M., & Graf, T. (2005). Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development, 132(1), 203–213. doi:10.1242/dev.01558.
Strom, D. K., Nip, J., Westendorf, J. J., Linggi, B., Lutterbach, B., Downing, J. R., et al. (2000). Expression of the AML-1 oncogene shortens the G(1) phase of the cell cycle. The Journal of Biological Chemistry, 275(5), 3438–3445.
Sun, J., Ramos, A., Chapman, B., Johnnidis, J. B., Le, L., Ho, Y. J., et al. (2014). Clonal dynamics of native haematopoiesis. Nature, 514(7522), 322–327. doi:10.1038/nature13824.
Tan, L., Wei, X., Zheng, L., Zeng, J., Liu, H., Yang, S., & Tan, H. (2016). Amplified HMGA2 promotes cell growth by regulating Akt pathway in AML. Journal of Cancer Research and Clinical Oncology, 142(2), 389–399. doi:10.1007/s00432-015-2036-9.
Taoudi, S., Gonneau, C., Moore, K., Sheridan, J. M., Blackburn, C. C., Taylor, E., & Medvinsky, A. (2008). Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell, 3(1), 99–108. doi:10.1016/j.stem.2008.06.004.
Tober, J., Yzaguirre, A. D., Piwarzyk, E., & Speck, N. A. (2013). Distinct temporal requirements for Runx1 in hematopoietic progenitors and stem cells. Development, 140(18), 3765–3776. doi:10.1242/dev.094961.
Toma, J. G., Akhavan, M., Fernandes, K. J., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R., & Miller, F. D. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell Biology, 3(9), 778–784. doi:10.1038/ncb0901-778.
Topley, G. I., Okuyama, R., Gonzales, J. G., Conti, C., & Dotto, G. P. (1999). p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proceedings of the National Academy of Sciences of the United States of America, 96(16), 9089–9094.
Trempus, C. S., Morris, R. J., Bortner, C. D., Cotsarelis, G., Faircloth, R. S., Reece, J. M., & Tennant, R. W. (2003). Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. The Journal of Investigative Dermatology, 120(4), 501–511. doi:10.1046/j.1523-1747.2003.12088.x.
Tseng, P. C., Hou, S. M., Chen, R. J., Peng, H. W., Hsieh, C. F., Kuo, M. L., & Yen, M. L. (2011). Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. Journal of Bone and Mineral Research, 26(10), 2552–2563. doi:10.1002/jbmr.460.
van Bragt, M. P., Hu, X., Xie, Y., & Li, Z. (2014). RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells. eLife, 3, e03881. doi:10.7554/eLife.03881.
Wang, Q., Stacy, T., Binder, M., Marin-Padilla, M., Sharpe, A. H., & Speck, N. A. (1996a). Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 93(8), 3444–3449.
Wang, Q., Stacy, T., Miller, J. D., Lewis, A. F., Gu, T. L., Huang, X., et al. (1996b). The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell, 87(4), 697–708.
Wang, C. Q., Jacob, B., Nah, G. S., & Osato, M. (2010). Runx family genes, niche, and stem cell quiescence. Blood Cells, Molecules & Diseases, 44(4), 275–286. doi:10.1016/j.bcmd.2010.01.006.
Wang, C. Q., Motoda, L., Satake, M., Ito, Y., Taniuchi, I., Tergaonkar, V., & Osato, M. (2013). Runx3 deficiency results in myeloproliferative disorder in aged mice. Blood, 122(4), 562–566. doi:10.1182/blood-2012-10-460618.
Wang, C. Q., Krishnan, V., Tay, L. S., Chin, D. W., Koh, C. P., Chooi, J. Y., et al. (2014). Disruption of Runx1 and Runx3 leads to bone marrow failure and leukemia predisposition due to transcriptional and DNA repair defects. Cell Reports, 8(3), 767–782. doi:10.1016/j.celrep.2014.06.046.
Wang, C. Q., Chin, D. W., Chooi, J. Y., Chng, W. J., Taniuchi, I., Tergaonkar, V., & Osato, M. (2015). Cbfb deficiency results in differentiation blocks and stem/progenitor cell expansion in hematopoiesis. Leukemia, 29(3), 753–757. doi:10.1038/leu.2014.316.
Worthley, D. L., Churchill, M., Compton, J. T., Tailor, Y., Rao, M., Si, Y., et al. (2015). Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell, 160(1–2), 269–284. doi:10.1016/j.cell.2014.11.042.
Wrone, D. A., Yoo, S., Chipps, L. K., & Moy, R. L. (2004). The expression of p63 in actinic keratoses, seborrheic keratosis, and cutaneous squamous cell carcinomas. Dermatologic Surgery, 30(10), 1299–1302. doi:10.1111/j.1524-4725.2004.30403.x.
Xie, M., Lu, C., Wang, J., McLellan, M. D., Johnson, K. J., Wendl, M. C., et al. (2014). Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature Medicine, 20(12), 1472–1478. doi:10.1038/nm.3733.
Yamamura, Y., Lee, W. L., Inoue, K., Ida, H., & Ito, Y. (2006). RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. The Journal of Biological Chemistry, 281(8), 5267–5276. doi:10.1074/jbc.M512151200.
Yang, A., Schweitzer, R., Sun, D., Kaghad, M., Walker, N., Bronson, R. T., et al. (1999). p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature, 398(6729), 714–718. doi:10.1038/19539.
Ye, M., Zhang, H., Amabile, G., Yang, H., Staber, P. B., Zhang, P., et al. (2013). C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nature Cell Biology, 15(4), 385–394. doi:10.1038/ncb2698.
Yokomizo, T., & Dzierzak, E. (2010). Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development, 137(21), 3651–3661. doi:10.1242/dev.051094.
Yokomizo, T., Ogawa, M., Osato, M., Kanno, T., Yoshida, H., Fujimoto, T., et al. (2001). Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes to Cells, 6(1), 13–23.
Yu, H., Lim, H. H., Tjokro, N. O., Sathiyanathan, P., Natarajan, S., Chew, T. W., et al. (2014). Chaperoning HMGA2 protein protects stalled replication forks in stem and cancer cells. Cell Reports, 6(4), 684–697. doi:10.1016/j.celrep.2014.01.014.
Zhang, Y. V., Cheong, J., Ciapurin, N., McDermitt, D. J., & Tumbar, T. (2009). Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell, 5(3), 267–278. doi:10.1016/j.stem.2009.06.004.
Zhou, F., Li, X., Wang, W., Zhu, P., Zhou, J., He, W., et al. (2016). Tracing haematopoietic stem cell formation at single-cell resolution. Nature, 533(7604), 487–492. doi:10.1038/nature17997.
Acknowledgements
We thank members of MD2 Vivarium, NUS, for mouse husbandry. We thank Tumbar T., Nishimura E. and Speck NA for their careful and critical reading of our manuscript. This work was supported by National Medical Research Council, Biomedical Research Council, A*STAR (Agency of Science, Technology and Research), the National Research Foundation Singapore, the Singapore Ministry of Education under its Research Centres of Excellence initiative, and JSPS Kakenhi Grant Numbers JP15H04312 and JP16K14613, Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wang, C.Q., Mok, M.M.H., Yokomizo, T., Tergaonkar, V., Osato, M. (2017). Runx Family Genes in Tissue Stem Cell Dynamics. In: Groner, Y., Ito, Y., Liu, P., Neil, J., Speck, N., van Wijnen, A. (eds) RUNX Proteins in Development and Cancer. Advances in Experimental Medicine and Biology, vol 962. Springer, Singapore. https://doi.org/10.1007/978-981-10-3233-2_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-3233-2_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3231-8
Online ISBN: 978-981-10-3233-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)