Abstract
Core–shell protein cluster comprising bovine hemoglobin (HbBv) in the core and human serum albumin (HSA) at the shell was created as an artificial O2 carrier designed for use as a red blood cell (RBC) substitute. The protein cluster was prepared by covalent linkage between the Cys-34 residue of HSA and the surface Lys amino groups of HbBv using heterobifunctional cross-linker. The average HSA/HbBv ratio of one cluster was determined as 3.0 ± 0.2; therefore we indicated this hemoglobin–albumin cluster as HbBv–HSA 3 . Human Hb A (HbA) can be also used for a core protein to synthesize HbA–HSA 3 cluster. The isoelectric point of HbBv–HSA 3 (pI = 5.1) was markedly lower than that of HbBv and almost identical to the value of HSA. SFM and TEM measurements revealed a triangular shape of HbBv–HSA3. The complete 3D structure based on TEM data was reconstructed. The clusters showed moderately higher O2 affinities than the native HbBv and HbA. Viscosity and blood cell counting measurements demonstrated that HbBv–HSA 3 has good compatibility with whole blood. Intravenous administration of HbBv–HSA 3 into anesthetized rats elicited no unfavorable increase in systemic blood pressure by vasoconstriction. The half-life of 125I-labeled cluster in circulating blood is longer than that of HSA. All results indicate that HbBv–HSA 3 has sufficient preclinical safety as an alternative material for RBC transfusion. Interestingly, clusters prepared under N2 atmosphere showed low O2 affinity resembling human RBC. Furthermore, the exterior HSA units possess a remarkable ability to bind antioxidant agent, such as Pt nanoparticle (PtNP). The peripheral HSA–PtNP shell prevents oxidation of the core HbBv, which enables the formation of an extremely stable O2 complex even in H2O2 solution. This chapter reviews the synthesis, structure, O2-binding property, and preclinical safety of hemoglobin–albumin cluster as a promising RBC substitute for practical use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Over the last few decades, hemoglobin (Hb)-based O2 carriers (HBOCs) of many kinds have been designed and developed as red blood cell (RBC) substitutes (Squires 2002; Jahr et al. 2011; Kluger and Lui 2013; Mondery-Pawlowski et al. 2013), such as intramolecularly cross-linked Hb (Snyder et al. 1987; Nagababu et al. 2002), polymerized Hb (Buehler et al. 2005; Pearce et al. 2006; Kluger and Zhang 2003; Hu and Kluger 2008), poly(ethylene glycol)-decorated Hb (Vandegriff et al. 2003; Manjula et al. 2003; Li et al. 2008, 2009), enzyme-conjugated Hb (D’Agnilloo and Chang 1998; Alagic et al. 2005), saccharide-linked Hb (Zhang et al. 2008), and nano-/microparticle-encapsulated Hb (Sakai 2012; Xiong et al. 2013). In any period, the social requests have promoted the development of the RBC substitute, for example, a need in battlefield, a concern to virus diffusion, and a primary measure for crisis management. Currently, the motive of the artificial O2 carrier is moving to a medical measure to supplement blood transfusion treatment. The declining birthrate and aging population make it difficult to retain a stable blood transfusion system. The number of old people will continue to increase, although the population of blood donors is expected to decrease. In fact, the Japanese Red Cross Society predicts a blood shortage equivalent to 890,000 people per year in 2027 (Ministry of Health, Labor and Welfare, Japan 2014). However, no HBOC product has been assigned yet for medical use (Jahr et al. 2011; Pearce et al. 2006; Natanson et al. 2008; Kluger 2010). The major concern of the Hb derivatives is vasoconstriction, which causes a mild increase in systemic blood pressure. This pressor response is inferred to be due to quick scavenging of nitric oxide (NO), the endothelial-derived relaxing factor, by Hb leaked into the extravascular space (Shultz et al. 1993; Rohlfs et al. 1998; Doherty et al. 1998).
Recently, we prepared a covalent core–shell structured protein cluster composed of Hb in the core and human serum albumin (HSA) at the shell as a unique HBOC (Fig. 9.1) (Tomita et al. 2013; Hosaka et al. 2014; Haruki et al. 2015). The average HSA/Hb ratio of one cluster was 3.0 ± 0.2. We indicate this hemoglobin–albumin cluster as Hb–HSA 3 . It is noteworthy that intravenous transfusion of Hb–HSA 3 does not elicit the acute increase in blood pressure (Haruki et al. 2015). This is attributed to the fact that Hb–HSA 3 is not eliminated from the vasculature walls because of the electrostatic repulsion between the negative surface net charges of the cluster and the glomerular basement membrane around the endothelial cells. This chapter reviews the synthesis, structure, O2-binding property, and preclinical safety of Hb–HSA 3 as a promising RBC substitute for practical use.
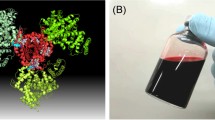
(A) Illustration of molecular structure of HbBv–HSA 3 in which an HbBv core is wrapped covalently by three HSAs (Tomita et al. 2013). (B) HbBv–HSA 3 solution (20 g/dL) in PBS (pH 7.4)
9.2 Synthesis and Structure of Hemoglobin–Albumin Cluster
HSA is a heart-shaped monomeric protein bearing one free sulfhydryl group of Cys at position 34 (Curry et al. 1998). Therefore, we used a heterobifunctional cross-linking agent, N-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC), as a connector between the Cys-34 residue of HSA and the surface Lys amino groups of Hb to create a core–shell cluster of Hb and HSA (Fig. 9.2) (Tomita et al. 2013; Hosaka et al. 2014). First, SMCC was reacted with bovine Hb (HbBv) in phosphate-buffered saline (PBS, pH 7.4) solution. Then the resulting maleimide-activated HbBv was added dropwise into the HSA solution, followed by stirring at 4 °C. Size-exclusion chromatography (SEC) of the resultant mixture showed distinct three peaks at the high molecular weight region. Native PAGE also exhibited three new bands above HSA (Tomita et al. 2013; Hosaka et al. 2014). Using gel filtration chromatography (GFC), unreacted HSA was removed and the major products were collected. Based on the Hb assay and protein assay, the HSA/HbBv ratio (mol/mol) of the cluster was determined to be 3.0 ± 0.2. We indicate this product as Hb–HSA 3 with italicized subscript 3. Reaction with human adult Hb (HbA) also generated similar protein cluster, HbA–HSA 3 (Kimura et al. 2015).
The CD spectrum of HbBv–HSA 3 fit perfectly with the sum of the HbBv spectrum and a threefold-enlarged HSA spectrum (Tomita et al. 2013; Hosaka et al. 2014). This result implies that (i) the HbBv:HSA ratio in one cluster is 1:3 (mol:mol) on average and (ii) the secondary structure of the individual protein unit remains constant after the cluster formation. The isoelectric points of HbBv–HSA 3 (pI: 5.1) were markedly lower than the value of native HbBv (pI, 7.0) and resembled to that of HSA (pI: 4.9). These results supported that the HbBv core is covalently wrapped by HSAs (Tomita et al. 2013; Hosaka et al. 2014).
Scanning probe microscopy (SPM) images of HbBv–HSA3 on a mica surface in PBS solution depicted clearly triangular shape of several entities (Fig. 9.3A) (Tomita et al. 2013). We were convinced of a triangular core–shell structure with HbBv in the center and three exterior HSAs are formed.
(A) SPM image of HbBv–HSA3 bound on mica surface in PBS (pH 7.4) solution at 25 °C. (B) A selected class sum image of HbBv–HSA3. (C) Spatial molecular view of HbBv–HSA3 derived from a 3D volume reconstruction. Color code: HbBv, red; HSAs, chartreuse, lemon, and pale green. Hemes (C, gray) in HbBv, Cys-34 (S, yellow) in HSA
Furthermore, the 3D reconstruction of HbBv–HSA3 based on transmission electron microscopy (TEM) images revealed a complete triangular structure (Tomita et al. 2013). The original TEM pictures of HbBv–HSA3 showed individual particles (diameter: approximately 10 nm), but detailed structure information was unavailable owing to the low contrast. Then we used additional image processing procedure (single-particle analysis). From the obtained class sum images with an enhanced signal-to-noise ratio (Fig. 9.3B) (Tomita et al. 2013; Kimura et al. 2015), the 3D volume of HbBv–HSA3 was reconstructed. We calculated a presentation of the protein moieties by fitting their PDB data into the reconstructed volume. The proposed geometries conferred a possible spatial arrangement of the HbBv interior and three HSA exterior. The fitting of three HSAs defined an arrangement of the Cys-34 of HSA, which suggested the potential binding Lys partners on HbBv (Fig. 9.3C). The HSA binding sites on HbA in HbA–HSA3 are almost the same as those of HbBv–HSA3 (Kimura et al. 2015).
9.3 O2-Binding Property of Hemoglobin–Albumin Cluster
The deoxy, oxy, and carbonyl forms of HbBv–HSA 3 in PBS solution under N2, O2, and CO atmospheres, respectively, showed identical absorption spectra to the corresponding forms of naked HbBv (Fig. 9.4) (Hosaka et al. 2014; Antonini and Brunori 1971). We reasoned that the electronic states of the prosthetic heme groups in HbBv were unaltered by the covalent linkages of HSAs.
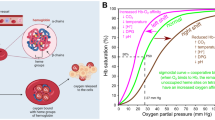
The O2 affinity (P 50: O2 partial pressure where Hb is half-saturated with O2) and the cooperativity coefficient (Hill coefficient: n) of the HbBv–HSA 3 were measured using an automatic recording system for blood O2 equilibrium curve (Hemox Analyzer). The P 50 of native HbBv was 23 Torr at 37 °C, whereas the value of HbBv–HSA 3 was 9 Torr (Table 9.1) (Hosaka et al. 2014). The n value decreased from 2.6 to 1.5. The results imply that the HbBv–HSA 3 shows higher O2 affinity than HbBv does. The P 50 and n value reductions were also seen in HbA–HSA 3 (P 50 = 8 Torr, n = 1.4) (Table 9.1) (Kimura et al. 2015). There are two possible explanations for the increase of O2 affinity and decrease in cooperativity. The first is the binding of the maleimide terminal of SMCC to Cys-93(β) in HbBv and HbA. Modification of the sulfhydryl group of Cys-93(β), which is located nearby the proximal His-92(β) coordinated to the heme (Mueser et al. 2000), is known to enhance the O2 affinity (Manjula et al. 2003; Li et al. 2009; Zhang et al. 2008). Furthermore, it reduces the available motion of the α 1 β 1/α 2 β 2 interface and induces disturbance of the quaternary structure of Hb from the Tense (T)-state to the Relaxed (R)-state (Zhang et al. 2008). In fact, the number of cysteinyl thiols per HbBv decreased from 2.0 to 0.2 after the SMCC reaction, indicating that two Cys-93(β) of HbBv are blocked by SMCC maleimide. The second reason is the modifications of surface Lys groups of HbBv or HbA by succinimide terminal of SMCC. They are needed to create the cluster, but the chemical modifications of Lys groups on Hb influence the O2 affinity (Kluger and Zhang 2003; Hu and Kluger 2008; Vandegriff et al. 2003). In particular, Lys-82(β) plays a key role to modulate the quaternary structural change from the T-state to R-state of Hb. Our 3D reconstruction suggested that Lys-82(β) is a binding partner of Cys-34 of HSA (Tomita et al. 2013; Kimura et al. 2015). It can be concluded that (i) the masking of Cys-93(β) increases the O2 affinity and (ii) modification of surface Lys groups locks the R-state configuration of the central HbBv or HbA, resulting in the decrease of O2-binding cooperativity. Interestingly, HbBv–HSA 4 , which is hemoglobin–albumin cluster bearing four HSA units (large-size variant), showed the same O2-binding parameters as HbBv–HSA 3 (Yamada et al. 2016). It implies that the central Hb conformation is independent of the binding number of HSA.
9.4 Preclinical Safety of Hemoglobin–Albumin Cluster
The viscosity of the HbBv–HSA 3 solution (20 g/dL, [Hb] = 5.0 g/dL) is dependent on the shear rate, namely, a Newtonian fluid (Haruki et al. 2015). The viscosity at 230 s−1, the shear rate in the human arterial wall, was 2.8 cP, which is lower than that of blood (3.8 cP). A mixture solution of freshly drawn whole blood and HbBv–HSA 3 (1/1, v/v) showed non-Newtonian viscosity, which obeyed a nonlinear correlation to the shear rate. The viscosity was reasonably high: 3.3 cP at 230 s−1. Furthermore, we counted the number of blood cell components [RBC, white blood cell (WBC), and platelet (PLT)] of the blood/HbBv–HSA 3 mixture solution in vitro (Haruki et al. 2015). The numbers of RBC, WBC, and PLT decreased in proportion to their respective dilution ratios. These results indicate that HbBv–HSA 3 has good compatibility with whole blood.
The HbBv–HSA 3 solution (20 g/dL) was injected into anesthetized rats (6 mL/kg) and observed their mean arterial pressure (MAP) (Haruki et al. 2015). Notably, a small transient alternation in MAP was observed after administration of HbBv–HSA 3 (Fig. 9.5). The slight elevation of ΔMAP (25.3 ± 2.9 mmHg) from the basal value was followed by a decrease to 10 mmHg and retained constant during the monitoring time. The response is almost identical to that observed after infusion of HSA (20 g/dL). On the contrary, the administration of ββ-cross-linked HbBv (XLHbBv, 5 g/dL) is associated with an acute increase in ΔMAP (55.5 ± 5.9 mmHg) and urinary excretion of Hb from 10 min after the injection.
Difference of mean arterial pressure (ΔMAP) from the basal value after intravenous administration of HbBv–HSA 3 , HSA, and XLHbBv solutions to rats. Each data point represents the mean ± SD (n = 4). **p < 0.01 vs. XLHbBv. Basal values are 84.5 ± 4.4 mmHg in the HbBv–HSA 3 group, 85.0 ± 7.3 mmHg in the HSA group, and 87.5 ± 5.1 mmHg in the XLHbBv group
This non-vasopressor response of HbBv–HSA 3 is attributed to the negative surface net charge and high molecular weight of the cluster. HSA shows low vascular permeability of less than 1/100 Hb because of the electrostatic repulsion between the albumin surface and the glomerular basement membrane around the endothelial cells (Haraldsson et al. 2008). The isoelectric point of HbBv–HSA 3 (pI = 5.1) is close to that of HSA. Furthermore, the molecular weight of HbBv–HSA 3 (26.4 kDa) is much greater than that of HSA (66.5 kDa). Thus, the release of HbBv–HSA 3 into the extravascular space was attenuated. In contrast, the small XLHbBv having neutral surface charge passes through the vascular endothelium and contributes to the consumption of NO. Moreover, XLHbBv passes through the renal glomerulus, thereby inducing excretion of Hb in urine.
The 125I-labeled HbBv–HSA 3 was injected into rats to evaluate blood retention (Haruki et al. 2015). The 125I-labeled native HbBv was cleared rapidly from circulation with the half-life (T 1/2) of 0.53 h (Fig. 9.6). On the one hand, the time course of HbBv–HSA 3 demonstrated very slow kinetics. The T 1/2 of HbBv–HSA 3 was significantly long (18.5 h) and 1.7-fold greater than that of HSA (T 1/2 = 11.0 h). The negative surface net charge and large molecular size of HbBv–HSA 3 prevent filtration by the renal glomerulus. We reasoned that the superior blood retention property of HbBv–HSA 3 is attributable to suppression of movement to the extravascular space and renal filtration. All parameters of HbBv–HSA 4 were comparable to those of HbBv–HSA 3 (Yamada et al. 2016). The HSA-binding number on Hb is ineffective to extend the circulation persistence.
All animals injected with HbBv–HSA 3 solution (20 g/dL, 6 mL/kg) were alive for 7 days (Haruki et al. 2015). No remarkable change was found in their appearance or behavior during the measurement time. The body weight increased gradually thereafter. The 26 analytes of the serum biochemical tests after 7 days from the administration showed almost identical data to those of the control groups (HSA injection group, sham-operated group). Microscopic observations of the stained specimens of major organs (liver, kidney, spleen, lungs, and heart) showed no histopathologic disorder in their tissues.
9.5 Various Hemoglobin–Albumin Cluster Derivatives
9.5.1 Low O2 Affinity Model
In general, HBOCs possess high O2 affinity compared to RBC (Nagababu et al. 2002; Kluger and Zhang 2003; Hu and Kluger 2008; Li et al. 2009). High O2 affinity is inferred (i) to prevent the transport of a sufficient amount of O2 to tissues under physiological conditions, but (ii) to avoid early O2 offloading on the arterial side of circulation, which may be beneficial for targeted O2 delivery to the hypoxic regions (Rohlfs et al. 1998; Intaglietta 2004; Winslow 2003; Zhang and Palmer 2010). One of the interesting challenges of artificial O2 carrier is to prepare a novel HBOC with controllable O2-binding affinity. It could become a promising RBC substitute and O2-providing therapeutic reagent for clinical situations.
The heterobifunctional cross-linker SMCC binds not only to the amino groups of Lys on HbA but also to the sulfhydryl group of Cys-93(β). As a result, the HbA–HSA 3 showed high O2 affinity as described before. In our synthesis, the HbA is kept in the carbonyl form to prevent the autoxidation of the hemes. It is known that the Cys-93(β) in deoxygenated T-state HbA is less accessible to cross-linking agents (Buehler et al. 2006). We found that SMCC cannot bind to the Cys-93(β) of deoxy HbA under N2 atmosphere. As expected, the cluster prepared in N2, HbA(T)–HSA 3 , showed lower O2 affinity (P 50 = 26 Torr) than the native HbA (12 Torr) does (Table 9.1) (Kimura et al. 2015). The n value of HbA(T)–HSA 3 was 1.2, indicating a loss of O2-binding cooperativity. We reasoned that the HbA center was locked in the T-state conformation by the binding of SMCC under N2 atmosphere. Interestingly, the cluster including an αα-cross-linked Hb with bis(3,5-dibromosalicyl)fumarate (DBBF), ααHbA(T), demonstrated markedly low O2 affinity (P 50 = 35 Torr): lower than that of human RBC (Table 9.1) (Kimura et al. 2015). We inferred that the T-state conformation of the ααHbA(T) core was preserved strongly by chemical modification of the surface Lys groups. These HbA–HSA 3 clusters with different O2 affinities can support a new generation of RBC substitute that is better tuned to a role in O2 delivery.
9.5.2 Antioxidation Model
If one can confer an additional functionality to the external HSA unit of Hb–HSA 3 , it would become a promising O2 carrier with high performance. In this context, we designed to add antioxidant property to HbBv–HSA 3 . Pt nanoparticle (PtNP) is known to act as an effective catalysis for both O2 •– and H2O2 dismutations (Kajita et al. 2007; Hamasaki et al. 2008; San et al. 2012) and shows almost no cytotoxicity against cells (Hamasaki et al. 2008). We found that small PtNP (diameter: approximately 1.8 nm) is incorporated into HSA. TEM images demonstrated the formation of equivalent complex of HSA and PtNP (Fig. 9.7) (Hosaka et al. 2014). Close inspections of TEM micrographs revealed that each PtNP is incorporated in the center of the protein. We reasoned that one PtNP binds to the positively charged cleft of HSA, forming a 1:1 HSA–PtNP complex. The obtained HSA–PtNP complex showed superoxide dismutase (SOD) (O2 •– dismutation) activity and catalase (H2O2 dismutation) activity with high efficiency (Hosaka et al. 2014). The IC50 value (the concentration of enzyme necessary to attain 50 % inhibition of the Cyt. c reduction) of the HSA–PtNP complex was 0.16 μM, which resembled the value of native Cu, Zn-SOD (Weser and Schubotz 1981). The HSA–PtNP complex possesses a strong capability to catalyze the dismutation of O2 •–. The catalase activity of the HSA–PtNP complex was determined by measuring the H2O2 decomposition. The T 50 value (time required for quenching half of H2O2) of HSA–PtNP was 19 min, which is two orders of magnitude larger than that of native catalase.
The HbBv–HSA 3 also possesses the capability of binding PtNP into the HSA shells (Hosaka et al. 2014). The K value and binding number of PtNP with the exterior HSA unit were 1.1 × 107 M−1 and 1.1. The resultant HbBv–HSA 3 (PtNP) cluster forms a very stable O2 adduct, even in aqueous H2O2 (20 μM) solution. We can conclude that the HSA–PtNP shell acts as an efficient scavenger for external H2O2 and achieves protection of the core HbBv.
The similar HbBv–HSA 3 derivative with high resistance toward oxidation reactions was prepared by incorporation of Mn(II)-protoporphyrin IX into the exterior HSA units (Daijima and Komatsu 2014). These artificial O2 carriers having triple functionalities (O2 transport, O2 •– dismutation, H2O2 dismutation) might be useful in clinical conditions with ischemia–reperfusion.
9.6 Conclusion
Covalently wrapping of HbBv or HbA with the most abundant plasma protein, HSA, generated a core–shell structured protein cluster as a promising O2 carrier for RBC substitute. The cluster was prepared by covalent linkage between Hb’s Lys and HSA’s Cys-34 using heterobifunctional cross-linker. Major products were isolated using gel filtration chromatography, and the average HSA/Hb ratio of the product was 3.0 ± 0.2. We designated the clusters as HbBv–HSA 3 and HbA–HSA 3 . The low isoelectric points (pI = 5.1) of the clusters were almost equal to that of HSA, proving the covering of Hb core by negatively charged HSA. The 3D reconstruction of HbBv–HSA3 based on TEM images revealed a complete triangular structure. The possible spatial arrangement of the HbBv center and HSA exteriors was determined. The HbBv–HSA 3 and HbA–HSA 3 showed higher O2 affinity (P 50 = 9 Torr) than the native Hbs. The viscosity measurements and blood cell counting measurements of the mixture solution of whole blood and HbBv–HSA 3 revealed the high blood compatibility of this O2-carrier protein. The administration of HbBv–HSA 3 to anesthetized rats caused a slight change in MAP, which is identical to that observed in the control group with HSA. This hemodynamic response contrasts against the acute hypertension occurred after infusion of XLHbBv. The T 1/2 of HbBv–HSA 3 was 1.7-fold longer than that of HSA. The non-vasopressor response and superior blood retention property of HbBv–HSA 3 are attributable to the negative surface net charge and larger molecular weight of the cluster. The serum biochemical parameters resembled those of the control groups. Histopathologic inspections proved that HbBv–HSA 3 gave no negative side effects in any major organ. These results support the preclinical safety of the HbBv–HSA 3 solution. Clusters prepared under N2 atmosphere showed low O2 affinity (P 50 = 26 Torr). Moreover, the cluster containing an αα-cross-linked HbA possessed markedly low O2 affinity (P 50 = 35 Torr). A PtNP binds within a cleft of HSA, yielding a stable HSA–PtNP complex. This platinated protein showed high O2 –• and H2O2 dismutation activities. The HbBv–HSA 3 also captured PtNP into the HSA units. The obtained HbBv–HSA 3 (PtNP) cluster formed very stable O2 complex even in aqueous H2O2 solution. All the results indicate that a series of hemoglobin–albumin clusters can be of tremendous medical importance as an alternative material to RBCs for transfusion in many clinical situations.
References
Alagic A, Koprianiuk A, Kluger R (2005) J Am Chem Soc 127:8036–8043
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. In: Neuberger A, Tatum EL (eds) North-Holland research monographs, vol 21, Frontiers of Biology. North-Holland, Amsterdam, pp 13–39
Buehler PW, Boyskins RA, Jia Y, Norris S, Freedberg DI, Alayash AI (2005) Structural and functional characterization of glutaraldehyde-polymerized bovine hemoglobin and its isolated fractions. Anal Chem 77:3466–3478
Buehler PW, Boykins RA, Norris S, Alayash AL (2006) Anal Chem 78:4634–4641
Curry S, Mandelkow H, Brick P, Franks N (1998) Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat Struct Biol 5:827–835
D’Agnilloo F, Chang TMS (1998) Nat Biotechnol 16:667–671
Daijima Y, Komatsu T (2014) Haemoglobin wrapped covalently by human serum albumin mutants containing Mn(III) protoporphyrin IX: an O2 complex stable in H2O2 solution. Chem Commun 50:14716–14719
Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD (1998) Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol 16:672–676
Elmer J, Zorc K, Rameez S, Zhou Y, Cabrales P, Palmer AF (2012) Hypervolemic infusion of Lumbricus terrestris erythrocruorin purified by tangential-flow filtration. Transfusion 52:1729–1740
Hamasaki T, Kashiwagi T, Imada T, Nakamichi N, Aramaki S, Toh K, Morisawa S, Shimakoshi H, Hisaeda Y, Shirahata S (2008) Kinetic analysis of superoxide radical-scavenging and hydroxyl radical-scavenging activities of platinum nanoparticles. Langmuir 24:7354–7364
Haraldsson B, Nyström J, Deen WM (2008) Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88:451–487
Haruki R, Kimura T, Iwsaki H, Yamada K, Kamiyama I, Kohno M, Taguchi K, Nagao S, Maruyama T, Otagiri M, Komatsu T (2015) Safety evaluation of hemoglobin-albumin cluster “HemoAct” as a red blood cell substitute. Sci Rep 5:12778, 1–9
Hosaka H, Haruki R, Yamada K, Böttcher C, Komatsu T (2014) Hemoglobin–albumin cluster incorporating a Pt nanoparticle: artificial O2 carrier with antioxidant activities. PLoS ONE 9:e110541, 1–9
Hu D, Kluger R (2008) Functional cross-linked hemoglobin bis-tetramers: geometry and cooperativity. Biochemistry 47:12551–12561
Intaglietta M (2004) Microvascular transport factors in the design of effective blood substitutes. In: Messmer K, Burhop KE, Hutter J (eds) Microcirculatory effects of hemoglobin solutions. Karger AG, Basel, pp 8–15
Jahr JS, Sadighi A, Doherty L, Li A, Kim HW (2011) Hemoglobin-based oxygen carriers: history, limits, brief summary of the state of the art, including clinical trials. In: Bettati S, Mozzarelli A (eds) Chemistry and biochemistry of oxygen therapeutics: from transfusion to artificial blood. Wiley, West Sussex, pp 301–316
Kajita M, Hikosaka K, Iitsuka M, Kanayama A, Toshima N, Miyamoto Y (2007) Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic Res 41:615–626
Kimura T, Shinohara R, Böttcher C, Komatsu T (2015) Core-shell clusters of human haemoglobin A and human serum albumin: artificial O2-carriers having various O2-affinities. J Mater Chem B 3:6157–6164
Kluger R (2010) Red cell substitutes from hemoglobin – do we start all over again? Curr Opin Chem Biol 14:538–543
Kluger R, Lui FE (2013) HBOCs from chemical modification of Hb. In: Kim HW, Greenburg AG (eds) Hemoglobin-based oxygen carriers as red cell substitutes and oxygen therapeutics. Springer, Berlin, pp 159–183
Kluger R, Zhang J (2003) Hemoglobin dendrimers: functional protein clusters. J Am Chem Soc 125:6070–6071
Li D, Hu T, Manjula BN, Acharya SA (2008) Non-conservative surface decoration of hemoglobin: influence of neutralization of positive charges at PEGylation sites on molecular and functional properties of PEGylated hemoglobin. Biochim Biophys Acta 1784:1395–1401
Li D, Hu T, Manjula BN, Acharya SA (2009) Extension arms facilitated pegylation of αα-hemoglobin with modifications targeted exclusively to amino groups: functional and structural advantages of free Cys-93(β) in the PEG-Hb adduct. Bioconjug Chem 20:2062–2070
Manjula BN, Tsai A, Upadhya R, Perumalsamy K, Smith K, Malavalli A, Vandegriff K, Winslow RM, Intaglietta M, Prabhakaran M, Friedman JM, Acharya AS (2003) Site-specific PEGylation of hemoglobin at Cys-93(β): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem 14:464–472
Ministry of Health, Labor and Welfare, Japan (2014) Proceedings of blood donation promotion committee, pharmaceutical affairs and food sanitation council on 2 December, 2014. http://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000067177.pdf. Accessed 30 Dec 2015
Mondery-Pawlowski CL, Tian LL, Pan V, Gupta AS (2013) Synthetic approaches to RBC mimicry and oxygen carrier systems. Biomacromolecules 14:939–948
Mueser TC, Rogers PH, Arnone A (2000) Interface sliding as illustrated by the multiple quaternary structures of liganded hemoglobin. Biochemistry 39:15353–15364
Nagababu E, Ramasamy S, Rifkind JM, Jia Y, Alayash AI (2002) Site-specific cross-linking of human and bovine hemoglobins differentially alters oxygen binding and redox side reactions producing rhombic heme and heme degradation. Biochemistry 41:7407–7415
Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM (2008) Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death. J Am Med Assoc 299:2304–2312
Pearce LB, Gawryl MS, Rentko VT, Moon-Massat PF, Rausch CW (2006) HBOC-201 (hemoglobin glutamer-250) (bovine), hemopure®): clinical studies. In: Winslow RM (ed) Blood substitutes. Elsevier, San Diego, pp 437–450
Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD Jr, Vandegriff KD, Winslow RM (1998) Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem 273:12128–12134
Sakai H (2012) Present situation of the development of cellular-type hemoglobin-based oxygen carrier (hemoglobin-vesicles). Curr Drug Discov Technol 9:188–193
San BH, Moh SH, Kim KK (2012) The effect of protein shells on the antioxidation activity of protein-encapsulated platinum nanoparticles. J Mater Chem 22:1774–1780
Shultz SC, Grady B, Cole F, Hamilton I, Burhop K, Malcolm DS (1993) A role for endothelin and nitric oxide in the pressor response to diaspirin cross-linked hemoglobin. J Lab Clin Med 122:301–308
Snyder SR, Welty EV, Walder RY, Williams LA, Walder JA (1987) HbXL99α: a hemoglobin derivative that is cross-linked between the α subunits is useful as a blood substitute. Proc Natl Acad Sci U S A 84:7280–7284
Squires JE (2002) Artificial blood. Science 295:1002–1005
Tomita D, Kimura T, Hosaka H, Daijima Y, Haruki R, Böttcher C, Komatsu T (2013) Covalent core–shell architecture of hemoglobin and human serum albumin as an artificial O2 carrier. Biomacromolecules 14:1816–1825
Vandegriff KD, Malavalli A, Wooldbridge J, Lohman W, Winslow RM (2003) MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion 43:509–516
Weser U, Schubotz LM (1981) Imidazole-bridged copper complexes as Cu2Zn2-superoxide dismutase models. J Mol Catal 13:249–261
Winslow RM (2003) Current status of blood substitute research: towards a new paradigm. J Intern Med 253:508–517
Xiong Y, Liu ZZ, Georgieva R, Smuda K, Steffen A, Sendeski M, Voigt A, Patzak A, Bäumler H (2013) Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano 7:7454–7461
Yamada K, Yokomaku K, Haruki R, Taguchi K, Nagao S, Maruyama T, Otagiri M, Komatsu T (2016) Influence of molecular structure on O2-binding properties and blood circulation of hemoglobin-albumin clusters. PLoS ONE 11:e0149526, in press
Zhang N, Palmer AF (2010) Polymerization of human hemoglobin using the crosslinker 1,11-bis(maleimido)triethylene glycol for use as an oxygen carrier. Biotechnol Prog 26:1481–1485
Zhang Y, Bhatt VS, Sun G, Wang PG, Palmer AF (2008) Site-selective glycosylation of hemoglobin on Cys 93. Bioconjug Chem 19:2221–2230
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Area (“Coordination Programming” Area 2107, No. 21108013) from MEXT Japan, a Chuo University Grant for Special Research, and a Joint Research Grant from the Institute of Science and Engineering, Chuo University. The author acknowledges Prof. Mitsutomo Kohno (Tokai University), Dr. Kazuaki Taguchi, Prof. Masaki Otagiri (Sojo University), and Prof. Toru Maruyama (Kumamoto University) for their great supports and valuable comments on animal experiments, and Dr. Christoph Böttcher (Freie Universität Berlin) for his skillful experiments related to TEM measurements and 3D reconstruction.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Komatsu, T. (2016). Hemoglobin–Albumin Clusters as a Red Blood Cell Substitute. In: Otagiri, M., Chuang, V. (eds) Albumin in Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-10-2116-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-2116-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2115-2
Online ISBN: 978-981-10-2116-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)