Abstract
In order to understand the transformation behaviors of selenium and cadmium during coal combustion, the kinetic studies were conducted by using an online analysis system of trace elements in flue gas. The analysis system is based on a modified inductively coupled plasma optical emission spectroscopy (ICP-OES) and can measure the concentrations of trace elements in flue gas quantitatively and continuously. The standard aerosol for calibration is produced by applying an ultrasonic nebulizer. The results of online analysis showed that selenium and cadmium concentrations in flue gas increase with a rise in temperature. Selenium is more easily released than cadmium during coal combustion. Based on the results of selenium and cadmium concentrations in flue gas, the release rates of selenium and cadmium during coal combustion were determined, respectively, at the particle level. The higher the temperature is, the higher the release rates of selenium and cadmium are, although the release rates are not proportional to the temperature. This indicates a strong influence of temperature on selenium and cadmium release kinetics.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Although the toxic trace elements selenium (Se) and cadmium (Cd) present in only small amounts in coal, the large use of coal in coal-fired power plants leads to significant accumulative emissions of Se and Cd [1]. It is essential to understand the release behaviors of Se and Cd during coal combustion for the control of their emissions.
Various experimental studies have been conducted to investigate the vaporization behaviors of Se and Cd during coal combustion. Tang et al. [2] studied the distribution of Se in coal-fired power plants by analyzing the solid residues. They found that the concentration of Se in fly ash was higher than that in bottom ash and feed coal, and thus, they suggested that Se was volatile during coal combustion and was likely to condense into smaller particles. Seames and Wendt [3] investigated the partitioning behaviors of Se and Cd between the vapor and solid phases during coal combustion. They suggested that the reactions of Se and Cd vapors on the surface or within the pores of an ash particle were essential for the transformations of Se and Cd vapors to particles. Methods analyzing the residues can give insight into the partitioning behaviors of Se and Cd between the vapor and solid phases during coal combustion. However, such methods need the taking of discrete samples and only give an average value for trace element emission over a long period. Consequently, they are difficult to obtain the information on trace element emission variation with time.
Yan et al. [4] employed thermodynamic calculation to determine Se and Cd species in flue gas during coal combustion. Nevertheless, the temporal concentrations of Se and Cd during coal combustion are still unclear. Such concentrations can be investigated only by kinetic studies.
Yu et al. [5] investigated the release behaviors of Cd, Pb, Zn, and Cu from impregnated mineral matrices (Al2O3 particles) by aspirating and analyzing the samples directly at given times. They aspirated Al2O3 particles at the interval of 10 min by using a vacuum pump since Al2O3 particles will not break during the process of thermal treatment. However, it is difficult to obtain the release kinetics of trace elements during coal combustion due to the difficulty in sampling burning solid. Thus, the concentration of trace elements in flue gas is the only measurable temporal data. Therefore, to continuously measure Se and Cd concentrations in flue gas is one of the key points of a kinetic study. Considerable efforts have been made previously to develop the reliable technologies for measuring the concentrations of trace elements in flue gas directly [6–8]. Among various technologies, the online analysis system based on inductively coupled plasma optical emission spectroscopy (ICP-OES) is regarded as an effective approach for measuring the concentrations of trace elements in flue gas quantitatively and continuously [9, 10]. The development of online analysis system of trace elements in flue gas is meaningful in both the understanding of industrial processes and the measurement of emissions for legislative purposes [6, 11].
Falcoz et al. [12] studied the vaporization kinetics of Cd, Pb, and Zn during realistic artificial waste incineration and determined the kinetic laws governing Cd, Pb, and Zn vaporization at different temperature range. In our previous studies [13], the kinetic laws of Cd vaporization during mineral matrices and realistic artificial waste thermal treatment at 850 °C were determined, respectively. A first-order kinetic law was determined for mineral matrices, and a second-order kinetic law was determined for realistic artificial waste. By far, dynamic characteristics of Se and Cd release during coal combustion are still unclear. Therefore, the release kinetics of Se and Cd are needed to be investigated for further clarifying their transformation behaviors during coal combustion.
In this work, the main objective was to investigate the kinetic characteristics of Se and Cd release during coal combustion. An online analysis system based on ICP-OES was developed to measure the concentrations of Se and Cd in flue gas quantitatively and continuously. The inverse method was applied to calculate the release rates of Se and Cd during coal combustion based on the results of online analysis.
2 Experimental Section
2.1 Fluidized Bed Reactor
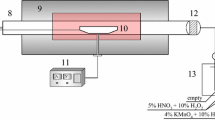
The experimental setup is shown in Fig. 1. The experiments were conducted using a bubbling fluidized bed. It is made up of Cr25Ni20 stainless steel with an inner diameter of 40 mm. The upper section of the reactor is designed for reaction (1100 mm), and the lower section of the reactor is for preheating (400 mm). The reactor is electrically heated by a three-section electric furnace. Thermocouples are immersed in each section to measure the temperature of the reactor. The bed material is composed of quartz sand with the particle diameter of 0.55–0.83 mm (initial bed height: 35 mm).
The experiments were conducted at different temperatures: 750, 800, and 850 °C. During the tests, the air was supplied from the bottom of the reactor and preheated through the preheating section. The coal (density: 1250 kg m−3) used in this study is mined from the Shenfu Coalfield, which is classified as bituminous coal. The proximate analysis (wt%, air-dried basis) for the coal sample is as follows: fixed carbon, 55.99; volatile matter, 27.98; ash, 5.84; moisture, 10.19. The ultimate analysis (wt%, air-dried basis) for the coal sample is as follows: C, 66.48; H, 4.31; N, 0.80; S, 0.33; O, 12.04. A given amount of coal (2 g) was injected when the desired temperature was reached and the operating conditions were maintained. The initial Se and Cd concentrations in the coal sample were measured by ICP-OES after acid digestion of the particles. The concentration of Se in coal was found to be 4.8 mg kg−1. The concentration of Cd in coal was 1.3 mg kg−1.
2.2 Online Analysis System
The concentrations of Se and Cd in flue gas were measured by an online analysis system, as shown in Fig. 1. This system is based on a modified inductively coupled plasma optical emission spectroscopy (ICP-OES, Spectro Arcos Sop, Germany). A quartz demountable torch was employed for measurement with an injector i.d. of 1.8 mm. This torch can realize a higher coolant gas flow, which is needed for a higher power to maintain the stability of plasma under atmospheric conditions. The emission signal intensities of Se and Cd were measured at 196.090 and 214.438 nm in this study, respectively.
A first isokinetic sampling stage aspirated the sample gas from the outlet of the reactor. A secondary sampling stage controlled the gas flow rate to the plasma at 0.14 l/min by applying a double-head peristaltic pump. The sample gas flow rate was optimized based on a balance between signal intensity and background noise. The sample line was maintained at 200 °C in order to avoid water condensation.
In this study, the standard aerosol was prepared in situ by nebulizing standard trace elements solutions covering the range from 0.1 to 2 μg l−1. An ultrasonic nebulizer (USN, Cetac U5000AT+) was used to provide dry standard aerosol and to normalize the water content of the sample aerosol. Part of the fluidization gas was used as nebulization gas, in order to keep constant the gas composition of standard aerosol and sample aerosol. The emission intensities of Se and Cd were measured by the ICP-OES. Since both the gas composition and the sampling flow rate are constant, the emission intensities of Se and Cd are proportional to the concentrations of elements. Then, the concentrations of Se and Cd in flue gas can be obtained after calibration. The detection limit obtained for Se in flue gas was 0.007 mg m−3. The detection limit obtained for Cd in flue gas was 0.001 mg m−3. The operating parameters of the online analysis system are shown in Table 1.
3 Results and Discussions
3.1 Temporal Concentration of Selenium in Flue Gas During Coal Combustion
The temporal concentrations of Se in flue gas during coal combustion are plotted in Fig. 2. The gaseous Se concentration reaches a peak almost instantaneously after the coal sample is injected into the reactor. Then, it slows down and levels off with time. The concentration of Se in flue gas increased with a rise in temperature. The release percent of Se during coal combustion increased with increasing temperature. The release percent of Se at 750 and 850 °C after combustion is about 45.2 and 60.1 %, respectively. This is because a higher reaction temperature improves the decomposition of coal particles and thus facilitates Se release during coal combustion [14].
The released Se during coal combustion can distribute between the vapor and solid phases in different proportions. The differences in Se species present in flue gas and the reactions of gaseous Se species with fly ash can affect the partitioning of Se between the vapor and solid phases [3, 15]. SeO2 is one of the dominant gaseous Se species in flue gas of coal combustion. SeO2 can react with Ca in fly ash with the formation of Se–Ca complexes [3, 16]. X-ray absorption near-edge structure spectrometry (XANES) result showed that Se4+ is dominant form of Se in fly ash [1]. The formation of stable Se–Ca compound in fly ash is owing to the following chemical reaction:
3.2 Release Kinetics of Selenium During Coal Combustion
The online analysis system developed in this study can measure the instantaneous concentration of Se in the flue gas during coal combustion quantitatively. This makes it possible to obtain the release rate (r) of Se during coal combustion by applying the inverse method [13]. The modified Kunii and Levenspiel model was adapted in the inverse method. The bed is assumed to be isothermal. Thus, the internal phenomenon within a solid particle, including heat and mass transfer as well as chemical reactions, would have no effect on the results. This macroscopic approach uses only the global flux of generation of species at the external surface of the particles. Therefore, it may be extended to other combustion systems in which solid–gas mass transfer occurs. Global release flux is related to the release rate in the inverse method. Therefore, the inverse method could predict the release rates of trace elements during coal combustion based on the results of online analysis. The detailed description of the inverse model can be found in our previous paper [13]. The values of various parameters employed in the inverse method are listed in Table 2.
Temporal release rates of Se during coal combustion are shown in Fig. 3. It is clear that the release rates of Se during coal combustion increased with a rise in temperature. This indicates that the temperature has a strong effect on release dynamics of Se during coal combustion. The release rate of Se at 850 °C is faster than that at 750 °C. This may be due to the fact that the high temperature suppressed the interactions of Se with mineral matters in coal. In addition, the release rate of Se during coal combustion may then affect the reactions of SeO2 (g) with CaO (s) in fly ash.
3.3 Temporal Concentration of Cadmium in Flue Gas During Coal Combustion
The quantitative results of Cd concentration in flue gas during coal combustion are plotted in Fig. 4. The concentration of Cd in flue gas exhibits a peak almost instantaneously after the coal sample is injected into the reactor. It can be observed that the higher the temperature, the higher the Cd concentration in flue gas. Although the total amount of Cd released is not proportional to the temperature, the release percent of Cd increases with a rise in temperature. The release percent of Cd after combustion is about 30.7 % at 750 °C and is about 48.6 % at 850 °C. The results of Cd concentration in flue gas showed that the temperature has a strong influence on Cd release during coal combustion. Temperature affects the release profile and the Cd release is governed by internal diffusion [17]. At the experimental temperature range (750–850 °C), the particle combustion controls the release of trace elements.
The conversion of released Cd into fly ash is the key factor affecting the distribution of Cd between the vapor and solid phases. It was considered that Cd in coal was oxidized to CdO after combustion. CdO in flue gas can react with mineral in fly ash. Cd release decreases with time because of the high-temperature chemical reactions [11]:
3.4 Release Kinetics of Cadmium During Coal Combustion
Cd release kinetics during coal combustion is shown in Fig. 5. It is clear that the higher the temperature, the higher the release rates of Cd during coal combustion. This indicates that the temperature has a strong effect on release kinetics of Cd during coal combustion. The release rate of Cd reaches a peak value at about 30 s for each temperature, which is about 10 s later than that of Se. This may be due to the differences in the occurrence form and content in coal of Se with Cd. In addition, the release rate of Cd during coal combustion may affect the reactions of CdO (g) with SiO2 (s) or Al2O3 (s) in fly ash.
4 Conclusions
An online analysis system was developed to measure the temporal concentrations of Se and Cd in flue gas quantitatively and continuously. Temperature significantly influences the release behavior of Se and Cd during coal combustion. The total amounts of Se and Cd released after coal combustion increased with a rise in temperature. About 60.1 % of Se and 48.6 % of Cd were emitted into the flue gas at 850 °C after combustion. The release percent of Se after combustion is higher than that of Cd, indicating that Se is more easily released than Cd during coal combustion. The release rates of Se and Cd during coal combustion were obtained based on the results of online analysis. The results showed that the release rates of Se and Cd during coal combustion increased with increasing temperature. The release rate of Se is faster than that of Cd and reaches a peak value earlier.
References
Shah P, Strezov V, Prince K, Nelson PF (2008) Speciation of As, Cr, Se and Hg under coal fired power station conditions. Fuel 87(10):1859–1869
Tang Q, Liu G, Yan Z, Sun R (2012) Distribution and fate of environmentally sensitive elements (arsenic, mercury, stibium and selenium) in coal-fired power plants at Huainan, Anhui, China. Fuel 95:334–339
Seames WS, Wendt JO (2000) Partitioning of arsenic, selenium, and cadmium during the combustion of Pittsburgh and Illinois# 6 coals in a self-sustained combustor. Fuel Process Technol 63(2):179–196
Yan R, Gauthier D, Flamant G (2001) Volatility and chemistry of trace elements in a coal combustor. Fuel 80(15):2217–2226
Yu J, Sun L, Xiang J, Hu S, Su S (2013) Kinetic vaporization of heavy metals during fluidized bed thermal treatment of municipal solid waste. Waste Manage 33(2):340–346
Poole D, Sharifi V, Swithenbank J, Argent B, Ardelt D (2007) On-line detection of metal pollutant spikes in MSW incinerator flue gases prior to clean-up. Waste Manage 27(4):519–532
Poole DJ, Sharifi VN, Swithenbank J, Ardelt D (2005) Identification of metal concentration fluctuations in waste-to-energy plant flue gases—a novel application for ICP-OES. J Anal At Spectrom 20(9):932–938
Clarkson P, Poole D, Sharifi V, Swithenbank J, Waarlo H-J, Ardelt D, Falk H (2004) Continuous measurement of mercury in flue gas using ICP-OES. J Anal At Spectrom 19(5):652–653
Wellinger M, Biollaz S, Wochele J, Ludwig C (2011) Sampling and online analysis of alkalis in thermal process gases with a novel surface ionization detector. Energy Fuels 25(9):4163–4171
Liu J, Liu X, Gauthier D, Abanades S, Flamant G, Qiu J, Zheng C (2010) Kinetics of heavy metal vaporization from coal in a fluidized bed by an inverse model. Asia Pac J Chem Eng 5(2):266–273
Liu J, Falcoz Q, Gauthier D, Flamant G, Zheng C (2010) Volatilization behavior of Cd and Zn based on continuous emission measurement of flue gas from laboratory-scale coal combustion. Chemosphere 80(3):241–247
Falcoz Q, Gauthier D, Abanades S, Flamant G, Patisson F (2009) Kinetic rate laws of Cd, Pb, and Zn vaporization during municipal solid waste incineration. Environ Sci Technol 43(6):2184–2189
Liu J, Abanades S, Gauthier D, Flamant G, Zheng C, Lu J (2005) Determination of kinetic law for toxic metals release during thermal treatment of model waste in a fluid-bed reactor. Environ Sci Technol 39(23):9331–9336
Zhou C, Liu G, Yan Z, Fang T, Wang R (2012) Transformation behavior of mineral composition and trace elements during coal gangue combustion. Fuel 97:644–650
Sterling R, Helble J (2003) Reaction of arsenic vapor species with fly ash compounds: kinetics and speciation of the reaction with calcium silicates. Chemosphere 51(10):1111–1119
Senior C, Van Otten B, Wendt JO, Sarofim A (2010) Modeling the behavior of selenium in Pulverized-Coal Combustion systems. Combust Flame 157(11):2095–2105
Mazza G, Falcoz Q, Gauthier D, Flamant G (2009) A particulate model of solid waste incineration in a fluidized bed combining combustion and heavy metal vaporization. Combust Flame 156(11):2084–2092
Acknowledgments
This work was supported by National Natural Science Foundation of China (51376072), National Basic Research Program of China (2014CB238904), and Natural Science Foundation of Hubei Province (2015CFA046).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore and Tsinghua University Press
About this paper
Cite this paper
Shen, F., Liu, J., Zhang, Z. (2016). Kinetics of Selenium and Cadmium Vaporization During Coal Combustion in a Fluidized Bed. In: Yue, G., Li, S. (eds) Clean Coal Technology and Sustainable Development. ISCC 2015. Springer, Singapore. https://doi.org/10.1007/978-981-10-2023-0_51
Download citation
DOI: https://doi.org/10.1007/978-981-10-2023-0_51
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2022-3
Online ISBN: 978-981-10-2023-0
eBook Packages: EnergyEnergy (R0)