Abstract
Present study describes the adsorptive removal of Cd(II) ions from aqueous solution using activated carbon prepared from sweet lime peels (a fruit processing industry waste) treated with zinc chloride (ZnCl2) in impregnation ratio 1:1 (w/w) followed by carbonization at 500 °C for 2 h. The ZnCl2-activated carbon obtained was characterized by proximate analysis, surface textural characterization, surface charge etc. An effect of pH, temperature and contact time on Cd(II) ion removal efficiency of activated carbon was investigated in batch mode to establish optimum adsorption conditions. Adsorption equilibrium data analysis for the temperature range 303–323 K showed best fit in Langmuir adsorption isotherm. Maximum adsorption capacity was observed to be 110.98 mg g−1 at 323 K. Adsorption of Cd(II) ions on activated carbon followed the Pseudo-second-order kinetics from which activation energy for Cd(II) ion adsorption was determined to be 31.27 kJ mol−1 and indicate the chemisorption phenomenon.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The natural surface and ground water sources are greatly contaminated by heavy metal ions released from various sources including industrial processes and mining activities. This has posed a serious environmental threat because of the potential toxicity and irreparable effects of the heavy metal ions on the environment. Cadmium, one of the potentially carcinogenic heavy metal, can enter into the ecosystem from effluents of electroplating, production of batteries, mining, smelting and alloy industries (Farooq et al. 2010; Purkayastha et al. 2014). Bioaccumulation of cadmium in liver causes the severe liver and reproductive damage (Hrudey et al. 1996). Moreover, higher doses of cadmium build up in kidney is also responsible for the malfunction in spilling proteins in urine and disrupting protein metabolism (Waalkes 2000). A number of physio-chemical methods have been used over the past few decades for the removal of cadmium from wastewater. These include chemical precipitation, coagulation–flocculation, flotation, membrane filtration, ion exchange and electro-dialysis (O’Connell et al. 2008; Sud et al. 2008). However, most of these methods have their own intrinsic limitations of high capital and operational cost, inefficiency to treat effluents of dilute metal concentrations and generation of toxic sludge which requires further treatment (Moussous et al. 2012; Purkayastha et al. 2014). In this concern, an efficient and economically viable process is needed to be developed for effective removal of cadmium from wastewater to environmentally safe levels.
Adsorption process using the low-cost and abundantly available renewable sources offers an attractive potential alternative to the conventional methods. An extensive literature is available on the utilization of different types of biomass as adsorbent for removal of heavy metal ions from wastewater. However, untreated biomass suffers with low adsorption efficiency, system contamination due to release of soluble organic compounds contained in the biomass that further requires the higher chemical and biological oxygen demand (Wan Ngah and Hanafiah 2008). To resolve this, biomass has to be either chemically treated and/or transformed to carbonized mass using thermal activation for the effective surface area and texture to achieve desired adsorbent reactivity. Comprehensive efforts have been made to develop the activated adsorbent material for efficient metal ion removal (Leyva-Ramos et al. 2005; Kula et al. 2008; Wang et al. 2010; Machida et al. 2012; Bohli et al. 2015). Nevertheless, there is still having scope to find the cost-effective alternative adsorbent for heavy metal ion removal from contaminated streams. India being an agrarian country, sweet lime peels are readily available as inexpensive waste from fruit processing industry. An appropriate treatment and activation of this waste biomass may result into the suitable adsorbent for metal ion removal from contaminated water resources.
Hence, aim of the present study is to utilize sweet lime peels as a cheap renewable material for preparation of activated carbon by chemical treatment with ZnCl2, followed by carbonization. The adsorption experiments in batch mode were performed to evaluate the potentiality of the prepared activated carbon for the adsorption of Cd(II) ions from aqueous solution. The effects of pH, temperature and contact time were investigated. Furthermore, the equilibrium experimental data were assessed using isotherm and kinetic sorption models to elucidate adsorption mechanism.
2 Experimental
2.1 Metal Solution and Reagents
All the reagents including the metal salt (3CdSO4·8H2O) were of AR grade and procured from Merck Scientific-India. Experimental stock solution of Cd(II) ion (1000 mg L−1) was prepared using double distilled water. Fresh dilutions of Cd(II) ion stock solution were used to prepare the synthetic water of different concentrations for experimental adsorption studies.
2.2 Preparation of Activated Carbon
The precursor (sweet lime peels) was obtained from M/S Mapro Foods Pvt. Ltd., Kangra (Himachal Pradesh, India). The preparation of activated carbon from the peels using ZnCl2 as activating agent was carried out according to the procedure described by Subha and Namasivayam (2010). 200 g of dried peels were impregnated (70 °C, 4 h) with concentrated solution of ZnCl2 (1:1, w/w). An impregnated whole mass was then set in a cotton filter to remove the excess solution, and oven-dried at 80 °C for 24 h. This impregnated dried mass was filled in a purpose made stainless still tubular reactor (L, 120 mm × Ø, 80 mm) and heated to carbonization temperature of 500 °C under N2 flow at the heating rate of 10 °C min−1 for 2 h. The carbonized mass thus prepared was cooled in N2 atmosphere and immersed in 200 ml of 2 M HCl solution at 80 °C for 1 h to leach out the excess ZnCl2. Acid treated carbonized mass was repeatedly washed with warm distilled water to remove the traces of ZnCl2 and other residue. An effective removal of ZnCl2 was monitored through silver nitrate addition. Obtained material was dried at 100 °C for 24 h and sieved through particle size ≤0.425 mm.
2.3 Characterization of Activated Carbon
The proximate analysis viz. moisture content, volatile matter and ash content of the activated carbon was determined using ASTM D 2867-99, ASTM D 5832-98, ASTM D 2866-94 (reapproved 1999) methods respectively, and fixed carbon content was obtained as difference. The apparent density was determined according to ASTM D 2854-96 (reapproved 2000). The point of zero charge (pHpzc) determination was carried out as per the method described by Cerovic et al. (2007). Specific surface area and pore volume were determined using multipoint surface area analyzer NOVA–1000 (Quantachrome Instruments, USA). Temperature of the system (−195.6 °C) was maintained using liquid nitrogen. Brunaur, Emmet and Teller (BET) method was followed to determine the specific surface area (S BET) over a relative pressure range of 0.01–0.3. Pore volume (V t) was estimated from the liquid volume of adsorbate (N2) at a relative pressure, p/p 0 = 0.99. The average pore diameter (D ap) was obtained using the relation of D ap = 4V t/S BET (Tsai et al. 2001). The surface morphology of the activated carbon was observed by Field Emission-Scanning Electron Microscope (FE-SEM) S4800 Type-II (Hitachi High-Technologies Corporation, Tokyo, Japan) coupled to Energy dispersive x-ray (EDX) elemental mapping facility. A thin layer of gold was sputter-coated on the carbon for charge dissipation during SEM imaging.
2.4 Batch Adsorption Study
Batch mode adsorption studies were performed in a series of 100 mL Erlenmeyer flasks for the parameters of specified set conditions. Rotation speed of the shaker was kept at 200 rpm (REMI CIS-24BL orbital shaker). An investigated pH range of Cd(II) ion aqueous solution was 2–10. The pH of each solution was adjusted using 0.1 M HCl or 0.1 M NaOH solutions. A fixed mass of adsorbent carbon (2.0 g L−1) was added to 25 mL Cd(II) ion solution (50 mg L−1) and shaken to achieve equilibrium condition. An equilibrated mass was then filtered through Whatman filter paper (no. 42) and extent of Cd(II) ion adsorbed was determined as difference between residual and initial Cd(II) ion concentrations obtained using S-series atomic absorption spectrometer (AAS) equipped with SOLAAR software (Thermo Fisher Scientific Inc., India).
The equilibrium adsorption isotherms were generated for varying concentrations (5 mg L−1 to 300 mg L−1) of Cd(II) ion solutions at optimized pH condition and at the temperatures 303, 313 and 323 K. Mass of the carbon was kept constant at 2.0 g L−1. All the experiments were replicated twice and the average results are reported.
Percent Cd(II) ion adsorption and adsorption capacity (q e, mg g−1) at equilibrium were calculated from the experimental results of the batch study using the Eqs. (1) and (2), respectively, as given below:
where C 0—initial Cd(II) ion concentrations (mg L−1), C t and C e—residual concentrations of Cd(II) ion (mg L−1) after adsorption at time t and at equilibrium time respectively, V—volume of solution treated (L) and M—mass of the adsorbent (g).
3 Result and Discussion
The preparation of ZnCl2—activated carbon has produced the yield of 27.1 and 40.8 % on the basis of dried untreated peel mass and ZnCl2 impregnated mass respectively. The yield is the relative measure of the vaporization of volatile products from the hydroaromatic structure of the precursor due to intensified dehydration and elimination reactions. The physio-chemical characteristics of the prepared activated carbon are presented in Table 1.
3.1 Effect of PH
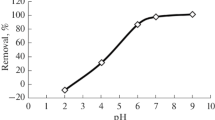
The pH of the solution medium to be treated for remediation of metal ion contaminated resources plays a worthy role in adsorption process. The variation in pH influences the surface charge on the binding sites of the adsorbent. Also, the ionization and solubility of metal species in the solution is affected (Remenarova et al. 2012). The Cd(II) ion removal efficiency as a function of varying pH in the range of 2–10 was investigated and the results are shown in Fig. 1. A gradual increase in Cd(II) ion adsorption from 32.7 to 52.5 % is evident with increasing pH from 2 to 5. Further increase in pH value above 5 has shown a steep rise in the Cd(II) ion removal till pH, 8 (from 52.5 to 95.9 %); thereafter almost a plateau regime with no significant increase was observed. These changes in the Cd(II) ion adsorption is related to surface charge properties of the activated carbon in solutions of different pH conditions. This can be elucidated well by point of zero charge (pHpzc) theory, which defines the pH at which the adsorbent surface gains a neutral charge in absence of specific sorption. The experimentally determined pHpzc of activated carbon is 5.8. At solution pH > pHpzc the active sites on the carbon surface became negatively charge favoring the better adsorption of cationic Cd(II) ions due to increased electrostatic attraction (Borai et al. 2007). Therefore, changing the solution pH from 5 to 8 resulted in higher Cd(II) uptake rate from 13.1 to 23.9 mg g−1. On the contrary, when the solution pH is maintained below pHpzc the surface charge on the carbon is positive and thus, would repulse more significantly Cd(II) ions in the solution (Srivastava et al. 2006). Therefore, the adsorptive uptake of Cd(II) ion decreased from 19.6 to 8.1 mg g−1 with decline in the pH from 6 to 2.
Besides, the adsorption process is also dependent upon the species distribution of the metal ions in the aqueous medium, which is strongly influenced by pH. At pH < 6, cadmium solely exists in the form of Cd2+ ionic species in the solution, while at pH below 8 the prevailing species are Cd2+ and Cd(OH)+ (Leyva-Ramos et al. 1997). The shift in the solution pH beyond 8 resulted into decreased Cd2+ concentration in solution, and concurrently cadmium could start precipitating at pH, 8.3 due to formation of insoluble Cd-hydroxy species, Cd(OH)2(s) (Ajmal et al. 2003). From Fig. 1, the majority of Cd(II) ion adsorption was seen at pH, 7 corresponding to adsorption efficiency of 92.7 % and the equilibrium capacity was 23.0 mg g−1. Therefore, the solution pH of 7 was chosen to be optimum, at which adsorption efficiency was greater than 90.0 % and is well below the pH levels of cadmium precipitation.
3.2 Effect of Temperature and Contact Time
The rate of Cd(II) ion adsorption on activated carbon was investigated at different temperatures in the range of 303–323 K and the results are shown in Fig. 2.
It has been observed that percent Cd(II) ion removal and adsorption capacity (q e) increased for temperature rise from 303 to 313 K, but further increase in temperature showed no significant contribution in sorption process. A rapid Cd(II) ion adsorption stage was seen initially within 180 min of contact time at different temperatures, and then subsequently much slower stage of adsorption before equilibrium was established. It can also be noted that the time required to reach sorption equilibrium was not affected by increasing the temperature from 303 to 323 K. In general, increase in temperature provides sufficient energy to overcome the resisting hydration (adsorbate solvation) energy acting on the diffusing metal ions, thereby increasing the mobility of metal ions towards adsorbent surface (Wang et al. 2012). However, a very slight change in q e values (23.0–23.6 mg g−1) was observed for activated carbon in the tested temperature range suggesting non-significant influence of temperature rise on adsorptive removal of Cd(II) ions.
3.3 Adsorption Isotherm
The fitment of experimental equilibrium data to one or more standard isotherm models is of immense importance to understand the interaction of adsorbate (metal ions) with solid adsorbent (activated carbon) and to elucidate the governing mechanism in sorption process (Arshadi et al. 2014). In this work, the two typical adsorption isotherm models viz. Freundlich isotherm and Langmuir isotherm were used to fit the equilibrium data. The non-linear form of the Freundlich and Langmuir isotherm models are given by Eqs. (3) and (4) respectively, as follows:
where q e—adsorption capacity of adsorbent at equilibrium (mg g−1), C e—equilibrium concentration of the adsorbate in solution (mg L−1), K F—Freundlich constant related to sorption capacity (mg1−1/n g−1 L1/n), (1/n)—dimensionless constant related to sorption intensity, q m—saturated monolayer adsorption capacity of the sorbent (mg g−1) and K L—Langmuir affinity constant (L mg−1).
The goodness of fit of these applied models was assessed using coefficient of determination (r 2) and non-linear chi-square (χ2) error function. An optimization approach was used for model parameter estimation and fitting of the isotherm curves. In the method, non-linear regression using repetitive attempts to succeed at maximizing the r 2 values between the experimental data and the predicted isotherms was performed using Solver add-in function facility in Microsoft excel. The optimized non-linear Freundlich and Langmuir isotherm fits for adsorption of Cd(II) ions on activated carbon at different temperatures are shown in Fig. 3.
The resultant Freundlich and Langmuir parameters and error function (χ2) values are listed in Table 2. The r 2 values of Langmuir isotherm obtained for activated carbon were extremely high (≥0.99) in comparison to that of Freundlich isotherm. In addition, the relatively very low χ2 values of Langmuir isotherm suggest that the Langmuir model is able to provide the perfect fit to the equilibrium data, confirming the monolayer adsorption of Cd(II) ions on activated carbon. The Langmuir constant, K L is related to adsorption energy. The rise in temperature from 303 to 323 K of sorption process resulted to give increasing K L values, signifying higher heat of adsorption associated with increasing temperature. Moreover, increase in q m values with temperature was observed. This indicated that higher temperatures favor the adsorption of Cd(II) ions on activated carbon, suggesting the sorption process to be endothermic.
3.4 Adsorption Kinetics
The study of rate kinetics of sorption process is of great significance in determination of the performance parameters, which subsequently forms a basis for effective designing and scaling of adsorption process (Elkady et al. 2011). The experimental kinetic data were fitted to reaction rate models viz. Pseudo-first-order and Pseudo-second-order kinetic models, the linear forms of which are given by the Eqs. (5) and (6) respectively, as follows:
where k 1—first-order rate constant (min−1), k 2—second-order rate constant (g mg−1 min−1), q e—equilibrium adsorption uptake at time t = ∞(mg g−1) and q t —adsorption uptake at time t (mg g−1).
The experimental data at different temperatures for the adsorption of Cd(II) ions on activated carbon was fitted to kinetics models using linear regression analysis of Eqs. (5) and (6). The calculated values of rate parameters (k 1, k 2), adsorption capacity (q e,cal) and the corresponding regression coefficients (R 2) are summarized in Table 3.
Pseudo-first-order model showed poor fit to the experimental data as R 2 values at tested temperatures were ≤0.95 and the calculated equilibrium sorption capacity values (q e,cal) were far lower than the experimentally determined values (q e,exp). As against, a highly linear relationship over the whole range of contact time with R 2 values close to unity (>0.99) was obtained for Pseudo-second-order kinetic plot at each temperature. Additionally, q e,cal from the second-order model at different temperature approximated well to q e,exp values. Therefore, the sorption kinetics of Cd(II) ion adsorption on activated carbon is more precisely described by the mechanism of surface site-adsorbate reaction of Pseudo-second-order model. The model is based on the assumption that the rate-limiting step is chemisorption, and thus may involving valence forces through sharing or exchange of electrons between the adsorbate, Cd(II) ions and adsorbent, activated carbon (Mohan et al. 2006). Similar findings were reported for the uptake of Cd(II) ions by multi-walled carbon nanotubes (Salam et al. 2012) and activated carbon prepared chemically from olive stones (Bohli et al. 2015).
These results further allowed determination of the activation energy (E a) using the Arrhenius equation in logarithmic form as follows:
where A—constant called the frequency factor (g mg−1 min−1), R—gas constant (J mol−1 K−1) and T—solution temperature (K).
Linear regression was applied to the plot of ln(k 2) versus (1/T) to determine the activation energy (E a) from the slope, (−E a/R). Besides, magnitude of E a is commonly taken as a measure to identify the underlying phenomenon of physical and chemical sorption in the process. Physical adsorption involves weaker electrostatic forces yielding usually less than 4.2 kJ mol−1 of E a. While chemical adsorption is specific, involving stronger forces and thus, requires larger E a from 8.4 to 83.7 kJ mol−1 (Saha and Chowdhury 2011). For the sorption of Cd(II) ions on activated carbon the E a was obtained to be 31.27 kJ mol−1. Positive value of E a signifies the energy that must be overcome for adsorption to occur. Also, it can be confirmed from E a value that the chemisorption is the primary governing mechanism in sorption of Cd(II) ions on activated carbon.
3.5 SEM-EDX Analysis
SEM micrographs and EDX spectra of activated carbon before and after Cd(II) ion adsorption are presented in Fig. 4. The carbon surface is shown to possess the porous structure with highly heterogeneous and rough texture. After Cd(II) ion adsorption, distinct alterations in surface morphology of carbon can be clearly visualized. The carbon surface shows filling of pores covered with precipitous layers formed upon complexation with Cd(II) ions. Furthermore, the carbon-metal interaction has shown appearance of a distinctive peak signal of Cd(II) ion in the EDX spectra (Fig. 4b), which confirms the ability of the activated carbon for Cd(II) ion binding. The elemental composition as determined by EDX analysis consist of C = 69.92 %, O = 8.16 % and Cd = 3.82 % on normalized wt percent basis.
4 Conclusion
The following important conclusions were drawn based on the results of the present study:
-
(i)
The pH of the aqueous medium is the controlling parameter for adsorption of Cd(II) ions on ZnCl2-activated carbon. The Cd(II) ion removal efficiency increased with increasing pH, and the optimized pH was found to be 7.
-
(ii)
Increasing the temperature, neither affected the time to reach equilibrium nor showed any substantial influence on Cd(II) ion adsorption capacity.
-
(iii)
Adsorption of Cd(II) ion is better described by Langmuir isotherm model (r 2 ≥ 0.99) suggesting the dominance of monolayer coverage of Cd(II) ions on activated carbon surface.
-
(iv)
Kinetics of Cd(II) ion adsorption obeyed the Pseudo-second-order model indicating the rate limiting step may be chemical sorption.
-
(v)
In overall, ZnCl2-activated carbon has shown its ability to effectively adsorb Cd(II) ions from aqueous solution.
References
Ajmal, M., Rao, R.A.K., Anwar, S., Ahmad, J., Ahmad, R.: Adsorption studies on rice husk: removal and recovery of Cd(II) from wastewater. Bioresour. Technol. 86, 147–149 (2003)
Arshadi, M., Amiri, M.J., Mousavi, S.: Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resources and Industry 6, 1–17 (2014)
Bohli, T., Ouederni, A., Fiol, N., Villaescusa, I.: Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. C. R. Chim. 18, 88–99 (2015)
Borai, E.H., El-Sofany, E.A., Morcos, T.N.: Development and optimization of magnetic technologies based processes for removal of some toxic heavy metals. Adsorption 13, 95–104 (2007)
Cerovic, L.S., Milonjic, S.K., Todorovic, M.B., Trtanj, M.I., Pogozhev, Y.S., Blagoveschenskii, Y., Levashov, E.A.: Point of zero charge of different carbides. Colloids Surf. A 297, 1–6 (2007)
Elkady, M.F., Abu-Saied, M.A., Rahman, A.A., Soliman, E.A., Elzatahry, A.A., Yossef, M.E., Eldin, M.M.: Nano-sulphonated poly (glycidyl methacrylate) cations exchanger for cadmium ions removal: effects of operating parameters. Desalination 279, 152–162 (2011)
Farooq, U., Kozinski, J.A., Khan, M.A., Athar, M.: Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour. Technol. 101, 5043–5053 (2010)
Hrudey, S.E, Chen, W., Rousseaux, C.G.: Bioavailability in environment risk assessment, pp. 91–100. CRC Press, Boca Raton, Florida (1996)
Kula, I., Ugurlu, M., Karaoglu, H., Celik, A.: Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour. Technol. 99, 492–501 (2008)
Leyva-Ramos, R., Rangel-Mendez, J.R., Mendoza-Baron, J., Fuentes-Rubio, L., Guerrero-Coronado, R.M.: Adsorption of Cadmium(II) from aqueous solution onto activated carbon. Water Sci. Technol. 35, 205–211 (1997)
Leyva-Ramos, R., Bernal-Jacome, L., Acosta-Rodriguez, I.: Adsorption of cadmium(II) from aqueous solution on natural and oxidized corncob. Sep. Purif. Technol. 45, 41–49 (2005)
Machida, M., Fotoohi, B., Amamo, Y., Ohba, T., Kanoh, H., Mercier, L.: Cadmium(II) adsorption using functional mesoporous silica and activated carbon. J. Hazard. Mater. 221, 220–227 (2012)
Mohan, D., Pittman Jr., C.U., Steele, P.H.: Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—a biosorbent. J. Colloid Interface Sci. 297, 489–504 (2006)
Moussous, S., Selatnia, A., Merati, A., Junter, G.A.: Batch Cadmium(II) biosorption by an industrial residue of macrofungal biomass (Clitopilus scyphoides). Chem. Eng. J. 197, 261–271 (2012)
O’Connell, D.W., Birkinshaw, C., O’Dwyer, T.F.: Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour. Technol. 99, 6709–6724 (2008)
Purkayastha, D., Mishra, U., Biswas, S.: A comprehensive review on Cd(II) removal from aqueous solution. Journal of Water Process Engineering 2, 105–128 (2014)
Remenarova, L., Pipiska, M., Hornik, M., Rozloznik, M., Augustin, J., Lesny, J.: Biosorption of cadmium and zinc by activated sludge from single and binary solutions: mechanism, equilibrium and experimental design study. J. Taiwan Inst. Chem. Eng. 43, 433–443 (2012)
Saha, P., Chowdhury, S.: Insight into adsorption thermodynamics. In: Tadashi, M. (ed.) Thermodynamics, pp. 349–364. InTech, Vienna, Austria (2011)
Salam, M.A., Al-Zhrani, G., Kosa, S.A.: Simultaneous removal of copper(II), lead(II), zinc(II) and cadmium(II) from aqueous solutions by multi-walled carbon nanotubes. C. R. Chim. 15, 398–408 (2012)
Srivastava, V.C., Mall, I.D., Mishra, I.M.: Equilibrium modelling of single and binary adsorption of cadmium and nickel onto bagasse fly ash. Chem. Eng. J. 117, 79–91 (2006)
Subha, R., Namasivayam, C.: ZnCl2-modified activated carbon from biomass coir pith for the removal of 2-Chlorophenol by adsorption process. Bioremediat. J. 14, 1–9 (2010)
Sud, D., Mahajan, G., Kaur, M.P.: Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–a review. Bioresour. Technol. 99, 6017–6027 (2008)
Tsai, W.T., Chang, C.Y., Lin, M.C., Chien, S.F., Sun, H.F., Hsieh, M.F.: Characterization of activated carbons prepared from sugarcane bagasse by ZnCl2 activation. Journal of Environmental Science and Health: Part B 36, 365–378 (2001)
Waalkes, M.P.: Cadmium carcinogenesis in review. J. Inorg. Biochem. 79, 241–244 (2000)
Wan Ngah, W.S., Hanafiah, M.A.K.M.: Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour. Technol. 99, 3935–3948 (2008)
Wang, C.P., Wang, B.L., Liu, J.T., Yu, L., Sun, H.W., Wu, J.Z.: Adsorption of Cd(II) from acidic aqueous solutions by tourmaline as a novel material. Chin. Sci. Bull. 57, 3218–3225 (2012)
Wang, F.Y., Wang, H., Ma, J.W.: Adsorption of cadmium(II) ions from aqueous solution by a new low-cost adsorbent–bamboo charcoal. J. Hazard. Mater. 177, 300–306 (2010)
Acknowledgments
The authors are very much thankful to the TEQIP-II, funded by MHRD, Government of India, Delhi (India) for providing financial support to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this paper
Cite this paper
Meshram, P.D., Bhagwat, S.S. (2016). Removal of Cd(II) Ions from Aqueous Solution by Adsorption on ZnCl2-Activated Carbon: Equilibrium and Kinetic Study. In: Regupathi, I., Shetty K, V., Thanabalan, M. (eds) Recent Advances in Chemical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-1633-2_14
Download citation
DOI: https://doi.org/10.1007/978-981-10-1633-2_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1632-5
Online ISBN: 978-981-10-1633-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)