Abstract
Spontaneous intracerebral hemorrhage (ICH) is a neurologic emergency. Patients presenting to the emergency department (ED) with ICH must be rapidly diagnosed, managed, and transported to an ICU or operating room. Emergency physicians must be prepared to stabilize and diagnose patients with ICH in a timely fashion and have knowledge about the underlying disease process and the evidence supporting various treatment modalities. In combination with rapid diagnosis, appropriate treatment of elevated blood pressure and coagulopathy, and preparedness for common pitfalls related to ICH, patient outcomes can be substantially improved. ICH occurs without warning, has a high mortality, and leaves many survivors with significant disability. Nevertheless, outcomes have improved over the last several decades due to advances in emergency and critical care. Although once thought of as a uniformly devastating disease, many patients have improved mortality and morbidity following ICH thanks to several recent advances and new studies that have been published. In particular, recent studies pertaining to the management of patients with ICH and concomitant antiplatelet medications, anticoagulation, and hypertension are critical for the practicing emergency physician. This chapter describes the current evidence as it pertains to emergent treatment of ICH.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Emergency Department
- Practicing Emergency Physician
- Intracerebral Hemorrhage
- Intracerebral Haemorrhage (ICH)
- Novel Oral Anticoagulants (NOACS)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Non-traumatic intracerebral hemorrhage (ICH) is defined as bleeding into the parenchyma of the brain that may extend into the ventricles and, in rare cases, the subarachnoid space [1]. Historically, the morbidity and mortality rate of ICH has been notoriously high; however, recent advances regarding the treatment of blood pressure as well as anticoagulation reversal have led to improved patient outcomes. ICH is the second most common subtype of stroke, following ischemic stroke [2]. Emergency physicians are typically the first physician contact with patients who have an ICH and, as such, can make an impactful difference in the care of these patients. Ongoing and future research is targeted both at preventing hematoma expansion and developing tools for endoscopic hematoma evacuation.
2 Identification and Triage of Stroke-Like Symptoms in the Emergency Department
2.1 Differential Diagnosis
A schematic diagram showing evaluation and management for patients with ICH is described in Fig. 7.1. The classical presenting signs and symptoms of ICH may include headache, vomiting, syncope, altered mental status, or abrupt onset of focal neurologic deficits which might include speech disturbances or weakness on half of the body. Patients with symptoms concerning for ICH have a wide differential diagnosis. Hypoglycemia should be considered in any patient with altered mental status presenting to the ED and finger-stick blood glucose performed immediately. Otherwise, the differential diagnosis should include ischemic stroke, seizure, complicated migraine, intracranial tumors, intracranial infections or encephalitis, and Todd’s paralysis. Depending on the location of hemorrhage, size of hemorrhage, intraventricular extension, comorbidities, and time since onset, the presentation may differ from patient to patient in the setting of ICH.
2.2 Emergency Department Evaluation and Workup
The vast majority of patients presenting to the ED with ICH will be undifferentiated, and it will be unknown whether their symptoms are secondary to ischemic or hemorrhagic stroke. As more and more hospital systems are moving toward protocoled rapid triage and imaging of patients with stroke-like symptoms, patients with ICH symptoms may ultimately go directly to the CT scanner for immediate diagnosis of ICH. Given the time-sensitive nature of both ischemic stroke and ICH, with the need for simultaneous blood pressure control and reversal of potential anticoagulation, history and physical should be performed in concert with preparation for imaging and both diagnosis and treatment modalities pursued at the same times as the initial history and physical are performed.
2.3 History and Physical Exam
Depending on the patient’s neurologic deficits, he or she may not be able to provide much, if any, of his or her medical history. In these instances, it is important to find family members or friends to determine history. The pertinent portions of history should include the last time the patient was without neurologic deficits and medications—especially anticoagulants and antiplatelets. If the patient uses antiplatelets or anticoagulants, it is important to determine the last time these medications were taken. The 2015 American Heart Association (AHA) guidelines also recommend that ED providers determine any vascular risk factors, recent trauma or surgery, alcohol or illicit drug use, past or current seizures, liver disease, cancer, or other hematologic disorders [3]. Additionally, it is beneficial to know if the patient has a known intracranial mass, arteriovenous malformation (AVM), recent ischemic stroke, or other intracranial pathology, as this will help in determining the cause of ICH.
The physical exam should ideally be completed at the same time the history is taken to expedite care. After assessing the patient’s respiratory status, mental status, and potential need for intubation prior to imaging, the exam should be focused on the neurologic symptoms. Although an entire neurologic exam consists of level of consciousness, cranial nerve exam, vision, motor, sensory, cerebellar findings, and language, the initial physical exam may be briefer such that a timely diagnosis is made. There is no neurologic exam specific to ICH. Even though the Glasgow Coma Scale was developed for the trauma setting, it is often utilized in the emergency department for ICH, given that it is so widely recognized among healthcare providers. Although the National Institutes of Health Stroke Scale (NIHSS) was originally developed for use in ischemic stroke, it provides an additional mechanism for reporting physical exam findings in ICH [4].
2.4 Imaging
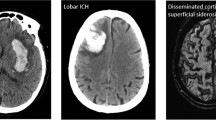
Until imaging has been obtained, it is unknown whether a patient has had an ischemic or hemorrhagic stroke. Thus, imaging should be performed quickly. The most efficient imaging method to diagnose ICH is with a non-contrast head CT (NCHCT). Non-contrast head CT should be performed as soon as safely possible in the ED [5]. On NCHCT, the hemorrhage has increased attenuation compared to brain parenchyma in the setting of the acute hemorrhage (Fig. 7.2a). When reviewing the NCHCT, it is important for emergency clinicians to evaluate for perihematomal edema, herniation, intraventricular hemorrhage, and midline shift, all of which can be visualized on initial head CT. Hematoma volume can easily be calculated as well on initial head CT. Since a higher hematoma volume is significantly associated with higher mortality, this information is important to evaluate. The ICH volume can be estimated using the ABC/2 formula, “where A is the greatest hemorrhage diameter by CT, B is the diameter 90° to A, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness.” [6].
(a) Demonstrates a right frontal intracerebral hemorrhage (ICH). Note the increased attenuation of hemorrhage in comparison to brain parenchyma. (b) Demonstrates an ICH with significant intraventricular hemorrhage (IVH). This IVH has required the placement of two external ventricular drains (EVDs). (c) Demonstrates a spot sign in a left parietal occipital ICH. The spot sign is noted by an arrow and demonstrates active contrast extravasation into the hemorrhage
ICH is frequently complicated by intraventricular hemorrhage (IVH), which occurs when blood leaks into the ventricles (Fig. 7.2b). When this happens, hydrocephalus may worsen rapidly, leading to brain herniation. One of the indications for acute neurosurgical intervention in the setting of ICH is the occurrence of IVH. An external ventricular drain (EVD) may need to be placed; thus neurosurgical consultation should be obtained immediately when IVH is recognized.
An important feature to note if vascular imaging is obtained in the ED is the significance of the “spot sign.” The spot sign, which is one or more areas of enhancement noted on contrasted images within the ICH, has been recognized as a marker of hemorrhagic expansion (Fig. 7.2c). It is demonstrative of active contrast extravasation occurring during the study, meaning that the hemorrhage is increasing in real time. The spot sign has been described in several prospective studies, including in a multicenter prospective observational cohort study of 268 patients with ICH. In this study, patients who were spot sign positive had a significantly higher amount of ICH expansion, defined as growth >6 mL or 33% at follow-up CT, as well as higher mortality at 3 months, and decreased functional status at 3 months [7].
Additionally, magnetic resonance imaging (MRI) may be necessary in certain patients with ICH. Typically, an MRI can be delayed until a patient is admitted or transferred and is generally not necessary in the ED in the setting of ICH. MRIs are time-consuming and are not safe for unstable patients or patients at risk of becoming unstable. The timing of subacute hemorrhages can be determined by MRI, as the appearance of hemorrhage changes in a predictable fashion. Gradient recalled echo (GRE) sequences may demonstrate microbleeds, assisting in the diagnosis of amyloid angiopathy, if multilobar. If microbleeds appear concentrated in deeper structures, this may be more suggestive of chronic hypertensive arteriopathy.
It is worthwhile to note that the ICH score developed in 2001, which has been derived and externally validated to predict 30-day mortality following ICH, hinges on key imaging findings from the NCHCT. Patients with higher scores on ICH score have a predicted worse outcome, and points are given for lower GCS, higher age, infratentorial location, higher ICH volume, and presence of IVH [8].
2.5 Lab Work
Patients with ICH should undergo a comprehensive lab workup in the ED. Any patient with a presentation of altered mental status should have an immediate finger-stick blood glucose obtained. Hypoglycemia is a rapidly treatable and correctable condition and should be diagnosed, ideally, in the prehospital setting. A complete blood count (CBC) should be performed, with particular attention to platelet count. Platelets should be transfused if under 100 K in the acute setting. Platelets should not be if patients are taking an antiplatelet drug and platelet count is normal. The recently published platelet transfusion in cerebral hemorrhage (PATCH) trial, a randomized controlled trial in which patients on antiplatelet medications were randomized to receive either standard care or standard care plus platelet transfusion, did not find a difference in patients who received platelet transfusions [9]. Coagulation status, measured by protime (PT) and activated prothrombin time (aPTT), should be obtained as well. Although the PT with conversion to the INR is an excellent measurement of the effects of warfarin, there is more ambiguity in the setting of novel oral anticoagulants [10]. A serum chemistry panel should be performed, in addition to a toxicology screen if concern for illicit drugs as an etiology for the hemorrhage. Women of childbearing age should also have a pregnancy test obtained. American Heart Association (AHA) guidelines also recommend a chest x-ray (CXR) and electrocardiogram (EKG) initially [3]. If possible, lab work should be completed point of care (POC), such that the results of coagulation status are available so that coagulopathy can be rapidly managed.
3 Organization of Care of Hemorrhagic Stroke
3.1 Emergency Department Management
Unlike the treatment of ischemic stroke, which may be treated with IV tPA and endovascular reperfusion in certain patients [11,12,13,14,15,16], there are no well-defined, targeted treatment modalities for ICH. Priorities for ED management are airway and hemodynamic stabilization, diagnosis, blood pressure treatment, anticoagulation reversal, and timely disposition.
3.2 Airway
Depending on the size and location of the hemorrhage, patients with ICH may require endotracheal intubation upon arrival to the ED secondary to decreased mental status and inability to manage secretions safely. In a prospective cohort study of 574 patients, 33% of patients with ICH required intubation either prior to arrival, during their ED stay, or within the first 24 h of admission [17]. In patients who do require intubation, both etomidate and ketamine are safe drug choices for induction during rapid sequence intubation (RSI). A 2009 randomized controlled trial demonstrated no difference between the two in critically ill ED patients requiring intubation [18]. Ideally, if neuromuscular blockade is performed, a short-acting paralytic agent, such as succinylcholine, should be used, so that the neurologic exam is not obscured for a lengthy period of time. Adequate analgesia and sedation should be given to intubated patients to prevent ICP elevation. In patients who do not require intubation initially, it is critical to monitor the patient’s airway status with serial exams, as the neurologic exam may deteriorate while the patient is in the ED, as 9.8% of patients in the previously described prospective cohort required intubation while in the ED [17]. Following intubation, providers should maintain eucapnia. Patients should not be artificially hyperventilated, as hyperventilation is a temporizing measure, meant only for patients who are about to receive definitive operative therapy. Although hyperventilation does decrease intracranial pressure for a short period of time, patients who are artificially hyperventilated for a longer time course have worse outcomes compared to patients maintained with eucapnia. This association has been well-demonstrated previously in the TBI literature and has even led to long-term poor outcomes in this patient population [19]. Patients with ICH who are not intubated should not eat or drink initially until further formal evaluation, as they are at risk of aspiration. Of patients who initially survive an ICH, approximately 9% will ultimately require percutaneous endoscopic gastrostomy (PEG) placement [20].
3.3 Blood Pressure
Once the patient’s airway has been assessed and managed and the diagnosis of ICH has been made, it is important for the emergency medicine physician to direct his or her attention to blood pressure management. If hypotension is noted or intravenous fluids are required, it is imperative to avoid hypotonic or dextrose containing fluids, as these may worsen cerebral edema. However, hypertension is significantly more common in patients with acute ICH.
Acute hypertension management in the setting of ICH has been the subject of several large recently published and ongoing studies. Elevated blood pressure in the setting of acute ICH is an independently associated measure of neurologic deterioration, hematoma expansion, and unfavorable long-term outcome in the setting of acute ICH; thus, controlling hypertension in acute ICH is important [21]. Unlike in ischemic stroke, where rapid substantial blood pressure reduction is unsafe and leads to decreased penumbra perfusion, it is generally well tolerated in patients with ICH, and aggressive blood pressure management does not lead to additional brain ischemia [22, 23]. Both the acute cerebral hemorrhage study (ATACH 1) and the intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT-1) did not note a difference in the safety when lowering systolic blood pressure (SBP) to <140 mmHg [21, 22]. Current American Heart Association (AHA) guidelines have given a Class 1; level of evidence A for acutely lowering blood pressure to 140 mmHg if SBP on arrival is between 150 and 220 mg as being safe [3]. INTERACT-2 randomized 2839 patients to either intensive blood pressure management, defined as SBP < 140 mmHg within 1 h, or standard blood pressure management, defined as a SBP < 180 mmHg. The endpoints of INTERACT-2 were 90-day death or disability. Fifty-two percent of patients in the treatment arm compared to 56% in the standard arm experienced the primary outcome (odds ratio 0.87; 95% CI 0.75–1.01, P = 0.06). INTERACT-2 demonstrated that the intensive blood pressure management was safe but did not overall reduce likelihood of death, improve functional outcome, or significantly reduce hematoma expansion [24]. ATACH II, currently ongoing, is comparing blood pressure management in ICH patients to a goal of <140 mmHg SBP versus <180 mmHg SBP [25]. With the results of INTERACT-2, the AHA has given a Class IIa, level of evidence B guideline that lowering SBP to 140 mmHg in the setting of acute ICH is effective for improving functional outcomes [3].
3.4 Anticoagulation/Antiplatelet Considerations/Reversal
Patients who are anticoagulated for any reason and found to have an ICH in the ED should have the anticoagulation reversed promptly. Indeed, emergency physicians need to be well-prepared for anticoagulation reversal. Anticoagulation is common in this patient population, with approximately one in five cases of ICH associated with anticoagulation use [26]. This particular knowledge basis has become much more complicated since the addition of novel oral anticoagulants (NOACS). Anticoagulant reversal and blood pressure management are the two most critical actions that must be taken in treating patients with ICH. For instance, when FFP is utilized to reverse warfarin, each 30-min delay in the start of FFP is associated with a 20% reduction in INR correction within the first 24 h [27]. Indeed, a 2015 retrospective study of 1176 anticoagulated patients with ICH demonstrated a substantial mortality reduction to patients who had both INR reversal to <1.3 and SBP controlled to <160 mmHg within 4 h of ED presentation [28].
Warfarin, a vitamin K antagonist (VKA), is the most commonly used anticoagulant, has been on the market for decades, and, thus, has the most data regarding its reversal in the setting of ICH. All patients on warfarin must receive vitamin K. It should be given intravenously in the ED for the most rapid absorption [29]. Vitamin K allows the patient to generate new clotting factors, but this may take hours to days. For immediate reversal, fresh frozen plasma (FFP) to replace all factors, or prothrombin complex concentrates (PCCs) which contain either three or four factors only, must be given as well to patients. PCCs may be preferable, given that there is less volume to infuse, are able to be stored at room temperature, and result in faster INR correction compared to FFP for the reversal of warfarin-associated ICH [30]. However, they are more expensive in many institutions and, as such, more restricted. PCCs have been in use in Europe for over 20 years but were only recently FDA approved in the United States. PCCs generally contain factors II, VII, IX, X, C, S, Z, and antithrombin 3, whereas FFP contains all plasma factors [31, 32]. When compared to one another, 62% of patients given PCCs achieve INR correction by 0.5 h after the end of the infusion, compared to 9.6% of patients who are given FFP, with similar safety events [30]. Frontera et al. conducted a prospective observational study of PCC vs. FFP vs. PCC + FFP in the setting of VKA-associated ICH. Patients who received PCCs had a significantly lower risk of death and severe disability at 3 months compared to FFP alone, without an increase of adverse events [33]. The fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial hemorrhage related to vitamin K antagonists trial (INCH trial) enrolled 54 patients but was terminated early due to safety concerns. Prior to termination, they found that PCCs were superior in normalizing INR and stated that the faster INR normalization was associated with smaller hematoma expansion [34]. Ultimately, current CHEST and AHA guidelines recommend that patients with VKA-associated major hemorrhage or ICH receive 4-factor PCC instead of FFP (Grade 2C—CHEST; Class IIb, level of evidence B—AHA) [3, 5, 10].
Newer anticoagulant classes have been approved for the management of non-valvular atrial fibrillation and thromboembolic disease. These classes of medications include direct thrombin inhibitors and factor Xa inhibitors and, as such, utilize different mechanisms within the clotting cascade to provide anticoagulant benefit. In patients who are taking direct thrombin inhibitors, first-line therapy for reversal should be idarucizumab, which is able to completely reverse the drug within minutes [35]. Direct thrombin inhibitors are cleared by the kidneys and, as such, can also be removed by hemodialysis [36]. However, starting hemodialysis in the ED poses many challenges, including the need for dialysis access, equipment, and hemodialysis monitoring capabilities. Factor eight inhibitor bypassing activity (FEIBA), also known as activated 4-factor PCC, may be another option for dabigatran reversal based on in vitro testing [37]. Factor Xa inhibitors may be best reversed with andexanet alfa. Andexanet alfa is a modified recombinant form of factor Xa, and thus it binds to factor Xa inhibitors [38]. It currently is in phase III trials for the reversal of factor Xa inhibitors but showed promise in a single-arm study of patients with acute bleeding [39]. In vitro testing has shown that 4-factor PCCs are appropriate for the reversal of factor Xa inhibitors, given that these drugs are protein bound and cannot be removed with hemodialysis [40, 41]. Recombinant factor VIIa may also be an option for reversal of NOACs, also based on in vitro animal testing [42,43,44].
Patients receiving antiplatelet therapy may also develop ICH, and the optimal treatment and reversal mechanisms for this class of patients are still under study. The recently published platelet transfusion in cerebral hemorrhage (PATCH) trial, a randomized controlled trial in which patients on antiplatelet medications were randomized to receive standard care or standard care plus platelet transfusion, failed to find a difference in patients who received platelet transfusions and even trended toward harm with platelet transfusion [9]. Desmopressin, or DDAVP, may be an option for reversal of antiplatelets. A small study of 14 patients who were on antiplatelets and had sustained an ICH showed that the drug was well tolerated and improved platelet function [45]. However, larger studies are necessary to determine if this will provide more clinically meaningful endpoints in ICH patients. More details about antithrombotic-induced bleeding will be discussed as a main topic in Chap. 14.
3.5 Hemostatic Agents
Hemostatic agents, such as recombinant factor VIIa, have been explored as a treatment for ICH both in anticoagulated patients and non-anticoagulated patients. The efficacy and safety of recombinant-activated factor VIIa for acute intracerebral hemorrhage (FAST) study was a randomized controlled trial of 841 patients with ICH. Although there was a significant difference in the size of hematoma growth, with the treatment group demonstrating less hematoma group, there was no difference in clinical outcomes [46]. Further data suggested that rFVIIa may be most beneficial in patients with spot sign noted on CT angiogram. There are two ongoing randomized placebo-controlled trials to test rFVIIa—the Spot Sign for Predicting and Treating ICH (STOP-IT) trial and the Spot Sign Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy (SPOTLIGHT) trial. Tranexamic acid (TXA) is another promising hemostatic agent. This drug has not yet been tested in ICH, but two randomized controlled trials in the TBI literature demonstrated statistically significant reduction of hemorrhage progression without clinically meaningful differences [47].
3.6 Seizure Management and Prophylaxis
Patients with clinical seizure-like activity after ICH should be treated with anti-seizure medications while in the ED and should be managed similar to any other patient with seizures [3]. The incidence of seizures following ICH in the immediate period is unknown; is likely dependent on ICH location, comorbidities, and size of hemorrhage; but may be as low as 1.7% in one series and as high as 17% in patients with supratentorial ICH [48, 49]. Seizure prophylaxis is not recommended and has been associated with poor outcomes when adjusted for age, initial hematoma volume, presence of intraventricular blood, initial GCS, and previous warfarin use [49]. However these studies demonstrating harm predominately utilized phenytoin for seizure prophylaxis. Newer data report that levetiracetam may be a safer option for seizure prophylaxis [50].
3.7 Management of Increased Intracranial Pressure
After ICH, patients may experience increased intracranial pressure. Much of the literature from traumatic brain injury has been extrapolated into the realm of ICH, especially for the role of ICP management. There is an association between elevated ICP and outcome in ICH however. In a study of 121 patients with ICH and ICP monitoring in place, there was a significant association with both poor functional outcome and mortality with ICP greater than 20 [51]. Simple maneuvers, such as raising the head of the bed to 30°, have previously demonstrated a decrease in intracranial pressure and improved cerebral perfusion pressure, without compromising cardiac output in other types of intracranial hemorrhages, although less specifically in ICH [52]. As long as there is no concern for concomitant spinal cord injury, the head of the bed should be raised to 30–45° as a basic maneuver to prevent elevated intracranial pressure. Both mannitol and hypertonic saline (3% or 23.4%) are used to manage increased intracranial pressure [53]. It is important to note that mannitol’s mechanism of action is via osmotic diuresis, so clinicians must be careful to monitor urine output and guard against hypotension. Hypertonic saline can be used as 3% or 23.4%; however, 23.4% cannot be used with a peripheral IV, and patients must have central venous access. 23.4% hypertonic saline provides an osmolar load in the serum with lower volume and shorter infusion time.
3.8 Role of Surgical Management
Two multicenter randomized controlled trials have been conducted to determine if there is a potential benefit of surgical intervention in supratentorial ICH—the STICH I and STICH II trials. Neither the STICH I nor STICH II trial demonstrated benefit in surgical management of ICH [54, 55]. However, these trials had high numbers of crossover from medical management into surgical management groups, so neurosurgeons may decompress some ICHs, depending on the indications. On the other hand, posterior fossa ICHs require surgical intervention much more frequently. Although there is no randomized controlled trial data, case series describe the benefit of posterior fossa decompression [56]. Due to the proximity of the fourth ventricle and brainstem, patients with posterior fossa ICH should have surgical decompression if they have any signs of deterioration, brainstem compression, or hydrocephalus. Since there is no equipoise regarding this topic, there is unlikely to be a trial conducted evaluating the utility of posterior fossa decompression [3]. Emergency medicine physicians should recognize this surgical indication and be able to communicate the findings of posterior fossa ICH to neurosurgical colleagues, as well as any signs of early deterioration that might mandate surgical intervention.
3.9 Prognostic Factors Associated with ICH
Prognosis following ICH is dependent on many factors. The ICH score, derived and externally validated, is the most common tool to determine prognosis [8]. However, the ICH score should not be the only factor used in determining prognosis. Recently, Morgenstern et al. described that a cohort of patients who were predetermined to have poor prognosis based on ICH score had a much lower mortality than expected (20% compared to 50% expected prognosis) simply by the avoidance of early DNR orders [57]. The ICH score ranges from 0 to 6, with higher score indicating worse prognosis. A score of 5 or 6 indicates 100% mortality, for instance. The ICH score factors in GCS, ICH volume, IVH, location of hemorrhage, and age [8]. Overall, the volume of ICH on head CT is the most important of these factors and weighs the heaviest in determining mortality [58]. Patients with brainstem hemorrhages and those with hematoma expansion also tend to have poorer prognosis [59, 60]. More details will be discussed as a main topic in Chap. 15.
3.10 Disposition
All patients with spontaneous ICH require admission to the hospital and in nearly all cases require admission to the intensive care unit (ICU). The ICU disposition is usually institution specific and may be managed by an internist, intensivist, neuro-intensivist, neurologist, or neurosurgeon. Interestingly, in a study of 13 ICUs, it was noted that after controlling for demographics, ICH severity, and institutional and ICU characteristics, patients admitted to a neuro ICU demonstrated an improvement in mortality rate when compared to general ICUs (odds ratio 3.4; 95% CI 1.65–7.6). A dedicated full-time neuro-intensivist also was associated with lower mortality rate (OR 0.388; 95% CI 0.22–0.67) [61].
3.11 DNR Status/Physician Pessimism
Early withdrawal of care is independently associated with mortality in ICH [62]. An early DNR, defined as <24 h after arrival, was associated with doubling in hazard ratio of death at 30 days (hazard ratio [HR] 2.17, 95% CI 1.38, 3.41) with adjustment made for age, gender, ethnicity, presenting Glasgow Coma Scale, ICH volume, intraventricular hemorrhage, and infratentorial hemorrhage [62]. Factors that have been notable for an association with DNR orders signed in the ED include increased age, ambulatory status before the event, CT findings with midline shift, intraventricular extension, larger hematoma size, and arrival to the ED with GCS ≤8 [63]. This has been described in the literature as the “self-fulfilling prophecy,” such that patients have care withdrawn when they may have otherwise had a potentially good outcome if exposed to aggressive care early in their course. Recent data also suggests that patients with ICH continue to have neurologic improvements beyond 6 months [64]. These studies have led to changes in the AHA guidelines, now giving a Class IIa recommendation to avoid signing a DNR order until the second full day of hospitalization. Thus, emergency physicians should provide maximal care to patients with ICH in the immediate period.
References
Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60.
Van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60.
Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–70.
Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–29.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–14.
Hemphill JC 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–13.
Samama MM, Guinet C. Laboratory assessment of new anticoagulants. Clin Chem Lab Med. 2011;49:761–72.
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29.
Berkhemer OA, Majoie CB, Dippel DW. Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2363.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Campbell BC, Mitchell PJ. Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2365–6.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Elmer J, Pallin DJ, Liu S, et al. Prolonged emergency department length of stay is not associated with worse outcomes in patients with intracerebral hemorrhage. Neurocrit Care. 2012;17:334–42.
Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300.
Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–9.
Faigle R, Bahouth MN, Urrutia VC, et al. Racial and socioeconomic disparities in gastrostomy tube placement after intracerebral hemorrhage in the United States. Stroke. 2016;47:964–70.
Sakamoto Y, Koga M, Yamagami H, et al. Systolic blood pressure after intravenous antihypertensive treatment and clinical outcomes in hyperacute intracerebral hemorrhage: the stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study. Stroke. 2013;44:1846–51.
Zazulia AR, Diringer MN, Videen TO, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–10.
Koch S, Romano JG, Forteza AM, et al. Rapid blood pressure reduction in acute intracerebral hemorrhage: feasibility and safety. Neurocrit Care. 2008;8:316–21.
Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65.
Qureshi AI, Palesch YY. Antihypertensive treatment of acute cerebral hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15:559–76.
Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–21.
Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–5.
Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–36.
Nee R, Doppenschmidt D, Donovan DJ, et al. Intravenous versus subcutaneous vitamin K1 in reversing excessive oral anticoagulation. Am J Cardiol. 1999;83:286–8.
Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–43.
Levy J, Tanaka K, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology. 2008;109:918.
Dusel CH, Grundmann C, Eich S, et al. Identification of prothrombin as a major thrombogenic agent in prothrombin complex concentrates. Blood Coagul Fibrinolysis. 2004;15:405–11.
Frontera JA, Gordon E, Zach V, et al. Reversal of coagulopathy using prothrombin complex concentrates is associated with improved outcome compared to fresh frozen plasma in warfarin-associated intracranial hemorrhage. Neurocrit Care. 2014;21:397–406.
Steiner T, Poli S, Griebe M, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol. 2016;15:566–73.
Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373:511–20.
Garcia DA, Baglin TP, Weitz JI, et al. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians. Evidence-based clinical practice guidelines. Chest. 2012;141:e24S–43S.
Khoo TL, Weatherburn C, Kershaw G, et al. The use of FEIBA(R) in the correction of coagulation abnormalities induced by dabigatran. Int J Lab Hematol. 2013;35:222–4.
Yates SW. Interrupting anticoagulation in patients with nonvalvular atrial fibrillation. P T. 2014;39:858–80.
Connolly SJ, Milling TJ Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375:1131–41.
Perzborn E, Heitmeier S, Laux V, et al. Reversal of rivaroxaban-induced anticoagulation with prothrombin complex concentrate, activated prothrombin complex concentrate and recombinant activated factor VII in vitro. Thromb Res. 2014;133:671–81.
Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significance of studies in vitro with circulating human blood. PLoS One. 2013;8:e78696.
Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–9.
Godier A, Miclot A, Le Bonniec B, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology. 2012;116:94–102.
Fukuda T, Honda Y, Kamisato C, et al. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost. 2012;107:253–9.
Naidech AM, Maas MB, Levasseur-Franklin KE, et al. Desmopressin improves platelet activity in acute intracerebral hemorrhage. Stroke. 2014;45:2451–3.
Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.
Zehtabchi S, Abdel Baki SG, Falzon L, et al. Tranexamic acid for traumatic brain injury: a systematic review and meta-analysis. Am J Emerg Med. 2014;32:1503–9.
Berger AR, Lipton RB, Lesser ML, et al. Early seizures following intracerebral hemorrhage: implications for therapy. Neurology. 1988;38:1363–5.
Messe SR, Sansing LH, Cucchiara BL, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11:38–44.
Sheth KN, Martini SR, Moomaw CJ, et al. Prophylactic antiepileptic drug use and outcome in the ethnic/racial variations of intracerebral hemorrhage study. Stroke. 2015;46:3532–5.
Sykora M, Steinmacher S, Steiner T, et al. Association of intracranial pressure with outcome in comatose patients with intracerebral hemorrhage. J Neurol Sci. 2014;342:141–5.
Schulz-Stubner S, Thiex R. Raising the head-of-bed by 30 degrees reduces ICP and improves CPP without compromising cardiac output in euvolemic patients with traumatic brain injury and subarachnoid haemorrhage: a practice audit. Eur J Anaesthesiol. 2006;23:177–80.
Qureshi AI, Wilson DA, Traystman RJ. Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: comparison between mannitol and hypertonic saline. Neurosurgery. 1999;44:1055–63.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97.
Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408.
Da Pian R, Bazzan A, Pasqualin A. Surgical versus medical treatment of spontaneous posterior fossa haematomas: a cooperative study on 205 cases. Neurol Res. 1984;6:145–51.
Morgenstern LB, Zahuranec DB, Sanchez BN, et al. Full medical support for intracerebral hemorrhage. Neurology. 2015;84:1739–44.
Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93.
Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23.
Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81.
Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635–40.
Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–7.
Fan JS, Huang HH, Chen YC, et al. Emergency department do-not-resuscitate order in patients with spontaneous intracerebral hemorrhage. Am J Emerg Med. 2017;35:1850–4.
Hanley DF, Awad IA, Vespa PM, et al. Hemorrhagic stroke: introduction. Stroke. 2013;44:S65–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Kreitzer, N., Woo, D. (2018). Overview of Hemorrhagic Stroke Care in the Emergency Unit. In: Lee, SH. (eds) Stroke Revisited: Hemorrhagic Stroke. Stroke Revisited. Springer, Singapore. https://doi.org/10.1007/978-981-10-1427-7_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-1427-7_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1426-0
Online ISBN: 978-981-10-1427-7
eBook Packages: MedicineMedicine (R0)