Abstract
Cell-based therapy is attracting attention not only for its regenerative property but also for its long therapeutic time window. A growing number of studies in animal models with brain injuries have shown that cell therapies are beneficial. Among the variety of cell types to be used for cell therapies, autologous umbilical cord blood cells (UCBCs) are the most feasible; UCB contains several types of stem cells, the collection of the UCB is totally noninvasive and no ethical issues are involved, and UCBCs have no tumorigenicity. More than 20 preclinical studies have examined the effects of human UCBCs in models of neonatal brain injury; the majority of the studies were conducted in a rodent model of hypoxia-ischemia. Systemic administration of mononuclear fraction of UCB is the most extensively explored, and most of the studies have shown beneficial effects. Intravenous infusion of autologous non-cryopreserved volume- and red blood cell-reduced UCB is the most feasible method for cell therapy, especially when used at the acute phase of acute onset diseases. Fewer than ten clinical studies, including ours, using UCB for newborns with acute brain injury have been reported or listed on open registration websites, and only a few of the studies have reported the results, proving safety and feasibility and implying efficacy. No randomized control studies have been reported with respect to cell therapies during the newborn period. Further preclinical studies to optimize the treatment protocol and clinical trials to prove efficacy are warranted.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others

Keywords
1.1 Background of Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy (HIE)
Acute brain injury may occur unexpectedly during the perinatal and neonatal periods. The conditions in which neonates present with acute brain dysfunction are collectively termed neonatal encephalopathy [1]. The signs and symptoms of brain dysfunction are recognized as an altered level of consciousness, weak muscle tone, impaired feeding, respiratory depression, and seizures. The vast majority of infants with neonatal encephalopathy are born with asphyxia, the causes of which are heterogeneous, such as low maternal blood pressure, premature placental abruption, compression of the umbilical cord, and severe congenital heart disease. The pathophysiology of neonatal encephalopathy is hypoxic-ischemic encephalopathy (HIE) in the majority of cases, up to 85% of neonatal encephalopathy cases, followed by neonatal stroke, which accounts for up to 10% of cases [2]. Children with severe neonatal HIE typically die or develop severe neurological sequelae such as cerebral palsy (CP), mental retardation, and epilepsy [3]. Children with moderate neonatal HIE suffer neurological sequelae in many cases; 81% have cognitive dysfunctions, and 30% have cerebral palsy when the survivors are assessed in their late teens [4].
Success in the translation of mild hypothermia was a landmark event for both neonatologists/pediatricians and neuroscientists. Before the introduction of mild hypothermia, treatment in neonatal intensive care units (NICUs) was merely supportive. Mild hypothermia was the first and is still the only treatment proven effective in large-scale randomized clinical trials [5, 6]. However, even if infants are treated with hypothermia, nearly half of them die or are left with moderate to severe neurological impairments. The therapeutic time window of hypothermia is within 6 h after birth. Therefore, there is an urgent need for the development of novel therapies for HIE. A review in year 2006 reported that nearly 700 therapies for acute stroke had proven effective in preclinical studies and that over 100 of them had been used in practice. Nevertheless, tissue plasminogen activator administered within 3 h after the onset of stroke was the only therapy proven effective in a clinical study [7]. Most of those therapies exert beneficial effects only when administered before the ischemic insult or immediately after the insult. Although some therapies demonstrate beneficial effects even when administered after certain periods of time, the therapeutic time window rarely exceed 6 h after the insult.
Cell-based therapy is attracting a lot of attention because not only of its regenerative property but also of the long therapeutic time window. Various studies in animal models with brain injuries have shown that cell therapies are beneficial. Those studies range from embryonic stem cells and induced pluripotent stem cells to mononuclear cell fractions of bone marrow and umbilical cord blood with respect to cell type, from culturing with several gene transfections and manipulations to simple separation of cells with gradient centrifugation with respect to cell preparation, from intracerebral transplantation to intravenous injection with respect to the administration route, and from xenotransplantation (from different species) to autologous transplantation (using one’s own cells) with respect to the origin of cells.
In this chapter, after we briefly review the history of cell therapies in both preclinical and clinical studies, we review cell therapies using umbilical cord blood.
1.2 History of Cell Therapies for Brain Injury
1.2.1 Preclinical Studies on Cell Therapies for Neonatal Brain Injury
During the early days of research in stem cell therapy, most researchers conceived of the concept of “regeneration,” in which transplanted stem cells proliferate and differentiate into new neurons and replace lost neurons. During the past decade, several other concepts have been added to the research, in which transplanted stem cells secrete a variety of trophic factors, suppress inflammation and modulate the immune response, and enhance endogenous neurogenesis and angiogenesis [8, 9]. A growing number of studies have demonstrated that the long-term survival of stem cells transplanted in the brain is not necessary [10,11,12]. Furthermore, many studies have shown that transition into the brain is not necessary in order that the administered cells exert beneficial effects on an injured brain [13, 14]. Currently conceived mechanisms of stem cell therapy are multimodal and not confined to regeneration. Hence the term “cell-based therapy” could be more appropriate than “regenerative therapy” at least in the field of acute brain injury.
More than 60 research articles on cell-based therapies for perinatal/neonatal brain injury have been published to date, and the number has been growing each year [15] (see Chap. 5 for details). The vast majority of those studies used rodent models of neonatal HIE. During the first decade since the first report in this field by [16], intracerebral transplantation of either the fetal brain tissue or neural stem cells (NSCs) was investigated. Systemic administration by intraperitoneal injection of cells was first reported by Meier et al. in [17]. Also, Meier et al. was the first to report the effects of umbilical cord blood (UCB) cells in neonatal models. Intravenous injection, a clinically feasible route of systemic administration, was first reported by Yasuhara et al. [18]. During the second decade, roughly equal numbers of studies using intracranial transplantation or systemic administration of stem cells have been reported in this research field. With respect to the donor cells, the mononuclear cell (MNC) fraction of human UCB and mesenchymal stem cells (MSCs) derived from rodent bone marrow (BM) have been most extensively investigated. MNCs are infused systematically and MSCs are transplanted intracranially in many of the studies.
Human UCB (hUCB) is the most extensively used cell source for preclinical research in neonatal brain injury. Over 20 studies have been reported in the literature [15, 19]. For details, refer to Chap. 5 in this textbook. Briefly, the majority of them have reported the beneficial effects of cell therapy in either morphological or behavioral evaluations or in both evaluations, although some reported no therapeutic effects. No adverse effects were noted. Two studies examined the effects of hUCB CD34+ cells (hematopoietic/endothelial progenitor cells); three studies examined the effects of MSCs derived from hUCB; one study examined the effects of total nucleated cells; and all the other studies examined the effects of MNC fraction of hUCB. With respect to the administration route, cells were transplanted into brains in three studies with MSCs and two studies with MNCs; cells were injected systemically in other studies, intraperitoneally in approximately one third of the studies, and intravenously in approximately two thirds of the studies. The effective cell dose varied from 1.5 × 104 cells up to 1 × 108. Similarly, effective timing for the cell administration varied from 6 h to 7 days after the insult. Few studies have examined the optimal cell dose and timing of cell administration. Taking these findings together, among cell therapies for neonatal brain injury, systemic administration of hUCB MNCs is the most vigorously studied, and it has proven effective with no serious adverse effects, although the optimal therapeutic protocol, such as the timing and dose of cells, is not known. This therapy, intravenous infusion of autologous UCB cells (UCBCs), is considered to have the lowest risk in clinical translation for infants with brain injury [20].
1.2.2 Clinical Use of Cell Therapy with Umbilical Cord Blood
Blood transfusion is the oldest cell therapy for mankind. Hematopoietic stem cell (HSC) transplantation, i.e., BM transplantation, is the first cell therapy to use the regenerative property of stem cells and a well-established standard therapy for patients with leukemia and other malignant diseases. BM transplantation exerts benefits for mitigating neurodegenerative progress in some inborn errors of metabolism, such as Hurler’s disease and adrenoleukodystrophy [21,22,23]. UCB contains a high concentration of HSCs; hence UCB transplantation is an excellent alternative for BM transplantation. UCB transplantation favorably alters the disease progression of some inborn errors of metabolism as well [24, 25]. The beneficial effect of HSC transplantation is primarily through enzyme replacement by the donor cells. Healthy donor cells engraft in the recipient and continue to excrete the enzyme that is defective in the recipient. Although HSC transplantation involves the intravenous administration of HSCs, transfused cells enter the donor brain and survive for months [26]. In addition to enzyme replacement, HSC transplantation is considered to exert a benefit through its effect on the immune system, such as the effect of donor-derived microglia in the brain [27]. One case study suggests that donor gene material reaches neurons and that these neurons contain some proteins of the donor gene product [28]. Taking these findings together, intravenous administration of HSCs has effects on the brain through a couple of mechanisms other than the hematopoietic potency. In addition, there is no risk of tumorigenicity in either UCB or BM transplantation.
1.3 Current Status of Cell Therapies for Brain Injury
Among the variety of cell types to be used for cell therapies, autologous UCBCs are the most feasible; the collection of the cells is totally noninvasive, and no ethical issues are involved as UCBs are usually discarded directly after birth. The safety and feasibility of intravenous infusion of cryopreserved autologous UCB have been reported in a study in children with neurological disorders, most of whom had CP [29]. Among 184 study participants, three patients experienced infusion reaction, which resolved after discontinuation of the infusion and medical therapy. No other adverse events were reported during the 12-month follow-up. Supported with positive results of systemic administration of hUCBCs in preclinical studies on neonatal encephalopathy, several institutions in several countries have started applying UCB transfusion for children with brain injury of non-metabolic origin.
1.3.1 Systemic Administration of UCBCs for Chronic Brain Injury
The chronic phase of brain injury is an easier target to treat with cell therapy compared with the acute phase of brain injury especially when the injury is sudden onset as in neonatal encephalopathy. Hence, CP has been the main target of UCBC therapy thus far. CP is a group of permanent disorders affecting motor development and posture resulting from various ischemic brain injuries that occur during the prenatal or neonatal period, of which neonatal HIE is the most conspicuous cause. Nearly 20 clinical trials are listed on the website of the US National Institute of Health (ClinicalTrials.gov) as a cell therapy for CP and related diseases (Table 1.1). Approximately half of the trials use UCB and the other half use cells derived from BM.
Of the 20 trials, 11 trials from six research groups use UCBCs. Only one of those trials has published the results thus far (NCT01193660) [30]. NCT01193660 is a randomized trial with 96 participants conducted at CHA University in South Korea. HLA-matched allogeneic UCB containing >3 × 107/kg total nucleated cells (TNCs) was intravenously administered to children with CP along with erythropoietin and immunosuppressive treatment. Compared with the control group and the group treated with erythropoietin only, the UCB-treated group had significantly higher scores on the Gross Motor Performance Measure and Bayley II Mental and Motor Scales at 6 months. The incidence of serious adverse events did not differ between groups. The same group compared allogeneic (three patients) and autologous (four patients) UCB transplantation in children with CP [31]. The allogeneic transplantation showed a better outcome than autologous transplantation.
Among the remaining ten trials, one trial (NCT01988584) uses either autologous UCB or BM-MNCs, three other trials (NCT01072370, NCT01147653, NCT02460484) use autologous UCB, five trials (NCT01528436, NCT01639404, NCT01991145, NCT02025972, NCT02599207) use allogeneic UCB, and one trial (NCT01929434) does not specify the source of UCB. Cells are administered intravenously in many of the trials but intra-arterially or intrathecally in some trials. NCT01929434 is the only phase III trial among them; the rest of them are either phase I or II trials. Regarding the cell types used, MSCs are used in a single trial (NCT01929434), MNCs are used in some trials (NCT01988584, NCT01072370), and the cell type used is not described in other trials.
Apart from the clinical studies listed on ClinicalTrials.gov, there are a few case reports of cell therapies in infants with brain injury. Jensen and Hamelmann reported the case of a boy with HI brain injury due to cardiac arrest at 2 years of age [32]. The boy received autologous intravenous UCB transfusion 9 weeks after the cardiac arrest. He demonstrated remarkable neurofunctional recovery from a vegetative state during the 2 months after the cell therapy. Jansen and Hamelmann attribute the recovery to the cell therapy. A pilot study of the intravenous infusion of autologous UCB was conducted in 20 children with CP with no control group [33]. Five children showed more improvements in neurodevelopmental evaluations than would normally be expected during the 6-month period after the infusion. Clinical studies using cells derived other than from either UCB or BM are limited. A randomized control trial of allogeneic transplantations of olfactory ensheathing cells (OECs) in 33 children with CP has been reported [34]. OECs were isolated from an aborted human fetal olfactory bulb, and the cells were injected into the frontal lobe of the patients. The OEC-treated children showed better Gross Motor Function Measure score than the controls. Although these results seem promising, it is difficult to interpret the efficacy as they are a case report and a clinical trial with no control group or with a small sample size.
1.3.2 Systemic Administration of UCBCs for Acute Brain Injury
Treating sudden onset diseases during their acute phase is difficult. Cells that require a preparation process with cell culture cannot be used during the acute phase, as cell culture takes from days to weeks. Hence, when culture work is required to prepare them, cells should be allogeneically prepared in advance (off-the-shelf). Autologous cells are advantageous over allogeneic cells in many respects; autologous cells have no or minimal risks on immune reactions and virus infections, no ethical issues related to the donors, and no shortage of donors. BM cells are a feasible autologous cell source for acute treatment in children and adults. However, collecting BM cells from a sick newborn is relatively invasive. In contrast, the collection of UCB is totally noninvasive for a newborn and his or her mother. For these reasons, autologous UCB is the most feasible cell source of autologous cells for treating acute onset diseases during the acute phase.
1.3.2.1 Clinical Trials of Systemic Administration of UCBCs for Acute Brain Injury
Seven clinical trials are listed on ClinicalTrials.gov as trials for newborns with neonatal encephalopathy to the best of our knowledge: NCT00593242, NCT02612155NCT01506258, NCT01649648, NCT02256618, NCT02434965, NCT02605018 (NCT02551003 seems identical to NCT02605018), and NCT02612155 (Table 1.2). All of them use autologous UCB. The registration websites of five of the trials do not specify the cell type used, most of which are assumed to be volume-reduced whole nucleated cells. The remaining two trials use either UCB CD34+ cells or UCB along with placenta-derived stem cells. Almost all of them are administered intravenously; two trials do not specify the administration route. Apart from one trial, in which cells are infused up to 7 days after birth, cells are administered within a few days after birth.
Only NCT00593242 (principal investigator, Dr. Cotten at Duke University, USA) has been completed, and the results have been published [35]. Cotten and colleagues enrolled 23 infants treated with hypothermia for HIE and intravenously infused non-cryopreserved volume- and red blood cell-reduced UCBCs: up to four doses (up to two doses in the current protocol), ~72 postnatal hours (~48 postnatal hours in the current protocol), and the mean number of cells after processing 4.1 × 108 cells/patient. No significant infusion reactions were noted. One-year neurodevelopmental outcomes were assessed with Bayley III, and 72% of UCBC treated infants had Bayley scores ≥85. Of infants who did not have available UCB and received standard treatments including hypothermia during the study period, 41% had Bayley scores ≥85. Of note, 26% of UCBC-treated infants were outborn (transported from an outside hospital after delivery), while 88% of infants with standard treatment were outborn. As outborn infants generally tend to have poorer outcome than inborn infants, caution should be exercised in interpreting the benefit of UCBCs. Nevertheless, the trial suggests that autologous UCBC infusion therapy for neonatal HIE is safe and feasible and may improve the outcome.
One of the seven trials is being conducted by us in Japan (NCT02256618, principal investigator, Dr. Shintaku at Osaka City University); our protocol is similar to the one at Duke University.
1.3.2.2 Our Clinical Trial of Systemic Administration of UCBCs for Acute Brain Injury
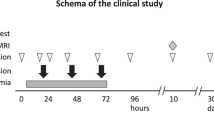
We, Neonatal Encephalopathy Consortium Japan, are currently conducting a phase I trial named “Autologous cord blood cell therapy for neonatal encephalopathy” (NCT02256618). This is a pilot study for testing the feasibility and safety of UCBC therapy in infants with neonatal HIE; the study is an open-label single group assignment. If a neonate is born with signs and symptoms of moderate to severe encephalopathy and meets the criteria for therapeutic hypothermia, the neonate is considered for entry of this clinical study; inclusion and exclusion criteria for the study are set in line with those of therapeutic hypothermia for term newborns with HIE (Table 1.3). Estimated enrollment is six cases. To make sure that UCB is properly collected without contamination, we exclude outborn infants from the trial. If an infant is born with severe asphyxia, the UCB is collected directly after the birth from an umbilical cord vein with special care to avoid contamination. We obtain parental consent before collecting UCB. UCB is volume- and red blood cell-reduced by centrifugation in a closed system using an automated machine named Sepax (Biosafe Inc. Switzerland). The volume- and red blood cell-reduced UCB contains all sorts of nucleated cells, including a variety of stem cells such as CD34+ hematopoietic stem/endothelial progenitor cells. The processed UCB is divided into three doses and stored at 4 °C until use. The cell dose is not adjusted. The total amount of UCB collected is used after the abovementioned simple centrifugation. Estimated cell doses administered would be approximately 6 × 108 cells/newborn. If the total amount of UCB is less than 40 mL, the newborn shall not be enrolled in the trial because the automated UCB process may not be reliable if the volume to be processed were less than 40 mL. We examined the quality of the processed and non-cryopreserved UCB using UCB collected from volunteers before commencement of this trial. At 72 h after the processing, there was no growth of bacteria or increase in potassium, and cell viability was well maintained. We obtain written informed consent from the parent(s) twice; first, when we consider the newborn with HIE meets the entry criteria after the initial assessment, which is normally a few hours after the birth, and, second, before the first administration of UCBC treatment for the newborn.
Autologous volume-reduced cord blood cells are administered intravenously at 12–24, 36–48, and 60–72 h after birth. Circulatory and respiratory status is closely monitored during and after the cell treatment. The primary outcome measure is the rate of adverse events. The combined rate of three adverse events at 30 days of age, death, continuous respiratory support, and continuous use of vasopressor, will be compared between the neonates with cell therapy and those with conventional therapy including hypothermia. The secondary outcome measure is efficacy. Neuroimaging at 12 months of age and neurodevelopmental function measured with Bayley III at 18 months of age will be compared between the cell recipients and neonates with conventional therapy. The infants will be followed for safety and neurodevelopmental outcome up to 10 years of age.
1.3.3 Systemic Administration of UCBCs for Brain Injury Associated with Preterm Birth
To the best of our knowledge, as few as one clinical trial (NCT01121328) is listed on ClinicalTrials.gov with respect to cell treatment for brain injury associated with preterm birth. The clinical trial focuses on premature infants born less than 34 weeks of gestation. Autologous UCB-MNCs are infused in the first 14 days after birth.
1.3.4 Issues to be Considered for UCBC Therapies
All those trials but one are small nonrandomized ones; therefore the efficacy of the cell therapies would not be known. The group led by Cotten is preparing a phase II clinical trial (NCT02612155) with an estimated enrollment of 160 cases.
The properties of hUCBCs may be altered by several factors, such as the gestational age and perinatal asphyxia [36, 37]. For example, Aly et al. reported that although the UCB-MNC count does not differ between healthy term newborns and term newborns with perinatal asphyxia, neuronal differentiation of hUCB-MSCs is more pronounced in the cells derived from newborns with asphyxia [38]. Lymphocyte counts are elevated in term infants with HIE, although the counts rapidly normalized [39]. The apoptosis of neutrophils is impaired in cord blood compared with adult peripheral blood, and the apoptosis is reduced by hypoxia [40]. Those alterations may become beneficial or detrimental to infants receiving UCB therapy.
Autologous UCBC treatment is the most feasible cell therapy for neonates with encephalopathy during the acute phase. Although the therapy has the lowest risk for clinical use, there are some drawbacks. The following two issues are critical. Firstly, autologous UCB may be difficult to collect in an urgent situation. Secondly, the risk of bacterial contamination is not negligible as the UCB is not cryopreserved for days, especially when a neonate is born via vaginal delivery at a small hospital with limited medical staff.
1.4 Conclusion
A growing number of preclinical studies suggest that systemic administration of UCBCs has the potential for ameliorating infant brain injury even when the treatment is started days after the insult. As having the lowest risk in clinical use for sick newborns, intravenous administration of autologous UCBCs without cell sorting and cell culturing has been tried in many institutions in several countries. There is, however, a paucity of preclinical data on the optimal treatment protocol for neonatal encephalopathy. Rigorous preclinical studies are needed to optimize the protocol as well as to clarify the mechanisms of action. At the same time, many patients and their parents are desperately seeking opportunities to receive cell therapies, as the current therapies for neonatal encephalopathy and its neurological sequelae offer limited hope. We believe it is important to proceed with clinical trials promptly under monitoring by the regulatory authorities.
References
Dammann O, Ferriero D, Gressens P. Neonatal encephalopathy or hypoxic-ischemic encephalopathy? Appropriate terminology matters. Pediatr Res. 2011;70:1–2.
Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol. 2012;72:156–66.
van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007;166:645–54.
Lindstrom K, Lagerroos P, Gillberg C, Fernell E. Teenage outcome after being born at term with moderate neonatal encephalopathy. Pediatr Neurol. 2006;35:268–74.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. National Institute of Child H, Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77.
Castillo-Melendez M, Yawno T, Jenkin G, Miller SL. Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Front Neurosci. 2013;7:194.
Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone Marrow Transplant. 2013;48:890–900.
Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–26.
Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8:1193–201.
Mendez-Otero R, de Freitas GR, Andre C, de Mendonca ML, Friedrich M, Oliveira-Filho J. Potential roles of bone marrow stem cells in stroke therapy. Regen Med. 2007;2:417–23.
Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–9.
Sarnowska A, Braun H, Sauerzweig S, Reymann KG. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue. Exp Neurol. 2009;215:317–27.
Tsuji M, Johnston MV. Cell-based therapies in neonatal stroke. In: Hess DC, editor. Cell therapy for brain injury. Switzerland: Springer International; 2015. p. 321–49.
Elsayed MH, Hogan TP, Shaw PL, Castro AJ. Use of fetal cortical grafts in hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 1996;137:127–41.
Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M, et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res. 2006;59:244–9.
Yasuhara T, Hara K, Maki M, Mays RW, Deans RJ, Hess DC, et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J Cereb Blood Flow Metab. 2008;28:1804–10.
Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Sato Y, Tsuda H, et al. Effects of intravenous administration of umbilical cord blood CD34+ cells in a mouse model of neonatal stroke. Neuroscience. 2014;263:148–58.
Bennet L, Tan S, Van den Heuij L, Derrick M, Groenendaal F, van Bel F, et al. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600.
Beam D, Poe MD, Provenzale JM, Szabolcs P, Martin PL, Prasad V, et al. Outcomes of unrelated umbilical cord blood transplantation for x-linked adrenoleukodystrophy. Biol Blood Marrow Transplant. 2007;13:665–74.
Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and hla-haploidentical related donor bone marrow transplantation in fifty-four children. The storage disease collaborative study group. Blood. 1998;91:2601–8.
Peters C, Charnas LR, Tan Y, Ziegler RS, Shapiro EG, DeFor T, et al. Cerebral x-linked adrenoleukodystrophy: tThe international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–8.
Aldenhoven H, Kurtzberg J. Cord blood is the optimal graft source for the treatment of pediatric patients with lysosomal storage diseases: clinical outcomes and future directions. Cytotherapy. 2015;17:765–74.
Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–9.
Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A. 2003;100:1364–9.
Moser HW, Mahmood A. New insights about hematopoietic stem cell transplantation in adrenoleukodystrophy. Arch Neurol. 2007;64:631–2.
Schönberger S, Roerig P, Schneider DT, Reifenberger G, Gobel U, Gartner J. Genotype and protein expression after bone marrow transplantation for adrenoleukodystrophy. Arch Neurol. 2007;64:651–7.
Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010;50:1980–7.
Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31:581–91.
Bae SH, Lee HS, Kang MS, Strupp BJ, Chopp M, Moon J. The levels of pro-inflammatory factors are significantly decreased in cerebral palsy patients following an allogeneic umbilical cord blood cell transplant. Int J Stem Cells. 2012;5:31–8.
Jensen A, Hamelmann E. First autologous cell therapy of cerebral palsy caused by hypoxic-ischemic brain damage in a child after cardiac arrest-individual treatment with cord blood. Case Rep Transplant. 2013:951827.
Lee YH, Choi KV, Moon JH, Jun HJ, Kang HR, Oh SI, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med. 2012;10:58.
Chen L, Huang H, Xi H, Xie Z, Liu R, Jiang Z, et al. Intracranial transplant of olfactory ensheathing cells in children and adolescents with cerebral palsy: a randomized controlled clinical trial. Cell Transplant. 2010;19:185–91.
Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164:973–9. e971
Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, et al. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64:68–73.
Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, et al. Sswitch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood. 2011;118:1699–709.
Aly H, Mohsen L, Badrawi N, Gabr H, Ali Z, Akmal D. Viability and neural differentiation of mesenchymal stem cells derived from the umbilical cord following perinatal asphyxia. J Perinatol. 2012;32:671–6.
Phelan JP, Korst LM, Ahn MO, Martin GI. Neonatal nucleated red blood cell and lymphocyte counts in fetal brain injury. Obstet Gynecol. 1998;91:485–9.
Hanna N, Graboski S, Laskin DL, Weinberger B. Effects of ibuprofen and hypoxia on neutrophil apoptosis in neonates. Biol Neonate. 2004;86:235–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tsuji, M., Shintaku, H. (2018). Clinical Trial of Autologous Cord Blood Cell Therapy for Neonatal Hypoxic Ischemic Encephalopathy (HIE). In: Shintaku, H., Oka, A., Nabetani, M. (eds) Cell Therapy for Perinatal Brain Injury. Springer, Singapore. https://doi.org/10.1007/978-981-10-1412-3_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-1412-3_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1411-6
Online ISBN: 978-981-10-1412-3
eBook Packages: MedicineMedicine (R0)