Abstract
Dialysis adequacy has conventionally been measured by small solute clearance such as Kt/V urea or creatinine clearance. Many guidelines agree on the minimum target of total weekly Kt/V urea of >1.7. However, increasing small solute clearance beyond this level does not guarantee better survival as evidenced by two previous large randomized controlled trials. In fact, dialysis adequacy cannot be merely determined by one biochemical marker. Instead, comprehensive understanding of dialysis adequacy is required to improve clinical outcomes. This should encompass adequate fluid balance, optimal blood pressure control, maintaining acid-base homeostasis, correction of anemia, malnutrition, and calcium-phosphorus mineral disturbances, decreasing inflammation, and improving middle molecule clearance. Residual renal function is involved in all these parameters of dialysis adequacy. Patients with preserved residual renal function are more likely to have well-controlled blood pressure, euvolemia, better nutritional status, less inflammation, and better phosphate and middle molecule clearance as compared to anuric patients. Maintaining euvolemia is also of paramount importance as fluid overload is associated with an increased cardiovascular risk such as high blood pressure, cardiac dilatation and hypertrophy, and congestive heart failure. Not surprisingly, residual renal function and fluid balance are indeed two most important determinants of clinical outcomes in dialysis patients. For this reason, these two should be incorporated into measures of dialysis adequacy.
This chapter details urea kinetic modeling, its use in clinical practice, current comprehensive concept of dialysis adequacy beyond small solute clearance, the importance and management of residual renal function, and maintenance of euvolemia.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Small Solute Clearance
- Middle Molecule Clearance
- Dialysis Adequacy
- Ultrafiltration Failure
- Peritoneal Ultrafiltration
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Urea Kinetic Modeling in Peritoneal Dialysis
Dialysis dose in peritoneal dialysis (PD) is measured by Kt/V urea or creatinine clearance (CrCl). In hemodialysis, it is not easy to add residual renal function (RRF) for the calculation of total Kt/V urea because Kt/V urea is expressed as a value per single dialysis session. To overcome this issue, “standard Kt/V” has recently been introduced (Leypoldt 2004; Daugirdas et al. 2010). It includes RRF and dialysis clearance and is expressed as weekly Kt/V as in PD. This equation enables us to compare dialysis adequacy between different dialysis modalities. However, it is a very complicated equation to calculate and thus is not widely used in clinical practice. Nowadays, it is generally performed for research purposes. In PD, RRF can be easily calculated and be added to peritoneal Kt/V urea, making weekly Kt/V urea. RRF is particularly important in PD because patients on PD can maintain urine output longer than those on hemodialysis. It is well known that RRF is an independent predictor of mortality in both PD and hemodialysis patients. In addition, patients on PD have better patient survival than those on hemodialysis up to 2 years after dialysis initiation. This is presumably attributed to more preserved RRF by PD. Therefore, RRF should be incorporated to Kt/V when it comes to PD adequacy.
1.1 Measurement of Kt/V Urea
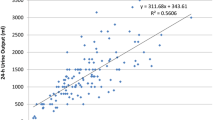
As aforementioned, Kt/V should encompass both peritoneal and renal clearance in PD. A schematic calculation is illustrated in Fig. 15.1. It is a dimensionless index determined by measuring fractional urea clearance. To calculate this, all drained dialysates should be collected over a 24-h period. Urea concentration is measured from the effluent and patient’s plasma. Kt represents urea clearance, which is calculated by total drained volume × (urea concentration in dialysate effluent/blood urea nitrogen level). For renal clearance , 24-h urine collection is also required. Similar to peritoneal Kt/V, urea concentration in the urine is divided by blood urea nitrogen level, and this value is then multiplied by urine volume, giving renal Kt. A total Kt is a sum of peritoneal Kt and renal Kt. This is then normalized to volume of distribution of urea (V). V represents total body water and urea has a volume of distribution equal to total body water. Various formulas have been established to estimate volume of distribution of urea using age, sex, height, and weight of the patient. Among these, the Watson or Hume-Weyers formula is most commonly used in clinical practice. Total Kt is then divided by V, giving a daily Kt/V. Weekly K/V is calculated as daily Kt/V multiplied by 7 (days). A summary of the equation is presented in Table 15.1.
Because CAPD is a continuous therapy, blood urea nitrogen concentration is relatively constant throughout the day, thus sampling timing is not a major concern. However, in intermittent PD such as CCPD and NIPD , plasma urea concentration differs between daytime and nighttime. The general rule regarding sampling time in patients treated with these modalities is to take blood sample in the middle of the non-cycling daytime period when urea concentration represents the average value of blood urea for a day. This is usually between 1:00 and 5:00 p.m.
1.2 Measurement of Creatinine Clearance (CrCl)
Molecular weight of creatinine is slightly higher than urea (113 D vs. 60 D). Although urea and creatinine are well dialyzed by diffusion , creatinine equilibration is relatively lower than urea equilibration (Fig. 15.2). Therefore, monitoring both Kt/V urea and CrCl is recommended in clinical practice. CrCl is calculated in the same manner as in the measurement of Kt/V. For peritoneal CrCl, creatinine concentration is measured in the effluent from the 24-h collection of dialysates and in the plasma. For renal CrCl , creatinine concentration is also measured in the 24-h collection of urine. However, it is well known that renal CrCl overestimates true GFR because creatinine is exceedingly secreted by the proximal tubules in advanced stages of CKD. Therefore, renal CrCl is generally expressed as an average of the urinary urea clearance and creatinine clearance . Total CrCl is calculated as a sum of peritoneal CrCl and renal CrCl and is then normalized for 1.73 m2 body surface area (BSA). BSA can be estimated using the DuBois formula, which is most commonly used worldwide. These are summarized in Table 15.1.
Urea and creatinine equilibration. Urea diffuses rapidly into PD solution compared with creatinine. Rate of entry of a larger molecule, vitamin B12, is slower than urea and creatinine. D/P ratio indicates dialysate concentration of urea, creatinine, and vitamin B12 divided by plasma urea concentration of each molecule
1.3 Clinical Examples of Measurement of Kt/V Urea and CrCl
PD adequacy can numerically be determined by Kt/V urea and CrCl. Therefore, physicians should be familiar with these formulas. Examples of how the formulas are calculated and how we put these into clinical practice are presented as follows:
-
1.
A 60-year-old female started PD due to hypertensive nephrosclerosis. She underwent three exchanges of a 1.5% 2 L glucose PD solution. A daily net peritoneal ultrafiltration and urine volume were 1 L per day and 500 mL per day, respectively. She was 165 cm tall and weighed 62 kg. Laboratory findings were as follows:
-
Dialysate (24-h collection): Urea, 55 mg/dL; creatinine, 6 mg/dL
-
Plasma: BUN, 75 mg/dL; creatinine, 8 mg/dL
-
Urine (24-h collection): Urea, 600 mg/dL; creatinine, 48 mg/dL
-
What is a total weekly Kt/V urea and CrCl?
1.3.1 Kt/V Urea
Step 1 Calculate peritoneal Kt/V.
-
Daily peritoneal Kt = total ultrafiltration × (dialysate urea nitrogen/serum BUN) = 7 (L) × (55/75) = 6.4 L per day.
-
Weekly peritoneal Kt = 7 (days) × 6.4 L/day = 44.8 L.
-
By the Watson formula, V = 30.8 L.
-
Weekly peritoneal Kt/V = 44.8/30.8 = 1.45.
Step 2 Calculate renal Kt/V.
-
Daily renal Kt = urine volume × (urinary urea nitrogen/serum BUN) = 0.5 (L) × (600/60) = 5.0 L per day.
-
Weekly renal Kt = 7 (days) × 5.0 L/day = 35 L.
-
Weekly renal Kt/V = 35/30.8 = 1.14.
Step 3 Calculate weekly Kt/V.
-
Weekly Kt/V = peritoneal Kt/V + renal Kt/V = 1.45 + 1.14 = 2.59.
1.3.2 CrCl
Step 1 Calculate peritoneal CrCl.
-
Daily peritoneal CrCl = total ultrafiltration × (dialysate creatinine/serum creatinine) = 7 (L) × (6/8) = 5.25 L per day.
-
Weekly peritoneal CrCl = 7 (days) × 5.25 L/day = 36.75 L.
-
By the DuBois formula,
-
BSA = 1.68.
-
BSA corrected to 1.73 m2 = 1.73/1.68 = 0.97.
-
Weekly peritoneal CrCl normalized to 1.73 m2 BSA = 36.75/0.97 = 37.9 L.
Step 2 Calculate renal CrCl.
-
Daily renal CrCl = urine volume × ([urinary urea nitrogen/serum BUN] + [urinary creatinine/serum creatinine])/2 = 0.5 (L) × (600/60 + 48/8)/2 = 4.0 L per day.
-
Weekly renal CrCl = 7 (days) × 4.0 L/day = 28 L.
-
Weekly renal CrCl normalized to 1.73 m2 BSA = 28/0.97 = 28.9 L.
Step 3 Calculate weekly CrCl.
-
Weekly CrCl = peritoneal CrCl + renal CrCl = 37.9 + 28.9 = 66.8 L/week
-
2.
She had been well maintained on CAPD without symptoms and signs of uremia until 1.5 years after commencing dialysis. At 2 years, urine volume was decreased to 100 mL per day, and she was not quite well and could not eat much. Her body weight decreased to 60 kg. Laboratory findings revealed dialysate urea of 72 mg/dL, serum BUN of 80 mg/dL, and urinary BUN of 350 mg/dL. Assuming the same net peritoneal ultrafiltration , calculate Kt/V and what should physicians do?
Using the same steps above, weekly total Kt/V is calculated as a sum of peritoneal and renal Kt/V: peritoneal Kt/V = 44.1/30.3 = 1.46; renal Kt/V = 3.08/30.3 = 0.1. Thus, weekly Kt/V is 1.56.
In this case, the patient does not meet the optimal target value of Kt/V as recommended by the current guidelines (see Sect. 15.2). Her symptoms and signs are most likely to be caused by inadequate dialysis. This is attributed to a significantly decreased RRF. She needs to do one more exchange of PD solution, four exchanges in total.
-
3.
A 55-year-old male patient had been treated with APD for 3 years. Using a cycler, he underwent 8.0 L during nighttime only, and the abdomen was dried during daytime. He became anuric 6 months ago. At that time, Kt/V urea and CrCl were 1.8 and 48 L/week per 1.73 m2. This time, laboratory tests showed dialysate urea of 55 mg/dL, serum BUN of 65 mg/dL, dialysate creatinine of 4.8 mg/dL, and serum creatinine of 7 mg/dL. Total mean drained volume was 9.0 L. He weighed 68 kg and was 172 cm tall. How would you like to adjust dialysis prescription?
Following the steps above, peritoneal Kt/V urea = 1.61 and peritoneal CrCl = 41.5 L/week per 1.73 m2. We have several options to increase Kt/V urea and CrCl to the target levels. These can be (1) adding daytime dwell, (2) increasing dwell volume during nighttime, or (3) increasing frequency of exchanges on a cycler. Strengths and problems of each strategy are discussed below in Sect. 15.3.2 .
2 Therapeutic Targets of Kt/V Urea and CrCl
Many studies have been conducted to find the optimal target values of small solute clearance by using Kt/V urea and CrCl. In1996, the Canada-USA (CANUSA) PD study group reported the results of the prospective cohort study of 680 patients commencing continuous PD in 14 centers in two countries (1996). They found a 5% increase in the relative risk of death in proportion to a decrease of 0.1 unit of Kt/V urea, suggesting the importance of small solute clearance. Two-year survival rates were 78 and 81% in patients with Kt/V urea of 2.1 and 2.3, whereas it was only 66% in patients with Kt/V urea of 1.5. Similarly, patients with CrCl of 80 L/week per 1.73 m2 had a higher 2-year survival rate than patients with CrCl of 40 L/week per 1.73 m2 (81% vs. 65%). In line with this finding, many observational studies consistently found a decreased risk of mortality as Kt/V urea or peritoneal CrCl increased. Encouraged by the results of the observational studies, two randomized controlled trials had been consecutively published in 2002 and 2003 (Table 15.2). Both studies aimed to evaluate whether higher target of small solute clearance could improve patient survival. The ADEMEX (Adequacy of PD in Mexico) study first addressed this issue in 965 patients from 24 centers in 14 Mexican cities (Paniagua et al. 2002). The participants were randomized into two arms, and a modified PD regimen to achieve a peritoneal CrCl of 60 L/week per 1.73 m2 was prescribed to the intervention group. Fifty-nine percent of the intervention group achieved this target value by either an increase in exchange volume or the addition of a nighttime exchange or both. Contrary to the findings of the previous observational studies, this study failed to show a better survival rate in patients with an increased peritoneal clearance than in control group. In the following year, another randomized controlled trial produced similar results. A total of 320 patients were recruited from six centers in Hong Kong and were randomized into three groups with total Kt/V targets of <1.7, 1.7–2.0, and >2.0 (Lo et al. 2003). The 2-year survival rates did not differ among the three groups (87.3% in patients with Kt/V of >2.0, 86.1% in patients with Kt/V of 1.7–2.0, and 81.5% in patients with Kt/V < 1.7). In addition, there were no significant differences in technique survival, nutritional score, and hospitalization rate. However, erythropoietin was more used in patients with the lowest Kt/V group, and more patients in that group dropped out from the study due to inadequate dialysis and inadequate ultrafiltration. Although survival rates were not statistically significant, p-value was 0.054 between patient with Kt/V of <1.7 and patients with Kt/V of 1.7–2.0, suggesting a poorer outcome in patients with lowest Kt/V.
Consequently, many guidelines proposed by the National Kidney Foundation (Peritoneal Dialysis Adequacy Work 2006), the International Society of PD (Lo et al. 2006), the UK Renal Association (http://www.renal.org/guidelines/modules/peritoneal-dialysis-in-ckd#sthash.Re0T4XBR.dpbs), the European Best Practice Working Group (Dombros et al. 2005), the Canadian Society of Nephrology (Blake et al. 2011), and the Australian Society of Nephrology (Johnson et al. 2005) adopted the findings of these two randomized controlled trials and agreed on the minimum target for Kt/V urea of at least 1.7 per week (Table 15.3). Some groups suggest a minimum target for CrCl depending on dialysis modality or transport types. In fact, there has been concern about inadequate dialysis with respect to CrCl in patients with a slow transport-type peritoneal membrane. In general, patients on APD use short and frequent exchanges, thus the target value of CrCl may not be achieved particularly in patients with a slow transport status. It takes only several hours for urea to equilibrate between plasma and peritoneal fluid (Fig. 15.2). D/P urea ratio typically is 0.7 at 2 h and 0.9 at 4 h after the dwell, where D and P represent dialysate and plasma, respectively. Creatinine equilibration is relatively lower than urea equilibration. Therefore, in slow transporters, creatinine may not be adequately removed particularly when short and frequent exchanges are used. This phenomenon becomes more evident once RRF is lost. In this regard, some guidelines additionally suggest targets of CrCl and recommend a regular monitoring. Nevertheless, compared to relatively strong evidence for Kt/V target supported by the two previous randomized controlled trials, CrCl targets for slow transporters have weak evidence. To date, there is no randomized controlled trial to support the minimum targets of CrCl in patients on APD or in patients with a slow transport-type peritoneal membrane. For this reason, it is acceptable to use the same targets as for CAPD in these patients.
2.1 Frequency of Measurement
Many guidelines suggest measurement of Kt/V urea and CrCl within the first month of dialysis initiation. These are typically done together with peritoneal equilibration test. As seen in Sect. 15.1, total Kt/V and CrCl are a sum of peritoneal and renal clearance . Thus, dialysis adequacy assessed by small solute clearance significantly relies on RRF during the initial period of PD. Because RRF gradually declines over time, it should be regularly monitored. It is generally recommended that a 24-h urine collection for urine volume and solute clearance measurement should be performed at a minimum of every 1–2 months for patients who have a significant residual urine volume. These can be monitored at longer intervals, for example, every 4–6 months if RRF is lost and patients do well without any significant deterioration in physical health. For patients without RRF, peritoneal Kt/V should be targeted to at least >1.7 by increasing frequency of exchanges or dialysis solution volume.
2.2 Kt/V Urea vs. CrCl
There is lack of evidence as to which parameter is superior in predicting adverse outcome. An early observation showed more experience and fewer methodological problems with Kt/V (Twardowski 1998). In addition, Kt/V urea is exclusively used for measurement of hemodialysis adequacy. In this regard, many physicians are more familiar with Kt/V urea than CrCl, and the former is more commonly used in clinical practice. As aforementioned, renal creatinine clearance overestimates true clearance and generally exceeds urea clearance. In contrast, creatinine slowly diffuses into the peritoneum due to higher molecular weight than urea; peritoneal CrCl is lower than urea clearance (Fig. 15.2). Therefore, CrCl should be interpreted with caution depending on PD modality and membrane types. Nevertheless, these two measures are small solute clearance and do not reflect middle molecule clearance. Given a variety of uremic toxins beyond small solute and disappointing results of the ADEMEX and the Hong Kong study, the targets for small solute clearance proposed by many guidelines should be understood as the minimum level to accomplish dialysis adequacy (Fig. 15.3).
Relationship between dialysis adequacy and survival. Once the minimum target of small solute clearance is achieved, survival rate does not increase. Beyond this point, other factors can play an important role in improving patient outcomes (see Sect. 15.4)
3 Factors Affecting Peritoneal Clearance
There are number of factors that determine peritoneal small solute clearance. As presented in Table 15.4, these can be classified into inherent and modifiable factors. Factors such as RRF, body size, and peritoneal membrane characteristics are inherent in individual patient, thus not easily modifiable. In contrast, we can adjust dwell volume, frequency of PD solution exchange, and use of high glucose concentration PD solution depending on patient’s inherent factors. Strategies for increasing small solute clearance are summarized in Fig. 15.4.
3.1 Inherent Factors
3.1.1 RRF
As noted above, total Kt/V urea and CrCl are largely dependent on RRF, while urine volume is maintained. It can contribute approximately up to 50% of total clearance during the initial period of dialysis. Accordingly, physicians should be alert on decline of RRF and consider increasing peritoneal clearance targeted to Kt/V of at least >1.7 when patients become anuric (Fig. 15.4 and Fig. 15.5). The importance of RRF has recently been highlighted in many aspects. It has been well demonstrated that RRF is more important than small solute clearance in determining clinical outcomes in dialysis patients. Therefore, much effort should be made to preserve RRF. This is discussed in detail in Sect. 15.5.
Relationship between total Kt/V, peritoneal clearance, and residual renalfunction over time. (a) Total clearance decreases without increasing peritoneal clearance as residual renal function declines. (b) Total clearance remains unchanged if peritoneal clearance increases corresponding to a decrease in residual renal function
3.1.2 Body Size
Body size can also affect total clearance because Kt and CrCl are normalized to volume of distribution of urea, which is equal to total body water, and BSA, respectively. Theoretically, patients with extremely large body size may not adequately maintain PD in terms of small solute clearance. If a patient undergoes four exchanges of 2.0 L PD solutions per day to meet the target of Kt/V of >1.7 and net ultrafiltration is 1.0 L, then V = D/P urea ratio × (7 days × 9.0 L)/1.7. Assuming a D/P urea ratio of 0.9, then V is 40.6 L, which approximately fits to a man with a standard body size, 170 cm in height, and 70 kg in weight. Thus, obese patients have extremely high V, it is difficult to achieve a Kt/V urea target of >1.7 when typical peritoneal dialysis prescription is given. Conversely, Kt/V can be interpreted with caution in patients with very low V. Because these patients are more likely malnourished and deprived of muscle, it is not fair to say they do well on dialysis even though Kt/V is >1.7.
3.1.3 Peritoneal Membrane Characteristics
Peritoneal membrane transport types can be determined by peritoneal equilibration test. It is important to define membrane transport status in dialysis prescription. As seen in Fig. 15.2, equilibration of creatinine or larger molecules is slower and lower than that of urea. This can raise a problem regarding dialysis adequacy in patients on APD. These patients use a cycler and typically do 4–5 exchanges during 8–10 h. This inevitably requires short and frequent exchanges, resulting in lack of enough time for larger molecules to equilibrate. Urea clearance is acceptable because urea rapidly equilibrates within several hours. However, larger molecules than urea are not adequately removed due to the slower diffusion. This becomes more pronounced in slow transporters (previously known as low transport). These patients exhibit much slower equilibration of solutes than fast transporters. For this reason, dialysis prescription can be tailored depending on transport types. In general, high-volume exchanges and long-duration dwells are recommended in slow transporters, whereas short-duration dwell is more effective in fast transporters.
3.2 Modifiable Factors
These factors are related to dialysis prescription. When the target of Kt/V or CrCl is not achieved, we can adjust dialysis prescription by increasing dwell volume, increasing exchange frequency, and using hypertonic PD solutions. This adjustment is largely dependent on inherent factors, particularly such as transport types.
3.2.1 Dwell Volume
As explained above, increasing the dwell volumes is commonly used to achieve the target of Kt/V > 1.7, particularly in slow transporters. This strategy is also more effective in patients with large body size than in those with small body size. By doing this, peritoneal clearance can be increased because total drained volume is increased, while urea and creatinine equilibration are slightly decreased, giving a small dip in D/P urea or creatinine. As a result, total Kt calculated as total drained volume multiplied by D/P urea is increased. In patients with small body size, clearance is unlikely to increase by increasing dwell volume because of the greater decrease in equilibration. It is generally known that, assuming four exchanges given, increasing volume from 2.0 to 2.5 L can induce an approximately 20% increase in peritoneal clearance. However, patients may feel uncomfortable with larger instilled volume and may complain of back pain, abdominal discomfort, or shortness of breath. In addition, inguinal, ventral, or diaphragm hernia can develop due to an increase in intra-abdominal pressure. This complication can cause peritoneal fluid leak into soft tissue, thus leading to localized edema.
3.2.2 Adjusting Dwell Duration and Frequency of Exchanges
In typical patients on CAPD with average peritoneal transport types, increasing frequency from four to five exchanges per day accompanied by shortened dwell time does not hamper urea equilibration, which remains at 85–90% as long as patients ensure adequate dwell time of at least 4 h. In slow transporters, increasing clearance can be achieved by increasing dwell duration because creatinine equilibration still rises 4 h after the initiation of the dwell. On the other hand, in fast transporters, osmotic gradient is dissipated soon after the dwell, thus increasing frequency of daily exchanges can be an option to increase peritoneal clearance in these patients. However, one more exchange can be burdensome and limit daily activities in patients who have active lifestyle. They do not want to be tied up with PD exchange procedure. In this regard, increasing dwell volume is more effective and preferred to enhance clearance.
In patients on APD, this strategy of adjusting dwell time and frequency of exchanges can be also applied in clinical practice. These patients use a cycler during nighttime, thus problems of increasing frequency of exchanges can be resolved by the aid of the machine, which is particularly helpful in fast transporters. However, as aforementioned, shortened dwell time caused by frequent exchanges may result in insufficient solute clearance. Loss of RRF can worsen this problem. Moreover, middle molecule clearance is time dependent. Therefore, in this case, many patients require one or more exchanges of daytime dwell to increase clearance. Another way to increase clearance is to increase dwell volume as in CAPD. APD patients are unlikely to complain of abdominal discomfort because intra-abdominal pressure is not increased in supine position. How to prescribe dialysis in APD is described in detail in Chap. 4.
3.2.3 The Use of High Concentration Glucose Solution
Because ultrafiltration is mainly derived from osmotic gradient, the use of high concentration glucose solution can increase ultrafiltration volume by inducing higher osmotic gradient. Accordingly, peritoneal clearance can increase as total drained volume is a determinant of Kt. However, there is much concern about glucose toxicity to the peritoneal membrane damage. In addition, glucose can be absorbed via peritoneal capillary beds, leading to systemic harmful effects such as hyperglycemia, hypertriglyceridemia, weight gain, inflammation, etc. For this reason, it is generally recommended to limit the use of high concentration glucose solutions unless volume overload should be controlled. Moreover, glucose-sparing strategies have recently prevailed to preserve peritoneal membrane and to prevent glucose toxicity-associated complication.
4 Comprehensive Understanding of Dialysis Adequacy
As seen in the ADEMEX study and the Hong Kong study, further increase in small solute clearance beyond some point did not improve clinical outcomes. In fact, urea and creatinine may be merely markers of kidney function, and there are more uremic toxins besides small solutes. Less importance of small solute clearance was also observed in patients undergoing hemodialysis. The Hemodialysis Study Group conducted a randomized controlled trial in 1846 patients to evaluate whether high dose of dialysis could improve survival compared with standard dose (Eknoyan et al. 2002). They failed to demonstrate that increasing Kt/V urea up to 1.7 was beneficial in decreasing mortality and morbidity. The findings of the randomized controlled trials in dialysis patients suggest that there are more other factors that can determine clinical outcomes, and thus much attention should be paid to these factors after the minimum targets of Kt/V urea and CrCl are achieved. Therefore, we should comprehensively understand dialysis adequacy beyond small solute clearance. Dialysis adequacy cannot be determined by one biochemical marker. More importantly, it should encompass the management of fluid overload, blood pressure, acid-base disturbances, anemia, malnutrition, calcium-phosphorus mineral disturbances, inflammation, and middle molecule clearance (Fig. 15.6). To achieve these goals, RRF should be preserved because kidney is involved in all aspects of dialysis adequacy. It is no wonder why RRF is more associated with clinical outcomes than any other parameters. To corroborate this view, the current guidelines put much emphasis on the preservation of RRF and fluid overload management rather than small solute clearance. These are discussed in detail in Sects. 15. 5 and 15.6.
5 RRF
5.1 Survival Benefit of RRF
Recently, the importance of RRF has been highlighted through a number of studies. Its significant impact on patient survival was first reported in 1995 by Maiorca et al. (1995). They observed 68 CAPD and 34 HD patients in a prospective observational study for 3 years and found that persistence of RRF was associated with improved survival. In the following year, the CANUSA study first demonstrated the survival benefit with an increase in small solute clearance assessed by Kt/V urea. Five years later, the group reanalyzed the CANUSA data to evaluate relative contribution of RRF and peritoneal clearance to dialysis adequacy (Bargman et al. 2001). The results showed that each 5 L/week per 1.73 m2 increment in GFR was associated with a 12% decrease in risk of death, but no association between peritoneal creatinine clearance and mortality was found. Furthermore, the original finding of the ADEMEX study indicated no improvement in patient survival by increasing small solute clearance. Interestingly, in the multivariable analysis, residual renal Kt/V urea and CrCl were significantly associated with a reduced risk of mortality, whereas peritoneal Kt/V urea and CrCl were not. Subsequently published studies from the European cohort and the Asian cohort consistently have shown the survival advantage of RRF compared with no effect of peritoneal clearance on survival. As in PD patients, loss of RRF was a significant predictor of death in HD patients (Shafi et al. 2010).
5.2 Mechanistic Link Between RRF and Better Survival
5.2.1 Fluid Status, Blood Pressure, Cardiac Hypertrophy, and RRF
There have been a number of studies to explain mechanisms responsible for the improved survival conferred by RRF. As seen in Fig. 15.6, RRF plays an important role in the regulation of a variety of disturbances in dialysis patients. Fluid overload is a significant determinant of adverse outcomes and is highly associated with increased blood pressure, cardiac hypertrophy, and congestive heart failure in these patients. Ates et al. evaluated the effects of fluid and sodium removal on mortality in 125 PD patients (Ates et al. 2001). They observed an increased patient survival rate in proportion to the amount of sodium and fluid removal. In particular, a 3-year patient survival rate was highest in patients with fluid removal >2035 mL/24 h/1.73 m2, whereas it was lowest in those with fluid removal <1265 mL/24 h/1.73 m2. Their findings highlight the harmful effects of fluid retention on patient survival. Not surprisingly, fluid overload can be more easily controlled in patients with RRF than in anuric patients. In a cross-sectional study by Konings et al. (2003a, b), extracellular water content was significantly and inversely correlated with residual GFR. In addition, patients with residual GFR < 2 mL/min had higher extracellular water than those with residual GFR > 2 mL/min. This finding suggests that presence of RRF is advantageous for fluid management. Accordingly, blood pressure can be more controlled by maintaining fluid balance in patients with preserved RRF. Menon et al. performed a retrospective observational study to identify factors associated with uncontrolled blood pressure in 207 PD patients (Menon et al. 2001). In their study, declining RRF was significantly associated with high blood pressure. Moreover, fluid overload can contribute to the development of left ventricular hypertrophy. The prevalence of left ventricular hypertrophy is remarkably high, up to 75–90% in patients who initiate dialysis (Wang et al. 2002a, b, 2004a, b, c). It is well known that it is an independent predictor of cardiovascular events and death in dialysis patients (Silberberg et al. 1989). In a cross-sectional study by Wang et al., left ventricular hypertrophy index was significantly lower in patients with preserved RRF than anuric patients. However, this association was not observed for peritoneal clearance. Interestingly, sodium and fluid removal differ between the kidney and the peritoneum depending on presence or absence of RRF in patients undergoing PD. Cheng et al. evaluated fluid status by using bioimpedance analysis in 195 CAPD patients and found that sodium and fluid removal was greater by the kidney than by the peritoneum in patients with urine volume > 400 mL per day (Cheng et al. 2006). Conversely, peritoneal clearance in sodium and fluid removal became more important as RRF declined. Taken together, all these findings suggest that in the absence of RRF, patients are more likely to have fluid overload, high blood pressure, and cardiac dilatation, which ultimately result in the increased cardiovascular morbidity and mortality.
5.2.2 Middle Molecule and Phosphate Clearance and RRF
Middle molecule clearance has recently emerged as an important therapeutic target in dialysis patients. Among many middle molecules, β2-microglobulin (β2MG) has widely been investigated. Many studies have consistently shown that β2MG is a strong and independent predictor of mortality in ESRD patients. RRF is also associated with removal of middle molecules. Bammens et al. conducted a longitudinal observational study to evaluate relative contribution of the peritoneum and the kidney to the clearance of small solutes and β2MG (Bammens et al. 2005). Renal clearance of urea and creatinine declined over time, while peritoneal clearance of these molecules increased. Interestingly, there was also a decline in renal clearance of β2MG, but its peritoneal clearance remained stable throughout the study period. These findings suggest that, for small solutes, peritoneal clearance can increase in compensation for loss of RRF. However, the elimination of middle molecule, β2MG, is less likely to be counterbalanced by the peritoneum.
Phosphate toxicity has been well recognized in CKD patients. In fact, phosphate promotes vascular calcification process, which in turn increases cardiovascular risk. Kidney is a major organ that regulates phosphate clearance. Accordingly, phosphate accumulates in the body as CKD progresses. It is well known that hyperphosphatemia is a significant predictor of mortality in CKD patients. Phosphate is a small molecule because its molecular weight is only 96 D. Phosphate itself is not water-soluble and thus theoretically cannot be removed by dialysis therapy. Interestingly, phosphate behaves like a middle molecule because it is surrounded by water molecules, making it water-soluble and thus dialyzable. Not surprisingly, RRF plays an important role in removal of phosphate (Wang et al. 2004a, b, c). Residual GFR is inversely correlated with serum phosphate levels in dialysis patients. Because middle molecule clearance is more affected by RRF than by the peritoneal clearance, phosphate control is much difficult in patients without RRF. Thus, presence of RRF has advantage of the elimination of phosphate and middle molecules.
5.2.3 Inflammation and RRF
Inflammation is another important predictor of adverse outcomes in dialysis patients. It is significantly associated with malnutrition, arteriosclerosis, and increased cardiovascular risk in these patients. Increased mortality rate is also observed in PD patients with increased CRP levels (Noh et al. 1998). There is evidence that loss of RRF can contribute to inflammation. In a cross-sectional study by Pecoits-Filho et al., residual GFR was inversely correlated with serum levels of inflammatory markers such as high-sensitive CRP and IL-6 (Pecoits-Filho et al. 2003). In addition, a prospective observational study by Wang et al. revealed that the combination of inflammation, loss of RRF, and cardiac hypertrophy was significantly associated with an increased risk of death in PD patients (Wang et al. 2004a, b, c). Although causality is uncertain, it can be presumed that loss of RRF and inflammation work together in a vicious cycle, eventually leading to the increased mortality.
5.2.4 Vascular Protection and RRF
The importance of RRF has also been recognized from a viewpoint of vascular protection. Endothelial dysfunction and arterial stiffness in CKD patients are of multifactorial origin including traditional and nontraditional risk factors. Many of these factors such as blood pressure, fluid overload, and inflammation are significantly associated with RRF as mentioned earlier. Thus, it can be presumed that vascular insufficiency is less severe in patients with preserved RRF compared to patients without RRF. In fact, endothelial dysfunction assessed by flow-mediated dilation was significantly correlated with residual GFR in PD patients (Han et al. 2012). In addition, another study demonstrated that brachial artery pulse wave velocity, a marker of arterial stiffness, was inversely associated with RRF (Huang et al. 2008). These findings suggest that preservation of RRF can be also helpful in protecting vascular function.
5.2.5 Nutritional Status and RRF
Malnutrition is a serious complication in dialysis patients. RRF is also associated with nutritional status. There are number of studies indicating significant association between RRF and parameters of nutritional status such as lean body mass, normalized protein catabolic rate, subjective global assessment score, dietary protein intake, serum albumin, and handgrip strength. The differential effects of RRF and peritoneal clearance on nutritional intake were demonstrated by Wang et al. In their findings, presence of RRF significantly contributed to better protein and calorie intake, whereas increased peritoneal solute clearance had no impact on nutritional intake (Wang et al. 2002a, b). RRF also showed an inverse relationship with resting energy expenditure (Wang et al. 2004a, b, c), suggesting disturbed protein metabolism as kidney function declined. As mentioned earlier, loss of RRF can accelerate inflammatory status. Of note, inflammation is an important mediator that aggravates muscle wasting and anorexia. Presumably, malnutrition can be worsened by loss of RRF via exacerbated inflammation.
5.2.6 Peritonitis and RRF
The potential association between RRF and PD-related peritonitis has also been suggested. Several studies have shown that risk of PD-related peritonitis was lower in patients with preserved RRF (Perez Fontan et al. 2005; Han et al. 2007). Conversely, there is an opposite direction of the relationship indicating that peritonitis can accelerate loss of RRF (Szeto et al. 2007) possibly as a result of infection itself or the use of nephrotoxic antibiotics. Whichever comes first, peritonitis is significantly associated with morbidity and mortality in PD patients, and thus it should be prevented by using available strategies.
5.3 Strategies to Preserve RRF
Even though declining residual function in dialysis patients is inevitable after commencing dialysis, the diseased kidney can still carry out numerous functions. Therefore, nephrologists should attempt to preserve RRF. Conventionally, as in CKD patients prior to dialysis, avoidance of the use of nephrotoxic drugs and volume depletion is highly recommended. Much attention should be paid to dialysis patients with presence of RRF before the use of contrast dye. Blood pressure should be well controlled given the relationship between uncontrolled blood pressure and CKD progression. PD-related peritonitis should also be prevented and be promptly managed if it occurs, given the accelerated decline in RRF after peritonitis. However, there is lack of evidence as to whether all the conventional strategies can indeed be of help in the preservation of RRF. The rationales for the application of these to dialysis patients are largely speculative based on the results of the studies in non-dialysis CKD population.
In addition to the general management, there are more available options to prevent loss of RRF: (1) PD as the first therapy when commencing dialysis, (2) the use of renin-angiotensin system (RAS) inhibitors, and (3) the use of new PD solutions (Fig. 15.7). These strategies have been studied mostly in PD patients, and thus there has been more evidence for utilization of these in this population compared with HD patients.
5.3.1 PD as the First Dialysis Modality
In general, PD is known to preserve RRF better than HD. Although this concept has long been accepted, relevant studies to this are not randomized controlled trials, but mostly observational in nature, and thus the findings of these studies should be interpreted with caution. Moist et al. analyzed factors associated with loss of kidney function in 1843 dialysis patients using the US Renal Data System (USRDS) data and found that PD was associated with a 65% risk reduction of developing a urine volume < 200 mL per day compared to HD (Moist et al. 2000). In line with this result, the European cohort study also observed a similar finding. The Netherlands Cooperative Study on the Adequacy of Dialysis phase-2 (NECOSAD-2) study involved 522 incident dialysis patients treated with PD or HD from 32 dialysis centers nationwide (Jansen et al. 2002). The results showed that residual GFR declined faster in HD patients than in PD patients during a 12-month observation period. The better preservation of RRF by PD is well reflected in survival benefit of PD over HD, particularly during early period after dialysis initiation. Heaf et al. first suggested the better survival rate in PD patients than in HD patients up to 2 years after dialysis initiation (Heaf et al. 2002). Since then, many epidemiologic studies have consistently shown similar findings (McDonald et al. 2009; Weinhandl et al. 2010; Choi et al. 2013; Kumar et al. 2014). This phenomenon may be attributed to unseen confounding factors between patients on HD and PD, but many researchers believe that preserved RRF plays a significant role in the survival advantage of PD over HD during initial period of dialysis. Based on these results, some groups proposed an “integrative care approach” when commencing dialysis. This includes sequential utilization of PD first and then HD, given the favorable effect of PD on RRF. In fact, in a single-center retrospective analysis, this approach was associated with better patient survival compared to patients who started and remained on PD alone or HD alone (Van Biesen et al. 2000). However, further large-scale studies are required to confirm this finding.
The exact mechanisms for better maintaining kidney function by PD are unclear. Renal ischemia is considered a key explanation for this. Because HD is intermittently provided compared with continuous nature of PD, the volume gained during interdialysis period should be removed during only 3–4 h of HD treatment. For this reason, not surprisingly, renal ischemia occurs more frequently in HD than in PD. In addition, inflammatory mediators are released from extracorporeal circulation during HD treatment and exhibit nephrotoxic effects on the kidney. These together can eventually lead to faster loss of RRF in HD patients. During the past decades, HD technology has greatly advanced. Accordingly, there has been hope that the disadvantage of HD regarding faster deterioration of RRF can be overcome by the new advanced HD modalities. Nowadays, cellulose membrane, which is known as a main culprit for inflammatory cytokine production, has been replaced by a new biocompatible HD membrane. In addition, hemodiafiltration can provide more hemodynamic stability and more removal of inflammatory cytokines than conventional HD. Interestingly, two previous studies showed that a gap in a decline rate in RRF between PD and HD was decreased or null since biocompatible polysulfone membrane was utilized (Lang et al. 2001; McKane et al. 2002). Unfortunately, these studies are limited by small sample size or observational nature, and no other studies have been conducted to examine this issue thereafter. Thus, evidence level is very low. Of note, recent randomized controlled trials failed to show that RRF was more preserved by hemodiafiltration as compared with conventional HD (Penne et al. 2010; Mostovaya et al. 2014). Further studies are required to investigate whether the use of new modalities can prevent rapid loss of RRF in HD patients.
5.3.2 The Use of RAS Blockers
RAS inhibitors have been proven to prevent progression of kidney disease in patients with CKD prior to dialysis. To date, there have been several randomized controlled trials to investigate whether these drugs could preserve RRF in dialysis patients. Most studies have been conducted in PD patients, whereas there was only one in HD patients. Li et al. conducted a prospective randomized controlled open-label trial in 60 PD patients and found that RRF declined slower in the angiotensin-converting enzyme inhibitor group than in the control group (Li et al. 2003). In addition, another randomized controlled open-label study of 34 PD patients showed that the use of an angiotensin receptor antagonist, valsartan, resulted in better preservation of RRF (Suzuki et al. 2004). However, a recent prospective randomized placebo-controlled double-blinded trial of 82 HD patients did not prove superiority of RAS inhibitor to placebo in the preservation of RRF (Kjaergaard et al. 2014). It is possible that hemodynamically negative effects of HD on RRF may overwhelm the renoprotective effects of RAS blockers. Nevertheless, given the importance of RRF, the use of RAS inhibitors can be incorporated as a therapeutic strategy to preserve RRF in dialysis patients.
5.3.3 The Use of New PD Solutions
During the past decades, there has been much concern on “glucose toxicity” of high glucose contents of PD solutions. These include deleterious effects of bioincompatible characteristics of the solutions on peritoneal membrane, systemic inflammation, and even RRF. Therefore, new neutral pH biocompatible solutions containing low glucose degradation products (GDPs), or non-glucose-based solutions such as icodextrin or amino acids, have been developed and commercially available. There is evidence to favor the use of the new solutions from a viewpoint of preserving RRF. The initial study by Fan et al. failed to show the beneficial effect of the new PD solutions on the preservation of RRF (Fan et al. 2008). However, in a prospective randomized controlled open-label trial by Kim et al., residual GFR and urine volume declined faster in the conventional solution group than in the biocompatible solution group during 1-year follow-up period (Kim et al. 2009). A subgroup analysis showed that the better preservation of RRF by biocompatible solutions was more pronounced in patients with residual GFR > 2 mL/min per 1.73 m2. A subsequent study by Haag-Weber et al. included patients with residual GFR ≥ 3 mL/min per 1.73 m2 and observed a similar finding (Haag-Weber et al. 2010). These findings were not entirely supported by the Balance in Australian and New Zealand (balANZ) trial (Johnson et al. 2010). This was a prospective randomized open-label multicenter study involving the largest number of patients regarding this issue. Overall decline rate in residual GFR did not differ between groups, but time to the development of anuria was significantly longer in the biocompatible solution group than in the conventional solution group. Interestingly, the biocompatible solution group also had significantly longer time to the first peritonitis episode compared to the control group. It is uncertain why biocompatible solutions exhibit the renoprotective effect on RRF. One potential mechanism is the decreased burden of GDPs due to the less systemic absorption via the peritoneum, which is considered the main factor that can generate inflammatory and oxidative damage to the kidney. In addition, as noted in the balANZ trial, delayed onset of peritonitis can also provide an alternative explanation for the beneficial effect of the biocompatible solutions, given the bidirectional relationship between RRF and peritonitis.
Icodextrin PD solution has also been developed with an aim to reduce glucose toxicity. Icodextrin is a glucose polymer and induces ultrafiltration by colloid osmosis. Since Davies et al. first suggested the renoprotective effect of this solution, many studies have produced conflicting results regarding this issue. Cho et al. performed a systemic review of randomized controlled trials that had evaluated the effects of icodextrin solution and showed comparable effects on residual GFR or urine volume between icodextrin solution and conventional solutions (Cho et al. 2013). However, a change in RRF was not a primary outcome in all studies. Of note, a recent prospective randomized controlled multicenter trial by Chang et al. primarily focused on RRF and found that residual urine volume was more preserved in the icodextrin group than in the control group despite no difference in a decline rate in residual GFR between groups (Chang et al. 2016). The underlying mechanism for this effect is uncertain, but one potential explanation includes the presence of high-molecular-weight icodextrin metabolites in plasma, which in turn may increase plasma oncotic pressure and hence preserve plasma volume and renal perfusion.
There are no guidelines that recommend the use of biocompatible or icodextrin solutions for the purpose of preserving RRF. A systematic review of the new PD solutions appeared to favor the renoprotective effects of the biocompatible solutions compared to conventional solutions, whereas the net effect of icodextrin on RRF was inconclusive (Cho et al. 2013).
6 Fluid Overload Management
The current guidelines recognize well the importance of RRF and fluid overload in dialysis patients and thus put much emphasis on preserving RRF and maintaining euvolemia to achieve dialysis adequacy. As aforementioned, fluid overload is significantly associated with high blood pressure, left ventricular hypertrophy, and congestive heart failure. All are well-known cardiovascular risk factors, and thus uncontrolled fluid overload will eventually result in the increased cardiovascular morbidity and mortality. Adequate fluid balance is particularly important in anuric patients. In the prospective observational European APD Outcome Study (EAPOS), baseline peritoneal ultrafiltration <750 mL per day was significantly associated with an increased risk of death in anuric patients on APD (Brown et al. 2003). In line with this, the NECOSAD study group also showed that increased peritoneal ultrafiltration was significantly associated with better survival (Jansen et al. 2005). These findings together highlight the importance of fluid balance in dialysis patients.
6.1 Factors Causing Fluid Overload
6.1.1 Loss of RRF
There are many factors that can cause fluid overload in PD patients (Table 15.5). Not surprisingly, loss of RRF is a major contributor to the fluid overload. The importance and the preservation of RRF are discussed in detail above (see Sects. 15.5.2 and 15.5.3). Fluid overload can become a serious concern while RRF declines over time. In general, most dialysis patients eventually become anuric in several years after commencing dialysis. Therefore, regular monitoring of RRF and fluid status is required to maintain euvolemia. A corresponding increase in dialysis dose by increasing either frequency or volume of PD solution exchanges should be considered in compensation for loss of RRF.
6.1.2 Excessive Salt and Fluid Intake
Excessive salt intake and inadequate peritoneal ultrafiltration are also important causative factors for fluid overload. Failure to dietary restriction is very common, up to 67% in PD patients (Griva et al. 2014a, b). Tzamaloukas et al. assessed clinical features of PD patients with fluid retention (Tzamaloukas et al. 1995). Symptomatic fluid retention was commonly observed in up to 30.7% of all patients and was associated with peripheral edema, pulmonary congestion, pleural effusion, and hypertension. Patients who did not adhere to dietary restriction and had excessive fluid and salt intake were more likely to have fluid retention and its associated problems. The authors also found that inadequate peritoneal ultrafiltration was another important factor associated with fluid retention. A previous study by Gunal et al. further substantiated these findings (Gunal et al. 2001). They performed a stepwise approach to achieve normal blood pressure in 47 hypertensive PD patients. Interestingly, 20 (42.5%) patients achieved a blood pressure of <140/90 mmHg after 4-week salt restriction alone of <4 g/day. Of the remaining 27 patients, increasing peritoneal ultrafiltration by using hypertonic glucose solutions combined with maintaining salt restriction additionally resulted in normal blood pressure in 17 (36.2%) patients. Together, 37 (78.7%) patients achieved a target blood pressure without adding antihypertensive medications. These findings highlight the importance of salt restriction and adequate peritoneal ultrafiltration in the management of fluid overload.
6.1.3 Insufficient Ultrafiltration
Non-compliance is another important cause of insufficient ultrafiltration. A recent systematic review study with respect to nonadherence issue reported the rates of nonadherence to dialysis exchanges to be approximately >20% of patients (Griva et al. 2014a, b). Factors associated with non-compliance are controversial between studies, but one small group study showed that patients who did not have a person to help exchange procedure were more non-compliant than those who were assisted by someone (Bernardini et al. 2000).
Inadequate peritoneal ultrafiltration can be also caused by mechanical complications and peritoneal membrane failure. Mechanical complications include peritoneal fluid leak associated with hernias and peritoneal-pleural shunt and catheter malfunction. These appear to occur more frequently during the early period after commencing dialysis (see Sect. 15.7, Complication of PD). Peritoneal membrane transport characteristics are an important determinant of peritoneal ultrafiltration. In this regard, PD prescription can be adjusted depending on membrane transport types. In general, patients with fast transport are vulnerable to fluid overload because osmotic gradient dissipates quickly after the dwell, and thus short and frequent exchanges are recommended in these patients. Membrane failure frequently occurs particularly in long-term PD patients. In clinical practice, peritoneal equilibration test should be done at least every 6 months to characterize and monitor membrane transport type. In fact, increasing D/P creatinine ratio at 4 h generally precedes the development of ultrafiltration failure (Davies et al. 1996). Therefore, much attention should be given to long-term PD patients who develop fluid overload without specific causes and exhibit a progressive increase in D/P creatinine ratio.
Insufficient ultrafiltration can occur as a result of excessive fluid absorption during the long-dwell exchanges. In this case, patients typically complain of the decreased net ultrafiltration after the night exchange in CAPD or the daytime exchange in APD.
6.1.4 Congestive Heart Failure
Cardiovascular disease is the most common cause of morbidity and mortality in dialysis patients. Not surprisingly, fluid management is difficult when heart failure occurs. A number of studies have shown that many circulating biomarkers and echocardiographic parameters could predict the future cardiovascular adverse outcomes even in asymptomatic dialysis patients. This led many physicians and researchers to acknowledge the need for monitoring of cardiac function. Accordingly, the KDOQI guidelines suggest that echocardiograms should be performed in all patients at the initiation of dialysis and at 3-yearly intervals thereafter (Evidence C).
6.2 Management of Fluid Overload
6.2.1 Regular Monitoring of Fluid Status
To maintain euvolemia, routine monitoring of RRF, fluid status, and peritoneal membrane function is mandatory. Detailed methods on evaluating RRF and peritoneal membrane function are described in other sections. To evaluate fluid status, physical examination and clinical assessment should be primarily performed, which are easily used in clinical practice. These include obtaining previous history and symptoms and examining signs of volume overload such as peripheral edema, elevated blood pressure, swollen jugular vein, and pulmonary congestion on chest X-ray. However, it is not easy to evaluate exact fluid status by physical examination alone because significant fluid overload can be present in the absence of detectable signs and symptoms.
To increase diagnostic accuracy of fluid overload, many assessment devices have been tested and validated such as trace dilution techniques, imaging studies, circulating biomarkers, and bioimpedance analysis. Theoretically, dilutional methods by using deuterium and bromide have high levels of reproducibility and accuracy and are considered gold standard methods to assess water contents of the body. However, these are very costly and cumbersome and thus have not been widely used in clinical practice.
Imaging studies such as intravenous collapse index and echocardiography can also be used, but have limitations in wide interpatient variability, operator dependency, and high variations depending on cardiac function. Circulating biomarkers such as atrial natriuretic peptide (ANP), N-terminal pro-brain natriuretic peptide (NT-proBNP), and cyclic guanidine monophosphate (cGMP) have been reported to predict adverse outcomes in dialysis patients. However, these markers also have wide interpatient variability and are mostly secreted by the kidney. In addition, there is much concern about inability of these markers to discriminate fluid status.
Bioimpedance analysis is a relatively easy technique to assess fluid status. It can measure both extracellular and intracellular water contents and assess nutritional status. In addition, fluid status assessed by this method well correlates with the results determined by dilutional methods and is highly reproducible. However, it can underestimate volume removed from trunk and temperature, and ion effects and recumbent position can affect water contents assessed by this method. To date, there is no single test to precisely represent fluid status. A variety of tests together can be applied to increase accuracy, but cost-effectiveness and inconvenience should be taken into account. Regardless of which assessment methods are used, regular and serial measurements using the same method seem to be the most helpful and reliable way to determine the fluid status in an individual patient.
6.2.2 Management of Fluid Overload
6.2.2.1 Preservation of RRF
Table 15.6 presents a summary of management of fluid overload in dialysis patients. RRF should be preserved given its important role in fluid status. Preventive strategies against loss of RRF are described in detail above (see Sect. 15.5.5.3).
6.2.2.2 High-Dose Loop Diuretics
The use of diuretics is also a useful option to manage fluid overload, as long as sufficient RRF exists. In general, high loop diuretics are recommended in advanced stages of CKD given its pharmacodynamics. Dialysis therapy does not influence removal of diuretics (Sica 2012). Studies to evaluate the effects of diuretics are lacking in ESRD population. Favorable effects of high-dose furosemide on preservation of RRF were first suggested by Medcalf et al. (2001). They conducted a randomized controlled trial in 61 incident PD patients and found that urine volume and sodium excretion were greater in patients assigned to furosemide 250 mg compared to control group. In agreement with this finding, reanalysis of the data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) showed diuretic users were more likely to have RRF at 1 year after entering the study than nonusers (Bragg-Gresham et al. 2007). In contrast, a small group study involving 62 PD patients alone failed to show beneficial effects of furosemide on preserving RRF (Flinn et al. 2006). Nevertheless, patients with presence of RRF have merits in many aspects. To date, the use of high-dose furosemide is widely accepted as a therapeutic option to maintain euvolemia.
6.2.2.3 Diet Control
Because excessive salt and water intake is the primary cause of fluid overload, dietary counseling can be helpful. Dietary restriction is not routinely recommended for all patients. However, it should be given to patients who have persistent edema and uncontrolled blood pressure. The effect of dietary restriction alone on normalization of blood pressure was demonstrated in small group studies as discussed above (Tzamaloukas et al. 1995).
6.2.2.4 Enhanced Compliance Via Education
Non-compliance to dietary restrictions or the prescribed peritoneal dialysis regimen can be improved through repeated education programs. Every PD facility should implement such program that can assess issues regarding inadequate dialysis. Home visit program is another good option to increase compliance and optimize dialysis effectiveness. This can be more effective particularly in patients who do not have someone to assist exchange procedure, elderly people, or physically disabled patients. In one small group case-control study in Italy, home visit program was associated with improved technique survival (Martino et al. 2014).
6.2.2.5 Increasing Peritoneal Ultrafiltration
Hypertonic PD solutions should be considered to use if fluid overload persists particularly in anuric patients. Some patients have concern about the use of high concentration glucose solution because of infusion pain, long-term deleterious effects of high glucose burden, or hyperglycemia in diabetic patients. Proper education should be delivered to these patients. Blood sugar levels in diabetic patients should be monitored and controlled because osmotic gradient across the peritoneal membrane induced by high concentration glucose solution use is required for fluid removal. PD prescription should be modified in patients with ultrafiltration failure depending on peritoneal membrane transport status. This is discussed in detail in Sect. 15.5.6.3 below.
6.2.2.6 Preservation of Peritoneal Membrane Function
To maintain consistently adequate ultrafiltration and clearance, strategies to preserve peritoneal membrane function should be employed. To this end, PD-related peritonitis should be prevented because peritonitis can directly give damage to the peritoneum. Severe and prolonged peritonitis which has not been resolved despite the proper use of antibiotics can eventually lead to structural and functional derangement of peritoneal membrane. Although hypertonic PD solutions are allowed for the management of fluid overload, avoidance of the frequent use of these solutions is recommended to minimize glucose toxicity to the peritoneum. It is well known that long-term exposure to glucose is a main cause of peritoneal membrane failure. In addition, glucose absorbed during PD may induce weight gain, hyperglycemia, lipid abnormalities, and insulin resistance and thus increase cardiovascular risk. For this reason, “glucose-sparing strategies” have been recently proposed to lessen glucose toxicity. These approaches focus on fluid management with the reduced need for hypertonic solution use, salt and water restriction, the use of high-dose loop diuretics in the presence of RRF, preservation of RRF, and the use of non-glucose-based solutions.
The harmful effects of the conventional glucose-based solutions are mainly attributed to “bioincompatibility,” which is characterized by GDPs, lactate, low pH, and high osmolality. Therefore, new neutral pH, bicarbonate-buffered biocompatible solutions containing low GDPs have recently been highlighted. In vitro and in vivo studies have demonstrated the strengths of the biocompatible solutions compared to conventional solutions with respect to the improved viability of peritoneal mesothelial and inflammatory cells, less accumulation of GDPs, reduced new vessel formation, and decreased fibrosis (Hoff 2003; Mortier et al. 2004; Fabbrini et al. 2006). However, whether these favorable findings from experimental studies can be translated into the better peritoneal membrane function is uncertain. Early studies showed that the use of new biocompatible solutions resulted in more peritoneal ultrafiltration volume than conventional solutions (Tranaeus 2000; Fan et al. 2008). However, these findings were not validated by many subsequent studies (Fang et al. 2008; Kim et al. 2009; Haag-Weber et al. 2010; Johnson et al. 2012; Lui et al. 2012). Interestingly, there may be reciprocal changes between RRF and peritoneal ultrafiltration; a decrease in RRF is compensated by an increase in peritoneal ultrafiltration or vice versa. Thus, it is possible that the effects of the biocompatible solutions on RRF or peritoneal ultrafiltration may not be caused by “biocompatibility” itself, but can simply vary depending on fluid status. However, the study duration was 1 or 2 years in most trials, thus further long-term studies are required to prove superiority of the new biocompatible solutions, particularly after RRF is lost.
Another option to decrease “glucose toxicity” is to use non-glucose-based solutions. In particular, icodextrin solution has been highlighted in terms of improving peritoneal ultrafiltration. Due to its high molecular weight (14–18 kD), icodextrin solution can induce peritoneal ultrafiltration by colloid osmosis, unlike conventional solutions by crystalloid osmosis. Ultrafiltration capacity of icodextrin solution is as much as that of 4.25% glucose solution in the long-dwell time up to 12–16 h. In fact, many studies have shown that the use of icodextrin solution improves fluid status by sustained increase in ultrafiltration (Posthuma et al. 1997, Plum et al. 2002, Davies et al. 2003, Konings et al. 2003a, b), even in patients with fast transport membrane (Lin et al. 2009). Possibly, it has also potential merits in preservation of RRF, improved lipid profiles, and less glucose burden.
The beneficial effects of these new solutions were adopted by the European Best Practice Guidelines. The committee suggests that the use of these solutions can be used particularly when there is concern on biocompatibility, glucose toxicity, or fluid balance (Evidence level B or C).
6.3 Fluid Management in Peritoneal Ultrafiltration Failure
There are three types of ultrafiltration failure depending on peritoneal membrane transport status. Therapeutic strategies should be individualized according to these types given the different pathophysiology of ultrafiltration failure (Fig. 15.8).
6.3.1 Fast Transport Status
This is known as type I ultrafiltration failure characterized by low ultrafiltration volume and fast transport status. It is most common among three types of ultrafiltration failure. High amount of glucose exposure and PD-related peritonitis are two main factors leading to this type of ultrafiltration failure. Due to early osmotic dissipation in fast transport status, short and frequent exchanges are generally recommended. Best program in this case is, for example, the combined use of short 1–2-h dwells using cycler during the nighttime and additional long dwell of icodextrin solution during the daytime. Icodextrin solution exerts its maximal ultrafiltration during the 12–16-h dwell; thus the use of this solution is an ideal approach. In fact, icodextrin solution has been demonstrated to prolong technique survival in patients with ultrafiltration failure and fast transport status (Takatori et al. 2011).
Resting the peritoneum for 4 weeks is another option for ultrafiltration failure with fast transport as peritoneal membrane function can be restored to some degree following a temporary cessation of PD. The mechanism for this is unclear, but it can be presumed that increased vascularity can be resolved during the PD-free period.
6.3.2 Slow Transport Status
This type of ultrafiltration failure is referred to type II ultrafiltration failure. Possible causes for this ultrafiltration failure are peritoneal adhesions and scarring after a severe peritonitis or other intra-abdominal complication. Unfortunately, short exchanges or icodextrin solution use is not effective in patients with type II ultrafiltration failure. Transfer to HD is generally recommended because it is difficult to maintain PD unless patients have significant RRF.
6.3.3 Average Transport Status
Type III ultrafiltration occurs in patients with average transport status. It is caused by aquaporin deficiency or increased lymphatic reabsorption. Because there are no reliable drugs to restore aquaporin function or to decrease lymphatic absorption, general approach such as salt and water restriction, use of diuretics, and other methods to increase ultrafiltration can be employed. Of note, icodextrin induces ultrafiltration by colloid osmosis, which is not involved in aquaporin channels. Therefore, as in type I ultrafiltration failure, long dwell of icodextrin solution with short and frequent exchanges by APD can be useful in type III ultrafiltration failure.
7 Nutritional Status
Malnutrition, which is now interchangeably referred to protein-energy wasting (PEW), is common in PD patients on dialysis and has been recognized as an important risk factor for adverse outcomes in these patients. Therefore, it is widely acknowledged that nutritional issue should be a part of dialysis adequacy. To correctly define PEW, recent criteria by a panel of experts from the International Society of Renal Nutrition and Metabolism (ISRNM) have been proposed and are steadily gaining acceptance (Fouque et al. 2008). Based on these criteria, PEW can be diagnosed when at least three out of the four listed categories should be met and at least one test in each of the selected category should be included; four categories are serum biochemistry, body mass, muscle mass, and dietary intake. There are number of factors that are involved in the development of PEW in dialysis patients. These include inflammation, inadequate dialysis, insufficient nutrient intake, loss of protein during dialysis, chronic acidosis, hypercatabolic illness, and comorbid conditions. Given the multifactorial and complicated pathogenesis of PEW, a multidisciplinary approach should be provided through careful nutritional assessment, dietary counseling, delivery of adequate dialysis dose, proper nutritional support, correction of acidosis, and management of comorbid conditions (Han and Han 2012). Besides such general care, there are more factors that should also be taken into account in PD patients. As discussed earlier, RRF is significantly associated with nutritional status, thus preservation of RRF can be a therapeutic strategy to improve PEW. Peritonitis should also be prevented because appetite and nutritional status are impaired by recurrent peritonitis. Encouraging dietary intake and adequate nutritional support is of paramount importance due to insufficient calorie intake in PD patients and the substantial protein loss into the dialysate. Unfortunately, nutritional intervention trials to date have produced inconclusive findings because these have not been well controlled and are limited by short follow-up duration and small sample sizes. Further long-term prospective, randomized, controlled trials are warranted to clarify the beneficial effects of nutritional interventions. In the meantime, patients with PEW should be treated by using currently available therapeutic strategies (Table 15.7).
8 Middle Molecule Clearance
Small solutes are not sole uremic toxins. Enhancing the elimination of middle molecules should be incorporated to a therapeutic target in dialysis patients. β2MG is a representative middle molecule with a molecular weight of 11,000 D. The harmful effects of β2MG are well recognized as in carpal tunnel syndrome and β2MG-related amyloidosis. These complications frequently occur particularly in patients on long-term dialysis. Recent studies have identified β2MG as an independent prognostic factor of morbidity and mortality in both HD and PD patients. Cheung et al. reanalyzed the HEMO study data and found that a 10 mg/L increase in serum β2MG level was significantly associated with an 11% increase in risk of all-cause mortality in HD patients (Cheung et al. 2006). Similar findings were also observed in PD patients. In a prospective cohort study in Korea (Koh et al. 2015), patients in the highest tertile of β2MG level had higher mortality rate than patients in the lowest tertile. PD has conventionally been thought to have better removal of larger molecular weight uremic toxins than intermittent HD. There are several reasons for this notion. First, peritoneal membrane is more porous than the cellulosic HD membrane. Second, middle molecule clearance is time dependent (Kim et al. 2001). Therefore, PD has advantage over intermittent HD in terms of removal of middle molecules. However, HD technology has consistently advanced, and convective therapy such as online hemodiafiltration has increasingly been used in clinical practice. These modalities have been reported to have superior removal of β2MG to conventional HD. There has been lack of studies to evaluate which dialysis modality between HD and PD is better in middle molecule clearance. Evenepoel et al. compared dialytic clearance of β2MG between high-flux HD and PD (Evenepoel et al. 2006). The results showed that β2MG clearance was significantly higher in patients receiving high-flux HD than in patients receiving PD. When different PD modalities were compared to HD, serum β2MG level was significantly lower in high-flux HD group than in APD group. However, there was no significant difference in serum β2MG level between high-flux HD group and CAPD group. Presumably, short and frequent exchanges in APD might result in insufficient removal of larger molecules. Because HD is an intermittent therapy, it is difficult to maintain consistently lower serum β2MG levels as compared to continuous PD therapy despite the superior capacity of middle molecule removal by high-flux dialysis. Considering the theoretical merits of PD with respect to removal of middle molecules, convective therapy using more porous HD membrane and extended duration of HD treatment are required to remove middle molecules more effectively. This notion is supported by a previous study by Raj et al. (2000). They compared β2MG clearance between conventional thrice a week HD and nocturnal HD. Two dialysis modalities used the same high-flux dialyzer. β2MG clearance was significantly higher, and serum predialysis β2MG level was lower in nocturnal HD group than in conventional HD group. To date, there is no randomized controlled trial to investigate the optimal level of β2MG or whether lowering β2MG level can result in better survival. In addition, the current guidelines do not recommend the routine monitoring of β2MG or other middle molecules in dialysis patients. Nevertheless, given the fact that β2MG exhibits deleterious effects and is a strong predictor of death, much effort should be made to increase β2MG clearance.
References
Ates K, Nergizoglu G, Keven K, Sen A, Kutlay S, Erturk S, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2001;60(2):767–76.
Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Time profiles of peritoneal and renal clearances of different uremic solutes in incident peritoneal dialysis patients. Am J Kidney Dis. 2005;46(3):512–9.
Bargman JM, Thorpe KE, Churchill DN, CPDS Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–62.
Bernardini J, Nagy M, Piraino B. Pattern of noncompliance with dialysis exchanges in peritoneal dialysis patients. Am J Kidney Dis. 2000;35(6):1104–10.
Blake PG, Bargman JM, Brimble KS, Davison SN, Hirsch D, McCormick BB, et al. Clinical practice guidelines and recommendations on peritoneal dialysis adequacy 2011. Perit Dial Int. 2011;31(2):218–39.
Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS). Am J Kidney Dis. 2007;49(3):426–31.
Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol. 2003;14(11):2948–57.
Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996;7(2):198–207.
Chang TI, Ryu DR, Yoo TH, Kim HJ, Kang EW, Kim H, et al. Effect of Icodextrin solution on the preservation of residual renal function in peritoneal dialysis patients: a randomized controlled study. Medicine (Baltimore). 2016;95(13):e2991.
Cheng LT, Chen W, Tang W, Wang T. Residual renal function and volume control in peritoneal dialysis patients. Nephron Clin Pract. 2006;104(1):c47–54.
Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17(2):546–55.
Cho Y, Johnson DW, Badve S, Craig JC, Strippoli GF, Wiggins KJ. Impact of icodextrin on clinical outcomes in peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2013;28(7):1899–907.
Choi JY, Jang HM, Park J, Kim YS, Kang SW, Yang CW, et al. Survival advantage of peritoneal dialysis relative to hemodialysis in the early period of incident dialysis patients: a nationwide prospective propensity-matched study in Korea. PLoS One. 2013;8(12):e84257.
Daugirdas JT, Depner TA, Greene T, Levin NW, Chertow GM, Rocco MV, et al. Standard Kt/V urea: a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77(7):637–44.
Davies SJ, Bryan J, Phillips L, Russell GI. Longitudinal changes in peritoneal kinetics: the effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant. 1996;11(3):498–506.
Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003;14(9):2338–44.
Dombros N,Dratwa M, Feriani M, Gokal R, Heimburger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 7 Adequacy of peritoneal dialysis. Nephrol Dial Transplant. 2005;20(Suppl 9):ix24–ix27.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–9.
Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y. Superior dialytic clearance of beta(2)-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int. 2006;70(4):794–9.
Fabbrini P, Zareie M, Ter Wee PM, Keuning ED, Beelen RH, van den Born J. Peritoneal exposure model in the rat as a tool to unravel bio(in)compatibility of PDF. Nephrol Dial Transplant. 2006;21(Suppl 2):ii8–11.
Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008;73(2):200–6.
Fang W, Mullan R, Shah H, Mujais S, Bargman JM, Oreopoulos DG. Comparison between bicarbonate/lactate and standard lactate dialysis solution in peritoneal transport and ultrafiltration: a prospective, crossover single-dwell study. Perit Dial Int. 2008;28(1):35–43.
Flinn A, Ledger S, Blake P. Effectiveness of furosemide in patients on peritoneal dialysis. CANNT J. 2006;16(3):40–4.
Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8.
Griva K, Kang AW, Yu ZL, Mooppil NK, Foo M, Chan CM, et al. Quality of life and emotional distress between patients on peritoneal dialysis versus community-based hemodialysis. Qual Life Res. 2014a;23(1):57–66.
Griva K, Lai AY, Lim HA, Yu Z, Foo MW, Newman SP. Non-adherence in patients on peritoneal dialysis: a systematic review. PLoS One. 2014b;9(2):e89001.
Gunal AI, Duman S, Ozkahya M, Toz H, Asci G, Akcicek F, et al. Strict volume control normalizes hypertension in peritoneal dialysis patients. Am J Kidney Dis. 2001;37(3):588–93.
Haag-Weber M, Kramer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010;25(7):2288–96.
Han SH, Han DS. Nutrition in patients on peritoneal dialysis. Nat Rev Nephrol. 2012;8(3):163–75.
Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, Lee TH, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant. 2007;22(9):2653–8.
Han SH, Lee SC, Kang EW, Park JK, Yoon HS, Yoo TH, et al. Reduced residual renal function is associated with endothelial dysfunction in patients receiving peritoneal dialysis. Perit Dial Int. 2012;32(2):149–58.
Heaf JG, Lokkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112–7.
Hoff CM. In vitro biocompatibility performance of physioneal. Kidney Int. 2003;Suppl(88):S57–74.
Huang WH, Chen KH, Hsu CW, Chen YC, Hung CC, Huang JY, et al. Residual renal function—one of the factors associated with arterial stiffness in peritoneal dialysis patients. Insight from a retrospective study in 146 peritoneal dialysis patients. Blood Purif. 2008;26(2):133–7.
Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–53.
Jansen MA, Termorshuizen F, Korevaar JC, Dekker FW, Boeschoten E, Krediet RT, et al. Predictors of survival in anuric peritoneal dialysis patients. Kidney Int. 2005;68(3):1199–205.
Johnson D, Brown F, Lammi H, Walker R, Caring for Australians with Renal Impairment. The CARI guidelines. Dialysis adequacy (PD) guidelines. Nephrology (Carlton). 2005;10(Suppl 4):S81–107.
Johnson DW, Clarke M, Wilson V, Woods F, Brown FG. Rationale and design of the balANZ trial: a randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrol. 2010;11:25.
Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol. 2012;23(6):1097–107.
Kim DJ, Do JH, Huh W, Kim YG, Oh HY. Dissociation between clearances of small and middle molecules in incremental peritoneal dialysis. Perit Dial Int. 2001;21(5):462–6.
Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant. 2009;24(9):2899–908.
Kjaergaard KD, Peters CD, Jespersen B, Tietze IN, Madsen JK, Pedersen BB, et al. Angiotensin blockade and progressive loss of kidney function in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2014;64(6):892–901.
Koh ES, Lee K, Kim SH, Kim YO, Jin DC, Song HC, et al. Serum beta2-microglobulin predicts mortality in peritoneal dialysis patients: a prospective cohort study. Am J Nephrol. 2015;42(2):91–8.
Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003a;63(4):1556–63.
Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003b;18(4):797–803.
Kumar VA, Sidell MA, Jones JP, Vonesh EF. Survival of propensity matched incident peritoneal and hemodialysis patients in a United States health care system. Kidney Int. 2014;86(5):1016–22.
Lang SM, Bergner A, Topfer M, Schiffl H. Preservation of residual renal function in dialysis patients: effects of dialysis-technique-related factors. Perit Dial Int. 2001;21(1):52–7.
Leypoldt JK. Urea standard Kt/V(urea) for assessing dialysis treatment adequacy. Hemodial Int. 2004;8(2):193–7.
Li PK, Chow KM, Wong TY, Leung CB, Szeto CC. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003;139(2):105–12.
Lin A, Qian J, Li X, Yu X, Liu W, Sun Y, et al. Randomized controlled trial of icodextrin versus glucose containing peritoneal dialysis fluid. Clin J Am Soc Nephrol. 2009;4(11):1799–804.
Lo WK, Ho YW, Li CS, Wong KS, Chan TM, Yu AW, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64(2):649–56.
Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int. 2006;26(5):520–2.
Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis. 2012;60(6):966–75.
Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, et al. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10(12):2295–305.
Martino F, Adibelli Z, Mason G, Nayak A, Ariyanon W, Rettore E, et al. Home visit program improves technique survival in peritoneal dialysis. Blood Purif. 2014;37(4):286–90.
McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20(1):155–63.
McKane W, Chandna SM, Tattersall JE, Greenwood RN, Farrington K. Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int. 2002;61(1):256–65.
Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59(3):1128–33.
Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant. 2001;16(11):2207–13.
Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11(3):556–64.
Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: Effects on the peritoneal membrane. Kidney Int. 2004;66(3):1257–65.
Mostovaya IM, Bots ML, van den Dorpel MA, Grooteman MP, Kamp O, Levesque R, et al. A randomized trial of hemodiafiltration and change in cardiovascular parameters. Clin J Am Soc Nephrol. 2014;9(3):520–6.
Noh H, Lee SW, Kang SW, Shin SK, Choi KH, Lee HY, et al. Serum C-reactive protein: a predictor of mortality in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1998;18(4):387–94.
Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–20.
Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41(6):1212–8.
Penne EL, van der Weerd NC, van den Dorpel MA, Grooteman MP, Levesque R, Nube MJ, et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis. 2010;55(1):77–87.
Perez Fontan M, Rodriguez-Carmona A, Garcia-Naveiro R, Rosales M, Villaverde P, Valdes F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25(3):274–84.
Peritoneal Dialysis Adequacy Work G. Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S91–7.
Plum J, Gentile S, Verger C, Brunkhorst R, Bahner U, Faller B, et al. Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. Am J Kidney Dis. 2002;39(4):862–71.
Posthuma N, ter Wee PM, Verbrugh HA, Oe PL, Peers E, Sayers J, et al. Icodextrin instead of glucose during the daytime dwell in CCPD increases ultrafiltration and 24-h dialysate creatinine clearance. Nephrol Dial Transplant. 1997;12(3):550–3.
Raj DS, Ouwendyk M, Francoeur R, Pierratos A. beta(2)-microglobulin kinetics in nocturnal haemodialysis. Nephrol Dial Transplant. 2000;15(1):58–64.
Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–58.
Sica DA. Diuretic use in renal disease. Nat Rev Nephrol. 2012;8(2):100–9.
Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–90.
Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis. 2004;43(6):1056–64.
Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant. 2007;22(2):552–9.
Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1337–44.
Tranaeus A. A long-term study of a bicarbonate/lactate-based peritoneal dialysis solution—clinical benefits. The Bicarbonate/Lactate Study Group. Perit Dial Int. 2000;20(5):516–23.
Twardowski ZJ. Relationships between creatinine clearances and Kt/V in peritoneal dialysis patients: a critique of the DOQI document. Dialysis Outcome Quality Initiative, National Kidney Foundation. Perit Dial Int. 1998;18(3):252–5.
Tzamaloukas AH, Saddler MC, Murata GH, Malhotra D, Sena P, Simon D, et al. Symptomatic fluid retention in patients on continuous peritoneal dialysis. J Am Soc Nephrol. 1995;6(2):198–206.
Van Biesen W, Vanholder RC, Veys N, Dhondt A, Lameire NH. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol. 2000;11(1):116–25.
Wang AY, Sea MM, Ip R, Law MC, Chow KM, Lui SF, et al. Independent effects of residual renal function and dialysis adequacy on dietary micronutrient intakes in patients receiving continuous ambulatory peritoneal dialysis. Am J Clin Nutr. 2002a;76(3):569–76.
Wang AY, Wang M, Woo J, Law MC, Chow KM, Li PK, et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002b;62(2):639–47.
Wang AY, Sea MM, Tang N, Sanderson JE, Lui SF, Li PK, et al. Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol. 2004a;15(12):3134–43.
Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004b;15(8):2186–94.
Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK. Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: what are the implications? Am J Kidney Dis. 2004c;43(4):712–20.
Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21(3):499–506.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Chang, T.I., Han, S.H. (2018). Kinetic Modeling and Adequacy in PD. In: Kim, YL., Kawanishi, H. (eds) The Essentials of Clinical Dialysis. Springer, Singapore. https://doi.org/10.1007/978-981-10-1100-9_15
Download citation
DOI: https://doi.org/10.1007/978-981-10-1100-9_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1099-6
Online ISBN: 978-981-10-1100-9
eBook Packages: MedicineMedicine (R0)