Abstract
Telocytes have been identified as a distinctive type of interstitial cells and have been recognized in most tissues and organs. Telocytes are characterized by having extraordinary long cytoplasmic processes, telopodes, that extend to form three-dimensional networks and commonly constitute specialized forms of cell-to-cell junctions with other neighboring cells. Telocytes have been localized in the stem cell niche of different organs such as the heart, lung, skeletal muscle, and skin. Electron microscopy and electron tomography revealed a specialized link between telocytes and stem cells that postulates a potential role for telocytes during tissue regeneration and repair. In this review, the distribution of telocytes in different stem cell niches will be explored, highlighting the intimate relationship between the two types of cells and their possible functional relationship.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Telocyte (TC) is a newly described type of interstitial cells which is characterized by having a small cell body and extremely long cytoplasmic prolongations that are called telopodes. Each telopode is composed of alternating thin segments (podomers) and dilated segments (podoms) [47].
Using electron microscopy (EM) is considered the ideal technique to identify TCs in most tissues. Meanwhile, double-positive immunohistochemistry for CD34/c-Kit (usually localized in TC cell body) or CD34/vimentin (localized in telopodes) is designated as a reliable method for TC visualization [48]. More recently, the emergence of cutting-edge technologies in subcellular three-dimensional (3D) imaging, such as FIB-SEM tomography, has provided more evidence-based approach for TC study with special emphasis on their relationships with neighboring cells [15].
Reviewing the literature establishes a growing body of evidence that TCs co-localize with stem and progenitor cells in different organs such as the heart [24], lungs [25], and skeletal muscles [53]. Furthermore, more emerging evidence has supported the notion of the potential role of TCs in tissue regeneration and/or repair after injury ([36, 41, 64, 69, 76]).
Being the fundamental cells for tissue regeneration, different categories of stem cells (SCs) have been identified in most adult human organs. However, resident SCs cannot survive only by their capacity of self-renewal. Therefore, SCs are strongly dependent on the microenvironment they live in each organ. Eventually, the term “stem cell niche” refers to the well-organized minute tissue areas housing SCs which ensure an appropriate microenvironment maintained with blood vessels, nerve endings, extracellular matrix, and supporting interstitial cells [19]. Stem cell niche commonly occurs at tissue intersections or transition zones, and it enables SCs to have spatial and dynamic interaction with neighboring cells as well as with remote cells through paracrine and endocrine signals [20].
Accordingly, several studies have confirmed the existence of TCs in SC niche microenvironment in a variety of tissues and organs such as the heart [2], lungs [51–53], skeletal muscle [6, 11, 64], skin [10], meninges and choroid plexus [54], liver [68, 71], eye [38], and aorta [74].
Moreover, TCs were found to express SC markers like c-kit, Sca-1, and Oct 4 [6, 23, 63]. Although the expression of particular SC markers by TCs varied among tissues, this finding suggests a major role of TCs in regeneration [14].
It seems prudent to indicate that no definite mechanism has been confirmed yet to explain the direct or indirect interaction between SCs and TCs. Meanwhile, several hypotheses have been proposed based on the morphological features of both types of cells in the microenvironment. For example, [57] suggested direct cell-to-cell signaling and close cross talk where TCs might provide antioxidant protective effect to SCs [78]. Another regenerative role of TCs was attributed to their expression of VEGF and PDGFR-b, both in situ and in vitro, speculating their potential effect in angiogenesis during tissue repair [51–53, 64].
Some investigators suggested a role of TCs in mesenchymal SC differentiation [10], while others [57] argued that TCs could be a subpopulation of mesenchymal SCs as they express some markers of stromal niche cells. In addition, TCs were proposed to play an important role in cell signaling, hence controlling the microenvironment in normal and malignant tissues [28].
In fact, increasing evidence reveals that TCs actively contribute to the coordination of cell signaling in SC microenvironment either through direct intercellular junctions or via shed vesicles that exert a paracrine effect [3, 26, 40].
In the following sections, tissue-specific localization of TCs in SC niche will be explored with special reference to the unique relationship between the two types of cells as evidenced by previous reports.
23.1 Telocytes in Cardiac Stem Cell Niche
TCs have been vigorously studied as a distinct type of cells in the heart interstitium [24, 27, 47, 60, 62]. More recently, 3D imaging using FIB-SEM tomography has added new evidence to the identification of cardiac TCs [15].
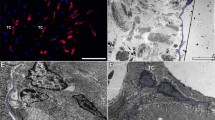
TCs were described as 3D network in the myocardium among cardiomyocytes, blood capillaries, nerve endings, and immune system cells [2, 3, 15]. In addition, telocytes were clearly identified under the pericardium in close association with cardiac SCs and progenitor cells (Fig. 23.1) [24].
(a) Electron microscopy of the subepicardial SC niche in the mouse showing the presence of putative cardiac stem cells (CSC), isolated or in small groups, cardiomyocyte progenitors (CMP), and cells with intermediate features (CSC–CMP). All these cells are placed in a loose extracellular matrix. (b) Higher magnification from area marked in (a) with a dotted square. The CMP have characteristic leptofibrils (arrow) and are closely assisted by telocyte processes (TCp) which contain few dense granules (asterisk) suggesting paracrine signaling. CM cardiomyocyte, macro macrophage, coll collagen fibers (Courtesy with permission from Gherghiceanu and Popescu [24])
The human myocardium is characterized by its extremely low ability to regenerate due to its limited number of cardiac SCs (0.01–1 %). Meanwhile, the number of cardiac TCs (0.5–1 %) exceeds that of cardiac SCs. Although TCs still represent a minute portion of human cardiac interstitial cells, their extremely long and extensive telopodes allow them to occupy more surface area that forms 3D reticulum probably for supporting other cells [55].
The exceptional relationship between cardiac TCs and SCs was best described as “tandem” where both types of cells integrate in a structural and functional manner [14, 24, 26]. Given such relationship, TCs were nominated to provide support for cardiac SCs [24, 26], guide cardiac progenitor cells [2, 50], and enhance neoangiogenesis [41, 76].
Different types of heterocellular junctions were described in adult murine and human heart tissue [25, 26] that have been localized between TCs in one side and cardiac SCs, cardiomyocytes, and other interstitial cells in the other side. The reported distance between junction cell membranes was 10–20 nm that goes well with transfer of macromolecules [26]. TCs and cardiomyocytes revealed electron-dense nanocontacts [25], while the junctions between TCs and cardiac SCs were described as stromal synapse or adherent junctions [26]. TCs were found also to have point and/or planar contacts with endothelial cells, pericytes, Schwann cells, and other interstitial cells such as fibroblasts, macrophages, and mast cells [26].
Similar types of junctions were described between cocultured cardiac TCs and SCs where cells exhibited heterocellular adherens junctions as well as nonclassical junctions such as the puncta adherentia (Fig. 23.2) and stromal synapses. The stromal synapse formed between TCs and SCs was frequently associated with small electron-dense structures (15 nm in length) that connect the two opposing cell membranes (Fig. 23.3) [56].
(a) Transmission electron microscopy images of TC–CSC culture after 48 h shows a telopode (Tp) in close contact with a cardiac stem cell (CSC). (b–d) Marked areas from image A are shown at higher magnification in the corresponding panels. A planar contact (stromal synapse) between TC and CSC can be seen associated with a number of electron-dense structures (arrows). A puncta adherentia junction (arrowhead) is visible between TC and CSC in image (c). Cp coated pit (Courtesy with permission from Popescu et al. [56])
Transmission electron microscopy shows similar intercellular connections (plain stromal synapses) between telocytes (TC) and cardiac stem cells (CSC) in culture (a) and in tissue (b). Telopodes (Tp) connect with a cardiac stem cell (CSC) through small electron-dense structures (arrows). Endo endothelial cell, P pericyte, N nerve ending, CM cardiomyocyte (Courtesy with permission from Popescu et al. [56])
Such intimate cross-cellular relationships in situ and in vitro might help cardiac SCs to proliferate and differentiate refuting the hypothesis of a solitary role of SCs during cardiac regeneration [55]. Thus, the lack of such nursing role of TCs was proposed to explain the failure of solely engrafted cardiac SCs to survive in the microenvironment of injured myocardium [56].
Special attention has been drawn to the epicardium as a novel source of cardiac SCs [7]. Cardiac TCs and SC niche in the subepicardial region were shown to express common surface markers such as PDGFR-b and c-kit (Popescu et al. 2010, [8, 73, 79]). This finding argued for proposing cardiac TCs per se as a subpopulation of epicardium-derived progenitor cells [2]. Another postulation was attributed to the possibility that TCs might be a potential source of cardiac mesenchymal cells [17]. Accordingly, cardiac TCs were similar to bone marrow-derived SCs in being positive for mesenchymal marker CD29 and negative for hematopoietic marker CD45 [5].
Reviewing literature, in vitro and in vivo studies revealed the possible modulating effect of cardiac TCs on cardiac development and repair [2, 21, 24]. In vitro studies demonstrated that cardiac TCs embrace cell populations of growing cardiomyocytes supporting the proposed nursing role [2]. A tissue-engineered heart model demonstrated networks of TCs with their telopodes communicating with growing cardiomyocytes through junctions and shed vesicles [80]. Interestingly, studies on zebra fish revealed that, upon myocardial regeneration following amputation of the ventricular apex, reorganized TCs with their telopodes were recognized in large number in close association with growing cardiomyocytes [32, 51].
In vivo transplantation of cardiac TCs in experimental rat model of myocardial infarction was found to decrease the size of infarction and to enhance myocardial function [75, 76]. Such improvement was attributed to the potential ability of TCs to promote angiogenesis and to reduce fibrosis following heart injury [4, 75, 76]. Another study revealed an improvement of myocardial infarction after transplantation of human iPS-derived mesenchymal SCs with specific networked arrangement of TCs in the interstitial space of infarction [42].
Thus, TCs might be critically involved in the integration of heterocellular communications that is crucial for proliferation, differentiation, and maturation of myocardial progenitor cells [4, 66]. Therefore, preconditioning of transplanted cardiac SCs with TCs should be considered as an alternative modality to regenerate cardiomyocyte instead of monocellular therapy protocols.
23.2 Telocytes in Skeletal Muscle Stem Cell Niche
The skeletal muscle is well known of its extraordinary capacity to regenerate after injury. Satellite cells are the primary cells involved in skeletal muscle regeneration and are found in specific satellite cell niche [33]. However, other non-satellite progenitor cell niche can be recognized in which other precursor cells exist such as bone marrow-derived cells and pericytes [16, 18, 34, 43].
It is worth mentioning that TCs have been well recognized in human adult skeletal muscle [51–53] where they closely communicate via telopodes with cells in both satellite cell niche and non-satellite progenitor cell niche suggesting a potential role for their proliferation and differentiation [6, 51–53].
TCs were found to constitute a 3D network in the interstitial tissue of skeletal muscle where they locate very close to blood capillaries, nerve fibers, myocytes, and satellite cells [6, 51–53]. Intimate contacts between TCs and both types of resident muscle SCs, which is satellite (Fig. 23.4) and non-satellite (Fig. 23.5), were clearly identified where telopodes were found in their vicinity [53]. Heterocellular junctions were also identified between TCs and the progenitor neighboring cells with shed vesicles and exosomes [4, 53].
Transmission electron micrographs show TC with Tps, podoms, and podomeres in between muscle fibers. Note the typical appearance of satellite cells. TCs (digitally blue colored) are positioned in the close vicinity of satellite cells. Two ultrastructural features are remarkable: the close spatial relationships of Tps with satellite cells and the fact that these Tps release shed vesicles (purple arrows). This may indicate that a transfer of chemical information flows from TC to satellite cells (Courtesy with permission from Popescu et al. [53])
Electron micrographs of human skeletal muscle show a TC (blue colored), which extends its Tps indicated by red arrows around a striated cell, in fact a (putative) progenitor cell. Note: the tandem TC–progenitor cell making a non-satellite (resident) progenitor stem cell niche. Inset: higher magnification of the progenitor cell shows incompletely differentiated features: unorganized myofilaments (mf), glycogen deposits (Gly), prominent Golgi complex (G). N nucleus, nc nucleolus (Courtesy with permission from Popescu et al. [53])
TCs in the skeletal muscle were postulated to have proliferative and angiogenic capabilities based on their expression of proliferative marker Ki67, pluripotency marker Oct4, and vascular proliferation marker VEGF [6]. In vitro studies demonstrated better differentiation capacity of skeletal muscle-derived SCs into adipocytes, chondrocytes, and osteoblasts when cocultured with TCs, a finding that reinforces the potential role of TCs in tissue regeneration and repair [6].
23.3 Telocytes in Liver Growth and Regeneration
As many other solid organs, TCs in the liver have been clearly identified by both transmission electron microscopy and double immunofluorescent staining for CD34 with c-kit/CD117, vimentin, or PDGFR-a/b [71]. TCs were localized in the space of Disse, in close proximity with hepatocytes, putative stem/progenitor cells, and endothelial cells (Fig. 23.6) [71].
Electron microscope images showing the ultrastructure of the liver (mice). (a) Telocytes (TCs) with telopodes (Tps) in the space of Disse (D) between endothelial cells (E) and hepatocytes (H). Note the upper telopode (Tp) which is more than 20 μm long. (b) Higher magnification of the field inside the rectangle in (a). Note in between the TC and hepatocytes (H) the presence of a putative stem cell (pSC) which has the features of a young cell (progenitor cell?); ER endoplasmic reticulum, N nucleus. (c) A TC with at least three Tps; H hepatocyte, m mitochondria. (d) A TC with a heterochromatic nucleus (N) at a higher magnification; (e) endothelial cell; RBC red blood cell; H hepatocyte, m mitochondria, Tp telopodes; scale bar = 5 μM (Courtesy with permission from Xiao et al. 2013)
The liver is characterized by its remarkable ability to regenerate after exposure to traumatic or ischemic injury [31]. Such regenerative capacity is attributed to the proliferation of hepatocytes and, in severe injuries, the activation and differentiation of hepatic stem/progenitor cells [69].
Some studies have evaluated the involvement of TCs in posttraumatic regeneration and physiological growth of the liver. The possible role of TCs in liver regeneration has been investigated using a mouse model of partial hepatectomy [68]. It was reported that the peak activity of hepatocyte proliferation was recorded after the first 2 days of partial hepatectomy followed by another, yet lower, peak after the third day that was concurrently associated with an elevation of both TCs and CK19-positive hepatic SCs [68]. These findings suggested an exclusive relationship between TCs and other progenitor cells involved in liver regeneration. TCs might contribute to the control of proliferating hepatocytes and/or the differentiating stem cells [4].
Furthermore, an important insinuation of TCs was postulated in pregnancy-induced hepatic proliferation [69]. The two peaks of hepatocyte proliferation during pregnancy in mice correlated with an increase in CD34/PDGFR-α positive TCs [69]. Further molecular mechanisms need to be investigated to explain the hepatic adaption in pregnancy with possible enrollment of TCs.
It is speculated that TCs contribute in liver regeneration through direct intercellular junctions or paracrine effect via shed vesicles [69]. However, further evidence is required to determine the functional relationship between TCs and other cells in the liver.
23.4 Telocytes and Skin Stem Cells
The skin is characterized by its astonishing competency to regenerate featuring it as an ideal model for studying SCs [29]. Multiple locations for SC niche have been recognized in the skin including the bulge of the hair follicle, dermal papillae, and perivascular spaces [22, 70].
Bulge SCs express specific markers such as nestin [35]. However, they can proliferate and differentiate only after receiving signals from specialized neighboring cells in the stroma which is known as the “bulge activation hypothesis” [65].
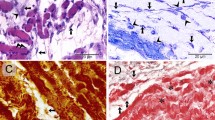
As per other organs and tissues, electron microscopy and immunofluorescence confirmed the presence of TCs in human dermis [10, 58]. TCs were found to border a round cluster of bulge SCs with apparently two types of TCs/SCs contacts, point and planar contacts, forming atypical heterocellular junctions [10].
Interestingly, studying scleroderma skin samples revealed that TCs were found to decrease in areas of SC niches [39]. In addition, SCs could not be observed in the perivascular space in diffuse cutaneous scleroderma [39]. The depletion of TCs might be implicated to the impaired reparative and regenerative function of the skin in cases of scleroderma [39]. Nevertheless, TCs were also found to be reduced in organs other than the skin of systemic scleroderma patients such as the myocardium, lung, and stomach [40]. The pattern of TC distribution in the skin augments the hypothesis that TCs are “nursing cells” where they may interact with resident cells in SC niche through indirect (chemical) and/or direct (junctional) contacts [10].
23.5 Telocytes and Stem Cells in the Lung and Respiratory Passages
TCs have been described in the human and mouse respiratory tree including alveolar ducts as well as terminal and respiratory bronchioles [52]. The tandem of TC–SC was clearly demonstrated in subepithelial niches of the bronchioles particularly at the bronchoalveolar junction. Using electron tomography, telopodes were found to connect with SCs via bridging nanostructures [52].
The direct connection between TCs and resident lung SCs through nanocontacts, shed vesicles, and exosomes [52] postulates a major supporting, cell guiding, and communicating role of TCs [51].
In order to specifically identify the genes that regulate lung TCs, the genetic profile of murine lung TCs was vigorously studied and compared to mesenchymal SCs and fibroblasts [77]. At least 46 genes were found to be functionally connected in TCs, mesenchymal SCs, and fibroblasts [77]. Consequently, multiple functional roles have been implicated for lung TCs including tissue development and regeneration, remodeling of the extracellular matrix, and neovascularization with special emphasis on their potential effect on keeping the integrity of vascular basement membrane [77]. TCs usually locate in close vicinity of small blood vessels and express angiogenesis markers such as VEGF and NO [41].
In addition, lung TCs were found to express pluripotency marker Oct4, which is usually expressed in embryonic SCs (Fig. 23.7), a finding that urged the supposition that TCs could be a subpopulation of SCs [23].
Isolated lung Oct4-GFPpos cells that were maintained in culture for 5 days. Cells were sorted based on GFP fluorescence and kept in culture at 37 °C in MEM medium. (a–c) Bright-field images show cells with a fusiform telocyte-like shape with thin prolongations (asterisks). Examples are provided for cells having one (a), two (b), or three (c) cell prolongations (telopodes) with a particular shape resembling a string of beads (arrows in c). (d) Co-expression of Oct4 and vimentin in telocytes. (e) Co-staining of telocytes with Oct4 and PDGFR-α. Note that telocytes do not express PDGFR-α; bar = 50 μm (Courtesy with permission from Galiger et al. [23])
23.6 Telocytes and Stem Cell Niche in the Eye
The limbus (corneoscleral junction) was described to host a rich SC niche [44, 59] that is essential for corneal regeneration [46]. TCs and SCs have been co-localized at the perivascular spaces of the limbus and the stroma of the iris (Fig. 23.8) among nerve endings. Heterocellular direct membranous junctions have been identified between TCs and SCs in the form of nanocontacts and planar contacts [38].
Transmission electron microscopy images of epithelial (a) and stromal (b, c) stem cell (SC) niches in the mouse eye. (a) Basal SC is sited on the basement membrane of limbus epithelium. A telopode (Tp1) runs parallel with the basement membrane, and a gap junction (arrowheads) connects it with another one (Tp2; higher magnification in inset). (b, c) Stem cells in the stromal SC niches located in the corneoscleral junction. Direct contacts (arrowheads) between a Tp and the SC are visible in (b). TC telocytes, Tp telopodes, Fb fibroblast, n nerve endings, L lumen of an arteriole. Scale bars: (a) – 2 μm; inset – 0.1 μm; (b) – 1 μm; (c) – 5 μm (Courtesy with permission from Luesma et al. [38])
By securing a strategic 3D networked position in the eye among blood vessels, nerve endings, and other stromal cells such as SCs, melanocytes, and macrophages, TCs reinforce their highly proposed cell nursing role [38]. TCs might be able to influence other neighboring cells via short-distance signals through direct contacts, exosomes, and shed vesicles [1]. Meanwhile, their extremely long telopodes could exert a long-distance macromolecular signaling effect through which transfer of proteins, membrane receptors, and mRNAs occurs [37, 45]. Moreover, a specific modulatory effect [61] as well as guiding role of TCs [49] has been proposed in the immune system. Nevertheless, TCs per se could be an integral part of the mesenchymal SC niche [9, 38]. Eventually, TCs are candidates that pave the way for SC migration during tissue regeneration of the eye [38] and might be a promising new treatment modality for degenerative eye diseases.
23.7 Telocytes and Hematopoietic Stem Cells in the Spleen
The spleen is one of the frequently reported sites for extramedullary hematopoiesis in adults [72]. Putative hematopoietic SCs have been identified adjacent to the sinusoidal endothelium of the spleen where they constitute SC niche that ensures a hypoxic environment sufficient for maintaining hematopoietic SC growth and proliferation [30].
The presence, characteristics, and distribution of TCs in the spleen have been recently reported [13]. TCs exhibited similar ultrastructural morphology to that described in other organs where they connected via their telopodes with leukocytes and red blood corpuscles in the red pulp of the spleen [13]. Moreover, splenic TCs formed a 3D network by their telopodes when cultured in vitro anchored by hetero- and homocellular junctions [13].
After 3 days of culture, splenic TCs formed circle-like structures in which their telopodes extended to support the circumference of these circles [13]. Double-labeling immunofluorescence revealed positive immuno-expression of vimentin with CD34, nanog, and Sca-1; however, splenic TCs were negative for c-kit [13].
Therefore, the expression of pluripotent SC marker nanog [12] and hematopoietic progenitor markers CD34 and Sca-1 [67] in splenic TCs suggests some role in regeneration [13]. Eventually, TCs were suggested to contribute in the control of splenic hematopoietic cell niche via signal transmission [13].
23.8 Summary and Conclusions
Morphological features and the characteristic distribution of TCs in SC niche remain the only current indicator that supports a structural relationship between the two types of cells. Therefore, more function-oriented studies need to be done in the future to prove a dual biological and structural liaison during migration, proliferation, and differentiation of SCs.
Moreover, genetic and proteomic analysis of TCs in different organs would clarify the functional relationship with other neighboring cells including SCs. The use of 3D culture techniques and lab-on-chip technology with microfluidic systems can help provide the ideal microenvironment in which TCs could effectively contribute to tissue repair and regeneration. In addition, coculture of SCs and TCs and the use of dual cell therapy protocols would enhance the proposed beneficiary effects in regenerative medicine.
Given the aforementioned techniques would make it possible to answer an open question about the exact nature of TC–SC tandem and whether TCs are a specific form of mesenchymal/progenitor SCs.
References
Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–4.
Bani D, Formigli L, Gherghiceanu M, Faussone-Pellegrini M. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J Cell Mol Med. 2010;14:2531–8.
Bani D, Nistri S. New insights into the morphogenic role of stromal cells and their relevance for regenerative medicine, lessons from the heart. J Cell Mol Med. 2014;18:363–70.
Bei Y, Wang F, Yang C, Xiao J. Telocytes in regenerative medicine. J Cell Mol Med. 2015;19(7):1441–54.
Bei Y, Zhou Q, Fu S, Lv D, Chen P, Chen Y, Wang F, Xiao J. Cardiac telocytes and fibroblasts in primary culture: different morphologies and immunophenotypes. PLoS One. 2015;10(2):e0115991.
Bojin FM, Gavriliuc OI, Cristea MI, Tanasie G, Tatu CS, Panaitescu C, Paunescu V. Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med. 2011;15:2269–72.
Bollini S, Smart N, Riley PR. Resident cardiac progenitor cells: at the heart of regeneration. J Mol Cell Cardiol. 2011;50:296–303.
Bollini S, Vieira JMN, Howard S, Dubè KN, Balmer GM, Smart N, Riley PR. Re-activated adult epicardial progenitor cells are a heterogeneous population molecularly distinct from their embryonic counterparts. Stem Cells Dev. 2014;23:1719–30.
Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011;15:2594–600.
Ceafalan L, Gherghiceanu M, Popescu LM, Simionescu O. Telocytes in human skin – are they involved in skin regeneration? J Cell Mol Med. 2012;16:1405–20.
Ceafalan LC, Popescu BO, Hinescu ME. Cellular players in skeletal muscle regeneration. Biomed Res Int. 2014:957014.
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining embryonic stem cells. Cell. 2003;113:643–55.
Chang Y, Li C, Gan L, Li H, Guo Z. Telocytes in the Spleen. PLoS One. 2015; 23:10(9):e0138851.
Cretoiu SM, Popescu LM. Telocytes revisited. Biomol Concepts. 2014;5(5):353–69.
Cretoiu D, Hummel E, Zimmermann H, Gherghiceanu M, Popescu LM. Human cardiac telocytes: 3D imaging by FIB-SEM tomography. J Cell Mol Med. 2014;18(11):2157–64.
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67.
Díaz-Flores L, Gutiérrez R, García MP, Sáez FJ, Díaz-Flores Jr L, Valladares F, Madrid JF. CD34+ stromal cells/fibroblasts/ fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol. 2014;29:831–70.
Doyonnas R, LaBarge MA, Sacco A, Charlton C, Blau HM. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc Natl Acad Sci. 2004;101:13507–12.
Drummond-Barbosa D. Stem cells, their niches and the systemic environment: an aging network. Genetics. 2008;180:1787–97.
Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9.
Faussone-Pellegrini MS, Bani D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J Cell Mol Med. 2010;14:1061–3.
Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–84.
Galiger C, Kostin S, Golec A, Ahlbrecht K, Becker S, Gherghiceanu M, Popescu LM, Morty RE, Seeger W, Voswinckel R. Phenotypical and ultrastructural features of Oct4-positive cells in the adult mouse lung. J Cell Mol Med. 2014;18:1321–33.
Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7.
Gherghiceanu M, Popescu LM. Heterocellular communication in the heart: electron tomography of telocyte-myocyte junctions. J Cell Mol Med. 2011;15(4):1005–11.
Gherghiceanu M, Popescu LM. Cardiac telocytes – their junctions and functional implications. Cell Tissue Res. 2012;348:265–79.
Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–4.
Horch RE, Boos AM, Quan Y, Bleiziffer O, Detsch R, Boccaccini AR, Alexiou C, Sun J, Beier JP, Arkudas A. Cancer research by means of tissue engineering–is there a rationale? J Cell Mol Med. 2013;17:1197–206.
Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–56.
Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012;49(3):508–23.
Kandilis AN, Koskinas J, Tiniakos DG, Nikiteas N, Perrea DN. Liver regeneration: focus on cell types and topographic differences. Eur Surg Res. 2010;44:1–12.
Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21.
Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31.
LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601.
Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci. 2003;100:9958–61.
Li L, Lin M, Li L, Wang R, Zhang C, Qi G, Xu M, Rong R, Zhu T. Renal telocytes contribute to the repair of ischemically injured renal tubules. J Cell Mol Med. 2014;18:1144–56.
Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11–5.
Luesma MJ, Gherghiceanu M, Popescu LM. Telocytes and stem cells in limbus and uvea of mouse eye. J Cell Mol Med. 2013;17:1016–24.
Manetti M, Guiducci S, Ruffo M, Rosa I, Faussone-Pellegrini MS, Matucci-Cerinic M, Ibba-Manneschi L. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013;17(4):482–96.
Manetti M, Rosa I, Messerini L, Guiducci S, Matucci-Cerinic M, Ibba-Manneschi L. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. J Cell Mol Med. 2014;18:253–62.
Manole CG, Cismaşiu V, Gherghiceanu M, Popescu LM. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15:2284–96.
Miao Q, Shim W, Tee N, Lim SY, Chung YY, Ja KP, Ooi TH, Tan G, Kong G, Wei H, Lim CH, Sin YK, Wong P. iPSC-derived human mesenchymal stem cells improve myocardial strain of infarcted myocardium. J Cell Mol Med. 2014;18:1644–54.
Mitchell KJ, Pann_erec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a nonsatellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–66.
Ordonez P, Di Girolamo N. Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells. 2012;30:100–7.
Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83(11):1484–94.
Pinnamaneni N, Funderburgh JL. Concise review: stem cells in the corneal stroma. Stem Cells. 2012;30:1059–63.
Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40.
Popescu LM, Nicolescu MI (2013) Telocytes and stem cells. In: Goldenberg RC dos S, Carvalho ACC de, editors. Resid Stem Cells Regen Ther. Oxford:Academic Press; 205–231.
Popescu LM, Gherghiceanu M, Cretoiu D, Radu E. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30.
Popescu LM, Gherghiceanu M, Manole CG, Faussone-Pellegrini MS. Cardiac renewing: interstitial Cajal- like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13(5):866–86.
Popescu LM, Gherghiceanu M, Kostin S. Telocytes and heart renewing. In: Wang P, Kuo CH, Takeda N, Singal PK, editors. Adaptation biology and medicine, vol 6. Cell adaptations and challenges. New Delhi: Narosa; 2011. p. 17–39.
Popescu LM, Gherghiceanu M, Suciu LC, Manole CG, Hinescu ME. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403.
Popescu LM, Manole E, Serboiu CS, Manole CG, Suciu LC, Gherghiceanu M, Popescu BO. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92.
Popescu BO, Gherghiceanu M, Kostin S, Ceafalan L, Popescu LM. Telocytes in meninges and choroid plexus. Neurosci Lett. 2012;516:265–9.
Popescu LM, Curici A, Wang E, Zhang H, Hu S, Gherghiceanu M. Telocytes and putative stem cells in ageing human heart. J Cell Mol Med. 2015;19(1):31–45.
Popescu LM, Fertig ET, Gherghiceanu M. Reaching out: junctions between cardiac telocytes and cardiac stem cells in culture. J Cell Mol Med. 2016;20(2):370–80.
Roatesi I, Radu BM, Cretoiu D, Cretoiu SM. Uterine telocytes: a review of current knowledge. Biol Reprod. 2015;93(1):10.
Rusu MC, Mirancea N, Manoiu VS, Vâlcu M, Nicolescu MI, Păduraru D. Skin telocytes. Ann Anat. 2012;194(4):359–67.
Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–64.
Sheng J, Shim W, Lu J, Lim SY, Ong BH, Lim TS, Liew R, Chua YL, Wong P. Electrophysiology of human cardiac atrial and ventricular telocytes. J Cell Mol Med. 2014;18:355–62.
Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–30.
Suciu L, Nicolescu MI, Popescu LM. Cardiac telocytes: serial dynamic images in cell culture. J Cell Mol Med. 2010;14:2687–92.
Suciu L, Popescu LM, Gherghiceanu M, Regalia T, Nicolescu MI, Hinescu ME, Faussone-Pellegrini MS. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39.
Suciu LC, Popescu BO, Kostin S, Popescu LM. Platelet-derived growth factor receptor-β-positive telocytes in skeletal muscle interstitium. J Cell Mol Med. 2012;16:701–7.
Sun TT, Cotsarelis G, Lavker RM. Hair follicular stem cells: the bulge-activation hypothesis. J Invest Dermatol. 1991;96:77S–8.
Vannucchi MG, Bani D, Faussone-Pellegrini MS. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr Stem Cell Res Ther. 2016;11(5):383–9.
Wagner-Souza K, Diamond HR, Ornellas MH, Gomes BE, Almeida-Oliveira A, Abdelhay E, Bouzas LF, Rumjanek VM. Rhodamine 123 efflux in human subpopulation of hematopoietic stem cells: comparison between bone marrow, umbilical cord blood and mobilized peripheral blood CD34+ cells. Int J Mol Med. 2008;22:237–42.
Wang F, Song Y, Bei Y, Zhao Y, Xiao J, Yang C. Telocytes in liver regeneration: possible roles. J Cell Mol Med. 2014;18:1720–6.
Wang F, Bei Y, Zhao Y, Song Y, Xiao J, Yang C. Telocytes in pregnancy-induced physiological liver growth cell. Physiol Biochem. 2015;36:250–8.
Wong VW, Levi B, Rajadas J, Longaker MT, Gurtner GC. Stem cell niches for skin regeneration. Int J Biomater. 2012;2012:926059.
Xiao J, Wang F, Liu Z, Yang C. Telocytes in liver: electron microscopic and immunofluorescent evidence. J Cell Mol Med. 2013;17:1537–42.
Xue Y, Chen F, Zhang D, Lim S, Cao Y. Tumor-derived VEGF modulates hematopoiesis. J Angiogenes Res. 2009;1:9.
Yang Y, Sun W, Wu SM, Xiao J, Kong X. Telocytes in human heart valves. J Cell Mol Med. 2014;18:759–65.
Zhang HQ, Lu SS, Xu T, Feng YL, Li H, Ge JB. Morphological evidence of telocytes in mice aorta. Chin Med J (Engl). 2015;128(3):348–52.
Zhao B, Chen S, Liu J, Yuan Z, Qi X, Qin J, Zheng X, Shen X, Yu Y, Qnin TJ, Chan JY, Cai D. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J Cell Mol Med. 2013;17:123–33.
Zhao B, Liao Z, Chen S, Yuan Z, Yilin C, Lee KK, Qi X, Shen X, Zheng X, Quinn T, Cai D. Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats. J Cell Mol Med. 2014;18:780–9.
Zheng Y, Zhang M, Qian M, Wang L, Cismasiu VB, Bai C, Popescu LM, Wang X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol Med. 2013;17(4):567–77.
Zheng Y, Cretoiu D, Yan G, Cretoiu SM, Popescu LM, Fang H, Wang X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J Cell Mol Med. 2014;18:1035–59.
Zhou J, Zhang Y, Wen X, Cao J, Li D, Lin Q, Wang H, Liu Z, Duan C, Wu K, Wang C. Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment. J Cell Mol Med. 2010;14:2641–5.
Zhou J, Wang Y, Zhu P, Sun H, Mou Y, Duan C, Yao A, Lv S, Wang C. Distribution and characteristics of telocytes as nurse cells in the architectural organization of engineered heart tissues. Sci China Life Sci. 2014;57(2):241–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
El Maadawi, Z.M. (2016). A Tale of Two Cells: Telocyte and Stem Cell Unique Relationship. In: Wang, X., Cretoiu, D. (eds) Telocytes. Advances in Experimental Medicine and Biology, vol 913. Springer, Singapore. https://doi.org/10.1007/978-981-10-1061-3_23
Download citation
DOI: https://doi.org/10.1007/978-981-10-1061-3_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1060-6
Online ISBN: 978-981-10-1061-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)