Abstract
Occupational and chronic exposure to silica particles causes pulmonary fibrosis known as silicosis, which is a typical form of pneumoconiosis. Although the respiratory complications of silicosis include pulmonary tuberculosis, pleurisy, bronchitis, and other disorders such as lung cancer, silicosis (SIL) patients often suffer from complications of autoimmune diseases such as rheumatoid arthritis (RA), systemic sclerosis (SSc), and other forms of these conditions. The mechanisms causing impairment of immunity by silica exposure were considered adjuvant effects of these particles, and we have been investigating the direct effects of particles on immune cells, particularly T cells, and found chronic activation of both responder and regulatory T helper cells. The results of chronic activation indicated that responder T cells obtain resistance against CD95/Fas-mediated apoptosis to survive longer, whereas regulatory T cells become prone to CD95/Fas-mediated apoptosis and die quickly. These findings suggest that the imbalance of these cells may initiate the observed autoimmune disorders. Furthermore, various autoantibodies were detected in the serum of SIL without any symptoms of autoimmune diseases. Further investigation is needed to study the effects of silica on other immune cell types such as Th17, dendritic, and B cells. Adequate preventive tools against the progression of immunological impairments are also required to improve occupational health.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Silica Exposure and Pulmonary Diseases
Silica-exposed patients suffer from pulmonary fibrosis known as silicosis, a typical form of pneumoconiosis [1–5]. Although silicosis is divided clinically into chronic, subacute, and acute forms, simple silicosis is characterized by the presence of small rounded shadows, which usually appear in the upper lobes and later are observed throughout the lungs [1–5]. This is a classic disease historically and was noted in the era of Hippocrates, as well as Ramazzini and Agricola in their books De Morbis Artificum Diatriba and De Re Metallica [1–3]. They wrote that miners developed dyspnea and suggested a positive relationship between rock-dust exposure and dyspnea. The occupational sources of exposure are mining, quarrying (granite, sandstone, slate, pumice, pumicite), tunneling, stone working, activities involving abrasives, glass manufacture, the agate industry and glass manufacture, the refractory brick industry, and many other sources [1–6].
The pulmonary complications of silicosis are typically tuberculosis, as well as pleuritis, pneumothorax, bronchitis, bronchiectasis, and lung cancer. Interestingly, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) categorized crystalline silica as a group I carcinogen when inhaled in the form of quartz and cristobalite from occupational sources [7–10].
Basically, there are no curative procedures for chronic progression of lung fibrosis designated as silicosis, and these patients suffer from dyspnea and other pulmonary symptoms [1–5].
2.2 Immune Complications
In addition to the pulmonary complications mentioned above, SIL suffer from autoimmune disorders [1–3, 11–15]. Caplan’s syndrome [16, 17] and rheumatoid arthritis (RA) are well-known complications of silicosis. Systemic sclerosis (SSc) [18–21] and systemic lupus erythematosus (SLE) [22–24] are also known as frequent complications of silicosis, although SLE appears to be limited to workers associated with sandblasting, grinding, and the handling of silica for use as a scouring powder or in patients with acute or accelerated silicosis [1–3]. More recently, anti-neutrophil cytoplasmic antibody (ANCA)-related vasculitis/nephritis have been reported as complications in SIL [25–28].
Basically, the cellular mechanisms responsible for the dysregulation of autoimmunity caused by silica exposure have been regarded as an adjuvant effect of the silica particles [29–31]. There are many self-antigens such as proteins, amino acids, and nucleotides derived from cell debris yielded by physiological cell apoptosis and other processes in the body. These candidate antigens may bind with the silica particles as adjuvants and subsequently exert strong antigenicity to induce the autoimmune diseases observed in SIL.
However, since inhaled silica particles remain in the lung and lymph nodes, these particles may recurrently and chronically encounter circulating immune cells [1–5]. It is therefore reasonable to consider the possible direct effects of these inhaled silica particles on the peripheral immune cells of the human body.
2.3 Alteration of Immune Cells and Functions
2.3.1 Responder T Cell Activation Caused by Silica
Although it is commonly thought that immunotoxicity may kill immune-competent cells through the action of certain substances, our investigations have revealed excess immune stimulation or reduction of certain immune levels due to environmental factors such as silica particles to result in representative phenotypes of immunotoxicity when considering that immune systems should remain physiologically neutral. Excess immune reactions cause allergies and autoimmune disorders, and a reduced immunological state may result in impairment of tumor immunity and transplantation immunity. Thus, a consideration of immune impairments caused by environmental substances suggests that altered activation and/or reduced function of immune cells should be regarded as an indication of immunotoxicity in the broad sense of the term.
In vitro experiments were performed initially to determine whether silica particles produced immunotoxic effects on human peripheral T cells [32]. When peripheral blood mononuclear cells (PBMCs) were cultured with or without silica particles, only T cells in PBMC gradually expressed CD69 as an early activation marker of T cells for 4–10 days, although the increased expression of CD69 was slower than that of standard mitogen such as phytohemagglutinin (PHA), which immediately activated T cells within 24 h followed by cessation within 2 or 3 days. Actually, chrysotile asbestos fibers did not allow T cells to express CD69 [32].
In addition, PD-1 expression as one of the chronically activated T cell markers was examined using peripheral blood T cells (CD4+ and CD25+ or CD4+ CD25- fractions) from SIL and compared with that of healthy volunteers (HV). Both fractions derived from SIL showed a significantly higher expression of PD-1 than that of HV [33].
Since the concentration of serum soluble interleukin (IL)-2 receptor (sIL-2R) has recently been considered an activation marker of T cells in autoimmune diseases, in addition to tumor biomarker for T cell leukemia and lymphoma [34, 35], serum sIL-2R levels in HV, SIL, and SSc were measured and compared [36]. Results revealed that sIL-2R levels in SSc were significantly higher than those in HV, and levels in SIL tended to be higher than those in HV. In addition, the level of sIL-2R in SIL was correlated with antinuclear antibody (ANA) titer, immunoglobulin (Ig) G, and anti-centromere/CENP-B antibody titer. Furthermore, factor analysis indicated that sIL-2R in SIL contributed to the subpopulation with a poorer immunological status without much impairment of respiratory parameters [36]. Thus, sIL-2R was also considered an indicator of the chronically activated status of T cells in SIL.
The overall findings suggested that responder T cells in SIL are chronically activated by recurrent encounters with silica particles in the circulation.
2.3.2 Inhibitory Molecules for CD95/Fas-Mediated Apoptosis in SIL
As mentioned above, responder T cells circulating in the peripheral blood of SIL are activated chronically. However, the reactions of activated T cells usually cease due to activation-induced cell death (AICD) mediated by CD95/Fas death receptor via autocrine or paracrine binding with Fas ligand produced by similarly activated T cells [37–39]. Examination of autoimmune diseases revealed that HV had lower serum levels of soluble Fas (sFas), which lacks the transmembrane domain of the Fas molecule as an alternatively spliced variant and is secreted from cells for binding with the auto- or para-produced Fas ligand at extracellular spaces to prevent Fas-mediated apoptosis/AICD [40–43]. If the chronically activated T cells in these diseases, which are recognizing the self-antigens, are avoiding AICD/Fas-mediated apoptosis, these self-recognizing T cells may survive longer and cause continuous impairment of autoimmunity to result in autoimmune disorders that last longer and progress to worsening states.
Serum sFas levels in SIL were also elevated, and examination of mRNA expression in PBMC revealed excess expression of the sFas message, which lacks the 63 bp of the transmembrane domain relative to the wild-type membrane and binds the Fas molecule [44, 45]. In addition, PBMC from SIL showed a higher expression relative to HV of other alternatively spliced variants of the Fas gene, all of which conserved the Fas-ligand binding domain but lacked the transmembrane domain [46]. Most of these variants may act in a manner similar to the typical variant, the sFas molecule, to prevent Fas-mediated apoptosis.
Similar to sFas, decoy receptor 3 (DcR3) was also found to prevent TNF-related apoptosis-inducing ligand (Trail)-mediated apoptosis by binding with Trail at extracellular spaces [47–49]. DcR3 molecules were first reported to be produced by lung and colon cancer cells for self-prevention from tumor attacking Trail secreted by T cells bearing tumor immunity [50]. Thereafter, serum DcR3 levels were found to be higher in various autoimmune diseases. In our analysis, PBMC from SIL showed a higher DcR3 expression level than that of HV [51]. We have been trying recently to measure serum DcR3 levels, as well as determine the role of DcR3 levels in the immunological pathophysiological status of SIL. We will report the findings of these investigations in the near future.
The overall results indicate that responder T cells chronically activated by silica particles avoid AICD/Fas-mediated apoptosis by self-producing inhibitory molecules.
2.3.3 Regulatory T Cells in Silicosis Peripheral Blood
When we consider the T cell status that tends to progress to autoimmune disorders, the balance between responder T cells and regulatory T cells (Treg) defined by CD4+ CD25+ and forkhead box P3 (FoxP3) transcription factor positive is important, and reduction of function and/or number of Treg may prolong the activation of responder T cells from various non-self or self-antigens and cause autoimmune disorders [52, 53]. In addition, T helper 17 (Th17) cells are also important in the formation of autoimmune disorders [54–56]. Cytokine levels, particularly those of IL-6 and transforming growth factor (TGF-)-β, influence the polarization between Treg and Th17 cells [57–59]. Although we have not investigated Th17 alteration following exposure to silica particles, we found an interesting change in Treg following exposure to silica particles, as well as alteration of peripheral Treg from SIL [60].
We first examined Treg function derived from the CD4+CD25+ fraction of SIL and compared it with that of HV [60]. Although the examined SIL did not show any symptoms of autoimmune diseases, the suppressive function that reduces the antigen-induced proliferation in responder (CD4+CD25-) T cells induced by a cocultured peripheral blood CD4+CD25+ fraction was reduced in SIL compared to HV. This result indicated that Treg function in the peripheral CD4+CD25+ fraction of SIL may be reduced or weakened by chronic exposure to silica particles [60].
Treg from SIL and HV showed higher CD95/Fas expression compared with that in responder T cells of the CD4+CD25- fraction [33]. However, it was significantly higher in SIL compared to HV. Since CD95/Fas expression in Treg is known as an activation marker for this cell type, chronic silica exposure may stimulate Treg in a manner similar to that of responder T cells as mentioned above. The results showing chronic activation of Treg with excess expression of CD95/Fas may indicate they are sensitive to Fas-mediated apoptosis [33]. In fact, Treg from SIL proceeded toward apoptosis more rapidly and with greater amounts compared to Treg from HV when these cells were cultured with agonistic anti-Fas antibody. Furthermore, cultures with silica particles and PBMCs from HV revealed that the CD4+CD25+ fraction as a marker of Treg and activated responder T cells did not change remarkably; however, the level of FoxP3+ in CD4+ decreased significantly during the culture period [33]. These results indicate that Treg in SIL are highly sensitive to Fas-mediated apoptosis and can be killed easily and may be recruited from the bone marrow [33].
The overall findings show that silica exposure chronically activates responder T cells and Treg. The former are resistant to Fas-mediated apoptosis and survive longer, whereas the latter are sensitive and progress toward cell death. An imbalance between these two types of T cells then occurs in SIL and subsequently results in the germination of impaired autoimmunity.
2.3.4 Autoantibodies in SIL
We have reported various autoantibodies that were found in SIL. For example, anti-CD95/Fas autoantibody was found in approximately one fourth of SIL [61]. This autoantibody was functional, i.e., when this autoantibody binds with membrane Fas, the cells proceed toward apoptosis. This was confirmed using sister human myeloma cell lines from pleural effusion and the bone marrow derived from the same patients [61, 62]. The cell line from the effusion showed scant Fas expression, whereas the line from the bone marrow exhibited strong expression. Additionally, the former line was not killed by serum from anti-Fas autoantibody positive SIL, but cells in the latter line died. These results indicated that Treg in SIL who are positive for this autoantibody are much more sensitive and likely to be killed.
We have also reported anti-caspase 8 [63, 64] and anti-desmoglein autoantibodies [65] in SIL. Moreover, anti-Scl-70 (topoisomerase I) autoantibody was detected in SIL [66, 67]. These autoantibodies are clinically significant and are often detected in SSc. The anti-Scl-70 autoantibody is seen in the diffuse SSc type with organ fibrosis such as the lung, whereas the anti-CENP-B (centromere) autoantibody is observed in the limited type of SSc with lesions in the esophagus and involving pulmonary hypertension. The clinical status of both antibodies was assayed in SIL patients that did not exhibit any symptoms of SSc. Recently, the titer index (Log10) of anti-CENP-B autoantibody in SIL was higher than that of HV, and that of SSc was higher than those of HV and SIL. This titer index was positively correlated with an assumed immune status of one for HV, two for SIL, and three for SSc. Moreover, factor analysis of SIL cases revealed that although the titer index of anti-CENP-B autoantibody formed the same factor with that of anti-Scl-70 autoantibody, the Ig G value and the age of patients and other factors extracted showed that the Ig A value and anti-Scl-70 antibody were positively related, but anti-CENP-B showed an opposite tendency [68]. These results indicated that the titer index of anti-CENP-B autoantibody may be a biomarker for dysregulation in SIL cases.
Our findings suggest that B cells, as the producer of autoantibodies, may be affected by chronic silica exposure and an alteration caused by long-surviving responder T cells. In addition, dendritic cells as the initial antigen-presenting cell may have their function modified by chronic exposure to silica, although we have not performed a detailed analysis of the cellular and molecular alterations of these cells following silica exposure.
2.4 Conclusion
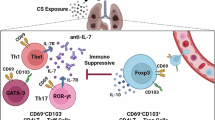
All the findings described above are summarized schematically in Fig. 2.1. Silica exposure causes a form of pulmonary fibrosis known as silicosis, a condition that progresses gradually to reduce the health of affected individuals. In addition, the pulmonary complications observed with this condition sometimes result in a severe pathological status, particularly when involving tuberculosis and lung cancer, which further burdens SIL patients with a difficult clinical course. However, silica particles not only cause respiratory impairments, but also immunological disorders, especially those involving autoimmune diseases [68–74]. Based on the adjuvant effect of silica particles, our investigations have elucidated the direct action of silica on immune-competent cells. Silica chronically activates responder and regulatory T cells to result in an imbalance of these two types of T cells, which makes individuals more prone to developing autoimmune disorders. Once autoimmune impairments appear, the pathological status also progresses and worsens gradually, never to return to the previous unimpaired condition. Although future studies are required regarding silica’s direct effects on Th17, dendritic, and B cells, investigations of preventive procedures using physiologically active substances or chemicals from plants and foods are necessary to inhibit the subclinical progression of immunological impairments and improve occupational health.
References
Weill H, Jones RN, Raymond Parkes W. Silicosis and related diseases. In: Raymond Parkes W, editor. Occupational lung disorders. 3rd ed. Oxford: Butterworth-Heinemann Ltd; 1994. p. 285–339.
Weissman DN, Banks DE. Silicosis. In: Schwarz MI, King Jr TE, editors. Interstitial lung disease. 4th ed. Hamilton: BC Decker; 2003. p. 387–401.
Kelley J. Occupational lung diseases caused by asbestos, silica, and other silicates. In: Baum GL, Crapo JD, Celli BR, Karlinsky JB, editors. Textbook of pulmonary diseases, vol. 1. 6th ed. Philadelphia: Lippincott-Raven Publishers; 1998. p. 659–82.
Cullinan P, Reid P. Pneumoconiosis. Prim Care Respir J. 2013;22(2):249–52. doi:10.4104/pcrj.2013.00055.
Leung CC, Yu IT, Chen W. Silicosis. Lancet. 2012;379(9830):2008–18. doi:10.1016/S0140-6736(12)60235-9.
Rees D, Murray J. Silica, silicosis and tuberculosis. Int J Tuberc Lung Dis. 2007;11(5):474–84.
Silica, some silicates, coal dust and para-aramid fibrils. In: IARC monographs on the evaluation of carcinogenic risks to humans. Volume 68. Lyon: WHO Press. 1997.
Steenland K, Ward E. Silica: a lung carcinogen. CA Cancer J Clin. 2014;64(1):63–9. doi:10.3322/caac.21214.
Pelucchi C, Pira E, Piolatto G, Coggiola M, Carta P, La Vecchia C. Occupational silica exposure and lung cancer risk: a review of epidemiological studies 1996–2005. Ann Oncol. 2006;17(7):1039–50.
Finkelstein MM. Silica, silicosis, and lung cancer: a risk assessment. Am J Ind Med. 2000;38(1):8–18.
Uber CL, McReynolds RA. Immunotoxicology of silica. Crit Rev Toxicol. 1982;10(4):303–19.
Steenland K, Goldsmith DF. Silica exposure and autoimmune diseases. Am J Ind Med. 1995;28(5):603–8.
Mayes MD. Epidemiologic studies of environmental agents and systemic autoimmune diseases. Environ Health Perspect. 1999;107 Suppl 5:743–8.
Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107 Suppl 5:793–802.
Hess EV. Environmental chemicals and autoimmune disease: cause and effect. Toxicology. 2002;181–182:65–70.
Caplan A. Certain unusual radiological appearances in the chest of coal-miners suffering from rheumatoid arthritis. Thorax 8(1): 29–37. doi:10.1136/thx.8.1.29.
Caplan A. Rheumatoid disease and pneumoconiosis (Caplan’s syndrome). Proc R Soc Med. 1959;52:1111–3.
Siltzbach LE. Diffuse pulmonary granulomatosis and fibroses. Mod Treat. 1964;15:290–306.
Rodnan GP, Benedek TG, Medsger Jr TA, Cammarata RJ. The association of progressive systemic sclerosis (scleroderma) with coal miners’ pneumoconiosis and other forms of silicosis. Ann Intern Med. 1967;66(2):323–34.
Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C. Silica-induced scleroderma. J Am Acad Dermatol. 1990;22(3):444–8.
Haustein UF, Anderegg U. Silica induced scleroderma – clinical and experimental aspects. J Rheumatol. 1998;25(10):1917–26.
Sanchez-Roman J, Wichmann I, Salaberri J, Varela JM, Nuñez-Roldan A. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann Rheum Dis. 1993;52(7):534–8.
Koeger AC, Lang T, Alcaix D, Milleron B, Rozenberg S, Chaibi P, Arnaud J, Mayaud C, Camus JP, Bourgeois P. Silica-associated connective tissue disease. A study of 24 cases. Medicine (Baltimore). 1995;74(5):221–37.
D’Cruz D. Autoimmune diseases associated with drugs, chemicals and environmental factors. Toxicol Lett. 2000;112–113:421–32.
Gregorini G, Tira P, Frizza J, D’Haese PC, Elseviers MM, Nuyts G, Maiorca R, De Broe ME. ANCA-associated diseases and silica exposure. Clin Rev Allergy Immunol. 1997;15(1):21–40.
Tervaert JW, Stegeman CA, Kallenberg CG. Silicon exposure and vasculitis. Curr Opin Rheumatol. 1998;10(1):12–7.
Mulloy KB. Silica exposure and systemic vasculitis. Environ Health Perspect. 2003;111(16):1933–8.
Bartůnková J, Pelclová D, Fenclová Z, Sedivá A, Lebedová J, Tesar V, Hladíková M, Klusácková P. Exposure to silica and risk of ANCA-associated vasculitis. Am J Ind Med. 2006;49(7):569–76.
Levine S, Sowinski R. Enhancement of allergic encephalomyelitis by particulate adjuvants inoculated long before antigen. Am J Pathol. 1980;99(2):291–304.
Stone OJ. Autoimmunity as a secondary phenomenon in scleroderma (and so-called human adjuvant disease). Med Hypotheses. 1991;34(2):127–30.
Rao TD, Frey AB. Administration of silica sensitizes lipopolysaccharide responsiveness of murine macrophages but inhibits T and B cell priming by inhibition of antigen presenting function. Immunol Investig. 1998;27(3):181–99.
Wu P, Hyodoh F, Hatayama T, Sakaguchi H, Hatada S, Miura Y, Takata-Tomokuni A, Katsuyama H, Otsuki T. Induction of CD69 antigen expression in peripheral blood mononuclear cells on exposure to silica, but not by asbestos/chrysotile-A. Immunol Lett. 2005;98(1):145–52.
Hayashi H, Miura Y, Maeda M, Murakami S, Kumagai N, Nishimura Y, Kusaka M, Urakami K, Fujimoto W, Otsuki T. Reductive alteration of the regulatory function of the CD4(+)CD25(+) T cell fraction in silicosis patients. Int J Immunopathol Pharmacol. 2010;23(4):1099–109.
Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediat Inflamm. 2005;2005(3):121–30.
Murakami S. Soluble interleukin-2 receptor in cancer. Front Biosci. 2004;9:3085–90.
Hayashi H, Maeda M, Murakami S, Kumagai N, Chen Y, Hatayama T, Katoh M, Miyahara N, Yamamoto S, Yoshida Y, Nishimura Y, Kusaka M, Fujimoto W, Otsuki T. Soluble interleukin-2 receptor as an indicator of immunological disturbance found in silicosis patients. Int J Immunopathol Pharmacol. 2009;22(1):53–62.
Kabelitz D, Janssen O. Antigen-induced death of T-lymphocytes. Front Biosci. 1997;2:d61–77.
Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol Cell Biol. 2002;80(2):131–7.
Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81.
Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263(5154):1759–62.
Tokano Y, Miyake S, Kayagaki N, Nozawa K, Morimoto S, Azuma M, Yagita H, Takasaki Y, Okumura K, Hashimoto H. Soluble Fas molecule in the serum of patients with systemic lupus erythematosus. J Clin Immunol. 1996;16(5):261–5.
Jodo S, Kobayashi S, Kayagaki N, Ogura N, Feng Y, Amasaki Y, Fujisaku A, Azuma M, Yagita H, Okumura K, Koike T. Serum levels of soluble Fas/APO-1 (CD95) and its molecular structure in patients with systemic lupus erythematosus (SLE) and other autoimmune diseases. Clin Exp Immunol. 1997;107(1):89–95.
Nozawa K, Kayagaki N, Tokano Y, Yagita H, Okumura K, Hasimoto H. Soluble Fas (APO-1, CD95) and soluble Fas ligand in rheumatic diseases. Arthritis Rheum. 1997;40(6):1126–9.
Tomokuni A, Aikoh T, Matsuki T, Isozaki Y, Otsuki T, Kita S, Ueki H, Kusaka M, Kishimoto T, Ueki A. Elevated soluble Fas/APO-1 (CD95) levels in silicosis patients without clinical symptoms of autoimmune diseases or malignant tumours. Clin Exp Immunol. 1997;110(2):303–9.
Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, Kawakami Y, Kusaka M, Ueki H, Kita S, Ueki A. Soluble Fas mRNA is dominantly expressed in cases with silicosis. Immunology. 1998;94(2):258–62.
Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, Isozaki Y, Hyodoh F, Kawakami Y, Kusaka M, Kita S, Ueki A. Detection of alternatively spliced variant messages of Fas gene and mutational screening of Fas and Fas ligand coding regions in peripheral blood mononuclear cells derived from silicosis patients. Immunol Lett. 2000;72(2):137–43.
Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274(20):13733–6.
Lin WW, Hsieh SL. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem Pharmacol. 2011;81(7):838–47. doi:10.1016/j.bcp.2011.01.011.
Siakavellas SI, Sfikakis PP, Bamias G. The TL1A/DR3/DcR3 pathway in autoimmune rheumatic diseases. Semin Arthritis Rheum. 2015;45(1):1–8. doi:10.1016/j.semarthrit.2015.02.007.
Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, Godowski PJ, Wood WI, Gurney AL, Hillan KJ, Cohen RL, Goddard AD, Botstein D, Ashkenazi A. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396(6712):699–703.
Otsuki T, Tomokuni A, Sakaguchi H, Aikoh T, Matsuki T, Isozaki Y, Hyodoh F, Ueki H, Kusaka M, Kita S, Ueki A. Over-expression of the decoy receptor 3 (DcR3) gene in peripheral blood mononuclear cells (PBMC) derived from silicosis patients. Clin Exp Immunol. 2000;119(2):323–7.
Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32.
Takahashi T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in maintaining immunologic self-tolerance and preventing autoimmune disease. Curr Mol Med. 2003;3(8):693–706.
Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148(1):32–46.
Chen Z, O’Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41(2):87–102. doi:10.1007/s12026-007-8014-9.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi:10.1146/annurev.immunol.021908.132710.
O’Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin Exp Immunol. 2010;159(2):137–47. doi:10.1111/j.1365-2249.2009.04040.x.
Girtsman T, Jaffar Z, Ferrini M, Shaw P, Roberts K. Natural Foxp3(+) regulatory T cells inhibit Th2 polarization but are biased toward suppression of Th17-driven lung inflammation. J Leukoc Biol. 2010;88(3):537–46. doi:10.1189/jlb.0110044.
Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121(13):2402–14. doi:10.1182/blood-2012-09-378653.
Wu P, Miura Y, Hyodoh F, Nishimura Y, Hatayama T, Hatada S, Sakaguchi H, Kusaka M, Katsuyama H, Tomita M, Otsuki T. Reduced function of CD4+25+ regulatory T cell fraction in silicosis patients. Int J Immunopathol Pharmacol. 2006;19(2):357–68.
Takata-Tomokuni A, Ueki A, Shiwa M, Isozaki Y, Hatayama T, Katsuyama H, Hyodoh F, Fujimoto W, Ueki H, Kusaka M, Arikuni H, Otsuki T. Detection, epitope-mapping and function of anti-Fas autoantibody in patients with silicosis. Immunology. 2005;116(1):21–9.
Ohtsuki T, Yawata Y, Wada H, Sugihara T, Mori M, Namba M. Two human myeloma cell lines, amylase-producing KMS-12-PE and amylase-non-producing KMS-12-BM, were established from a patient, having the same chromosome marker, t(11;14)(q13;q32). Br J Haematol. 1989;73(2):199–204.
Ueki A, Isozaki Y, Tomokuni A, Hatayama T, Ueki H, Kusaka M, Shiwa M, Arikuni H, Takeshita T, Morimoto K. Intramolecular epitope spreading among anti-caspase-8 autoantibodies in patients with silicosis, systemic sclerosis and systemic lupus erythematosus, as well as in healthy individuals. Clin Exp Immunol. 2002;129(3):556–61.
Ueki A, Isozaki Y, Kusaka M. Anti-caspase-8 autoantibody response in silicosis patients is associated with HLA-DRB1, DQB1 and DPB1 alleles. J Occup Health. 2005;47(1):61–7.
Ueki H, Kohda M, Nobutoh T, Yamaguchi M, Omori K, Miyashita Y, Hashimoto T, Komai A, Tomokuni A, Ueki A. Antidesmoglein autoantibodies in silicosis patients with no bullous diseases. Dermatology. 2001;202(1):16–21.
Ueki A, Isozaki Y, Tomokuni A, Tanaka S, Otsuki T, Kishimoto T, Kusaka M, Aikoh T, Sakaguchi H, Hydoh F. Autoantibodies detectable in the sera of silicosis patients. The relationship between the anti-topoisomerase I antibody response and HLA-DQB1*0402 allele in Japanese silicosis patients. Sci Total Environ. 2001;270(1–3):141–8.
Tomokuni A, Otsuki T, Sakaguchi H, Isozaki Y, Hyodoh F, Kusaka M, Ueki A. Detection of anti-topoisomerase I autoantibody in patients with silicosis. Environ Health Prev Med. 2002;7(1):7–10. doi:10.1007/BF02898059.
Maeda M, Nishimura Y, Kumagai N, Hayashi H, Hatayama T, Katoh M, Miyahara N, Yamamoto S, Hirastuka J, Otsuki T. Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol. 2010;7(4):268–78. doi:10.3109/1547691X.2010.512579.
Lee S, Hayashi H, Maeda M, Chen Y, Matsuzaki H, Takei-Kumagai N, Nishimura Y, Fujimoto W, Otsuki T. Environmental factors producing autoimmune dysregulation – chronic activation of T cells caused by silica exposure. Immunobiology. 2012;217(7):743–8. doi:10.1016/j.imbio.2011.12.009.
Lee S, Matsuzaki H, Kumagai-Takei N, Yoshitome K, Maeda M, Chen Y, Kusaka M, Urakami K, Hayashi H, Fujimoto W, Nishimura Y, Otsuki T. Silica exposure and altered regulation of autoimmunity. Environ Health Prev Med. 2014;19(5):322–9. doi:10.1007/s12199-014-0403-9.
Kumagai N, Hayashi H, Maeda M, Miura Y, Nishimura Y, Matsuzaki H, Lee S, Fujimoto W, Otsuki T. Immunological effects of silica and related dysregulation of autoimmunity. In: Mavragani CP, editor. Autoimmune disorders – pathogenetic aspects. Rijeka: InTech Open Access Publisher; 2011. p. 157–74.
Hayashi H, Nishimura Y, Hyodo F, Maeda M, Kumagai N, Miura Y, Kusaka M, Uragami K, Otsuki T. Dysregulation of autoimmunity caused by silica exposure: fas-mediated apoptosis in T lymphocytes derived from silicosis patients. In: Petri M, editor. Autoimmune disorders: symptoms, diagnosis and treatment. Hauppauge: Nova Science Publishers; 2011. p. 293–301.
Takei-Kumagai N, Lee S, Matsuzaki H, Hayashi H, Maeda M, Nishimura Y, Otsuki T. Silica, immunological effects. In: Uversky VN, Kretsinger RH, Permyakov EA, editors. Encyclopedia of metalloproteins. New York: Springer Science+Business Media; 2013. p. 1956–71.
Lee S, Maeda M, Hayashi H, Matsuzaki H, Kumagai-Takei N, Nishimura Y. Otsuki Immunostimulation by silica particles and the development of autoimmune dysregulation. In: Guy Huynh Thien D, editor. Immune response activation. Rijeka: InTech publisher; 2014. p. 249–65.
Acknowledgments
The authors thank former colleagues in the Department of Hygiene, Kawasaki Medical School, namely, Prof. Ayako Ueki, Drs. Fuminori Hyodoh, Akiko Takata-Tomokuni, Yasuhiko Kawakami, Takaaki Aikoh, Shuko Murakami, and Yoshie Miura. We also appreciate the technical assistance of Ms. Haruko Sakaguchi, Naomi Miyahara, Minako Katoh, and Yumika Isozaki. We express special thanks to Drs. Masayasu Kusaka and Kozo Urakami for coordinating the collection of clinical samples. Part of the experimental results in this article was supported by the Special Coordination Fund for Promoting Science and Technology (H18-1-3-3-1, “Comprehensive approach on asbestos-related diseases”), KAKENHI grants (18390186, 19659153, 20390178, and 25460825), Kawasaki Medical School Project grants (20-410I, 23S5, 24S6, 25B65, and 27B06), the Sumitomo Foundation Grant (053027), the Yasuda Memorial Foundation Grant (H18), funding from the Takeda Science Foundation (I-2008) and Young Investigator Activating Grant from the Japanese Society of Hygiene (H18), the Ryobi Teien Memorial Foundation (H24), and the Kawasaki Foundation for Medical Science and Medical Welfare (H24).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Lee, S. et al. (2017). Silica-Induced Immunotoxicity: Chronic and Aberrant Activation of Immune Cells. In: Otsuki, T., Petrarca, C., Di Gioacchino, M. (eds) Allergy and Immunotoxicology in Occupational Health. Current Topics in Environmental Health and Preventive Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-10-0351-6_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-0351-6_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-0349-3
Online ISBN: 978-981-10-0351-6
eBook Packages: MedicineMedicine (R0)