Abstract
Transient receptor potential canonical (TRPC) channels mediate the influx of different types of cations through the cell membrane and are involved in many functions of the organism. Evidences of involvement of TRPC channels in neuronal development suggest that this family of proteins might play a role in certain neurological disorders. As reported, knockout mice for different TRPC channels show alterations in neuronal morphological and functional parameters, with behavioral abnormalities, such as in exploratory and social behaviors. Although mutations in TRPC channels could be related to mental/neurological disorders, there are only a few cases reported in literature, indicating that this correlation should be further explored. Nonetheless, other functional evidences support the implication of these channels in neurological diseases. In this chapter, we summarize the main findings relating TRPC channels to neurological disorders, such as autism spectrum disorders, bipolar disorder, and intellectual disability among others.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Nervous system development is an intricate process that involves a series of steps, including proliferation of progenitor cells, cellular migration, neurite development and guidance, and establishment of synapses. All these steps have to be strictly regulated in order to enable the correct configuration of neuronal networks and adequate brain functioning. Disruption in such processes can lead to abnormal development or functioning of neuronal circuits, contributing to abnormal regulation of neuronal plasticity, which ultimately results in psychiatry/neurological diseases.
The TRPC channels have important roles in different cellular processes in several tissues, including nervous system. They are divided in three main groups according to their sequence similarity: TRPC1/4/5, TRPC3/6/7, and TRPC2 which, in humans, is actually a pseudogene. TRPC channels are highly expressed in the brain, and their roles on neuronal development have been intensively investigated. Some examples of TRPC channels functions are summarized below.
TRPC1 is reported to have an important role in neural progenitor cell proliferation [11, 23, 49, 62]. TRPC3 induces long-term depression in Purkinje cells [42] and participates in the regulation of hippocampal neuronal excitability, affecting the memory formation [59]. In conjunction with TRPC6, TRPC3 affects axonal path finding induced by BDNF [51] and has a protective effect in neuronal survival [34]. Moreover, TRPC channels are also important for synaptogenesis, for example, TRPC proteins could interact with postsynaptic membrane scaffolding proteins [87]. TRPC6 has a role in excitatory synapse formation [90], and both TRPC3 and TRPC6 are necessary for BDNF-induced increase in dendritic spine density [2].
Different TRPC channels even have opposite roles in the regulation of neurite outgrowth. In PC12 cells, TRPC1 induces neurite outgrowth through a mechanism that is independent of Ca2+ influx, while TRPC5 has an opposite effect [31]. Activation of TRPC4 by Gαi2 inhibits neurite growth and dendritic arborization of hippocampal neurons [33]. Neurotrophin-3 induces Ca2+ influx through TRPC5 and inhibits neurite outgrowth in rat hippocampal neurons, while neurotrophin-4 promotes neurite growth through TRPC6 activation [30]. Growth cone collapse induced by Semaphorin-3A is reduced in hippocampal neurons from TRPC5 knockout mice [35]. Finally, TRPC6 promotes dendritic growth via a Ca2+-CaMKIV-dependent mechanism in rat neurons [79]. It is worthy to highlight that most of the function of TRPC in neurons are mediated by Ca2+ influx, although they are nonselective cation channels. In fact, Ca2+ signaling is a crucial mechanism for brain development (reviewed in [73]).
In summary, TRPC channels contribute to the regulation of neuronal development and function. Malfunction of these channels might have an impact on such processes and thus contribute to psychiatric/neurological disorders. Here we enumerate some human disorders for which there are evidences of involvement of TRPC channels in their pathophysiology. For some of them, direct disruption of the sequence of a -trpc gene was found in the patients, while for some other disorders, the relationship between TRPC channels and disease is based on indirect evidences.
12.2 TRPC6 and Autism
Autism spectrum disorders (ASD) are defined as a group of neurodevelopmental disorders characterized by repetitive behaviors as well as impairments in communicative and social interaction skills. Although environmental factors might play a role in ASD etiology, evidences support a major contribution of genetic factors to these disorders. However, these genetic factors are heterogeneous across autistic individuals, and in fact, dozens of genes have already been implicated in ASD.
trpc6 is one of the genes that have been recently implicated in ASD etiology. The relation between trpc6 and ASD is originally based on the work of Griesi-Oliveira et al. [28] that has identified an autistic patient with a chromosomal translocation between chromosomes 3 and 11. Translocation mapping indicated that the sequence of genes vprbp on chromosome 3 and trpc6 on chromosome 11 are disrupted, leading to haploinsufficiency. Previous studies have shown that TRPC6 overexpression in rat neurons leads to an enhancement on dendritic growth, dendritic spine density, and excitatory synapse formation, both in vitro and in vivo [79, 90]. Based on these evidences, the authors invested in the analysis of the consequences of such disruption in the function of TRPC6 and its downstream signaling pathway in the cells of the reported patient. The role of TRPC6 on neuronal development is mainly related to the calcium influx through the channel, leading to CREB phosphorylation [79], which in turn mediates the regulation of expression of many genes. Promoting the activation of the channel using hyperforin, a specific activator of TRPC6, will be discussed later, and authors demonstrated that CREB phosphorylation is reduced in patient’s dental pulp cells compared to control cells. Accordingly, a global expression analysis suggests that a significant number of putative CREB target genes are dysregulated in patient’s dental pulp cells.
Griesi-Oliveira et al. generated induced pluripotent stem cells (iPSC) from the patients and healthy controls through cell reprogramming to investigate trpc6 disruption consequences on targeted cortical neuronal cells derived from the iPSC. Again, after inducing TRPC6 activation with hyperforin, the authors showed that calcium influx through neuronal progenitor cells is reduced in the patient’s sample. In addition, patient’s cortical neurons are found to have a reduced dendritic arborization, shorter neurites, and reduced density of dendritic spines and glutamatergic vesicles compared to controls (cortical neurons derived from non-ASD, healthy individuals). All these abnormalities are in accordance to previous findings on rat neurons overexpressing TRPC6, which have the opposite characteristics [79]. Moreover, the authors perform gain and loss of function experiments in neurons. Reducing TRPC6 expression in control cells leads to the same neuronal alterations seen in the patient’s neurons. On the contrary, complementation of TRPC6 expression in patient’s cells rescues the neuronal phenotypes. These results corroborate that the alterations in patient’s neurons are related to trpc6 disruption and consequent haploinsufficiency. Behavioral studies with TRPC6 knockout mice, although they do not indicate any alteration in social or repetitive behavior, have a reduced exploratory behavior, which is associated to clinical signs of ASD, including the autistic patients used by Griesi and colleagues [8, 28]. Finally, the patient’s neuronal abnormalities is rescued by treating the neurons with hyperforin, raising the hypothesis that autistic patients with trpc6 mutations, or other genes mutated in this pathway, could benefit from this chemical. However, the number of patients with truncating mutations in trpc6 is only around 0.2%. Thus, an effective clinical trial would benefit from a previous stratification of the ASD individuals based on the genetic alterations in trpc6 or its downstream genes. The idea to use genome sequencing and functional tests using cell reprogramming to stratify a heterogeneous population for clinical trials is recently proposed for ASDs [54].
12.3 TRPC Channels, BDNF, and Rett Syndrome
Rett syndrome is an X-linked neurodevelopmental disorder caused mostly by loss-of-function mutations in mecp2 (methyl-CpG-binding protein) gene and affects primarily females. Male patients usually die early in development. The disease is characterized by a period of apparently normal development until 6–18 months of age followed by a progressive regression of developmental and motor skills, seizures, and hypotonia, and the majority of the patients can also present autistic features. MeCP2 protein influences neuronal development in several ways. MeCP2 presents a wide range of actions on transcriptional regulation, through its binding to CpG-methylated islands, where it recruits other transcriptional repressors and histones acetylases. In addition, MeCP2 has been shown to regulate TRPC channels expression in mouse brain, and Ca2+ signals elicited by TRPC3/6 activation are impaired in neurons of male mecp2 mutant mice [10, 50]. Furthermore, MeCP2 is found in association with trpc6 promoter region in human neurons, and in accordance, neurons expressing a loss-of-function allele of mecp2 presented deregulation of trpc6 expression [28]. Interestingly, neurons from RTT patients present a series of abnormalities, such as shorter neurites, reduced dendritic arborization, and lower spine density, which is also found in trpc6 mutant autistic patient’s neurons described in the previous section [53, 54]. In accordance, Nageshappa et al. [58] show opposite effects in neuronal cells from patients with mecp2 duplication. MeCP2 also seems to exert an indirect action in regulating TRPC channels, through the control of the expression of another target, the bdnf gene [12, 55]. Involvement of BDNF on pathophysiology of Rett syndrome is widely documented (reviewed by Katz [36]). This gene codes for a neurotrophin that acts as a chemoattractant molecule for neuronal growing process and supports neuronal survival and maturation. Studies suggest that these roles of BDNF on synapse formation are mediated, at least in part, by TRPC channels. The first evidence comes from the observation that exogenous BDNF triggers membrane cation currents resembling those that occur through TRPC channels [48]. Later, it is demonstrated that Ca2+ currents induced by BDNF are in fact abolished by inhibition of TRPC channels via pharmacological inhibition or downregulation of their expression [51]. Such inhibition also blocks BDNF-induced cone growth. The protective effect of BDNF on neuronal survival as well as its induction of dendritic spine formation is also dependent on TRPC channel expression [2, 34]. Finally, Fortin et al. [24] show that pharmacological blocking of TRPC channel activity prevents BDNF-induced translation and incorporation of GluA1-containing AMPA receptors in synaptic membrane. Thus, it would be of interest to investigate whether Rett syndrome patients could also benefit from chemicals that can modulate TRPC channels activity.
12.4 TRPC5 and X-Linked Intellectual Disability
Intellectual disability (ID) is a clinical phenotype characterized by significant limitations in cognitive function and in adaptive behavior, i.e., social and practical skills needed for everyday life activities, such as communication, self-care, and socialization. ID is associated with many different genetic syndromes and genetic alterations and can also be caused by pre- or postnatal environmental factors, such as alcohol abuse by the mother, exposure to pathogens during pregnancy, problems at the time of birth, or diseases in early childhood such as meningitis. It is estimated that ID affects about 3% of the population [56]. Prevalence of ID is biased toward males, indicating that X-linked genes have an important contribution to cognitive development. In fact, many different X-linked genes were already identified as being the causative gene for ID, especially in pedigrees in which this phenotype segregates in an X-linked pattern [14, 65, 72]. Recently, Mignon-Ravix et al. [57] use a custom high-resolution CGH array to investigate the presence of copy number variations (CNV) in the X chromosome, in a cohort of 54 male individuals presenting ID. In this study, the group finds a patient with a 47 kb deletion in Xq23 region involving the first exon of trpc5 gene. A CNV that is considered to be probably pathogenic since it has not been previously identified in any control sample and since trpc5 is highly expressed in the brain. The deletion is also found in the mother, who is unaffected and presented a 50:50 ratio of X chromosome inactivation in her lymphocytes. The individual is reported to present autistic features such as repetitive and stereotyped behavior. TRPC5 is shown to be important for the control of neurite extension and growth cone morphology [27, 30, 31, 35]. Downregulation of TRPC5 in rat neuronal progenitor cells reduced the Ca2+ currents through the cells and blocked the neuronal differentiation [76]. Finally, TRPC5 knockout mice present abnormally high branched dendrites in cerebellar neurons and reduced LTP [64, 67]. These animals present motor deficits and diminished innate fear levels in response to aversive stimuli [67, 68]. These data support a role of TRPC5 in nervous system development and a possible involvement in intellectual disability.
12.5 TRPC3, TRPC7, and Bipolar Disorder
Bipolar disorder (BD), also known as maniac-depressive disorder, is a mental illness characterized by unusual shifts in mood, swinging from periods of maniac episodes, when the individual experiences an overexcited state, being abnormally happy, energetic or irritable, and periods of depressive mood, in which the individual presents sadness and poor interest and pleasure in most activities. As with most of the neurological disorders previously discussed, BD is a disorder with heterogeneous etiology, in which genetic and environmental factors play a role [40]. Many genes have already been associated with BD based on exome studies, case-control cohorts, or genetic studies of families in which BD segregates throughout generations [39, 77]. Evidences from functional studies have also brought support for involvement of some genes in BD, as are the cases of trpc3 and trpc7. Reports of abnormalities in basal and agonist-stimulated Ca2+ concentrations in different cell types of BD individuals suggest that Ca2+ homeostasis plays a significant role in BD pathophysiology [18, 20], especially considering the importance of Ca2+ signaling for neuroplasticity [26]. Moreover, a series of studies using lithium treatment (one of the most widely used drug treatment for BD) show that this chemical affects Ca2+ signaling in neurons and in non-excitable cells such as platelets, lymphocytes, and glial cells, providing another line of evidence supported for the involvement of Ca2+ signaling in BD [13, 17, 81, 85]. The evidences that Ca2+ signaling abnormalities in non-excitable cells from BD individuals prompt scientists to investigate mechanisms involved in Ca2+ influx in these types of cells, particularly in the glial cells [82]. Among the molecular mechanisms responsible for Ca2+ dynamics regulation in non-excitable cells, store-operated calcium entry is proposed to be involved in BD pathogenesis [32, 84], which directs the attention to channels from TRP family. TRPC7 expression was reduced in a subgroup of BD patients [86]. Interestingly, this reduction was inversely correlated to Ca2+ basal levels in B lymphoblasts cell lines (BLCLs) from BD subjects. Andreopoulos et al. [6] report that chronic lithium treatment significantly reduces TRPC3 protein levels in BLCLs of BD individuals. Decreased levels of TRPC3 proteins are also reported in cerebral cortex of rats chronically treated with lithium [88]. Interestingly, neither of the studies detects decreases in TRPC3 mRNA levels. A faster Ca2+ influx is observed in BLCLs of BD individuals upon lysophosphatidic acid (LPA) stimulation [63]. In this model, the authors suggested that LPA-mediated Ca2+ influx is probably mediated by TRPC3, since LPA is structurally homologue to 1-oleoyl-2-acetyl-sn-glycerol (OAG), a known activator of TRPC3/6/7, but only TRPC3 is expressed in BLCLs [69], suggesting a role of TRPC3 in BD pathogenesis. Oxidative stress has also been implicated in BD [5, 60, 83]. Based on the fact that TRPC channels can be sensitive to redox state of the cells, Roedding and collaborators [70] investigate whether TRPC3 expression and function could be affected by oxidative stress in BLCLs from BD individuals and controls. The authors found that chronic mitochondrial-generated oxidative stress reduces TRPC3 protein levels as well as the Ca2+ influx through these channels, though this reduction is equally found in controls and BD individuals. A similar reduction is detected in primary rat cortical neurons exposed to stressor conditions [71]. Why no differences between subjects and controls were found regarding TRPC3 expression and function response to stressor conditions tested and why these conditions induced a decrease in protein levels (which is an opposite consequence expected considering the effects of lithium in similar cellular models) are questions that deserve further investigation.
12.6 TRPC3 and Williams–Beuren Syndrome
Williams–Beuren syndrome (WBS) is a multisystem disorder caused by a deletion on chromosome 7, specifically at 7q11.23 region. WBS patients present characteristic facial features (such as wide mouth, flattened nasal bridge, and widely spaced teeth), connective tissue problems, and cardiovascular malformations. They also present neurodevelopmental problems that include moderate mental retardation, difficulty with visual-spatial tasks, in contrast to strong verbal skills and a friendly personality, demonstrating an extreme interest in other people. Patients with WBS can also have hypercalcemia, which is most frequently present during childhood [4, 43]. The region deleted in WBS harbors 26–28 genes. Their relative contribution to the phenotype is still poorly understood, except for elastin gene, which is related to the cardiovascular problems presented by the patients [21]. One of the genes in 7q11.23 is gtf31 (general transcription factor IIi). This gene codes for the transcription factor TFII-I, which is thought to be one of the main causative factors for cognitive dysfunctions in WBS based on the definition of a shared region always present between patients with different sizes of the deletion [7]. Although TFII-I is primarily a transcription factor, it may also have a role outside the nucleus, as it is evidenced by its presence in cytosol, for example, of B lymphocytes [61] and in dendritic trees of Purkinje cells [16]. In human B lymphocytes, it has been described that TFII-I suppresses accumulation of TRPC3 in cell surface, thus, decreasing Ca2+ entry into the cells [9]. Based on this observation, Letavernier and colleagues [45] investigate whether TRPC3 expression is altered in a WBS individual reported to have hypercalcemia. By using immunohistochemistry, they found an increased protein expression of TRPC3 in lymphocytes and epithelial intestinal cells from this patient compared to control samples. Although TRPC3 mRNA levels seems not to be altered in neuronal cells derived from iPSC of WBS individuals [1, 41, 44], we cannot disregard that TFII-I might be acting at the protein level as it is seen in B lymphocytes [9].
12.7 TRPC Channels, Hyperforin, and Depression
Depression is a mental disorder which etiology and pathology remain largely unknown, but it is hypothesized to be associated with reduced function of monoamine chemicals, such as serotonin and norepinephrine (reviewed by Dale et al. [15]). In fact, the most successful approaches to treat major depressive disorder (MDD) target the monoamine system by either the selective serotonin reuptake inhibitors (SSRI) or by serotonin and norepinephrine reuptake inhibitors (SNRI) increasing monoamine transmission [15]. However, only about 50% of patients diagnosed with MDD evolve into clinical remission under these treatments [74]. In this regard, hyperforin, one of the main bioactive compounds of the medicinal plant Saint John’s wort (SJW), has shown to present unique antidepressant effect and to be significantly more effective than those in the first line of antidepressant drugs [22, 25, 78]. In contrast to conventional drugs, hyperforin acts as a nonselective neurotransmitter reuptake inhibitor, blocking the uptake of serotonin, norepinephrine, dopamine, and other neurotransmitters [89]. Part of hyperforin’s antidepressant effects has been attributed to its property to activate TRPC6 channel. TRPC6 channels are permeable to sodium in the presynaptic membrane and its activation contributes to decreasing the sodium gradient that drives the neurotransmitters reuptake through the transporters, contributing to increasing the levels of the monoamine neurotransmitters in the synaptic cleft [46, 47, 80].
Depression is also commonly associated with decreased levels of BDNF in the hippocampus [38, 75]. As BDNF is a neurotrophic factor fundamental to the modulation of dendritic architecture and synapse [37], it is also hypothesized that depression is associated with loss of hippocampal synapses and dendritic spines contributing to dysfunction of synaptic plasticity [19, 29, 66]. As previously mentioned, synaptogenic properties of BDNF is thought to be in part mediated by calcium transients evoked by TRPC channels [2, 3]. Similarly, the potential effectiveness of hyperforin in the treatment of depression may also be attributed to the calcium influx through TRPC6 channel, which can contribute to the modulation of dendritic spine density and morphology [47].
Although hyperforin has shown to present exciting antidepressant properties and now to be commonly prescribed for the treatment of mild to moderate depression [52], studies in vivo demonstrating the potential effectiveness of this compound are still lacking and require further investigation. In summary, although there are no genetic findings directly correlating TRPC channels with depression, the studies mentioned above show how these channels (in particular, TRPC6) modulate etiopathological mechanisms involved in this disorder and how these channels represent potential therapeutic targets, not only for depression but also for other neurological diseases with dysfunction of these channels.
12.8 Conclusions and Future Perspectives
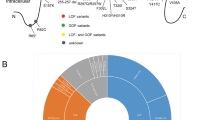
Genetic and functional evidences have indicated the participation of TRPC channels in the pathophysiology of several mental disorders (as summarized in Fig. 12.1). Further investigation of such relationships would be of great value since these proteins are ion channels and, thus, can be directly targeted to regulate their activity, which may lead to an effective therapeutic effect.
TRPC channels are mediators of sensory signals with marked effects on neuronal functions. TRPC3 proteins can form heteromeric interactions with TRPC6 and regulate neuronal survival, axonal growth cone guidance, and dendritic spine formation. Disturbances in the functioning of these proteins and in the cation influx through the channels they form have been implicated in the pathophysiology of several neurological diseases, such as ASD, Rett syndrome, and Williams–Beuren syndrome. Similarly, X-linked intellectual disability pathophysiology has been attributed to disturbances in the calcium influx elicited through TRPC5 channels and altered neurite outgrowth. Finally, altered calcium influx, possibly through TRPC3 or TRPC7, has been associated with Bipolar disorder
As illustrated by the case of trpc6 disruption in an ASD individual, the use of cell reprogramming to obtain neuronal cells derived from pluripotent stem cells from individuals with neurological disorders is a powerful tool to explore possible roles of TRPC channels in the brain. Furthermore, considering that TRPC channels are one of the major classes of channels responsible for ion influx in non-excitable cells, their involvement in the pathophysiology of neurological disorders should be explored not only in neurons but also neuronal progenitor cells and glial cells. For instance, it would be interesting to validate the results found by the expression and functional studies conducted in BLCLs of patients with BD in neuronal cells derived from these patients using such approach. If these studies point to a participation of TRPC channels in the etiology of the disease, the same system can also be used to screen for drugs that can modulate TRPC channel activity in target cell types in an attempt to rescue cellular function and, possibly, contributing to the treatment of the diseases.
References
Adamo A, Atashpaz S, Germain PL, Zanella M, D’Agostino G, Albertin V, Chenoweth J, Micale L, Fusco C, Unger C, Augello B, Palumbo O, Hamilton B, Carella M, Donti E, Pruneri G, Selicorni A, Biamino E, Prontera P, McKay R, Merla G, Testa G (2015) 7q11.23 dosage-dependent dysregulation in human pluripotent stem cells affects transcriptional programs in disease-relevant lineages. Nat Genet 47(2):132–141. doi:10.1038/ng.3169
Amaral MD, Pozzo-Miller L (2007a) TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci 27(19):5179–5189. doi:10.1523/JNEUROSCI.5499-06.2007
Amaral MD, Pozzo-Miller L (2007b) BDNF induces calcium elevations associated with IBDNF, a nonselective cationic current mediated by TRPC channels. J Neurophysiol 98(4):2476–2482. doi:10.1152/jn.00797.2007
Amenta S, Sofocleous C, Kolialexi A, Thomaidis L, Giouroukos S, Karavitakis E, Mavrou A, Kitsiou S, Kanavakis E, Fryssira H (2005) Clinical manifestations and molecular investigation of 50 patients with Williams syndrome in the Greek population. Pediatr Res 57(6):789–795. doi:10.1203/01.PDR.0000157675.06850.68
Andreazza AC, Shao L, Wang J-F, Young LT (2010) Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 67(4):360–368. doi:10.1001/archgenpsychiatry.2010.22
Andreopoulos S, Wasserman M, Woo K, Li PP, Warsh JJ (2004) Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharm J 4(6):365–373. doi:10.1038/sj.tpj.6500266
Antonell A, Del Campo M, Magano LF, Kaufmann L, de la Iglesia JM, Gallastegui F, Flores R, Schweigmann U, Fauth C, Kotzot D, Pérez-Jurado LA (2010) Partial 7q11.23 deletions further implicate GTF2I and GTF2IRD1 as the main genes responsible for the Williams-Beuren syndrome neurocognitive profile. J Med Genet 47(5):312–320. doi:10.1136/jmg.2009.071712
Beis D, Schwarting RKW, Dietrich A (2011) Evidence for a supportive role of classical transient receptor potential 6 (TRPC6) in the exploration behavior of mice. Physiol Behav 102(2):245–250. doi:10.1016/j.physbeh.2010.11.002
Caraveo G, van Rossum DB, Patterson RL, Snyder SH, Desiderio S (2006) Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science 314(5796):122–125. doi:10.1126/science.1127815
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320(5880):1224–1229. doi:10.1126/science.1153252
Chen HC, Wang CH, Shih CP, Chueh SH, Liu SF, Chen HK, Lin YC (2015) TRPC1 is required for survival and proliferation of cochlear spiral ganglion stem/progenitor cells. Int J Pediatr Otorhinolaryngol 79(12):2290–2294. doi:10.1016/j.ijporl.2015.10.027
Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME (2003) Depression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302(5646):885–889. doi:10.1126/science.1086446
Chen Y, Hertz L (1996) Inhibition of noradrenaline stimulated increase in [Ca2+]i in cultured astrocytes by chronic treatment with a therapeutically relevant lithium concentration. Brain Res 711(1–2):245–248. doi:10.1016/0006-8993(95)01199-4
Chiurazzi P, Schwartz CE, Getz J, Neri G (2008) XLMRgenes: update 2007. Eur J Hum Genet 16(4):422–434. doi:10.1038/sj.ejhg.5201994
Dale E, Bang-Andersen B, Sánchez C (2015) Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol 95(2):81–97. doi:10.1016/j.bcp.2015.03.011
Danoff SK, Taylor HE, Blackshaw S, Desiderio S (2004) TFII-I, a candidate gene for Williams syndrome cognitive profile: parallels between regional expression in mouse brain and human phenotype. Neuroscience 123(4):931–938. doi:10.1016/j.neuroscience.2003.08.038
Dubovsky SL, Lee C, Christiano J, Murphy J (1991) Lithium decreases platelet intracellular calcium concentration in bipolar patients. Lithium 2:167–174
Dubovsky SL, Murphy J, Thomas M, Rademacher J (1992) Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 149(1):118–120. http://dx.doi.org/10.1176/ajp.149.1.118
Duman RS (2002) Pathopshysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 17(Suppl 3):306–310. doi:10.1016/S0924-9338(02)00654-5
Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A, Warsh JJ (1997) High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am J Psychiatry 154(7):976–982. http://dx.doi.org/10.1176/ajp.154.7.976
Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, Stock AD, Leppert M, Keating MT (1993) Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet 5(1):11–16. doi:10.1038/ng0993-11
Fava M, Alpert J, Nierenberg AA, Mischoulon D (2005) A double-blind, randomized trial of St. John’s wort, fluoxetine, and placebo in major depressive disorder. J Clin Psychopharmacol 25(5):441–447. doi:10.1097/01.jcp.0000178416.60426.29
Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL (2005) Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci 25(10):2687–2701. doi:10.1523/JNEUROSCI.0951-04.2005
Fortin DA, Srivastava T, Dwarakanath D, Pierre P, Nygaard S, Derkach VA, Soderling TR (2012) Brain-derived neurotrophic factor activation of CaM-Kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J Neurosci 32(24):8127–8137. doi:10.1523/JNEUROSCI.6034-11.2012
Gastpar M, Singer A, Zeller K (2005) Efficacy and tolerability of hypericum extract STW3 in long-term treatment with a once-daily dosage in comparison with sertraline. Pharmacopsychiatry 38(2):78–86. doi:10.1055/s-2005-837807
Ghosh A, Greenberg ME (1995) Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science 268(5208):239–247. doi:10.1126/science.7716515
Greka A, Navarro B, Oancea E, Duggan A, Clapham DE (2003) TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6(8):837–845. doi:10.1038/nn1092
Griesi-Oliveira K, Acab A, Gupta AR, Sunaga DY, Chailangkarn T, Nicol X, Nunez Y, Walker MF, Murdoch JD, Sanders SJ, Fernandez TV, Ji W, Lifton RP, Vadasz E, Dietrich A, Pradhan D, Song H, Ming G, Gu X, Haddad G, Marchetto MCN, Spitzer N, Passos-Bueno MR, State MW, Muotri AR (2015) Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol Psychiatry 20(11):1350–1365. doi:10.1038/mp.2014.141
Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS (2009) Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry 65(5):392–400. doi:10.1016/j.biopsych.2008.09.031
He Z, Jia C, Feng S, Zhou K, Tai Y, Bai X, Wang Y (2012) TRPC5 channel is the mediator of neurotrophin-3 in regulating dendritic growth via CaMKIIa in rat hippocampal neurons. J Neurosci 32(27):9383–9395. doi:10.1523/JNEUROSCI.6363-11.2012
Heo DK, Chung WY, Park HW, Yuan JP, Lee MG, Kim JY (2012) Opposite regulatory effects of TRPC1 and TRPC5 on neurite outgrowth in PC12 cells. Cell Signal 24(4):899–906. doi:10.1016/j.cellsig.2011.12.011
Hough C, Lu S-J, Davis CL, Chuang D-M, Post RM (1999) Elevated basal and thapsigargin-stimulated intracellular calcium of platelets and lymphocytes from bipolar affective disorder patients measured by a fluorometric microassay. Biol Psychiatry 46(2):247–255. doi:10.1016/S0006-3223(98)00308-4
Jeon JP, Roh SE, Wie J, Kim J, Kim H, Lee KP, Yang D, Jeon JH, Cho NH, Kim IG, Kang DE, Kim HJ, So I (2013) Activation of TRPC4β by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium 54(4):307–319. doi:10.1016/j.ceca.2013.07.006
Jia Y, Zhou J, Tai Y, Wang Y (2007) TRPC channels promote cerebellar granule neuron survival. Nat Neurosci 10(5):559–567. doi:10.1038/nn1870
Kaczmarek JS, Riccio A, Clapham DE (2012) Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc Natl Acad Sci U S A 109(20):7888–7892. doi:10.1073/pnas.1205869109
Katz DM (2014) Brain-derived neurotrophic factor and Rett syndrome. Handb Exp Pharmacol 220:481–495. doi:10.1007/978-3-642-45106-5_18
Kellner Y, Gödecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M (2014) The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci 6(5):1–17. doi:10.3389/fnsyn.2014.00005
Kerman IA (2012) New insights into BDNS signaling: relevance to major depression and antidepressant action. Am J Psychiatry 169(11):1137–1140. doi:10.1176/appi.ajp.2012.12081053
Kerner B (2014) Genetics of bipolar disorder. Appl Clin Genet 7:33–42. doi:10.2147/TACG.S39297
Kerner B (2015) Toward a deeper understanding of the genetics of bipolar disorder. Front Psychiatry 6:105. doi:10.3389/fpsyt.2015.00105
Khattak S, Brimble E, Zhang W, Zaslavsky K, Strong E, Ross PJ, Hendry J, Mital S, Salter MW, Osborne LR, Ellis J (2015) Human induced pluripotent stem cell derived neurons as a model for Williams-Beuren syndrome. Mol Brain 8(1):77. doi:10.1186/s13041-015-0168-0
Kim SJ (2013) TRPC3 channel underlies cerebellar long-term depression. Cerebellum 12(3):334–337. doi:10.1007/s12311-013-0455-1
Kruse K, Pankau R, Gosch A, Wohlfahrt K (1992) Calcium metabolism in Williams-Beuren syndrome. J Pediatr 121(6):902–907. doi:10.1016/S0022-3476(05)80336-1
Lalli MA, Jang J, Park JC, Wang Y, Guzman E, Zhou H, Audouard M, Bridges D, Tovar KR, Papuc SM, Tutulan-Cunita AC, Huang Y, Budisteanu M, Arghir A, Kosik KS (2016) Haploinsufficiency of BAZ1B contributes to Williams syndrome through transcriptional dysregulation of neurodevelopmental pathways. Hum Mol Genet 25(7):1294–1306. doi:10.1093/hmg/ddw010
Letavernier E, Rodenas A, Guerrot D, Haymann J-P (2012) Williams-Beuren syndrome hypercalcemia: is TRPC3 a novel mediator in calcium homeostasis? Pediatrics 129(6):e1626–e1630. doi:10.1542/peds.2011-2507
Leuner K, Kazanski V, Müller M, Essin K, Henke B, Gollasch M, Harteneck C, Müller WE (2007) Hyperforin – a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J 21(14):4101–4111. doi:10.1096/fj.07-8110com
Leuner K, Li W, Amaral MD, Rudolph S, Calfa G, Schuwald AM, Harteneck C, Inoue T, Pozzo-Miller L (2013) Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca(2+)-permeable TRPC6 channels. Hippocampus 23(1):40–52. doi:10.1002/hipo.22052
Li H, Xu XS, Montell C (1999) Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24(1):261–273. doi:10.1016/S0896-6273(00)80838-7
Li M, Chen C, Zhou Z, Xu S, Yu Z (2012) A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium 51(6):486–496. doi:10.1016/j.ceca.2012.04.014
Li W, Calfa G, Larimore J, Pozzo-Miller L (2012) Activity-dependent BDNF release and TRPC signaling is impaired in hippocampal neurons of Mecp2 mutant mice. Proc Natl Acad Sci U S A 109(42):17087–17092. doi:10.1073/pnas.1205271109
Li Y, Jia Y-C, Cui K, Li N, Zheng Z-Y, Wang Y-Z, Yuan X-B (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434(7035):894–898. doi:10.1038/nature03477
Linde K, Mulrow CD, Berner M, Egger M (2005) St John’s wort for depression. Cochrane Database Syst Rev 2:CD000448. doi:10.1002/14651858.CD000448.pub2
Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR (2010) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143(4):527–539. doi:10.1016/j.cell.2010.10.016
Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria KC, Beltrao-Braga PC, Trujillo CA, Mendes AP, Padmanabhan K, Nunez Y, Ou J, Ghosh H, Wright R, Brennand KJ, Pierce K, Eichenfield L, Pramparo T, Eyler LT, Barnes CC, Courchesne E, Geschwind DH, Gage FH, Wynshaw-Boris A, Muotri AR (2016) Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. doi:10.1038/mp.2016.95
Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE (2003) DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302(5646):890–893. doi:10.1126/science.1090842
Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S (2011) Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 32(2):419–436. doi:10.1016/j.ridd.2010.12.018
Mignon-Ravix C, Cacciagli P, Choucair N, Popovici C, Missirian C, Milh M, Mégarbané A, Busa T, Julia S, Girard N, Badens C, Sigaudy S, Philip N, Villard L (2014) Intragenic rearrangements in X-linked intellectual deficiency: results of a-CGH in a series of 54 patients and identification of TRPC5 and KLHL15 as potential XLID genes. Am J Med Genet A 164A(8):1991–1997. doi:10.1002/ajmg.a.36602
Nageshappa S, Carromeu C, Trujillo CA, Mesci P, Espuny-Camacho I, Pasciuto E, Vanderhaeghen P, Verfaillie CM, Raitano S, Kumar A, Carvalho CM, Bagni C, Ramocki MB, Araujo BH, Torres LB, Lupski JR, Van Esch H, Muotri AR (2016) Altered neuronal network and rescue in a human MECP2 duplication model. Mol Psychiatry 21(2):178–188. doi:10.1038/mp.2015.128
Neuner SM, Wilmott LA, Hope KA, Hoffmann B, Chong JA, Abramowitz J, Birnbaumer L, O’Connell KM, Tryba AK, Greene AS, Savio Chan C, Kaczorowski CC (2015) TRPC3 channels critically regulate hippocampal excitability and contextual fear memory. Behav Brain Res 281:69–77. doi:10.1016/j.bbr.2014.12.018
Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11(6):851–876. doi:10.1017/S1461145707008401
Novina CD, Kumar S, Bajpai U, Cheriyath V, Zhang K, Pillai S, Wortis HH, Roy AL (1999) Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton’s tyrosine kinase. Mol Cell Biol 19(7):5014–5024. doi:10.1128/MCB.19.7.5014
Paez PM, Fulton D, Spreuer V, Handley V, Campagnoni AT (2011) Modulation of canonical transient receptor potential channel 1 in the proliferation of oligodendrocyte precursor cells by the golli products of the myelin basic protein gene. J Neurosci 31(10):3625–3637. doi:10.1523/JNEUROSCI.4424-10.2011
Perova T, Wasserman MJ, Li PP, Warsh JJ (2008) Hyperactive intracellular calcium dynamics in B lymphoblasts from patients with bipolar I disorder. Int J Neuropsychopharmacol 11(2):185–196. doi: http://dx.doi.org/10.1017/S1461145707007973
Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F (2013) Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol 83(2):429–438. doi:10.1124/mol.112.082271
Piton A, Redin C, Mandel JL (2013) XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am J Hum Genet 93(2):368–383. doi:10.1016/j.ajhg.2013.06.013
Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33(1):88–109. doi:10.1038/sj.npp.1301574
Puram SV, Riccio A, Koirala S, Ikeuchi Y, Kim AH, Corfas G, Bonni A (2011) A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev 25(24):2659–2673. doi:10.1101/gad.174060.111
Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Van’t Veer A, Meloni EG, Carlezon WA, Bolshakov VY, Clapham DE (2009) Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137(4):761–772. doi:10.1016/j.cell.2009.03.039
Roedding AS, Li PP, Warsh JJ (2006) Characterization of the transient receptor potential channels mediating lysophosphatidic acid-stimulated calcium mobilization in B lymphoblasts. Life Sci 80(2):89–97. doi:10.1016/j.lfs.2006.08.021
Roedding AS, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ (2012) Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord 14(2):151–161. doi:10.1111/j.1399-5618.2012.01003.x
Roedding AS, Tong SY, Au-Yeung W, Li PP, Warsh JJ (2013) Chronic oxidative stress modulates TRPC3 and TRPM2 channel expression and function in rat primary cortical neurons: relevance to the pathophysiology of bipolar disorder. Brain Res 1517:16–27. doi:10.1016/j.brainres.2013.04.025
Ropers HH (2010) Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet 11:161–187. doi:10.1146/annurev-genom-082509-141640
Rosenberg SS, Spitzer NC (2011) Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol 3(10):a004259. doi:10.1101/cshperspect.a004259
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163(11):1905–1917
Sen S, Duman R, Sanacora G (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analysis and implications. Biol Psychiatry 64(6):527–532. doi:10.1016/j.biopsych.2008.05.005
Shin HY, Hong YH, Jang SS, Chae HG, Paek SL, Moon HE, Kim DG, Kim J, Paek SH, Kim SJ (2010) A role of canonical transient receptor potential 5 channel in neuronal differentiation from A2B5 neural progenitor cells. PLoS ONE 5(5):e10359. doi:10.1371/journal.pone.0010359
Shinozaki G, Potash JB (2014) New developments in the genetics of bipolar Disorder. Curr Psychiatry Rep 16(11):493. doi:10.1007/s11920-014-0493-5
Szegedi A, Kohnen R, Dienel A, Kieser M (2005) Acute treatment of moderate to severe depression with hypericum extract WS5570 (St John’s wort): randomized controlled double blind non-inferiority trial versus paroxetine. BMJ 330(7490):503–507. doi: http://dx.doi.org/10.1136/bmj.38356.655266.82
Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, Wang Y (2008) TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci 121:2301–2307. doi:10.1242/jcs.026906
Treiber K, Singer A, Henke B, Müller WE (2005) Hyperforin activates nonselective cation channels (NSCCs). Br J Pharmacol 145(1):75–83. doi:10.1038/sj.bjp.0706155
Varney MA, Godfrey PP, Drummond AH, Watson SP (1992) Chronic lithium treatment inhibits basal and agonist-stimulated responses in rat cerebral cortex and GH3 pituitary cells. Mol Pharmacol 42(4):671–678
Verkhratsky A, Orkand RK, Kettenmann H (1998) Glial calcium: homeostasis and signaling function. Physiol Rev 78(1):99–141
Wang J-F, Shao L, Sun X, Young LT (2009) Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord 11(5):523–529. doi:10.1111/j.1399-5618.2009.00717.x
Wasserman MJ1, Corson TW, Sibony D, Cooke RG, Parikh SV, Pennefather PS, Li PP, Warsh JJ (2004) Chronic lithium treatment attentuates intracellular calcium mobilization. Neuropsychopharmacology 29(4):759–769. doi:10.1038/sj.npp.1300400
Yamaji T, Kagaya A, Uchitomi Y, Yokota N, Yamawaki S (1997) Chronic treatment with antidepressants, verapamil, or lithium inhibits the serotonin-induced intracellular calcium response in individual C6 rat glioma cells. Life Sci 60(11):817–823. doi:10.1016/S0024-3205(97)00010-6
Yoon I-S, Li PP, Siu K-P, Kennedy JL, Macciardi F, Cooke RG, Parikh SV, Warsh JJ (2001) Altered TRPC7 gene expression in bipolar-I disorder. Biol Psychiatry 50(8):620–626. doi:10.1016/S0006-3223(01)01077-0
Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF (2003) Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114(6):777–789. doi:10.1016/S0092-8674(03)00716-5
Zaeri S, Farjadian S, Emamghoreishi M (2015) Decreased levels of canonical transient receptor potential channel 3 protein in the rat cerebral cortex after chronic treatment with lithium or valproate. Res Pharm Sci 10(5):397–406
Zanoli P (2004) Role of hyperforin in the pharmacological activities of St. John’s Wort. CNS Drug Rev 10(3):203–218. doi:10.1111/j.1527-3458.2004.tb00022.x
Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y (2008) Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 11(7):741–743. doi:10.1038/nn.2127
Acknowledgments
This work was supported by grants from the California Institute for Regenerative Medicine (CIRM) TR2-01814 and TR4-06747; the National Institutes of Health through the P01 NICHD033113; NIH Director’s New Innovator Award Program 1-DP2-OD006495-01, R01MH094753, R01MH103134, and U19MH107367; and a NARSAD Independent Investigator.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Griesi-Oliveira, K., Suzuki, A.M., Muotri, A.R. (2017). TRPC Channels and Mental Disorders. In: Wang, Y. (eds) Transient Receptor Potential Canonical Channels and Brain Diseases. Advances in Experimental Medicine and Biology, vol 976. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1088-4_12
Download citation
DOI: https://doi.org/10.1007/978-94-024-1088-4_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1086-0
Online ISBN: 978-94-024-1088-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)