Abstract
Polybrominated diphenyl ethers (PBDEs) are globally dispersed throughout the environment, and the levels of some PBDEs in the environment may still be increasing. Previous studies showed that BDE 209 exerted neurodevelopmental and neurobehavioral effects in humans and animals. Oxidative stress is a common mechanism reported in PBDEs-induced neurotoxicity. Taurine, as an antioxidant, whether it is effective in alleviating BDE 209-induced neurotoxicity is still unknown. PC12 cells were exposed to various concentrations of BDE 209 (6.25, 12.5, 25, 50, and 100 μM). 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was used to assess the cell viability. 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) detector was used to explore the production of ROS. Acridine orange was used to reflect the permeation of lysosomal membrane. Rhodamine 123 was used to reflect the permeation of mitochondrial membrane. Lactate dehydrogenase and catalase in PC12 cells exposed to BDE 209 were examined by kits. The results showed that taurine could significantly reverse the decreased viability, the serious oxidative stress and abnormal autophagy in PC12 cells exposed to BDE 209. Collectively, our results indicated that taurine could protect PC12 cells from BDE 209-induced neurotoxicity by alleviating oxidative stress.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Polybrominated diphenyl ethers (PBDEs) are flame retardants that were widely used in many consumer products such as electronic equipment, textiles, upholstered furniture and plastics (Rahman et al. 2001). PBDEs could be easily released into the environment, such as emissions from the manufacture of PBDE-containing products and from the products themselves. Consequently, these chemicals are detected in samples of house dust and air extensively. PBDEs revealed a potential exposure for human (Frederiksen et al. 2009). Of the 209 possible PBDE congeners, penta-BDE, octa-BDE and deca-BDE are typically detected in the environment (La Guardia et al. 2006). Though the lower brominated forms are highly toxic and are presently not in commercial use, the highest brominated (2,2′,3,3′,4,4′,5,5′,6,6′-decabromodiphenyl ether, BDE 209) remains in use and can debrominate to more toxic lower brominated congeners. As a congener of PBDEs, BDE 209 had been found in biological samples, and the level of which has been increasing in human breast milk and blood serum (Sarkar et al. 2015). It was reported that BDE 209 could induce the toxic effect on endocrine and central nervous system (CNS), and especially the neurobehavioral effects. Human epidemiological evidence and animal studies both showed that BDE 209 could induce neurodevelopmental and neurobehavioral effects (Costa and Giordano 2011). Hence, it is urgent to find substitutions or prevention to protect biological systems from toxicity induced by BDE 209.
Oxidative stress is a common mechanism reported in PBDEs-induced neurotoxicity both in vivo and in vitro. In particular, several PBDEs have been shown to cause oxidative stress in neurons, leading to apoptotic neuronal death (Tagliaferri et al. 2010). In addition to apoptosis, autophagy is another way of program cell death, which is important in eliminating toxic protein aggregates, defective organelles and pathogens from cells, as well as intracellular pathogens. It is reported that diverse stimuli can induce autophagy including nutritional depletion, endoplasmic reticulum (ER) stress, hypoxia, hyperoxia, mitochondrial damage, oxidative stress. Previous study in our lab showed that BDE 47 could induce autophagy related to oxidative stress in HepG2 cells (Liu et al. 2015). However, autophagy had not been reported in the neurotoxicity induced by BDE 209.
Taurine (2-aminoethanesulfonic acid) is an amino acid, and could be synthesized from cysteine, which is toxic in mammals. It was reported that taurine is important for brain by the facts that it not only protects neurons against pollutants-induced cytotoxicity, and also benefits neuronal proliferation and synaptogenesis (Sayed et al. 2012). As an antioxidant, whether taurine is effective in alleviating BDE 209-induced neurotoxicity is still unknown.
In the present study, the role of taurine in BDE 209-induced oxidative stress in PC-12 cells was evaluated by the indexes of cell viability, ROS production, mitochondrial membrane potential, lysosomal membrane permeability, lactate dehydrogenase and catalase.
2 Methods
2.1 Chemicals
BDE 209 (99% purity) was supplied by Chem Service (PuertoArmuelles, USA). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA), acridine orange, Rhodamine 123 and the kits for lactate dehydrogenase and catalase were purchased from Beyotime Institute of Biotechnology in China. All the other chemicals were analytical degrade.
2.2 Cell Culture
The rat pheochromocytoma cell line (PC12) was purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai Institute of Cell Biology, Chinese Academy of Sciences. PC12 cells were cultured and grown in antibiotic-free Dulbecco’s modified Eagle medium (DMEM) (Hyclone, USA) supplemented with 10% horse serum and 5% fetal bovine serum (FBS) (Hyclone, USA). Cells were trypsinized with 0.125% Trypsin-EDTA (Beyotime, Chine), sub-cultured, and maintained in a humid incubator (37 °C, 5% CO2).
2.3 Determination of Cell Viability
The cell viability was determined by MTT (tetrazolium) assay (Tang et al. 2004). PC12 cells were seeded at the density of 1 × 104 cells per well in 96-well plates. After the cells were treated with PFOS, 100 μL (0.5 mg/mL) 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was added to the cell for a further 2 h incubation at 37 °C, 5% CO2. Then the medium was discarded and 100 μL dimethyl sulfoxide (DMSO) was added to dissolve the formazan blue formed by mitochondria reducing in the cell. The cell viability was determined by measuring the absorbency of the DMSO-dissolved solution at 570 nm with ELISA Reader (DG-I, China). At least three independent experiments were performed to determine the percentage of viable cells.
2.4 Measurement of Reactive Oxygen Species (ROS) Production
The level of intracellular ROS was quantified by Reactive Oxygen Species Assay Kit. DCFH-DA, a fluorescent probe, is oxidized by reactive oxygen species in viable cells to 2′,7′-dichlorofluorescein (DCF). With designated treatment, the same density of PC12 cells were harvested by cell counting. The cells were then incubated with 100 μM DCFH-DA (dissolved in DMSO) for 30 min at 37 °C. After three times washes with PBS, the relative levels of fluorescence were quantified by a multi-detection microplate reader (485 nm excitation and 535 nm emission).
2.5 Mitochondrial Membrane Potential (MMP)
A fluorescent dye, Rhodamine 123, was used to determine the effect of BDE 209 on MMP in PC12 cells as described elsewhere (Shaki et al. 2012). Rhodamine 123 is a specific fluorescent cationic dye, and could be readily sequestered by active mitochondria with dependence of transmembrane potential, and therefore, the decrease in the fluorescence can directly reflect the decrease in MMP. Briefly, PC12 cells were cultured in a 6-well plate at a density of 2 × 105 mL−1 overnight. Then, the cells were treated with BDE 209. After being washed with PBS, the cells was resuspended in 2 mL of fresh medium containing1.5 μM Rhodamine 123, and incubated at 37 °C for 15 min. Then, the cells were washed with PBS to remove the unbound dye and measured at 490 ex/520 em in a fluorescence spectrophotometer.
2.6 Lysosomal Membrane Permeability (LMP)
The effect of BDE 209 on lysosomal membrane stability in PC12 cells was evaluated by acridine orange (AO). AO is a fluorescent dye used to reflect changes of pH by reversibly accumulating into acidified membrane-bound compartments, such as lysosomes. Briefly, PC12 cells were treated with BDE 209. Then the cells were washed twice with PBS and incubated with AO at a final concentration of 5 μM at 37 °C in the dark for 15 min. The cells were washed with PBS to remove the fluorescent dye and resuspended in PBS. The changes in lysosomal membrane stability were measured by a fluorescence spectrophotometer at 495 ex/530 em.
2.7 Lactate Dehydrogenase and Catalase
Lactate dehydrogenase (LDH) is a stable cytoplasmic enzyme that could be rapidly released into the cell culture medium when the integrity of plasma membrane is damaged. In brief, the culture medium was collected after BDE 209 exposure for 24 h. And LDH reagent was added. The release of LDH into culture medium was measured at 490 nm, and was defined as 100%. CAT is an important antioxidant enzyme in alleviating oxidative stress. Hence, the activity of CAT was also evaluated in the present study. For CAT activity, cell extract samples were measured with hydrogen peroxide to generate N-4-antipyryl-3-chloro-5-sulfonate-p-benzoquinone monoimine, which absorbed maximally at 520 nm.
2.8 Statistical Analysis
All results were expressed as mean ± standard error, and statistical analysis was performed by one-way analysis of variance (ANOVA), followed by LSD and Dunnett’s T3 test using SPSS 13.0 (SPSS Inc, Chicago, IL). Differences were considered statistically significant at P < 0.05.
3 Results
3.1 Effect of Drugs Treatment on Cytotoxicity in Neuronal Differentiated PC12 Cells
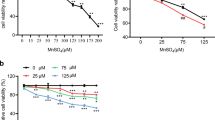
As shown in Fig. 1, BDE 209 at concentrations of 25 μM or over could significant decrease the viability of PC12 cells, compared with the control group (P < 0.05). For example, about 20% of PC12 cell population was decreased in 50 μM BDE 209 exposed groups. Taurine pretreatment could increase the viability of PC12 cells exposed to 50 μM BDE 209 significantly (P < 0.05).
3.2 Effect of Drugs Treatment on ROS Production in Neuronal Differentiated PC12 Cells
DCFH-DA dye was used in the present study to detect the production of ROS. And the result in Fig. 2 showed that 50 μM BDE 209 could increase the production of ROS significantly, compared with the control group (P < 0.05). And pretreatment with taurine could decrease the production of ROS induced by BDE 209 exposure alone.
3.3 Effect of Drugs Treatment on MMP in Neuronal Differentiated PC12 Cells
The results in Fig. 3 showed that 50 μM BDE 209 exposure could decrease MMP of PC12 cells significantly, compared with the control (P < 0.05). Pretreatment with taurine, MMP in PC12 cells exposed to BDE 209 alone was increased significantly (P < 0.05).
3.4 Effect of Drugs Treatment on Autophagy in Neuronal Differentiated PC12 Cells
The dye acridine orange is a weak base that can enter autophagic vacuoles (and other acidic compartments) and become protonated thereby emitting fluorescence in the red range. In Fig. 4, the red fluorescence intensity was increased with the concentration of BDE 209 increased. However, the fluorescence intensity was decreased in PC12 cells pretreated with taurine.
3.5 Effect of Drugs Treatment on Lactate Dehydrogenase and Catalase in Neuronal Differentiated PC12 Cells
As shown in Fig. 5a, 50 or 100 μM BDE 209 increased the leakage of LDH significantly, compared with the control (P < 0.05). Then, PC12 cells pretreated with 80 μM taurine could decrease the leakage of LDH induced by BDE 209 exposure alone significantly (P < 0.05). In Fig. 5b, 50 or 100 μM BDE 209 decreased the activity of CAT significantly, compared with the control (P < 0.05). Then, PC12 cells pretreated with 80 μM taurine could increase the activity of CAT induced by BDE 209 exposure alone significantly (P < 0.05).
4 Discussion
As a group of synthetic organic flame retardants, polybrominated diphenyl ethers (PBDEs) have been widely used for several decades. Among 209 congeners, pentabromodiphenyl ethers (penta-BDEs), octabromodiphenyl ethers (octa-BDEs) and decabromodiphenyl ethers (BDE 209) have been produced for commercial use. And it was reported that BDE 209 comprised 82% of the PBDEs present in electronic products, electrical appliances and industry products globally (Liang et al. 2010). PBDEs are lipophilic, persistently exist in the environment, and easily bio-accumulated in the food chain. Consequently, more attention has been focused on the potential toxicity of BDE 209, especially the neurotoxicity. The present studies showed that BDE 209 could decrease the viability and arouse oxidative stress in PC12 cells. Then, taurine pretreatment could alleviate the toxic effect induced by BDE 209.
ROS is mostly produced in mitochondria and a sensitive index in oxidative stress. ROS has been known to be a protector for bacterial invasion, while the stimulated production of ROS was originally called “the respiratory burst” because of the increased consumption of oxygen (Suzuki et al. 2011). Increased production of ROS could exhibit oxidative stress, inducing damage in DNA, lipids and proteins (Apel and Hirt 2004). CAT is one of the enzyme in defensing for these damage. In accordance with these mentioned mechanisms, the result showed that BDE 209 increased the production of ROS, caused the leakage of LDH, and decreased the activity of CAT in PC12 cells. And decreased mitochondria membrane potential in PC12 cells induced by BDE 209 was also companied, which further supported the destruction of oxidative stress. However, the toxic effects could be reversed in PC12 cells pretreated with taurine.
It was reported that oxidative stress leading to cell death is an acceptable mechanism for the neurotoxicity induced by BDE 209, though death-receptor pathway is also reported (Chen et al. 2016). Autophagy is another way of programmed cell death. Recently, autophagy is reported to be involved in pollutant-induced abnormal apoptosis caused by ROS production (Bodas et al. 2016). Autophagy is recognized as an evolutionary conserved pathway, keeping cellular homeostasis through removal and recycling of damaged macromolecules and organelles. However, increased autophagy is a way of leading to cell death. Our previous study showed that BDE 47 could induce autophagy in HepG2 cells depicted by increased lysosomal membrane permeability (Liu et al. 2015). In the present study, autophagy could also be observed in PC12 cells exposed to BDE 209. And taurine could alleviate the autophagy induced by BDE 209.
Considering the possible role of oxidative stress in apoptosis and autophagy, these aforementioned results indicated that oxidative stress exerted by BDE 209 could also be intervened by taurine, similar as N-acetylcysteine (Zhang et al. 2010).
5 Conclusion
In conclusion, the developmental neurotoxicity induced by BDE 209 has been received much concerns. Hence, finding the substitutions or prevention to protect biological systems from toxicity induced by BDE 209 is necessary. In the present study, the role of taurine in BDE 209-induced oxidative stress was investigated. And the results found that decreased cell viability, aggravated oxidative stress and autophagy could be alleviated in PC12 cells exposed to BDE 209 by taurine pretreatment. The finding that taurine is an effective antioxidant in BDE 209-induced oxidative stress supply a clue in improving the toxicity induced by BDE 209.
Abbreviations
- CAT:
-
Catalase
- LDH:
-
Lactate dehydrogenase
- PBDEs:
-
Polybrominated diphenyl ethers
- ROS:
-
Reactive oxygen species
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bodas M, Van Westphal C, Carpenter-Thompson R, Mohanty DK, Vij N (2016) Nicotine exposure induces bronchial epithelial cell apoptosis and senescence via ROS mediated autophagy-impairment. Free Radic Biol Med 97:441–453
Chen H, Tang X, Zhou B, Xu N, Wang Y (2016) Mechanism of Deca-BDE-induced apoptosis in Neuro-2a cells: role of death-receptor pathway and reactive oxygen species-mediated mitochondrial pathway. J Environ Sci (China) 46:241–251
Costa LG, Giordano G (2011) Is decabromodiphenyl ether (BDE-209) a developmental neurotoxicant? Neurotoxicology 32:9–24
Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE (2009) Human internal and external exposure to PBDEs—a review of levels and sources. Int J Hyg Environ Health 212:109–134
La Guardia M J, Hale R C, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures[J]. Environmental science and technology 40(20):6247–6254
Liang SX, Gao HX, Zhao YY, Ma XM, Sun HW (2010) Effects of repeated exposure to decabrominated diphenyl ether (BDE-209) on mice nervous system and its self repair. Environ Toxicol Pharmacol 29:297–301
Liu X, Wang J, Lu C, Zhu C, Qian B, Li Z, Liu C, Shao J, Yan J (2015) The role of lysosomes in BDE 47-mediated activation of mitochondrial apoptotic pathway in HepG2 cells. Chemosphere 124:10–21
Rahman F, Langford KH, Scrimshaw MD, Lester JN (2001) Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ 275:1–17
Sarkar D, Chowdhury JP, Singh SK (2016) Effect of polybrominated diphenyl ether (BDE-209) on testicular steroidogenesis and spermatogenesis through altered thyroid status in adult mice. Gen Comp Endocrinol 239:50–61. doi:10.1016/j.ygcen.2015.11.009
Sayed RH, Salem HA, El-Sayeh BM (2012) Potential protective effect of taurine against dibromoacetonitrile-induced neurotoxicity in rats. Environ Toxicol Pharmacol 34:849–857
Shaki F, Hosseini MJ, Ghazi-Khansari M, Pourahmad J (2012) Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim Biophys Acta 1820:1940–1950
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG (2010) Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol In Vitro 24:116–122
Tang MZ, Wang ZF, Shi YL (2004) Involvement of cytochrome c release and caspase activation in toosendanin-induced PC12 cell apoptosis. Toxicology 201:31–38
Zhang C, Liu F, Liu X, Chen D (2010) Protective effect of N-acetylcysteine against BDE-209-induced neurotoxicity in primary cultured neonatal rat hippocampal neurons in vitro. Int J Dev Neurosci 28:521–528
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81302400/H2601 to X.L.; 81273031/H2601 to J.S.); and the startup funding from Dalian Medical University under Talent Introduction Program (201069 to J.S.).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this paper
Cite this paper
Liu, Q. et al. (2017). Role of Taurine in BDE 209-Induced Oxidative Stress in PC12 Cells. In: Lee, DH., Schaffer, S.W., Park, E., Kim, H.W. (eds) Taurine 10. Advances in Experimental Medicine and Biology, vol 975. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1079-2_71
Download citation
DOI: https://doi.org/10.1007/978-94-024-1079-2_71
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1077-8
Online ISBN: 978-94-024-1079-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)