Abstract
Defining the physical-chemical determinants of protein folding and stability, under normal and pathological conditions has constituted a major subfield in biophysical chemistry for over 50 years. Although a great deal of progress has been made in recent years towards this goal, a number of important questions remain. These include characterizing the structural, thermodynamic and dynamic properties of the barriers between conformational states on the protein energy landscape, understanding the sequence dependence of folding cooperativity, defining more clearly the role of solvation in controlling protein stability and dynamics and probing the high energy thermodynamic states in the native state basin and their role in misfolding and aggregation. Fundamental to the elucidation of these questions is a complete thermodynamic parameterization of protein folding determinants. In this chapter, we describe the use of high-pressure coupled to Nuclear Magnetic Resonance (NMR) spectroscopy to reveal unprecedented details on the folding energy landscape of proteins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The phenomenon of spontaneous protein folding underlies nearly all key biological processes, yet despite decades of intense research and significant progress in both experiment and theory (Wolynes et al. 2012; Dill and McCallum 2012) the mechanisms and determinants of folding remain incompletely understood. A protein is a hetero-polymer, the basic motif for which is one of the 20 natural amino acids. The chaining of these motives constitutes the sequence of the protein. Individual protein sequences have evolved in order to adopt a tridimensional (3D) structure that confers the level of stability, cooperativity and conformational dynamics required for their optimal function under the conditions in which the organism must survive. However, we have yet to reveal the subtle rules by which sequence determines these properties. Protein folding energy landscapes remain to be experimentally mapped. A general description of folding transition states and routes cannot be predicted for arbitrary amino acid sequences. Likewise, a protein’s propensity to aggregate cannot be deduced from its sequence. Finally, we do not know how sequence encodes the conformational fluctuations and heterogeneity required for function in specific contexts. Nevertheless such a high level of understanding will be required for further progress in the conception and modulation of proteins, enzymes and small molecules for therapeutic or biotechnological applications. Importantly, numerous “conformational” diseases (Alzheimer, Parkinson, Prion diseases) are associated with a deregulation of the folding/unfolding equilibrium for a specific protein (Dobson 2005), which justifies – if needed – the efforts dedicated to the study of this phenomenon.
Most of the information gathered on this phenomenon comes from folding/unfolding experiments performed in vitro. Several methods are possible to unfold a protein: adding chaotropic reagents (urea, guanidinium chloride…), modifying the physicochemical parameters of the sample (P, T, pH)… In spite of difficulties for implementation, pressure is a method of choice to unfold a protein: it is a “soft” method, generally reversible, that give access to a large panel of thermodynamic parameters, specific to the folding/unfolding reaction (Kamatari et al. 2004; Akasaka et al. 2013). It is generally used in conjunction with spectroscopic methods, like Circular Dichroism, fluorescence or IR spectroscopy (Dellarole and Royer 2013), which bring global information on the evolution of the tertiary or secondary structure during protein unfolding. Associated to a method which provided access to local information, such as Nuclear Magnetic Resonance (NMR), pressure can yield extremely detailed, residue specific information on protein folding. The combination of high pressure and NMR constitutes a powerful tool that can lead to a new knowledge about the role of residue packing in protein stability, of conformational fluctuations in water penetration, or that can be used to describe folding intermediates or other details of the energy landscape, otherwise invisible when using other approaches.
2 Unfolding Protein with High Hydrostatic Pressure
As noted in other contributions to this volume, the physical basis for the effects of pressure on protein structure and stability remains controversial, in contrast to a relatively clear physical understanding of temperature effects on protein structure (Kauzmann 1959; Privalov and Gill 1988; Murphy et al. 1990). The fundamental observation of pressure effects has been that over most of the accessible temperature range, the application of pressure leads to the unfolding of proteins, indicating that the volume change upon unfolding is negative, i.e., the specific molar volume of the unfolded state is smaller that that of the folded state (reviewed in Royer 2002). This observation is rather counter-intuitive, as one might expect that pressure would simply compress the folded state of the protein. While this compression does occur, pressure eventually unfolds most proteins, due to this negative volume change upon unfolding and Le Chatelier’s principle, which states that that application of any perturbation will shift a system in equilibrium toward the state that alleviates the perturbation. In the case of pressure perturbation, this is the state that occupies the smallest volume, i.e., the unfolded state.
The relation establishing the difference of free energy between the native and the unfolded states at a given pressure (∆G(p)) depends on the volume difference (∆V, first order term) and the difference in compressibility (∆β, second order term):
where ∆G 0 stands for the difference in free energy between native and unfolded states at atmospheric pressure (p 0). The difference in compressibility between the native and unfolded states is typically found to be negligible compared to the experimental error in ∆G(p) measurement, such that Eq. 13.1 is usually simplified to:
As noted previously (Royer 2002), and in other contributions to this volume, the origin of ∆V remains matter of debate. Several effects have been put forward: (i) a difference in compressibility between the native and the unfolded states of proteins (Chalikian and Macgregor 2009), (ii) pressure induced changes in the structure of bulk water (Grigera and McCarthy 2010), (iii) density difference for water molecules close to polar or apolar amino acids (Mitra et al. 2006), (iv) lost of dehydrated cavities inside the native structure of a protein, or a combination of these different effects. Recently, using a model protein (the Staphyloccocal Nuclease hyper-stable variant: ∆ + PHS SNase) and several of its mutants, we have shown that the main contribution to ∆V was the presence of residual cavities inside the native 3D structure (Fig. 13.1) (Roche et al. 2012a; Rouget et al. 2011).
Unfolding of SNase and of its cavity mutants: a fluorescence study. (a) SNase 3D structure (hyper-stable variant ∆ + PHS): the native cavity is colored in light blue, the mutated residues in red. (b) Pressure dependence of the SNase tryptophan (Trp140) emission fluorescence. At low pressure, the fluorescence emission is intense, with a frequency located in the «blue» region of the visible light spectrum, indicative of a tryptophan residue buried in the hydrophobic core of a folded protein. At high pressure, the emission drop and shifts to «red», suggesting a solvent (water) exposed tryptophan residue, as expected in an unfolded protein. (c) The center of mass of the fluorescence spectra reported in (b) are plotted as a function of the pressure. The 3 curves stand for 3 different samples with 3 different guanidinium chloride concentrations. Addition of this chaotropic reagent is necessary to unfold SNase in the pressure range experimentally available (≤300 MPa). The sigmoidal behavior of these curves suggests a two-states equilibrium between the native and the unfolded protein. Note the similar slope within the three curves, suggesting that addition of guanidinium chloride has little effect on ∆V. (d) Measured ∆V on ∆ + PHS SNase and 10 of its cavity mutants: introducing an additional cavity (or enlarging the original native cavity) brings a concomitant increase in ∆V. Modified from results published (Roche et al. 2012a)
The ∆V values accessible through techniques like fluorescence spectroscopy represent model dependent global values and as such are not thermodynamic quantities, per se. Techniques allowing atomic resolution, such as NMR, provide not one spectroscopic probe, but dozens, which are site-specific, allowing observation of pressure effects over the entire sequence and structure of the protein. Comparing the ∆V values from all of these observables reveals deviations from the two state model. Indeed, if unfolding is truly 2-state, then the pressure unfolding NMR profiles from all of the site-specific resonances should be identical within experimental error. Resonances with significantly different profiles can reveal the existence of intermediates and what is more, provide information about their structure.
3 NMR Spectroscopy: A Method of Investigation at a “Local” Scale
With X-Ray crystallography, NMR is the only technique available for solving the three-dimensional structure of a molecule at atomic resolution. An NMR structure is obtained “indirectly”, by building a 3D model from inter-atomic distances deduced from the quantification of magnetic interactions between the magnetically active nuclei of the molecule. Thus, the classical strategy used to solve the 3D structure of a protein can be divided into two steps: the resonance assignment of the active nuclei (1H, 13C and 15N), then the measurement of the interactions between these nuclei. These interactions, essentially the dipolar through-space interactions, are translated into distance restraints used within molecular dynamics algorithms for the calculation of the 3D structure. Resonance assignment is an important step, requiring the exploitation of state-of-the-art spectroscopy techniques that can be grouped together under the generic term of “correlation spectroscopy”. Given the number of nuclei concerned, protein spectra are eminently complex. Thus, correlation spectroscopy is generally coupled to multidimensional spectroscopy, allowing distributing the information initially contained in a 1D spectrum along two or three (sometimes more!) dimensions, yielding a considerable gain in resolution and facilitating their interpretation.
For a biophysicist, the interest of NMR to study protein folding lies in the close relationship between the resonance frequencies and the chemical, but also tridimensional, structure of the protein: a proton covalently bound to a nitrogen atom has a different resonance frequency than a proton covalently bound to a carbon atom, and protons borne by the same chemical group (methyl groups, for instance…) will have a different resonance frequency if similar chemical groups are involved in different structures (β-sheet, α-helix…), if they “see” a different environment. Thus, the ∆ + PHS SNase 2D correlation spectrum reported in Fig. 13.2 concerns nuclei (1H and 15N) belonging to similar chemical groups: the amide group (HN) that contributes to the peptide bond along the protein backbone. From a residue to another, their resonance frequencies differ, since they are involved in different environments inside the protein 3D structure.
2D [1H-15 N] HSQC spectrum recorded on a ∆ + PHS SNase sample. This spectrum reveals heteronuclear scalar (through-bond) correlations between the proton and the nitrogen nuclei from a given amide group (HN). The cross-peaks are labeled with the «one-letter» code for amino acids. 1H and 15N resonance frequencies are displayed on the horizontal and the vertical axes, respectively. Instead of the absolute frequency (in Hz), the nuclei resonances are reported in ppm, a relative measurement that allows ignoring the magnetic induction delivered by the magnet used for the measurement
Each amino acid bears an HN group, with the exception of proline, generally a minority residue in the composition of soluble proteins (<3 %), and will give rise to a specific correlation in the 2D [1H-15N] HSQC spectrum of the protein. This HSQC spectrum (Bodenhausen and Ruben 1980) can be seen as a protein’s “finger-print”: any “chemical” (mutation) or structural modification will bring about a change in the amide resonance frequencies in the region concerned by the modification. Thus, these resonances constitute local probes, allowing detection of any perturbation in the 3D structure of the protein.
While protein unfolding does not bring any chemical modification of its sequence, it considerably modifies its 3D structure. This perturbation can be measured locally, residue-per-residue, by following the evolution of the correlation peaks on HSQC experiments recorded with increasing pressures (Fig. 13.3). In a two-state model of the unfolding/refolding equilibrium, the exchange between the native and unfolded forms occurs on a time scale slower than the time scale of the NMR measurement. Thus, we do not observe chemical shift variations (a displacement of the cross-peaks on the HSQC spectra, indicative of fast exchange) as a function of pressure, but the disappearance of correlation peaks belonging to the native form, with the concomitant appearance of new peaks (centered at 8.5 ppm on the proton chemical-shift axis) that correspond to the spectrum of the unfolded species. The weak spectral dispersion of these cross-peaks corresponds to an unfolded protein: the native 3D structure turns into a “random coil-like” structure, yielding a similar environment for all residues, hence for all amide groups. The residual dispersion comes from a weak “chemical” effect due to the different nature of the side chain between different the 20 natural amino acids used in a protein.
Coupling high-pressure and NMR raises complicated technological issues. The experimental device should of course support high pressure, but must be non-magnetic and permeable to radiofrequency. Among all the alternatives proposed (reviewed in Fourme et al. 2012), a satisfying commercial solution has emerged, based on the use of ceramic tubes (zirconium oxide). These tubes can support up to 300 MPa (3 kbar), usually enough to unfold protein. If proteins are too stable to be unfolded by pressure over this range, one can adjust the protein stability, generally by adding low concentration of chaotropic reagents. The ceramic high pressure NMR tubes can be used in combination with any commercial NMR probe, and maintain a sensitivity of ∼50 % relative to that obtained with classical 3 mm Borosilicate or Pyrex glass tubes (Fig. 13.4).
Experimental device installed at the Centre de Biochimie Structurale (Montpellier, France) for High Pressure NMR. From right to left. – Avance III 600 MHz NMR Bruker spectrometer used for this study. This spectrometer is equipped with a classical «hot» double-resonance (BBI) probe, with Z gradients. – Deadalus Innovation™ High Pressure automatic pump. – Deadalus Innovation™ ceramic tube with its titan manifold. This system is connected to the pump by a stainless steel pipe (ø = 0.25′), and pressurized via a pushing fluid: a light paraffin wax (Sigma™), immiscible with the aqueous buffer used to dissolve the protein sample
4 High-Pressure NMR and Protein Unfolding
In combination with high pressure, NMR is a technique able to bring unprecedented details, residue-per-residue, on protein unfolding (Roche et al. 2012b, 2013a). Equilibrium measurements give access to thermodynamic parameters such as the free energy difference between the folded and unfolded states of the protein, and ∆V. As noted, comparison of the values from the different residues informs on the cooperativity of the unfolding/folding reaction. Moreover, given the volumetric properties of protein folding transition states, kinetic measurements using multi-dimensional NMR are also possible (See also Chap. 4 by Royer in this volume).
4.1 High-Pressure NMR and Protein Unfolding: Steady-State Study
4.1.1 Measurement of “Local” Thermodynamic Parameters
Steady-state measurements are recorded when the equilibrium between native population/unfolded population has been reached after a pressure jump. Reaching equilibrium after a change in pressure requires a variable period of time (the relaxation time). Because the activation volume for protein folding is invariably positive, relaxation times can be quite long, ranging from seconds to a few minutes (∆ + PHS SNase L125A variant or WT SNase for example), up to several hours (∆ + PHS SNase). Classically, steady-state measurements consist in recording 2D [1H-15 N] HSQC – type NMR experiments as a function of pressure, as reported in Fig. 13.3. One follows the evolution of the residue specific cross-peak intensities (or volumes), as a function of the applied pressure. As the native population decreases compared to the unfolded one, the intensity of the cross-peaks corresponding to the native state decreases with pressure, following a sigmoidal curve that can be fitted with the characteristic equation for a two-state equilibrium:
where I is the cross-peak intensity at a given pressure, I F the cross-peak intensity at atmospheric pressure (folded protein, I F), and I U the residual cross-peak intensity when the protein is fully unfolded (I U). The four parameters I F, I U, ∆G 0 and ∆V have to be fitted to the experimental cross-peak intensities measured at variable pressure. This equation has been obtained from the following relations:
where [U] and [F] stands for the protein unfolded and folded populations, respectively.
Contrary to fluorescence spectroscopy, for instance, which gives a “global” value for the parameters ∆G 0 and ∆V, NMR yields “local” residue specific values of ∆G 0 and ∆V (Fig. 13.5). For our model reference protein (∆ + PHS SNase), unfolding is a nearly cooperative phenomenon: most of the residues “see” a similar ∆V of ≈ 80 ml mol−1, a value close to the “global” value measured with fluorescence spectroscopy. Nevertheless, in some areas of the protein, the measured residue-specific ∆V values fall below this average value (<30 ml mol−1), skewing the distribution in Fig. 13.5, and suggesting the presence of folding intermediates, i.e. partially folded conformers having some degree of stability, in the protein energy landscape.
From left to right. – Sigmoidal decrease of the intensity measured for 4 cross-peaks in the native 2D [1H-15N] HSQC spectrum of ∆ + PHS SNase (see Figs. 13.2 and 13.3) as a function of pressure. The lines come from the fit of the experimental intensities with the characteristic equation for a 2-states equilibrium (Eq. 13.3). – ∆V values obtained through the fit of the intensity decrease of the 2D [1H-15N] HSQC cross-peaks with pressure, plotted versus the protein sequence. – Histogram of the ∆V values measured for ∆ + PHS SNase. This histogram has been fitted with a Gaussian function (in red) that gives an average value for ∆V close to the one measured with fluorescence. Modified from results published (Roche et al. 2012a)
4.1.2 Structural Characterization of Folding Intermediates
High pressure NMR measurements highlight differences in local stability for ∆ + PHS SNase: some elements of structure unfold before others. That means that partially unfolded structures exist along the coordinate axis of the folding/unfolding reaction. These partially unfolded structures are mandatory steps in the folding pathway of a protein.
We used the following procedure to extract structural and energetic information about the folding intermediates from the pressure-dependent multi-dimensional NMR data. After normalizing the residue-specific denaturation (Fig. 13.6a) curves obtained from the amide cross-peak intensity decays measured on the HSQC experiments recorded at variable pressure, the value of one for a given cross-peak (I = I F = 1) can be associated with a probability of one (100 %) for the corresponding residue to be in the native state, with all the native contacts present. Similarly, a residue for which, at the same pressure, the corresponding cross-peak has disappeared (I = I U = 0) from the HSQC spectrum has a probability equal to zero to be in a native state: it belongs to an unfolded state where all the native contacts are lost.
Tracking folding intermediates with high pressure NMR. (a) Residue-specific normalized denaturation curves can be used to calculate contact probabilities between two residues (here between residue 38 and 117) at a given pressure (80 MPa). (b) Contact probabilities are reported with a color code (above the diagonal) in a contact map. This map will be used to define the lists of native contacts at a given pressure (80 MPa). (c) These contact lists will be translated into restraint lists for use by molecular dynamics program allowing the modeling of the corresponding conformation. (d) Conformation analysis: on the graph, the populations (Ln(P)) are plotted as a function of the residual native contacts (Q), at five different pressures. The red curve shows the result for a pressure of 80 MPa. It presents three minima: the most populated (Q = 0.85) corresponds to the population of native conformers at this specific pressure; the minimum at Q = 0.1 corresponds to the population of unfolded states; the minimum (shoulder) at Q = 0.55 corresponds to the population of an intermediate states which structure is represented in the insert. Modified from results published (Roche et al. 2012a)
Now, we consider two residues i and j, in an intermediate situation where the probability to be in a folded states are p(i) = 0.99 and p(j) = 0.68, respectively, at a given pressure (80 MPa in the example displayed in Fig. 13.6a). For two residues that are in contact in the native state, we assume the probability p(i,j) to be in contact at pressure of 80 MPa in this example to be given by the product of the individual probabilities p(i,j) = p(i) x p(j) = 0.67. Pressure dependent contact maps can be constructed based on the contact map of the folded protein. These folded state contact maps are constructed from the 3D crystal or NMR structure of the protein by measuring all contacts between different atoms: usually, only the distances between Cα atoms of the different residues are used, and a “contact” between two residues i and j is defined by a distance Cα i – Cα j ≤ 4 Å. These contacts are then plotted in a diagonal diagram with the protein sequence numbering on the X and Y axes (Fig. 13.6b). It is now possible to use a color code (for example) in order to report in this contact map the pressure dependent probability of contact, as defined above (Fig. 13.6b).
Given the folded state contact map and our contact probabilities for each contact pair at each pressure we work backwards to obtain structural models and population probabilities for the folding intermediates. Indeed, if it is easy to calculate a contact map from a known 3D structure, it is also possible to calculate a 3D structure from a contact map, using the contacts as restraints in a molecular modeling program. This is the classical method used by NMR spectroscopists to build a protein 3D structure from inter-nuclear distances deduced from magnetic interactions between nuclei. Similarly, a restraint list can be obtained from a contact map and used for molecular modeling. It is also possible to “weight” the restraint list with contact probabilities calculated at a given pressure. In this manner, several (usually 100) restraint lists are generated, instead of one. A contact between two residues associated to a probability of 0.8 will be randomly added to 80 lists over 100, if the probability is only 0.4, the corresponding restraints will be in 40 lists over 100, etc.
Then we model, class and analyze the 3D structures obtained from coarse grained simulations using the 100 different restraint lists. In the case of our model protein ∆ + PHS SNase, this analysis allowed us to identify a population of conformers corresponding to a folding intermediate where the C-terminal α-helix is unfolded, whereas the N-terminal β-barrel maintains its native structure (Fig. 13.6d). It is worth noting that this folding intermediate has been also identified through other high-pressure NMR experiments, concerning the measurement of amide proton/deuteron exchange (Roche et al. 2012b). These experiments allow probing the evolution of the H-bond network involved in the stabilization of the protein 3D structure. This approach has been developed in detail elsewhere (Fuentes and Wand 1998) and will not be discussed here further.
4.2 High-Pressure NMR and Protein Unfolding: Kinetics Study
A complete understanding of the protein folding/unfolding phenomenon requires, in addition to the spatial, structural description of the energy landscape, a temporal description of the sequence of events along the folding pathway followed by the protein. Such a description relies on the measurement of kinetic parameters, after perturbation of the thermodynamic equilibrium between the folded and unfolded conformers of the protein. This perturbation can be achieved by a fast mixing with chaotropic reagent, a pH jump, a temperature jump… and of course a pressure jump (P-jump)!
In addition to kinetic parameters, these measurements allow characterizing the transition state associated to a first order kinetics, commonly used to describe an equilibrium reaction between two states. Thus, using pressure to unfold the protein will give access to folding (k f) and unfolding (k u) rates, as well as to the volume of the transition state ensemble (TSE): the transition state of a protein being relatively heterogeneous, it is better described as an ensemble of (close) states rather than an unique conformer (Fig. 13.7).
Protein folding and kinetics measurements. (a) Unfolding reaction coordinates as a function of the free energy. The constants k f and k u stand for the folding and unfolding rates, respectively, and τ is the experimentally measured relaxation time as depicted in Fig. 13.8. (b) Scheme of the volumetric diagram of the folding/unfolding reaction. F, TSE and U represents the folded, transition, and unfolded state, respectively. (c) Experimental volumetric diagrams obtained for ∆ + PHS SNase and 10 of its “cavity” variants. ∆V et stands for the volume difference between the folded and unfolded states as measured at thermodynamic equilibrium (steady state measurements), ∆V f ‡ is the activation volume between the folded and transition states obtained from kinetics measurements. Modified from results published (Roche et al. 2013b)
The return to a new equilibrium after perturbation can be monitored by different spectroscopy techniques that give access to a “global” measurement of the kinetic parameters for the folding/unfolding reaction (Fluorescence, IR…). A “local” description of the kinetic parameters and of the transition state ensemble implies the use of a technique combining spatial resolution, allowing a precise local description of the time evolution of the structure of the protein, and a sufficient time resolution. At atmospheric pressure the return to thermodynamic equilibrium after a perturbation can be relatively fast (few ms seconds to a few minutes, generally at most). NMR has high spatial resolution, but its time resolution is limited: the recording time of the 2D [1H-15N] HSQC spectra described above ranges from 10 to 40 min, depending on the sample concentration and the expected spectral resolution. Thus, such experiments can be used only in the case of proteins having extremely slow relaxation times.
Pressure, because of the positive activation volume for folding, has the unique feature to slow down the folding reaction: a reaction completed in few seconds at atmospheric pressure will take several minutes to few hours at higher pressure, making P-jump NMR monitoring feasible. For example, ∆ + PHS SNase at pressure above 1 kbar, exhibits residue-specific relaxation times greater than 10 h! (see for instance Fig. 13.9b). This is all the more true since methodological advances have been realized during the last decade in the field of “real-times” measurement of NMR multidimensional experiments (Frydman et al. 2002; Gal et al. 2007). Now, 2D correlation experiments can be acquired in few tens of seconds (sometimes even in less than one second!) instead of few tens of minutes, with a good sensitivity and enough spectral resolution. For example for following faster P-jump kinetics we have used the so-called 2D [1H-15 N]- “SOFAST-HMQC” experiments (Schanda and Brutscher 2005), recorded in only 25 s (Fig. 13.8), to monitor the unfolding reaction kinetics of the L125A variant of ∆ + PHS SNase, exhibiting relaxation times shorter than 10 min after P-jump of 20 MPa (Roche et al. 2013b). In the case of proteins exhibiting even faster relaxation times, it is possible to combine NMR measurements with stop-flow techniques where the sample evolution is “frozen” at different times along the exponential decay of the cross-peak intensities (Balbach et al. 1996). This approach is easy to implement when using addition of denaturing reagents, or pH-jump to unfold the protein, but has severe technological limitations when combined to high pressure.
High-pressure NMR and kinetics measurements. (a) Series of 2D [1H-15N] HSQC spectra used to sample a positive P-jump of 20 MPa (100 to 120 MPa) on a sample of ∆ + PHS SNase. Individual measuring time for each HSQC experiment was 20 min, for a protein concentration of 1 mM. (b) Times evolution of the amide cross-peak intensity for 4 residues. The curves were obtained by exponential fitting of the intensity values, giving the value of τ = 1/(k u + k f)
Practically, P-jump kinetics measurements consist in recording a series of 2D HSQC (or SOFAST-HMQC) after a pressure jump, in order to correctly sample the exponential decay of the cross-peak intensity during unfolding (in the case of a “positive” P-jump, where pressure is increased) (Fig. 13.9), but can be done as well for negative pressure jumps. Several P-jumps are realized in the pressure range where unfolding appears (typically between 40 and 200 MPa for ∆ + PHS SNase and its variants). P-jump amplitude should be enough to get a measurable intensity decay for the amide cross peaks, but should remain moderate to avoid any imbalance between the folding and the unfolding reactions: an excessive positive P-jump, for instance, will favor the unfolding reaction at the expense of the folding reaction. In the present study, we have used pressure jumps of 20 MPa, which is about 10 % of the pressure range needed to fully unfold the protein ∆ + PHS SNase and its variants (200 MPa).
The fit of the amide cross-peak decays with exponential functions gives a residue-specific exponential time t for each P-jump, equal to the inverse of the sum of the folding and unfolding rates (t = 1/(k u + k f)). It is thus possible to obtain the value of the activation volume (TSE volume) for the unfolding reaction at atmospheric pressure by the fit of the evolution of t values at different pressure with the following equation:
where ∆V et = ∆V f – ∆V u and K eq = k f/k u stand for the volume difference between the folded and unfolded states and the equilibrium constant measured at thermodynamic equilibrium. Only two variables need to be fitted: k u0 (the unfolded rate at atmospheric pressure) and ∆V ‡ u0 (the activation volume for unfolding at atmospheric pressure) because of the equilibrium constraints (Fig. 13.10).
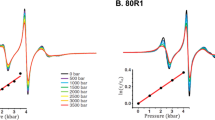
High-pressure NMR kinetics measurements. (a) Examples for the evolution of the residue-specific relaxation time τ values as a function of the final pressure for the pressure jump. Curves are obtained with the fit of the experimental values with Eq. 13.5 and allow determining the value of the activation volume for the folding reaction “see” by each residue. (b) Comparison of ∆V at equilibrium (dark blue) and ∆V f ‡ activation volumes (light blue) as a function of the protein sequence. Modified from results published (Roche et al. 2013b)
Analogous to the steady state measurements, combining high pressure and NMR spectroscopy in relaxation kinetics allows obtaining a local dynamic view of the folding pathway being taken by each residue, through the measurement of the kinetic parameter τ and the extraction of the activation volume for each residue. It is worth noting that when looking to the activation volumes, average values obtained for the reference protein ∆ + PHS SNase and its mutants, very different results are obtained: the reference protein shows an activation volume ∆V ‡ f close to the equilibrium ∆V value, indicating that the molar volume of the transition state is quite close to that of the folded state, suggesting a “dehydrated” TSE where most of the native cavities are still present. In contrast, some mutants (I92A, V66A…) show very different values for these two parameters, with a TSE volume close to that of the unfolded state, reflecting a more “hydrated” TSE where most of the native cavities have disappeared and are hydrated (Fig. 13.7) (Roche et al. 2013b).
5 Conclusions
Combining modern NMR techniques with high pressure gives unprecedented details on protein folding. The spatial and temporal resolution of NMR spectroscopy is necessary to describe the folding pathway at a residue level, giving both a structural description of the pathway, bringing to light the existence of intermediate states, and a dynamic description informing on the rates of local rearrangements involved in the phenomenon. Thus, we have obtained a fairly complete structural and energetic description of the folding landscape for our model protein (Fig. 13.11) and several of its mutants. Even more information can be obtained on the protein folding/unfolding mechanism by combining high-pressure NMR with other perturbations: temperature for instance, or addition of denaturing agents, allowing in-depth mapping of folding free energy landscapes and how they can be modulated.
Beyond the fundamental interest of studying the protein folding/unfolding mechanism, a better understanding of this phenomenon will find applications in many fields. Is it necessary to recall that the so-called “conformational” neurodegenerative diseases (Alzheimer, Parkinson, prion diseases…) are due to misfolding of proteins having originally a physiological function? A better understanding of the folding/unfolding mechanism for each of these specific proteins may allow exploring new avenues for drug rational design. Similarly, understanding phenomenon underlying protein stability is a major issue for the design of industrial enzymes or other biotechnological products, able to function at high pressure or high temperature. Thus, understanding how a protein can accommodate mutations to gain stability, keeping intact its function, could have a major economic impact. The challenge is huge, but combining NMR with high pressure may prove to be extremely useful.
References
Akasaka K, Kitahara R, Kamatari YO (2013) Exploring the folding energy landscape with pressure. Arch Biochem Biophys 531:110–115
Balbach J, Forge V, Lau WS, van Nuland NA, Brew K, Dobson CM (1996) Protein folding monitored at individual residues during a two-dimensional NMR experiment. Science 274:1161–1163
Bodenhausen G, Ruben DJ (1980) Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem Phys Lett 69:185–189
Chalikian TV, Macgregor RB Jr (2009) Origins of pressure-induced protein transitions. J Mol Biol 394:834–842
Dellarole M, Royer C (2013) High-pressure fluorescence applications. In: Engelborghs Y, Visser T (eds) Methods in molecular biology, Springer, pp 53–74
Dill KA, MacCallum JL (2012) The protein-folding problem, 50 years on. Science 338:1042–1046
Dobson CM (2005) An overview of protein misfolding diseases. In: Buchner J, Kiefhaber T (eds) Protein folding handbook. Wiley-VCH Verlag GMBH & Co. KgaA, Weinheim, pp 1093–1113
Fourme R, Hamel G, Loupiac C, Perrier-Cornet J-M, Pin S, Roche J (2012) La biologie sous pression: de la molecule au procédé. Hautes Pressions: les Nouveaux Enjeux. Ed.: Publications Mission Ressources et Compétences Technologiques 2012.
Frydman L, Scherf T, Lupulescu A (2002) The acquisition of multidimensional NMR spectra within a single scan. Proc Natl Acad Sci U S A 99:15858–15862
Fuentes EJ, Wand AJ (1998) Local stability and dynamics of apocytochrome b562 examined by the dependence of hydrogen exchange on hydrostatic pressure. Biochemistry 37:9877–9883
Gal M, Schanda P, Brutscher B, Frydman L (2007) UtraSOFAST HMQC NMR and repetitive acquisition of 2D protein spectra at Hz rates. J Am Chem Soc 129:1372–1377
Grigera JR, McCarthy AN (2010) The behavior of the hydrophobic effect under pressure and protein denaturation. Biophys J 98:1626–1631
Kamatari YO, Kitahara R, Yamada H, Yokoyama S, Akasaka K (2004) High-pressure NMR spectroscopy for characterizing folding intermediates and denatured states of proteins. Methods 34:133–143
Kauzmann W (1959) Some factors in the interpretation of protein denaturation. Adv Protein Chem 14:1–63
Mitra L, Smolin N, Ravindra R, Royer CA, Winter R (2006) Pressure perturbation calorimetric studies of the solvation properties and the thermal unfolding of proteins in solution: experiments and theoretical interpretation. Phys Chem Chem Phys 8:1249–1265
Murphy KP, Privalov PL, Gill SJ (1990) Common features of protein unfolding and dissolution of hydrophobic compounds. Science 247:559–561
Privalov PL, Gill SJ (1988) Stability of protein structure and hydrophobic interaction. Adv Protein Chem 39:191–234
Roche J, Caro JA, Norberto DR, Barthe P, Roumestand C, Schlessman JL, Garcia AE, Garcia-Moreno B, Royer CA (2012a) Cavities determine the pressure unfolding of proteins. Proc Natl Acad Sci U S A 109:6945–6950
Roche J, Dellarole M, Caro JA, Guca E, Norberto DR, Yang YS, Garcia AE, Roumestand C, García-Moreno B, Royer CA (2012b) Remodeling of the folding free-energy landscape of staphylococcal nuclease by cavity-creating mutations. Biochemistry 51:9535–9546
Roche J, Caro JA, Dellarole M, Guca E, Royer CA, García-Moreno B, Garcia AE, Roumestand C (2013a) Structural, energetic and dynamic responses of the native state ensemble of staphylococcal nuclease to cavity-creating mutations. Proteins 81:1069–1080
Roche J, Dellarole M, Caro JA, Norberto DR, Garcia AE, García-Moreno B, Roumestand C, Royer CA (2013b) J Am Chem Soc 135:14610–14618
Rouget JB, Aksel T, Roche J, Saldana JL, Garcia AE, Barrick D, Royer CA (2011) Size and sequence and the volume change of protein folding. J Am Chem Soc 133:6020–6027
Royer CA (2002) Revisiting volume changes in pressure-induced protein unfolding. Biochim Biophys Acta 1595:201–209
Schanda P, Brutscher B (2005) Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J Am Chem Soc 127:8014–8015
Wolynes PG, Eaton WA, Fersht AR (2012) Chemical physics of protein folding. Proc Natl Acad Sci U S A 109:17770–17771
Acknowledgements
Authors are indebted to our long and ongoing collaborations with J.A. Caro, B. Garcia-Moreno (John Hopkins University, Baltimore, USA) and Angel E. Garcia (Rensselaer Polytechnic Institute, Troy, U.S.A.), in the work that led to the publications reviewed here. High-pressure NMR equipment (except the spectrometers) used in the work of the original manuscripts cited here was funded by the French National Agency for Research (ANR, PiriBio 09–455024; Project Coordinator: C.A. Royer). This work was also supported by the French Infrastructure for Integrated Structural Biology (FRISBI) ANR-10-INSB-05-01.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Roche, J., Dellarole, M., Royer, C.A., Roumestand, C. (2015). Exploring the Protein Folding Pathway with High-Pressure NMR: Steady-State and Kinetics Studies. In: Akasaka, K., Matsuki, H. (eds) High Pressure Bioscience. Subcellular Biochemistry, vol 72. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9918-8_13
Download citation
DOI: https://doi.org/10.1007/978-94-017-9918-8_13
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9917-1
Online ISBN: 978-94-017-9918-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)