Abstract
Through the selected examples of different classes of secondary metabolites two general approaches in the analysis of complex mixtures of natural products are presented. The first one involves isolation of the constituents using optimized semi-prep LC in combination with classical column chromatography (CC) and the second one represents direct (on-line) analysis of complex plant extracts prior to isolation of the constituents. Thus the isolation of mg-quantities of jatrophanes the bioactive constituents of Euphorbia dendroides, with their structure determination on the basis of spectroscopic data interpretation (IR, NMR, MS), is described. The direct analysis of the plant extracts is demonstrated by the characterization of antioxidative polyphenols, i.e. oligomeric proanthocynidins from the MeOH extracts of grape Vitis vinifera seeds by the application of hyphenated methods (LC/UV/MS) in combination with 13C NMR spectroscopy, as well as tandem mass spectrometry (MS/MS) and MSn in combination with and without LC for identification of oligomeric proanthocynidins from other sources. The potential of another hyphenated instrumentation, LC/UV/SPE-NMR is shown by on-line identification of the various constituents of the crude extract of Hypericum perforatum. The possibilities and limitations of the NMR technique named diffusion ordered spectroscopy (DOSY) for direct analysis of the complex mixture, such as e.g. methanolic extract of Gentiana lutea, is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Natural products (secondary metabolites) have always been among the most fascinating objects of the practicing chemist. In fact, in many definitions of chemistry the isolation, purification and structural elucidation of natural products play a central role. For centuries, drugs were entirely of natural origin and composed of herbs, animal products and inorganic materials. The chemistry of natural products has always excited the interest of the scientists and it provided the early stimulus for foundation of organic chemistry as a separate discipline. The serious study of natural products, lasting for a long time, has been greatly facilitated by the advent of modern spectroscopic techniques, namely nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS), as well as separation techniques, such as gas and liquid chromatography (GC and LC). Nowadays more rapid strategies for chemical characterizations of phytoconstituents of natural products as well as assessing the bioactivities of the natural products are available. The coupling of spectrometers to chromatographs resulted in very powerful so-called hyphenated (coupled) techniques enabling on-line direct analysis of complex mixtures of natural products without prior isolation.
The GC/MS, developed during the 1950s, after being originated by James and Martin in 1952 (James and Martin 1952) was practically the only heavily used hyphenated method for half a century. But its application is rather limited to thermally stable non-polar volatiles, e.g. essential oils, fatty acid methyl esters and similar samples. The breakthrough, responsible for introducing the combination LC/MS applicable to the variety of polar non-volatiles has occurred in the second half of the twentieth century by development of MS ionic sources compatible with LC: APCI (atmospheric pressure chemical ionization) by J. Henion (Henion et al. 1982; Covey et al. 1986) and ESI (electrospray ionization) by J. Fenn, (Fenn et al. 1989) the latter was awarded a share of the Nobel Prize in chemistry in 2002 for this achievement. Today, the various hyphenated methods involving coupling LC and MS are becoming routine, e.g. LC/UV- photodiode array detection/MS (LC/UV/MS), LC-tandem MS (LC/MS/MS) and LC-multiple stage MS (LC/MSn).
The combination LC/UV/MS affords on-line UV and mass spectra of the eluted components, as well the liquid chromatograms measured as the variation in time of the absorbance (mAU, miliabsorbance units) at the selected wavelength (PDA) andor total ion current, TIC (%∑I) with MS detector (see the example presented in Fig. 4.3).
LC coupled to a NMR spectrometer (LC/NMR) has first appeared in the late 1970 through demonstration of both stop-flow and continuous-flow LC/NMR, and until recently, been little used mainly because of its lack of sensitivity. However, development of suitable flow cells and techniques to optimize NMR acquisition conditions, introduction of pulse field gradients (PFG) and solvent signal suppression sequences, together with improvements in probe technology (development of cryoprobe) and the construction of high field magnets have alleviated many of the initial problems of this technique. LC/NMR has important potential for on-line structure identification of natural products (Wolfender et al. 2001). Indeed, NMR spectroscopy is by far the most powerful spectroscopic technique for obtaining detailed structural information about organic compounds in solution. The development of solid phase extraction (SPE) and the recent addition of an automated SPE unit to an LC/NMR system for peak trapping results in an improved NMR signal-to-noise ratio (S/N) and also has other practical advantages. This indirect hyphenation technique (LC/SPE NMR) involving multiple collections of the separated compounds on SPE cartridges, followed by drying with nitrogen and their subsequent transfer to the NMR flow cells using deuterated solvents (Fig. 4.1) is nowadays the most efficient technique in comparison with the previous ones (on-flow and stop-flow) leading to foundation for today’s commercial HPLC-NMR instruments (Jaroszewski 2005). The advantages of LC/SPE NMR are the following:
LC/UV/MS/SPE NMR device (Ref. Jaroszewski (2005))
-
LC separation with cheap, non-deuterated solvents
-
Additives non-compatible with NMR are possible.
-
The complete peak can be trapped: concentration effect.
-
This leads to a higher sensitivity. Multiple trapping feasible
-
Desorption with 100 % deuterated solvents is possible.
-
Choices! No or less solvent suppression is necessary
-
Choice of peaks that should be analysed
-
Easy comparison with literature spectra
-
Measurements at another NMR spectrometer are possible.
-
Easy 2D NMR mesurements (COSY, NOESY, HSQC, HMBC etc.)
At the same time, due to the high sensitivity of the mass spectrometry, a small portion of the effluent after LC can be transferred in the same run via splitter to the mass spectrometer (Fig. 4.1) thus affording online UV, MS and NMR spectra in the same experiment. Combining spectral data from MS, NMR and UV can considerably decrease the time for structural elucidation of secondary metabolites. Identification of a compound often is very difficult based on NMR spectral data only; hence, MS data also are essential. Therefore, LC based hyphenation of NMR and MS detectors to combine sets of spectral information of secondary metabolites has already been used for several years.
In addition, other LC-combinations using different spectrometers as chromatographic detectors have also developed: LC-infrared spectroscopy (LC/IR), and LC-circular dichroism (LC/CD). The application of LC/CD enables chiroptical online stereoanalysis of natural products directly from stereoisomeric mixtures, thus giving the full absolute stereostructures of novel compounds without the necessity of isolation and purification. The combination of the high separation efficiency of LC with these different detectors has made possible the acquisition of on-line complementary spectroscopic data, e.g. LC-MS/MS-NMR-CD “triad”, on an LC peak of interest within a complex mixture (Bringmann and Lang 2003).
In further text two general approaches to the analysis of the plant extracts are discussed: (i) the “traditional” one involving isolation of the components and (ii) identification of the components in the crude extracts without prior isolation.
2 Disscusion
2.1 The “Traditional” Procedure of the Analysis of the Plant Extracts
The rather simplified presentation of the “traditional” procedure being used in the identification of constituents of the plant is presented in Fig. 4.2. Unfortunately, extraction, which is usually the first step in the analysis of the plant constituents, rarely results in the form of pure compounds. More often rather complex mixtures containing quite a few components are obtained. The complete characterization of constituents has to rely on spectral analyses of their purified form. Therefore, rational and efficient isolation procedures have to be developed. Historically, separation and isolation of components from complex mixtures has occupied (and still does) a central role in the activity of researchers in the field of natural products research. Consequently, most of the working time is devoted to the separation/purification steps and not to the structural determination.
The following drawbacks of this (traditional) methods are encountered:
-
Large amounts of the rare biological material are sometimes needed.
-
Expensive tools and supplies, like adsorbents and eluents
-
Long lasting process (several days....)
-
Unstable compounds may decompose already during the preparative separation and thus may escape the analysis.
Recently, a development of the very efficient automated LC semi-prep chromatographic methods, which could be performed on the analytical LC-devices made the preparative step much more efficient, enabling collecting mg quantities of the pure compounds ready for structure determination in one run. The versatile semi-prep systems perform sample injection, fraction collection and re-injection of the collected fractions. The difference between the analytical and preparative HPLC is the amount of sample applied to the column. Semi-preparative chromatography is the link between analytical HPLC and preparative LC. Even though the chromatographic systems used for semi-preparative LC do not reach the size of preparative LC systems, the objectives remain the same:
-
Purification and isolation of maximum sample quantity
-
Savings in time and costs.
In the analytical LC the applied sample amount is very small compared to the amount of stationary phase in the column (less than 1:10,000). Therefore, very good separations can be achieved. To purify higher amounts of sample in a single run the loadability of a column has to be increased. This can be done either by concentration or volume overloading, depending on the application. Consequently, the scale-up from analytical LC (microgram quantity) to semi-prep one (milligram quantities) requires the increase column dimensions from ca. 4–8 to 10–20 mm ID, as well as the flow rate from ca. 0.4–2 to 2–10 mL/min, respectively. In addition, in last two decades some of the inherent insensitivity of the NMR spectroscopy which is the major tool for structure elucidation, has been overcome by improvements in spectrometer hardware and development of new multipulse sequences, considerably reducing the required quantity of the sample to be analysed down to the sub-microgram range.
Example #1
Isolation of jatrophanes, bioactive diterpenoids from Euphorbia dendroides (Tree Spurge) using optimized semi-prep LC in combination with classical column chromatography (CC)
The application of this improved purification step involving semi-prep LC in combination with the classical chromatographic methods used for preliminary purification and enrichment of the interesting fractions in the analysis of the bioactive constituents of the Montenegrin spurge Euphorbia dendroides as a part of the comprehensive study of this species has been carried out recently in our laboratory (Aljančić et al. 2011; Pesic et al. 2011; Jadranin et al. 2013). In course of these investigations, two types of samples were prepared: 60 % aqueous ethanolic extract of the aerial parts (Aljančić et al. 2011) and n-hexane fraction of the lyophilized latex (Jadranin et al. 2013). Before the preparative isolation both samples were subjected to LC/UV/ESI TOF MS as well as the 1H NMR analysis (not shown), revealing rather complex mixtures with diterpenoids as the major constituents (see LC/UV/ESI MS chromatograms of the lyophilized latex, Fig. 4.3).
LC/UV/ESI TOF MS (+ mode) chromatogram of the lyophilized crude latex of Euphorbia dendroides: LC/MS total ion current (TIC) (upper trace ) and LC/PDA (λ = 260 nm) (lower trace); Experimental conditions: Zorbax Eclipse Plus C18 column (150 × 4.6 mm i.d.; 1.8 μm), mobile phase (0.6 mL/min): A (0.20 % formic acid in MilliQ water) and B (acetonitrile), gradient mode of elution, 5 % B (0–5 min), 5–95 % B (5–26 min), 95 % B, (26–35 min); HPLC instrument (Agilent 1200 Series) and a photodiode-array detector (PDA) coupled with a 6210 Time-of-Flight LC/MS system (Agilent Technologies)
Before the semi-prep LC, the isolation of the extract constituents involved a combination of the classical prep chromatographic techniques on Si-gel, i.e. DCFC/prep TLC (Aljančić et al. 2011), or DCFC/CC (Jadranin et al. 2013) (see Fig. 4.4).
Isolation scheme used in isolation of pure compounds from the liophilized crude latex of Euphorbia dendroides (Jadranin et al. 2013)
The isolation scheme used for the purification of latex components (Fig. 4.4) involving in the first step DCFC on Si-gel (n-hexane/EtOAc in three different proportions, 60 %, 80 %, and 100 % EtOAc) afforded fractions A – C, respectively, containing, according to the 1H NMR and LC/UV/ESI TOF MS spectra, jatrophane diterpenoids as the main constituents. In the second step these fractions were divided by CC on silica gel eluting with EtOAc/n-hexane (Fig. 4.4), into subfractions A 1−3 , B 1−2 and C 1−2 and subjected to the semi-prep LC. The example of semi-prep LC chromatogram of fraction A 2 containing four diterpenes (1, 2, 6 and 9) is presented in Figs. 4.5 and 4.6.
Semi-Prep LC of fraction A 2 : on the Agilent Technologies 1100 Series analytical instrument equipped with autosampler under the following conditions: injection volume 1,000 μL (c ~ 20 mg/mL, MeOH), Zorbax Eclipse XDB-C18 column (250 × 9.4 mm; 5 μm), column temp. 25 °C, mobile phase (4.00 mL/min): A (0.20 % formic acid in MilliQ water) and B (acetonitrile), gradient mode of elution, 55–90 % B (0–25 min), 90 % B (25–35 min). The molecular formula are based on the precise mass measurement on LC/ESI TOF MS
From both extracts (aerial parts and latex) nineteen jatrophane diterpenoids (1–19) and two epimeric tiglianes (20, 21) have been isolated (Fig. 4.7). With exception of tigliane 21, named Euphorbia factor Pr2 by Wu et al. (1994), all isolated compounds were new and their structures were elucidated by spectroscopic methods, including HRESIMS, 1D and various 2D NMR techniques. Compounds 1, 2 and 16 were found in both extracts. It also should be noted that the biological activity of compounds 1–5, 16 and 20 was studied against four human cancer cell lines. The most effective jatrophane-type compound (2) and its structurally closely related derivative (1) were evaluated for their interactions with paclitaxel and doxorubicin using a multidrug-resistant cancer cell line. Both compounds exerted a strong reversal potential resulting from inhibition of P-glycoprotein transport (Aljančić et al. 2011; Pesic et al. 2011). Among the jatrophanes isolated from latex, the assessment of the P-glycoprotein (P-gp) inhibiting activities of the representative set of compounds 1, 2, 6–10 and 15–19 revealed the activity of jatrophanes 6 and 9 as the most powerful in inhibition of P-gp, even higher than R(+)-verapamil and tariquidar in colorectal multi-drug resistant (MDR) cells (DLD1-TxR) (Jadranin et al. 2013).
2.2 Identification of the Components in the Crude Extracts Without Prior Isolation
There are two general approaches in the analysis of the crude extracts without prior isolation: hyphenated (coupled) techniques involving the combination of chromatographic and spectroscopic techniques and direct spectroscopic measurement of the extract, as well as their combination (Fig. 4.7).
Example #2
Proanthocynidins from grape ( Vitis vinifera ) seed extracts
The hyphenated techniques involving LC are now extensively used for investigation of various polar biologically active secondary metabolites such as, for example, antioxidant phytochemicals. Most of them are polyphenols widely present in the plant kingdom. Very important representatives of these natural products are proanthocyanidins (PAs), also known as condensed tannins. They are ubiquitous and one of the most abundant group of natural polyphenols and can be detectable in a wide variety of food sources, such as fruits, nuts, beans, apples, and red wine (Prior and Gu 2005). PAs in foods are of interest in human nutrition and medicine due to their antioxidant capacities and beneficial effects on human health in reducing the risk of chronic diseases, such as cardiovascular and cancer. They are oligomers and polymers composed of flavan-3-ol units linked mainly through C(4)-C(8) (Fig. 4.8a) and less frequent through C(4)-C(6) bond (Fig. 4.8b), both denoted as B-type. The flavan-3-ol units can also be doubly linked by an additional ether C(2)-O(7) which is assigned as A-type (Fig. 4.8c).
The monomeric units of the oligomeric and polymeric PAs are afzelechin, catechin, gallocatechin and their 3β-epimers (Fig. 4.9). The size of PAs is described by its degree of polymerization (DP). Oligomers are those with DP = 2 ~ 7, whereas polymers exhibit DP = 8 ~ 24 or more.
The most common classes of the proanthocyanidins are the procyanidins, which are chains of catechin, epicatechin, and their gallic acid esters, and the prodelphinidins, which consist of gallocatechin, epigallocatechin, and their galloylated derivatives as the monomeric units. The proanthocyanidins containing afzelechin as the subunits are named propelargonidins.
Several methods for the analysis of polyphenols have been presented in the literature (Gu 2012). Most of them are based on liquid chromatography (LC) coupled with either a photodiode array (PDA) detector and/or a mass spectrometer (MS). This is a very effective and highly sensitive method for characterizing procyanidins from complex matrices (Flamini 2003; Reed et al. 2005). In recent years, electrospray ionization (ESI) has been shown to be suitable for the analysis of such polar compounds in aqueous solutions without any previous sample derivatisation (Gaskell 1997). ESI permits the identification of the molecular weight of procyanidins with different degrees of polymerisation. Both normal and reverse phase LC techniques are applied. The normal- phase technique effects the separation of the proanthocyanidin oligomers according different degrees of polymerisation (DP) (Gu et al. 2003) whereas reversed-phase C18 columns have the ability to separate different isomeric oligomers with equivalent molecular mass. However reverse-phase analysis of higher oligomeric proanthocyanidins (i.e. ≥ tetramers) is not feasible due to the fact that, with increasing degrees of polymerization, the number of isomers also increases. This effect results in a retention time overlap of isomers containing differing degrees of polymerization, causing the large unresolved peak (Guyot et al. 1997). Catechins and proanthocyanidins are usually detected at 280 nm using a UV detector. However, peak intensity is rather low, and many other phenolic compounds also absorb light at 280 nm. Much better sensitivity is obtained using fluorescence with excitation and emission set to 230 and 321 nm, respectively (Gu 2012; Gómez-Alonsoa et al. 2007).
The application of the LC/MS method for the analysis of such compounds, together with 13C NMR spectroscopy, is demonstrated in our laboratory by direct analysis of polyphenol constituents of grape seed MeOH extracts (GSE) (Stanković et al. 2008). Grape seeds are known as an abundant source of procyanidins consisting of both (+)-catechin and (−)-epicatechin forming units that, in the particular case of (−)-epicatechin, can appear galloylated or not (Cantos et al. 2002). The use of a rapid resolution HPLC column and the appropriate gradient program afforded the separation of seventeen phenolic compounds in less than 30 min (see the example shown in Figs. 4.10 and 4.11) (Stanković et al. 2008). The exact mass measurements of the of molecular and fragment ions of analytes with a time-of-flight (TOF) mass spectrometer in positive and in negative polarity mode revealed the structures of seventeen polyphenols in our extract comprising catechin and epicatechin monomers, i.e. procyanidin oligomers, and their gallates (Fig. 4.11).
LC PDA of GSE (V. vinifera L., cv. Cabernet Sauvignon, Montenegro) chromatograms (λ = 280 nm), measured using Zorbax Eclipse XDB-C18 column (5 μm, 150 × 4.6 mm). For the chromatographic conditions (see Ref. Stanković et al. (2008))
Gross structures of the identified compounds in GSE (numbered according to LC-elution order, Fig. 4.11). In addition, monogallates of proantocyanidin trimer (10) and dimer (14), (−) – epicatechin monogallate (17) and proantocynaidin dimer digallate (16) were detected
The procyanidin dimers, trimers, tetramers and pentamers (B-type) have molecular masses of 578, 866, 1,154 and 1,442, respectively and their gross structures were identified in the positive mode by the abundant [M + H]+ and, in some cases by [M + Na] + and [2M + Na]+ sodium adducts (positive mode). All identified compounds exhibited [M-H]− in the negative mode, as well as [2M-H]− ions for some of them, confirming molecular mass. In addition, procyanidin dimers and epicatechin gallate possessed high affinity to sodium ions, giving adducts such as [M + Na-2H]− and [2M + 2Na-3H]−. Doubly charged [M-2H]2− species were observed for procyanidin epicatechin gallate possessed high affinity to sodium ions, giving adducts such as [M + Na-2H]− and [2M + 2Na-3H]−. Doubly charged [M-2H]2− species were observed for procyanidin trimers.
It should also be noted that such identification, based solely on precise mass measurement of quasimolecular ions, of the gross structure of procyanidin oligomers is tentative. This method allows only elucidation of chain length, and the chemical constitution of the individual chains, but not the stereochemistry at the chiral centers C-2 and C-3. In order to get more information regarding the structure of procyanidin oligomers detected in the extract, the 13C NMR spectroscopy was applied (Fig. 4.12). The 13C NMR spectrum of the GSE was measured by a pulse sequence allowing the total relaxation of all carbons, as well as the suppression of the nuclear Overhauser effect (NOE effect) which enables quantitative measurements based on peak intensities. Assignments of the resonances (Fig. 4.12) were based on comparison with those reported in the literature (Newman et al. 1987; Behrens et al. 2003).
13C 125 MHz NMR spectrum of the GSE in acetone-d6/D2O + tris(2,4-pentadionato)chromium(III) (CrAcac) at conc. 50 mM (relaxation reagent), recorded with inverse gated decoupling pulse sequence for NOE suppression, 8-supst – substituted position 8; 3ext-, 3term – position 3 at extender and terminal flavan-3-units (Ref. Stanković et al. (2008))
This spectrum provided structural and stereochemical details on subunit composition. The signals of the 13C-NMR spectrum indicated the presence of catechin, epicatechin and B-type linked procyanidins, with some traces of gallate unit. There were no other major signals present in GSE, ruling out the presence of some other types of flavonoid molecules, or proanthocyanidins based on other units. The signals at δ 82 and 79 ppm were diagnostic for the 2,3-trans and 2,3-cis stereochemistry of the heterocyclic ring respectively, indicating that both stereoisomers (catechin/epicatechin) are present. Comparison of the areas of these resonances, gave catechin/epicatechin ratio of 1:0.74.
The same HPLC UV/ESI MS method was also applied subsequently in screening of the GSE from eight grape cultivars (Vitis vinifera) growing in Serbia for their polyphenolic composition (Gođevac et al. 2010). The study revealed 34 phenolic compounds belonging to the following groups: flavan-3-ol monomers, procyanidin dimers and trimers, flavonoids, hydroxycinnamic acid and hydroxybenzoic acid derivatives. The quantities of the main constituents were determined using LC PDA. Qualitative and quantitative differences among the cultivars were observed.
In the above examples only oligomeric proanthocyanidin chains of B-type formed from the same monomeric flavanol building blocks, i.e. (+)-catechin and its (−)-epimer, i.e. procyanidins, were encountered. However, there are numerous examples of heterogeneous proanthocynidins containing in addition to (epi)catechin other monomer units such as (epi)afzelechin and/or gallocatechin, as well as A-type linkage. Such type of mixed chains could be analysed by combination of LC with tandem mass spectrometry, e.g. (LC/MS/MS) or LC-multiple stage MS (LC/MSn). Tandem mass spectrometry (MS/MS) can give more information about the structural details of the different molecules which is based on their characteristic fragmentation patterns. The MS/MS technique used for this analysis is mostly product ion scan involving selection of the quasi-molecular ion, such as [M-H]− or [M + H]+ by the first analyzer, its fragmentation by the collisional activation, followed by the analysis of product ions. The proanthocynidin mixtures can also be analysed directly, without chromatographic separation using matrix-assisted laser desorption ionization in combination with time of flight mass spectrometry (MALDI/TOF MS) using the so-called post source decay (PSD) fragmentation (Behrens et al. 2003). Another example of direct analysis, without chromatographic separation of the condensed tannins is a direct flow injection electrospray ionization ion trap tandem mass spectrometry (ESI-IT-MS/MS) which was used to investigate the polyphenolic compounds present in an infusion from the barks of Hancornia speciosa Gom. (Apocynaceae), a native Brazilian plant popularly known as ‘mangabeira’, used as a source of nutrition and against gastric disorders (Rodrigues et al. 2007).
In the negative mode MS the proanthocynaidins exhibit three main MS fragmentation pathways assigned as quinone methide (QM), the heterocyclic ring fission (HFR) and the retro-Diels-Alder (RDA) mechanisms (Fig. 4.13), that can be useful in structure determination, namely the assignment of the connection sequence of different monomeric units (Gu 2012; Gu et al. 2003).
Main fragmentation pathways of B-type proanthocyanidins illustrated for the dimer identified in the infusion from the barks of Hancornia speciosa (Ref. Rodrigues et al. (2007))
The QM, HFR and RDA fragmentation patterns are also encountered in the positive mode MS of proanthocyanidins and are also used for sequencing of the proanthocyanidins (Li and Deinzer 2007). The A-type linkage could be easily recognized from the molecular mass. Introduction of one A-type linkage leads to its reduction of mass by two mass units in comparison with that of the B-type. Thus, procyanidin trimer gave rise to [M-H]− m/z 865, whereas the corresponding trimer with one A-type linkage yielded [M-H]− m/z 863. Contrary to the B-type, A-type interflavan bond doesn’t undergo QM cleavage, which an additional difference between them used for the sequencing.
Example #3
Major constituents of Hypericum perforatum (St. John’s Wort) by LC/SPE-NMR and LC/MS (Tatsis et al. 2007)
The hyphenated instrumentation of LC/UV/SPE-NMR and LC/UV/ESI MS techniques have been applied for separation and structure verification of the major known constituents present in Greek Hypericum perforatum extracts. The chromatographic separation was performed on a C18 column (Fig. 4.14). Acetonitrile-water was used as a mobile phase.
Chromatographic profile of Hypericum perforatum extract at 270 nm as obtained by LC-PDE/SPE-NMR instrumentation. The peaks under study, which are denoted 1–12, were trapped three times each in the same SPE cartridge (Ref. Tatsis et al. (2007))
For the on-line NMR detection, the analytes eluted from column were trapped one by one onto separate SPE cartridges, and hereafter transported into the NMR flow-cell. LC/UV/SPE-NMR and LC/UV/MS allowed the characterization of constituents of Greek H. perforatum, mainly naphtodianthrones (hypericin, pseudohypericin, protohypericin, protopseudohypericin), phloroglucinols (hyperforin, adhyperforin), flavonoids (quercetin, quercitrin, isoquercitrin, hyperoside, astilbin, miquelianin, I3,II8-biapigenin) and phenolic acids (chlorogenic acid, 3-O-coumaroylquinic acid) (Fig. 4.15). Two phloroglucinols (hyperfirin and adhyperfirin) were detected for the first time, which have been previously reported to be precursors in the biosynthesis of hyperforin and adhyperforin (compounds 9 and 10, Fig. 4.15).
Structures of the major compounds present in Greek H. perforatum extract identified by LC/UV/SPE-NMR and LC/UV/ESI MS (Ref. Tatsis et al. (2007))
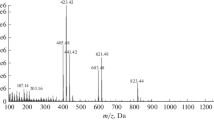
The identification of novel phloroglucinols 9 and 10 (named hyperfirin and adhyperfirin, respectively) was based on measured m/z values of 467 and 481 of [M-H]− ions, respectively. The mass data and especially the MS/MS results showed that these phloroglucinols are homologues of hyperforin (11) and adhyperforin (12). The MS/MS fragmentation pattern analysis indicated the absence of a prenyl chain compared to those of 11 and 12. The 1H NMR spectra obtained by the LC/SPE-NMR (Fig. 4.16) were fully compatible with the structures of acylphloroglucinols 9 and 10, which were reported as biosynthetic intermediates of hyperforin and adhyperforin (Adam et al. 2002; Bystrov et al. 1975).
Selected regions of the 400 MHz 1H NMR after triple trapping on the same cartridge of (a) 9, hyperfirin, and (b) 11, hyperforin. The asterisk denotes an unknown peak (Ref. Tatsis et al. (2007))
The prenylated phloroglucinol hyperforin is thought to be an essential component for the anti-depressant activity of St. John’s Wort (Hypericum perforatum), but this molecule is highly unstable undergoing to facile oxidative degradation (Trifunović et al. 1998; Verotta et al. 1999; Vugdelija et al. 2000; Vajs et al. 2003). The degradation products of hyperforin were the subject of numerous studies involving different LC-hyphenated techniques, mostly LC/MS (Wang et al. 2004; Ang et al. 2004; Fuzzati et al. 2001; Wolfender et al. 2003). In one of them (Wolfender et al. 2003) the application of on-flow and stop-flow LC/NMR and LC/MS/MS of the extract revealed the structures of few products of oxidative degradation of hyperforin.
Example #4
An attempt to analyze a mixture of secondary metabolites (secoiridoids, xanthones and C -glucoflavones) of Gentianceae species by application of diffusion-ordered spectroscopy (DOSY) (unpublished results)
Nowadays, and thanks to the developments of NMR, it is not always necessary to separate the components of a mixture in order to obtain spectroscopic information from its constituents. The mixture could be directly submitted to NMR and the acquisition is implemented in a way that allows the data from the components to be obtained with no previous separation. The most important procedures are based on differences in the translational diffusion and NMR (diffusion ordered sectroscopy, DOSY) and relaxation times (so called relaxation editing) of the components in the mixture. The survey of these methods is presented in a review article by R. Novoa-Carballal et al. (2011). Unlike other physical methods used for mixture analysis, such as LC/MS or LC/NMR, DOSY does not require a prior separation. DOSY has been utilized as a non-invasive technique which involves the separation of the NMR peaks corresponding to individual components of mixtures based on differences of their diffusion coefficients, and therefore differences in the size and shape of the molecule, as well as physical properties of the surrounding environment such as viscosity, temperature, etc. (Morris 2002). The two dimensional DOSY spectrum represents chemical shifts and diffusion coefficients in two orthogonal directions, and the signals of the individual components should be effectively resolved in the diffusion dimension. One of the recent examples involving application of the technique in the field of the natural product chemistry is a flavonoid mixture analysis by DOSY (Cassani et al. 2012). However, as it was pointed out in this paper, the method struggles when diffusion coefficients are very similar and/or when spectra are highly overlapped. Nearly in all (except the simplest) mixtures, some signals overlap. In heavily overlapped spectra, interpretation of the data can be prevented (see Fig. 4.17).
In course of our studies of secondary metabolites of wild-growing plant species from the central part of Balkan belonging to Gentianaceae family three classes of compounds were encountered typical for the family: secoiridoids, C-glucoflavones and xanthones (Aljančić et al. 2008). In these studies the “tradititional” method involving the application of various preparative chromatographic techniques for isolation of pure compounds was applied. In order to develop more efficient method for the investigation of such extracts, preliminary DOSY experiments were carried out on the MeOH extract of leaves of the very well known medicinal plant Gentiana lutea (Yellow Gentian), containing the above mentioned three classes of secondary metabolites (Šavikin et al. 2009). As it could be seen, the DOSY of this extract (Fig. 4.17) is the typical example of the severe overlap which doesn’t allow the interpretation of the data.
At the same time, the DOSY spectrum of the artificial mixture of three constituents, mangiferin (C-glucoxanthone), gentiopicroside (secoiridoid) and isoorientin (C-glucoflavone) (Fig. 4.18), detected in the G. lutea extract using LC UV/ ESI MS (not shown), was recorded (Fig. 4.19).
With exception of the majority of glycosidic signals and a vinyl proton at δ 7.40 the resonances of gentiopicroside are well resolved on the diffusion axis. Due to the very similar diffusion coefficients, the signals of C-glucosides mangiferin and isoorientin are overlapped.
At the same in DOSY spectrum of a binary mixture of mangiferin and gentiopicroside, only some sugar signals are not resolved on the diffusion axis (Fig. 4.20).
There are some processing techniques for overcoming the problem of signals overlap, such as multi-exponential fitting (Nilsson et al. 2006) and multivariate methods (Nilsson and Morris 2008). Another approach to significantly reduce the overlap is extending the 2D DOSY experiment to 3D DOSY using HMQC, (Barjat et al. 1998) COSY, (Wu et al. 1996) TOCSY, (Viel and Caldarelli 2008) or NOESY (Gozansky and Gorenstein 1996). Unfortunately, much longer data acquisition times and more complicated processing are required for 3D experiments, which can still fail to resolve complex mixtures. Peak overlap can also be efficiently reduced by suppressing the multiplet structure, as in method called pure shift NMR (Nilsson and Morris 2007). It has been also shown that performing DOSY in a matrix with which the analytes interact differentially can resolve signals from similar compounds that would otherwise show the same diffusion. In this method, called matrix-assisted DOSY the interaction of the analytes with the matrix modulates the average diffusion coefficients, as different mixture components bind to the matrix to different extents.
Adding matrix to the mixture can also sometimes helps to resolve the spectral overlap by causing differential chemical shift changes (Tormena et al. 2010). It has been demonstrated that the component spectra of a mixture of isomers with nearly identical diffusion coefficients (catechol, resorcinol, and hydroquinone) can be resolved using matrix-assisted DOSY, in which diffusion is perturbed by the addition of a surfactant. The isomeric species are then resolved due to their different degrees of interaction with micelles or reversed micelles formed by the addition of a surfactant (Evans et al. 2009).
Another example is dissolving the two naturally-occurring epimers of naringin in an aqueous solution of β-cyclodextrin which caused both shift and diffusion changes, allowing the signals of the epimers to be distinguished. Chiral matrix-assisted DOSY has the potential to allow simple resolution and assignment of the spectra of epimers and enantiomers (Adams et al. 2011).
According to this, matrix-assisted DOSY method has great potential for the analysis of complex mixtures, such as those common in natural product chemistry.
3 Conclusion
As shown in the examples given, hyphenated techniques – in which chromatographic separations are coupled with powerful spectroscopic techniques such as MS and NMR, as well as diffusion-ordered NMR (DOSY) experiments provide a great deal of information about the content and nature of constituents of the crude plant extracts. These methods are playing an increasingly important role as strategic tools for screening or secondary metabolite profiling aimed at distinguishing between already known compounds (dereplication) and new molecules directly in crude plant extracts. They are very useful when dealing with large numbers of extracts because unnecessary isolation of known compounds is avoided. At the same time, after the preliminary analyses using the hyphenated methods, the isolation of pure compounds, for complete structural elucidation and biological or pharmacological testing, is still needed.
References
Adam P, Arigoni D, Bacher A, Eisenreich W (2002) Biosynthesis of hyperforin in Hypericum perforatum. J Med Chem 45:4786–4793
Adams RW, Aguilar JA, Cassani J, Morris GA, Nilsson M (2011) Resolving natural product epimer spectra by matrix-assisted DOSY. Org Biomol Chem 9:7062–7064
Aljančić IS, Vajs VE, Tešević VV, Milosavljević SM (2008) Some wild-growing plant species from Serbia and Montenegro as the potential sources of drugs. Curr Pharm Des 14:3089–3105, and references therein
Aljančić IS, Pešić M, Milosavljević SM, Todorović NM, Jadranin M, Milosavljević G, Povrenović D, Banković J, Tanić N, Marković ID, Ruždijić S, Vajs VE, Tešević VV (2011) Isolation and biological evaluation of jatrophane diterpenoids from Euphorbia dendroides. J Nat Prod 74:1613–1620
Ang CYW, Hu L, Heinze TM, Cui Y, Freeman JP, Kozak K, Luo W, Mattia A, Dinovi M (2004) Instability of St. John’s Wort (Hypericum perforatum L.) and degradation of hyperforin in aqueous solutions and functional beverage. J Agric Food Chem 52:6156–6164
Barjat H, Morris GA, Swanson AG (1998) A three-dimensional DOSY-HMQC experiment for the high-resolution analysis of complex mixtures. J Magn Reson 131:131–138
Behrens A, Maie N, Knicker H, Kögel-Knabner I (2003) MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry 62:1159–1170
Bringmann G, Lang G (2003) Full absolute stereostructures of natural products directly from crude extracts: the HPLC-MS/MS-NMR-CD ‘triad’. In: Müller WEG (ed) Marine molecular biotechnology. Springer, Berlin/Heidelberg, pp 89–116
Bystrov NS, Chernov BK, Dobrynin VN, Kolosov MN (1975) The structure of hyperforin. Tetrahedron Lett 32:2791–2794
Cantos E, Espin JC, Tomas-Barberan FA (2002) Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J Agric Food Chem 50:5691–5696
Cassani J, Nilsson M, Morris GA (2012) Flavonoid mixture analysis by matrix-assisted diffusion-ordered spectroscopy. J Nat Prod 75:131–134
Covey TR, Lee ED, Henion JD (1986) High-speed liquid chromatography/tandem mass spectrometry for the determination of drugs in biological samples. Anal Chem 58:2453–2460
Evans R, Haiber S, Nilsson M, Morris GA (2009) Isomer resolution by micelle-assisted diffusion-ordered spectroscopy. Anal Chem 81:4548–4550
Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM (1989) Electrospray ionization for mass spectrometry of large biomolecules. Science 246(4926):64–71
Flamini R (2003) Mass spectrometry in grape and wine chemistry. Part I: polyphenols. Mass Spectrom Rev 22:218–250
Fuzzati N, Gabetta B, Strepponi I, Villa F (2001) High-performance liquid chromatography–electrospray ionization mass spectrometry and multiple mass spectrometry studies of hyperforin degradation products. J Chromatogr A 926:187–198
Gaskell SJ (1997) Electrospray: principles and practice. J Mass Spectrom 33:677–688
Gođevac D, Tešević V, Veličković M, Vajs V, Milosavljević S (2010) Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J Serb Chem Soc 75:1641–1652
Gómez-Alonsoa S, García-Romeroa E, Hermosín-Gutiérrez I (2007) HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J Food Comp Anal 20:618–626
Gozansky EK, Gorenstein DG (1996) DOSY-NOESY: diffusion-ordered NOESY. J Magn Reson B 111:94–96
Gu L (2012) Analysis methods of proanthocyanidins. In: Xu Z, Howard LR (eds) Analysis of antioxidant-rich phytochemicals. Wiley-Blackwell, Chichester, pp 247–274, and references therein
Gu L, Kelm MA, Hammerstone JF, Zhang Z, Beecher G, Holden J, Haytowitz D, Prior RL (2003) Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J Mass Spectrom 38:1272–1280
Guyot S, Doco T, Souquet JM, Moutounet M, Drilleau JF (1997) Characterization of highly polymerized procyanidins in cider apple (Malus sylvestris var. Kermerrien) skin and pulp. Phytochemistry 44:351–357
Henion JD, Thomson BA, Dawson PH (1982) Determination of sulfa drugs in biological fluids by liquid chromatography/mass spectrometry/mass spectrometry. Anal Chem 54:451–456
Jadranin M, Pešic M, Aljančić IS, Milosavljević SM, Todorović NM, Podolski-Renić A, Banković J, Tanić N, Marković I, Vajs VE, Tešević VV (2013) Jatrophane diterpenoids from the latex of Euphorbia dendroides and their anti-P-glycoprotein activity in human multi-drug resistant cancer cell lines. Phytochemistry 86:208–217
James AT, Martin AJP (1952) Gas–liquid partition chromatography: the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem J 50:679–680
Jaroszewski JW (2005) Hyphenated NMR methods in natural products research, part 2: HPLC-SPE-NMR and other new trends in NMR hyphenation. Planta Med 71:795–802
Li H-J, Deinzer ML (2007) Tandem mass spectrometry for sequencing proanthocyanidins. Anal Chem 79:1739–1748
Morris GA (2002) Advances in NMR. In: Grant DM, Harris RK (eds) Encyclopedia of nuclear magnetic resonance, vol 9. Wiley, Chichester, p 35
Newman RH, Porter LJ, Foo LY (1987) High-resolution 13C NMR studies of proanthocyanidin polymers (condensed tannins). Magn Reson Chem 25:118–124
Nilsson M, Morris GA (2007) Pure shift proton DOSY: diffusion-ordered 1H spectra without multiplet structure. Chem Commun 933–935
Nilsson M, Morris GA (2008) Speedy component resolution: an improved tool for processing DOSY data. Anal Chem 80:3777–3782
Nilsson M, Connell MA, Davis AL, Morris GA (2006) Biexponential fitting of diffusion-ordered NMR data: practicalities and limitations. Anal Chem 78:3040–3045
Novoa-Carballal R, Fernandez-Megia E, Jiménez C, Riguera R (2011) NMR methods for unravelling the spectra of complex mixtures. Nat Prod Rep 28:78–98
Pesic MM, Bankovic JZ, Aljancic IS, Todorovic NM, Jadranin MB, Vajs VE, Tesevic VV, Vuckovic IM, Momcilovic MB, Markovic ID, Tanic NT, Ruzdijic SD (2011) New anti-cancer characteristics of jatrophane diterpenes from Euphorbia dendroides. Food Chem Toxicol 49:3165–3173
Prior RL, Gu L (2005) Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry 66:2264–2280
Reed JD, Krueger CG, Vestling MM (2005) MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 66:2248–2263
Rodrigues CM, Rinaldo D, dos Santos LC, Montoro P, Piacente S, Pizza C, Hiruma-Lima CA, Souza Brito Alba RM, Vilegas W (2007) Metabolic fingerprinting using direct flow injection electrospray ionization tandem mass spectrometry for the characterization of proanthocyanidins from the barks of Hancornia speciosa. Rapid Commun Mass Spectrom 21:1907–1914
Šavikin K, Menković N, Zdunicć G, Stević T, Radanović D, Janković T (2009) Antimicrobial activity of Gentiana lutea L. extracts. Z Naturforsch C 64:339–342
Stanković M, Tešević V, Vajs VE, Todorović N, Milosavljević SM, Godjevac DM (2008) Antioxidant properties of grape seed extract on human lymphocyte oxidative defence. Planta Med 74:730–735
Tatsis EC, Boeren S, Exarchou V, Troganis AN, Vervoort J, Gerothanassis IP (2007) Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 68:383–393
Tormena CF, Evans R, Haiber S, Nilsson M, Morris GA (2010) Matrix-assisted diffusion-ordered spectroscopy: mixture resolution by NMR using SDS micelles. Magn Reson Chem 48:550–553
Trifunović S, Vajs V, Macura S, Juranić N, Djarmati Z, Jankov R, Milosavljević S (1998) Oxidation products of hyperforin from Hypericum perforatum. Phytochemistry 49:1305–1310
Vajs V, Vugdelija S, Trifunović S, Karadžić I, Juranić N, Macura S, Milosavljević S (2003) Further degradation product of Hypericum perforatum (St. John’s Wort). Fitoterapia 74:439–444
Verotta L, Appendino G, Belloro E, Jakupovic J, Bombardelli E (1999) Furohyperforin, a prenylated phloroglucinol from St. John’s Wort (Hypericum perforatum). J Nat Prod 62:770–772
Viel S, Caldarelli S (2008) Improved 3D DOSY-TOCSY experiment for mixture analysis. Chem Commun 2013–2015
Vugdelija S, Vajs V, Trifunović S, Djoković D, Milosavljević S (2000) A new hetereocyclization product of Adhyperforin from Hypericum perforatum (St. John’s Wort). Molecules 5:M158
Wang Z, Ashraf-Khorassani M, Taylor LT (2004) Air/light-free hyphenated extraction/analysis system: supercritical fluid extraction on-line coupled with liquid chromatography-UV absorbance/electrospray mass spectrometry for the determination of hyperforin and its degradation products in Hypericum pertoratum. Anal Chem 76:6771–6776
Wolfender JL, Ndjoko K, Hostettmann K (2001) The potential of LC-NMR in phytochemical analysis. Phytochem Anal 12:2–22
Wolfender J-L, Verotta L, Belvisi L, Fuzzati N, Hostettmann K (2003) Structural investigations of isomeric oxidised forms of hyperforin by HPLC-NMR and HPLC-MS. Phytochem Anal 14:290–297
Wu D, Sorg B, Hecker E (1994) Oligocyclic and macrocyclic diterpenes in Thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China) 6. Tigliane type diterpene esters from latex of Euphorbia prolifera. Phytother Res 8:95–99
Wu D, Chen A, Johnson CS (1996) Three-dimensional diffusion-ordered NMR spectroscopy: the homonuclear COSY–DOSY experiment. J Magn Reson Ser A 121:88–91
Acknowledgements
This research was supported by the Ministry of Education and Science of the Republic of Serbia (Grant no. 172053).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Gođevac, D., Jadranin, M., Aljančić, I., Vajs, V., Tešević, V., Milosavljević, S. (2015). Application of Spectroscopic Methods and Hyphenated Techniques to the Analysis of Complex Plant Extracts. In: Máthé, Á. (eds) Medicinal and Aromatic Plants of the World. Medicinal and Aromatic Plants of the World, vol 1. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9810-5_4
Download citation
DOI: https://doi.org/10.1007/978-94-017-9810-5_4
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9809-9
Online ISBN: 978-94-017-9810-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)