Abstract
Based on three main reasons, the biosafety of hydrogen is very high: The first is evidence from medical research on hydrogen diving, the second is that hydrogen is an endogenous gas, and the third is direct research on the biosafety of hydrogen. Research on hydrogen diving, combined with human trials on hydrogen diving, proved that hydrogen is very safe for humans to breathe. Since a certain level of hydrogen is produced by Escherichia coli in the large intestine of normal humans, hydrogen can be considered an endogenous gas. So far, no clinical evidence has been found that hydrogen can be harmful for the human body. Published data from the EU and the US government on the biosafety of hydrogen showed that hydrogen has no acute or chronic toxicity on the human body under normal pressure. Despite this, any substance that can produce biological effects on the human body has the potential of destroying homeostasis, which may be harmful. Although the biosafety of hydrogen is very high, we still cannot assure that hydrogen has no side effects on the human body.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Studies in Diving Medicine
Hydrogen was first named by the famous French chemist Lavoisier, who was also the forerunner of the study of the physiology of hydrogen. Since hydrogen and oxygen can be chemically combined into water, Lavoisier named hydrogen using Hydro as the root which means elements of water. In Japanese and Korean languages, hydrogen is translated directly into elements of water. So in the market, elements of water equal hydrogen water. Our chemistry named hydrogen as “Qing,” since it has a pronunciation similar to light in Chinese and it is a kind of gas.
As early as 1789, the famous chemist Lavoisier and Sequin performed animal studies using hydrogen as breathing medium. In the experiment, Lavoisier put guinea pigs into a bell-shaped glass container; hydrogen was added into the container while nitrogen and oxygen were maintained above a certain level to keep them alive. No adverse effect was found after 8–10 h. [1].
In 1937, English scientists Case and Haldane carried out a human study in which hydrogen was added to breathing gas during diving. The subjects were exposed under a pressure of 1.1 MPa when breathing hydrogen–oxygen mixed gas for 5 min, and no adverse effect was found [2]. In 1941, the former Soviet Union scientist Lazarev conducted an experiment on mice under a pressure of 9.1 MPa; mice breathed a gas mixture of hydrogen, nitrogen, and oxygen for 3 min, and then survived after 1-h decompression [3]. These early studies could draw a preliminary conclusion that it is safe for humans and animals to breathe a gas mixture of hydrogen and oxygen.
In the early stage of diving experiments on breathing oxygen and helium conducted by US scientists, a hydrogen mixture was attempted in diving studies in many countries since it was very difficult to obtain helium at that time. The most famous example was the experiment conducted by Arne Zetterstrom (Fig. 3.1), a diving technical engineer in the Sweden navy, who tried a gas mixture of hydrogen and oxygen during diving [4].
Hydrogen–oxygen mixed gas is explosive, but no explosion will occur when oxygen concentration is lower than 4 %. Under normal pressure, life will not survive when oxygen concentration is 4 %. However, the partial pressure of oxygen can be elevated to 16 KPa. In 1944, Zetterstrom invented a way of producing hydrogen and oxygen mixture from air; the concentration of oxygen in this mixture is 4 % and explosion can be avoided. The hydrogen and oxygen mixture was then applied in diving. Zetterstrom first replaced used air by a nitrogen and oxygen (4 %) mixture as breathing gas at a depth of 30 m, then nitrogen was replaced by the same percentage of hydrogen, and he successfully dived to a depth of 100 m. He became extraordinarily excited at this depth (anesthetic effect of high-pressure hydrogen), and his voice also changed obviously, which influenced the communication with people on the surface.
On August 7, 1945, to prove that hydroxide diving techniques play an important role in submarine rescue, Zetterstrom successfully dived to a depth of 161 m in the biggest submarine rescue ship Belos by breathing hydroxide gas. Unfortunately, since there was a lack of a standard decompression procedure and faulty operation by surface staff, Zetterstrom directly ascended to the surface without decompression in the 50-m decompression stop. Zetterstrom died due to severe anoxia and decompression sickness [5].
Since 1960, diving medicine, especially the improvement of saturated diving technology, promoted animal experiments on hydroxide diving to be conducted again by US, English, French, Russian, and Swedish scientists [6]. Between 1960 and 1970, the improvement of hydrogen-producing technology had a greater price advantage; replacing hydrogen with helium aroused high attention again. During this period of research on hydroxide diving, animal experimentation had reached a depth of 1000 m and breathing hydrogen exposure time up to 24 h. Human experimentation has reached a depth of 60 m and exposure time 10–20 min. In the 1970s, Edel conducted simulation diving experiments on hydroxide. He not only studied the effects of hydrogen on the body but also included hydroxide diving safety procedures, decompression plans, and respiratory gas conversion technology [7]. The greatest achievement at that time was the finding that hydrogen could prevent high-pressure nervous syndrome (HPNS), which is one of the most important advantages of breathing hydrogen. Breathing helium at a depth over 300 m could cause HPNS, which is one of the biggest obstacles during increasing diving depth [8].
With continuous ocean development, greater depths of diving operations are needed, which promote the study of hydroxide diving. Throughout the world, France has been in the leading position with respect to hydroxide diving study. COMEX is a French company that once led research on diving medicine and technology. In the early 1980s, the company began to carry out a dive plan using a deep-sea gas mixture Hydra (Hydra means hydrogen, as hydrogen was the main gas in the mixture). The dive plan included animals, human simulation, and field experiments, consisting of three sections which were security check, medical physiology, and developing diving equipment. Hydra III–VII simulation experiments were conducted in which a routine dive at a depth of 75–90 switches to hydroxide saturation diving at a depth of 520 m. During Hydra V, hydrogen was first used in saturation diving at a depth of 450 m; the divers completed mechanical connection, underwater cutting, and several diving operation tasks [9, 10].
In June 1983, COMEX started the Hydra plan. Under the leadership of Deulaze, president and concurrently diver of the company, Hydra III diving experiments were conducted at a depth of 91 m near Marseille; the breathing hydrogen gas mixture consisting of 95 % oxygen was 5 %. No anesthetic effects of hydrogen were found, heat dissipation effects were similar to helium, and breathing resistance was lower than helium. In November 1983, Hydra IV human simulation experiments were conducted at a depth of 300 m, six subjects breathing a gas mixture of hydrogen, oxygen, and helium, the gas ratio of which was 74:24:2, respectively. Psychology examination found that the visual reaction time, calculating ability, and memory effects were less than those at a depth of 80-m air diving as well as those at a depth of 240-m hydroxide diving (gas ratio of hydrogen to oxygen less than 98:2). At a depth of 180 m, breathing a gas mixture of hydrogen to oxygen (98:2), the anesthetic effect began to appear; hydrogen anesthesia was different from nitrogen narcosis, illusion being the main symptom, the anesthesia level of which was also lower than nitrogen narcosis. Changes in heart rate during hydroxide diving are less than in helium–oxygen diving. After hydrogen simulation diving, indicators of blood, urine, nervous system, and respiratory function were normal. Simultaneously, hydroxide diving animal experiments were conducted; 40 mice were exposed at a depth of 600 m for 40 h, after decompression to ambient pressure, and no abnormalities were found on heart, liver, and lung histology examination [11].
In May 1985, Deulaze carried out the Hydra V experiment; the subjects were divided into two groups, with exposure to a depth of 450 m. The physiological role of hydrogen was studied, underwater capabilities of divers were tested, and the gas mixture ratio was systematically studied at the same time. It was found that the optimal ratio of hydrogen to helium and oxygen was 54:45:1, and during the operation breathing was smooth and no pressurized joint pain appeared [12].
These series of experiments further confirmed that hydrogen is not harmful to the body, while establishing the best safety standards for hydrogen gas mixture preparation.
In November 1986, Fructus et al. carried out a Hydra VI simulated dive, in which eight divers participated in simulated diving at a depth of 520 m 25 times while the water temperature was 4 ºC [10]. In February 1988, COMEX conducted Hydra VIII in the Mediterranean applying hydrogen mixed gas in saturation diving experiments. Six divers took part in the experiment. In February 21, compression started until a depth of 520 m was reached; in the following 6 days, six divers conducted several diving operations [13]. On September 1989, the COMEX company conducted Hydra IX in which divers conducted saturation diving simulation for 14 consecutive days. The purpose of the experiments was to investigate the minimum and maximum depth limit in using hydrogen, the long-term (49 days) effect of high-pressure hydrogen environment exposure on human physiological function, long-time isolated confinement effects on the behavior of the human spirit, and so on. This item completed research plans including diving medicine, neurophysiology, psychology, ventilation and cardiovascular function, biochemical, thermal, and fluid balance, diving decompression procedures, expert systems, etc. [14].
In the 1990s, COMEX successively carried out experiments of saturation diving under 680 m with excursion diving under 701 m (Hydra X) and simulated human saturation diving under 750 m (Hydra XI) [15]. The maximum depth recorded on human exposure to high pressure was 750 m. Currently, COMEX Hydra XII experiments are being carried out; divers dive to a depth of 210 m for 28 dives, including four times for helium–oxygen diving and four for hydrogen helium–oxygen diving. Throughout the experiment, divers breathed heliox in the deck saturation chamber, while breathing a hydroxide mixture during underwater operations. These experiments explored the feasibility of existing equipment for hydroxide diving and closed respiratory system for underwater breathing gas conversion (helium gas mixture converted to hydrogen gas mixture) situation. Each dive lasted for 2–6 h; both analytical thinking and operating capacity of the divers were normal when they breathed the hydrogen gas mixture [16].
In the Hydra plans, Gardette successfully conducted experiments on 110 mice using a hydrogen and helium–oxygen mixture, exposed to a condition of 2000 m for 12 continuous days [10]. It was found that when mice were pressurized with hydroxide at 1800 m, HPNS appeared obviously, but no such syndrome appeared when they used a hydrogen and helium–oxygen mixture instead. These results indicated that hydrogen could alleviate HPNS.
In recent years, more countries have begun to pay close attention to hydroxide diving gradually. The US Navy studied the physiological effects of hydrogen on the human body under high pressure; it was confirmed that hydrogen is harmless, and that it could not only effectively reduce breathing resistance under high pressure but also alleviate the HPNS. US scientists also carried out a special experiment related to hydroxide diving: the biology decompression method for hydroxide diving. Methanogenic bacteria could be capable of converting carbon dioxide and hydrogen into methane and water, through which the partial pressure of hydrogen in vivo could be reduced promptly. They used the above principle to accelerate the decompression of hydroxide diving. In 1998, Kayar reported that decompression time can be effectively shortened through injecting methanogenic bacteria into animal gut; meanwhile, the incidence of decompression sickness was also reduced [17]. More importantly, the following study found that the methanogenic bacteria belong to the normal intestinal flora, and decompression time can be shortened to a certain extent by itself in normal circumstances [18]. If we implement this technology in future diving, decompression time will be further shortened, which is an important direction for future diving medical research.
Since there exist many technical obstacles, hydrogen diving is currently at the research stage. However, the important progress we have made, particularly the knowledge of the physiology of hydrogen diving, remains very valuable.
3.1.1 High-Pressure Hydrogen Gas Has No Toxic Effects
The results of the present study clearly show that hydrogen has no toxic effects on the body at any pressure. Since Lavoisier began to study the role of hydrogen on the body from 1789, almost all research involves the safety issue of hydroxide diving. Although it was once thought that hyperbaric hydrogen may have toxic effects on the body, it turned out to be wrong in later research. Especially when the series of human hydroxide diving experiments were carried out by Sweden, France, etc. successfully, such as the experiments conducted by France from 1988 to 1989, human hydroxide diving time added up to 7200 h. These experiments further explained that it is safe for humans to breathe hydroxide when diving. Since high-pressure hydrogen has no toxic effects, a small dose of hydrogen gas would be safer.
3.1.2 High-Pressure Hydrogen Gas Has a Certain Narcotic Effect
Physiologically inert gases tend to have a certain narcotic effect at high pressure, most notable of which is the narcotic effect of nitrogen. As long as the ambient pressure increases by four times, the narcotic effect will occur in many people. When the pressure increases up to ten times, a very serious narcotic effect will occur in most people, which is also an important reason why air diving should not exceed 60 m. Animal experiments suggest that high-pressure hydrogen also has a certain narcotic effect, which can resist HPNS to a certain extent. This happens to be a problem helium diving could not solve, which becomes one of the advantages of hydrogen toward deep sea diving. The narcotic effect of hydrogen ranks between nitrogen and helium, the relative narcotic effect of nitrogen, hydrogen, and helium being 4.3:2.3:1. The narcotic effect of hydrogen is about 54 % of nitrogen, but higher than helium and neon. People took advantage of this characteristic of hydrogen to reverse the excessive excitatory effects on the body caused by high pressure. Recently, animal and human studies showed that HPNS could be antagonized by hydrogen. HPNS will not occur in humans when they breathe 4.6-MPa (46-atm) hydrogen, nor is working ability affected [19, 20].
The main signs of the narcotic effect of high-pressure hydrogen are hallucinogenic effects, which have a high impact on the cognitive ability of divers; even when the body is exposed to 1.9-MPa high-pressure hydrogen environment, this effect will occur. In 1974, Edel performed the first study on the narcotic effect of hydrogen, in which four subjects dived once, under the conditions of 0.7-MPa breathing nitroxide, heliox, and hydroxide for 120 min. Results of intelligence and operational skills showed that nitroxide has the most obvious influence on operational skills, while no significant effect of heliox and hydroxide was found. In the experiments of Hydra IV, Hydra A, and Hydra V, conducted in 1984, Carioz once investigated the narcotic effect of hydrogen on human bodies; the results showed no obvious changes in operation skills and visual reaction time when breathing 2.45-MPa hydrogen. Human studies also showed that there exist obvious different narcotic sensitivities among individuals [21, 22].
3.1.3 The Diffusion Rate of Hydrogen Is Very Fast
Since the diffusion rate of hydrogen is faster than air, it is easier for hydrogen to pass through narrow pores which is beneficial for the pressure adjustment between inner and outer side of gas chamber such as eardrum room. Hydrogen’s high diffusion ability provides a guarantee for exerting its biological effects; hydrogen can enter into any sites of the body, and any parts of the cell, such as the nucleus and mitochondria. Hydrogen possesses this advantage over many other drugs.
3.1.4 Body Conducts Heat Faster Under Hydrogen Condition
Both thermal conductivity and heat capacity of hydrogen are larger than nitrogen and helium. Therefore, when breathing hydroxide gas, more heat is lost easily from the lungs, which is a major limiting factor for hydrogen diving. Experiments show that at 310-m depth in a hydroxide environment, body heat loss is very fast; even if engaged in heavy physical operation, metabolic heat production cannot compensate the loss of body heat. So hydroxide diving requires external heating. In a hydroxide environment, it is appropriate to maintain cabin temperature within 34 ºC. In addition, the breathing gas should be heated to a certain extent to make divers feel comfortable. However, there exists a debate over the optimum temperature. Smith et al. found that, under the condition of 1.1–10.1 MPa, the speed of respiratory cooling and convection cooling of breathing hydroxide is 38 % and 32 % higher than that of helium oxide, respectively. They proposed that a comfortable temperature range of hydroxide diving should be 31.1–31.6 ºC [23].
3.1.5 Effects of Breathing Hydrogen on Voice
Current researches focus on the voice effect of helium; only a few researches have reported on the voice effect of hydrogen. In the high-pressure hydrogen environment, the human voice changes significantly, such as higher tone, with nasal voice, lower clarity, and being difficult to understand, and so on. During the early hydrogen diving experiments conducted by Swedish engineer Arne Zetterstrom, voice change problem was found which significantly affected the language communication with the surface. His later death accident was caused indirectly by the voice change.
3.1.6 Effects of High-Pressure Hydrogen on Respiratory System
Hydrogen gas is the least dense gas in the universe. When breathing hydroxide, the respiratory resistance is lower; therefore, the work of breathing and physical exertion reduced which helped improve diver’s operational efficiency. Dougherty found that the maximum voluntary ventilation of breathing pure oxygen was equivalent to that of 40 % helium–oxygen; other respiratory indicators changed significantly, mainly due to higher relative density of oxygen, suggesting that ventilation should be larger when breathing hydrogen mixed gas [24, 25]. However, from 4.6-MPa helium oxygen diving simulation experiments conducted by Giry, no significant improvement of ventilation function was found; on the contrary, ventilation function was reduced which might be caused by the narcotic effect of high-pressure hydrogen on the respiratory center [26]. Dahlback found that under 1.3-MPa conditions, functional lung impedance, when breathing 98 % hydrogen and 2 % oxygen, was decreased by 35 % compared to that of 98 % helium and 2 % oxygen, which could reduce the load of the respiratory muscles and then improve the work capacity of deep divers. In addition, breathing hydroxide under the conditions of 1.3 MPa, lung capacity slightly increased, but Giry’s experimental results did not find this [27].
3.1.7 Effects of High-Pressure Hydrogen on Circulation System
Gennser et al. studied the effect of hydrostatic pressure, hydrogen, nitrogen, and helium on rat atrial rate at the cellular level. The results showed that 15-MPa hydrostatic pressure reduced the isolated rat atrial spontaneous frequency by 30.6 ± 7.2 %; if perfused with high-pressure hydrogen (hydrogen partial pressure: 4.9, 9, and 14 MPa) saturated solution, the rat atrial spontaneous frequency increases with the increase in hydrogen partial pressure [28]. Some researchers also found nitrogen to be twice as strong as hydrogen over the effect of alleviating bradycardia, but hydrogen is five times stronger that helium. The same effect was found in 5-MPa nitrogen and 9-MPa hydrogen toward reversing bradycardia. Hydra V experiments showed that hydrogen could resist the effect of hydrostatic pressure. Giry believes that the cardiovascular changes found when breathing high-pressure hydrogen gas mixture might be caused by inhibition of the excitability of the parasympathetic nervous system by hydrogen [29].
Diving medicine investigated the biological effects of hydrogen through exposing animals and humans to very high pressures. Since there exists a relationship between the effect of gas and the concentration of dissolved gas, and the concentration of dissolved gas is related to its partial pressure, the biological effects of high-pressure hydrogen focus on the effects of hydrogen under high pressure, which is different from our current concept of the biological effects of hydrogen. However, by reviewing the relevant knowledge from diving medicine, we can clearly know that even at very high-pressure conditions, hydrogen gas is still very safe for the human body. So when the dosage of breathing hydrogen is far below the diving condition thousands of times, the biosecurity of hydrogen can be assured.
3.2 Hydrogen Is an Endogenous Gas
Bacteria of human large intestine can produce hydrogen, which at least proves that hydrogen is an endogenous gas existing in the normal environment of human cells. Although the evidence is not enough to prove the safety of hydrogen, it provides some clues.
3.2.1 Studies of Intestinal Gas
Studies on gas production and composition of the large intestine have a long history; the composition and sources of gas were the main focus in early times. Even though we know little about the specific details of the gas sources of the large intestine, data about bacteria of the large intestine, which produce hydrogen, are adequate.
According to the literature, the first attempt was to measure gas of the large intestine in 1868. Ruge E, a German scholar, founder of the large intestine gas discipline, utilized a special chair to confine subjects; the anus was connected to a glass tube and gas was collected through the draining water by the gathering of gas law [30]. In 1942, Beazell and Ivey measured gas collected from 24 healthy people in 24 h; they found that 1 day’s gas production of one person was about 380–655 ml. Later, Kirk proved cellulose in foods can increase gas production [31]. Steggerda collected gases with an average of 360 ml every day from some food (boiled eggs, beef, and fine apple juice), which was originally thought not capable of producing gas; further analysis found that it was composed of 7.4 % methane and 19.8 % hydrogen, which initially proved that methane and hydrogen are from coliform bacteria. In the following 7-day test, researchers observed the effects of different types of food in the body gas produced through adding different types of legumes into food, without changing the total energy intake from overall protein, fat, and carbohydrates [32]. The results found that small-molecule carbohydrates such as monosaccharides, disaccharides, and oligosaccharide can contribute to gas production. For example, if choosing pork and soy as the main food, gas production can be increased to 4.2 times. Using a constant perfusion technique, Levitt et al. proved that hydrogen can be produced by all healthy human large intestines, most of the hydrogen generated being entirely dependent on the bacterial fermentation of food components [33].
Gas produced by human colon is mainly composed of hydrogen, carbon dioxide, methane, nitrogen, oxygen, and other trace gases. Nitrogen and oxygen are mainly taken in from the mouth through swallowing into the digestive tract. Gas concentrations of hydrogen in the large intestine can be up to 74 %. Carbon dioxide can be produced by coliform bacteria, which digest dietary ingredients escaping from the human digestive enzymes or endogenous “food” dropping off the colonic mucosa. Some trace gases are really rare. For instance, one research found that the concentration of hydrogen sulfide in the human large intestine was only 1.06 μM, the concentration of methyl mercaptan only 0.21 μM, and the concentration of dimethyl sulfide only 0.08 μM. These trace gases are not only highly diffusible but also easily degradable, resulting in the final concentration of these trace gases released through the anus perhaps being lower than that in the large intestine.
The gas composition is not only affected by the bacterial species in the large intestine but also closely related to the physiological state of the host. As much as 30–40 % of carbon dioxide, hydrogen, and methane can be absorbed by colon mucosa into the circulatory system, and then released by the skin and respiration outside the body. Remaining gases such as hydrogen can be used for other bacteria, or eventually released through the anus. Therefore, the concentration of hydrogen may reflect the ratio of hydrogen producing and hydrogen-consuming bacteria [34].
Less than 20 % of carbohydrates in a typical western diet cannot be directly absorbed by the body; theoretically, 340 ml of hydrogen can be produced by bacteria from 1 g of glucose. Therefore, we can reckon that the maximum amount of hydrogen produced by bacteria in the human body is 13 l. But results from Strocchi and Levitt’s test, perfusion with glucose, showed that each gram of glucose can only produce 80 ml of hydrogen instead of 340 ml [35]. So energy metabolism in bacteria is not so simple; there exist metabolic pathways that do not produce hydrogen while consuming energy substances. Hammer found that ingestion of 12.5 g lactulose will generate 50–200 ml of hydrogen per 6 h [36]. In fact, there is a huge difference in the amount of hydrogen produced according to different diet compositions and individuals. This difference reflects the very complex relationship and influence of factors existing between the human body and bacteria, for example, food type, composition and the amount of food, carbohydrate utilization ability of microorganisms in the large intestine, different number and location distribution of hydrogen producing or hydrogen consuming bacteria, efficiency of intestinal motility, pH, sulfide, and other environmental factors.
The National Aeronautics and Space Administration (NASA) once conducted a comprehensive study during the Apollo program on gas composition of the large intestine [37]. Most people might doubt the motivation of studying gas composition of the large intestine by NASA. In fact, this is the major obstacle related to security management inside the spaceship, since a high proportion of combustible gases are produced by astronauts themselves. If the gases are not properly handled, this could cause an explosion or fire in the spacecraft when these gases happen to ignite under certain circumstances. In addition, some ingredients in these gases are toxic, which may be harmful to the health of astronauts if no appropriate action has been taken. After careful analysis, NASA researchers found that gas compositions of the human large intestine are up to 400 species. The main gas components are nitrogen, hydrogen, carbon dioxide, methane, oxygen, and other odorless gases, plus some trace gases including ammonia, hydrogen sulfide, indole, skatole, volatile amines, volatile fatty acids, and other gases. Putting together all the malodorous gases, the volume can be no more than 1 % of the total volume. In the gas components, except nitrogen and oxygen, the vast majority of the gases are produced by intestinal bacteria.
3.2.2 The Biological Effects of Hydrogen in the Large Intestine
In the past, people knew little about hydrogen in the large intestine; most people thought that hydrogen was metabolic wastes from the large intestine bacterial metabolism and had no special effects. However, with the discovery of the biological effects of the hydrogen molecule, people gradually consider inducing colonic bacteria to generate hydrogen gas, which could exert a therapeutic effect on certain diseases.
Ohta’s research group from the Nippon Medical School first proposed the issue; they tested breathing gas composition in healthy people after orally taking acarbose, and found that the concentration of hydrogen in the breathing gas was significantly increased but there was no effect on methane concentration. Acarbose is a class of drugs for treating diabetes whose mechanism is inhibition of intestinal epithelial absorption of glucose. Since the intestinal absorption of glucose is reduced, glucose will be transported to the large intestine through intestinal motility; bacteria of the large intestine can utilize glucose and produce a large amount of hydrogen. Thus, the common side effects of these drugs are abdominal distention and and excessive Farting. Early in the acarbose study, significant cardioprotective effects had been found, the molecular mechanism of which was not very clear. Since taking acarbose orally can promote the generation of hydrogen, and hydrogen has a protective effect against heart disease, they speculated that the reason why acarbose has cardioprotective effects was the generation of hydrogen [38]. Although this is very likely, this hypothesis still needs to be proved.
Since protective effects of endogenous hydrogen gas have been confirmed, then whether additional protective effect will appear after the producing of hydrogen is strengthened? Recently, researchers have found that orally administered cellulose or starch has a protective effect on the liver ischemia through inducing large intestinal bacteria to produce hydrogen [39]. Researchers provided rat cellulose and starch which cannot be digested directly for seven continuous days, and then detected the transaminase activity, which represents liver function or damage, the ratio of total glutathione and oxidized glutathione, which represents the degree of oxidative stress in liver. They found that the oral administration of cellulose or starch could promote the generation of hydrogen, improve liver function, and reduce oxidative stress after liver ischemia. The results proved that disease could be treated by promoting the generation of hydrogen gas. Dietary fiber was proved to be able to treat diseases many years ago, but the mechanism underlying this is yet to be found. There are a lot of resistant starch products in the market, although there are many explanations, but none is recognized. Promoting the large intestine to produce hydrogen is one mechanism that can clearly explain the effect of dietary fiber and resistant starch.
It remains controversial whether hydrogen of the large intestine has a biological effect. The body produces a huge amount of hydrogen, e.g., normal human bacteria have been reported to be capable of producing hydrogen 140 ml or even more which equals 10 l of hydrogen-saturated water. In many animal and clinical studies, hydrogen was given through injection or drinking at a dosage of 10–20 % of the above dosage; why is its effect not covered by that of endogenous hydrogen? There are two questions to be explained in this phenomenon. First, does endogenous hydrogen have an effect? Second, in which way does the relatively low dose of exogenous hydrogen exert its effect? This is a contradiction, which remains to be solved in the field of hydrogen research.
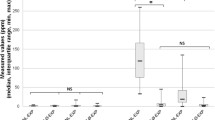
A recent study provides a reasonable explanation. The study observed the effect of oral lactulose-induced hydrogen produced by colonic bacteria in the animal model of Parkinson’s disease (PD), healthy people, and PD patients [40]. Lactulose is one kind of disaccharide that cannot directly be absorbed by the body, but can be used to produce hydrogen gas in the large intestine by bacteria. Lactulose is widely used in clinical practice to treat constipation and hepatic encephalopathy. End-alveolar breath hydrogen concentrations were measured in 28 healthy subjects and 37 PD patients, as well as in 9 rats after taking hydrogen water or lactulose. Six-hydroxydopamine (6-OHDA)-induced hemi-PD model was stereotactically generated in rats. The authors analyzed the effects of continuous and intermittent administration of 2 % hydrogen gas.
For intermittent administration of 2 % hydrogen gas, rats were supplied serially with 2 % hydrogen gas for 15 min and then room air for 45 min using a time controller. The 1-h cycle was repeated 12 times from 6 pm to 6 am to recapitulate the habit of drinking water once every hour in the dark. Each hydrogen administration protocol was started 1 week before the surgery. The control group was supplied with 2 % hydrogen gas continuously.
The results showed that lactulose increased breath hydrogen levels monophasically in nine rats.
However, oral lactulose and continuous administration of 2 % hydrogen gas had no obvious effect on ameliorating 6-OHDA-induced PD, whereas intermittent inhalation had effects on prevention of PD in rats. This study suggested that intermittent inhalation of hydrogen had a better effect than that of continuous inhalation. Marking effects of ad libitum administration of hydrogen water in a rat model of PD were found. Although lactulose also increases hydrogen levels in rats, lactulose and continuous inhalation of 2 % hydrogen gas have marginal effects on the prevention of 6-OHDA-induced PD.
Notably, since the release of hydrogen from the body is very rapid, drinking water is similar to intermittent inhalation of hydrogen. This study may explain why the human body can produce a large amount of hydrogen itself, but drinking hydrogen water or short inhalation of hydrogen may have a better effect, because the study found that intermittent administration of hydrogen had a better effect than that of continuous administration. Hydrogen is continuously produced in the body and its concentration is relatively stable, but the effect of drinking hydrogen water, intermittent inhalation, or injection of hydrogen are caused by rapid increasing of hydrogen concentration. Although the authors did not provide evidence at the molecular level, this is a relatively reasonable explanation.
Evidence of the treatment effect of endogenous hydrogen is from research by Harvard University, Boston Children’s Hospital. This study examined whether H(2) released from intestinally colonized bacteria could affect Concanavalin A (ConA)-induced mouse hepatitis [41]. Systemic antibiotics significantly decreased the level of H(2) in both liver and intestines along with the suppression of intestinal bacteria. As determined by the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), tumor necrosis factor (TNF)-alpha, and interferon (IFN)-gamma in serum, suppression of intestinal bacterial flora by antibiotics increased the severity of ConA-induced hepatitis, while reconstitution of intestinal flora with H(2)-producing E. coli, but not H(2)-deficient mutant E. coli, downregulated the ConA-induced liver inflammation. Furthermore, in vitro production of both TNF-alpha and IFN-gamma by ConA-stimulated spleen lymphocytes was significantly inhibited by the introduction of H(2). These results indicated that H(2) released from intestinal bacteria has biological effects.
References
Acott C. A brief history of diving and decompression illness. SPUMS J. 1999;29:98–109.
Case E, Haldane JBS. Human physiology under high pressure: I. effects of nitrogen, carbon dioxide, and cold. J Hyg. 1941;41:225–49.
Lazarev N, Lyublina E, Madorskaya R. Biological actions of gases under pressure. Leningrad: VMMA; 1941.
Zetterstrom A. Deep-sea diving with synthetic gas mixtures. Mil Surg. 1948;103:104–6.
Bjurstedt H, Severin G. The prevention of decompression sickness and nitrogen narcosis by the use of hydrogen as a substitute for nitrogen, the Arne Zetterstrom method for deep-sea diving. Mil Surg. 1948;103:107–16.
Hengyi Tao, Xuejun Sun et al. Diving medicine. Shanghai: Shanghai Science and Technology Press; 2010.
Edel P. Sea-Space Research Co., Inc. Harvey, La 70058 since hydrogen is the lightest of all gases, we might expect it to offer the lowest resistance in a laminar flow system which should promote more rapid diffusion of O2 and CO2 within the gas exchange units of the, hydrogen as a diving gas: Proceedings of the Thirty-third Undersea and Hyperbaric Medical Society Workshop. Undersea and Hyperbaric Medical Society; 1987. p. 275.
Bennett P, Towse E. The high pressure nervous syndrome during a simulated oxygen-helium dive to 1500 ft. Electroencephalogr Clin Neurophysiol. 1971;31:383–93.
Gardette B, Gortan C. Mice and monkeys deep dives in heliox, hydrox and hydreliox gas mixtures-synthesis of COMEX “Hydra” programme, Basic and applied high pressure biology. Rochester: University Press of Rochester; 1994. p. 173–84.
Abraini J, Gardette-Chauffour M, Martinez E, Rostain J, Lemaire C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J Appl Physiol. 1994;76:1113–3.
Gardette B, Gortan C, Delauze HG. Helium in-hydrogen out. A new diving technique. Proceedings of the 23rd Annual Scientific Meeting of the European. Bled: Underwater and Baromedical Society; 1997.
Gortan C, Delauze H. Hydra V hydrogen experimental dive to 450 meters, offshore technology conference. Offshore technology conference, 1986.
Imbert J, Gortan C, Fructus X, Ciesielski T, Gardette B. Hydra 8: pre-commercial hydrogen diving project, submersible technology: adapting to change. Advances in Underwater Technology, Ocean Science and Offshore Engineering. 1988;14:107–16.
Joulia F, Barthèlemy P, Guerrero F, Jammes YT. Wave changes in humans and dogs during experimental dives. J Appl Physiol. 1992;73:1708–12.
Gardette B, Massimelli J, Comet M, Gortan C, Delauze H, Comex S, France M. Hydra 10: a 701 msw onshore record dive using “Hydreliox.”, XIXth annual meeting of European undersea biomedical society on diving and hyperbaric medicine. Trondheim: SINTEF UNIMED; 1993.
Imbert C, Colton J, Long W, Grossman Y, Moore H. A system for saturating in vitro preparations with high pressure O2, He, H2, and mixtures. Undersea Biomed Res. 1992;19:49–53.
Kayar SR, Miller TL, Wolin MJ, Aukhert EO, Axley MJ, Kiesow LA. Decompression sickness risk in rats by microbial removal of dissolved gas. Am J Physiol-Regul Integr Comp Physiol. 1998;275:R677–82.
Kayar S, Fahlman A, Lin W, Whitman W. Increasing activity of H2-metabolizing microbes lowers decompression sickness risk in pigs during H2 dives. J Appl Physiol. 2001;91:2713–19.
Brauer RW, Way RO. Relative narcotic potencies of hydrogen, helium, nitrogen, and their mixtures. J Appl Physiol. 1970;29:23–31.
Brauer R, Way R, Perry T. Narcotic effects of helium and hydrogen and hyperexcitability phenomenon at simulated depths of 1500 to 4000 ft of sea water, toxicity of anesthetics. Baltimore: Williams and Wilkins; 1968. p. 241–55.
George M, Craig C, William R, Kevin L, Bernard R. Performance effects with repeated exposure to the diving environment. J Appl Psychol. 1981;66:502.
Rostain JC, Balon N. Recent neurochemical basis of inert gas narcosis and pressure effects. Undersea Hyperb Med. 2006;33(3):197–204.
Kayar SR, Parker EC, Harabin AL. Metabolism and thermoregulation in guinea pigs in hyperbaric hydrogen: effects of pressure. J Thermal Biol. 1997;22:31–41.
Dougherty J Jr. Use of H2 as an inert gas during diving: pulmonary function during H2-O2 breathing at 7.06 ATA. Aviat Space Environ Med. 1976;47:618–26.
Dougherty JH Jr, Schaefer KE. Pulmonary functions during saturation-excursion dives breathing air. Aerosp Med. 1968 39(3):289–92.
Lenoir P, Jammes Y, Giry P, Rostain J, Burnet H, Tomei C, Roussos C. Electromyographic study of respiratory muscles during human diving at 46 ATA. Undersea Biomed Res. 1990;17:121–37.
Ornhagen H, Warkander D, Dahlback G. [abstract] Respiratory mechanics during hydrox breathing at 13 ATM, (1984).
Gennser M, Ornhagen H. Effects of hydrostatic pressure, H2, N2, and He, on beating frequency of rat atria. Undersea Biomed Res. 1989;16:153–64.
Ornhagen H, Adolfson J, Gennser M, Gustavson M, Muren A. [abstract] Performance during hydrogen breathing at 13 atm, (1984).
Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122–7.
Askevold F. Investigations on the influence of diet on the quantity and composition of intestinal gas in humans. Scand J Clin Lab Invest. 1956;8:87–94.
Steggerda F. Gastrointestinal gas following food consumption. Ann N Y Acad Sci. 1968;150:57–66.
Levitt MD. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N Engl J Med. 1971;284:1394–98.
Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:504–18.
Strocchi A, Corazza G, Ellis CJ, Gasbarrini G, Levitt MD. Detection of malabsorption of low doses of carbohydrate: accuracy of various breath H2 criteria. Gastroenterology. 1993;105(5):1404–10.
Hammer HF. Colonic hydrogen absorption: quantification of its effect on hydrogen accumulation caused by bacterial fermentation of carbohydrates. Gut. 1993;34:818–22.
Gall L. The role of intestinal flora in gas formation. Ann N Y Acad Sci. 1968;150:27–30.
Suzuki Y, Sano M, Hayashida K, Ohsawa I, Ohta S, Fukuda K. Are the effects of α-glucosidase inhibitors on cardiovascular events related to elevated levels of hydrogen gas in the gastrointestinal tract? FEBS Lett. 2009;583:2157–59.
Nishimura N, Tanabe H, Sasaki Y, Makita Y, Ohata M, Yokoyama S, Asano M, Yamamoto T, Kiriyama S. Pectin and high-amylose maize starch increase caecal hydrogen production and relieve hepatic ischaemia—reperfusion injury in rats. Br J Nutr. 2012;107:485–92.
Ito M, Hirayama M, Yamai K, Goto S, Ito M, Ichihara M, Ohno K. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med Gas Res. 2012;2:15.
Kajiya M, Sato K, Silva MJ, Ouhara K, Do PM, Shanmugam K, Kawai T. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun. 2009;386:316–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Sun, Q., Han, W., Nakao, A. (2015). Biological Safety of Hydrogen. In: Sun, X., Ohta, S., Nakao, A. (eds) Hydrogen Molecular Biology and Medicine. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9691-0_3
Download citation
DOI: https://doi.org/10.1007/978-94-017-9691-0_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9690-3
Online ISBN: 978-94-017-9691-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)