Abstract
High mountain regions may be perceived as areas of high environmental quality, yet many contaminants are ubiquitous on the planet through long-range atmospheric transportation. Mountain lake sediments record and archive how this global contamination is proceeding and have developed throughout history, particularly since rapid industrialization in the 1950s. Action against diffuse atmospheric contamination transported far away from the sources requires the development of national and international protocols, which must be based on reliable scientific evidence. Research on mountain lake sediments aims to provide long-term references, models for interpretation of results, and sound understanding of the mechanisms that lie behind the observed patterns of contamination. Mountains offer an excellent setting for environmental research because short distances may provide marked physical gradients (e.g., air temperature), and ecosystems are relatively amenable to observation and modelling. The lake sediment contributions are important complements to other observational approaches used in global change research. This chapter focuses on trace metals, polycyclic aromatic hydrocarbons (PAHs) and organohalogen compounds (OHCs). After a short introduction regarding contaminants and the several operative ways to examine the sediment archive, the main features of contaminant distribution in mountain lake sediments are described, followed by a section on the understanding of the processes behind the patterns (e.g., atmospheric transport, catchment interactions, air-water exchange, water column dynamics and eventual sediment archiving), and finishes with a section on biological assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Global change
- Diffuse pollution
- Contaminants cold-trapping
- Legacy contaminants
- Mining archaeology
- Mercury
- Persistent organic pollutants
- Alpine lakes
Introduction
Diffuse, Background Pollution
Long-range atmospheric transport (LRAT) of acidifying substances on lakes and forests was the first indication that human activity can change the state of ecosystems situated far away from the site where the contaminant originated (Beamish and Harvey 1972). While the atmospheric increase of carbon dioxide was still a matter of academic debate, evidence of acidic deposition impact in remote areas, due to the industrialization emissions, cut across societal sectors in the early 1970s. As a result, international agreements to control emissions and mitigate the effects of acidification were undertaken both at national and international levels. The archiving of acidification proxies by the mountain lake sediments will not be addressed here because there are recent reviews (e.g., Catalan et al. (2013) and references there in). Nonetheless, acidification merits mention in this introduction because it was the beginning of the international awareness that human atmospheric pollution was changing the nature of background atmospheric deposition across large parts of the planet with highly uncertain consequences for the biosphere and humankind (Rockstrom et al. 2009a).
Contaminants are substances of synthetic origin (or natural of origin but enriched by human activity) that persist sufficiently in the environment to cause health problems to humans or to other living beings and, eventually, interfering with natural ecosystem dynamics. Monitoring all kinds of contaminants at large scales using remote sensing or deploying instrumental stations in a sufficiently dense network across large and scarcely inhabited areas is not feasible; therefore, natural registers, such as lake sediments, provide an extremely valuable source of information to track long-range distribution of atmospheric contaminants (Camarero et al. 1995). Occasionally, these natural registers can also provide evidence of ecosystem responses to the contamination pressure.
Mountains offer an excellent setting for environmental research because short distances may provide marked physical gradients (e.g., air temperature). Mountain regions are distributed worldwide and many of them contain lakes whose sediments are archives of regional deposition of contaminants. Because of the mountain geomorphology, the ratio between the lake area and its watershed is low and hence favors sediment archives with atmospherically dominated variability. That is, direct atmospheric deposition and short distance transport by surface and subsurface flows dominate matter loads to the lakes, particularly in glacial cirques and former volcanos. In contrast, the sediment archive variability in lakes and reservoirs in the main valleys and at low altitude plains is highly influenced by the way the fluvial transport organize and fluctuate over large areas and time (Fan et al. 2010). Lakes without direct human influences in the catchment are usually more appropriate for studying atmospheric pollution. In some cases, however, the atmospheric origin of some contaminants can be determined comparing lakes without human occupations in the watershed with those with some impact (Datta et al. 1998; Schmid et al. 2007). The mountain regions represent topographic barriers to air transport and, as a consequence, provide a more regionalized view of the background planetary pollution compared to remote high-latitude areas (e.g., Arctic), which may be closer to a hemispheric average. Several high mountain ranges are imbedded within regions of high emissions.
In this chapter, I will examine: (i) LRAT of contaminants tracked using lake sediments in mountain regions; (ii) the main patterns of current and past contaminant distributions; (iii) the processes to consider in the interpretations of the sediment records; and (iv) the extent to which toxic impacts can be inferred from the same sediment sequences. Advantages, shortcomings and complementarities of sediments with respect to other observational matrices such as direct air sampling, deposition, snowpack, soils and vegetation will be also addressed.
The Contaminants
Three groups of contaminants that differ in their origin, transport characteristics, bioaccumulation features and potential toxicity will be considered (Table 1): trace metals, polycyclic aromatic hydrocarbons (PAHs) and organohalogen compounds (OHCs).
Trace Metals
The term trace metal is increasingly used to include heavy metals , metalloids and organometals (e.g., Pb, As, MeHg) that fall along a continuum of biogeochemical behaviors and share the potential to be toxic to biota; thus the term has more of an environmental connotation rather than chemical (Luoma and Rainbow 2008). The term “trace” refers to the usual concentrations observed within organisms. The concentration in a specific medium, particularly in the sediments, can be far beyond what is considered analytically a “trace concentration”.
Some trace metals are required by living organisms in small doses; they are referred as essential metals. The affinity of essential metals for sulfur and nitrogen has been evolutionary exploited in metabolic pathways, which are common to most living forms. Non-essential trace metals are not necessarily more toxic than essential ones, and the thresholds at which trace metal availability become toxic may be lower for some essential metals than for non-essential ones. An approximate order of toxicity for trace metal inorganic forms is Hg > Ag > Cu > Cd > Zn > Ni > Pb > Cr > Sn, although toxic effects vary between organisms (Luoma and Rainbow 2008).
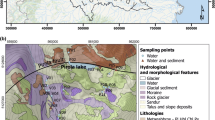
Mountain lake sediments are naturally rich in trace metals because of the weathering of fresh rocks in their catchments. Therefore, it becomes mandatory to take into account these natural metal loads for assessing current atmospheric contamination and also that soil characteristics may modify the transport towards the lake (Navas and Lindhorfer 2005). Comparing the concentration in the sediment upper levels with concentrations in older sediment sequences corresponding to pre-industrial periods is a simple approximation. For some trace metals, the distinction between on-site and atmospheric sources can be made using stable isotope ratios, which usually differ between the two sources (Fig. 1). For instance, lead isotope ratios (i.e., 206Pb/207Pb and 208Pb/206Pb, or 206Pb/204Pb and 208Pb/204Pb) have been used to distinguish between alternative potential pollution sources related to mining (Camarero et al. 1998) and ore smelting areas (Reynolds et al. 2010). The Pb isotope ratios are useful also for investigating the different pathways (e.g., organic matter association, horizon distribution) of endogenous and exogenous sources in the catchment soils and the eventual implication for transport to lakes (Semlali et al. 2001) .
Lead profiles in the sediments of Lake Redòn (Pyrenees). Solid circles, total Pb; empty circles, Pb from watershed sources as estimated using Al concentrations; inverse triangles, 206Pb/207Pb isotopic ratio. Atmospheric Pb deposition started to be significant during the Roman period and has been present since there, with a maximum at 680 AD and a second large input during post-1950s period. The Pb isotopic ratio varied according to the flux changes and indicates mining sources even at recent times. From Camarero et al. (1998) with kind permission from Springer Science and Business Media
Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) , also discussed elsewhere in this book (e.g. Korosi et al. this volume, Rose and Ruppel this volume), are organic compounds with two or more fused aromatic rings. PAHs have received increased attention in air pollution studies because some are highly carcinogenic or mutagenic (Srogi 2007). PAHs are widespread contaminants resulting from incomplete combustion of organic materials. In the atmosphere, they can undergo photodecomposition when exposed to UV light from solar radiation and react with oxides yielding diones, nitro- and dinitro-PAHs, and sulfonic acids. They are highly lipophilic, thus they easily attach to organic particles in water. In the water column of lakes, differential algal scavenging can change the PAHs profile between atmospheric deposition and sediment deposition (Wang et al. 2011). In soils and lake sediments, PAHs may be degraded by microorganisms (Haritash and Kaushik 2009). All these processes increase the complexity of the interpretation of the PAH sediment archive. In addition, air-soil PAH exchanges vary seasonally because of the volatilization features of these compounds; this variation adds to the seasonality of the emissions (Wang et al. 2011). Therefore, measurements integrated over several years are more environmentally meaningful than point assessments. In that sense, the sediment record is of some advantage.
There are many PAH compounds of natural and artificial origin. Environmental studies usually report 16 priority PAHs initially established by the US Environmental Protection Agency. In palaeolimnological studies, the number analyzed is usually lower and changes from one study to another. Any comparison of total PAHs should be aware of this variability in the analytical practice, yet there are usually a few compounds that account for much of the PAH total at each site. In some cases, instead of total PAHs, sums of certain compounds are reported; for instance, parent PAHs, excluding derived and diagenetic forms, or pyrolytic PAHs, focusing on compounds produced at high temperature combustion. The PAH profile differs according to the combustion source, therefore the composition at remote sites has been used to try to infer the relative contribution of different sources, mainly for distinguishing between low temperature combustions of biogenic fuels (wood, biofuels, dung cakes, etc.), coal and fossil fuel. PAHs can also be compared with other proxies indicative of combustion, such as black carbon (Gustafsson et al. 2001) and spheroidal carbonaceous particles (SCP) (Rose and Rippey 2002, Rose and Ruppel, this volume), for instance. The latter is produced solely by high temperature combustion of coal and oil, whereas PAHs and black carbon are produced in any combustion of organic matter. There are still a limited number of studies comparing these proxies in mountain lakes (Muri et al. 2006) .
Organohalogen Compounds
Many synthetic OHCs, with cyclic or aromatic structure, have been produced for agricultural, urban, and industrial applications. Some of them, due to their persistence and volatility, are spreading globally and have been jointly named as persistent organic pollutants (POPs). The Stockholm Convention on Persistent Organic Pollutants (2001) is a global treaty to protect human health and the environment from these kinds of chemicals, and maintains an official list of compounds included in this category that is periodically revised (http://chm.pops.int/). The chemical stability of POPs is due to the halogen substituents: primarily chlorine, but also bromine and fluorine. Many OHCs have been used as insecticides (e.g., dichlorodiphenyltrichloroethane (DDT) , hexachlorocyclohexanes (HCHs), aldrin, toxaphenes, chlordane, mirex, endosulfan, etc.) during different periods since the first half of the twentieth century. Hexachlorobenzene (HCB) was used as a fungicide, and today is a by-product in the manufacturing of various chlorinated organic solvents. Polychlorobiphenyls (PCBs) were synthesized for use as dielectrics in transformers, fire retardants, high thermal stability oils and other applications. The production was stopped in the 1970s, but there is still a large stock in some countries to be disposed of; due to their scarce reactivity and resistance they are found worldwide. Whilst some of these compounds were synthesized as pure products, they were often produced and used as mixtures, as in the case of PCBs, HCHs, and toxaphenes. Hence, numerous compounds were introduced into the environment. In some cases, the initial product is transformed into other contaminants (e.g., DDT transforms into DDE and DDD). Dioxins and dibenzofurans are not manufactured; rather, they are generated through processes such as the combustion of organic materials that contain chlorine atoms.
In the 1970s polybrominated diphenyl ethers (PBDEs) were introduced, designed as flame retardants. Similar to PCBs, they were initially produced as mixtures, which have been increasing in the number of brominated sites (tetra-, penta-, octo-) until the fully brominated form BDE-209 was produced. The volatility of this latter compound is extremely low, and its hydrophobicity high, even compared to other organohalogens. Hence, it was expected that it will not diffuse away through the atmosphere or water. Surprisingly, it has been found in remote areas as other PBDEs (Bartrons et al. 2011; Breivik et al. 2006). The extremely high production and fast increase of background levels of PBDEs in a few years caused concern and progressive substitution for other flame retardants (Law et al. 2006).
Recently, the surfactants perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) are receiving attention due to their ubiquitous presence in humans and the environment, and their potential for bioaccumulation (Houde et al. 2011). The stability of PFOS and perfluorinated carboxylic acids (PFCAs) limits their degradation. High water solubility and low Henry’s Law constant render them susceptible to wet deposition, making LRAT in the vapor phase unlikely. However, these perfluorinated compounds have been recorded in water of mountain lakes (Loewen et al. 2008). It seems that volatile precursors (fluorotelomer alcohols, FTHOs), intermediates in the production of polymers primary used in water resistant coatings, are atmospherically transported to remote areas where their degradation becomes the source for PFCAs. Identifying sources and pathways involved in transporting PFCAs to remote locations may help to follow how environmental stocks respond to recent changes in fluorochemical production practices and phase-out initiatives.
Many OHCs are toxic to humans and are now banned or heavily restricted, but others are still produced or in use across the planet or at least in some parts (e.g., DDT against mosquitoes carrying malaria). Recent studies have indicated that dicofol may be a potential source of fresh DDT for high mountain regions worldwide (Meire et al. 2012b); the o, p’-DDT/p, p’-DDT ratio have been used as an indication of new vs. old DDT (Li et al. 2006). DDT impurities of up to 0.1 % can be found in technical dicofol formulations, but can reach up to 20 % with some poor production techniques. Stocks in use and awaiting destruction (e.g. PCBs) may still be large even decades after production stopped. In Brazilian mountains, PCB levels in the air are proportional to the surrounding human population (Meire et al. 2012a). Therefore, existing POPs will be a matter of environmental surveillance and research for many years. The environmental consequences of the addition of new products to this legacy of contaminants, forming “pollution cocktails” of increasing complexity, are uncertain.
Different Ways to Study the Sediment Archive
Alpine mountain lakes (i.e., those located above tree line) are usually relatively small, morphologically simple and relatively deep compared to their surface areas, and located in catchments of relatively low sediment export. Therefore, the sedimentation patterns across the lake, excluding most littoral zones and inflows, are relatively constant, and one can assume that the sediment sequence corresponds to a temporal series without hiatus or temporal discordances. For comparisons, it is generally correct that within-lake variation is lower than among lakes. In contrast, lakes in the main valley of large watersheds and located below tree line tend to show complex sedimentation patterns and, therefore, a single core may not be representative of the overall accumulation rate. On the other hand, lakes located at high elevation plateaus (e.g., Andes, Himalayas), particularly if they are situated in dry areas, differ from the conventional paradigm of mountain lakes. In this chapter, I will make only occasional reference to plateau lakes. In any case, there are alternative ways to study and evaluate the sediment record of contaminants. The appropriate methodology depends on the question being addressed and the trade-off between the accuracy required and the operational cost (time, logistics, and analytical capacity).
Time Series
The canonical approach is to interpret sediment archive as a historical sequence. Therefore, the core must be dated to obtain a chronological sequence. Lakes with varved sediments, i.e. with distinguishable annual layers of sediment deposition (couplets or varves), are valuable because of the chronological accuracy (Brännvall et al. 1999), but are rarely found. In most cases, one has to rely on indirect dating methods. For the most recent period (< 200 years), dating using 210Pb, complemented with 137Cs and other radioisotopes , may provide excellent age-depth models if the sediment is finely sliced (Appleby et al. 1986). When interest goes beyond the last two centuries (for instance, looking for metallurgy pollution of ancient cultures; see Cooke and Bindler, this volume), dating relies on 14CAMS techniques and age-depth modelling (Blaauw and Heegaard 2012). Although this technique has improved enormously over the last few decades, it performs better at ages older than 500 years. Therefore, the age period from 200 to 500 years sometimes is covered with extrapolations of 210Pb and 14C age models (Camarero et al. 1998).
Obviously, dating becomes critical when the main target is not ‘how much’ but ‘exactly when’. For instance, the deposition of spheroidal carbonaceous particles (SPC), which are produced in high temperature combustion, vary many orders of magnitude throughout the European mountains. However, the timing for an exponential increase in the 1950s is coherent all over the European continent indicating a synchrony in industrialization despite the marked differences in intensity among countries (Rose et al. 1999).
Mountain lakes exhibit sediment focusing. Therefore, contaminant concentrations have to be converted into atmospheric deposition fluxes with caution (Van Metre and Fuller 2009). Knowledge of 210Pb deposition in the catchment may help to correct for focusing (Appleby 2008, see also Kuzyk et al. this volume) or some lithogenic elements may be used to standardize the measurements through time and across the lake (see below). The question investigated determines how much uncertainty in the estimation of the deposition fluxes the study can tolerate, and sampling has to be tailored accordingly. It is not uncommon to be examining changes in a range of more than one order of magnitude. In any case, knowledge about the local sedimentation process is always useful.
Total Inventories
Occasionally, the main interest of a study is the total inventory of the contaminant during a certain period. For instance, for synthetic organic contaminants, the target could be comparison among sites of the accumulation since the substance was first produced. It would not be efficient to measure many samples in great detail along each core. The key point is just to assure that the core section measured covers the entire temporal period of use of that contaminant. As a general guide, for mountain lakes above tree line and without permanent significant streams, the first 15 cm of sediment usually reach more than 50 years ago (Battarbee et al. 2002). This core length is sufficient to evaluate the total inventory of synthetic substances made during the last few decades (e.g., OHCs). Again, if the lakes being compared differ in the degree of sediment focusing, the relative difference in total inventory may not reflect the actual differences in atmospheric flux. A way to standardize the comparison, within an area of similar atmospheric deposition characteristics, is to use the total amount of 210Pb. Correction for focusing may notably reduce apparent differences in atmospheric contaminant deposition among nearby lakes (Fernandez et al. 2000).
Top-Bottom Approach and Enrichment Factors
For contaminants that exist naturally, one may choose to compare current changes with a reference in the past over large areas. For instance, one may need to assess the impacts of the last industrialization burst in a region compared to the historical background levels. In these cases, a so-called “top-bottom” palaeolimnological approach may be instructive (Smol 1995). This method involves comparing contaminant levels of an upper (top) sediment section, assumed representative of the present, with those at some sediment depth representing an arbitrary past (e.g., top and 15 cm depth sections in mountain lakes (Camarero et al. 2009)). This approach can be criticized in many ways as there is a poor substitution of well-dated sequences (e.g., see chapter by Boyle et al., this volume). However, the advantage relies in the cost efficiency. Therefore, it is appropriate for studies considering a large number of sites, in which the synoptic evaluation is more valuable than each individual case. The differences between the two sediment levels measured are expressed as enrichment factors (EF). There is a high risk to obtain misleading results as changes in erosion rates or sediment focusing patterns between the two periods may have occurred at each site. Therefore, it is highly preferred to normalize the values using some lithogenic tracer that may account for these processes. The selection of the tracer may depend on the nature of the catchment bedrock, and the analytical capability. Different elements have been used (e.g., Ti, Zr, Al, and Rb) (Boes et al. 2011). Comparisons with EF calculated from Pb isotopes indicate variability between 10–30 % depending on the element; therefore, it is advisable to try multiple reference elements and critically evaluated the differences when possible (Boes et al. 2011). Even when using several elements as reference, EF methods are not completely free of misinterpretation (see chapter by Boyle et al., this volume); therefore, scientific pros and cons in the evaluation of a particular environmental problem have to be considered before application. The top-bottom approach should not be used for compounds that undergo significant diagenetic transformation within the sediments (see Outridge and Wang, this volume).
Whole-Lake Budgets
The deepest central lake area may not accurately represent the whole-lake basin (Yang et al. 2002c). For certain studies (e.g., whole-watershed contaminant mass balance), a precise and accurate estimation of the whole-lake accumulation rate is required. Cores from several sites are necessary to take into account current and past lake focusing heterogeneity. The number of sites may vary according to the sedimentary characteristics of the lake. Progressing deltas in the inflows, high rugosity of the shoreline and other geomorphologic complexities will demand coring a large number of sites, but 5–10 sites, distributed to proportionally cover the main zones (axes) of variability, are sufficient in many small to medium size lakes (Rippey et al. 2008). In order to minimize cost, contaminants can be measured in one central site, and the spatial variability on accumulation rates taken into account by extrapolating these measurements based on some indicative, but easy to analyze, variable (e.g., organic matter content). Sometimes, the depositional correlation between cores is quite low and then analyzing multiple cores becomes prescriptive. This is the case, for instance, when the contaminant transport towards the lake is bound to large and heterogeneous particles, such as plants debris (Yang et al. 2002a).
Altitudinal and Other Geographical Variability
Under current dynamic environmental conditions, assessments over large areas and comparison between different regions are of primary interest. The temporal perspective that a single sediment archive can provide is always valuable, but the interpretation may benefit from contextualization within a geographical framework. Environmental conditions in mountains change with altitude, and this variability has to be considered when planning for sampling and in comparisons among sites. In fact, altitude is a factor that is a local surrogate for many variables, yet the relationship with some of these variables changes with latitude (Körner 2007). When comparing regions across continents, altitude is not a correct normalizing factor and other variables (e.g., air temperature) have to be considered according to the features of the contaminant. There are factors that change smoothly with altitude, such as temperature, but others distribute in belts. Vegetation is the best example for the latter. Particularly noteworthy is the distinction between truly alpine lakes (those above three-line) and lakes located in forested areas. The form and quantity of carbon (dissolved and particulate) cycling through these two types of lakes can markedly differ, with strong implications for the transport and retention of some of the contaminants. High mountains are also topographic barriers, the depositional contrast among nearby sites at the same altitude may be astonishing, depending on the range morphology and its orientation respect to the air mass circulation. Finally, when dealing with LRAT, it is necessary to consider that geographical distance to sources is not equivalent to the likelihood for transport from them. The air mass trajectories can effectively shorten (or lengthen) the geographical distances so that the meaning of local and background sources is diffuse without a study of the airshed of each lake.
It is worthwhile to consider altitudinal and geographical issues beforehand, when planning the sampling surveys, not only as factors in the posterior data analysis. Most environmental sciences face the issue of how to deal with geographical, physical, ecological and socio-economical co-variability. One can try to block geographical differences in emissions, for instance, by sampling within relatively small areas, but then it becomes difficult to find a large gradient of the factor under study (e.g. air temperature, organic matter, etc.), yet there are exceptional locations (Landers et al. 2010). This is a methodological problem that environmental chemistry has not fully addressed compared to other environmental sciences that face similar problems (e.g., geochemistry, ecology, etc.). Whereas dynamic models are relatively easily accepted as a methodological tool, despite the uncertainties that the parameterization may carry, relatively complex statistical analysis of data are also necessary. At the end, there is no way to disassociate entirely the influence of geography, history and dynamical processes using sampling designs; therefore, there is a need to include in the study of patterns more sophisticated statistical methods, just as other earth and environmental science have done (Legendre et al. 2002; Peres-Neto et al. 2006).
Contaminant Distribution
The nineteenth century Industrial Revolution increased the atmospheric emissions of contaminants, yet the tipping point of long-distance pollution occurred when massive use of fossil fuel reserves started in the 1950s. Combustions were a primary source of contaminants, but the availability of low cost energy triggered the development of massive production of new products that revolutionized agriculture and industry . Some of the processes for fertilizer and pesticide production were known before that time, but had not been fully exploited because they were energetically too costly. Since then, industrialization and environmental safety measures have not developed at the same pace worldwide. Historical, socio-economic and political factors lie behind geographical and temporal variation in the production and emissions of contaminants. Tracking LRAT of contaminants using mountain lake sediments can contribute not only to the assessment of current contamination levels but also to the development of the environmental history, particularly for regions where scarce documentary information exists.
Release of contaminants is coincident with technological progress. Although the 1950s were a tipping point, particularly in the variety of contaminants produced, trace metal atmospheric pollution started with early metallurgy and contaminant transport to real long-distance is evident at least since the Roman Empire in Europe (Renberg et al. 2001). In central China, the concentrations of Cu, Ni, Pb, and Zn increased gradually from about 3000 BC; enormous inputs of these metals corresponded to China’s Warring States Period (475–221 BC) and the early Han Dynasty (206 BC–220 AD) (Lee et al. 2008). Many ores are located in mountain areas or nearby. In addition to background contamination , mountain lake sediments also may document unexpected high emissions from these locations, and contribute to an environmental history of mining (Cooke et al. 2011; Cooke and Bindler, this volume).
Many mountain ranges around the world have lakes with similar ecosystem features. Therefore, comparisons among mountain regions worldwide may resolve basic questions about LRAT of contaminants. Existing information is highly biased towards North America and Europe, yet the information is notably increasing for Asia and South America in recent years. Since our planet is immersed in an on-going global change , further studies will be necessary on broad geographic scales, including areas that have been previously studied as many relevant investigations were performed more than a decade ago. The situation is continuously changing because the new production and use of compounds and the shifting long-range atmospheric trajectories due to climate change . We should expect that increasing awareness of the long-range transport of pollution (Rockstrom et al. 2009b) will foster investigations of the mountain lake sediments throughout the planet. In this section, I summarize the main worldwide patterns of contaminants, as revealed through mountain lake sediments. It does not aim to be a comprehensive review but to illustrate the range of variation, and the geographical and historical main features found up to present.
Trace Metals
Trace metal contamination is the oldest airborne atmospheric pollution recognized. In Europe about half of the cumulative burden of atmospheric Pb contamination was deposited before industrialization (Bindler et al. 2008) . While some Scandinavian and Greenland records provide evidence of long-range atmospheric pollution (Renberg et al. 2001) the mountain sediment archive probably has a stronger contribution of nearby ore exploitations, as indicated by Pb isotopes in the case of the Pyrenees (Camarero et al. 1998). In that sense, mountain lake sediment archives have much potential for regional mining and metallurgy history, complementing other archives, such as peat bogs (Bindler et al. 2011). In Sweden, the first traces of atmospheric lead pollution recorded in lake sediments date to about 3500 years ago (Bindler et al. 2008). Signals in the sediments reflecting Roman metallurgy are found from Scandinavia boreal forest lakes (Brännvall et al. 1999) to Southern Europe mountain lakes (Camarero et al. 1998). Lead pollution comparable to levels found since the Industrial Revolution (~ 1800 A.D.) were found during Medieval times (1200–1600 A.D.) in the Austrian Alps (Schmidt et al. 2008) and even earlier, during post-Roman times (~ 600 A.D) in the Pyrenees (Camarero et al. 1998) (Fig. 1). Archaeological studies are indicating early occupation of the mountains in the Neolithic, and soon after the mid-Holocene (5000 year B.P.), fire was used to increase pasture lands and early mining and metallurgy impacts occurred in mountain valleys, either to use mineral resources or wood as fuel for metallurgy (Bal et al. 2011). Signatures from Bronze Age may also be recorded in other parts of the world as there is evidence from lakes in alluvial planes in China (Lee et al. 2008). In fact, there is a broad scope of historical circumstances for investigation: the high mountain impacts of Medieval times in Europe (Camarero et al. 1998; Schmidt et al. 2008); China’s Warring States period (Lee et al. 2008); Andean cultures and colonial period mining (Strosnider et al. 2011; Cooke et al. 2011; Cooke et al. 2009); and west colonial occupation in North America and settlement of smelters (Baron et al. 1986; Mast et al. 2010), among many others. At each region, this historical background becomes the reference for assessing the trace metal pollution during the last two centuries of industrialization, and particularly the last five decades of intensive use of fossil fuels .
The decades after the surge in industrialization following the 1940s and 1950s are usually the target of current environmental concern. Studies covering large territories are particularly valuable to provide a general assessment. This was the case of a pan-European study of trace metals (Pb, Cd, Zn, Cu, As, Hg and Se) in mountain lake sediments that was performed in the year 2000 AD across the most important mountain ranges in Europe including the Pyrenees, Alps, the Rila Mountains, Retezat, Julian Alps, the Tatras and, Scottish mountains (Camarero et al. 2009). A top-bottom palaeolimnological approach was applied to compare current levels with pre-industrial concentrations. The concentrations of trace metals in many locations were comparable to those reported in aquatic sediments receiving high direct contamination loads. This is largely the consequence of high natural loads due to rock weathering in these low vegetated catchments, in which fresh exposed rock surface is produced continuously by geomorphic processes related to freezing of water. Therefore, Ti was used to normalize for lithogenic inputs and focus on atmospheric loads. The EFs for most elements were well above 1.5 in many lakes, indicating post 1950s atmospheric contamination. Pb was the element that showed the highest contamination level at the European scale (median EF of 2.3), followed by Hg and As. Zn, Cd, Cu and Se contamination was detectable to a lower degree. An indication of the generalized increase of diffuse atmospheric contamination is the exceptional increase of correlation among the current concentration of the different trace metals respect to the correlation in the pre-industrialization reference (Fig. 2). The only exception was As, indicating that the increase of this element has been due to other causes.
Correlation between trace metals in top (0–2 cm) and bottom (15–17 cm) sediment samples from 75 mountain lakes of the Pyrenees [data from Camarero (2003)]. Note the increase in the correlations in recent times due to increase atmospheric contamination. It was assumed that the bottom layers corresponded to pre-1950s periods
The Tatra Mountains and Scotland have been the most affected by trace metal pollution (Camarero et al. 2009). Natural mechanisms leading to the formation of highly organic, metal-binding sediments may be the cause of the high levels in Scotland (Yang et al. 2002a), whereas those in the Tatra Mountains are due to elevated deposition (Kopáček et al. 2006), which may be a general feature in Eastern Central Europe in the early post-industrialization decades. Metal enrichments in the Rila Mountains in Bulgaria are comparable to those in the Tatras, but concentrations are much lower. In the Alps, enrichments in Pb, Hg and Zn are higher in southern than in central areas suggesting a flux of these pollutants from the south, the highly industrialized area of Northern Italy. In the Pyrenees, the conspicuous feature is the high natural levels of As (Camarero 2003). The Retezat in the Carpathian Mountains of Romania is among the least contaminated regions in Europe (Camarero et al. 2009). Although high resolution sediment studies in Lacul Negru (Rose et al. 2009) provided indications of Zn and Cd contamination from as early as the sixteenth century and from the eighteenth century for lead Pb, Cd and Hg. The level of contamination in the sediments of Lacul Negru is typically higher than in mountain lakes of Scandinavia but lower than that found in the Tatra Mountains, the Central Alps and the UK .
Top-bottom palaeolimnological approaches make sense when studying a large number of lakes over large areas; thus they are rather exceptional. Most existing studies evaluate contaminant time series from a single or a few sites. This approach is highly informative but is lengthy for the evaluation of contamination over large territories (e.g., continents); it depends on the progressive accumulation of studies across the territory. This is the case for the Asian continent, for which there is still insufficient data for a general assessment as has been done in Europe, but there are some valuable studies offering an initial view. In the Khamar Daban Mountain Range (Siberian Central Asia), the lake sediments of Lake Kholodnoye (1760 m a.s.l.) provided evidence of Pb and Zn pollution from the 1920s (Flower et al. 1997), the contamination tendency stepped up in the early 1970s and at the time of the study had not declined. According to the SCP levels, contamination was estimated to be about 100 times lower than in acidified UK sites. However, whether the recent contamination in the Khabar Daban Mountains (Siberia) is derived mainly from background atmospheric contamination of the Northern Hemisphere or from regional emissions was not determined. At Southern latitudes, in Daihailake (Inner Mongolia, China), there is no clear evidence of the current increase of trace metal atmospheric pollution (Han et al. 2007). Air-deposited Cu and Pb were evaluated using Ga as a normalizing element of fluvial transport and lithogenic origin. The Cu and Pb pollution was related to monsoon variability. Further south, the record of Lake Shudu (Yunnan province, China) also indicated scarce trace metal pollution during the last decades (Jones et al. 2012). Therefore, despite the scarce number of studies in north-central Asia, it seems that current background trace metal pollution is low compared with Europe. Nonetheless, the number of studies is still low in relation to the vast (mountain) territory of the continent. Cd atmospheric contamination has been found in soils of the Gongga Mountain in the eastern edge of the Tibetan Plateau (Wu et al. 2011); and recent studies from lake sediments in the Tibetan Plateau (Yang et al. 2010a) has shown that the first indication of Hg pollution is much earlier than the onset of the Industrial Revolution in Europe, but the most significant pollution increase is from the 1970s, followed by a further marked and continuous increase from the 1990s synchronous with the recent economic development in Asia (especially China and India). As most of the sites are exceptionally remote and situated above the atmospheric boundary layer, these results underline the need to understand the local Hg cycle in both regional and global context.
For some time, it was believed that preindustrial gold/silver mining in the Americas dominated the inventory of Hg global emissions. Recently, the evaluation of Hg deposition history over the last millennium, from a suite of lake-sediment cores collected from remote regions of the globe, indicates that atmospheric Hg emissions from early mining in America were modest as compared to recent industrial emissions (Engstrom et al. 2014). Most of the large quantities of Hg employed in the gold and silver mines was not volatilized, but rather was immobilized in mining waste. In North America, the Baron et al. (1986) early work in subalpine lakes of the Rocky Mountains indicated that Pb atmospheric pollution started in the second half of the nineteenth century, probably related to mining activities in Colorado. There was no clear evidence of increased deposition related to recent industrialization. Mining in the past ~ 150 years was also the main source of trace metal pollution (Ag, As, Cd, Cu, In, Pb, Bi, Sb, Sn, Te, Zn and S) arriving to Uinta Mountains (Utah) (Reynolds et al. 2010) . Lead concentrations declined in sediment deposited since about the mid-1970s, probably because of the cessation of smelting, temporary halt to mining for establishing better emission control systems, and the phase-out of leaded gasoline. In the Western US National Parks, Pb, Cd, SCP in lake sediment indicate deposition due to high-temperature combustion of fossil fuels (Landers et al. 2010). SCP increased in lake sediments in the conterminous 48 states during the twentieth century, peaking around the 1960s. However, in recent decades, sediment Pb, Cd, and SCP have declined substantially, reflecting source reductions due to regulations. Atmospheric deposition of Hg began to increase around 1900 in the Rocky Mountains, reaching a peak sometime after 1980 (Mast et al. 2010). The average ratio of modern to preindustrial fluxes was 3.2, which is similar to remote lakes elsewhere in North America. Current-day measurements of wet deposition are lower than the modern sediment-based estimates, perhaps owing to inputs of dry-deposited Hg to the lakes, although catchment processes may play also a role. In the Western US National Parks, deposition of anthropogenic Hg, indicated by temporal patterns in sediment cores, increased in nearly all parks during the twentieth century (Landers et al. 2010), starting between 1975 and ~ 1990 depending on the area. Currently, Hg sediment flux appears to be generally increasing or showing no change in some lakes and decreasing in others. This finding reflects a complex array of factors consistent with decreasing regional contaminant sources, increasing global contributions, and influences of individual watersheds on sediment Hg loading (Landers et al. 2008) .
Tropical and southern hemisphere mountain lakes have been less studied for trace metal contamination than those in the Northern Hemisphere. Recent studies in the Rwenzori Mountains of Uganda show that the lakes have been contaminated by Hg from atmospheric deposition starting at least by the late nineteenth century (Yang et al. 2010b). The Hg accumulation increased by about 3-fold since the mid-nineteenth century, similarly to other remote regions worldwide. High Hg levels in the pristine lacustrine ecosystems of the Nahuel Huapi National Park, a protected zone situated in the Andes of Northern Patagonia (Argentina) have been related to local volcanic activity since many-fold concentrations above the background values were observed underlying some tephra layers in the sediment cores (Guevara et al. 2010). Extended fires might be another potential atmospheric source because the earlier Hg peaks coincide with reported charcoal peaks, whereas the upper Hg peaks coincided with evidence of extended forest fires from tree-ring data and historical records.
Trace metal emission controls started several decades ago in North America and Europe. It was expected to find a quick trace metal decline in the remote lake sediment records, as was found in precipitation (Pacyna et al. 2009). There were examples of that decline (Muir et al. 2009), but there were exceptions due to the ‘legacy contamination’ stored in the lake’s catchments. In fact, soils have been accumulating trace metals for decades and these contaminants are now slowly being released. In Scotland, Pb fluxes to the lakes have been steady despite emission reductions (Yang et al. 2002b). In the Pyrenees, a net export of Pb and As from the catchments to the lakes was found, whereas Zn atmospheric contamination was largely retained in the catchment (Bacardit and Camarero 2010c). Nearly all Pb entering the lakes was retained in the sediments, whereas 5–38 % of As and Zn was lost through the outflow.
Polycyclic Aromatic Hydrocarbons
The PAHs are probably the clearest indicators of the Industrial Revolution based on fossil fuels and the environmental carelessness (ignorance) of the first decades of their massive use . PAHs also exemplify the success of emission controls, as declines in the sediment records have closely followed these actions. Differences in concentrations and loads were extremely high among regions (Fig. 3), mostly depending on the distance from fuel burning areas (Fernandez et al. 2000). Fluxes between 50 and 150 µg m−2 yr−1 were common throughout remote mountain lakes of Europe during the last decades of the twentieth century (e.g., Alps, Pyrenees), even in scarcely industrialized areas. PAH fluxes ranged from up to 1000–2000 µg m−2 y−1 in some lakes of the Tatra Mountains (Central Europe) and Julian Alps (Slovenia), to 5 µg m−2 y−1 in Arctic islands (e.g., Svalbard) (Fernandez et al. 1999). The highest fluxes were similar to those found in lakes situated near urban or industrial areas. In general, the European mountain lake sediments show an industrialization fingerprint screened through a long-distance transport; the composition is dominated by benzo[b + j]fluoranthenes, indeno[1,2,3,-cd]pyrene, chrysene + triphenylene, benzo[k]fluoranthene and fluoranthene, among other less abundant PAHs of pyrolytic origin. Throughout Europe, the increase of PAH fluxes in mountain sediments started during the last decades of the nineteenth century and achieved the maximum between 1960 and 1990 (Fernandez et al. 2000; Muri et al. 2006). The ratio between the maximum flux and the background levels was 12–34 in the broad central geographical core (Alps, Tatras, Pyrenees) and < 7 in the periphery, both Northern (Scandinavia, Arctic) and Southern (Iberian Peninsula mountains). The exceptional high concentrations and fluxes in the Tatra Mountains correlated with the average sulfate deposition in the area before the 1990s (Fernandez et al. 1999). PAHs decline in many sediment records since the mid-1990s in response to an international agreement to reduce combustion emissions. In Lake Ladove, in the Tatras, PAHs concentration declined to half from the early 1990s to 2000 (Van Drooge et al. 2011). There are no European studies to evaluate the decline progress during the twenty-first century.
PAH fluxes and PAH concentrations normalized to total organic carbon (TOC) in sediments from high altitude mountain lakes during the last decades. 1, Escura (Serra Estrela, Portugal); 2, Cimera (Sierra Gredos, Spain); 3, La Caldera (Sierra Nevada, Spain); 4. Redòn (Pyrenees, Spain); 5. Noir, (Alps, France); 6. Schwarsee ob Sölden (Alps, Austria); 7. Gossenköllesee, (Alps, Austria); 8. Dlugi (Tatras, Poland); 9. Starolesnianske (Tatra, Slovakia); 10, Maam (Donegal, Ireland); 11. Øvre Neådalsvatn (Caledonian, Norway); 12. Arresjöen (Danskoyab, Norway). Reprinted with permission from (Fernandez et al. 1999). Copyright 1999 American Chemical Society
The number of studies investigating PAHs variability within lake districts that consider a large number of lakes is limited. A top-bottom palaeolimnological approach was followed by Van Drooge et al. (2011) in the Tatra Mountains. A northwest to southeast gradient was found, which is consistent with EMEP (European Monitoring and Evaluation Programme) estimates that identified a large area in southern Poland as a focus of high emission, just west of the Tatras. A handicap of the top-bottom approach is that fluxes are more difficult to estimate without dating or at least complementary data to evaluate the differences in accumulation rates and focusing degree among lakes. However, as a large number of lakes were evaluated at each sub-region, diagnostics at a sub-range scale should be acceptable. Different standardization procedures (e.g., lake size, organic carbon content, titanium, 210Pb amount) may help in the interpretation. Some high mountain lakes may receive contaminants from a point source rather than regional background pollution, when the source is not far away and there are no topographic barriers in between. PAHs loadings may stand out over similar places not directly affected by orders of magnitude (e.g., Snyder Lake catchment of Glacier National Park affected by a local aluminum smelter (Usenko et al. 2010)) .
The information from Asian mountain areas comes mostly from studies in China and usually not from archetypical mountain lakes. In fact, lakes in high elevation plateaus or in exceptionally large mountain valleys are extremely different from a typical alpine lake. Sometimes they are in arid areas and become saline; in others, with more suitable conditions, human populations may settle in the catchments. All in all, studies in this type of lakes have provided some indications of the background PAHs emissions for some parts of this vast territory. Compared to Europe, the records show an increasing tendency of PAHs deposition that started a decade or two later than in Europe and is still rising (Guo et al. 2010) (Fig. 4). On the other hand, there is a higher contribution of biomass burning and domestic coal combustion, although the contribution of high temperature combustions is growing. In some records, PAHs show a high correlation with Hg, Pb and other trace metals, indicating a common source related to progressive industrialization (Wang et al. 2010b). This was also a feature in Europe. The shift from low to high temperature combustion sources is reflected in the decreasing proportion of the simplest PAHs, with two and three rings, from 1860s to the present. In fact, the sediment PAHs records reflect the industrialization avatars in China: increased deposition since the “Westernization movement” in the 1860s; short interruption during the period of the “Cultural Revolution” (1966–1976); and steep increase during the last 30 years of unprecedented industrial and urban growth (Wang et al. 2010b).
Concentration and flux profiles of PAH7 (sum of benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benz[a]pyrene, dibenzo[ah]anthracene and indeno[1,2,3,-cd]pyrene) in lake sediments of Western China: QH Qinghai Lake; BS Bosten Lake; SG Sugan Lake; EH Erhai Lake; CH Chenghai Lake. Solid circles indicate fluxes and open circles: concentrations. Reprinted from Guo et al. (2010), Copyright (2010), with permission from Elsevier
Phenanthrene is a PAH that is abundant (even dominant) in the air and deposition measured in mountain sites, but it usually plays a secondary role in the sediments (Fernandez et al. 2003). The underrepresentation of phenanthrene in the sediments may be related to the water-particle partition in the water column. Low temperature may mitigate the discrepancy between air and sediments. Phenanthrene is the main PAH in the sediments of lakes in the Svalbard islands in the Arctic [Arresjöen Lake, (Fernandez et al. 1999); Ny-Alesund Lake (Jiao et al. 2009)]. There, the source was unclear. Biogenic origins, enhanced condensation because of the low temperatures, and local sources have been suggested as possible alternative (Rose et al. 2004). Phenanthrene was also dominant in the highest southernmost lake of Europe (La Caldera, 3050 m a.s.l., Sierra Nevada (Fernandez et al. 1999)), where temperature is high in summer .
Retene is common in most mountain lake sediment profiles, although in low quantities, and usually do not correlate with the dominant pyrolytic PAHs. Wood combustion is a source of retene, but it also has a diagenetic origin (Grimalt et al. 2004b), for instance, from abietic acid, which is found in conifer resin and other plant lipids. Conifer forest may cover a large proportion of some mountain slopes. Retene has been identified as the main PAH in soils of the montane stage in the Pyrenees (Quiroz et al. 2011). One way to investigate the origin of retene is to compare its sediment profile with that of the ratio between 1,7-dimethylphenanthrene and 2,6-dimethylphenanthrene, which is an indicator of wood combustion. There are sites where they closely match (e.g., Noir (French Alps), Escura (Serra d’Estrela, Portugal), Gossenköllesee and Schwarsee ob Sölden (Tyrolian Alps, Austria)), indicating a wood combustion origin, and others where they substantially differ (e.g. Redòn (Pyrenees), Starolesnianske pleso and Dlugi (Tatra Mountains), Øvre Neådalsvatn (Norway), Cimera (Sierra de Gredos, Spain), indicating the diagenetic origin of a large proportion (Fernandez et al. 2000). In Planina Lake (Julian Alps, Slovenia), retene was linked to forest fires in the lake vicinities (Muri et al. 2003), concentrations reaching exceptional values (ca. 1000 ng g−1 DW). The retene potential as a mountain forest fire indicator has not been explored sufficiently yet. It could be used complementing other fire indicators (e.g. charcoal particles).
Perylene sediment profiles also tend to differ from pyrolytic PAHs. At some sites, it suddenly increases at relatively deep sediment layers, as a result of diagenesis. In some areas, barely affected by industrial and urban pollution, it is largely dominant in the sediment profile (e.g., some Chilean mountain lakes; Barra et al. 2006), which has been related to coniferous signatures.
Organohalogen Compounds
Organohalogen compounds are numerous and diverse in their properties, applications and periods of use. Recent results from the Global Atmospheric Passive Sampling (GAPS) network (Pozo et al. 2009) indicate that the latitudinal distribution of OHCs reflect the estimated spatial variability of global emissions, with the highest concentrations observed at mid-latitudes of the northern hemisphere, including PCBs, which have been long banned.
PCBs can be viewed as a benchmark set for OHCs because different congeners cover a broad range of physicochemical properties and their distribution can reflect regional background conditions as its production stopped in the 1970s. They are found everywhere. The lowest PCB total concentrations found in remote mountain lake sediments are around 1 ng g−1 DW (Guzzella et al. 2011). Within a region, PCBs tend to increase with altitude in lake sediments, particularly the less volatile congeners (penta- and hexa-PCB). This was first observed by Grimalt et al. (2001) in a survey of European high mountains lakes covering different ranges to achieve a larger temperature gradient. In this study, it could be criticized that the highest altitudes (lower air temperatures) corresponded to areas of central Europe, which were potentially closer to regions of higher emissions (Daly and Wania 2005). However, the article showed that, within the subset of lakes in the Alps, although the gradient was shorter, the relationship was also significant. In addition, the variation of each compound was partitioned into air temperature and location influences using appropriate statistical techniques (Borcard et al. 1992), being the explicative power markedly in favor of temperature for the less semi-volatile compounds. Later, altitudinal PCB patterns in lakes have been found in sediments (Guzzella et al. 2011) and fish (Gallego et al. 2007; Felipe-Sotelo et al. 2008; Demers et al. 2007). More interestingly, comparison of OHC composition in atmospheric deposition and sedimentary records in geographically distant sites demonstrates a selective trapping of the less volatile compounds. Trapping efficiencies increase at decreasing air temperatures of lacustrine systems (Carrera et al. 2002). Studies in the Chilean Andean lakes show the highest PCB fluxes in the sediments between 1991 and 1998 (Pozo et al. 2007), which differ from the main peaks in rural Canadian and European lakes, which tend to be in the period 1955–1970.
DDT is usually found in mountain lake sediments as its derivates (e.g., DDE), which indicates that they do not correspond to recent use, and sometimes are reported together as DDTs. They also tend to show a local altitudinal increase as the less volatile PCBs (Grimalt et al. 2001). Although not in use in many countries, in others this insecticide is still a primary element in the fight against malaria. There is little knowledge on how it redistributes with LRAT. In lake sediments at Mt. Sagarmatha (Himalayas), between 4893 and 5293 m a.s.l., p, p’-DDE average levels were 0.19 ± 0.27 ng g−1 DW, and no other form was measured above the detection limit (Guzzella et al. 2011). These values are similar to those found in the Arctic and Subarctic and may be considered representative of background concentrations in remote cold areas.
HCHs were used initially as a technical mixture of α-HCH and γ-HCH, and later it was commercialized as lindane (γ-HCH) until it was banned in 2009. The HCHs distribution mainly reflected its broad application in agriculture. For instance, HCHs levels compared to other OHCs indicate whether a mountain range is located in an industrial or agricultural region in Europe (Carrera et al. 2002). In the near future, the recent band of HCHs should be reflected in the sediment archive as they are compounds sufficiently volatile to achieve local equilibrium between different environmental compartments quickly (Liu et al. 2010). In Mt. Sagarmatha (Himalayas) lake sediments, HCHs were below detection limits (< 0.1 ng g−1 DW) in samples collected in 2007 (Guzzella et al. 2011). HCB, which is no longer used but results as a by-product of certain industrial processes, shows distribution patterns similar to HCHs in remote sites because of the similarity in the physiochemical properties between these compounds. In remote Himalayan lakes, the average levels were 0.08 ± 0.04 ng g−1 DW (Guzzella et al. 2011).
Other pesticides have had a more regional use than DDT or HCHs. Toxaphene has been found in Lochnagar sediments in Scotland (Rose et al. 2001), which could be evidence of transcontinental contaminant transport, because it was mainly used in North America. The profile showed two peaks, one in the 1970s and another in the 1990s (PCB had a unimodal distribution centered at 1973). The earlier peak agrees with the U.S. source curve; the second could be related to late use in eastern and south-eastern Europe, although air circulation from these regions only exceptionally arrives to Scotland. The toxaphene issue in European remote sites has not been sufficiently explored. Sediment concentrations in Lochnagar were equivalent for those reported in the Great Lakes, where there were fluvial inputs, and higher than untreated sites in the Canadian Arctic.
PBDEs have been used in different technical mixtures along time, with increasing bromination (penta-, octa-, deca-). The most recent consisted of a molecule fully brominated (BDE-209), with low sub-cooled vapor pressure (10−8.3 Pa) and high hydrophobicity, according to its octanol-water partition coefficient (Kow = 108.7), which, consequently, was not expected to be found in remote sites. However, it has reached mountain lakes far away from urban areas (Bartrons et al. 2011) becoming an example of how difficult is to predict the environmental performance of new synthetic substances. The changes in production and use of PBDE mixtures have been rapid and diverse throughout continents, and so have been registered in lake sediments. The presence of some brominated compounds (e.g., BDE-47, 99, 100) could be interpreted straightforward as an indication of the source technical mixture. However, there are indications that PBDEs are biodegraded in anoxic sediments faster than other persistent OHCs (Bartrons et al. 2011). On the other hand, they are also affected by temperature-dependent selective trapping. Specific patterns of PBDEs have been observed in the sediments of the Svalbard lakes (Arctic) (Jiao et al. 2009), showing higher concentrations of lower brominated compounds such as BDE-7, 17 and 28. In temperate mountains, PBDEs levels increase with altitude in lake sediments (Bartrons et al. 2011). Comparisons between PCBs and PBDEs distribution have been made (Fig. 5) because of their similar richness in different congeners, which cover a broad range of volatility, and because PBDE production and use raised fast to levels equivalent to PCBs (Usenko et al. 2007).
Focus-corrected flux profiles of current and historic-use of semivolatile organic compounds (SOCs) in Lone Pine Lake (3024 m a.s.l) and Mills Lake (3030 m a.s.l) sediment cores. The lakes are respectively located at west and east of the Continental Divide in the Rocky Mountain National Park (Colorado). Westerly winds predominate in the area, which together with the topographic barrier produce a contrasting pattern of contaminant fluxes between the two slopes. Solid lines indicate U.S. registered use date, dashed lines (red) indicate U.S. restriction date, and * marks indicate below method detection limit. Reprinted with permission from Usenko et al. (2007). Copyright 2007 American Chemical Society
The number of studies examining perfluorinated compounds in mountain lake sediments is low. In the Canadian Rocky Mountains, the isomer profiles and the temporal trend data suggest that FTOH oxidation is the dominant atmospheric source of PFCAs to high alpine lakes (Benskin et al. 2011). Alpine lakes from Austria presented similar profiles (Clara et al. 2009). Further investigations are required in other parts of the world.
Understanding the Sediment Archive
Mountain lake sediments are recording systems of regional background air contaminants. However, contaminants undergo a complex pathway from sources to the lake and within the lake before being archived in the sediments (Fig. 6). The LRAT of the contaminant can be in gas or particle phase. In this partition, the origin may have some influence but as soon as the travelled distance is sufficiently long, the physicochemical properties of the substance are the primary conditioning factor determining the gas-particle partition. The phase in which a substance is travelling has influence on reaction (e.g., photo-oxidation by high irradiance) and scavenging rates. High mountains are barriers to circulating air masses; particles may deposit in dry conditions by simple intersection by vegetation, rocks and soils, or by adiabatic cooling that may favor rainfall and concurrent scavenging by rain or snow fall. Once deposited, particles will be more or less efficiently transported by water flows to lakes and eventually will settle to the sediments. In water, a new partition between water and particle will take place, under different constraints than the air-particle partition. On the other hand, substances reaching the mountains in gas form will exchange with water according to their solubility properties and temperature conditions. In the water column, the substances will interact with the particles present. These particles are quite different from those present in the atmosphere. In fact, many of them are living organisms, from microbes to large fish. In this case, the water-particle partition will be highly conditioned by the hydrophobicity of the compound, which can be extremely high for some organic contaminants. Sinking particles accelerate the transport to the sediments. Bioaccumulation across the food web may delay the deposition of part of the contaminants to the sediment, but eventually, sooner or later, if the suspended matter is not exported down flow, particles with attached contaminants will settle to the sediment surface. Beyond the lake, a large proportion of the contaminants deposited can be stored in soils, vegetation or other catchment reservoirs (e.g., glaciers, peat lands). Depending on the circumstances, these reservoirs are permanent sinks for contaminants or may become a delayed source to the atmosphere or to the runoff.
These complex contaminant pathways to the sediments are not fully understood yet. Some aspects respond to general concepts of contaminant LRAT (Macdonald et al. 2000b) and others require the understanding of processes particular to mountains and mountain lakes. Mountains offer an ideal setting for environmental research as in short distances there are marked physical gradients (e.g., air temperature) and ecosystems are relatively amenable to observation and modelling, particularly mountain lakes. However, the complexity of air circulation at different spatial scales has to be considered.
Atmospheric Transport to the Watershed: The Airshed
Atmospheric transport of contaminants is the ultimate cause and, thus, a key process for understanding the sediment archive. Compared to the watershed, the airshed of lakes—the area that supplies the materials for deposition- does not have defined boundaries and may be changing through time as climate changes. Methods for determining air mass back-trajectories and increased understanding of mountain meteorology have notably improved the capacity for linking sources and sinks in the atmospheric transport of contaminants.
Atmospheric samples from European high mountain areas show that most semi-volatile OHCs are predominantly found in the gas phase (Van Drooge et al. 2004). For instance, HCB, the dominant compound in these samples, was exclusively present in the gas phase. Only the less volatile are predominant in the particulate phase (e.g., PCB congeners 149, 118, 153, and 180), and those with extremely low vapor pressure are assumed to be transported exclusively sorbed to particles (e.g., BDE-209). Generally, for a given site, there is an agreement between the amount of particles and amount of contaminants typically associated with them (Fernandez et al. 2003), indicating a relatively homogenous background of contamination . However, the correlation may be lost in areas with contrasting air mass trajectories in which one of them is crossing areas of dust emission. If contaminants are directly measured in the air or in single deposition episodes, the contaminant profile of the different air mass directions can be defined accurately. Unfortunately, in this respect, sediment samples integrate deposition over months, usually years. Therefore, the way to disentangle distinct geographical sources of contaminants has to be by using specific indicators. This has been mainly applied to PAHs, for instance, for distinguishing between low temperature combustions (wood, domestic coal, etc.) and high temperature ones (vehicles, electric stations, etc.) (Guo et al. 2010). When some potential specific point source (e.g., smelter, pesticide factory) adds to the background contamination, the indicators extend to specific trace metals, isotopes or synthetic compounds (e.g. lindane) (Usenko et al. 2010).
Contaminants associated with particle transport are generally better preserved in the sediment record than gas phase components, which tend to have more complex dynamics before being permanently archived. For instance, there is an approximate agreement (e.g., at logarithmic scales) between total PAHs in the air and soils or sediments of remote sites above the planetary boundary layer (Van Drooge et al. 2011). However, sediment PAHs composition generally agrees most closely with the air particulate phase in sites where both have been studied. The air gas phase is largely dominated by lighter, more volatile compounds, such as phenanthrene and fluorene (Van Drooge et al. 2010), which usually have a minor representation in the sediment record, compared to compounds attached to air particles (Van Drooge et al. 2011). This fact may change at exceptionally low temperatures, such as those that can be achieved at high latitudes or extraordinarily high altitudes, where those compounds typically associated to the air gas phase may condense and largely increase in the sediment record (Fernandez et al. 1999).
Atmospheric transport occurs under oxidizing conditions (e.g., UV radiation, free radicals), and some contaminants are more reactive than others. Generally, those compounds carried in the gas phase are more exposed to photo-oxidation and proportionally decay more than those attached to particles. Also, among PAHs and OHCs, there are compounds chemically more stable. Alkylated PAHs are more reactive than the corresponding parent PAHs. Their life time in the air is short; thus, their presence indicates nearby sources (Kalberer et al. 2004). In general, a certain indication of the relative distance of transport can be assessed by the proportion of short life compounds. For instance, benz[a]anthracene and benz[a]pyrene are more chemically labile than benz[e]anthracene and benz[e]pyrene, respectively. Ratios between these compounds have been used as relative indicators of distance to sources (Van Drooge et al. 2010). In endosulfan, the ratio between Endo I and Endo II increases after application because the latter is less stable in the atmosphere. The proportion of endosulfan sulfate may indicate re-emission since it is believed that microbial oxidation of endosulfan in soils is one main source of this form (Pozo et al. 2009). In any case, endosulfan sulfate is the only form usually found when the source of endosulfan is exclusively the long range atmospheric transport, and there are not regional sources (Carrera et al. 2002).
Single point air measurements are not representative of contamination even at remote sites. There is seasonality in the contaminant levels of the mountain air for several reasons. Part of the seasonal change may be related to current use of some compounds (HCHs, endosulfans) (Blais et al. 2001b). High altitude sites in South America appear to be affected by the large use of endosulfans in Brazil, and the concentrations during the summer can be an order of magnitude higher than in winter (Meire et al. 2012b). In the Tibetan Plateau, high summer values of HCHs and DDT-related compounds are due to more contaminated air being blown into the region from the Chengdu Plain, presumably either due to higher pesticide usage in summer or due to higher temperatures leading to higher evaporation in source regions (Liu et al. 2010). In Europe, compounds that have not been manufactured (PCBs) or drastically restricted in their use (4,4′-DDT related compounds) also may show seasonality due to the higher contribution during warm periods of the continental inputs, compared to a more maritime origin during cold periods (Van Drooge et al. 2004). In European mountains, air masses that have been travelling in the high troposphere (> 6000 m) carry 4–10 times less background OHCs (e.g., ca. 9 pg m−3 PCB and 0.4 4,4′-DDE) than the overall average (Van Drooge et al. 2004). In contrast, in the same areas, PAHs showed an opposed seasonal trend, concentrations in air were higher during the winter. The PAHs seasonality was higher in the particulate fraction, indicating higher winter emission but also the temperature dependence of gas-particle partitioning (Fernandez et al. 2002), which is reasonably well predicted in the case of air-soot partition.
Precipitation
Rain has a large capacity for scavenging particles from the atmosphere and thus their associated contaminants. In addition, water drops can also incorporate contaminants from the gas phase according to its solubility (air-water partition) and temperature. As temperature decreases with altitude, the levels of dissolved contaminants can increase (Wania et al. 1998). This feature, combined with higher precipitation with increasing altitude, has been suggested as a main mechanism for higher contaminant concentrations and selective cold trapping of contaminants at high altitude in mountains (Wania and Westgate 2008). Nevertheless, it has to be taken into account that the increase in precipitation with altitude is not a global general feature in the mountains (Körner 2007). The increase of precipitation with altitude is more marked at latitudes between 40 and 60°, where most of the studies have taken place up to now. The tendency is less marked between 30 and 40°, and there is not such a trend in the rest of the world. In subtropical zones (10–30°), the maximum of precipitation occurs at intermediate altitudes (ca. 1500 m) and decline rapidly towards higher altitudes. At equatorial (0–10°) and polar latitudes (> 60°), precipitation decreases with altitude, although with contrasting absolute values, high in the former, and low in the latter, respectively. The spatial heterogeneity can also be large within the same region. Whereas local correlation between altitude and air temperature is always high (r2 > 0.80), the correlation with precipitation is often statistically non-significant (Kirchner et al. 2009). This lack of correlation may be due either to contrasting orientation of the sites respect to the dominant trajectory of the wet air masses (e.g., different slopes in the Andes) or to the micrometeorological complexity occurring in massive mountains. In monsoon areas, the gradient and absolute values of precipitation are higher the lower the average altitude of the mountains in the range. Even locally, precipitation levels are difficult to model (and forecast) from valley to valley (Thompson et al. 2009). Altitudinal gradients of deposition have not been directly studied and sometimes the effect is indirectly evaluated by the concentrations of contaminants in soils, and whether they correlate or not with precipitation (Tremolada et al. 2008). As soils are also dynamically complex, studies of direct measurements of precipitation along altitudinal gradients will be valuable, yet the distribution of sampling points without other influential factors than altitude (and precipitation differences) is not that easy.
In high mountains, a large proportion of total precipitation is in snow form. Snow is even a better scavenger of particles than rain (Franz and Eisenreich 1998), including compounds that are usually in the gas phase (e.g., phenanthrene; Carrera et al. 2001). The highly hydrophobic and scarcely volatile BDE-209 has been recently found in snow (Arellano et al. 2011). Correlation of the OC concentration in snow versus mean annual winter temperature shows higher accumulation at lower temperatures. In the Tatra Mountains, the less volatile PCBs exhibited higher temperature dependencies than the more volatile congeners (Grimalt et al. 2009); contrarily, in sites of western Canada, the more volatile compounds increased preferentially with altitude (Blais et al. 1998). This may be related to the specific range of winter temperature in each area of study.
The quality and quantity of contaminants in precipitation change seasonally, because emissions from urban, industrial and agricultural sources and the stratification of the atmosphere vary from summer to winter. Therefore, a few months of deposition measurements are not representative of the annual load. In addition, snow introduces an added complexity. The snowpack has a complex dynamics, and it cannot be assumed that the load of contaminants that it contains will end-up into the soils and runoff (Finizio et al. 2006). Direct interpretation that higher levels of a contaminant in the snow will contribute to a higher load into the lake sediments is not necessarily correct.
Dust
In mountain lakes close to dust source areas, dust-transport and deposition become key factors for understanding contaminant deposition (e.g., some areas of Central Asia; Wang et al. 2010b). Dust can also be critical for understanding the contaminant deposition patterns in areas affected sporadically by air-masses that bring dust from far away sources. In general, dust has a buffering (Psenner and Catalan 1994) and fertilizing effect on mountain lakes (Camarero and Catalan 2012), which are usually acid-sensitive and oligotrophic. Trace metals are commonly associated with dust (Reynolds et al. 2010) but other contaminants have also been related to dust transport when the source of contaminants and dust are nearby (Wang et al. 2010b). In areas receiving sporadic (but intense) dust loads, dust contribution may jeopardize the correlations between particles and less volatile contaminants (e.g. PBDEs; Arellano et al. 2014), which is normally found when the source of particles is in urban or industrial zones.
Air Mass Trajectories and Distance to Source
Defining the lake’s airshed is not a simple task. When considering background contamination in mountain sites, the geographical distance is not necessarily a valid indicator of the potential contribution of a certain source area. Air mass trajectories can make distant sources close and, on the contrary, nearby sources far away.
Generally, high mountain lakes are located above the planetary boundary layer (full theoretical depth ca. 2000 m; nocturnal depth ca. 300 m at mid-latitudes) and are not regularly exposed to contamination from low lands. Therefore, high altitude lakes can be representative of background, long-range atmospheric contamination. However, this is not the case when the lakes are located in inhabited high altitude plateaus (Bogdal et al. 2011). On the other hand, convectional instabilities can develop in warm periods and mix the atmosphere up to several kilometers. Consequently, it has to be locally (regionally) evaluated, whether the lakes studied are permanently above the planetary boundary or not. In the Pyrenees, for instance, researchers have found evidence of trace metal local contamination up to about 2000–2300 m a.s.l. both in soils (Bacardit and Camarero 2010b) and the winter snowpack (Bacardit and Camarero 2010a).
A mountain lake rarely receives all its precipitation from the same direction and usually the different sources have distinctive chemical features; for instance, according to a more maritime (Cl, Na) or continental (Ca) contribution of inorganic compounds (Camarero and Catalan 1996). The cases in which single deposition events are analyzed are rare; more commonly, the samples are a composite of precipitation events covering a certain time. Then, the association between contaminants and air mass trajectories is made based on the relative contribution of different back-trajectories to precipitation of the sampling period (Bacardit and Camarero 2009). There are cases in which the air mass trajectories change seasonally. For instance, seasonality in semi-volatile organic compounds levels in the air of the European mountain is mainly due to a higher occurrence of air masses with strong continental inputs in the warm periods than in the cold periods (Van Drooge et al. 2004). HCHs deposition increased during the monsoon season in East Rongbuk Glacier (6572 m a.s.l.) in the Himalayas (Kang et al. 2009). If there is a geographical segregation of dominant sources (e.g., wood combustion vs. pyrolytic sources for PAHs) the air mass back-trajectories can help to confirm their relative contributions.
Air trajectories from different directions may also travel preferentially at certain altitudes and among complex terrains found in mountain areas, introducing additional sources of variability. Contaminant measurements at high altitude in oceanic islands have been proposed as representative of hemispheric free-troposphere conditions. At the Izaña Atmospheric Observatory (2367 m a.s.l.) on Teide Mountain (Canary islands, Atlantic), air PAHs are an order of magnitude lower than at any remote mountain site in the European continent (Van Drooge et al. 2010). OHCs in mountain ice cores have been related to some main climatic descriptors: DDT and HCH in the Mt. Everest ice to the El Niño-Southern Oscillation, DDT in Mt. Muztaga (Eastern Pamirs) to the Siberia High pattern and DDT in Snow Dome (Rocky Mountains) to North Atlantic Oscillation (Wang et al. 2010a).
Topographical Barriers
High mountains are topographical barriers even for tropospheric circulation. This may result in contrasting patterns between sites at the same altitude but different slopes. For instance, PAHs at high altitude soils showed similar relative composition but contrasting concentrations between northern and southern slopes in the central Italian Alps (Tremolada et al. 2009). As the air mass is forced to ascend to clear the barrier, air cools and precipitation becomes more likely; contrasting precipitation between slopes occurs if some direction dominates the regional air mass trajectories. Disparity in PCB sediment fluxes between lakes in the western and eastern Andes slopes of Chile has been attributed to differences in rainfall and air mass origin (Pozo et al. 2007). Similarly, from a study of 98 semi-volatile organic compounds in two lakes of the Rocky Mountain National Park, one west of the continental divide and another east, Usenko et al. (2007) conclude that regional upslope wind directions and site location with respect to regional sources and topographic barriers were determinant in the composition and magnitude of the sediment fluxes (Fig. 5).
Grasshopper Effect
Mountains are not only sinks for contaminants. Short-term changes in conditions may determine that deposited contaminants during cold periods are re-emitted during warm phases. This affects mostly the more volatile compounds. For instance, PCB summer release in the Alps pastures have been described (1900 m a.s.l.), which can be tracked in seasonal patterns in the contaminants of cow milk (Tato et al. 2011). Concentrations and enrichment in the less volatile congeners were higher in the Northern slope. The remission at low (warmer altitude) may become a supply for high (cold) altitudes. This jumping transport (the so-called Grasshopper Effect) may occur also between mountains (from lower to higher ones) and latitude (temperate mountains towards polar regions).
Catchment to Lake Transport
Ideally remote lakes will act as passive collectors of atmospheric deposition. Even in mountains, with the exception of volcano lakes, the catchment usually occupies a larger part of the watershed than the lake. Therefore, there is always some part of the annual contaminant load to the sediments that will originate from the catchment. The catchment is a reactive surface and, depending on its characteristics, will modify more or less the atmospheric profile of contaminants. Quick superficial runoff, in principle, should be more similar to atmospheric deposition than soil seepage. Soils act as filters for some substances, as has been recorded by comparing trace metals in snow and artesian flows (Shotyk et al. 2010). For lakes with a water budget that includes a significant contribution from catchment runoff, understanding the sediment archive requires understanding of what may happen in the catchment compartments. There have not been many attempts to quantify the direct and catchment-mediated contributions of contaminants [e.g., trace metals (Bacardit and Camarero 2010c), OHCs (Blais et al. 2001b)].
Snowpack
The snowpack is not a simple accumulation of snow that eventually melts. Throughout the cold months, the snowpack develops and changes in some physical properties. There are substances that can be re-emitted to the atmosphere or washout selectively during thaw periods. Emissions from the snowpack back to the air are more likely for the more volatile compounds (Hansen et al. 2006). They can be trapped at very low temperatures, but when the snowpack warms, a fraction of them may return to the gas phase. This is another mechanism of selective trapping of the less volatile compounds. Snow specific surface area, which determines the maximum amount of each organic compound that can be sorbed by snow, has proved useful for describing the distribution of the more volatile compounds (e.g., α-HCH and HCB) in the Tatra Mountains (Arellano et al. 2011).
The snowflakes undergo metamorphic change following deposition. In a simplistic view, flakes transform into grains with an ice core of pure water coated by ice with more solutes and particles. When conditions warm up, the coating ice melts first and, as a consequence, there is a differential elution of substances along the thaw period. The initial runoff from the snowpack is richer in substances than the final one, which is close to distilled water. This was first studied for inorganic compounds (Catalan 1989), but it has also been found for organic contaminants. In Bow Lake (Alberta), the first snowmelts exhibit high concentrations of organic contaminants relative to the snowpack, including the more hydrophobic compounds (Dieldrin, DDTs, and PCBs) (Lafrenière et al. 2006). There are areas of the world where two separate phases of snow accumulation and melting are clearly established, but in other parts, melting and freezing can alternate during many months, increasing the complexity of the process (Felip et al. 1999).
The ice and snow cover of the mountain lakes generally remain until a large part of the snowpack is melted (Catalan 1989). Water entering the lake circulates close to the ice cover, due to the inverse stratification of the water column. This determines that the first flush of snow runoff, which is rich in substances and particles, goes through the lake without much contribution to the sediments if the lake is relatively deep. The late thaw runoff usually does mix with the water column as the lake experiences spring turnover (Catalan 1992). Therefore, the relative contribution of catchment’s snow deposition to the contaminants sediment load may vary considerably from lake to lake and year to year. Necessarily, the contribution will differ depending on the geomorphologic and hydrologic setting: lake size and depth; ratio between lake area and catchment area, north and south facing, etc. The differences between contaminants may arise whether they are sorbed to large particles, which may sink quickly, or to fine-particles, or dissolved, which will pass through towards the outflow.
Glaciers
Some mountain lakes receive runoff from glaciers. They are not ideal systems within which to track atmospheric contamination because the glacier’s complexity as collectors and archives of contaminants complicates the interpretation (Villa et al. 2003; Blais et al. 2001b). Even under relatively constant climatic conditions, glaciers are in a dynamic equilibrium. Therefore, they are releasing contaminants during the warm season. The relative contribution of glaciers to runoff may be a relevant source of contaminant inter-annual variability in streams (Lafrenière et al. 2006). Under the current global warming regime, it is interesting to investigate whether and how contaminants archived for decades in the glacier ice are now released with the glacier retreat (Blais et al. 2001a). For some Switzerland glaciers, modelling projections indicate that time for release of the same quantity of contaminants can decrease 10-fold (Bogdal et al. 2010a). Oberaar Glacier is currently increasing the release of OHCs, which account for the observed OHCs increase in the lake of the same name (Bogdal et al. 2010a). In Lake Iseo, one of the large southern Alpine Italian lakes, the PCBs maximum in the lake sediments corresponds to the 1970s (the period of the highest PCB production) whereas DDT shows a sharp increase from the early 1990s, long after their agricultural use was banned in Italy (Bettinetti et al. 2011). This has been attributed to the retreat of the glaciers in the zone. In two lakes of the Bernese Alps (Switzerland), located only 8 km apart from each other, PCBs and DDT levels showed the highest levels in the sediments 30–50 years ago; however, in the one fed with glacier melting water, the peak was followed by a second increase in the 1990s (Schmid et al. 2011).
Soils
Soils play a dual role in the context of contaminant recording in mountain catchments. On one hand, they are an alternative archive to which the sediment register can be compared. On the other hand, similar to glaciers, they may act as a transient storage deposit for contaminants that eventually are released to the lakes. Compared to glaciers, however, dynamics of contaminants in soils may be more diverse and complex. As archive, soils differentiate from sediments by presenting higher bioturbation , lower accumulation rates (hence, there is less chronological control as archive), higher spatial (and seasonal) heterogeneity and different preservation conditions (Kirchner et al. 2009).
PAHs in lake sediments and nearby soils were compared for sites in the Pyrenees and Tatra Mountains (Grimalt et al. 2004b). Both archives showed airborne combustion mixtures refractory to photo-oxidation and chemical degradation. However, the degree of preservation compared to air samples was different (Fig. 7). Soil surface preserved the original air mixture better than the sediment surface. This indicates a PAH differential degradation, either during the water column transport or in the upper sediment before the ‘fossilization’ of the deposition signature. In contrast, lake sediments exhibited higher preservation of the more labile PAHs, indicating a lower degree of post-depositional oxidation than soils. The formation of some PAHs by diagenetic transformation also differs between soils and sediments; perylene usually increases in deep sediment sections, whereas phenanthrene enrichments in deep soils have been observed, which can result from the aromatization of diterpenoids. In several tropical regions, soils have a higher relative abundance of the lighter PAHs than in temperate regions, which likely reflects a higher contribution from biomass burnings (Daly et al. 2007a).
PAH in soils and sediments from Lake Redòn (Pyrenees). A and B refer to two different sediment and soil cores analyzed from this site. 1, fluorene; 2,phenanthrene; 3, anthracene; 4, fluoranthene; 5, pyrene; 6, retene; 7, benz[a]anthracene; 8, chrysene + triphenylene; 9, benzo[b + j]fluoranthenes; 10, benzo[k]fluoranthene; 11, benzo[e]pyrene; 12, benzo[a]pyrene; 13, perylene; 14, indeno[1,2,3-cd]pyrene; 15, benzo[ghi]perylene; 16, dibenz[a, h]anthracene, 17, coronene. PAH units in ng/g. The PAH composition in the upper layers of both compartments exhibits a high similarity with atmospheric aerosols collected at this site (Fernandez et al 2002). Reprinted from (Grimalt et al. 2004b), Copyright (2004), with permission from Elsevier
Soils are the major terrestrial reservoir of persistent organic pollutants (Meijer et al. 2003) and play a control on background atmospheric concentrations. For volatile compounds, there can be direct soil-air exchange (Cabrerizo et al. 2011). Depending on the soil characteristics, the maximum reservoir capacity may differ in many orders of magnitude among compounds (Dalla Valle et al. 2005). The reservoir capacity may change seasonally (and diurnally) with air temperature [e.g., PCBs (Guazzoni et al. 2011), DDT (Tremolada et al. 2011)]. The soils become net emission sources when maximum reservoir capacity is achieved. For semi-volatile compounds, mountain soils at mid-latitudes may be part of a stepwise mechanism of transport to high colder latitudes. Within a mountain range, soils at lower altitudes may become secondary sources for soils at higher altitude (grasshopping mechanism). For instance, soils in the Tibetan Plateau are secondary sources for HCB, HCHs, and low molecular PCBs; whereas they are still sinks for DDE and DDT (Liu et al. 2010; Wang et al. 2012). In the Czech Republic, the highest levels of HCHs and HCB were found in arable soils, but the highest for PCBs, DDTs and PAHs were found in high altitude forest soils, which reflected the legacy from the past high deposition (Holoubek et al. 2009).
Organic matter (OM) content increases the soil’s capacity to retain OHCs (Cabrerizo et al. 2011; Wang et al. 2012; Zheng et al. 2012) and particularly the most hydrophobic fraction (Tremolada et al. 2012). However, for PBDEs a better normalization of altitudinal gradients has been found using clay content rather than organic matter in soils (Yuan et al. 2012). Soil total organic matter (as total lipids in organisms) is a heterogeneous catchall that usually provides statistical significance for normalizing organic contamination, yet it may be too crude of a measure for understanding key processes. If the percentage of OM is used for normalizing OHC concentrations among samples, it is always advisable to report the OM values for each sample, so that one can calculate backwards to the original concentrations. This comment also applies to sediments.
The transport of contaminants from soils to streams and, eventually to lakes, is still poorly understood (Bergknut et al. 2011). It is an intermittent process since many of the contaminants are transported with particles or colloidal matter. Soil erosion is highly variable and dependent on multiple factors. In the Pyrenees, the anthropogenic trace metals accumulated in the catchments are three orders of magnitude higher than current atmospheric loads. The Zn soil exportation constitutes ca. 70 % of the lake inputs, yet soils are still a net sink for atmospheric deposition of Zn. In contrast, Pb atmospheric deposition is lower than Zn, and soils have become a net source for Pb, which contribute > 90 % of the lakes Pb loading (Bacardit et al. 2012; Bacardit and Camarero 2010b). In Lochnagar (Scotland) similar contributions have been found for Pb and about 78 % for Hg (Yang et al. 2002b). Consequently, the trace metal contamination legacy from soils may be a general environmental feature in European mountains. The net soil contribution to streams and lakes may last for at least decades (Bindler et al. 2008). This will delay the restoration of the lake system, despite reductions in emissions to the atmosphere, and may confound any evaluation of the reduction in trace metal deposition based on lake sediments if not taken into account. In Flin Flon (Manitoba, Canada), contradictory results between peat and sediment archives identified the soil legacy contribution of Hg and Zn (Outridge et al. 2011).
Plants, Forest
Bare soils and meadows are the common land cover of many high elevation mountain lake catchments. In rainy regions, or descending in altitude, more complex vegetation is often found. Cold and humid areas develop heathlands and peatlands, where plant debris may play a pivotal role in storing and transferring contaminants to the sediments. This has been mainly studied for trace metals, but it should be expanded to the study of other contaminants. In Lochnagar (Scotland), plant debris is the main transport mechanisms for trace metals from the catchment to the lake, a fact that increases the variability from cores taken in the same lake due to the clustered sedimentation of debris (Yang et al. 2002a). Scarcely vegetated catchments are more adequate for studying the chronological sequence of trace metal atmospheric deposition using lake sediments; whereas catchments with thick soils may be more interesting for studying the release of legacy contamination.
A forest has a filter effect on contaminants deposition (e.g., PCB deposition; Nizzetto et al. 2006). On one hand, a forest increases the scavenging of particles and associated contaminants (Davis et al. 2006), due to the large deposition surface that trees offer when air masses circulate through them. In deciduous forests, the filtering capacity may decrease after the autumnal leaf fall (Jaward et al. 2005) and the leaf litter becomes a vector of contaminants to soils, streams and lakes. On the other hand, tree leaves retain volatile contaminants from the gas phase depending on the air temperature. The temperature dependence implies seasonal fluctuations as observed for PCBs (Nizzetto et al. 2008). However, the contaminant concentrations in leaves may (Grimalt and van Drooge 2006) or may not be in equilibrium with average air temperature (Davidson et al. 2003), depending on the patterns of temperature fluctuations.
Confounding Processes
The catchment may be a legacy source for some contaminants, but also may release contaminants from natural sources. This is especially the case for trace metals. The need to always consider the natural sources related to rock weathering has already been mentioned. Alternative historical archives (e.g., ombrotrophic peats) in some cases may track aerial contamination more reliably than lake sediments (Koinig et al. 2003; Outridge et al. 2011). However, in the case where the main interest is in investigating the biological impacts of contaminants in the lakes, one has to consider that organisms do not bother whether trace metals are originating far away or in the same catchment, but it is the level of concentrations in the media that matters. Lakes in catchments with bedrocks rich in metals can achieve naturally high metal concentrations, which will likely fluctuate with climate change . This is the case for high As levels in the lake sediments of the Pyrenees (Camarero et al. 2009), for instance. Rock glacier enrichment in trace metals (i.e., Zn) can cause massive release during climate warming phases in metamorphic bedrocks (Thies et al. 2007), yet not related to atmospheric contamination (Ilyashuk et al. 2014). There can also be natural sources of PAHs. Coniferous forests are relevant sources in mountains, yet there is no confounding effect with pyrolytic PAHs. In Lake Stein (Swiss Alps), high levels of black carbon and PAHs have been attributed to the geological presence of graphite in its catchment area (Bogdal et al. 2011); this may be a quite exceptional case, but warns about the need to locally critically consider any apparent atmospheric contamination.
Air-Water Exchange
Direct exchange between air and lake water may occur in three ways: exchange with the gas phase for volatile compounds; dry deposition of particles; and scavenging by rain or snow. Rain and snow are particularly efficient in effecting the flux towards the lake. Direct air-water exchange has been less studied. Volatile compounds that arrive in the gas phase are exchanged with lake water according to their solubility. Temperature and wind control the kinetics of the process, and the exchange can go both ways, depending on conditions. The relevance of air-water exchange may depend on water residence time. In lakes with short renewal time, water flow will be more relevant (Wilkinson et al. 2005); whereas in deep lakes, with water residence time of several years, air-water exchange may be relevant (Meijer et al. 2006). Patterns that may reflect the temperature control of air-water exchange of volatile compounds in gas phase are: decreasing concentrations with depth of endosulfan, HCB, low molecular weight PCBs and PAHs found in deep lakes (Fernandez et al. 2005), and higher concentrations of PAHs in the dissolved phase of the water column below 6–7 °C in European mountain lakes (Vilanova et al. 2001b), whereas PAHs in particles did not show any temperature relationship. It is usually assumed that there is no exchange during the period in which the lakes are covered with ice and snow.
Water Column-Sediment Exchange
The OHCs distributions in the atmospheric deposition of the three remote sites geographically distant (Redòn, Pyrenees; Gossenköllesee, Austrian Alps; Øvre Neådalsvatn, Norway) were rather uniform and highly enriched in compounds with volatilities larger than 0.0032 Pa. However, more than 90 % of these compounds were not retained in the sediments (Carrera et al. 2002). This is an indication of the profound implications of in-lake processes for understanding OHCs distribution in lake sediments; particularly of the water-particle and water-sediment exchanges, which may determine a selective trapping of the less volatile compounds in the sediments (Meijer et al. 2006).
Both trace metals and organic contaminants are highly insoluble in water. Partition between water and particles are highly biased towards the latter, although differences among distinct compounds are enormous. Particles in suspension in mountain lakes are mainly organic (i.e., plankton) ; therefore, hydrophobic substances, such as organic pollutants, tend to adsorb to them, and the sinking of these particles will largely control the transport towards sediments (Meijer et al. 2006). In the sediments, the particulate fraction is in contact with porewater. If the solid fraction becomes enriched beyond equilibrium, there is a release of the compound to porewater and, eventually, it diffuses back to the water column. Among OHCs, more volatile compounds achieve equilibrium faster than semi-volatile compounds. In fact, semi-volatile compounds get buried before approaching equilibrium concentrations. Therefore, the latter may appear enriched in the sediment record with respect to deposition. During periods of complete water column overturn, particles may be resuspended from the sediments to the water column and—depending on the relevance of the water flow through the lake at this moment- contaminants can be exported downstream with the particulate matter. Phytoplankton growth and distribution and mixing patterns are extremely seasonal (Catalan et al. 2002); therefore, the pathways of exchange between the water column and the sediment are difficult to be accurately measured. Modelling has indicated that the plankton scavenging to the sediments can be a key factor for understanding selective trapping of some contaminants (Meijer et al. 2006). Unfortunately, accurate field studies of the water-column dynamics are lacking. Some data do not indicate the elevated seasonal variability in OHCs concentrations suggested by the models (Vilanova et al. 2001a; Vilanova et al. 2001b), but the information is too limited for being conclusive.
Active and Fossil Sediments
At certain depth, buried sediments achieve a state of nearly no significant changes in their composition; strictly, this ‘fossilized signature’ is what the sediment archive is about. In fact, there are substances that, from deposition at the top of the sediments to the final buried state, do not suffer any change, but others may experience transformations and/or relocation . The effective ‘fossilization’ takes from years to decades, depending on the sedimentation rates , which is not a problem for studies interested in long-term dynamics (e.g., over the Holocene) but may interfere with the interpretation of recent changes. Some deposited substances in the top sediment may diffuse back to the water column, may be resuspended with particles, may react with other substances, or may be biologically transformed or turbated. The occurrence of each of these potential changes depends on both the properties of the substance and the environmental conditions in the sediments.
Chemodynamics of trace metal in sediments is complex. The seasonal variation differs among trace metals (Anshumali et al. 2009). For instance, Fe and Mn are highly affected by redox changes, but Pb, Zn, Cr, Ni are less affected (Steiner et al. 2000). Cd adsorption by the sediments is pH dependent (Fu and Allen 1992) and thus sensitive to acidification (Borg et al. 1989; Yang et al. 2002a). Nevertheless, the relevance of this complexity appears be secondary compared to the contamination levels generally achieved. The prevalence of the contamination loading upon differential trace metal behaviors is shown by the higher correlation among trace metals concentrations in the most contaminated layers of the lake sediments with respect to their correlation in layers of natural background trace metal concentrations (Camarero et al. 2009). The atmospheric deposition of trace metals from contamination sources is usually rich in several metals and, consequently, increases the trace metal similarity among the sediments of the lakes affected. If contamination is very high, this effect may compensate for local differences in rock composition .
Diagenesis of PAHs in the sediments is still poorly studied, and this includes compounds clearly related to diagenetic processes, such as perylene. Some suggestions of differential preservation have been made based on comparisons between sediment layers. For instance, pyrene, benz[a]anthracene and benzo[ghi]perylene decline in deeper layers compared to their isomeric pairs, chrysene, fluoranthene, and indeno[1,2,3,-c, d]pyrene, respectively (Van Drooge et al. 2011). The latter are usually among the most abundant pyrolytic PAHs in the sediments; therefore, in terms of the reliability of the pollution record, this differential diagenesis is barely relevant.
Sediments usually become anoxic after a few millimeters in the sedimentary profile, even in unproductive lakes. Anaerobic dechlorination of some OHCs may occur (Grimalt et al. 2004c). Recent results indicate that PBDEs profiles can be particularly modified by anaerobic debromination (Bartrons et al. 2011). Higher abundance of anaerobic sites in more productive lakes can increase the proportion of less brominated compounds, with respect the initial deposited mixture. Therefore, the proportion of more brominated compounds at high altitude increases since lake productivity and organic matter content declines with altitude.
Generally, mountain lakes are oligotrophic and, as a consequence, water transparency is high and algal growth can take place along a large part of the bottom, from littoral to deep parts (Buchaca and Catalan 2007). As a consequence, most of the lake is covered with an active biofilm, within which strong gradients can develop in a few millimeters. Recently, it has been shown that even in relatively thin rock biofilms there is evidence of anaerobic microbial activity (Bartrons et al. 2012a). This is in agreement with the changes in PBDE composition from the purely autotrophic biofilms, such as colonies of the cyanobacterium Nostoc, common in mountain lakes, which contain OHCs profiles similar to precipitation, to the littoral and deep sediments that show increasing proportion of debrominated compounds (Bartrons et al. 2011). The understanding of OHCs biodegradation in sediments is poor and generally assumed irrelevant. This recent study indicates that, at least for some compounds, it cannot be ignored; otherwise, it will compromise the interpretation of selective accumulation and source attribution of PBDEs, for instance.
Modeling
Air mass trajectories can be traced back confidently at present (Draxler and Hess 1998). This is useful for determining the source areas of some deposition or air transport events (Killin et al. 2004) but, for sediment archives, they have to be applied in statistical terms. It is possible to investigate average back-trajectories for a site, how trajectories change seasonally, and what kind of sources are likely associated with certain trajectories. Weekly air measurements in a remote mountain site in south-western China indicated that HCHs and DDTs were brought preferentially by air masses from northern India, whereas high levels of γ-HCH and trans-chlordane were associated to air masses from southern China and the north of south-eastern Asian countries (Xu et al. 2011). In fact, current computational power can provide increased insights in source apportionment. Monte Carlo techniques allow for exploring the probability space more realistically and increase the characterization of the uncertainty associated with source apportionment (Sheesley et al. 2011).
Beyond the investigation of the routes of atmospheric transport to the mountain sites, modelling can help in the interpretation of the sediment patterns of contaminants. Ideally, a model mechanistically linking from sediments back to deposition would be the target. However, currently, this appears unrealistic as the complexity is overwhelming and uncertainties are too high. Even a comprehensive model from sources to sink appears extremely challenging. However, models targeting specific key steps of the contaminants pathway are quite informative and improve the interpretation of the patterns found. The transport and accumulation of non-volatile compounds (e.g., trace metals) in lake sediments can be modelled on the basis of a conceptual model of the catchment/lake system of a simplified number of compartments and input and output fluxes (Bacardit and Camarero 2010c). This can provide insight into the current influence of the catchment in the total sediment loads compared to atmospheric inputs. Even this simple model requires a significant effort in atmospheric field measurements, which is rarely achievable in many sites.
Trace metal, PAHs and OHCs include substances with a wide range of physicochemical characteristics. The parameters that define these properties are sufficiently known to allow exploring how the dynamics differ across the pathway to the sediment archive. Rather than trying to reproduce the whole process, it is useful to conduct numerical experiments to understand the sensitivity of the final archive to the different parts involved (Bogdal et al. 2010b). The initial step is the air transport and scavenging from the atmosphere (Breivik et al. 2006). Mountains provide a stimulating research area for modelers because of the altitudinal and the seasonal changes in air temperature and precipitation. For OHCs, differential accumulation of compounds with altitude and season appear as a result of their chemical properties (Wania and Westgate 2008). At this point, the key issue becomes understanding precipitation patterns across sites, and whether the rest of the pathway to the sediments is critical to distort or preserve the patterns generated with the precipitation scavenging.
The specific compartments in the lake catchments are each of interest because they serve as an aid in tracking long-range contamination. There are examples of modelling for PCB gas-phase changes between air and snowpack (Hansen et al. 2006), PAH air-soil exchange (Wang et al. 2011), PBDE and PAH uptake by vegetation (St-Amand et al. 2007, 2009), among others. Because of the interest of these compartments, the missing issue is their connection with the lake and eventually the sediment record. In that respect, evaluating the relevant constraints on how much of the contaminants in the snowpack might end up buried in the sediments is a challenge. Multimedia mass balance models have been developed to simulate whole-catchment processes. For the Lake Oberaar catchment, Bogdal et al. (2010a) combined a lake model, with a model of emissions at low land and advective transport to the lake catchment, and a model on the residence time of contaminants in the Oberaar Glacier after the surface deposition. They investigated the current release of legacy OHCs from the glacier and projections under climate change .
PCBs are excellent candidates for OHCs general biogeochemical analysis. The large number of congeners offers a range of physicochemical properties, and the lack of current use prevents from trying to reproduce patterns too locally conditioned. PCB transport in the water column has been modelled for Lake Redòn (Pyrenees) conditions (Meijer et al. 2006). The model assumes that only direct exchange with the atmosphere is relevant, the catchment component is ignored. The simulation clearly shows a selective transport of the less volatile congeners towards the sediment. Also, it shows that there is a re-emission of the more volatile compounds towards the water column, as they achieve equilibrium, which is not the case for less volatile ones, for which the system still has a large uptake capacity. However, the model predicts a highly seasonal and spatially variable distribution of PCBs in the water column that does not correspond with the limited existing observations (Vilanova et al. 2001a). It is an example on how modelling and observation are complementary and foster each other. PBDE and PCB modelling for Lake Thun (Switzerland) also indicated high sensitivity of the whole lake balance on the water-particle partition in the water column (Bogdal et al. 2010b).
There are still many opportunities for investigation using numerical experiments by dynamic modelling. Techniques may involve combinations of statistical, chemical and mechanistic modelling (Boyle and Birks 1999). For global assessments, including a contaminant module in climatic models is important for obtaining long-term projections (e.g., CliMochem; Wegmann et al. 2006), although initial results have challenged observations. Up-scaling may be the only possible way to make assessments over large mountain ranges (Andes, Himalayas, Rocky Mountains, Alps), when the interest is in the mountain themselves as, for instance, for conservation issues (e.g. national parks). Even partial models require a lot of parameters, initial data and certain capacity of validation. There is no substitute for real data, but with the lack of data or with partial information, model sensitivity analysis may provide insights into which can be the relevant processes.
The Mountain Altitudinal Gradients and Contaminants
The first principles behind the dynamics driving contaminant patterns are essentially the same everywhere. However, there is an environmental feature intrinsic to mountains, the altitudinal change in physical conditions, which justifies the specific interest for mountain studies (Fig. 8). The distribution of organic contaminants is frequently investigated with respect to altitudinal patterns. There are features of physical change in the mountains that occur globally, but other features vary according the latitude or the morphological characteristics of the range (Körner 2007). The ‘universal’ mountain gradient includes the decline with altitude of temperature (average lapse rate 5.5 ° C km−1), atmospheric pressure (ca. 11 % km−1) and available land area, and the increase of radiation, the proportion of UV-B ((λ 290–320 nm), and the size of the airshed to which sites are exposed. The patterns of altitudinal change of precipitation are not the same throughout the planet, and neither the seasonality of this precipitation (e.g., winter, and summer precipitation in the western, and eastern Andean slopes, respectively). Finally, the length of the growing season above tree line changes with latitude.
Altitudinal gradients of contaminants have been found measuring different matrices in addition to sediments: air passive samplers (Pozo et al. 2009; Loewen et al. 2008; Estellano et al. 2008; Meire et al. 2012a; Meire et al. 2012b; Liu et al. 2010; Li et al. 2006; Shunthirasingham et al. 2011); mosses and lichen (Liu et al. 2005; Grimalt et al. 2004a; Daly et al. 2007c); soils (Chen et al. 2008; Daly et al. 2007c); plant leaves (Wang et al. 2006; Davidson et al. 2003); snowpack (Arellano et al. 2011; Blais et al. 1998); fish (Vives et al. 2004; Blais et al. 2006; Gallego et al. 2007; Yang et al. 2010c; Vives et al. 2005b; Demers et al. 2007); and other aquatic organisms (Blais et al. 2003; Blais et al. 2006). However, there is not necessarily a unique mechanism behind altitudinal gradients of contaminants. The environmental altitudinal gradient in mountains is multivariate as described at the beginning of this section. The physical drivers may generate altitudinal gradients of contaminants by mechanisms such as differential air (or water) compound oxidation, differential compound solubilisation, differential particle loading (i.e., rain scavenging) and combinations thereof. In addition, the changes in physical conditions imply changes in the nature of the land cover (e.g., forest, meadows, bedrock, wetlands, etc.) and biological activity in general, which adds more opportunities for selective trappings across altitudes (e.g. organic matter in soils, forest filtering, etc.).
Complementary to new studies for investigating proposed hypotheses, the repeated observation of similar patterns throughout large geographical areas (or even worldwide) may help in discerning the most likely mechanisms. For instance, in the Bolivian Andes, air samples indicated increasing gas phase concentration of HCHs and endosulfans across an altitudinal gradient from 1820 to 5200 m a.s.l. The differences were attributed to different airsheds (Estellano et al. 2008): the high-altitude sites were exposed to larger airsheds susceptible to long-range transport from more distant (contaminating) regions. A similar altitudinal gradient for endosulfan has been found in Chile; in this case both long-range transport and cold trapping have been suggested as the key factors (Shunthirasingham et al. 2011). Recently, Meire et al. (2012b) found the same increasing pattern of endosulfan with altitude in the Brazilian tropical and subtropical mountains. Endosulfan was also found as a dominant organochlorine pesticide in the air of Mt. Everest region (4400–5000 m a.s.l) (Li et al. 2006). Such general and consistent patterns point towards a cold-trapping mechanism for endosulfans. Interestingly, PCBs did not show an increase with altitude in the Mt. Everest study, as the values were in a similar range (< 12 pg m −3) as measurements made in the mountain air of Chile (Pozo et al. 2004). In the case of lake sediments, there is a scarcity of repeated studies in nearby or complementary areas concerning organic contaminants yet, with some exceptions (Landers et al. 2010).
At first, processes driven by temperature are likely to provide more ‘universal’ altitudinal patterns than those driven by precipitation or any other physical factor that do not change so regularly across the mountain altitudinal gradient. For volatile and semi-volatile compounds, temperature appears to be key factor to consider. Rather than only plotting contaminant levels against altitude, it is worthwhile to plot the log transformed concentrations against the reciprocal of the average air absolute temperature at the corresponding sites and calculate the pseudo-enthalpies (Grimalt et al. 2001; Davidson et al. 2003). The comparison with theoretical enthalpies (e.g., vaporization and solubilisation) may inform whether the process is mainly temperature driven. This is the case, for instance, of the altitudinal gradient shown by some PCBs in lake sediments (Grimalt et al. 2001) and pine needles (Grimalt and van Drooge 2006; Davidson et al. 2003). On the contrary, the lack of minimum agreement between the observed and expected enthalpies may indicate that there is another relevant process behind those considered. For instance, PBDEs showed 3- to 4-fold higher pseudo-enthalpies than expected in the sediments of the lakes of the Pyrenees. The increased availability of anaerobic microsites at lower altitudes was suggested as a mechanism increasing degradation (Bartrons et al. 2011). Differential loss by degradation also has been suggested for soil altitudinal gradients (Daly et al. 2007b).
The altitudinal pattern does not have to be monotonic. Studies of PCBs and PBDEs in soils of Mt Qomolangma in the Tibetan Plateau from 3689 to 6378 m showed negative relationships with altitude below ca. 4500 m and positive dependence above (Wang et al. 2009). Below 4500 m, the distance to source may be the driver. Above 4500 m, physical gradients may dominate. Concentrations of heavier congeners (lower subcooled liquid vapor pressure) increased more with altitude than more volatile congeners, although the latter still were the main contributors to the total. This is consistent with what was found in Western Sichuan (China) by Chen et al. (2008) and the simulations of Wania and Westgate (2008), but opposite from the observation of OHCs in snow of mountains in the western Canada (Blais et al. 1998). Although the temperature gradient is more or less the same throughout, the compounds showing selective trapping may be different because absolute temperatures also matter. Those that are not selectively accumulated at certain temperatures may be affected at lower temperatures. This may explain discrepancies in the compounds selective trapped among snowpacks located at latitudes with different absolute temperature.
The role of wet deposition has been emphasized by some authors (Wania and Westgate 2008), due to its efficiency in scavenging contaminants from the atmosphere. The lower the air temperature, the higher is the partitioning towards the condensed phase. Hence, wet deposition scavenging is more efficient at high altitudes and in winter time, and for compounds less volatile and more hydrophobic. Consequently, relative mountain contamination could be predicted from the respective water-air (Kwa) and octanol-air (Koa) partition coefficients of the compounds and precipitation amount. This appears as a reliable mechanism for soils, and the patterns found provide observational support (Chen et al., 2008). Large spatial variability within the same altitude in a geographical area may be related to the soils heterogeneity (e.g. organic content conditioning re-emission properties) but also to the spatial and temporal heterogeneity of wet deposition. In contrast, lake sediments show less variability than soils. This fact points to the question of whether the driving mechanism of selective trapping is the same between sediments and soils. Meijer et al. (2006) have shown that selective trapping can be explained without any connection to catchment processes. Again, differences in the Kwa and Koa of the compounds are the key parameters determining the selective trapping. Consequently, it is very difficult to determine whether catchment or in-lake processes can be distinguishable from the statistical analysis of organic contaminants in the sediment patterns. Specific studies in the water column and deposition are required.
Biological Impact and Ecological Risk Assessments
The assessment of the potential impact of air-born contaminants on lakes, mountain ecosystems in general, or on some organisms in particular, can be a complementary goal to studying the LRAT and accumulation of contaminants . This is still an incipient field and different approaches have been tried.
A simple way to tentatively estimate the toxicity of sediments is the use of toxic equivalent factors (TEF) that relate the toxicity of the compound to that of some reference substance. Rather than actual toxicological assessments, TEF are a way to undertake normalized comparisons of the potential toxicity amongst sites. The method consists in weighting the concentration by some toxic equivalency for each compound and adding all the values to obtain an overall degree of toxicity for the sediment sample (e.g. PAH toxicity; Quiroz et al. 2010). A simpler version is to check the contaminant levels respect to some numerical sediment quality guidelines (SQGs) provided by different national or international institutions (MacDonald et al. 2000a). The aim of these guidelines is to rank areas that warrant study on the occurrence of adverse effects. They are useful for comparing the degree of contamination among sub-regions, and to identify which chemicals are elevated in concentration (Han et al. 2011). In general, organisms in high mountain lakes are naturally submitted to harsh conditions (e.g., high radiation, low temperatures, diluted waters, high metal concentration in the sediments). As a consequence, their toxicological response is likely to be different from organisms in low lands. Local adaptation of organisms to some natural stressor tends to also increase resistance to pollutants that may affect similar physiological processes. For instance, frogs show genotoxic co-tolerance to UV and PAH stress, according to the natural exposure to the former (Marquis et al. 2009); those at higher altitude are more resistant to both UV-B and PAH pollution.
Correlation is the simplest way of exploring potential contaminant impacts. Unambiguous statistical associations between pollutants and organism community composition are rare [e.g., diatoms, Moser et al. (2010); chironomids, Brooks et al. (2005)]. When contaminants are combined with another ecosystem stressor (e.g., acid-base conditions, eutrophication), then correlations are more likely to be found, such as trace metals and acidification (diatom changes) or trace metals and oligotrophication (chironomids; Il’yashuk and Il’yashuk 2000), yet the direct toxicity of the contaminants cannot be proven. More informative is the comparison of physiological biomarkers with contaminant levels in the sediments; for instance, Cyp1A gene expression in fish correlated with HCB and 4,4′-DDE levels in the sediments of European mountain lakes (Quiros et al. 2007), which were later confirmed in fish muscle tissue (Jarque et al. 2010). Cytochrome P450 1A (Cyp1A) is an established biomarker for xenobiotics, particularly for the so-called dioxin-like compounds including some PAHs (e.g., benz[a]pyrene) and coplanar PCB congeners. Sometimes contaminants in the sediments are used in ‘forensic approaches’. Cascade frog declines in the Mount Lassen area (California) and its potential connection to organic contaminants were investigated measuring 73 semi-volatile contaminants including pesticides, polychlorinated biphenyls (PCBs) , and polycyclic aromatic hydrocarbons (PAHs) in sediments and frog tadpole tissue at 31 sites where Cascades frogs had disappeared or are still present across the Lassen and Klamath regions (Davidson et al. 2012). This approach is more useful for rejecting possible causes than confirming them, as it was the case for Cascades frogs, where no connection was found with the contaminants that were measured.
The distribution of contaminants in the lake’s food web is generally closer to atmospheric deposition than to concentrations in the sediments. For instance, in Lake Redòn (Pyrenees), phenanthrene is the dominant PAH in both aerial deposition and food web organisms (Vives et al. 2005a), whereas it ranks 7th in the sediments (Fernandez et al. 1999). If the aim is not to investigate a time series, but carry out an assessment of contaminants throughout a large number of lakes in a region, littoral biofilms may more closely reflect the current characteristic of atmospheric deposition than deep sediments (e.g., PBDEs; Bartrons et al. 2011), and sampling is much easier. Fish are the most common organism sampled for contaminant assessment in mountain lakes (Blais et al. 2006), although they are often alien species to mountain lakes (Miró and Ventura 2013), as most mountain lakes were originally fishless due to their steep outlets, produced by glacial erosion, that fish cannot swim upwards or the lack of outlet in seepage and many volcanic lakes. Contaminants in fish may follow similar altitudinal patterns as in sediments (Grimalt et al. 2001), as they integrate food items from different lake habitats (Catalan et al. 2004). This is not the case for any other kind of organism. For instance, PAHs are higher in the organisms inhabiting the littoral habitat than the deep-sediment and the pelagic habitats, although deep living organisms are enriched in those PAHs with higher molecular weight (Vives et al. 2005a). Besides habitat effects, different contaminant composition among organisms also indicate a large variability in the capacity of metabolic degradation, which for PBDEs have been related to the evolutionary trophic history of the studied organisms (Bartrons et al. 2012b).
Bioassays are a direct assessment of toxicity for a specific organism, including humans. Whole sediment and sediment elutriate toxicity tests with fish, amphipods and cladocerans have been used in studies of pollution by acid-mine drainage (Finlayson et al. 2000). Molecular techniques allow for direct evaluation of estrogenic activity using recombinant yeast (Fig. 9), based on a human estrogen receptor, which allow for processing of a large number of sediment samples (Garcia-Reyero et al. 2005) or fish muscle extracts (Garcia-Reyero et al. 2007). If pollutants are measured in the same samples, then the compounds responsible for the potential human endocrine disruption can likely be identified.
Evaluation of the endocrine-disrupting potential of lake sediments in 83 mountain lakes across Europe. A recombinant yeast assay (RYA) was used that harbors a human estrogen receptor to which estrogenic endocrine-disrupting compounds binds. The results were classified into three estrogenicity classes (A, non-estrogenic; B, low estrogenicity; and C, high estrogenicity). The proportion of lakes belonging to each category is indicated in the plot for the mountain ranges studied. Reprinted with permission from Garcia-Reyero et al. (2005). Copyright 2005 American Chemical Society
Beyond physiological effects on specific organisms, which may be non-native to the system (e.g. stocked fish), there is a need to increase the understanding on how contaminants may modify biogeochemical cycles, particularly, the microbial driven pathways. With increasing molecular facilities, there is a large potential in this field awaiting exploration (Xie et al. 2011; Sanchez-Andrea et al. 2011).
Looking Forward: Research Perspectives in a Changing World
Developing an understanding of long-range transport of contaminants in the current framework of climatic change will demand the merging of various approaches including monitoring, experimentation, palaeolimnology, and modelling, and will require effective international collaboration. It has become apparent that the recovery of lakes from acidification is closely linked with the responses to, and interactions with, other large-scale components of global change (Keller 2009). This has to also be the case for contaminants, such as those considered in this chapter. Mountain lakes provide sites for monitoring and surveillance, but also case studies for understanding mechanisms. In fact, lakes are not absolutely necessary for establishing a network for monitoring atmospheric contaminants at remote sites. Nevertheless, they can provide a unique historical perspective and provide integration of whole catchment processes. With this aim, palaeolimnological studies should merge with the studies of biogeochemical cycling (Bindler et al. 2008) and the fate of organic contaminants in nature (Nizzetto et al. 2010).
Trace metal signatures of air contamination are well recorded in mountain lakes with airsheds influenced by mining activities. The use of mined materials has been increasing at a faster pace than world population. So far, there is no evidence that the growth of global materials use is slowing down (Krausmann et al. 2009). Therefore, although ores have been exhausted in some parts of the world, mining and smelting will continue in others (e.g., Sub-Andean Amazon, Peru; Lindell et al. 2010). The last chapters of the environmental history of mining have not yet been written. The level of mercury (Hg) species in ambient air and precipitation will be of interest in the years to come. Mercury in the atmosphere is largely (~ 95 %) elemental mercury [Hg(0)]. It is a gaseous and relatively insoluble form, which is subject to long-range transport, with a global average residence time for Hg(0) generally considered to be 6 months to 2 years (Schroeder and Munthe 1998). Following the discovery of atmospheric Hg depletion events in polar regions, a significant research effort was made to assess the physicochemical mechanisms behind the rapid conversion of atmospheric gaseous Hg(0) into reactive and water-soluble forms which are potentially bioavailable. The large increase in Hg emissions in rapidly developing countries (i.e., China, India) over the last decade, due primarily to energy production from coal combustion, are not currently reflected in the long-term measurements of total gaseous mercury in ambient air and precipitation at continuous monitoring sites in either Northern Europe or North America. The discrepancy between observed gaseous Hg concentrations (steady or decreasing) and global Hg emission inventories (increasing) has not yet been explained, though the potential oxidation of the atmosphere during the last decade is increasing (Sprovieri et al. 2010). Understanding of the atmospheric Hg fate is a main topic, particularly because projections indicate that Hg emissions will increase in the future, with maximum estimates of a doubling for 2050 respect current values. In addition, the relative emissions of divalent Hg will increase, implying a shift from long-range transport of elemental Hg to local deposition of Hg compounds (Streets et al. 2009). Mountain lake observations may complement latitudinal studies (e.g. Arctic; Muir et al. 2009) to investigate how the issue evolves.
PAHs are extensively used for tracking combustion contamination, yet their actual indicator value still has not been fully exploited. Used with other materials related to combustions, such as SCPs, black carbon, micro and macro charcoal rests and other organic compounds, they can provide an indication on how distinct types of combustion changed trough time (Muri et al. 2006), including the potential for assessing forest fire dynamics in the past.
Among the highest uncertainties regarding advancing global change as a multi-component phenomenon is the question of what will happen with the increasing cocktail of synthetic substances released to the environment that have the potential for interfering with biochemical pathways, many of which are essential to life. OHCs play a primary role in this question. There is a global toxicological puzzle that no one can yet evaluate, neither the magnitude nor the consequences (Rockstrom et al. 2009b). The establishment of the Global Atmospheric Passive Sampling (GAPS) network (Pozo et al. 2009), which mainly focuses on remote sites, will provide global baseline values, and will foster the development and validation of global transport models. Mountain lake sediments can complement the observations with a window to the past. In addition, there is a need to track long range contamination patterns and, simultaneously, toxicological susceptibility from organisms to ecosystems. Remote sites, such as high mountain lakes, provide a less complex setting to evaluate potential interference of synthetic substances with nature, which is now a challenging task under the generalized climatic and environmental changes (Noyes et al. 2009).
References
Anshumali, Ramanathan AL, Singh G, Ranjan R, Tripathi P (2009) Chemodynamics of trace metal fractions in surface sediments of the Pandoh Lake, Lesser Himalaya, India. Environ Geol 57:1865–1879
Appleby PG (2008) Three decades of dating recent sediments by fallout radionuclides: a review. Holocene 18:83–93
Appleby PG, Nolan PJ, Gifford DW, Godfrey MJ, Oldfield F, Anderson NJ, Battarbee RW (1986) Pb-210 Dating by low background gamma-counting. Hydrobiologia 143:21–27
Arellano L, Fernandez P, Tatosova J, Stuchlik E, Grimalt JO (2011) Long-range transported atmospheric pollutants in snowpacks accumulated at different altitudes in the Tatra Mountains (Slovakia). Environ Sci Technol 45:9268–9275
Arellano L, Fernández P, López JF, Rose NL, Nickus U, Thies H, Stuchlik E, Camarero L, Catalan J, Grimalt JO (2014) Atmospheric deposition of polybromodiphenyl ethers in remote mountain regions of Europe. Atmos Chem Phys 14:4441–4457
Bacardit M, Camarero L (2009) Fluxes of Al, Fe, Ti, Mn, Pb, Cd, Zn, Ni, Cu, and As in monthly bulk deposition over the Pyrenees (SW Europe): the influence of meteorology on the atmospheric component of trace element cycles and its implications for high mountain lakes. J Geophys Res-Biogeo 114, G00D02, doi:10.1029/2008JG000732
Bacardit M, Camarero L (2010a) Atmospherically deposited major and trace elements in the winter snowpack along a gradient of altitude in the Central Pyrenees: the seasonal record of long-range fluxes over SW Europe. Atmos Environ 44:582–595
Bacardit M, Camarero L (2010b) Major and trace elements in soils in the Central Pyrenees: high altitude soils as a cumulative record of background atmospheric contamination over SW Europe. Environ Sci Pollut Res Int 17:1606–1621
Bacardit M, Camarero L (2010c) Modelling Pb, Zn and As transfer from terrestrial to aquatic ecosystems during the ice-free season in three Pyrenean catchments. Sci Total Environ 408:5854–5861
Bacardit M, Krachler M, Camarero L (2012) Whole-catchment inventories of trace metals in soils and sediments in mountain lake catchments in the Central Pyrenees: apportioning the anthropogenic and natural contributions. Geochim Cosmochim Acta 82:52–67
Bal M-C, Pèlachs A, Pérez-Obiol R, Julià R, Cunill R (2011) Fire history and human activities during the last 3300 cal yr BP in Spain’s Central Pyrenees: the case of the Estany de Burg. Palaeogeogr Palaeoclimatol Palaeoecol 300:179–190
Baron J, Norton SA, Beeson DR, Herrmann R (1986) Sediment diatom and metal stratigraphy from Rocky-mountain lakes with special reference to atmospheric deposition. Can J Fish Aquat Sci 43:1350–1362
Barra R, Popp P, Quiroz R, Treutler HC, Araneda A, Bauer C, Urrutia R (2006) Polycyclic aromatic hydrocarbons fluxes during the past 50 years observed in dated sediment cores from Andean mountain lakes in central south Chile. Ecotoxicol Environ Saf 63:52–60
Bartrons M, Grimalt JO, Catalan J (2011) Altitudinal distributions of BDE-209 and other polybromodiphenyl ethers in high mountain lakes. Environ Pollut 159:1816–1822
Bartrons M, Catalan J, Casamayor EO (2012a) High bacterial diversity in epilithic biofilms of oligotrophic mountain lakes. Microb Ecol 64:860–869
Bartrons M, Grimalt JO, de Mendoza G, Catalan J (2012b) Pollutant dehalogenation capability may depend on the trophic evolutionary history of the organism: PBDEs in freshwater food webs. Plos One 7:e41829
Battarbee RW, Grytnes JA, Thompson R, Appleby PG, Catalan J, Korhola A, Birks HJB, Heegaard E, Lami A (2002) Comparing palaeolimnological and instrumental evidence of climate change for remote mountain lakes over the last 200 years. J Paleolimnol 28:161–179
Beamish RJ, Harvey HH (1972) Acidification of La Cloche mountain lakes, Ontario, and resulting fish mortalities. J Fish Res Board Can 29:1131–1143
Benskin JP, Phillips V, St Louis VL, Martin JW (2011) Source elucidation of perfluorinated carboxylic acids in remote alpine lake sediment cores. Environ Sci Technol 45:7188–7194
Bergknut M, Laudon H, Jansson S, Larsson A, Gocht T, Wiberg K (2011) Atmospheric deposition, retention, and stream export of dioxins and PCBs in a pristine boreal catchment. Environ Pollut 159:1592–1598
Bettinetti R, Galassi S, Guilizzoni P, Quadroni S (2011) Sediment analysis to support the recent glacial origin of DDT pollution in Lake Iseo (Northern Italy). Chemosphere 85:163–169
Bindler R, Renberg I, Klaminder J (2008) Bridging the gap between ancient metal pollution and contemporary biogeochemistry. J Paleolimnol 40:755–770
Bindler R, Segerstrom U, Pettersson-Jensen IM, Berg A, Hansson S, Holmstrom H, Olsson K, Renberg I (2011) Early medieval origins of iron mining and settlement in central Sweden: multiproxy analysis of sediment and peat records from the Norberg mining district. J Archaeol Sci 38:291–300
Blaauw M, Heegaard E (2012) Estimation of age-depth relationships. In: Birks HJB, Lotter AF, Juggins S, Smol JP (eds) Tracking environmental changes using lake sediments: data handling and numerical techniques. Developments in paleoenvironmental research, vol 5. Springer, Dordrecht, pp 379–414.I
Blais JM, Schindler DW, Muir DCG, Kimpe LE, Donald DB, Rosenberg B (1998) Accumulation of persistent organochlorine compounds in mountains of western Canada. Nature 395:585–588
Blais JM, Schindler DW, Muir DCG, Sharp M, Donald D, Lafreniere M, Braekevelt E, Strachan WMJ (2001a) Melting glaciers: a major source of persistent organochlorines to subalpine Bow Lake in Banff National Park, Canada. Ambio 30:410–415
Blais JM, Schindler DW, Sharp M, Braekevelt E, Lafreniere M, McDonald K, Muir DCG, Strachan WMJ (2001b) Fluxes of semivolatile organochlorine compounds in Bow Lake, a high-altitude, glacier-fed, subalpine lake in the Canadian Rocky Mountains. Limnol Oceanogr 46:2019–2031
Blais JM, Wilhelm F, Kidd KA, Muir DCG, Donald DB, Schindler DW (2003) Concentrations of organochlorine pesticides and polychlorinated biphenyls in amphipods (Gammarus lacustris) along an elevation gradient in mountain lakes of western Canada. Environ Toxicol Chem 22:2605–2613
Blais JM, Charpentié S, Pick F, Kimpe LE, Amand AS, Regnault-Roger C (2006) Mercury, polybrominated diphenyl ether, organochlorine pesticide, and polychlorinated biphenyl concentrations in fish from lakes along an elevation transect in the French Pyrenees. Ecotoxicol Environ Saf 63:91–99
Boes X, Rydberg J, Martinez-Cortizas A, Bindler R, Renberg I (2011) Evaluation of conservative lithogenic elements (Ti, Zr, Al, and Rb) to study anthropogenic element enrichments in lake sediments. J Paleolimnol 46:75–87
Bogdal C, Nikolic D, Luthi MP, Schenker U, Scheringer M, Hungerbuhler K (2010a) Release of legacy pollutants from melting glaciers: model evidence and conceptual understanding. Environ Sci Technol 44:4063–4069
Bogdal C, Scheringer M, Schmid P, Blauenstein M, Kohler M, Hungerbuhler K (2010b) Levels, fluxes and time trends of persistent organic pollutants in Lake Thun, Switzerland: combining trace analysis and multimedia modeling. Sci Total Environ 408:3654–3663
Bogdal C, Bucheli TD, Agarwal T, Anselmetti FS, Blum F, Hungerbuehler K, Kohler M, Schmid P, Scheringer M, Sobek A (2011) Contrasting temporal trends and relationships of total organic carbon, black carbon, and polycyclic aromatic hydrocarbons in rural low-altitude and remote high-altitude lakes. J Environ Monitor 13:1316–1326
Borcard D, Legendre P, Drapeau P (1992) Partialing out the spatial component of ecological variation. Ecology 73:1045–1055
Borg H, Andersson P, Johansson K (1989) Influence of acidification on metal fluxes in Swedish forest lakes. Sci Total Environ 87–8:241–253
Boyle JF, Birks HJB (1999) Predicting heavy metal concentrations in the surface sediments of Norwegian headwater lakes from atmospheric deposition: an application of a simple sediment-water partitioning model. Water Air Soil Pollut 114:27–51
Brännvall ML, Bindler R, Renberg I, Emteryd O, Bartnicki J, Billström K (1999) The medieval metal industry was the cradle of modern large scale atmospheric lead pollution in northern Europe. Environ Sci Technol 33:4391–4395
Breivik K, Wania F, Muir DCG, Alaee M, Backus S, Pacepavicius G (2006) Empirical and modeling evidence of the long-range atmospheric transport of decabromodiphenyl ether. Environ Sci Technol 40:4612–4618
Brooks S, Udachin V, Williamson B (2005) Impact of copper smelting on lakes in the southern Ural Mountains, Russia, inferred from chironomids. J Paleolimnol 33:229–241
Buchaca T, Catalan J (2007) Factors influencing the variability of pigments in the surface sediments of mountain lakes. Freshw Biol 52:1365–1379
Cabrerizo A, Dachs J, Jones KC, Barcelo D (2011) Soil-air exchange controls on background atmospheric concentrations of organochlorine pesticides. Atmos Chem Phys 11:12799–12811
Camarero L (2003) Spreading of trace metals and metalloids pollution in lake sediments over the Pyrenees. J Phys IV 107:249–253
Camarero L, Catalan J (1996) Variability in the chemistry of precipitation in the Pyrenees (northeastern Spain): dominance of storm origin and lack of altitude influence. J Geophys Res-Atmos 101:29491–29498
Camarero L, Catalan J (2012) Atmospheric phosphorus deposition may cause lakes to revert from phosphorus limitation back to nitrogen limitation. Nat Commun 3:1118–1118
Camarero L, Catalan J, Pla S, Rieradevall M, Jimenez M, Prat N, Rodriguez A, Encina L, CruzPizarro L, Castillo PS, Carrillo P, Toro M, Grimalt J, Berdie L, Fernandez P, Vilanova R (1995) Remote mountain lakes as indicators of diffuse acidic and organic pollution in the Iberian peninsula (AL:PE 2 studies). Water Air Soil Pollut 85:487–492
Camarero L, Masque P, Devos W, Ani-Ragolta I, Catalan J, Moor HC, Pla S, Sanchez-Cabeza JA (1998) Historical variations in lead fluxes in the Pyrenees (Northeast Spain) from a dated lake sediment core. Water Air Soil Pollut 105:439–449
Camarero L, Botev I, Muri G, Psenner R, Rose N, Stuchlik E (2009) Trace elements in alpine and arctic lake sediments as a record of diffuse atmospheric contamination across Europe. Freshw Biol 54:2518–2532
Carrera G, Fernandez P, Vilanova RM, Grimalt JO (2001) Persistent organic pollutants in snow from European high mountain areas. Atmos Environ 35:245–254
Carrera G, Fernandez P, Grimalt JO, Ventura M, Camarero L, Catalan J, Nickus U, Thies H, Psenner R (2002) Atmospheric deposition of organochlorine compounds to remote high mountain lakes of Europe. Environ Sci Technol 36:2581–2588
Catalan J (1989) The winter cover of a high-mountain Mediterranean lake (Estany Redó, Pyrenees). Water Resour Res 25:519–527
Catalan J (1992) Evolution of dissolved and particulate matter during the ice-covered period in a deep. high-mountain lake. Can J Fish Aquat Sci 49:945–955
Catalan J, Ventura M, Brancelj A, Granados I, Thies H, Nickus U, Korhola A, Lotter AF, Barbieri A, Stuchlik E, Lien L, Bitusik P, Buchaca T, Camarero L, Goudsmit GH, Kopacek J, Lemcke G, Livingstone DM, Muller B, Rautio M, Sisko M, Sorvari S, Sporka F, Strunecky O, Toro M (2002) Seasonal ecosystem variability in remote mountain lakes: implications for detecting climatic signals in sediment records. J Paleolimnol 28:25–46
Catalan J, Ventura M, Vives I, Grimalt JO (2004) The roles of food and water in the bioaccumulation of organochlorine compounds in high mountain lake fish. Environ Sci Technol 38:4269–4275
Catalan J, Pla-Rabés S, Wolfe AP, Smol JP, Rühland KM, Anderson NJ, Kopáček J, Stuchlík E, Schmidt R, Koinig KA, Camarero L, Flower RJ, Heiri O, Kamenik C, Korhola A, Leavitt PR, Psenner R, Renberg I (2013) Global change revealed by palaeolimnological records from remote lakes: a review. J Paleolimnol 49:513–535
Chen D, Liu W, Liu X, Westgate JN, Wania F (2008) Cold-trapping of persistent organic pollutants in the mountain soils of Western Sichuan, China. Environ Sci Technol 42:9086–9091
Clara M, Gans O, Weiss S, Sanz-Escribano D, Scharf S, Scheffknecht C (2009) Perfluorinated alkylated substances in the aquatic environment: an Austrian case study. Water Res 43:4760–4768
Cooke CA, Balcom PH, Biester H, Wolfe AP (2009) Over three millennia of mercury pollution in the Peruvian Andes. Proc Natl Acad Sci U S A 106:8830–8834
Cooke CA, Balcom PH, Kerfoot C, Abbott MB, Wolfe AP (2011) Pre-colombian mercury pollution associated with the smelting of argentiferous ores in the Bolivian Andes. Ambio 40:18–25
Daly GL, Wania F (2005) Organic contaminants in mountains. Environ Sci Technol 39:385–398
Daly GL, Lei YD, Castillo LE, Muir DCG, Wania F (2007a) Polycyclic aromatic hydrocarbons in Costa Rican air and soil: a tropical/temperate comparison. Atmos Environ 41:7339–7350
Daly GL, Lei YD, Teixeira C, Muir DCG, Castillo LE, Wania F (2007b) Accumulation of current-use pesticides in neotropical montane forests. Environ Sci Technol 41:1118–1123
Daly GL, Lei YD, Teixeira C, Muir DCG, Wania F (2007c) Pesticides in western Canadian mountain air and soil. Environ Sci Technol 41:6020–6025
Dalla Valle M Jurado E Dachs J Sweetman AJ Jones KC (2005) The maximum reservoir capacity of soils for persistent organic pollutants: implications for global cycling. Environ Pollut 134:153–164
Datta S, McConnell LL, Baker JE, Lenoir J, Seiber JN (1998) Evidence for atmospheric transport and deposition of polychlorinated biphenyls to the Lake Tahoe basin, California—Nevada. Environ Sci Technol 32:1378–1385
Davidson DA, Wilkinson AC, Blais JM, Kimpe LE, McDonald KM, Schindler DW (2003) Orographic cold-trapping of persistent organic pollutants by vegetation in mountains of western Canada. Environ Sci Technol 37:209–215
Davidson C, Stanley K, Simonich SM (2012) Contaminant residues and declines of the Cascades frog (Rana cascadae) in the California Cascades, USA. Environ Toxicol Chem 31:1895–1902
Davis RB, Anderson DS, Dixit SS, Appleby PG, Schauffler M (2006) Responses of two New Hampshire (USA) lakes to human impacts in recent centuries. J Paleolimnol 35:669–697
Demers MJ, Kelly EN, Blais JM, Pick FR, St. Louis VL, Schindler DW (2007) Organochlorine compounds in trout from lakes over a 1600 m elevation gradient in the Canadian Rocky Mountains. Environ Sci Technol 41:2723–2729
Draxler RR, Hess GD (1998) An overview of the HYSPLIT_4 modeling system of trajectories, dispersion, and deposition. Aust Meteorol Mag 47:295–308
Engstrom DR, Fitzgerald WF, Cooke CA, Lamborg CH, Drevnick PE, Swain EB, Balogh SJ, Balcom PH (2014) Atmospheric Hg emissions from preindustrial gold and silver extraction in the americas: a reevaluation from lake-sediment archives. Environ Sci Technol doi:10.1021/es405558e
Estellano VH, Pozo K, Harner T, Franken M, Zaballa M (2008) Altitudinal and seasonal variations of persistent organic pollutants in the Bolivian Andes Mountains. Environ Sci Technol 42:2528–2534
Fan C-W, Yang T-N, Kao S-J (2010) Characteristics of sedimentary polycyclic aromatic hydrocarbons (PAHs) in the subtropical Feitsui Reservoir, Taiwan. J Hydrol 391:217–222
Felip M, Camarero L, Catalan J (1999) Temporal changes of microbial assemblages in the ice and snow cover of a high mountain lake. Limnol Oceanogr 44:973–987
Felipe-Sotelo M, Tauler R, Vives I, Grimalt JO (2008) Assessment of the environmental and physiological processes determining the accumulation of organochlorine compounds in European mountain lake fish through multivariate analysis (PCA and PLS). Sci Total Environ 404:148–161
Fernandez P, Vilanova RM, Grimalt JO (1999) Sediment fluxes of polycyclic aromatic hydrocarbons in European high altitude mountain lakes. Environ Sci Technol 33:3716–3722
Fernandez P, Vilanova RM, Martinez C, Appleby P, Grimalt JO (2000) The historical record of atmospheric pyrolytic pollution over Europe registered in the sedimentary PAH from remote mountain lakes. Environ Sci Technol 34:1906–1913
Fernandez P, Grimalt JO, Vilanova RM (2002) Atmospheric gas-particle partitioning of polycyclic aromatic hydrocarbons in high mountain regions of Europe. Environ Sci Technol 36:1162–1168
Fernandez P, Carrera G, Grimalt JO, Ventura M, Camarero L, Catalan J, Nickus U, Thies H, Psenner R (2003) Factors governing the atmospheric deposition of polycyclic aromatic hydrocarbons to remote areas. Environ Sci Technol 37:3261–3267
Fernandez P, Carrera G, Grimalt JO (2005) Persistent organic pollutants in remote freshwater ecosystems. Aquat Sci 67:263–273
Finizio A, Villa S, Raffaele F, Vighi M (2006) Variation of POP concentrations in fresh-fallen snow and air on an Alpine glacier (Monte Rosa). Ecotoxicol Environ Saf 63:25–32
Finlayson B, Fujimura R, Huang ZZ (2000) Toxicity of metal-contaminated sediments from Keswick Reservoir, California, USA. Environ Toxicol Chem 19:485–494
Flower RJ, Politov SV, Rippey B, Rose NL, Appleby PG, Stevenson AC (1997) Sedimentary records of the extent and impact of atmospheric contamination from a remote Siberian highland lake. Holocene 7:161–173
Franz TP, Eisenreich SJ (1998) Snow scavenging of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in Minnesota. Environ Sci Technol 32:1771–1778
Fu GM, Allen HE (1992) Cadmium adsorption by oxic sediment. Water Res 26:225–233
Gallego E, Grimalt JO, Bartrons M, Lopez JF, Camarero L, Catalan J, Stuchlik E, Battarbee R (2007) Altitudinal gradients of PBDEs and PCBs in fish from European high mountain lakes. Environ Sci Technol 41:2196–2202
Garcia-Reyero N, Pina B, Grimalt JO, Fernandez P, Fonts R, Polvillo O, Martrat B (2005) Estrogenic activity in sediments from European mountain lakes. Environ Sci Technol 39:1427–1435
Garcia-Reyero N, Grimalt JO, Vives I, Fernandez P, Pina B (2007) Estrogenic activity associated with organochlorine compounds in fish extracts from European mountain lakes. Environ Pollut 145:745–752
Grimalt JO, van Drooge BL (2006) Polychlorinated biphenyls in mountain pine (Pinus uncinata) needles from Central Pyrenean high mountains (Catalonia, Spain). Ecotoxicol Environ Saf 63:61–67
Grimalt JO, Fernandez P, Berdie L, Vilanova RM, Catalan J, Psenner R, Hofer R, Appleby PG, Rosseland BO, Lien L, Massabuau JC, Battarbee RW (2001) Selective trapping of organochlorine compounds in mountain lakes of temperate areas. Environ Sci Technol 35:2690–2697
Grimalt JO, Borghini F, Sanchez-Hernandez JC, Barra R, Garcia CJT, Focardi S (2004a) Temperature dependence of the distribution of organochlorine compounds in the mosses of the Andean mountains. Environ Sci Technol 38:5386–5392
Grimalt JO, van Drooge BL, Ribes A, Fernandez P, Appleby P (2004b) Polycyclic aromatic hydrocarbon composition in soils and sediments of high altitude lakes. Environ Pollut 131:13–24
Grimalt JO, van Drooge BL, Ribes A, Vilanova RM, Fernandez P, Appleby P (2004c) Persistent organochlorine compounds in soils and sediments of European high altitude mountain lakes. Chemosphere 54:1549–1561
Grimalt JO, Fernandez P, Quiroz R (2009) Input of organochlorine compounds by snow to European high mountain lakes. Freshw Biol 54:2533–2542
Guazzoni N, Comolli R, Mariani L, Cola G, Parolini M, Binelli A, Tremolada P (2011) Meteorological and pedological influence on the PCBs distribution in mountain soils. Chemosphere 83:186–192
Guevara SR, Meili M, Rizzo A, Daga R, Arribere M (2010) Sediment records of highly variable mercury inputs to mountain lakes in Patagonia during the past millennium. Atmos Chem Phys 10:3443–3453
Guo J, Wu F, Luo X, Liang Z, Liao H, Zhang R, Li W, Zhao X, Chen S, Mai B (2010) Anthropogenic input of polycyclic aromatic hydrocarbons into five lakes in western China. Environ Pollut 158:2175–2180
Gustafsson Ö, Bucheli TD, Kukulska Z, Andersson M, Largeau C, Rouzaud JN, Reddy CM, Eglinton TI (2001) Evaluation of a protocol for the quantification of black carbon in sediments. Glob Biogeochem Cycle 15:881–890
Guzzella L, Poma G, De Paolis A, Roscioli C, Viviano G (2011) Organic persistent toxic substances in soils, waters and sediments along an altitudinal gradient at Mt. Sagarmatha, Himalayas, Nepal. Environ Pollut 159:2552–2564
Han Y, Jin Z, Cao J, Posmentier ES, An Z (2007) Atmospheric Cu and Pb deposition and transport in lake sediments in a remote mountain area, Northern China. Water Air Soil Pollut 179:167–181
Han YM, Cao JJ, Kenna TC, Yan BZ, Jin ZD, Wu F, An ZS (2011) Distribution and ecotoxicological significance of trace element contamination in a similar to 150 year record of sediments in Lake Chaohu, Eastern China. J Environ Monito 13:743–752.
Hansen KM, Halsall CJ, Christensen JH (2006) A dynamic model to study the exchange of gas-phase persistent organic pollutants between air and a seasonal snowpack. Environ Sci Technol 40:2644–2652
Haritash AK, Kaushik CP (2009) Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Holoubek I, Dusek L, Sanka M, Hofman J, Cupr P, Jarkovsky J, Zbiral J, Klanova J (2009) Soil burdens of persistent organic pollutants—their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environ Pollut 157:3207–3217
Houde M, De Silva AO, Muir DCG, Letcher RJ (2011) Monitoring of perfluorinated compounds in aquatic biota: an updated review pfcs in aquatic biota. Environ Sci Technol 45:7962–7973
Il’yashuk BP, Il’yashuk EA (2000) Paleoecological analysis of chironomid assemblages of a mountain lake as a source of information for biomonitoring. Russ J Ecol 31:353–358
Ilyashuk BP, Ilyashuk EA, Psenner R, Tessadri R, Koinig KA (2014) Rock glacier outflows may adversely affect lakes: lessons from the past and present of two neighboring water bodies in a crystalline-rock watershed. Environ Sci Technol. doi:10.1021/es500180c
Jarque S, Gallego E, Bartrons M, Catalan J, Grimalt JO, Pina B (2010) Altitudinal and thermal gradients of hepatic Cyp1A gene expression in natural populations of Salmo trutta from high mountain lakes and their correlation with organohalogen loads. Environ Pollut 158:1392–1398
Jaward FM, Di Guardo A, Nizzetto L, Cassani C, Raffaele F, Ferretti R, Jones KC (2005) PCBs and selected organochlorine compounds in Italian mountain air: the influence of altitude and forest ecosystem type. Environ Sci Technol 39:3455–3463
Jiao L, Zheng GJ, Minh TB, Richardson B, Chen L, Zhang Y, Yeung LW, Lam JCW, Yang X, Lam PKS, Wong MH (2009) Persistent toxic substances in remote lake and coastal sediments from Svalbard, Norwegian Arctic: levels, sources and fluxes. Environ Pollut 157:1342–1351
Jones RT, Cook CG, Zhang EL, Langdon PG, Jordan J, Turney C (2012) Holocene environmental change at Lake Shudu, Yunnan Province, southwestern China. Hydrobiologia 693:223–235
Kalberer M, Henne S, Prevot ASH, Steinbacher M (2004) Vertical transport and degradation of polycyclic aromatic hydrocarbons in an Alpine Valley. Atmos Environ 38:6447–6456
Kang J-H, Choi S-D, Park H, Baek S-Y, Hong S, Chang Y-S (2009) Atmospheric deposition of persistent organic pollutants to the East Rongbuk Glacier in the Himalayas. Sci Total Environ 408:57–63
Keller W (2009) Limnology in northeastern Ontario: from acidification to multiple stressors. Can J Fish Aquat Sci 66:1189–1198
Killin RK, Simonich SL, Jaffe DA, DeForest CL, Wilson GR (2004) Transpacific and regional atmospheric transport of anthropogenic semivolatile organic compounds to Cheeka Peak Observatory during the spring of 2002. J Geophys Res-Atmos 109(D23):S15
Kirchner M, Faus-Kessler T, Jakobi G, Levy W, Henkelmann B, Bernhoeft S, Kotalik J, Zsolnay A, Bassan R, Belis C, Kraeuchi N, Moche W, Simoncic P, Uhl M, Weiss P, Schramm KW (2009) Vertical distribution of organochlorine pesticides in humus along Alpine altitudinal profiles in relation to ambiental parameters. Environ Pollut 157:3238–3247
Koinig KA, Shotyk W, Lotter AF, Ohlendorf C, Sturm M (2003) 9000 years of geochemical evolution of lithogenic major and trace elements in the sediment of an alpine lake—the role of climate, vegetation, and land-use history. J Paleolimnol 30:307–320
Kopáček J, Borovec J, Hejzlar J, Kotorova I, Stuchlik E, Vesely J (2006) Chemical composition of modern and pre-acidification sediments in the Tatra Mountain lakes. Biologia 61:61S65–S76
Körner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574
Krausmann F, Gingrich S, Eisenmenger N, Erb KH, Haberl H, Fischer-Kowalski M (2009) Growth in global materials use, GDP and population during the 20th century. Ecol Econ 68:2696–2705
Lafrenière MJ, Blais JM, Sharp MJ, Schindler DW (2006) Organochlorine pesticide and polychlorinated biphenyl concentrations in snow, snowmelt, and runoff at Bow Lake, Alberta. Environ Sci Technol 40:4909–4915
Landers DH, Simonich S, Jaffe D, Geiser L, Campbell DH, Schwindt A, Schreck C, Kent M, Hafner W, Taylor HE, Hageman K, Usenko S, Ackerman L, Schrlau J, Rose N, Blett T, Erway MM (2008) The fate, transport, and ecological impacts of airbone contaminants in Western National Parks (USA). Western Airborne Contaminants Assessment project final report, vol 1. Corvallis, Oregon
Landers DH, Simonich SM, Jaffe D, Geiser L, Campbell DH, Schwindt A, Schreck C, Kent M, Hafner W, Taylor HE, Hageman K, Usenko S, Ackerman L, Schrlau J, Rose N, Blett T, Erway MM (2010) The Western Airborne Contaminant Assessment Project (WACAP): an interdisciplinary evaluation of the impacts of airborne contaminants in western U.S. National Parks. Environ Sci Technol 44:855–859
Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA (2006) Levels and trends of brominated flame retardants in the European environment. Chemosphere 64:187–208
Lee CSL, Qi SH, Zhang G, Luo CL, Zhao LYL, Li XD (2008) Seven thousand years of records on the mining and utilization of metals from lake sediments in central China. Environ Sci Technol 42:4732–4738
Legendre P, Dale MRT, Fortin MJ, Gurevitch J, Hohn M, Myers D (2002) The consequences of spatial structure for the design and analysis of ecological field surveys. Ecography 25:601–615
Li J, Zhu T, Wang F, Qiu XH, Lin WL (2006) Observation of organochlorine pesticides in the air of the Mt. Everest region. Ecotoxicol Environ Saf 63:33–41
Lindell L, Astroem ME, Sarenbo S (2010) Effects of forest slash and burn on the distribution of trace elements in floodplain sediments and mountain soils of the Subandean Amazon, Peru. Appl Geochem 25:1097–1106
Liu X, Zhang G, Jones KC, Li XD, Peng XZ, Qi SH (2005) Compositional fractionation of polycyclic aromatic hydrocarbons (PAHs) in mosses (Hypnum plumaeformae WILS.) from the northern slope of Nanling Mountains, South China. Atmos Environ 39:5490–5499
Liu W, Chen D, Liu X, Zheng X, Yang W, Westgate JN, Wania F (2010) Transport of semivolatile organic compounds to the Tibetan plateau: spatial and temporal variation in air concentrations in mountainous Western Sichuan, China. Environ Sci Technol 44:1559–1565
Loewen M, Wania F, Wang F, Tomy G (2008) Altitudinal transect of atmospheric and aqueous fluorinated organic compounds in Western Canada. Environ Sci Technol 42:2374–2379
Luoma SN, Rainbow PS (2008) Metal contamination in aquatic environments. Science and lateral management. Cambridge University Press, Cambridge
MacDonald DD, Ingersoll CG, Berger TA (2000a) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
MacDonald RW, Barrie LA, Bidleman TF, Diamond ML, Gregor DJ, Semkin RG, Strachan WMJ, Li YF, Wania F, Alaee M, Alexeeva LB, Backus SM, Bailey R, Bewers JM, Gobeil C, Halsall CJ, Harner T, Hoff JT, Jantunen LMM, Lockhart WL, Mackay D, Muir DCG, Pudykiewicz J, Reimer KJ, Smith JN, Stern GA, Schroeder WH, Wagemann R, Yunker MB (2000b) Contaminants in the Canadian Arctic: 5 years of progress in understanding sources, occurrence and pathways. Sci Total Environ 254:93–234
Marquis O, Miaud C, Ficetola GF, Bocher A, Mouchet F, Guittonneau S, Devaux A (2009) Variation in genotoxic stress tolerance among frog populations exposed to UV and pollutant gradients. Aquat Toxicol 95:152–161
Mast MA, Manthorne DJ, Roth DA (2010) Historical deposition of mercury and selected trace elements to high-elevation National Parks in the Western US inferred from lake-sediment cores. Atmos Environ 44:2577–2586
Meijer SN, Ockenden WA, Sweetman A, Breivik K, Grimalt JO, Jones KC (2003) Global distribution and budget of PCBs and HCB in background surface soils: implications or sources and environmental processes. Environ Sci Technol 37:667–672
Meijer SN, Dachs J, Fernandez P, Camarero L, Catalan J, Del Vento S, van Drooge B, Jurado E, Grimalt JO (2006) Modelling the dynamic air-water-sediment coupled fluxes and occurrence of polychlorinated biphenyls in a high altitude lake. Environ Pollut 140:546–560
Meire RO, Lee SC, Targino AC, Torres JPM, Harner T (2012a) Air concentrations and transport of persistent organic pollutants (POPs) in mountains of southeast and southern Brazil. Atmos Pollut Res 3:417–425
Meire RO, Lee SC, Yao Y, Targino AC, Torres JPM, Harner T (2012b) Seasonal and altitudinal variations of legacy and current-use pesticides in the Brazilian tropical and subtropical mountains. Atmos Environ 59:108–116
Miró A, Ventura M (2013) Historical use, fishing management and lake characteristics explain the presence of non-native trout in Pyrenean lakes: implications for conservation Biol Conserv 167:17–24
Moser KA, Mordecai JS, Reynolds RL, Rosenbaum JG, Ketterer ME (2010) Diatom changes in two Uinta mountain lakes, Utah, USA: responses to anthropogenic and natural atmospheric inputs. Hydrobiologia 648:91–108
Muir DCG, Wang X, Yang F, Nguyen N, Jackson TA, Evans MS, Douglas M, Kock G, Lamoureux S, Pienitz R, Smol JP, Vincent WF, Dastoor A (2009) Spatial trends and historical deposition of mercury in Eastern and Northern Canada inferred from lake sediment cores. Environ Sci Technol 43:4802–4809
Muri G, Wakeham SG, Faganeli J (2003) Polycyclic aromatic hydrocarbons and black carbon in sediments of a remote alpine lake (Lake Planina, northwest Slovenia). Environ Toxicol Chem 22:1009–1016
Muri G, Wakeham SG, Rose NL (2006) Records of atmospheric delivery of pyrolysis-derived pollutants in recent mountain lake sediments of the Julian Alps (NW Slovenia). Environ Pollut 139:461–468
Navas A, Lindhorfer H (2005) Chemical partitioning of Fe, Mn, Zn and Cr in mountain soils of the Iberian and Pyrenean ranges (NE Spain). Soil Sediment Contam 14:249–259
Nizzetto L, Cassani C, Di Guardo A (2006) Deposition of PCBs in mountains: the forest filter effect of different forest ecosystem types. Ecotoxicol Environ Saf 63:75–83
Nizzetto L, Pastore C, Liu X, Camporini P, Stroppiana D, Herbert B, Boschetti M, Zhang G, Brivio PA, Jones KC, Di Guardo A (2008) Accumulation parameters and seasonal trends for PCBs in temperate and boreal forest plant species. Environ Sci Technol 42:5911–5916
Nizzetto L, Macleod M, Borga K, Cabrerizo A, Dachs J, Di Guardo A, Ghirardello D, Hansen KM, Jarvis A, Lindroth A, Ludwig B, Monteith D, Perlinger JA, Scheringer M, Schwendenmann L, Semple KT, Wick LY, Zhang G, Jones KC (2010) Past, present, and future controls on levels of persistent organic pollutants in the global environment. Environ Sci Technol 44:6526–6531
Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Int 35:971–986
Outridge PM, Rausch N, Percival JB, Shotyk W, McNeely R (2011) Comparison of mercury and zinc profiles in peat and lake sediment archives with historical changes in emissions from the Flin Flon metal smelter, Manitoba, Canada. Sci Total Environ 409:548–563
Pacyna JM, Pacyna EG, Aas W (2009) Changes of emissions and atmospheric deposition of mercury, lead, and cadmium. Atmos Environ 43:117–127
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 87:2614–2625.
Pozo K, Harner T, Shoeib M, Urrutia R, Barra R, Parra O, Focardi S (2004) Passive-sampler derived air concentrations of persistent organic pollutants on a north-south transect in Chile. Environ Sci Technol 38:6529–6537
Pozo K, Urrutia R, Barra R, Mariottini M, Treutler H-C, Araneda A, Focardi S (2007) Records of polychlorinated biphenyls (PCBs) in sediments of four remote Chilean Andean Lakes. Chemosphere 66:1911–1921
Pozo K, Harner T, Lee SC, Wania F, Muir DCG, Jones KC (2009) Seasonally resolved concentrations of persistent organic pollutants in the global atmosphere from the first year of the GAPS study. Environ Sci Technol 43:796–803
Psenner R, Catalan J (1994) Chemical composition of lakes in crystalline basins: a combination of atmospheric deposition geologic backgrounds, biological activity and human action. In: Margalef R (ed) Limnology now. A paradigm of planetary problems. Elsevier, Amsterdam, pp 255–314.I
Quiros L, Jarque S, Lackner R, Fernandez P, O. Grimalt J, Pina B (2007) Physiological response to persistent organic pollutants in fish from mountain lakes: analysis of CYP1A gene expression in natural populations of Salmo trutta. Environ Sci Technol 41:5154–5160
Quiroz R, Grimalt JO, Fernandez P (2010) Toxicity assessment of polycyclic aromatic hydrocarbons in sediments from European high mountain lakes. Ecotoxicol Environ Saf 73:559–564
Quiroz R, Grimalt JO, Fernandez P, Camarero L, Catalan J, Stuchlik E, Thies H, Nickus U (2011) Polycyclic aromatic hydrocarbons in soils from european high mountain areas. Water Air Soil Pollut 215:655–666
Renberg I, Bindler R, Brannvall ML (2001) Using the historical atmospheric lead-deposition record as a chronological marker in sediment deposits in Europe. Holocene 11:511–516
Reynolds RL, Mordecai JS, Rosenbaum JG, Ketterer ME, Walsh MK, Moser KA (2010) Compositional changes in sediments of subalpine lakes, Uinta Mountains (Utah): evidence for the effects of human activity on atmospheric dust inputs. J Paleolimnol 44:161–175
Rippey B, Anderson NJ, Renberg I, Korsman T (2008) The accuracy of methods used to estimate the whole-lake accumulation rate of organic carbon, major cations, phosphorus and heavy metals in sediment. J Paleolimnol 39:83–99
Rockstrom J, Steffen W, Noone K, Persson A, Chapin FS, III, Lambin E, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sorlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley J (2009a) Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc 14
Rockstrom J, Steffen W, Noone K, Persson A, Chapin FS III, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sorlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley JA (2009b) A safe operating space for humanity. Nature 461:472–475
Rose NL, Rippey B (2002) The historical record of PAH, PCB, trace metal and fly-ash particle deposition at a remote lake in north-west Scotland. Environ Pollut 117:121–132
Rose NL, Harlock S, Appleby PG (1999) The spatial and temporal distributions of spheroidal carbonaceous fly-ash particles (SCP) in the sediment records of European mountain lakes. Water Air Soil Pollut 113:1–32
Rose NL, Backus S, Karlsson H, Muir DCG (2001) An historical record of toxaphene and its congeners in a remote lake in western Europe. Environ Sci Technol 35:1312–131
Rose NL, Rose CL, Boyle JF, Appleby PG (2004) Lake-sediment evidence for local and remote sources of atmospherically deposited pollutants on Svalbard. J Paleolimnol 31:499–513
Rose NL, Cogalniceanu D, Appleby PG, Brancelj A, Camarero L, Fernandez P, Grimalt JO, Kernan M, Lami A, Musazzi S, Quiroz R, Velle G (2009) Atmospheric contamination and ecological changes inferred from the sediment record of Lacul Negru in the Retezat National Park, Romania. In: Catalan J, Curtis CJ, Kernan M (eds) Patterns and factors of biota distribution in remote European Mountain Lakes, vol 62. Advances in Limnology. pp 319–350.I
Sanchez-Andrea I, Rodriguez N, Amils R, Sanz JL (2011) Microbial Diversity in Anaerobic Sediments at Rio Tinto, a Naturally Acidic Environment with a High Heavy Metal Content. Appl Environ Microbiol 77:6085–6093
Schmid P, Kohler M, Gujer E, Zennegg M, Lanfranchi M (2007) Persistent organic pollutants, brominated flame retardants and synthetic musks in fish from remote alpine lakes in Switzerland. Chemosphere 67:67S16–S21
Schmid P, Bogdal C, Bluthgen N, Anselmetti FS, Zwyssig A, Hungerbuhler K (2011) The Missing piece: sediment records in remote Mountain lakes confirm glaciers being secondary sources of persistent organic pollutants. Environ Sci Technol 45:203–208
Schmidt R, Roth M, Tessadri R, Weckstroem K (2008) Disentangling late-Holocene climate and land use impacts on an Austrian alpine lake using seasonal temperature anomalies, ice-cover, sedimentology, and pollen tracers. J Paleolimnol 40:453–469
Schroeder WH, Munthe J (1998) Atmospheric mercury—an overview. Atmos Environ 32:809–822
Semlali RM, van Oort F, Denaix L, Loubet M (2001) Estimating distributions of endogenous and exogenous Pb in soils by using Pb isotopic ratios. Environ Sci Technol 35:4180–4188
Sheesley RJ, Andersson A, Gustafsson O (2011) Source characterization of organic aerosols using Monte Carlo source apportionment of PAHs at two South Asian receptor sites. Atmos Environ 45:3874–3881
Shotyk W, Krachler M, Aeschbach-Hertig W, Hillier S, Zheng JC (2010) Trace elements in recent groundwater of an artesian flow system and comparison with snow: enrichments, depletions, and chemical evolution of the water. J Environ Monitor 12:208–217
Shunthirasingham C, Barra R, Mendoza G, Montory M, Oyiliagu CE, Lei YD, Wania F (2011) Spatial variability of atmospheric semivolatile organic compounds in Chile. Atmos Environ 45:303–309
Smol JP (1995) Paleolimnological approaches to the evaluation and monitoring of ecosystem health: providing a history for environmental damage and recovery. Evaluating and monitoring the health of large-scale ecosystems. Springer, pp 301–318.I
Sprovieri F, Pirrone N, Ebinghaus R, Kock H, Dommergue A (2010) A review of worldwide atmospheric mercury measurements. Atmos Chem Phys 10:8245–8265
Srogi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett 5:169–195
St-Amand AD, Mayer PM, Blais JM (2007) Modeling atmospheric vegetation uptake of PBDEs using field measurements. Environ Sci Technol 41:4234–4239
St-Amand AD, Mayer PM, Blais JM (2009) Modeling PAH uptake by vegetation from the air using field measurements. Atmos Environ 43:4283–4288.
Steiner B, Hanselmann KW, Krahenbuhl U (2000) Dating and heavy metal contents of sediment cores of a high-alpine, remote lake: Jorisee (Switzerland). Int J Environ Anal Chem 78:131–148
Streets DG, Zhang Q, Wu Y (2009) Projections of global mercury emissions in 2050. Environ Sci Technol 43:2983–2988
Strosnider WHJ, Lopez FSL, Nairn RW (2011) Acid mine drainage at Cerro Rico de Potosi I: unabated high-strength discharges reflect a five century legacy of mining. Environ Earth Sci 64:899–910
Tato L, Tremolada P, Ballabio C, Guazzoni N, Parolini M, Caccianiga M, Binelli A (2011) Seasonal and spatial variability of polychlorinated biphenyls (PCBs) in vegetation and cow milk from a high altitude pasture in the Italian Alps. Environ Pollut 159:2656–2664
Thies H, Nickus U, Mair V, Tessadri R, Tait D, Thaler B, Psenner R (2007) Unexpected response of high alpine lake waters to climate warming. Environ Sci Technol 41:7424–7429
Thompson R, Ventura M, Camarero L (2009) On the climate and weather of mountain and sub-arctic lakes in Europe and their susceptibility to future climate change. Freshw Biol 54:2433–2451
Tremolada P, Villa S, Bazzarin P, Bizzotto E, Comolli R, Vighi M (2008) POPs in mountain soils from the Alps and Andes: suggestions for a ‘precipitation effect’ on altitudinal gradients. Water Air Soil Pollut 188:93–109
Tremolada P, Parolini M, Binelli A, Ballabio C, Comolli R, Provini A (2009) Seasonal changes and temperature-dependent accumulation of polycyclic aromatic hydrocarbons in high-altitude soils. Sci Total Environ 407:4269–4277
Tremolada P, Comolli R, Parolini M, Moia F, Binelli A (2011) One-year cycle of DDT concentrations in high-altitude soils. Water Air Soil Pollut 217:407–419
Tremolada P, Guazzoni N, Smillovich L, Moia F, Comolli R (2012) The effect of the organic matter composition on pop accumulation in soil. Water Air Soil Pollut 223:4539–4556.
Usenko S, Landers DH, Appleby PG, Simonich SL (2007) Current and historical deposition of PBDEs, pesticides, PCBs, and PAHs to Rocky Mountain National Park. Environ Sci Technol 41:7235–7241
Usenko S, Smonich SLM, Hageman KJ, Schrlau JE, Geiser L, Campbell DH, Appleby PG, Landers DH (2010) Sources and deposition of polycyclic aromatic hydrocarbons to western US National Parks. Environ Sci Technol 44:4512–4518
Van Drooge BL, Grimalt JO, Camarero L, Catalan J, Stuchlik E, Garcia CJT (2004) Atmospheric semivolatile organochlorine compounds in European high-mountain areas (Central Pyrenees and High Tatras). Environ Sci Technol 38:3525–3532
Van Drooge BL, Fernandez P, Grimalt JO, Stuchlik E, Torres Garcia CJ, Cuevas E (2010) Atmospheric polycyclic aromatic hydrocarbons in remote European and Atlantic sites located above the boundary mixing layer. Environ Sci Pollut Res Int 17:1207–1216
Van Drooge BL, Lopez J, Fernandez P, Grimalt JO, Stuchlik E (2011) Polycyclic aromatic hydrocarbons in lake sediments from the High Tatras. Environ Pollut 159:1234–1240
Van Metre PC, Fuller CC (2009) Dual-core mass-balance approach for evaluating mercury and Pb-210 atmospheric fallout and focusing to lakes. Environ Sci Technol 43:26–32
Vilanova RM, Fernandez P, Grimalt JO (2001a) Polychlorinated biphenyl partitioning in the waters of a remote mountain lake. Sci Total Environ 279:51–62
Vilanova RM, Fernandez P, Martinez C, Grimalt JO (2001b) Polycyclic aromatic hydrocarbons in remote mountain lake waters. Water Res 35:3916–3926
Villa S, Vighi M, Maggi V, Finizio A, Bolzacchini E (2003) Historical trends of organochlorine pesticides in an Alpine glacier. J Atmos Chem 46:295–311.
Vives I, Grimalt JO, Catalan J, Rosseland BO, Battarbee RW (2004) Influence of altitude and age in the accumulation of organochlorine compounds in fish from high mountain lakes. Environ Sci Technol 38:690–698
Vives I, Grimalt JO, Ventura M, Catalan J (2005a) Distribution of polycyclic aromatic hydrocarbons in the food web of a high mountain lake, Pyrenees, Catalonia, Spain. Environ Toxicol Chem 24:1344–1352
Vives I, Grimalt JO, Ventura M, Catalan J, Rosseland BO (2005b) Age dependence of the accumulation of organochlorine pollutants in brown trout (Salmo trutta) from a remote high mountain lake (Redo, Pyrenees). Environ Pollut 133:343–350
Wang X-p, Yao T-d, Cong Z-y, Yan X-l, Kang S-c, Zhang Y (2006) Gradient distribution of persistent organic contaminants along northern slope of central-Himalayas, China. Sci Total Environ 372:193–202
Wang P, Zhang Q, Wang Y, Wang T, Li X, Li Y, Ding L, Jiang G (2009) Altitude dependence of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in surface soil from Tibetan Plateau, China. Chemosphere 76:1498–1504
Wang X, Gong P, Zhang Q, Yao T (2010a) Impact of climate fluctuations on deposition of DDT and hexachlorocyclohexane in mountain glaciers: evidence from ice core records. Environ Pollut 158:375–380
Wang X, Yang H, Gong P, Zhao X, Wu G, Turner S, Yao T (2010b) One century sedimentary records of polycyclic aromatic hydrocarbons, mercury and trace elements in the Qinghai Lake, Tibetan Plateau. Environ Pollut 158:3065–3070
Wang W, Simonich S, Giri B, Chang Y, Zhang Y, Jia Y, Tao S, Wang R, Wang B, Li W, Cao J, Lu X (2011) Atmospheric concentrations and air-soil gas exchange of polycyclic aromatic hydrocarbons (PAHs) in remote, rural village and urban areas of Beijing-Tianjin region, North China. Sci Total Environ 409:2942–2950
Wang X-p, Sheng J-j, Gong P, Xue Y-g, Yao T-d, Jones KC (2012) Persistent organic pollutants in the Tibetan surface soil: spatial distribution, air-soil exchange and implications for global cycling. Environ Pollut 170:145–151
Wania F, Westgate JN (2008) On the mechanism of mountain cold-trapping of organic chemicals. Environ Sci Technol 42:9092–9098
Wania F, Haugen JE, Lei YD, Mackay D (1998) Temperature dependence of atmospheric concentrations of semivolatile organic compounds. Environ Sci Technol 32:1013–1021
Wegmann F, Scheringer M, Hungerbuhler K (2006) First investigations of mountainous cold condensation effects with the CliMoChem model. Ecotoxicol Environ Saf 63:42–51
Wilkinson AC, Kimpe LE, Blais JM (2005) Air-water gas exchange of chlorinated pesticides in four lakes spanning a 1205 m elevation range in the Canadian Rocky Mountains. Environ Toxicol Chem 24:61–69
Wu YH, Bin HJ, Zhou J, Luo J, Yu D, Sun SQ, Li W (2011) Atmospheric deposition of Cd accumulated in the montane soil, Gongga Mt., China. J Soils Sediments 11:940–946
Xie J, He Z, Liu X, Liu X, Van Nostrand JD, Deng Y, Wu L, Zhou J, Qiu G (2011) GeoChip-based analysis of the functional gene diversity and metabolic potential of microbial communities in acid mine drainage. Appl Environ Microbiol 77:991–999
Xu Y, Zhang G, Li J, Chakraborty P, Li H, Liu X (2011) Long-range atmospheric transport of persistent organochlorinated compounds from south and mainland south-eastern Asia to a remote mountain site in south-western China. J Environ Monitor 13:3119–3127
Yang HD, Rose NL, Battarbee RW (2002a) Distribution of some trace metals in Lochnagar, a Scottish mountain lake ecosystem and its catchment. Sci Total Environ 285:197–208
Yang HD, Rose NL, Battarbee RW, Boyle JF (2002b) Mercury and lead budgets for Lochnagar, a Scottish mountain lake and its catchment. Environ Sci Technol 36:1383–1388
Yang HD, Rose NL, Battarbee RW, Monteith D (2002c) Trace metal distribution in the sediments of the whole lake basin for Lochnagar, Scotland: a palaeolimnological assessment. Hydrobiologia 479:51–61
Yang HD, Battarbee RW, Turner SD, Rose NL, Derwent RG, Wu GJ, Yang RQ (2010a) Historical Reconstruction of Mercury Pollution Across the Tibetan Plateau Using Lake Sediments. Environ Sci Technol 44:2918–2924
Yang HD, Engstrom DR, Rose NL (2010b) Recent changes in atmospheric mercury deposition recorded in the sediments of remote equatorial lakes in the Rwenzori Mountains, Uganda. Environ Sci Technol 44:6570–6575
Yang R, Wang Y, Li A, Zhang Q, Jing C, Wang T, Wang P, Li Y, Jiang G (2010c) Organochlorine pesticides and PCBs in fish from lakes of the Tibetan Plateau and the implications. Environ Pollut 158:2310–2316
Yuan G-L, Han P, Xie W, Che X-C, Wang G-H (2012) Altitudinal distribution of polybrominated diphenyl ethers (PBDEs) in the soil along Central Tibetan Plateau, China. Sci Total Environ 433:44–49
Zheng X, Liu X, Jiang G, Wang Y, Zhang Q, Cai Y, Cong Z (2012) Distribution of PCBs and PBDEs in soils along the altitudinal gradients of Balang Mountain, the east edge of the Tibetan Plateau. Environ Pollut 161:101–106
Acknowledgments
Writing this chapter has benefited from years of collaboration with Lluís Camarero, Joan Grimalt, Pilar Fernández, Mireia Bartrons, Montserrat Barcardí, Neil Rose and Peter Appleby, among others. I am grateful to have learnt from them. I thank the support of the project CUL-PA (Ref. 998/2013) from the Spanish National Parks research program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Catalan, J. (2015). Tracking Long-Range Atmospheric Transport of Trace Metals, Polycyclic Aromatic Hydrocarbons, and Organohalogen Compounds Using Lake Sediments of Mountain Regions. In: Blais, J., Rosen, M., Smol, J. (eds) Environmental Contaminants. Developments in Paleoenvironmental Research, vol 18. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9541-8_11
Download citation
DOI: https://doi.org/10.1007/978-94-017-9541-8_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9540-1
Online ISBN: 978-94-017-9541-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)