Abstract
Low temperature is a major environmental factor that limits plant growth, productivity, and distribution. To ensure optimal growth and survival, plants must respond and adapt to cold stress using a variety of biochemical and physiological processes. Currently, the most thoroughly understood cold-signalling pathway is the C-repeat binding factor/DRE-binding factor (CBF/DREB) transcriptional regulatory cascade. Abscisic acid (ABA) is an important stress hormone in plants that has been demonstrated to be involved in the cold stress response through regulation of a set of specific stress-responsive genes. The current consensus is that both ABA-dependent and ABA-independent pathways are involved in plant responses to cold stress. This chapter summarises recent progress made in our understanding of cold signalling and the role of ABA in cold stress, and we also address cross talk between ABA and several classical phytohormones that integrate with cold signalling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
As sessile organisms, plants suffer from a variety of abiotic environmental stresses, including low temperature, which can limit plant growth and the geographic distribution of a plant species, potentially impacting the reproduction of economically important crops. To adapt to adverse environments, plants have evolved a number of complex mechanisms to avoid or tolerate cold stress. Cold acclimation is one of the most thoroughly understood mechanisms contributing to increased cold tolerance in plants. During cold acclimation, plants modulate multiple morphological and physiological processes in order to establish a new state of cellular and metabolic homeostasis, which also involves in changes in phytohormone homeostasis, allowing for adaptation to stressful conditions (Hirayama and Shinozaki 2010; Theocharis et al. 2012; Zhu et al. 2007).
Abscisic acid (ABA) is a well-known stress hormone that plays a crucial role in dehydration stress. However, it has also been suggested that ABA plays a role in cold acclimation by triggering specific cellular and molecular osmotic responses. This chapter highlights the latest advances in our understanding of cold-response mechanisms that are either directly or indirectly influenced by ABA signalling. In particular, we focus on the regulatory networks used for signal perception, signal transduction, and the regulation of stress-responsive genes. Furthermore, both antagonistic and synergistic cross talk between ABA and other plant hormones involved in cold signalling will be discussed.
17.2 Cold-Signal Sensing and Transduction Pathways
Low temperatures can severely disrupt the metabolism and physiological homeostasis of plant cells and can even lead to plant death. Cold stress can be divided into non-freezing chilling stress (below 15 °C and above freezing point) and freezing stress (below freezing point). Chilling stress inhibits the activities of enzymes involved in photosynthesis, respiration, and biochemical processes such as reactive oxygen species (ROS) scavenging, leading to oxidative damage that can result in toxic compounds accumulation and the inhibition of metabolic reactions (O’Kane et al. 1996; Yang et al. 2005). Freezing stress results in the formation of intracellular ice crystals that induce cellular dehydration and osmotic stress, leading to membrane damage, and ultimately, to death of tissues (Uemura et al. 1995; Webb and Steponkus 1993). Most temperate plants are able to tolerate freezing stress following prior exposure to chilling, non-freezing temperatures, which is referred to as cold acclimation (Thomashow 1999). During cold acclimation, plants initiate global transcriptome changing and become tolerant to freezing temperatures by increasing the accumulation of osmolytes (such as soluble sugars) and antifreezing proteins, as well as by altering membrane composition, which together protect plant cells from dehydration and metabolic disruption (Yamada et al. 2002).
It has been noted that a reduction in the fluidity of the plasma membrane appears to be a primary event for the cold signalling sensing (Yamada et al. 2002). For example, in Synechocystis, histidine kinases (Hiks) are used to percept decreased levels of unsaturated fatty acids in the plasma membrane and activate the cold-induced des genes, which are responsible for the feedback maintenance of membrane lipid composition to modulate cold tolerance (Suzuki et al. 2000). Lipid composition of membranes, especially the portion of galactolipids containing unsaturated fatty acids, is crucial for high plant species during cold acclimation. In Arabidopsis, the expression of FAD2, which encodes an enzyme that is essential for polyunsaturated lipid synthesis. fad2 mutants show irregular membrane composition and cannot survive at low temperature (Miquel et al. 1993). Similar chilling-sensitive phenotypes are also observed in loss-of-function fad5 (Hugly and Somerville 1992). A recent study showed that a lipid desaturase, acyl-lipid desaturase2 (ADS2), is required for chilling and freezing tolerance in Arabidopsis and functions by altering membrane lipid composition (Chen and Thelen 2013). Furthermore, the Arabidopsis sensitive to freezing2 (SFR2) protein was identified as a galactolipid-remodelling enzyme that is localised to the outer chloroplast membrane, and it is essential for membrane lipid remodelling of chloroplast envelope under freezing stress (Moellering et al. 2010; Fourrier et al. 2008).
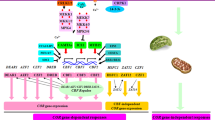
Under cold stress, membrane rigidity triggers second-messenger molecules such as Ca2+ and to activate complex signaling pathways involved in protein kinases or transcription-factor cascades (Viswanathan and Zhu 2002). However, the underlying mechanisms that plants use to perceive and transduce cold signals remain elusive. Currently, the most thoroughly understood cold-signalling pathway is the ICE–CBF–COR transcriptional cascade, which plays a crucial role in the activation of multiple downstream cold-regulated (COR) genes (Thomashow 2010) (Fig. 17.1). Acting as central nodes in the cold acclimation pathway, the C-repeat binding factors (CBFs) gene family is conserved in many plant species. In Arabidopsis, three CBF transcription factors belonging to the AP2/ERF (apetala 2/ethylene-responsive factor) superfamily have been identified. CBFs are also known as dehydration-responsive element binding factors (DREBs), which recognise the conserved cold- and dehydration-responsive C-repeat/DRE DNA motifs found within the promoter regions of COR genes (Liu et al. 1998). CBF1 overexpression triggers constitutive expression of the COR genes and induces freezing tolerance in Arabidopsis (Jaglo-Ottosen et al. 1998). Expression of the CBFs is rapidly induced by cold temperatures, and a growing number of studies have demonstrated that transcriptional regulation of the CBF genes is controlled by multiple mechanisms. In Arabidopsis, CBF2 is a negative regulator of the expression of CBF1 and CBF3, and it plays an important role in freezing tolerance (Novillo et al. 2004; Novillo et al. 2007). To date, several key upstream regulators of the CBF genes have been identified and characterised. CAMTA3 encodes a calmodulin-binding protein and acts upstream to activate CBF2 expression by binding to the CG-element in its promoter region in response to cold signals (Doherty et al. 2009). The MYC-type, basic helix-loop-helix transcriptional activator inducer of CBF expression 1 (ICE1) has been identified as a transcriptional activator that binds to MYC cis-elements within the CBF3 promoter, and the expression of CBF3 and its target COR genes is impaired in ice1-mutant plants during cold acclimation. In addition, overexpression of ICE1 in Arabidopsis results in increased freezing tolerance, supporting a pivotal role for ICE1 in the cold stress response (Chinnusamy et al. 2003). The transcription factor EIN3 was shown to negatively regulate expression of the CBFs by directly binding to CBF promoters to modulate the cold stress response in plants. Furthermore, a loss-of-function ein3 mutant shows enhanced freezing tolerance, whereas overexpression of EIN3 leads to decreased freezing tolerance (Shi et al. 2012). Using a genetic screening strategy involving changes in the expression of a luciferase construct under the control of a stress-inducible RD29A promoter, high expression of osmotically responsive genes1 (HOS1), which encodes a RING-type ubiquitin E3 ligase, was isolated as an upstream negative regulator of the CBFs. HOS1 interacts with and ubiquitinates ICE1 to negatively regulate the stability of the ICE1 protein (Dong et al. 2006). The R2R3-type MYB transcription factor AtMYB15 (MYB domain protein 15) was found to physically interact with ICE1. MYB15 overexpression leads to reduced expression of the CBF genes, whereas amyb15 mutant shows increased CBF expression. MYB15 binds to the CBF3 promoter to repress its CBF expression and negatively regulates freezing tolerance (Agarwal et al. 2006). Subsequently, a SUMO E3 ligase, SAP and Miz (SIZ1), was identified as a positive regulator of the ICE1 protein. SIZ1 can sumoylate ICE1 and repress the polyubiquitination of ICE1, which, in turn, enhances ICE1 stability (Miura et al. 2007). Therefore, regulation of the ICE1-CBF cascade at both transcriptional and post-translational levels demonstrates the existence of a complex network of CBF-dependent cold-signalling pathways.
Schematic illustration of the cold and dehydration response regulatory networks in Arabidopsis. IP3, Ca2+, and ROS act as second messengers in signalling networks to transduce signals through protein kinases or transcription-factor cascades. CBFs and AREB/ABFs transcription factors are responsible for the regulation of COR genes containing CRT/DRE (CCGAC) and ABRE (ACGT) motifs in their promoters, respectively. In Arabidopsis, approximately 10 % of the cold-induced transcriptome contains both CRT and ABRE motifs in their promoter regions, which are upregulated by both cold and dehydration stresses. CBFs are activated by ICE1 and CAMTA transcription factors, whereas repressed by MYB15. HOS1 and SIZ1 encode RING E3 ligase and SUMO E3 ligase, respectively, which antagonistically regulate the abundance of ICE1 protein. Cold activates ICE1 protein and AREB/ABFs are induced by ABA-mediated dehydration signalling pathway. IP 3 inositol 1,4,5-triphosphate, ROS reactive oxygen species, CPK calcium-dependent protein kinase, MAPK Ras-mitogen-activated protein kinase, Ub ubiquitin moiety, SUMO Small ubiquitin-related modifier, Pi phosphoryl group
17.3 The Role of ABA Biosynthesis and Signalling in Cold Stress
Investigations into the role of ABA in cold stress were originally based on observations made in the 1960s in woody species, showing that application of the gibberellic acid (GA) inhibitor dormin—which was later identified as ABA-resulted in increased freezing tolerance in trees, equivalent to that observed in plants that had undergone cold acclimation (Chrispeels and Varner 1967; Thomas et al. 1965). It has been shown that cold tolerance is usually accompanied by increased endogenous ABA levels in various plant species (Daie and Campbell 1981; Lang et al. 1994; Mantyla et al. 1995). Continuous application of ABA induces chilling tolerance in chilling-sensitive plant species, such as maize, rice, cucumber, and pepper. Furthermore, exogenous ABA application in temperate plants such as poplar, barely, wheat, and Arabidopsis can partially mimic cold acclimation and enhance freezing tolerance (Kadlecová et al. 2000; Smoleńska-Sym et al. 1995; Zhu et al. 2000; Thomashow 1999). Transcriptome analyses in Arabidopsis showed that a number of ABA-responsive genes can be induced by cold treatment (Zeevaart and Creelman 1988). However, it has also been shown that ABA application in several plant species has little effect on freezing tolerance, or if there are any changes, they are much less significant than those induced by cold acclimation (Gusta et al. 1982; Fayyaz et al. 1978; Holubowicz et al. 1982), suggesting the natural diversity of ABA responses in different plant species.
The primary source of increased ABA levels under stress conditions is a de novo biosynthesis pathway that converts carotenoids into bioactive ABA. ABA biosynthesis pathway may be required for full development of the cold response, as defects in both basal and acquired freezing tolerance have been observed in ABA-deficient mutants. For instance, ABA1 and ABA3 are identified as genes encoding enzymes involved in ABA biosynthetic pathway. Cold induction of the COR genes is reduced in the aba3 mutants aba3/los5/frs1 (Llorente et al. 2000; Xiong et al. 2002b). Cold acclimation was shown to be impaired in an aba1 mutant, which has also reduced expression of specific COR genes (Mantyla et al. 1995). The expression of none of the ABA biosynthesis genes is affected by cold treatment in Arabidopsis (Lee et al. 2005). Therefore, ABA biosynthesis is not an early event in response to cold stress. Consistent with this, foliar application of ABA to a wide range of plant species does not induce cold hardness or freezing tolerance (Gusta et al. 2005). A recent study showed that cold stress specifically activate the expression of ABA biosynthesis genes in reproductive organs, such as the inflorescence meristem, with only slightly increased expression in the leaves and vegetable organs (Baron et al. 2012). This finding supports the notion of ABA function in stimulating and hastening plant harvests under adverse environmental conditions. Taken together, ABA may function late in the development of cold-induced metabolic changes and is required for determining the maximum levels of cold tolerance in plants.
ABA activates a wide array of genes associated with stress response, seed dormancy, and stomatal movement through the ABA signalling pathway (Yamaguchi-Shinozaki and Shinozaki 2006). The PYR/PYL/RCAR family of proteins are ABA receptors that can inhibit the activity of the type-2C protein phosphatase PP2C in the presence of ABA, relieving the repression of the SnRK2 kinases activity, which in turn phosphorylates other downstream regulators (Furihata et al. 2006; Ma et al. 2009; Park et al. 2009). Overexpression of TaSnRK2.3 in Arabidopsis results in enhanced root-system architecture and significantly enhances the tolerance of this species to drought, salt, and freezing stresses (Tian et al. 2013), whereas downregulation of PP2CA proteins increases ABA sensitivity and accelerates cold acclimation in transgenic plants (Tahtiharju and Palva 2001). Promoter analysis found that some DRE/CRT motif-containing COR genes are induced by cold and dehydration and impaired in ABA-deficient mutants (Thomashow 1999; Shinozaki and Yamaguchi-Shinozaki 2000). As mentioned earlier, CBFs/DREB1s and their homolog, CBF4, were shown to bind to CRT/DRE motifs in vivo. CBF4 expression is induced by drought and ABA, but not by cold, which is quite distinctive from CBF1–CBF3. Overexpression of CBF4 results in increased freezing and drought tolerance (Haake et al. 2002). It appears that at least three separate regulatory signalling pathways exist that are mediated by the binding of transcription factors to CRT/DRE motifs. Among these, cold-induced CBF1–CBF3 and drought-induced DREB2 are the primary transcription factors that control gene expression through the ABA-independent pathway, whereas CBF4 acts as a regulator of the ABA response (Thomashow 1999; Shinozaki and Yamaguchi-Shinozaki 2000). In addition to the CRT/DRE motif, many cold-response gene promoters harbour ABRE cis-elements that can be activated in vivo by ABA through direct binding of bZIP transcription-factor proteins (AREBs/ABFs, ABRE-binding proteins/factors) (Uno et al. 2000). This evidence explains that expression of the COR genes is regulated by both ABA-dependent and ABA-independent pathways (Fig. 17.1).
17.4 ABA-Dependent Signalling Pathway for Cold Acclimation
Genetic and physiological analyses indicate that ABA-dependent signalling pathway plays an essential role in stress-responsive gene expression during cold-induced osmotic stress. ABA biosynthesis genes contribute to the enrichment of COR genes, such as RD29A, RD22, COR15A, COR47, and P5CS via activity of their ABRE cis-element (Xiong et al. 2001a, 2002b). In the study on Arabidopsis, four AREB/ABF transcription factors are found to be required for the activation of ABA-mediated signalling (Uno et al. 2000). Among them, ABF1 expression is significantly induced by cold, but not by osmotic stress (Choi et al. 2000), whereas AREB1/ABF2, AREB2/ABF4, and ABF3 are specifically induced by ABA and drought stress (Abdeen et al. 2010; Kang et al. 2002; Fujita et al. 2005). AREB proteins do not appear to play a role in CBF-dependent cold signalling; however, one study showed that ABF2, ABF3, and ABF4 interact with DREB2C in vitro (Lee et al. 2010). In addition, approximately 10 % of ABA-responsive genes are also responsive to cold stress (Kreps et al. 2002). Thus, although ABA- and cold stress-induced transcription factors show distinct regulation, some of the COR genes appear to be induced by both of these stimuli. Therefore, one could speculate that limited, cold-induced ABA production could enhance COR gene activation through the ABA-dependent pathway during cold acclimation.
In addition to AREBs, other bZIP transcription factors belonging to the ABI5 subfamily have been characterised as ABRE-binding proteins and were shown to affect seed germination as well as the cold stress response. For example, ectopic expression of the seed-specific transcriptional activator ABI3 confers the ability to express COR genes in vegetative tissues and enhances freezing tolerance in Arabidopsis (Tamminen et al. 2001). ABI5 has been shown to act downstream of and interact with ABI3 (Suzuki and McCarty 2008; Finkelstein and Lynch 2000, and it can be sumoylated and degraded by the SUMO E3 ligase SIZ1, which attenuates ABA signalling (Miura et al. 2009). Indeed, SIZ1-mediated SUMO conjugation is also required for the expression of CBF3 by modulating the activity of ICE1 (Miura et al. 2007). siz1 mutants are impaired in cold acclimation and are sensitive to ABA during seed germination (Miura et al. 2007, 2009). A recent study revealed that SIZ1 is able to sumoylate and stabilise the negative regulator of ABA signalling MYB30 (Zheng et al. 2012). Therefore, SIZ1 appears to be an integration node that mediates ABA and cold responses through post-transcriptional modifications.
Several MYC and MYB family genes have been reported to be important regulators of ABA-responsive gene expression under cold stress. For instance, MYB96 is induced by ABA and drought, and it enhances ABA-mediated drought and freezing tolerance (Guo et al. 2013; Seo et al. 2009). Expression of OsMYB3R-2 in rice is induced by cold, drought, and salt stresses, and overexpression of OsMYB3R-2 increases tolerance to freezing, drought, and salt stresses in transgenic Arabidopsis (Dai et al. 2007). Recent studies suggest that some NAC-domain family members are involved in stress responses (Olsen et al. 2005). For example, expression of a rice NAC gene, OsNAC5, is induced by osmotic stress and ABA, and overexpression of OsNAC5 increases stress-induced proline and soluble-sugar levels and enhances tolerance to cold, salt, and drought stresses (Takasaki et al. 2010; Song et al. 2011). Thus, it can be assumed that cold induction of some ABA-responsive genes is regulated by different kinds of transcription factors.
17.5 Second Messengers Integrate Cold and ABA Signalling
Both cold stress and ABA treatment induce the production and accumulation of IP3, Ca2+, and ROS inside the cell. These molecules act as second messengers in signalling networks to amplify and transduce signals through activating protein kinases or transcription-factor cascades. Increased inositol 1,4,5-triphosphate (IP3) levels, which are catalysed by phospholipase C (PLC) under cold stress, are known to release Ca2+ from vacuoles into the cytosol (Allen and Sanders 1995; Munnik et al. 1998). In Arabidopsis, FRY1 encodes an inositol polyphosphate 1—phosphatase that is involved in IP3 metabolism. fry1 mutant plants show significantly higher IP3 levels when treated with ABA, and they display defects in cold acclimation and hypersensitivity to osmotic stress, with lowered expression of certain stress-responsive genes (Xiong et al. 2001b).
In plants, Ca2+ is a widely used and important signalling molecule during early responses to abiotic stresses, as it functions by activating protein kinase cascades, which in turn can activate transcription factors and stress-responsive genes. More specifically, a transient influx of cytoplasmic Ca2+ occurs in response to cold shock, and it has been suggested that low-temperature-induced changes in membrane fluidity are the primary thermosensor signal that activates the Ca2+ influx in plants (Knight et al. 2004). Furthermore, calcium is required for the full expression of the COR genes in Arabidopsis. CAX1, which encodes a vacuolar membrane-located Ca2+/H+ antiporter, participates in the regulation of cold acclimation. Loss-of-function mutations in CAX1 increase CBF expression and enhance freezing tolerance following acclimation in Arabidopsis (Catala et al. 2003). One calmodulin proteins in Arabidopsis, AtCML9, has recently been demonstrated to be responsive to ABA, cold, and salt stress through an ABA-dependent pathway. The cml9 mutant exhibits hypersensitivity to ABA and enhanced tolerance to stress and drought (Magnan et al. 2008). CaM methylation is also involved in the early growth of Arabidopsis and responds to ABA, cold, and heat stress during germination (Banerjee et al. 2013). As mentioned earlier, the calmodulin-binding transcriptional activator CAMTA3 is involved in the regulation of freezing tolerance (Doherty et al. 2009). More importantly, a recent study suggested that calcium channels may involve in temperature perception in land plants, based on the observation that plants lacking the membrane protein cyclic nucleotide gated calcium channel (CNGC) showed constitutively high levels of Ca2+ influx and impaired thermoperception in a moss and in Arabidopsis (Finka et al. 2012). Moreover, a homologue of the synaptic Ca2+-binding protein synaptotagmin (SYT1), which is a calcium sensor that can prevent Ca2+-dependent membrane, damages in Arabidopsis protoplasts under freezing conditions (Yamazaki et al. 2008). These results suggest the central role of cytosolic calcium to trigger cold signalling. Ca2+ may function through calmodulin or Ca2+ sensors to modulate the activation of downstream calcium-dependent protein kinases (CPKs). Calcineurin B-like proteins (CBLs), one kind of calcium sensors, were also shown to regulate expression of the CBF genes, based on the observations that overexpression of CBL1 resulted in reduced freezing tolerance, whereas cbl1 null mutants showed increased cold-induced expression of stress genes and enhanced freezing tolerance (Albrecht et al. 2003; Cheong et al. 2003).
Several CBL-interacting protein kinases (CIPKs) and CPKs have been implicated in the responses to ABA, cold, and high-salt stress. In Arabidopsis, the expression of CIPK3 is strongly induced by cold stress and ABA treatment, but not by drought stress. In addition, the expression of various cold-induced marker genes is all significantly delayed in a cipk3 mutant, and the induction of ABA-responsive genes is also significantly inhibited (Kim et al. 2003). Therefore, CIPK3 may function as a cross talk node between signalling pathways for ABA, cold, and other abiotic stresses. It has also been suggested that CIPK1 is a convergence point for the ABA-dependent and ABA-independent stress responses. For example, CIPK1 can interact with CBL1 and CBL9, resulting in the formation of CBL1/CIPK1 and CBL9/CIPK1 complexes that mediate the ABA-independent and ABA-dependent responses, respectively, during osmotic stress (D’Angelo et al. 2006). Rice OsCDPK7 is a cold- and salt-inducible gene, and overexpression of OsCDPK7 confers cold and salt/drought tolerance on rice plants (Abbasi et al. 2004). Collectively, these results indicate a central role for calcium in sensing and transducing the cold and ABA signals.
17.6 ABA and ROS Under Cold Stress
The induction of ROS-scavenging systems is common to many stress pathways including ABA and cold stress (Fujita et al. 2006). The roles of ROS in cold stress are considered in two ways. On the one hand, excessive ROS levels are toxic and can lead to lipid peroxidation, and ultimately, to oxidative stress. On the other hand, increased ROS levels can also play a benefit signalling role and activate scavenging enzyme systems to eliminate excess ROS in plants subjected to low temperatures. Numerous studies have shown that ROS-scavenging enzymes such as superoxide dismutase (SOD), ascorbate peroxidise (APX), catalase (CAT), and glutathione peroxidise (GPX) are required for protection against cold-induced oxidative damage (Burdon et al. 1996; O’Kane et al. 1996). For example, overexpression of antioxidant-defensive genes enhances chilling tolerance in maize and soybean (Van Breusegem et al. 1999; Kocsy et al. 2001), whereas repression of catalase gene expression reduces chilling tolerance (Kerdnaimongkol and Woodson 1999). Arabidopsis chs (chilling-sensitive) mutants display increased sensitivity to chilling temperature, which is at least partially due to the excessive accumulation of H2O2 in chs mutant seedlings (Huang et al. 2010a, b; Wang et al. 2013; Yang et al. 2010). Recent studies also show that ROS act as signal molecules mediating cold stress-signal transduction. For instance, the Arabidopsis mutant frostbite1 (fro1) has a defective form of mitochondrial complex I NADH dehydrogenase, leading to constitutively lower ROS levels compared with the wild type, and this mutant shows reduced expression of cold stress-responsive genes and decreased cold acclimation (Lee et al.2002). ZAT12 encodes a ROS-responsive zinc-finger transcription factor that functions as a positive regulator of the cold-response pathway. Overexpression of ZAT12 results in enhanced freezing and oxidative tolerance (Davletova et al. 2005), which suggests that ROS signalling plays a beneficial role in mediating cold acclimation in plants.
It has been shown that the application of exogenous ABA effectively alleviates the symptoms of cold injury in many species. One explanation for this phenomenon is that ABA signalling is able to induce the transcription of ROS-scavenging enzymes. Indeed, one study showed that stress-induced ABA accumulation stimulates ROS scavenging to help maintain the cellular redox state (Guan et al. 2000). Furthermore, ABA-dependent proline accumulation under abiotic stress is regulated by the ROS-scavenging-mediated cellular redox state, which is also important for the cold response (Kishor et al. 2005). In particular, ABA treatment enhances ROS accumulation by activating plasma membrane-bound NADPH oxidases (Kwak et al. 2003). ABO5 and ABO6, which encode a PPR (pentatricopeptide repeat) protein and DEXH box RNA helicase, respectively, are required for ABA-induced ROS production in mitochondria (He et al. 2012; Liu et al. 2010). The activities of ABI1 and ABI2 are inhibited by H2O2 in vitro (Meinhard and Grill 2001; Meinhard et al. 2002), and further studies demonstrated that ABI2 interacts with glutathione peroxidase 3 (GPX3), which is an ROS-scavenging enzyme that functions as a redox transducer and scavenger to modulate ABA signalling (Miao et al. 2006). Glucosamine (GlcN) is a naturally occurring amino sugar that inhibits plant growth by significantly increasing the production of ROS. Ectopic overexpression of GlcN induces cell death, whereas scavenging of endogenous GlcN can enhance tolerance to oxidative, drought, and cold stresses in Arabidopsis (Chu et al. 2010).
As well-known signalling components downstream of ROS, several mitogen-activated protein kinases (MAPKs) have been shown to be activated by cold and ABA. ABA application induces MAPK activation within only a few minutes (Knetsch et al. 1996). The MAPK cascade is an important signalling pathway that enables the transmission of environmental and hormone signals to activate regulatory components within the cytoplasm and initiate cellular-responsive processes. In vivo assays data revealed that the MKK2–MPK4/MPK6 complex functions downstream of MEKK1 during salt and cold stress. MPK4 and MPK6 are strongly phosphorylated by MKK2 in response to cold and salt treatment, and null allele of mkk2 is impaired in ability of cold acclimation (Teige et al. 2004). Direct evidence of MAPK activation by ABA was observed in guard cells. MPK9 and MPK12 function downstream of ROS as positive regulators of ABA signalling, and mpk9 mpk12 double mutants are partially but specifically impaired in cold-induced stomatal closure, suggesting that they play a role in the cold-mediated ABA signalling cascade (Jammes et al. 2009). Arabidopsis NDP kinase 2 (NDPK2) was shown to interact with two oxidative stress-related MAPKs, MPK3, and MPK6, and overexpression of NDPK2 enhances cold tolerance (Moon et al. 2003). Taken together, these findings raise the possibility that the MAPK cascade acts downstream of ROS in cold and ABA signalling.
17.7 The Effects of Circadian Signals on Cold and ABA Signalling
The circadian clock is regulated by a central oscillator consisting of transcription/translation feedback loops that are entrained to light and temperature cues. The MYB transcription factors circadian clock associated1 (CCA1) and late elongated hypocotyl (LHY) can directly bind to the promoters of the evening-loop components timing of CAB expression1 (TOC1), pseudo response regulator (PRR7), and PRR9 to repress their expression after dawn; conversely, PRR7 and PRR9 act as negative regulators of CCA1 and LHY expression in the morning (Harmer 2009). Interestingly, CBF gene expression is under circadian regulation, and the cold induction of CBFs is gated by the circadian clock (Fowler et al. 2005). It has been shown that CCA1 and LHY are positive regulators of CBF expression that directly bind to CBF promoters (Lee and Thomashow 2012; Dong et al. 2011). In the morning, CBF expression increases following induction of CCA1 and LHY, and CBF transcript levels peak shortly after those of CCA1 and LHY. It has also been suggested that CAMTA3 and ICE1 act synergistically with CCA1 and LHY to induce CBF expression during the day (Dong et al. 2011). During the evening, the evening element TOC1 interacts with phytochrome interacting factor 7 (PIF7) to repress expression of CBF2 by directly binding to its promoter. Therefore, the circadian-gated regulation of the CBF genes appears to involve the action of CCA/LHY complex and PIF7/TOC1 evening complex (Dong et al. 2011). A recent study showed that TOC1 functions as a molecular switch that connects the circadian clock to the ABA-mediated drought stress response (Legnaioli et al. 2009). ABA signalling is closely related with circadian oscillator period, for example, ABA-mediated stomatal movement varies during day and night (Tallman 2004). It was proposed that the circadian clock may modulate stomatal closure under high-temperature conditions when water supplies are limited in order to optimise water-usage efficiency (Robertson et al. 2009). TOC1 has been found to negatively regulate the expression of ABA-related genes by directly interacting with ABI3 (Kurup et al. 2000). Moreover, TOC1 can also bind to the promoter of ABAR, a chloroplastic ABA binding protein, to control its circadian expression, and the gated-induction of TOC1 by ABA is abolished in ABAR RNAi plants. Furthermore, overexpression of TOC1 causes significant changes in plant responses to ABA and drought stress, which are indicative of a feedback mechanism linking the circadian clock to the ABA response (Legnaioli et al. 2009). Collectively, these findings illustrate the mechanisms by which the circadian clock regulates the cold and ABA responses.
17.8 Post-Transcriptional Modification Mediates Cold and ABA Signalling
RNA splicing and transport can lead to tissue-specific differences in gene expression as well as affect mRNA stability and turnover via nonsense-mediated decay (McGlincy and Smith 2008). By performing a genetic screen for deregulated expression of RD29A promoter-driven luciferase reporter gene, several genes encoding RNA processing proteins were identified. Loss of osmotic responsiveness 4 (LOS4) encodes a putative DEAD-box RNA helicase that has been implicated in nucleo-cytoplasmic mRNA transport. The recessive mutations los4-1 and los4-2 affect cold-induced CBF transcription and lead to changes in chilling and freezing tolerance (Gong et al. 2002, 2005), suggesting that mRNA translocation may also be a control point in cold acclimation. It was also found that a los4-2 mutant was hypersensitive to ABA (Gong et al. 2005). A recent study identified a regulator of CBF2 gene expression1 (rcf1-1) mutant that was hypersensitive to cold stress. RCF1 also encodes a cold-inducible DEAD-box RNA helicase. However, unlike LOS4, RCF1 maintains the proper splicing of COR pre-mRNAs under cold stress conditions (Guan et al. 2013). Another pre-mRNA splicing factor Stabilized1 (STA1) which is required for pre-mRNA splicing and mRNA turnover is upregulated by cold stress, but not by ABA or NaCl (Lee et al. 2006). A sta1 mutant displays a chilling-sensitive phenotype and hypersensitivity to ABA-induced root growth, as well as defects in splicing of the cold-induced COR15A gene (Lee et al. 2006). Arabidopsis FIERY2 (FRY2), which encodes an RNA polymerase II C-terminal domain (CTD) phosphatase, may function in mRNA processing. FRY2 plays a negative role in regulating salt stress and the ABA response during seed germination, and it has been shown to be a repressor of the CBF transcription factors. However, a fry2 mutant was shown to be hypersensitive to freezing stress (Xiong et al. 2002a). Sensitive to ABA and drought2 (SAD2) was found to be involved in nucleo-cytoplasmic trafficking during ABA treatment, and sad2 mutant plants show a hypersensitive response to ABA and enhanced expression of stress genes in response to ABA, salt, and low temperatures (Verslues et al. 2006). Therefore, post-transcriptional regulation also serves as a major mechanism in the global control of the ABA and cold responses.
17.9 Epigenetic Modifications in Cold and ABA Signalling
DNA and histones are epigenetically modified through acetylation and methylation following exposure to stress (Kim et al. 2010; Feng and Jacobsen 2011). HOS15 encodes a protein similar to the human WD-40 repeat protein TBL1 (transducin-like protein-1), which is involved in histone deacetylation. Both ABA and cold induce the expression of HOS15, and hos15-1 mutants exhibit hypersensitivity to ABA-induced inhibition of germination and freezing stress. Furthermore, HOS15 interacts with histone H4, and levels of acetylated histone H4 are higher in hos15 mutants than in the wild type (Zhu et al. 2008). Therefore, HOS15 is involved in the regulation of ABA and the cold stress response through H4 deacetylation-dependent chromatin remodelling in Arabidopsis. Consistently, increased levels of acetylated histone H4 are consistently associated with increased expression of RD29A in a hos15 mutant compared with the wild type under cold stress. On the other hand, histone deacetylation results in a non-permissive chromatin conformation that represses transcription. In Arabidopsis, histone deacetylase 6 (HDA6) has been reported to be involved in the ABA response and low-temperature-mediated flowering (Luo et al. 2012; Chen and Wu 2010). In Arabidopsis, HDA6 encodes an RPD3-type histone deacetylase, and a loss-of-function mutation inHDA6, axe1–5, displays hypersensitivity to ABA and salt stress (Luo et al. 2012). Interestingly, HOS1 was discovered as a chromatin remodelling factor for cold-induced transcriptional silencing of the FLC gene through an interaction with HDA6, thereby antagonising the actions of FVE and resulting in the inhibition of flowering in these plants (He and Amasino 2005; Jung et al. 2013). These findings suggest that plant flowering or stress response processes might be epigenetically controlled through similar mechanisms.
17.10 Cross Talk Between Phytohormones and the Cold Response
Emerging evidence indicates that cold acclimation is influenced by changes in the homeostasis of various hormones and that hormone signalling is crucial for regulation of the CBF pathway. Genetic analyses of Arabidopsis mutants with compromised ABA biosynthesis or signalling have identified a complex interplay between ABA and other phytohormone-signalling pathways. Generally, when plants are challenged by stress conditions, the stress hormones ABA and jasmonate acid (JA) inhibit growth by modulating the actions of auxin, GA, and cytokinin (Achard et al. 2006; Wolters and Jurgens 2009; Peleg and Blumwald 2011). The growth inhibition decreases the capacity for energy utilisation, which in turn, results in cold acclimation processes.
17.10.1 GA and Cold Stress
As an important plant phytohormone, GA affects plant abiotic stress responses, including those to salt, oxidative, and cold stresses during germination and seedling development. Acting as GA-signalling repressors, DELLA proteins have been identified as the central modulators of growth under a variety of stress conditions (Achard et al. 2006). ABA promotes the accumulation of DELLA proteins, which induces growth repression in plant lateral roots under salt stress through an ABA-dependent pathway (Achard et al. 2006). Intriguingly, GA treatment rescues normal growth and abolishes the late-flowering phenotype of CBF1-ox plants (Achard et al. 2008). Further investigation revealed that cold stress causes increased expression of the biosynthetic enzyme genes AtGA2ox3 and AtGA2ox6, which reduce endogenous levels of bioactive GA. Consistently, overexpression of CBFs represses plant growth via the accumulation of DELLA proteins (Achard et al. 2008). ABA is known to act as an antagonist of GA by inhibiting the expression of GA20ox and GA3ox to reduce GA levels during seed germination (Razem et al. 2006) (Fig. 17.2a). It has been demonstrated that the existence of a novel mechanism through which CBFs may regulate dormancy in parallel to their functions in CBF-mediated cold signalling, by regulation of DOG1 expression, which is a positive regulator of GA catabolism and ABA biosynthesis that promotes seed maturation at cool temperatures (Chiang et al. 2011; Kendall et al. 2011). A recent study showed that ABI3 and ABI5 interact with DELLA proteins to inhibit seed germination by repressing the expression of high-temperature-induced genes (Lim et al. 2013). Thus, DELLA-dependent growth restraint is necessary for cold acclimation and temperature-mediated seed germination.
Models of multiple interactions between ABA and other plant hormones in cold stress response. There are multiple points of interaction between ABA and other plant hormones including GA (a), cytokinin (b), ethylene (c), JA (d), and SA (e) in regulation of plant response to cold stress. CTK cytokinin, ETH ethylene, DELLA DELLA protein, AHK Arabidopsis histidine kinase, AHP Arabidopsis histidine phosphor-transferase, ARR Arabidopsis response regulator, EIN3 Ethylene insensitive3, JAZ jasmonate-zim-domain protein, MYC2 MYC-related transcriptional activator2
17.10.2 Cytokinin and Cold Stress
Cytokinin signalling belongs to the two-component signalling system, which involves plant adaptation to environmental stress (Argueso et al. 2009; Nishimura et al. 2004). The accumulated body of evidence indicates that cytokinin acts as an antagonist of ABA during environmental stress responses (Peleg and Blumwald 2011; Hwang et al. 2012). Based on studies involving mutants or transgenic plants with altered cytokinin biosynthesis, it has been postulated that cytokinin acts as a negative regulator to modulate abiotic stress signalling (Nishiyama et al. 2011). In Arabidopsis, the cytokinin receptor histidine kinases AHK2, AHK3, and CRE1 have been shown to play important roles in the regulation of plant abiotic responses in both ABA-dependent and ABA-independent signalling pathways (Tran et al. 2007); on the other hand, the homologous gene AHK1 was identified as an osmotic stress sensor that positively regulates abiotic stress (Wohlbach et al. 2008; Kumar et al. 2013; Tran et al. 2007). Increasing evidence suggests a negative role for cytokinin signalling in cold- and ABA-mediated abiotic stress. For instance, ahk2 ahk3 double mutants are highly tolerant to cold, drought, and salt stress and show strong expression of ABA-responsive genes (Jeon et al. 2010; Tran et al. 2007). However, whether cytokinin receptors can perceive stress signals is still unclear. AHP2, AHP3, and AHP5 function as redundant negative regulators of the drought stress response in both ABA-dependent and ABA-independent manners (Nishiyama et al. 2013). These studies support the notion that AHK2–AHK4 and AHPs play negative roles under unfavourable environmental conditions and may integrate with ABA signalling to modulate stress responses (Fig. 17.2b). Type-A ARR genes are negative response regulators in cytokinin signalling pathway. They are induced by cytokinin as well as by cold stress (To et al. 2004). Overexpression of type-A ARRs enhances freezing tolerance in Arabidopsis (Shi et al. 2012). In contrast, another study demonstrated that type-A ARRs are negative regulators of the cold response (Jeon et al. 2010). Although it can be assumed that the cytokinin signalling pathway plays a negative regulatory role in freezing tolerance, at least partially through inhibition of the ABA response, further study will be necessary to define the exact role of cytokinin signalling, particularly for the ARRs, in the regulation of cold signalling.
17.10.3 Ethylene and Cold Stress
Ethylene plays an important role in regulating plant responses to drought, flooding, and biotic stress (Wilkinson and Davies 2010). However, the function of ethylene in plant responses to cold stress appears to be complex and species dependent. Increased ethylene biosynthesis has been correlated with enhanced chilling and freezing tolerance in several plant species, including tomato (Lycopersicones culentum), cucumber (Cucumis sativus), and tobacco (Nicotiana tabacum cv. NC89) (Wang and Adams 1982; Ciardi et al. 1997; Zhang and Huang 2010). In contrast, improved cold tolerance was associated with the suppression of ethylene biosynthesis in mung bean (Vignaradiata) and Arabidopsis (Collins et al. 1995; Shi et al. 2012). In Arabidopsis, application of the ethylene precursor ACC decreases freezing tolerance, whereas application of the ethylene biosynthesis inhibitor AVG and the ethylene receptor antagonist AgNO3 promote freezing tolerance. Consistent with these findings, several ethylene-insensitive mutants, including etr1-1, ein4-1, ein2-5, ein3-1, and ein3 eil1, exhibit enhanced freezing tolerance. Genetic and biochemical analyses revealed that ethylene negatively regulates cold signalling, at least partially, through direct transcriptional control of the COR CBFs and type-A ARR genes via EIN3 (Shi et al. 2012) (Fig. 17.2c).
It is known that ethylene can promote seed germination and repress seed dormancy by antagonising ABA (Wilkinson and Davies 2010). Previous studies indicated that etr1-1 and ein2-5 mutants show enhanced seed dormancy, and their germination is hypersensitive to ABA (Chiwocha et al. 2005; Wang et al. 2007). As ABA accumulates in etr1 and ein2 mutants (Ghassemian et al. 2000; Chiwocha et al. 2005; Wang et al. 2007), ethylene may negatively regulate ABA biosynthesis during germination. Consistent with this notion, microarray data showed that a group of genes involved in ABA signalling are upregulated in an ein3 eil1 double mutant (Shi et al. 2012). Interestingly, a recent study revealed the mechanism of how ABA inhibits root growth by increasing ethylene biosynthesis in Arabidopsis. ABA-activated calcium-dependent protein kinases, CPK4 and CPK11 can phosphorylate ethylene biosynthesis synthase ACS6 and enhance its stability, thus promoting ethylene production (Luo et al. 2014). Although the mechanisms underlying the complex cross talk between ethylene, ABA, and cold signalling remain unclear, it appears likely that the upregulation of ABA-responsive genes also contributes to the freezing tolerance of ethylene-insensitive mutants.
17.10.4 JA and Cold Stress
JA has recently been found to positively modulate cold tolerance. In Arabidopsis, exogenous application of JA significantly improves freezing tolerance, whereas blockage of JA biosynthesis and signalling decreases freezing tolerance. Further study revealed that JAZ1 and JAZ4 proteins physically interact with the ICE1-proteins to repress their transcriptional activity, thereby repressing expression of CBFs and their regulons (Hu et al. 2013) (Fig. 17.2d). Therefore, it appears that JA modulates cold stress primarily through an ABA-independent pathway. However, an antagonistic interaction between ABA and JA signalling has also been reported. In particular, the transcription factor MYC2 was shown to act as a node for the integration of ABA and JA signalling. MYC2 can directly interfere with defence signalling through JAZ-mediated repression, whereas MYC2 acts as an important positive regulator of the ABA-dependent signalling pathway (Abe et al. 2003; Fernandez-Calvo et al. 2011) (Fig. 17.2d). Furthermore, recent studies showed that an ABA-inducible bHLH transcription factor, JAM1, acts as a repressor that negatively regulates JA signalling in Arabidopsis (Nakata et al. 2013).
17.10.5 SA and Cold Stress
Salicylic acid (SA) is important for the activation of plant defence responses (Raskin 1992). Multiple studies have demonstrated a link between cold signalling and the SA-mediated defence response. Cold stress induces the accumulation of SA in Arabidopsis, whereas SA-deficient mutants show increased growth in response to cold (Scott et al. 2004). Gain of function of R/R-like protein mutants that overproduce SA, such aschs1, chs2, and chs3, display chilling-sensitive phenotypes that are partially dependent upon SA (Huang et al. 2010a; Yang et al. 2010; Wang et al. 2013). Indeed, there appears to be an optimal threshold of endogenous SA that is crucial for the chilling-dependent inhibition of plant growth. Nevertheless, the exact role of SA in freezing tolerance remains unclear. For example, several SA-overproducing mutants, including cpr1, cpr5, and slh1, show enhanced freezing tolerance (Yang et al. 2010), whereas overexpression of DEAR1 (DREB and EAR motif protein) induces SA accumulation and freezing sensitivity in Arabidopsis (Tsutsui et al. 2009). The master regulator of cold signalling, ICE1, was shown to directly regulate the expression of BON1-associated protein (BAP1), which encodes a C2-domain protein that negatively mediates the SA-dependent defence responses (Zhu et al. 2011; Yang et al. 2006). CAMTA3/AtSR1 recognises the CBF2 promoter to positively regulate CBF2 expression during cold stress, but it also interacts with the EDS1 promoter to repress SA-dependent disease resistance (Kim et al. 2013; Doherty et al. 2009; Du et al. 2009).
ABA has been shown to play a broad role in the regulation of plant defence responses. For example, ABA acts a positive regulator of the defence response during pathogen invasion, whereas it acts as a negative regulator of the defence response following pathogen invasion (Yasuda et al. 2008; Ton et al. 2009; Robert-Seilaniantz et al. 2011). A recent study revealed that ABA deficiencies antagonise the high-temperature inhibition of disease resistance by enhancing the nuclear accumulation of the resistance proteins SNC1 and RPS4 in Arabidopsis (Mang et al. 2012). Therefore, ABA and SA may act antagonistically to affect plant development and the defence response, as well as the cold stress response (Fig. 17.2e).
17.11 Conclusions
Cold acclimation is a complex physiological process that is affected by various developmental and environmental factors, including growth stage, tissue type, photoperiod, hydration status, and low temperatures. ABA has long been thought to play an important role in mediating the cold response in plants, and accumulation of ABA may indeed be required for full cold acclimation. However, the extent of its role in vivo has been controversial. It appears that ABA biosynthesis is not a major aspect of the early cold stress response but rather that it contributes to the maximum induction of cold-responsive genes during later stages. It is also possible that ABA plays diverse roles in the regulation of multiple physiological processes that are affected by chilling or freezing stress. However, the majority of the molecular mechanisms underlying how ABA affects freezing tolerance remain to be investigated. In particular, elucidating whether the key components of ABA signalling are involved in CBF-regulated cold acclimation should be a priority for those studying the role of ABA in cold signalling. In addition, understanding the signalling networks between ABA and the other relevant plant hormones during the cold stress response will be crucial for determining any potential applications for ABA in enhancing plant tolerance to cold stress.
References
Abbasi F, Onodera H, Toki S, Tanaka H, Komatsu S. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced by cold and gibberellin in rice leaf sheath. Plant Mol Biol. 2004;55(4):541–52.
Abdeen A, Schnell J, Miki B. Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3. BMC Genom. 2010;11:69.
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15(1):63–78.
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311(5757):91–4.
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008;20(8):2117–29.
Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem. 2006;281(49):37636–45.
Albrecht V, Weinl S, Blazevic D, D’Angelo C, Batistic O, Kolukisaoglu U, Bock R, Schulz B, Harter K, Kudla J. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J. 2003;36(4):457–70.
Allen GJ, Sanders D. Calcineurin, a type 2B protein phosphatase, modulates the Ca2+-permeable slow vacuolar ion channel of stomatal guard cells. Plant Cell. 1995;7(9):1473–83.
Argueso CT, Ferreira FJ, Kieber JJ. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell Environ. 2009;32(9):1147–60.
Banerjee J, Magnani R, Nair M, Dirk LM, DeBolt S, Maiti IB, Houtz RL. Calmodulin-mediated signal transduction pathways in Arabidopsis are fine-tuned by methylation. Plant Cell. 2013;25(11):4493–511.
Baron KN, Schroeder DF, Stasolla C. Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant Sci. 2012;188:48–59.
Van Breusegem F, Slooten L, Stassart JM, Moens T, Botterman J, Van Montagu M, Inze D. Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol. 1999;40(5):515–23.
Burdon RH, O’Kane D, Fadzillah N, Gill V, Boyd PA, Finch RR. Oxidative stress and responses in Arabidopsis thaliana and Oryza sativa subjected to chilling and salinity stress. Biochem Soc Trans. 1996;24(2):469–72.
Catala R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM, Salinas J. Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell. 2003;15(12):2940–51.
Chen M, Thelen JJ. ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell. 2013;25(4):1430–44.
Chen LT, Wu K. Role of histone deacetylases HDA6 and HDA19 in ABA and abiotic stress response. Plant Signal Behav. 2010;5(10):1318–20.
Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell. 2003;15(8):1833–45.
Chiang GC, Bartsch M, Barua D, Nakabayashi K, Debieu M, Kronholm I, Koornneef M, Soppe WJ, Donohue K, De Meaux J. DOG1 expression is predicted by the seed-maturation environment and contributes to geographical variation in germination in Arabidopsis thaliana. Mol Ecol. 2011;20(16):3336–49.
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17(8):1043–54.
Chiwocha SD, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross AR, Kermode AR. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 2005;42(1):35–48.
Choi H, Hong J, Ha J, Kang J, Kim SY. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 2000;275:1723–30.
Chrispeels MJ, Varner JE. Hormonal control of enzyme synthesis: on the mode of action of gibberellic acid and abscisin in aleurone layers of barley. Plant Physiol. 1967;42(7):1008–16.
Chu SH, Noh HN, Kim S, Kim KH, Hong SW, Lee H. Enhanced drought tolerance in Arabidopsis via genetic manipulation aimed at the reduction of glucosamine-induced ROS generation. Plant Mol Biol. 2010;74(4–5):493–502.
Ciardi JA, Deikman J, Orzolek MD. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol Plant. 1997;101(2):333–40.
Collins GG, Nie X, Saltveit ME. Heat shock increases chilling tolerance of mung bean hypocotyls tissues. Physiol Plant. 1995;89(1):117–24.
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007;143(4):1739–51.
Daie J, Campbell WF. Response of tomato plants to stressful temperatures: increase IN abscisic acid concentrations. Plant Physiol. 1981;67(1):26–9.
Davletova S, Schlauch K, Coutu J, Mittler R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005;139:847–56.
Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21(3):972–84.
Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA. 2006;103(21):8281–6.
Dong MA, Farre EM, Thomashow MF. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc Natl Acad Sci USA. 2011;108(17):7241–6.
Du L, Ali GS, Simons KA, Hou J, Yang T, Reddy AS, Poovaiah BW. Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature. 2009;457(7233):1154–8.
D’Angelo C, Weinl S, Batistic O, Pandey GK, Cheong YH, Schultke S, Albrecht V, Ehlert B, Schulz B, Harter K, Luan S, Bock R, Kudla J. Alternative complex formation of the Ca-regulated protein kinase CIPK1 controls abscisic acid-dependent and independent stress responses in Arabidopsis. Plant J. 2006;48(6):857–72.
Fayyaz MM, McCown BH, Beck GE. Effect of temperature, photoperiod and several growth substances on the cold hardiness of Chrysanthemum morifolium rhizome. Physiol Plant. 1978;44:73–6.
Feng S, Jacobsen SE. Epigenetic modifications in plants: an evolutionary perspective. Curr Opin Plant Biol. 2011;14(2):179–86.
Fernandez-Calvo P, Chini A, Fernandez-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, Pauwels L, Witters E, Puga MI, Paz-Ares J, Goossens A, Reymond P, De Jaeger G, Solano R. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell. 2011;23(2):701–15.
Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell. 2012;24(8):3333–48.
Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12(4):599–609.
Fourrier N, Bedard J, Lopez-Juez E, Barbrook A, Bowyer J, Jarvis P, Warren G, Thorlby G. A role for sensitive to freezing2 in protecting chloroplasts against freeze-induced damage in Arabidopsis. Plant J. 2008;55(5):734–45.
Fowler SG, Cook D, Thomashow MF. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 2005;137(3):961–8.
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9(4):436–42.
Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17(12):3470–88.
Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1988–93.
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12(7):1117–26.
Gong Z, Dong CH, Lee H, Zhu J, Xiong L, Gong D, Stevenson B, Zhu JK. A DEAD Box RNA helicase is essential for mRNA export and important for development and stress responses in Arabidopsis. Plant Cell. 2005;17(1):256–67.
Gong Z, Lee H, Xiong L, Jagendorf A, Stevenson B, Zhu JK. RNA helicase-like protein as an early regulator of transcription factors for plant chilling and freezing tolerance. Proc Natl Acad Sci USA. 2002;99(17):11507–12.
Guan Q, Wu J, Zhang Y, Jiang C, Liu R, Chai C, Zhu J. A DEAD Box RNA helicase is critical for Pre-mRNA splicing, cold-responsive gene regulation, and cold tolerance in Arabidopsis. Plant Cell. 2013;25(1):342–56.
Guan LM, Zhao J, Scandalios JG. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000;22(2):87–95.
Guo L, Yang H, Zhang X, Yang S. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J Exp Bot. 2013;64(6):1755–67.
Gusta LV, Fowler DB, Tyler NJ. The effect of abscisic acid and cytokinins on the cold hardiness of winter wheat. Can J Bot. 1982;60:301–5.
Haake V, Cook D, Riechmann J, Pineda O, Thomashow MF, Zhang JZ. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol. 2002;130(2):639–48.
Harmer SL. The circadian system in higher plants. Annu Rev Plant Biol. 2009;60:357–77.
He Y, Amasino RM. Role of chromatin modification in flowering-time control. Trends Plant Sci. 2005;10(1):30–5.
He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z. DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell. 2012;24(5):1815–33.
Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010;61(6):1041–52.
Holubowicz T, Cummins JN, Forsline PL. Responses of Malus clones to programmed low-temperature stresses in late winter. J Am Soc Hortic Sci. 1982;107:492–6.
Hu Y, Jiang L, Wang F, Yu D. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013;25(8):2907–24.
Huang X, Li J, Bao F, Zhang X, Yang S. A gain-of-function mutation in the Arabidopsis disease resistance gene RPP4 confers sensitivity to low temperature. Plant Physiol. 2010a;154:796–809.
Huang X, Li Y, Zhang X, Zuo J, Yang S. The Arabidopsis LSD1 gene plays an important role in the regulation of low temperature-dependent cell death. New Phytol. 2010b;187(2):301–12.
Hugly S, Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 1992;99(1):197–202.
Hwang I, Sheen J, Muller B. Cytokinin signaling networks. Annu Rev Plant Biol. 2012;63:353–80.
Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280(5360):104–6.
Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA. 2009;106(48):20520–5.
Jeon J, Kim NY, Kim S, Kang NY, Novak O, Ku SJ, Cho C, Lee DJ, Lee EJ, Strnad M, Kim J. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J Biol Chem. 2010;285(30):23371–86.
Jung JH, Park JH, Lee S, To TK, Kim JM, Seki M, Park CM. The cold signaling attenuator HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE1 activates FLOWERING LOCUS C transcription via chromatin remodeling under short-term cold stress in Arabidopsis. Plant Cell. 2013.
Kadlecová Z, Faltus M, Prášil I. Relationship between abscisic acid content, dry weight and freezing tolerance in barley cv. Lunet. J Plant Physiol. 2000;157(3):291–7.
Kang JY, Choi HI, Im MY, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell. 2002;14(2):343–57.
Kendall SL, Hellwege A, Marriot P, Whalley C, Graham IA, Penfield S. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell. 2011;23(7):2568–80.
Kerdnaimongkol K, Woodson WR. Inhibition of catalase by antisense RNA increases susceptibility to oxidative stress and chilling Injury in transgenic tomato plants. J Am Soc Hortic Sci. 1999;124(4):330–6.
Kim KN, Cheong YH, Grant JJ, Pandey GK, Luan S. CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell. 2003;15(2):411–23.
Kim Y, Park S, Gilmour SJ, Thomashow MF. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75(3):364–76.
Kim SY, Zhu T, Sung ZR. Epigenetic regulation of gene programs by EMF1 and EMF2 in Arabidopsis. Plant Physiol. 2010;152(2):516–28.
Kishor PK, Sangam S, Amrutha R, Laxmi PS, Naidu K, Rao K, Rao S, Reddy K, Theriappan P, Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005;88(3):424–38.
Knetsch M, Wang M, Snaar-Jagalska BE, Heimovaara-Dijkstra S. Abscisic acid induces mitogen-activated protein kinase activation in Barley aleurone protoplasts. Plant Cell. 1996;8(6):1061–7.
Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol. 2004;135(3):1710–7.
Kocsy G, Toth B, Berzy T, Szalai G, Jednakovits A, Galiba G. Glutathione reductase activity and chilling tolerance are induced by a hydroxylamine derivative BRX-156 in maize and soybean. Plant Sci. 2001;160(5):943–50.
Kreps JA, Wu Y, Chang H-S, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130(4):2129–41.
Kumar MN, Jane WN, Verslues PE. Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol. 2013;161(2):942–53.
Kurup S, Jones HD, Holdsworth MJ. Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J. 2000;21(2):143–55.
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22(11):2623–33.
Lang V, Mantyla E, Welin B, Sundberg B, Palva ET. Alterations in water status, endogenous abscisic acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol. 1994;104(4):1341–9.
Lee BH, Henderson DA, Zhu J-K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell. 2005;17(11):3155–75.
Lee SJ, Kang JY, Park HJ, Kim MD, Bae MS, Choi HI, Kim SY. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol. 2010;153(2):716–27.
Lee BH, Kapoor A, Zhu J, Zhu JK. STABILIZED1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell. 2006;18(7):1736–49.
Lee BH, Lee H, Xiong L, Zhu JK. A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell. 2002;14(6):1235–51.
Lee CM, Thomashow MF. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2012;109(37):15054–9.
Legnaioli T, Cuevas J, Mas P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 2009;28(23):3745–57.
Lim S, Park J, Lee N, Jeong J, Toh S, Watanabe A, Kim J, Kang H, Kim DH, Kawakami N, Choi G. ABA-INSENSITIVE3, ABA-INSENSITIVE5, and DELLAs interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell. 2013;25(12):4863–78.
Liu Y, He J, Chen Z, Ren X, Hong X, Gong Z. ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J. 2010;63(5):749–65.
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10(8):1391–406.
Llorente F, Oliveros JC, Martinez-Zapater JM, Salinas J. A freezing-sensitive mutant of Arabidopsis, frs1, is a new aba3 allele. Planta. 2000;211(5):648–55.
Luo X, Chen Z, Gao J, Gong Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014;79:44–55.
Luo M, Wang YY, Liu X, Yang S, Lu Q, Cui Y, Wu K. HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J Exp Bot. 2012;63(8):3297–306.
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–8.
Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008;56(4):575–89.
Mang HG, Qian W, Zhu Y, Qian J, Kang HG, Klessig DF, Hua J. Abscisic acid deficiency antagonizes high-temperature inhibition of disease resistance through enhancing nuclear accumulation of resistance proteins SNC1 and RPS4 in Arabidopsis. Plant Cell. 2012;24(3):1271–84.
Mantyla E, Lang V, Palva ET. Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 1995;107(1):141–8.
McGlincy NJ, Smith CWJ. Alternative splicing resulting in nonsense-mediated mRNA decay: what is the meaning of nonsense? Trends Biochem Sci. 2008;33(8):385–93.
Meinhard M, Grill E. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 2001;508(3):443–6.
Meinhard M, Rodriguez P, Grill E. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta. 2002;214(5):775–82.
Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18(10):2749–66.
Miquel M, James D Jr, Dooner H, Browse J. Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc Natl Acad Sci USA. 1993;90(13):6208–12.
Miura K, Jin JB, Lee J, Yoo CY, Stirm V, Miura T, Ashworth EN, Bressan RA, Yun DJ, Hasegawa PM. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19(4):1403–14.
Miura K, Lee J, Jin JB, Yoo CY, Miura T, Hasegawa PM. Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc Natl Acad Sci USA. 2009;106(13):5418–23.
Miyazono KI, Miyakawa T, Sawano Y, Kubota K, Kang H-J, Asano A, Miyauchi Y, Takahashi M, Zhi Y, Fujita Y. Structural basis of abscisic acid signalling. Nature. 2009;462(7273):609–14.
Moellering ER, Muthan B, Benning C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science. 2010;330(6001):226–8.
Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak SS, Kim DH, Nam J, Bahk J, Hong JC, Lee SY, Cho MJ, Lim CO, Yun DJ. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA. 2003;100(1):358–63.
Munnik T, Irvine RF, Musgrave A. Phospholipid signalling in plants. Biochim Biophys Acta. 1998;1389(3):222–72.
Nakata M, Mitsuda N, Herde M, Koo AJ, Moreno JE, Suzuki K, Howe GA, Ohme-Takagi M. A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell. 2013;25(5):1641–56.
Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16(6):1365–77.
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmulling T, Tran LS. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 2011;23(6):2169–83.
Nishiyama R, Watanabe Y, Leyva-Gonzalez MA, Ha CV, Fujita Y, Tanaka M, Seki M, Yamaguchi-Shinozaki K, Shinozaki K, Herrera-Estrella L, Tran LS. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc Natl Acad Sci USA. 2013;110(12):4840–5.
Novillo F, Alonso JM, Ecker JR, Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2004;101(11):3985–90.
Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA. 2007;104(52):21002–7.
Olsen AN, Ernst HA, Leggio LL, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005;10(2):79–87.
O’Kane D, Gill V, Boyd P, Burdon R. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta. 1996;198(3):371–7.
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–71.
Peleg Z, Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol. 2011;14(3):290–5.
Raskin I. Role of salicylic acid in plants. Annu Rev Plant Biol. 1992;43(1):439–63.
Razem FA, Baron K, Hill RD. Turning on gibberellin and abscisic acid signaling. Curr Opin Plant Biol. 2006;9(5):454–9.
Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–43.
Robertson FC, Skeffington AW, Gardner MJ, Webb AA. Interactions between circadian and hormonal signalling in plants. Plant Mol Biol. 2009;69(4):419–27.
Scott IM, Clarke SM, Wood JE, Mur LA. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004;135(2):1040–9.
Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009;151(1):275–89.
Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell. 2012;24(6):2578–95.
Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol. 2000;3(3):217–23.
Smoleńska-Sym G, Gawrońska H, Kacperska A. Modifications of abscisic acid level in winter oilseed rape leaves during acclimation of plants to freezing temperatures. Plant Growth Regul. 1995;17(1):61–5.
Song SY, Chen Y, Chen J, Dai XY, Zhang WH. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta. 2011;234(2):331–45.
Suzuki I, Los DA, Murata N. Perception and transduction of low-temperature signals to induce desaturation of fatty acids. Biochem Soc Trans. 2000;28(6):628–30.
Suzuki M, McCarty DR. Functional symmetry of the B3 network controlling seed development. Curr Opin Plant Biol. 2008;11(5):548–53.
Tahtiharju S, Palva T. Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J. 2001;26(4):461–70.
Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K, Nakashima K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol Genet Genomics. 2010;284(3):173–83.
Tallman G. Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? J. Exp. Bot. 2004;55:1963–76.
Tamminen I, Makela P, Heino P, Palva ET. Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana. Plant J. 2001;25(1):1–8.
Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15(1):141–52.
Theocharis A, Clement C, Barka EA. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235:1091–105.
Thomas T, Wareing P, Robinson P. Chemistry and physiology of ‘dormins’ in sycamore: action of the sycamore ‘dormin’ as a gibberellin antagonist. Nature. 1965;205:1270–2.
Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Biol. 1999;50(1):571–99.
Thomashow MF. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 2010;154(2):571–7.
Tian S, Mao X, Zhang H, Chen S, Zhai C, Yang S, Jing R. Cloning and characterization of TaSnRK2.3, a novel SnRK2 gene in common wheat. J Exp Bot. 2013;64(7):2063–80.
To JP, Haberer G, Ferreira FJ, Deruere J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell. 2004;16(3):658–71.
Ton J, Flors V, Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009;14(6):310–7.
Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(51):20623–8.
Tsutsui T, Kato W, Asada Y, Sako K, Sato T, Sonoda Y, Kidokoro S, Yamaguchi-Shinozaki K, Tamaoki M, Arakawa K, Ichikawa T, Nakazawa M, Seki M, Shinozaki K, Matsui M, Ikeda A, Yamaguchi J. DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis. J Plant Res. 2009;122(6):633–43.
Uemura M, Joseph RA, Steponkus PL. Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 1995;109(1):15–30.
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA. 2000;97(21):11632–7.
Verslues PE, Guo Y, Dong CH, Ma W, Zhu JK. Mutation of SAD2, an importin beta-domain protein in Arabidopsis, alters abscisic acid sensitivity. Plant J. 2006;47(5):776–87.
Viswanathan C, Zhu JK. Molecular genetic analysis of cold-regulated gene transcription. Philos Trans R Soc Lond B Biol Sci. 2002;357(1423):877–86.
Wang CY, Adams DO. Chilling-induced ethylene production in cucumbers (Cucumis sativus L.). Plant Physiol. 1982;69:424–7.
Wang Y, Liu C, Li K, Sun F, Hu H, Li X, Zhao Y, Han C, Zhang W, Duan Y, Liu M, Li X. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol Biol. 2007;64(6):633–44.
Wang Y, Zhang Y, Wang Z, Zhang X, Yang S. A missense mutation in CHS1, a TIR-NB protein, induces chilling sensitivity in Arabidopsis. Plant J. 2013;75(4):553–65.
Webb MS, Steponkus PL. Freeze-induced membrane ultrastructural alterations in Rye (Secale cereale) leaves. Plant Physiol. 1993;101(3):955–63.
Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell Environ. 2010;33(4):510–25.
Wohlbach DJ, Quirino BF, Sussman MR. Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell. 2008;20(4):1101–17.
Wolters H, Jurgens G. Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet. 2009;10(5):305–17.
Xiong L, Ishitani M, Lee H, Zhu JK. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell. 2001a;13(9):2063–83.
Xiong L, Lee B, Ishitani M, Lee H, Zhang C, Zhu JK. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001b;15(15):1971–84.
Xiong L, Lee H, Ishitani M, Zhu JK. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem. 2002;277(10):8588–96.
Xiong L, Lee H, Ishitani M, Tanaka Y, Stevenson B, Koiwa H, Bressan RA, Hasegawa PM, Zhu JK. Repression of stress-responsive genes by FIERY2, a novel transcriptional regulator in Arabidopsis. Proc Natl Acad Sci USA. 2002a;99(16):10899–10904.
Yamada T, Kuroda K, Jitsuyama Y, Takezawa D, Arakawa K, Fujikawa S. Roles of the plasma membrane and the cell wall in the responses of plant cells to freezing. Planta. 2002;215(5):770–8.
Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803.
Yamazaki T, Kawamura Y, Minami A, Uemura M. Calcium-dependent freezing tolerance in Arabidopsis involves membrane resealing via synaptotagmin SYT1. Plant Cell. 2008;20(12):3389–404.
Yang MT, Chen SL, Lin CY, Chen YM. Chilling stress suppresses chloroplast development and nuclear gene expression in leaves of mung bean seedlings. Planta. 2005;221(3):374–85.
Yang H, Li Y, Hua J. The C2 domain protein BAP1 negatively regulates defense responses in Arabidopsis. Plant J. 2006;48(2):238–48.
Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. Plant J. 2010;63(2):283–96.
Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, Maruyama-Nakashita A, Kudo T, Shinozaki K, Yoshida S, Nakashita H. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell. 2008;20(6):1678–92.
Zeevaart J, Creelman R. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39(1):439–73.
Zhang Z, Huang R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol. 2010;73(3):241–9.
Zheng Y, Schumaker KS, Guo Y. Sumoylation of transcription factor MYB30 by the small ubiquitin-like modifier E3 ligase SIZ1 mediates abscisic acid response in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2012;109(31):12822–7.
Zhu B, Choi D-W, Fenton R, Close T. Expression of the barley dehydrin multigene family and the development of freezing tolerance. Mol Gen Genet. 2000;264(1–2):145–53.
Zhu J, Dong CH, Zhu JK. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr. Opin. Plant Biol. 2007;10:290–5.
Zhu J, Jeong JC, Zhu Y, Sokolchik I, Miyazaki S, Zhu JK, et al. Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4945–50.
Zhu Y, Yang H, Mang HG, Hua J. Induction of BAP1 by a moderate decrease in temperature is mediated by ICE1 in Arabidopsis. Plant Physiol. 2011;155(1):580–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Shi, Y., Yang, S. (2014). ABA Regulation of the Cold Stress Response in Plants. In: Zhang, DP. (eds) Abscisic Acid: Metabolism, Transport and Signaling. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9424-4_17
Download citation
DOI: https://doi.org/10.1007/978-94-017-9424-4_17
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9423-7
Online ISBN: 978-94-017-9424-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)