Abstract
Fleshy fruits represent an important component of the human diet in many parts of the world and often provide valuable sources of vitamins and antioxidant compounds. Much of their nutritional value results from complex biochemical processes that occur during ripening, including changes in color, texture, flavor and aroma. The regulation of fruit development and subsequent ripening is controlled by several phytohormones, of which perhaps the most characterized has been the gaseous hormone ethylene, which triggers the ripening program in climacteric fruits. However, recent studies have reinforced the importance of abscisic acid (ABA) biosynthesis, catabolism, and signaling in both the development and ripening of fleshy fruits. Here, we provide an overview of these advances and summarize the insights that they have provided into the molecular mechanisms for ABA from production to action, with particular emphasis on strawberry fruit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Ripened fleshy fruits play important roles in our food supply, nutrition, and health. Fleshy fruits are generally divided into climacteric and non-climacteric types on the basis on variations in respiration intensity and ethylene production during ripening. Many of the complex biochemical, molecular and physiological processes that occur during the development and ripening of fleshy fruits have been shown to be regulated by plant hormones. Of these, there has been particular focus on the central role played the gaseous hormone ethylene in controlling ripening in climacteric fruits, such as tomato (Solanum lycopersicum) (Alexander and Grierson 2002; Adams-Phillips et al. 2004; Prasanna et al. 2007; Gapper et al. 2013; Seymour et al. 2013; Perotti et al. 2014). However, there has been a recent increase in the number of studies that have addressed the roles of other phytohormones in fruit biology, as well as the extent and nature of hormonal cross-talk. This is exemplified by studies of abscisic acid (ABA), which has been demonstrated to play an important role in regulating the ripening of non-climacteric and climacteric fleshy fruits (Zhang et al. 2009; Gambetta et al. 2010; Jia et al. 2011; Chai et al. 2011; Akagi et al. 2012; Sun et al. 2012a, b; Romero et al. 2012; Li et al. 2013; Nicolas et al. 2014). Indeed, it has been proposed that, in addition to ethylene, ABA is a fundamentally important regulator of fruit maturation and senescence (Zhang et al. 2009).
It is well established that ABA is extremely important in the adaptation of plants to adverse environmental conditions, as well as regulating various aspects of plant growth and development, such as seed/bud dormancy and growth in both the seedling, and root (Leung and Giraudat 1998; Finkelstein et al. 2002; Himmelbach et al. 2003; Hirayama and Shinozaki 2007; Gapper et al. 2013; Seymour et al. 2013; Perotti et al. 2014). Moreover, much is now known about the molecular processes associated with ABA metabolism, signaling and downstream responses. ABA receptors, which were sought for by scientists for several decades, have now been identified (Fujii et al. 2009; Ma et al. 2009; Miyazono et al. 2009; Melcher et al. 2009; Nishimura et al. 2009; Santiago et al. 2009; Shang et al. 2010), and studies using the experimental model plant Arabidopsis thaliana have led to the discovery of two core ABA signaling pathways: ABA-PYR1-PP2C-SnRK2 (Fujii et al. 2009) and ABA-ABAR-WRKY40-ABI5 (Shang et al. 2010). In addition to these breakthroughs in elucidating ABA perception and signal transduction, much progress has also been made toward understanding ABA signaling specifically in fleshy fruits (Zhang et al. 2009; Gambetta et al. 2010; Jia et al. 2011; Chai et al. 2011; Akagi et al. 2012; Sun et al. 2012a, b; Romero et al. 2012; Li et al. 2013; Nicolas et al. 2014). In this review, we summarize some current ideas and observations regarding the roles of ABA in the regulation of fleshy fruit development and ripening and, where appropriate, recent insights into the underlying molecular mechanisms.
14.2 Effects of ABA on Fleshy Fruit Development and Ripening
Earlier studies of the significance of ABA in fleshy fruit development and ripening primarily targeted the berries of grape (Vitis vinifera), a non-climacteric fruit, where evidence was uncovered of a relationship between ABA and the veraison stage of development, which is marked by a berry color change at the onset of ripening (Coombe and Hale 1973; Coombe 1976; Inaba et al. 1976; Scienza et al. 1978; Palejwala et al. 1985; Cawthon and Morris 1982; Davies et al. 1997). Endogenous ABA levels are minimal 1 week prior to veraison and the application of exogenous ABA to grape berries can markedly accelerate the ripening process, including the rapid accumulation of sugars and anthocyanins (Coombe and Hale 1973; Inaba et al. 1976; Palejwala et al. 1985; Kataoka et al. 1982; Matsushima et al. 1989; Jeong et al. 2004). It is now widely accepted that ABA is a key factor in the regulation of diverse events during grape berry ripening including coloration, sugar accumulation, acid decline and flesh softening (Yu et al. 2006; Wheeler et al. 2009; Gambetta et al. 2010; Koyama et al. 2010; Giribaldi et al. 2010; Gagné et al. 2011; Nicolas et al. 2014).
In addition to studies in grape berries, considerable progress has been made recently in understanding the role of ABA in other non-climacteric fruits and environmental factors that alter ABA levels. For example, water deficit treated strawberry (Fragaria x ananassa) fruits have higher ABA levels, as well as reduced berry size, altered sugar/acid ratios, which affect the taste and levels of health-related compounds (phenolic and antioxidant compounds, Terry et al. 2007). Indeed, there is considerable physiological and molecular evidence to suggest an important role for ABA in the regulation of strawberry fruit ripening: (1) exogenous application of ABA or dimethyl sulfoxide (DMSO, an ABA biosynthesis accelerator) significantly promotes fruit ripening, whereas fluridone (an ABA biosynthesis inhibitor) markedly inhibits fruit ripening; (2) silencing of FaNCED1, a key ABA biosynthesis gene in strawberry fruit, results in a reduction in endogenous ABA levels and delayed ripening; (3) exogenous ABA rescues the uncolored phenotype of FaNCED1-RNAi fruits, in which FaNCED1 expression has been suppressed using an RNA interference (RNAi) strategy (Jia et al. 2011). The importance of ABA in strawberry fruit development was also confirmed by silencing of the β-glucosidase gene FaBG3, which is involved in ABA synthesis, since the encoded protein hydrolyzes an ABA glucose ester to release active ABA (Li et al. 2013). These findings together with other studies indicate that ABA as a key regulator of strawberry fruit ripening (Kano and Asahira 1981; Manning 1994; Perkins-Veazie 1995; Jiang and Joyce 2003; Terry et al. 2007; Jia et al. 2011; Li et al. 2013). However, it should be noted that in addition to its role in grape berry and strawberry ripening, ABA has been found to influence diverse processes of characteristics in other fleshy fruit species, including the development of astringency in persimmon (Diospyros kaki) fruit (Akagi et al. 2012), citrus peel development (Romero et al. 2012), and cherry (Prunus species) fruit maturation (Ren et al. 2010).
In addition to non-climacteric fruits, it is also now clear that ABA can regulate fruit development and ripening in climacteric fruits. For example, it was reported that application of ABA to detached tomato fruits substantially advances the onset of ripening (Mizrahi et al. 1975), which raises that possibility that ABA may also act to promote tomato fruit ripening in vivo. A role for ABA regulating tomato fruit development was indicated by studies of an ABA deficient tomato mutant (high-pigment 3) that accumulates 30 % more carotenoids, has an increased number of fruit plastids and a higher lycopene content (Galpaz et al. 2008) than wild type frutis. In addition, the results of targeted studies with transgenic tomato suggest that ABA regulates the degree of pigmentation, carotenoid composition, and fruit firmness during ripening (Sun et al. 2012a, b). Furthermore, the ABA-deficient notabilis/flacca (not/flc) tomato double mutant has been used to demonstrate that ABA stimulates cell enlargement and increases fruit size (Nitsch et al. 2012).
In addition, a recent report revealed that ABA may also be involved in suberization-based wound healing processes in tomato fruit stem scar tissue (Leide et al. 2011). Other studies have shown that ABA is involved in the regulation of avocado fruit growth (Cowan et al. 1997) and peach fruit sugar accumulation (Kobashi et al. 2001), suggesting that ABA is likely a common signaling molecule that modulates many processes in both climacteric and non-climacteric fleshy fruits.
14.3 ABA Metabolism in Fleshy Fruits
In land plants, ABA levels in a specific tissue are determined by the balance between ABA biosynthesis, catabolism and conjugation. Biosynthesis results from the oxidative cleavage of carotenoids, where the activity of the enzyme 9-cis-epoxycarotenoid dioxygenase (NCED) represents a major rate limiting step. Conversely, ABA catabolism results mainly from 8′-hydroxylation reactions catalyzed by 8′-hydroxylase enzymes, which are encoded by a small family of P450 monooxygenase CYP707A genes (Zeevaart et al. 1989; Nambara and Marion-Poll 2005). In addition, ABA conjugation can result from the action of ABA-induced glucosyltransferases (GTs), which convert free ABA to an ABA-glucosylester (ABA-GE; Xu et al. 2002). Earlier reports suggested that ABA-GE represents an inactive end product of ABA catabolism (Lehmann and Schutte 1984; Zeevaart 1999). However, this idea is called into question as a result of the identification of Arabidopsis beta-glucosidase1 (AtBG1), an enzyme that catalyzes the conversion of ABA-GE back into the pool of biologically active ABA, allowing a rapid change in ABA levels (Lee et al. 2006). A contemporary model might therefore involve the control of plant cellular ABA levels by NCEDs and CYPs in a synthesis/degradation pathway or/and by GTs and BGs in a conjugation/dissociation pathway. Potentially, the one-step pathway catalyzed by GTs or BGs allows rapid dynamic changes in ABA levels to meet developmental and adaptive requirements.
This foundation of knowledge resulting from studies of A. thaliana and model crop species has been valuable in elucidating ABA biosynthesis and catabolism in fleshy fruits and in identifying conserved mechanisms of ABA metabolism (Li et al. 2011, 2013; Jia et al. 2011; Sun et al. 2012b). For example, down regulation of FaNCED1 expression in strawberry using virus induced gene silencing (VIGS) resulted in a significant decrease in ABA levels, as well as uncolored fruits, demonstrating that FaNCED1 is a key gene for ABA biosynthesis in fruits and plays an important role in fruit ripening (Jia et al. 2011). Moreover, Li et al. (2013) reported the importance of BG enzymes in strawberry, showing that FaBG3-RNAi-treated fruit has reduced ABA levels and fruit color development and softening is inhibited, indicating a key role of β-glucosidases in regulation of ripening (Li et al. 2013). Interestingly, similar results were generated through studies of tomato fruit, where ABA levels are regulated by SlNCED1 and SlCYP707A1 (Nitsch et al. 2009; Zhang et al. 2009). It was observed that suppressing the expression of the SlNCED1using RNAi lead to a 20–50 % decrease in both ABA accumulation and SlNCED1 transcript compared with control fruit, and prolonged fruit shelf life by up to 3 weeks (Sun et al. 2012b).
In addition to these important breakthroughs made through observations of strawberry and tomato fruits (Li et al. 2011, 2013; Jia et al. 2011; Sun et al. 2012b), similar studies have been reported of ABA biosynthesis and catabolism in other fruits. Water deficit results in a nearly two-fold increase in ABA concentration in berries of a red-wine grape Cabernet Sauvignon, the same stress induces a decrease in ABA levels in the Chardonnay white-wine grape at veraison and shortly thereafter (Deluc et al. 2009). The higher transcript levels of VvNCED1 and VvBG1 together with lower transcript abundance of VvCYP1 and VvGT contribute to a continuous accumulation of ABA, especially VvBG1 transcripts increased more rapidly than that of VvNCED1 during berry red-coloring. An incubation test in vitro indicated that the Escherichia coli-expressed VvBG1 protein had high enzymatic activity, validating that β-glucosidase (VvBG1) has high enzymatic activity and might play a role in berry ripening (Sun et al. 2014). In avocado fruits, PaNCED1 and PaNCED3, but not PaNCED2, were strongly induced as the fruit ripened, and a correlation with water stress was also established, since PaNCED1 was induced under these conditions. Furthermore, recombinant PaNCED1 and PaNCED3, but not PaNCED2 could cleave 9-cis-xanthophylls into xanthoxin and C (25)-apocarotenoids in vitro, indicating that ABA biosynthesis in avocado is regulated at the level of carotenoid cleavage (Chernys and Zeevaart 2000). In citrus fruit, the accumulation of both ABA and ABA-conjugates occurs during fruit maturation (Harris et al. 1986). In citrus peels, high levels of ABA are detected and CsNCED1 is likely to play a primary role in ABA biosynthesis (Rodrigo et al. 2014). Moreover, in the flavedo and juice sacs, expression of CitNCED2 and/or CitNCED3 increases coincident with a substantial accumulation of ABA (Kato et al. 2006). In watermelon fruit, the expression of ClBG1, ClNCED4 and ClCYP707A1 has been shown to increase rapidly along with ripening, and reaches the highest levels at the stage of harvest. This trend was show to be consistent with ABA accumulation, which was also shown to be modulated by the described dynamic balance between biosynthesis and catabolic processes via these genes, indicating the importance of glucosidase genes in the ripening process (Li et al. 2012). Similarly, it was shown that the endogenous ABA content of sweet cherry fruit is regulated by PacNCED1, PacCYP707A1 and PacCYP707A3 transcripts during maturation (Ren et al. 2010), and in apple fruits, the major portion of the ABA has been shown to pool is conjugated to β-D-glucopyranosyl abscisate (ABA-GE) which leads to markedly lower ABA levels (Rock and Zeevaart 1990). The peach PpNCED1 gene has been shown to initiate ABA biosynthesis at the onset of fruit ripening (Zhang et al. 2009). These reports again reinforce the idea that ABA accumulation is involved in the regulation of ripeness and senescence in a broad range of fleshy fruits.
In all, the detection of key ABA biosynthesis and catabolism genes in fleshy fruit tissues suggests that although ABA could be transported from the leaves to the fruits via the phloem (Shiozaki et al. 1999), it can also be synthesized in fleshy fruits in situ. ABA levels in fleshy fruits are regulated mainly by NCED1 and CYP707A1, the accumulation of ABA-GE catalyzed by GT enzymes, contributes to a rapid response by one-step dissociation of ABA-EG catalyzed by BG in response to developmental and stress cues. During later stages of fruit development, beta-glucosidases (BGs) appear to play a more important role in ripening.
14.4 ABA Signaling in Fleshy Fruits
The presence of both transmembrane and cytosolic ABA receptors in plants was suggested by early studies using experimental model plants (Hornberg and Weiler 1984; Allan et al. 1994; Schwarz and Schroeder 1998), such as the identification of multiple receptors, secondary messengers, protein kinases and phospholipases, transcription factors, cis-elements, and target genes in A. thaliana. Known ABA receptors include the plasma membrane localized GTG1/GTG2 (Pandey et al. 2009), a class of cytosolic PYR/PYL/RCAR (Park et al. 2009; Ma et al. 2009) proteins and a plastid/chloroplast magnesium-chelatase H subunit ABAR/CHLH (Shen et al. 2006; Wu et al. 2009; Shang et al. 2010). Signalling components include G proteins, phospholipases, and various protein kinases, such as receptor-like kinases, SNF1-related protein kinases (SnRKs), calcium dependent protein kinases (CDPKs), calcineurin B-like protein kinases (CIPKs), and mitogen-activated protein kinases (MAPKs).Protein phosphatases of type-2C/A protein phosphatase (PP2C/A), various classes of transcription factors, including MYBs/MYCs, B3 domain transcription factors, APETALA2 domain transcription factors, bZIP domain transcription factor, and WRKY transcription factors, are also among important regulators of ABA levels (Nambara and Marion-Poll 2005; Hirayama and Shinozaki 2007; Verslues and Zhu 2007; Wang and Zhang 2008, and Cutler et al. 2010). To date, two core ABA signalling pathways in A. thaliana have been proposed: the ‘ABA-PYR/PYL/RCAR-PP2C-SnRK2’ pathway (Fujii et al. 2009), and the‘ABA-ABAR-WRKY40-ABI5’pathway (Shang et al. 2010). At the mechanistic level, a detailed gate latch-lock mechanism has been identified, in which ABA promotes the interaction of PYR1 and PP2C, resulting in PP2C inhibition and SnRK2 activation. This transduces ABA signals through phosphorylation of downstream factors such as AREB/ABF, ion channels, and NADPH oxidases (Park et al. 2009; Ma et al. 2009; Fujii et al. 2009).
14.4.1 ABA Perception in Flesh Fruits
In addition to pioneering studies in model experimental plants, there has also been much interest in understanding the molecular basis of ABA detection by specific receptors and subsequent downstream signalling in fleshy fruits. In studies of grape berry microsomes, a high proportion of specific ABA receptors (which had a measured Kd value of 17.5–50 nM) were shown to be localized mainly in the endomembranes and not in the plasma membrane or in the cytoplasm (Zhang et al. 1999). In contrast, ABA binding activity was scarcely detectable in microsomes derived from the flesh of developing apple fruit, but high ABA binding activity was detected in the cytosolic fraction with both high affinity (Kd 2.3 nM) and low affinity sites (Kd 58.8 nM; Zhang et al. 2001). Progress has also been made in understanding ABA perception in strawberry. Using a newly established tobacco rattle virus (TRV)-induced gene silencing technique in strawberry fruit, down-regulation of the expression levels of FaPYR1 or FaCHLH/ABAR, homologous to the A. thaliana ABA receptor genes, was shown to inhibit ripening (Jia et al. 2011; Chai et al. 2011). On the basis of these results, a model was proposed for ABA perception and signalling transduction during non-climacteric fruit ripening (Li et al. 2011). More recently, the Type 2C protein phosphatase FaABI1 was demonstrated to be a negative regulator of strawberry fruit ripening (Jia et al. 2013) and it was suggested that the ‘ABA-FaPYR1-FaPP2C-FaSnRK2’ signalling pathway, involving the positive regulatory effect of FaPYR1 in combination with the negative regulatory effect of FaABI1, represents a core mechanism by which ABA regulates strawberry fruit ripening (Jia et al. 2013).
Recently, the ABA perception mechanisms and core signalling systems in other fruit tree species have also been studied. The V. vinifera proteins VvRCAR6 and VvRCAR5 may be the major receptors involved in ABA perception and signalling in grape, mainly through VvPP2C4 (Boneh et al. 2012), while VvPYL1 may be an ABA receptor that modulates ABA signalling by inhibiting type PP2C activity (Li et al. 2012). In tomato, SlPYL1 and SlPYL2 are expressed at high levels throughout fruit development and ripening, and SlPP2C1 and SlPP2C5 are both strongly expressed during the breaker stage, while SlSnRK2.2, SlSnRK2.3, SlSnRK2.4, and SlSnRK2C are highly expressed at all maturity stages (Sun et al. 2011).
14.4.2 Downstream Components of ABA Signaling in Fleshy Fruits
14.4.2.1 Protein Kinases and Phosphatases
Plant protein kinases and phosphatases include CDPKs, SNF1-related kinases (SnRKs), MAPKs, a receptor-type kinase (RPK1), and protein phosphatase PP2Cs (Hirayama and Shinozaki 2007). As described above, reversible protein phosphorylation has been demonstrated to play key roles in ABA signal transduction through the protein kinase SnRK2 and the phosphataseABI1 (Fujii et al. 2009).
In grape berries, earlier studies revealed high activities of both the calcium-dependent protein kinase (CDPK) and the mitogen-activated protein kinase (MAPK) in the lag phase of fruit growth, prior to the ripening stage (Shen et al. 2004). The subsequent experiments lead to the identification and purification of a 58-kD ABA-stimulated CDPK, ACPK1, which localizes to both the plasma membranes and chloroplasts/plastids. The fact that ACPK1 is expressed in a developmental stage-dependent manner, and that it positively regulates the plasma membrane H+-ATPase in vitro, together with the observation that ABA stimulates ACPK1 in a dose-dependent manner, suggest that ACPK1 may be involved in the ABA-signalling pathway during berry development (Yu et al. 2006). Similar studies found that an apple MAPK signalling cascade, MdMKK1-MdMPK1, is involved in ABA signalling, and that MdMPK1 phosphorylates the ABI5 protein through a unique residue, Ser314, making ABI5 a potential direct downstream component of MAPK in ABA signalling (Wang et al. 2010). In grape berries, a glycogen synthase kinase 3 protein kinase, VvSK1, is strongly expressed when the berries accumulate glucose, fructose, and ABA, and overexpression of VvSK1 results in an upregulation of the transcripts of four monosaccharide transporters (VvHT3, VvHT4, VvHT5, and VvHT6), an up to 5-fold increase in the rate of glucose uptake, and a doubling in the amount of glucose and sucrose accumulation,. This indicates that VvSK1 controls sugar uptake and accumulation by a sugar/ABA-inducible protein kinase (Lecourieux et al. 2010). More importantly, the core phosphorylation signaling of PP2C-SnRK2 has been established in response to ABA during strawberry fruit ripening (Jia et al. 2013).
14.4.2.2 Transcription Factor, Cis-Elements and Target Genes
Many transcription factors, cis-elements and target genes related to ABA signaling have been identified in developing fleshy fruit. For example, in persimmon fruit, DkbZIP5 was shown to recognize ABA-responsive elements in the promoter region of DkMyb4 and to act as a direct regulator of DkMyb4 in an ABA-dependent manner. It is reported to suggest that ABA signals may be involved in proanthocyanidin (PA) biosynthesis via DkMyb4 activation by DkbZIP5 (Akagi et al. 2012). In tomato fruit, the higher hexose and acid concentration in SlAREB1-overexpressing lines can be correlated with an increased expression of genes encoding a vacuolar invertase and a sucrose synthase, suggesting that an AREB-mediated ABA signal affects the metabolism of acid and sugar in tomato fruit development (Bastas et al. 2011). ABA can also affect cell wall catabolism during tomato fruit ripening via down-regulation of the expression of major catabolic genes (SlPG, SlPME, SlTBG, SlXET, SlCels, and SlExp; Sun et al. 2012b), but an ABA biosynthesis inhibitor can increase transcript levels corresponding to enzyme activities associated with other aspects of fruit ripening, as is the case with an alcohol dehydrogenase from mango (Singh et al. 2010) and an indole-3-acetic acid-amido synthetase, DlGH3.2, in longan (Kuang et al. 2011). It has been proposed that the expression of FaASR, a member of the ABA-, stress- and ripening-induced (ASR) set of genes might partially contribute to the acceleration of the fruit ripening (Chen et al. 2011). Alterations in strawberry FaABI1 expression were reported to regulate the transcripts of a set of both ABA-responsive and ripening-related genes, including ABI3, ABI4, ABI5, SnRK2, ABRE1, CHS, PG1, PL, CHI, F3H, DFR, ANS, and UFGT (Jia et al. 2013).
Taken together, much progress has been made toward understanding ABA signaling, including the identification of ABA receptors, protein kinases, protein phosphatases, transcription factors, cis-elements, and target genes in fleshy fruit. These include the identification of core signaling components controlling the key links to fleshy fruit development, including strawberry ‘ABA-FaPYR1-FaPP2C-FaSnRK2’, grape ‘ABA-VvACPK1-H+-ATPase’ and ‘ABA-VvSK1-VvHTs’, apple ‘ABA-MdMKK1-MdMPK1-ABI5’, and persimmon ‘ABA- DkbZIP5- DkMyb4-PA’. To date, the target genes include those involved in fruit softening (PG, PL, PME, TBG, XET, Cels, and Exp)-, sugar metabolism (VvHTs, MiADH2)-, pigmentation (PA, CHS, CHI, F3H, DFR, ANS, and UFGT), and ripening (FaASR, DlGH3.2)-related genes.
14.5 Understanding of the Mechanisms for ABA Regulation of Fleshy Fruit Ripening
There is considerable interest at present in the relationship between ABA, ethylene and sugar content in ripening fleshy fruits. Two previous reports suggested that ABA facilitates the initiation and progression of ethylene-mediated ripening events, possibly by enhancing the sensitivity to ethylene (Jiang et al. 2000) or ethylene levels (Riov et al. 1990). In grape berries, sugar and ABA signaling orthologs are activated at the onset of the ripening, including the putative sucrose sensor SUT2, core G-protein signaling components, GPA1 and RGS1, hexose kinases (Hxk), PP2C protein phosphatases, Snf1-related kinases (SnRK), the sugar-related WRKY, ABA-related homeodomain–leucine zipper, as well as homeobox (HB) transcriptions factors, ABRE-binding factor (ABF), and AP2 transcription factors (Gambetta et al. 2010). Conserved changes in the dynamics of metabolic processes during fruit development and ripening across species were found (Klie et al. 2014). During tomato fruit growth, ABA stimulates cell enlargement by suppressing ethylene synthesis (Nitsch et al. 2012; Sun et al. 2012b) and during the ripening, a significant reduction in ABA levels is thought to promote coloring and firmness, resulting in the carbon that normally channels to ABA biosynthesis and catabolism being targeted to compounds upstream of ABA biosynthesis, including lycopene, carotene, and pectin (Sun et al. 2012a, b). Interestingly, in citrus fruit, ABA my induce its own biosynthesis at the transcriptional level and the feedback regulation of ABA has been shown to lead to decreases in carotenoid content in citrus juice sacs in vitro (Zhang et al. 2012). In strawberry fruit, sucrose may serve as a signalling molecule to promote mRNA expression levels of FaNCED1, as well as playing an important role in ABA accumulation and fruit ripening (Jia et al. 2011, 2013).
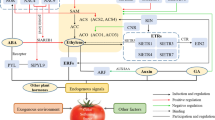
Fleshy fruit can synthesize ABA in response to developmental and environmental cues and the accumulation of ABA in fleshy fruit is controlled by four main enzymes classes that are fundamentally important in ABA biosynthesis and catabolism: NCEDs, CYP707As, GTs, and BGs. In the ripening of fleshy fruit, the interaction between ABA, ethylene and sugars occurs at physiological and molecular levels. The initiation of ABA action is triggered by the perception by its receptors, including PYRs and ABAR, causing signaling to be relayed by reversible protein phosphorylation of protein kinases and phosphatases, including ABI1, SnRK2, ACPK1, SK1, MKK1, and MPK1. The signalling cascade then involves transcription factors and cis-elements, including bZIP5, MYb4, ABI3, ABI4, ABI5, ABRE, triggering ripening-related genes, which are involved in modulating softening, and accumulation of sugars and pigments. To a large extent, the processes of which could be cooperated by the interaction of ABA with sugar and ethylene (Fig. 14.1).
A model for ABA metabolism and signaling in fleshy fruits. In response to developmental and environmental cues, fleshy fruit can synthesize ABA mainly by NCEDs and BGs while catabolize ABA by CYP707As and GTs. ABA levels in fleshy fruits are determined by the balance between ABA biosynthesis and catabolism. One-step dissociation of ABA-EG by BGs may rapidly accumulate ABA. The accumulated ABA in fleshy fruit can be perceived by its receptors, at least including PYR and ABAR. In turn, the initial signals may be relayed by the way of reversible protein phosphorylation, which involves ABI1, SnRK2, ACPK1, MKK1, and MPK1. Subsequently, the cascade signaling of phosphorylation can activate downstream transcript factors and corresponding cis-elements, including bZIP5, MYb4, ABI3, ABI4, ABI5, ABRE, then promote the expression of the ripening-related genes: softening-related genes include PG, PL, PME, TBG, XET, Cels, and Exp; sugar metabolism-related genes include SUT1, HTs, ADH2, and H +-ATPase, and coloring-related genes include CHS, CHI, F3H, DFR, ANS, and UFGT. Finally these expressed genes accelerate fleshy fruit ripening, to a large extent, the processes of which could be cooperated by the interaction of ABA with sugar and ethylene. Abbreviations: NCDEs, 9-cis-epoxycarotenoid dioxygenases; CYP707As, P450 monooxygenases; GTs, glucosyltransferases; BGs, beta-glucosidases; ABA-GE, ABA-glucosylester; PYR, pyrabactin resistance; ABAR, ABA receptor; SnRK2: sucrose non-fermenting 1-related protein kinase 2; ACPK1, ABA-stimulated calcium-dependent protein kinase1; MKK1, mitogen-activated protein kinase kinase 1; MPK1, mitogen-activated protein kinase 1; bZIP5, basic leucine-zipper 5; MYB4, dehydration responsive element-related transcription factor 4; ABI3-5, abscisic acid insensitive 3-5; ABRE, ABA response element; PG, polygalacturonase gene; PL, pectate lyase gene; PME, pectin methyl esterase gene; TBG, β-galactosidase gene; XET, xyloglucan endotransglycosylase gene; Cels, endo-1,4-β-cellulose gene; Exp, expansin gene; SUT1, sucrose transporter 1 gene; HTs, monosaccharide transporter genes; ADH2, alcohol dehydrogenase 2 gene; H +-ATPase, proton-pump ATPase gene; CHS, chalcone synthase gene; CHI, chalcone isomerase gene; F3H, flavanone 3-hydroxylase gene; DFR, dihydroflavonol 4-reductase gene; ANS, anthocyanidin synthase gene; UFGT, UDP-glucose:flavonoid 3-O-glucosyltransferase gene
14.6 Conclusion and Perspective
Fleshy fruit ripening involves marked physiological and metabolic changes in sugar metabolism, softening, and color development, and it is clear that these processes are substantially regulated by plant hormones. While the role of ethylene in climacteric fruit ripening has been definitively established at the molecular level for many years, it is now emerging that ABA is not only involved in the regulation of climacteric fruit development and ripening, but also in the developmental processes of non-climacteric fruit. Much progress has been made toward understanding the molecular mechanisms involving ABA biosynthesis and signaling in strawberry, a model plant for non-climacteric fruit research. However, given the complexity of non-climacteric fruit ripening, the studies concerning the interaction of ABA with ethylene, of ABA with sugar levels, and of sugars with ethylene, is an important area of future research work.
References
Adams-Phillips L, Barry C, Giovannoni J. Signal transduction systems regulating fruit ripening. Trends Plant Sci. 2004;9:331–8.
Akagi T, Katayama-Ikegami A, Kobayashi S, Sato A, Kono A, Yonemori K. Seasonal abscisic acid signal and a basic leucine zipper transcription factor, DkbZIP5, regulate proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2012;158:1089–102.
Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–55.
Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell. 1994;6:1319–28.
Bastas A, Lpez-Climent M, Valcrcel M, Rosello S, Gmez-Cadenas A, Casaretto JA. Modulation of organic acids and sugar content in tomato fruits by an abscisic acid-regulated transcription factor. Physiol Plant. 2011;141:215–26.
Boneh U, Biton I, Zheng C, Schwartz A, Ben-Ari G. Characterization of potential ABA receptors in Vitis vinifera. Plant Cell Rep. 2012;31:311–21.
Cawthon D, Morris J. Relationship of number and maturity to berry development, fruit maturation, hormonal changes and uneven ripening of “Concord” (Vitis labrusca L.) grapes. J Am Soc Hort Sci. 1982;107:1097–104.
Chai YM, Jia HF, Li CL, Dong QH, Shen YY. FaPYR1 is involved in strawberry fruit ripening. J Exp Bot. 2011;62:5079–89.
Chen JY, Liu DJ, Jiang YM, Zhao ML, Shan W, Kuang JF, Lu WJ. Molecular characterization of a strawberry FaASR gene in relation to fruit ripening. PLoS ONE. 2011;6:e24649.
Chernys JT, Zeevaart JA. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000;124:343–53.
Coombe B. The development of fleshy fruits. Annu Rev Plant Physiol. 1976;27:507–28.
Coombe BG, Hale CR. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiol. 1973;51:629–34.
Cowan AK, Moore-Gordon CS, Bertling I, Wolstenholme BN. Metabolic control of avocado fruit growth (isoprenoid growth regulators and the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme a reductase). Plant Physiol. 1997;114:511–8.
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–79.
Davies C, Boss P, Robinson S. Treatment of grape berries, a nonclimateric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997;115:1155–61.
Deluc LG, Quilici DR, Decendit A, Grimplet J, Wheatley MD, Schlauch KA, Merillon JM, Cushman JC, Cramer GR. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of cabernet sauvignon and chardonnay. BMC Genom. 2009;10:212.
Finkelstein R, Gampala S, Rock C. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:S15–45.
Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Aatoni R, Park SY, Cutler SR, Sheen J, Rotrigues PL, Zhu JK. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462:660–4.
Gagné S, Cluzet S, Merillon JM, Gény L. ABA initiates anthocyanin production in grape cell cultures. J Plant Growth Regul. 2011;30:1–10.
Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008;53:717–30.
Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta. 2010;232:219–34.
Gapper NE, McQuinn RP, Giovannoni JJ. Molecular and genetic regulation of fruit ripening. Plant Mol Biol. 2013;82:575–91.
Giribaldi M, Geny L, Delrot S, Schubert A. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. J Exp Bot. 2010;61:2447–58.
Harris MJ, Dugger WM. The occurrence of abscisic acid and abscisyl-beta-d-glucopyranoside in developing and mature citrus fruit as determined by enzyme immunoassay. Plant Physiol. 1986;82:339–45.
Himmelbach A, Yang Y, Grill E. Relay and control of abscisic acid signaling. Curr Opin Plant Biol. 2003;6:470–9.
Hirayama T, Shinozaki K. Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 2007;12:343–51.
HornbergC Weiler EW. High-affinity binding sites for abscisic acid on the plasmalemma of Vicia faba guard cells. Nature. 1984;310:321–4.
Inaba A, Ishida M, Sobajima Y. Changes in endogenous hormone concentrations during berry development in relation to the ripening of Delaware grapes. J Jap Soc Hort Sci. 1976;45:245–62.
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011;157:188–99.
Jia HF, Lu D, Sun JH, Li CL, Xing Y, Qin L, Shen YY. Type 2C protein phosphatase ABI1 is a negative regulator of strawberry fruit ripening. J Exp Bot. 2013;64:1677–87.
Jiang Y, Joyce DC. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003;39:171–4.
Jiang Y, Joyce DC, Macnish AJ. Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J Plant Growth Regul. 2000;19:106–11.
Jeong S, Goto-Yamamoto N, Kobayashi S, Esaka M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004;167:247–52.
Kano Y, Asahira T. Roles of cytokinin and abscisic acid in the maturing of strawberry fruits. J Jap Soc Hort Sci. 1981;50:31–6.
Kataoka I, Sugiura A, Utsunomiya N, Tomana T. Effect of abscisic acid and defoliation on anthocyanin accumulation in Kyoho grapes. Vitis. 1982;21:325–32.
Kato M, Matsumoto H, Ikoma Y, Okuda H, Yano M. The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit. J Exp Bot. 2006;57:2153–64.
Klie S, Osorio S, Tohge T, Drincovich MF, Fait A, Giovannoni JJ, Fernie AR, Nikoloski Z. Conserved changes in the dynamics of metabolic processes during fruit development and ripening across species. Plant Physiol. 2014;164:55–68.
Kobashi K, Sugaya S, Gemma H, Iwahori S. Effect of abscisic acid (ABA) on sugar accumulation in the flesh tissue of peach fruit at the start of the maturation stage. Plant Growth Regul. 2001;35:215–23.
Koyama K, Sadamatsu K, Goto-Yamamoto N. Abscisic acid stimulated ripening and gene expression in berry skins of the cabernet sauvignon grape. Funct Integr Genom. 2010;10:367–81.
Kuang JF, Zhang Y, Chen JY, Chen QJ, Jiang YM, Lin HT, Xu SJ, Lu WJ. Two GH3 genes from longan are differentially regulated during fruit growth and development. Gene. 2011;485:1–6.
Lecourieux F, Lecourieux D, Vignault C, Delrot S. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in grapevine cells. Plant Physiol. 2010;152:1096–106.
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang J. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126:1109–20.
Lehmamm H, Schutte HR. Abscisic-acid metabolism in intact wheat seedlings under normal and stress conditions. J Plant Physiol. 1984;117:201–9.
Leide J, Hildebrandt U, Hartung W, Riederer M, Vogg G. Abscisic acid mediates the formation of a suberized stem scar tissue in tomato fruits. New Phytol. 2011;194:402–15.
Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222.
Li CL, Jia HF, Chai YM, Shen YY. Abscisic acid perception and signaling transduction in strawberry: a model for non-climacteric fruit ripening. Plant Signal & Behav. 2011;6:1950–3.
Li Q, Li P, Sun L, Wang Y, Ji K, Sun Y, Dai S, Chen P, Duan C, Leng P. Expression analysis of glucosidase genes that regulate abscisic acid homeostasis during watermelon (Citrullus lanatus) development and under stress conditions. J Plant Physiol. 2012;169:78–85.
Li Q, Ji K, Sun Y, Luo H, Wang H, Leng P. The role of FaBG3 in fruit ripening and B. cinerea fungal infection of strawberry. Plant J. 2013;76:24–35.
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–8.
Manning K. Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta. 1994;94:62–8.
Matsushima J, Hiratsuka S, Taniguchi N, WSada R, Suzaki N. Anthocyanin accumulation and sugar content in the skin of grape cultivar “Olympia” treated with ABA. J Jap Soc Hort Sci. 1989;58:551–5.
Melcher K, Ng LM, Zhou XE, Soon FF, Xu Y, Suino-Powell KM, Park SY, Weiner JJ, Fujii H, Chinnusamy V, Kovach A, Li J, Wang Y, Li J, Peterson FC, Jensen DR, Yong EL, Volkman BF, Cutler SR, Zhu JK, Xu HE. A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature. 2009;462:602–8.
Miyazono K, Miyakawa T, Sawano Y, Kubota K, Kang HJ, Asano A, Miyauchi Y, Takahashi M, Zhi Y, Fujita Y, Yoshida T, Kodaira KS, Yamaguchi-Shinozaki K, Tanokura M. Structural basis of abscisic acid signalling. Nature. 2009;462:609–14.
Mizrahi Y, Dostal HC, McGlasson WB, Cherry JH. Effects of abscisic acid and benzyladenine on fruits of normal and rin mutant tomatoes. Plant Physiol. 1975;56:544–6.
Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–85.
Nicolas P, Lecourieux D, Kappel C, Cluzet S, Cramer GR, Delrot S, Lecourieux F. The bZIP transcription factor VvABF2 is an important transcriptional regulator of ABA-dependent grape berry ripening processes. Plant Physiol. 2014;164:365–83.
Nishimura N, Hitomi K, Arvai AS, Rambo RP, Hitomi C, Cutler SR, Schroeder JI, Getzoff ED. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science. 2009;326:1373–9.
Nitsch LM, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen WH. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1. Planta. 2009;229:1335–46.
Nitsch L, Kohlen W, Oplaat C, Charnikhova T, Cristescu S, Michieli P, Wolters-Arts M, Bouwmeester H, Mariani C, Vriezen WH, Rieu I. ABA-deficiency results in reduced plant and fruit size in tomato. J Plant Physiol. 2012;169:878–83.
Palejwala V, Himanshu P, Modi P, Modi V. The role of abscisic acid in the ripening of grapes. Physiol Plant. 1985;65:498–502.
Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–48.
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–71.
Perkins-Veazie P. Growth and ripening of strawberry fruit. Hort Rev. 1995;17:267–97.
Perotti VE, Moreno AS, Podestá FE. Physiological aspects of fruit ripening: the mitochondrial connection. Mitochondrion. 2014;17C:1–6.
Prasanna V, Prabha TN, Tharanathan RN. Fruit ripening phenomena–an overview. Crit Rev Food Sci Nutr. 2007;47:1–19.
Ren J, Sun L, Wu J, Zhao S, Wang C, Wang Y, Ji K, Leng P. Cloning and expression analysis of cDNAs for ABA 8′-hydroxylase during sweet cherry fruit maturation and under stress conditions. J Plant Physiol. 2010;67:1486–93.
Riov J, Dagan E, Goren R, Yang SF. Characterization of abscisic acid-induced ethylene production in citrus leaf and tomato fruit tissues. Plant Physiol. 1990;92:48–53.
Rock CD, Zeevaart JA. Abscisic (ABA)-Aldehyde is a precursor to, and 1′, 4′-trans-ABA-Diol a catabolite of, ABA in apple. Plant Physiol. 1990;93:915–23.
Rodrigo MJ, Alquezar B, Zacarias L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot. 2014;57:633–43.
Romero P, Rodrigo MJ, Alfrez F, Ballester AR, Gonzlez-Candelas L, Zacaras L, Lafuente MT. Unravelling molecular responses to moderate dehydration in harvested fruit of sweet orange (Citrus sinensis L. Osbeck) using a fruit-specific ABA-deficient mutant. J Exp Bot. 2012;63:2753–67.
Santiago J, Dupeux F, Round A, Antoni R, Park SY, Jamin M, Cutler SR, Rodriguez PL, Márquez JA. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature. 2009;462:665–8.
SchwarzM SchroederJI. Abscisic acid maintains S-type anion channel activity in ATP-depleted Vicia faba guard cells, indicating phosphatase inhibition. FEBS Lett. 1998;428:177–82.
Scienza A, Mieavalle R, Visai C, Fregoni M. Relationships between seed number, gibberellin and abscisic acid levels and ripening in cabernet sauvignon grape berries. Vitis. 1978;17:361–8.
Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C. Fruit development and ripening. Annu Rev Plant Biol. 2013;64:219–41.
Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, Wu FQ, Wang XF, Jiang T DuSY, Zhang XF, Zhao R, Sun HL, Liu R, Yu YT, Zhang DP. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22:1909–35.
Shen YY, Duan CQ, Liang XE, Zhang DP. Membrane-associated protein kinase activities in the developing mesocarp of grape berry. J Plant Physiol. 2004;161:15–23.
Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Zhang DP. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–6.
Sun L, Wang YP, Chen P, Ren J, Ji K, Li Q, Li P, Dai SJ, Leng P. Transcriptional regulation of SlPYL, SlPP2C, and SlSnRK2 gene families encoding ABA signal core components during tomato fruit development and drought stress. J Exp Bot. 2011;62:5659–69.
Sun L, Yuan B, Zhang M, Wang L, Cui M, Wang Q, Leng P. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and carotene contents in tomato fruit. J Exp Bot. 2012a;63:3097–108.
Sun L, Sun Y, Zhang M, Wang L, Ren J, Cui M, Wang Y, Ji K, Li P, Li Q, Chen P, Dai S, Duan C, Wu Y, Leng P. Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol. 2012b;158:283–98.
Sun JH, Dong YH, Li CL, Shen YY. Transcription and enzymatic analysis of beta-glucosidase VvBG1 in grape berry ripening. Plant Growth Regul. 2014;. doi:10.1007/s10725-014-9932-x.
Shiozaki S, Kamata Y, Ogata T, Horiuchi S, kawase K. Localization of abscisic acid in grape berry by immunohistochemical techniques. J Jap Soc Hortic Sci. 1999;68:1–9.
Singh RK, Sane VA, Misra A, Ali SA, Nath P. Differential expression of the mango alcohol dehydrogenase gene family during ripening. Phytochemistry. 2010;71:1485–94.
Terry LA, Chope GA, Bordonaba JG. Effect of water deficit irrigation and inoculation with Botrytis cinerea on strawberry (Fragaria x ananassa) fruit quality. J Agric Food Chem. 2007;55:10812–9.
Verslues PE, Zhu JK. New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol. 2007;10:447–52.
Wheeler S, Loveys B, Ford C, Davies C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust J Grape Wine Res. 2009;15:195–204.
Wang XF, Zhang DP. Abscisic acid receptors: multiple signal-perception sites. Ann Bot. 2008;101:311–7.
Wang XJ, Zhu SY, Lu YF, Zhao R, Xin Q, Wang XF, Zhang DP. Two coupled components of the mitogen-activated protein kinase cascade MdMPK1 and MdMKK1 from apple function in ABA signal transduction. Plant Cell Physiol. 2010;51:754–66.
Wu FQ, Xin Q, Cao Z, Liu ZQ, Du SY, Mei C, Zhao CX, Wang XF, Shang Y, Jiang T, Zhang XF, Yan L, Zhao R, Cui ZN, Liu R, Sun HL, Yang XL, Su Z, Zhang DP. The Mg-chelatase H subunit binds abscisic acid and functions in abscisic acid signaling: new evidence in Arabidopsis. Plant Physiol. 2009;150:1940–54.
Xu ZJ, Nakajima M, Suzuk Y, Yamaguchi I. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol. 2002;129:1285–95.
Yu XC, Li MJ, Gao GF, Feng HZ, Geng XQ, Peng CC, Zhu SY, Wang XJ, Shen YY, Zhang DP. Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol. 2006;140:558–79.
Zhang DP, Zhang ZL, Chen J, Jia WS. Specific abscisic acid-binding sites in mesocarp of grape berry: properties and subcellular localization. J Plant Physiol. 1999;155:324–31.
Zhang DP, Chen SW, Peng YB, Shen YY. Abscisic acid-specific binding sites in the flesh of developing apple fruit. J Exp Bot. 2001;52:2097–103.
Zhang M, Yuan B, Leng P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot. 2009;60:1579–88.
Zhang L, Ma G, Kato M, Yamawaki K, Takagi T, Kiriiwa Y, Ikoma Y, Matsumoto H, Yoshioka T, Nesumi H. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro. J Exp Bot. 2012;63:871–86.
Zeevaart JAD. Abscisic acid metabolism and its regulation. In: Hooykaas PPJ, Hall MA, Libbenga KR, editors. Biochemistry and molecular biology of plant hormones. Amsterdam: Elsevier Science; 1999. p. 189–207.
Zeevaart JA, Heath TG, Gage DA. Evidence for a universal pathway of abscisic acid biosynthesis in higher plants from o incorporation patterns. Plant Physiol. 1989;91:1594–601.
Acknowledgments
The research work in Shen’s laboratory was supported by the National Key Basic Research ‘973’ Program of China (grant no. 2012CB126306), the National Science Foundation of China (grant no 31272144), and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (grant no. IDHT20140509).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Shen, YY., Rose, J.K.C. (2014). ABA Metabolism and Signaling in Fleshy Fruits. In: Zhang, DP. (eds) Abscisic Acid: Metabolism, Transport and Signaling. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9424-4_14
Download citation
DOI: https://doi.org/10.1007/978-94-017-9424-4_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9423-7
Online ISBN: 978-94-017-9424-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)