Abstract
The expansion of the pine processionary moth with climate warming is likely to modify the interactions with its associated arthropod and vertebrate communities of parasitoids, predators and competitors. A first section details the response of some egg parasitoids to moth expansion. Then, we investigate how insectivorous vertebrates (specialist birds, generalist birds and generalist bats) may or not be efficient predators of T. pityocampa on the range expansion gradients. Finally, we discuss whether the expansion of the moth in inner Alpine valleys may become a serious threat to the endangered Spanish moon moth by competing for the same pine needle resource.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
This chapter aims at analyzing how the expansion of the pine processionary moth with climate warming is expected to change its trophic interactions with the associated arthropod and vertebrate communities of parasitoids, predators and competitors (i.e., other pine-feeding Lepidoptera). Although parasitoids and predators are known to influence moth outbreak dynamics, the evolution of host-parasitoid and predator-prey interactions under ongoing climate change is still subject to considerable uncertainty and may impede biocontrol efficiency. Moreover, the pine processionary moth expansion may also cause unprecedented damages on biodiversity if it rapidly spreads in pine forests with high conservation value outside of its previous range. Despite a large amount of mostly empirical literature available on predators and parasitoids of Thaumetopoea pityocampa, there had been no previous attempt to synthesize that information and replace it in the context of the climate-driven expansion of the moth. There is for example a lack of studies on the differential parasitism rates experienced by the moth in core areas of its former distribution range compared to newly-colonized areas. A first section presents some recent results obtained about the response of egg parasitoids to moth expansion. Then, we investigate how insectivorous vertebrates may or not be efficient predators of T. pityocampa on the range expansion gradients, by successively quantifying numerical and functional responses of specialist birds, generalist birds and generalist bats to T. pityocampa density for several moth life stages, including late-instar larvae, pupae and imagos. Finally, we will discuss whether the expansion of the moth in inner Alpine valleys may become a serious threat to the endangered Spanish moon moth Actias (=Graellsia isabellae) by competing for the same pine needle resource.
2 Expansion of Pine Processionary Moth and Parasitoid Responses
2.1 Introduction
The “Enemy Release Hypothesis” suggests that the success of invasive species could be linked to the absence of their natural enemies in the invaded environment (Colautti et al. 2004; Keane and Crawley 2002; Liu and Stiling 2006). It was primarily developed in the context of biological invasions caused by individuals transported from remote areas, where the probability of transport of natural enemies together with the host is quite low (Colautti et al. 2004; Nicholls et al. 2010). Indeed, Cornell and Hawkins (1993) showed that phytophagous insects had reduced parasite species richness and parasitism rates in invaded areas compared to their native range. Following colonization, the subsequent expansion capacities of the invasive species would thus be enhanced because they are released from natural enemy pressures (Keane and Crawley 2002; Mitchell and Power 2003). Some recent studies suggest that the Enemy Release Hypothesis could also apply to species increasing their natural range by gradual geographic expansion, even without long-distance colonization (Menendez et al. 2008). A strong relationship between parasitism rate, parasitoid species richness and time since the colonization date of a given area by the host was shown for several expanding Lepidoptera (Grabenweger et al. 2010; Grobler and Lewis 2008). In Europe, the example of Cameraria ohridella Deschka and Dimic (Lepidoptera: Gracillariidae) that feeds on the horse-chestnut (Aesculus hippocastanum L.) is particularly meaningful. This species has spread over 20 years almost everywhere its main host is present at a speed of 60 km per year (Augustin et al. 2009a, b). The success and expansion speed of this species could be explained among other reasons by quite low predation and parasitism rates (Augustin et al. 2009a), which even decreased in the recently colonized areas (Hernández-López et al. 2011). Moreover, the observed parasitism in recently colonized areas was mostly due to the shift of local parasitoids (Hernández-López et al. 2011), rather than to the tracking by natural enemies from the native range which appeared largely delayed compared to host expansion (Gebiola et al. 2014). On average, native parasitoids shifted to this new host after ca. two decades (Grabenweger et al. 2010).
As regards the pine processionary moth, its present and rapid geographical expansion (see Roques et al. 2014, Chap. 3, this volume) is likely to affect the distribution of its associated enemies. Consistent with the “host-tracking hypothesis” (Kohnen et al. 2012; Stone et al. 2012), interacting species can be expected to follow the distribution shifts of their host species, either concomitantly or with a temporal delay due for instance to lower dispersion capacities, or limited ability to detect the host when it is still at low densities. In this latter case, parasitism rates will be significantly reduced near the new boundaries of the host range. Moreover, the different parasitoids associated with a given species may not all be able to track their host at the same speed or with the same success, therefore altering the community composition and structure along the expansion routes. Conversely, parasitoid richness may also increase with time either because the invading host continuously encounters new parasitoid species during its spread (geographic spread-hypothesis) or because local parasitoids need different periods of time to adapt to the novel host (adjustment-hypothesis) (Grabenweger et al. 2010). In any case, geographic expansions and/or host shifts experienced by the associated parasitoids are expected to impact their genetic diversity and spatial genetic structure. Depending on the type of interaction and the level of specialization, these phenomena can result in differentiated populations along the expansion corridor (in case of repeated founder effects due to long distance dispersal), or in a continuous distribution with an isolation by distance pattern (in case of diffusive dispersal). Parallel evolution and diversification can generate synchronism between spatio-temporal phylogeographic patterns under the “Contemporary Host-tracking Hypothesis” (Becerra 2003), while temporal discrepancies can occur under the “Delayed Host-tracking Hypothesis”, in which the natural enemy can display more recent diversification patterns than its host (Kohnen et al. 2012; Stone et al. 2012).
All over the Mediterranean basin, a rich community of insect parasitoids and predators is associated to the pine processionary moth (see Battisti et al. 2014, Chap. 2, this volume). Most of these species specifically attacks a particular developmental stage of the host, i.e., embryos in eggs, any of the five instar larvae in the nest, mature larvae in procession, pupae, or adults. Among the parasitoids, the hymenopteran chalcids infesting pine processionary moth eggs have extensively been studied because they may act as biocontrol agents modulating pine processionary moth eruptive dynamics through an induced decrease in the number of larvae per nest and in the subsequent damage (Arnaldo and Torres 2006; Mirchev et al. 2004; Perez-Contreras and Soler 2004).
The many studies conducted around the Mediterranean basin on pine processionary moth egg parasitoids (Mirchev et al. 1998, 2004; Mirchev and Tsankov 2000; Schmidt et al. 1999; Tsankov et al. 1995, 1998, 2006) revealed an overall dominance of two thelytokous chalcid species, the eulophid Baryscapus servadeii Domenichini, considered as a pine processionary moth specialist, and the encyrtid Ooencyrtus pityocampae Mercet, which is supposed to be a generalist parasitoid attacking various lepidopterans and hemipterans (Battisti 1989; Géri 1980; Lopez-Sebastian et al. 2004). Two more chalcid species were usually found but at much lower densities, namely the eupelmid Anastatus bifasciatus Geoffroy and the trichogrammatid Trichogramma sp., sometimes identified as Trichogramma embryophagum Hartig (Tsankov and Mirchev 2003). These four species are the only pine processionary moth egg parasitoids commonly found in France, where they can present two generations per year. In Central France, adults of the generalist species, O. pityocampae, emerge in spring, well before the period of pine processionary moth egg laying but they can survive for long periods and use intermediate hosts before attacking pine processionary moth eggs (Battisti et al. 1988). In contrast, adults of the specialist species, B. servadeii, emerge in summer concomitantly with the pine processionary moth egg-laying and they immediately oviposit in the freshly laid eggs (Biliotti 1958; Géri 1980). The larvae of both species then develop at the expense of the young host embryos, and a new generation of adult parasitoids emerge before processionary moth larvae hatch. They immediately parasitize again pine processionary moth eggs but at a more advanced embryonic stage. The parasitoid larvae of this second generation then enter diapause to overwinter and the subsequent adults emerge the following spring or summer. However, a variable proportion of these larvae may remain in prolonged diapause for 1 more year (Biliotti 1958; Géri 1980).
Ancient data already suggested that parasitism rates and species richness of parasitoid communities are reduced near moth range boundaries. Hence, Biliotti (1958) observed a very low rate of egg parasitism, which was only attributed to Trichogramma sp., at the northern edge of pine processionary moth distribution whilst Géri (1980) noticed a similar phenomenon in a 1,300 m-high mountain pass in Corsica. These lower parasitism rates may favour pine processionary moth expansion because colony size may be subsequently higher near the expanding front, thereby increasing individual survival in this gregarious species (Perez-Contreras et al. 2003). In order to confirm and quantify egg parasitoids responses to pine processionary moth expansion northwards and upwards, and to compare parasitoids and host population structures, the variation in parasitism rates and species composition has been assessed along three gradients corresponding to the main pine processionary moth expansion pathways in Western Europe, from the historical native range to recently established populations on the leading front edge. The macroclimatic variations along such gradients can be viewed as natural laboratories to study the possible impact of climate warming on both pine processionary moth and parasitoids. The gradients were designed to compare latitudinal and altitudinal expansion, elevational gradients presenting the advantage to include a steep temperature variation over short geographical distances, thus reducing the confounding effects of different local environmental conditions. The genetic structure and diversity of the two main parasitoid species were simultaneously investigated along these gradients.
2.2 Species Composition and Parasitism Rates: Do Expanding Pine Processionary Moth Populations Escape Egg Parasitoid Pressures?
2.2.1 Definition of the Altitudinal and Latitudinal Gradients Used in the Survey of Parasitoid Responses
The three studied expansion gradients are shown in Fig. 7.1. The south-north gradient, corresponding to the pine processionary moth latitudinal expansion, was drawn from Spain (Eastern Iberian chain corresponding to a putative glacial refugia of pine processionary moth, see Kerdelhué et al. 2014, Chap. 4, this volume) to the current expanding front in the Paris basin in north-central France, including the pioneer populations identified above the front (see Roques et al. 2014, Chap. 3, this volume). One of the altitudinal gradients stretched along the Durance valley in the Southern French Alps, while the other was located in the South-Western part of the French Massif Central. In each gradient, sampling sites were selected in (i) the “core area”, i.e. the historical native range of the pine processionary moth, (ii) the “newly colonized area”, reached by the pine processionary moth between 1990 and 2000, and (iii) the “front area”, invaded by the pine processionary moth less than 5 years ago. For each gradient, whenever possible, egg batches were sampled on the same tree species to avoid any host plant effect (Arnaldo and Torres 2006). Emerging adult parasitoids were collected every week, identified and stored in alcohol for future genetic studies, while already emerged individuals were identified following Tanzen and Schmidt (1995). The rearing and identification methods are detailed in Imbert (2012). Species composition and parasitism rates could thus be reliably estimated. The study was conducted for 2 consecutive years, corresponding to egg batches laid in 2008 and 2009, in order both to assess the inter-annual variability in parasitism rate. The results were also compared with those from previous surveys done along the same gradients in the Alps (2003 and 2004) and the Massif Central (2007).
2.2.2 Variation of Total Egg Parasitism Along the Expansion Gradients
Parasitism rates of pine processionary moth egg batches are known to be highly variable in space and time over the moth’s distribution range. It typically ranged from 6 % to ca. 40 % in previous studies conducted in Southern Europe (Mirchev et al. 1999; Schmidt et al. 1999; Tiberi 1990; Tsankov et al. 1996, 1998).
Consistently, the parasitims rates measured along the three studied gradients varied both between sites and between years (Fig. 7.2.). Yet, spatial and temporal patterns could be noted. In all three regions, total parasitim rates were higher in 2009 than in 2008. Egg batches were always more parasitized in the Alps than along the other two gradients. However, whatever the year or the gradient total parasitism was significantly lower at the front edge than in the core and the newly- colonized areas.
Variation in total parasitism of pine processionnary egg batches by chalcid parasitoids along gradients from core areas to front edge and pionneer, isolated colonies beyond the front. (a) Latitudinal gradient, from glacial refugia in Spain to Paris basin in 2008 (no. analyzed batches from 25 to 51 according to area) and 2009 (n = 50–114); (b) Altitudinal gradient in the Southern Massif Central in 2007 (n = 18–127), 2008 (n = 12–49) and 2009 (n = 33–62); (c) Altitudinal gradient in the Southern French Alps in 2003 (n = 54–91), 2004 (n = 15–30) 2008 (n = 11–20) and 2009 (n = 29–60); Different letters show significant differences between locations during the same year and the same gradient following a Kruskal-Wallis test (P < 0.05)
The most recent, expanding populations were therefore always less affected by natural enemies, while the parasitism rates were usually already rather high in the areas colonized between 1990 and 2000. Unpublished data gathered by the Authors in 2003–2004 (i.e., 5 years earlier) over the same Alpine gradient already showed a similar trend but with a much lower parasitism at the front edge. Thus, parasitism of egg batches at the front averaged 7.3 % in 2003 and dropped to 1.5 % in 2004 whereas it reached 11.5 % in 2008 and up to 19.9 % in 2009. The survey of the annual variations in moth populations at the front edge using both qualitative visual estimations of nest densities and pheromone trappings of male moths revealed that nest density significantly increased between 2003 and 2004, simultaneously to a threefold increase in male captures (from an average of 27 per trap in 2003 to 82 in 2004). This could explain the observed decrease in the percentage of parasitized egg batches in 2004. In contrast, nest density strongly decreased between 2008 and 2009 but moth captures in 2009 (33 moths per trap) as well as nest densities were quite similar to the values observed in 2003 whereas these of 2008 (96 moths per trap) matched the 2004 observations. The much higher rate of parasitism observed in 2008–2009 compared to 2003–2004 may suggest that egg parasitoids tend to follow the expansion of the pine processionary moth but with a short time lag allowing the moth to escape from its main enemies during the first steps of the expansion. However, larger samplings are needed to confirm this hypothesis because host egg masses are highly clustered in space, especially in the expansion areas, and it is likely that the relation between host density and parasitism rates is not linear. An alternative explanation could be a de-synchronisation between the life cycles of pine processionary moth and its main egg parasitoids due to specific environmental conditions prevailing at the front edge (see below). As our data also show that the embryos’ mortality due to other factors is similar between sites, we conclude that more pine processionary moth larvae successfully hatch per egg batch at the front edge of the expansion areas, if the sizes of the egg masses are similar. In addition, pine processionary moth colony size is associated with the capacity to resist winter temperatures. A high density of larvae in the nest both increases individual survival to low temperatures (Huchon and Démolin 1971) and enhances growth rate (Perez-Contreras et al. 2003). The observed decrease of parasitism rate near the expanding front could thus lead to nests containing more larvae, and to a higher than expected individual survival in spite of harsher climatic conditions in the northernmost and upmost populations.
2.2.3 Variation in Parasitoid Communities Along the Gradients
As expected, the four species of pine processionary moth egg parasitoids known from France were present along the three gradients. The majority of sampled individuals belonged to either the pine processionary moth specialist, Baryscapus servadeii, or to the generalist, Ooencyrtus pityocampae. Anastatus bifasciatus and Trichogramma sp. were found occasionally but never at high frequencies. A few additional specimens, which may belong to the genus Eupelmus according to their barcode sequence (Auger-Rozenberg, personal observation), were observed in 2009 but they could not be identified at species level.
Species richness of parasitoids and relative abundance of the sampled species were highly variable between years and localities, and the observed patterns were not consistent between gradients (Fig. 7.3).
Baryscapus servadeii was predominant all along the latitudinal gradient except in the refugial area of Spain in 2009 where most emerging parasitoids were O. pityocampae (Fig. 7.3A). Interestingly, B. servadeii was even dominating the few specimens emerging from the pioneer colonies located beyond the front edge that seemed until recently free from egg parasitoids (Robinet et al. 2012). These results suggest that the specialist species could track its host with a limited delay, and that the generalist species did not efficiently shifted to this new resource in the Paris basin. Along the altitudinal gradient studied in the Massif Central, B. servadeii was mostly found in the core region, while O. pityocampae was the main species sampled at higher elevations (Fig. 7.3B). This is consistent with ecological hypotheses suggesting that O. pityocampae would be adapted to cooler climates while B. servadeii would be more susceptible to frost (Masutti 1964), which could impede its local expansion. Yet, this pattern was not confirmed in the second altitudinal gradient studied in the Alps, where the dominant species was different between years in most sites, especially in the recently colonized areas (Fig. 7.3C).
2.3 Host-Parasite Synchronisation and Survival in Different Environmental Conditions
As pine processionary moth is currently expanding towards higher latitudes and elevations, it reaches regions were climatic conditions tend to be more severe than in the historical range. For instance, we measured a difference of 1.7 °C in average winter temperatures between the core and the front areas in the Southern French Alps. Because warming temperatures may affect differently the phenology of host and parasitoids, this can alter the synchrony between interacting species (Parmesan and Yohe 2003; Root et al. 2003; Visser and Both 2005). This could explain the lower parasitism rates in the front areas documented above. It is also possible that parasitoids are more susceptible to low temperatures than pine processionary moth, and cannot survive the environmental conditions in the front areas. To test these hypotheses, in situ cross-translocation experiments were carried out in the Southern French Alps in order to compare (i) the emergence curves of adult parasitoids in the core vs. the front areas; (ii) the emergence curves of adult parasitoids issued from eggs laid in the core area that were artificially moved to the front edge before winter, and vice-versa (hereafter, “translocated parasitoids”); (iii) the pine processionary moth flight periods using in situ male pheromone trapping at both sites (Imbert 2012).
Indeed, the two major parasitoid species did not emerge simultaneously with pine processionary moth emergence peak, as already documented by Dulaurent et al. (2011) in South-Eastern France. Individuals of the generalist O. pityocampae emerged 3 weeks to a month before their host, while the emergence of the specialist B. servadeii started about a week before egg laying of pine processionary moth and continued throughout the pine processionary moth flight period (Fig. 7.4).
Flight period of parasitoids and pine processionary moth in the two sampling sites in the Southern French Alps (front and core areas): Bold lines represent peaks emergence, solid lines represent mass emergence, and dotted lines represent sparse emergence. For each site, native species correspond to species which emerged from egg-batches sampled in the same sites and translocated species to egg-batches sampled in one site but moved in the other site for winter
Both species naturally emerged about 2 weeks earlier in the core area than at the front edge. Translocated parasitoids of both species emerged as adults at the same time as their native conspecifics. This suggests that the difference in parasitoid phenology observed between core and front areas is mostly due to a plastic response to local winter conditions.
Actually a smaller gap between parasitoid and pine processionary moth emergence was noticed at the front edge compared to the core area. Thus, in the front area, the emergence of the two major parasitoids is still (and even better) synchronized with the egg laying period of the moth. Moreover, Dulaurent et al. (2011) showed that egg parasitoids may survive longer by feeding, e.g. on honeydew, and can thereby compensate a delay in host availability. Since the translocated parasitoids are capable to survive overwintering and emerge as adults at the front area, they are able to resist cooler temperatures than in the historical range. The decrease of parasitism rates along the expansion pathways of the pine processionary moth are thus probably not due to a de-synchronisation with their host, nor to a very high mortality due to harsher conditions.
Therefore, it could be so far hypothesized that the delay in parasitoid expansion as compared to the pine processionary moth may be explained by a lower dispersal capacity, or by difficulties in locating their host in the newly colonized areas, where the egg batches are probably more scattered in the landscape. Nevertheless, it cannot be excluded a sampling bias due to low densities of parasitoid populations in processionary moths expansion areas.
2.4 Parasitoid Genetic Diversity Along Pine Processionary Moth Expansion Pathways
Range expansions may have consequences on richness, abundance or phenology of species, but also on the genetic diversity of their populations. Just as we studied the variations of genetic diversity of the pine processionary moth along its main expansion pathways (Kerdelhué et al. 2014, Chap. 4, this volume), we also used molecular data to examine the genetic consequences of the expansion on egg parasitoids. As observed in several cases, invasive or expanding populations may exhibit reductions in genetic diversity along the colonization routes (Excoffier et al. 2009; Rousselet et al. 2010; Smith et al. 2011) due to founder effects, or to the fact that a fraction of the existing population contribute to the expansion at each generation. Contemporary processes and species life history and ecological traits (reproductive system, generation time, fecundity, dispersal modes, etc.) can affect the spatial distribution of genetic diversity in various ways. As explained in Kerdelhué et al. (2014, Chap. 4, this volume) for the processionary moth, dispersal potential can lead to various patterns of genetic variation and structure. Long-distance dispersal events can result in large patches of homozygosity and conversely, diffusive processes can lead to the maintenance of genetic diversity, usually with a spatial pattern of isolation by distance. Moreover, the egg parasitoid species studied here have an asexual reproduction (thelitokous parthenogenesis), which can also affect the genetic structure because it strongly affects gene flow.
In order to evaluate if the expansion affects the patterns of genetic diversity in pine processionary moth-associated parasitoids, and to compare the host/parasitoid patterns, the variability in mitochondrial DNA sequences was analysed along two of the gradients presented above. In the future, attempts will be made to develop highly polymorphic nuclear markers such as microsatellites, which are relevant to study genetic structure at regional spatial scales. Yet, this will necessitate species-specific development, and a much higher sampling effort.
A total of 13 sites ranging from northern Spain to Paris basin could be considered for the latitudinal gradient, and 6 sites in the Southern French Alps for the altitudinal one. In the Massif Central, the too limited number of emerged parasitoids allowed molecular analysis only in a single site of the core area. Following species identification using adult morphology, DNA was extracted from five individuals per species and per sampling site, reared from different egg masses and different pine trees to reduce the risk of collecting siblings. For each individual, a part of the Cytochrome Oxidase I (COI) mitochondrial gene was sequenced. We then could analyse the distribution of haplotypes and the spatial genetic diversity found for each species, and a parsimony network was built to infer gene genealogies.
The specialist species B. servadeii showed 13 haplotypes along in the latitudinal gradient whereas 12 were observed along the altitudinal Alpine gradient. However, no haplotype was shared between these two gradients, indicating a strong spatial genetic structure (Fig. 7.5). In the single site studied in the Massif Central, three haplotypes were observed, among which two were also found in the latitudinal gradient but none was also present in the Alps. In both gradients, the genetic diversity was significantly higher in the core areas, with ten haplotypes found in the Spanish sites and eight in the core area of the Alpine gradient. In contrast, only three haplotypes were observed in the newly-colonised areas and at the front edge of the latitudinal gradient whilst it decreased to a single haplotype in the corresponding areas of the altitudinal gradient. The patterns observed in the specialist egg parasitoids are thus consistent with a loss of genetic diversity along both northwards and upwards expansion routes. These results are quite similar to the patterns exhibited by the host (Kerdelhué et al. 2014, Chap. 4, this volume), and consistent with a diffusive dispersion of the parasitoid tracking its host. In addition, the spatial structure discovered for that species, with specific haplotypes found in both studied gradients, suggests that B. servadeii exhibits a strong phylogeographic structure, with a high genetic diversity retained in the southern part of its natural distribution.
The generalist O. pityocampae presented only five haplotypes throughout the two gradients. One of these haplotypes was present at high frequencies. It corresponded to the major haplotype along the latitudinal gradient, it was the only one found in the altitudinal one and it also occurred in the Massif Central. Three other haplotypes were restricted to the core area of the latitudinal gradient, and one was only found in the Massif Central. These results indicate that O. pityocampae exhibits a quite limited genetic diversity compared to B. servadeii, despite the fact that they have both asexual reproduction (thelytoky), and that this species is not significantly structured at the studied geographical scale (Fig. 7.5). The low variability of the considered mitochondrial marker did not allow to assess if the genetic patterns are consistent with a recent expansion of the parasitoid, or could correspond to a shift from local populations already present in the regions recently colonized by the pine processionary moth but developing upon other hosts. Range-wide studies of mitochondrial genetic diversity will be necessary to confirm these observations and fully understand the patterns observed (Torres-Leguizamon et al. submitted). Moreover, a fine scale study of the genetic variation along the gradients will be developed using highly polymorphic microsatellites markers in both species to better analyse the changes in genetic patterns along the expansion routes.
2.5 Conclusion and Perspectives
The studies presented here showed that four species of parasitoids are present throughout the distribution range of the pine processionary moth, no new species being observed in newly colonized areas. A specialist species, Baryscapus servadeii, and a generalist species, Ooencyrtus pityocampae, were by far the most frequent species. Quantification of parasitoid species richness and frequencies along three expansion gradients showed that parasitism rates were much lower at the front edges, which may be due to a delay in host tracking because parasitism rate significantly increased with time in this area whatever the moth population dynamics. This time lag may allow the host to experience significant population growth, which can further enhance its expansion rate. Moreover, the generalist parasitoid could be present beyond the pine processionary moth expansion front and associated there to other hosts. Thus, the parasitism due to O. pityocampae in newly colonized areas could be due to a local shift of this species to the pine processionary moth host rather than to parasitoid populations expanding from the core areas. In this case, the delay observed would be due to the time necessary for the parasitoid to adapt to the new host.
New research will now be necessary to fully understand the processes acting along the expansion routes on the pine processionary moth-parasitoid relationships. In particular, little is known about the environmental requirements and ecological niches of the different parasitoid species. The direct effects of climate warming and land use changes upon parasitoids remain unknown, which impedes understanding the host-tracking mechanisms. Data are also needed about host preference and host localization of the generalist parasitoids to infer their capacity to shift to the new, expanding hosts near the front edge. Range wide studies of phylogeographic patterns and genetic diversity will also help understanding the present-day situation by shedding light on the evolutionary histories of the multiple partners.
3 Numerical and Functional Responses of Predatory Birds and Bats to the Pine Processionary Moth
3.1 Introduction
3.1.1 Numerical and Functional Responses of Predators to Outbreaking Insects
The strength of predator-prey interactions is the main factor shaping the numerical and functional responses of predators to prey abundance (Abrams and Ginzburg 2000). While the numerical response of the predator can be defined as an increase in abundance with prey density, the functional response is the increase in prey consumption by predators with prey density. The efficiency of the biological control of pest insects by predators is therefore directly related to the shape and magnitude of their functional responses (Abrams and Ginzburg 2000). The most widespread functional response is the ‘type II’, in which consumption rate first increases with prey density and then decreases or remains constant irrespective of prey density. For outbreaking herbivorous insects, several Authors have highlighted the beneficial influence of predators on prey outbreak frequency and magnitude (Klemola et al. 2002; Barbosa et al. 2012). However, there is still considerable uncertainty regarding the evolution of predator-prey relationships under ongoing climate change (Berggren et al. 2009; Bretagnolle and Gillis 2010). In particular, spatial and temporal mismatches between a given predator and its prey may impede the future efficiency of biocontrol in agro-ecosystems (Thomson et al. 2010). The current range expansion of Thaumetopoea pityocampa with climate warming (Battisti et al. 2005), therefore questions the potential role of vertebrate predators, together with parasitoids and other mortality factors, on pine processionary moth dynamics in time and space.
3.1.2 Biological Control of Pest Insects by Birds and Bats
The key functional role played by predatory vertebrates feeding on forest pest insects is acknowledged for a long time (Morris et al. 1958; Buckner 1967). Insectivorous birds and small mammals are especially efficient predators showing numerical and functional responses to pest insect abundance (Buckner 1967; Dempster 1983; Glen 2004). Among efficient predators of forest arthropods, birds and bats are together considered to provide a valuable ecosystem service of pest insect biocontrol (Whelan et al. 2008; Kunz et al. 2011). While forest birds have long-term been considered key insect predators, the role of insectivorous bats as natural enemies is less documented, although bats have large diet spectrum and foraging tactics (Vaughan 1997; Schnitzler and Kalko 2001) and can affect insect populations as much or more than birds in cross-taxa experiments (Kalka et al. 2008; Williams-Guillén et al. 2008; Karp and Dialy 2014). The role of bat echolocation in the evolutionary responses of tympanate moths to escape bat predation is actually well-known (Schnitzler and Kalko 2001; Waters 2003; Windmill et al. 2006). The predation by insectivorous vertebrates allows maintaining prey populations at low densities but this effect becomes negligible during prey outbreaks because of a predominant type II functional response (Crawford and Jennings 1989; Glen 2004). However, generalist and specialist predators do not exert the same biological control on pest insects because their functional responses to prey abundance differ in intensity and shape (Klemola et al. 2002). Specialists and generalists generally feed on different prey life stages and their spatio-temporal predation patterns are highly complementary within complex food webs (Hanski et al. 2001; Symondson et al. 2002). There is consequently a need for maintaining the functional diversity of insectivorous vertebrates at the landscape scale, including both generalist and specialist predators, to allow long-term biological control of this urticating moth (Tscharntke et al. 2007; Philpott et al. 2009).
3.1.3 Pine Processionary Moth as a Special Case of Predator–Prey Interactions
Recent studies have brought evidence for a rapid range expansion of the pine processionary moth upwards and northwards in Europe (Battisti et al. 2005), and for a correlation between the magnitude of moth outbreaks and the NAO index (Hódar et al. 2012). This climate-driven expansion has been proven to depend on the number of feeding hours allowed to overwintering larvae, or its surrogate, daily mean minimum temperature during the coldest period (Buffo et al. 2007). The moth expansion is strengthened by a highly efficient strategy of predation avoidance due to both morphological and behavioural adaptations: eggs are covered with scales by the female, late-instar larvae are covered by urticating setae and overwinter in silk nests where they hide during the day, and pupae are buried into the soil (Halperin 1990; Battisti et al. 2000). Moreover, adults of T. pityocampa are among the moth species that have evolved tympanic organs adapted to detect efficiently bat echolocations (Sierro and Arlettaz 1997; Waters 2003; Windmill et al. 2006). Predatory birds and bats have developed counter-efficient strategies to feed on urticating Lepidoptera such as T. pityocampa with morphological and behavioural adaptations to avoid prey defences (Waters 2003; Barbaro and Battisti 2011). Moth species experience vertebrate predation throughout their seasonal life-stages, although mortality at late-instar larval and pupal stages is the most likely to impact moth population dynamics on the long-term (Dempster 1983; Crawford and Jennings 1989; Tanhuanpää et al. 2001). Four main predation periods succeeding in time and space can be distinguished for T. pityocampa, corresponding to main moth life-stages and associated predator strategies (Barbaro and Battisti 2011): (i) generalist passerines, including mixed-species flocks led by Paridae tits, prey on eggs and early-instar larvae in late summer and early autumn; (ii) a small generalist, the great tit Parus major, and two large specialists, the great spotted cuckoo Clamator glandarius and the common cuckoo Cuculus canorus, prey on urticating late-instar larvae, especially in late winter and early spring; (iii) the specialist Eurasian hoopoe Upupa epops prey on below-ground pupae in late spring and early summer (Fig. 7.6) and (iv) nocturnal predation of adult moths occurs in summer during moth emergence period, by generalist bats (at least Pipistrellus kuhlii and Eptesicus serotinus), European nightjar Caprimulgus europaeus and red-necked nightjar C. ruficollis (Table 7.1).
A breeding adult hoopoe carrying a pine processionary moth pupa during the breeding season. Aquitaine, France, 29th May 2011 (Alain Laborde). Breeding hoopoes can consume up to 74 % of buried pupae (Battisti et al. 2000)
3.2 Predation Strategies Used by Vertebrates to Feed on Thaumetopoea pityocampa
3.2.1 Strategies of Specialist Insectivorous Birds
Large insectivorous birds feeding on aposematic and toxic insects, including those carrying urticating setae, can be considered as dietarily specialized, i.e., they have both homogeneous diets and particular foraging behaviours (Sherry 1990). These species have evolved efficient strategies to feed on urticating Lepidoptera, including T. pityocampa, for example by banging late-instar larvae on branches to discard the head capsule and the integument, and eat only the viscera (Gill 1980; Gonzalez-Cano 1981). The late-instar larvae can also be cleaned from their setae by cuckoos and hoopoes rubbing them on the ground before ingesting the prey (Barbaro and Battisti 2011). Moreover, cuckoos of several genera (Cuculus, Chrysococcyx, Clamator and Coccyzus spp.) are well-adapted to feed on urticating larvae (Gill 1980). Their gizzard inner layer has evolved towards a soft, thick and non-keratinoid structure that allows the larva setae to be kept inserted in the gizzard wall and to be regurgitated as mixed pellets of mucous membrane, setae and gut contents, including pine needle fragments.
Cuckoos (Coccyzus spp., Clamator glandarius, Cuculus spp.) are also known for their post-migratory nomadic phase when returning from wintering grounds in early spring, during which they are likely to locate moth outbreak areas (including Lymantria dispar, Malacosoma spp. or T. pityocampa) before establishing their breeding territory (Hoyas and Lopez 1998; Barber et al. 2008). Great spotted Clamator glandarius and common cuckoos Cuculus canorus can locally specialized on urticating fifth instar larvae of T. pityocampa, typically preyed on during pre-pupation processions in early spring (Valverde 1971; Hoyas and Lopez 1998). Another high predator specialization on T. pityocampa pupae during below-ground spring diapause is found in the Eurasian hoopoe Upupa epops. Hoopoes’ long and curved bill allows the birds to dig out buried cocoons, separate the pupae from the urticating skins of the last larval moult, and ingest or carry out pupae for chicks (Battisti et al. 2000; Barbaro et al. 2008; Fig. 7.6). Finally, adult T. pityocampa moths are likely preyed on during their summer emergence by two specialists, nocturnal aerial feeders, European nightjar Caprimulgus europaeus and red-necked nightjar C. ruficollis, where they geographically co-occur with the moth in their respective ranges (Cuadrado and Dominguez 1996; Sierro et al. 2001; Table 7.1).
3.2.2 Strategies of Generalist Insectivorous Birds
Generalist insectivorous forest passerines such as tits (family Paridae) can aggregate in areas with high food availability, including outside the breeding season (Diaz et al. 1998). The great tit Parus major is particularly able to forage on temporarily available food resources provided by defoliating Lepidoptera, including some urticating species such as T. pityocampa (Pimentel and Nilsson 2007; Garcia-Navas et al. 2013). Empirical evidence for great tit predation on the pine processionary moth, including regular feeding on urticating late-instar larvae, is acknowledged for a long time (Biliotti 1958; Geroudet 1963). Great tits can feed both upon egg clusters, early-instar and late-instar larvae of T. pityocampa throughout the moth distribution range from south-western Europe to North africa and the Middle East (Gonzalez Cano 1981; Halperin 1990; Pimentel and Nilsson 2007; Barbaro and Battisti 2011). To feed on overwintering fifth instar larvae of T. pityocampa, great tits can dig holes in winter nests to collect larvae and remove their head capsule and most of the integument carrying the urticating setae to eat only inner parts of the larval body (Gonzalez Cano 1981). Most predation events occur in winter, when numerical responses of great tits to moth density can be observed (Barbaro et al. 2013). A previous study in central Spain has shown that up to 90 % of great tit stomachs and faeces contained residues of T. pityocampa larvae in winter (Gonzalez Cano 1981; Table 7.1).
The coal tit Periparus ater and the crested tit Lophophanes cristatus are two conifer-dwelling species that are also able to feed on eggs and early-instar larvae of T. pityocampa in summer and autumn in central Spain (Gonzalez Cano 1981). The same study provided evidence for predation of the coal tit on late-instar larvae of T. pityocampa using holes made by great tits in winter nests. Although the coal tit is known to feed mainly on pine seeds ouside the breeding season (Brotons and Herrando 2003), up to 66 % of its stomachs and faeces contained pine processionary moth with peak predation periods in October–December and April–May (Gonzalez Cano 1981). Finally, two deciduous forest specialists, the blue tit Cyanistes caeruleus and the long-tailed tit Aegithalos caudatus have also been reported as occasional, and maybe regular, predators of T. pityocampa eggs and early-instar larvae in fall and winter (Gonzalez Cano 1981). However, there is a lack of knowledge regarding the vertebrate and invertebrate predators of these early life-stages of the moth, potentially involving several insectivorous bird species, but also orthopterans and coleopterans (Hódar et al. 2013).
3.2.3 Strategies of Insectivorous Forest Bats
Using mostly echolocation to locate their prey, European bats (Microchiroptera) form a guild of nocturnal insectivores foraging on night-flying invertebrates in various habitats, including forests and their edges (Schnitzler and Kalko 2001; Kunz et al. 2011). Bats can consume over half of their body mass in insects nightly and each foraging guilds use different hunting techniques to feed on a wide spectrum of invertebrate prey, including forest moths (Kalka et al. 2008). Bats, especially open-habitat foragers, are also able to concentrate on locally abundant food resource by aggregative responses to increasing prey density (Müller et al. 2012). Adult moths are mainly active at night and therefore constitute a significant part of bat diets, albeit moths can also be predated by bats during their larval stage (Wilson and Barclay 2006). Numerical responses of bats have been studied to date by comparing total insect availability and bat activity or by nocturnal exclusion experiments (Kalka et al. 2008; Williams-Guillén et al. 2008; Müller et al. 2012; Karp and Dialy 2014). Functional responses of bats have been mostly demonstrated through faeces analysis (Vaughan 1997; Goiti et al. 2003; Kervyn and Libois 2008; Ashrafi et al. 2011).
Among the main bat species occurring in circum-Mediterranean pine forests, two species have particularly specialized foraging methods and diets on nocturnal Lepidoptera, the barbastelle bat Barbastella barbastellus (Sierro and Arlettaz 1997) and the grey long-eared bat Plecotus austriacus (Ashrafi et al. 2011). Both species are moth specialists with Lepidoptera representing up to 90 % of the diet (Vaughan 1997), which is similar to the proportion found on another nocturnal moth specialist, the European nightjar (Sierro et al. 2001). Two horseshoe bat species (Rhinolophus ferrumequinum and R. hipposideros) are also significant predators of Lepidoptera (20–90 % of the diet; Vaughan 1997), but they seldom use pine forests for foraging and are thus unlikely to feed on T. pityocampa. Three other species, the brown long-eared bat Plecotus auritus (Ashrafi et al. 2011), the Kuhl’s pipistrelle Pipistrellus kuhlii (Goiti et al. 2003) and the serotine bat Eptesicus serotinus (Kervyn and Libois 2008) are generalist predators whose foraging methods allows them to feed on moths within pine forest or edge habitats. As opportunistic feeders, their diet is composed of a significant part of Lepidoptera (Table 7.1) that are likely consumed in proportion with their availability. The latter two species, Kuhl’s pipistrelle and serotine bat, showed significant numerical and functional responses to experimentally increased density of pine processionary moths in pine plantations of south-western France (Charbonnier et al. 2014). Moreover, the strongest numerical and functional responses to T. pityocampa density were found in the Kuhl’s pipistrelle (Fig. 7.7), a Mediterraneo-atlantic bat that experienced a recent expansion of its distribution range with climate warming (Sachanowicz et al. 2006; Rebelo et al. 2010).
An echolocation sequence including a typical ‘buzz’ signal emitted by a Kuhl’s pipistrelle when capturing a prey such as a pine processionary moth during summer emergence. Kuhl’s pipistrelles were responsible for ca 75 % of bat foraging activity recorded during a study in south-western France (Charbonnier et al. 2014)
3.3 Functional and Numerical Responses of Predatory Birds and Bats
3.3.1 Functional Responses of a Pupae Specialist Bird
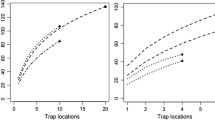
The Eurasian hoopoe Upupa epops is a specialist insectivore foraging on below-ground invertebrates by ground probing in short sward structures including patches of bare ground (Barbaro et al. 2008; Tagmann-Ioset et al. 2012). Hoopoes are local specialists of T. pityocampa late-instar larvae and especially below-ground pupae in circum-Mediterranean pine forests (Battisti et al. 2000; Barbaro et al. 2008). A quantitative study of hoopoes’ functional responses to the moth has been conducted in pine forests of northern Italy, showing that hoopoes can consume up to 74 % of buried moth pupae (Battisti et al. 2000). Quantifying pupae predation was based on the number of depredated cocoons left on the ground by hoopoes after pulling out the pupae (Battisti et al. 2000; Fig. 7.6). A telemetry study of foraging adult hoopoes during the breeding season has been performed in pine plantations of south-western France (Barbaro et al. 2008). The study revealed that breeding hoopoes selected pine plantation edges with short sward structures (<7 cm) and important bare sand cover (>25 %) to forage mainly on pine processionary moth pupae and Gryllus campestris (Barbaro et al. 2008). Hoopoe foraging micro-habitats matched narrowly with those used by T. pityocampa for below-ground pupation. As a result, we found a significant increase in hoopoe foraging intensity and nesting success during 3 consecutive years with increasing winter nest density of T. pityocampa measured along pine stand edges (Barbaro et al. 2008). The functional response of foraging hoopoes to moth density was analyzed by means of a linear mixed model with pine plantation edge as a random intercept effect and the 3 consecutive years of the study and pine processionary moth nest abundance as fixed effects (Zuur et al. 2009). The year effect on hoopoe foraging intensity was not significant but moth abundance significantly increased hoopoe foraging irrespective of year, indicating a marked functional response of U. epops to T. pityocampa (t1,67 = 4.95; P < 0.0001; Fig. 7.8).
Linear mixed model linking the log-transformed abundance of foraging hoopoes to winter nest abundance of pine processionary moth along pine plantation edges in south-western France (Modified from Barbaro et al. 2008)
We further estimated the efficiency of hoopoe predation on moth survival by pheromone trapping in paired stand edges located inside or outside hoopoes’ foraging areas during two summers in 2004 and 2006 (Fig. 7.9). We calculated the ratio of trapped moth abundance on winter nest counts in a given pine stand edge to estimate moth mortality due to hoopoe predation. There was a trend for a lower moth survival in preyed than in unpreyed stand edges, although the effect of hoopoe predation on the emergent moth/winter nest ratio was not significant (Fig. 7.9). However, the effect of hoopoe predation might be blurred by confounding effects of uncontrolled predation and parasitism and/or incertitude on true abundance of moth population estimated by male pheromone trapping.
3.3.2 Numerical Responses of Generalist Insectivorous Birds
The numerical responses of insectivorous bird assemblages to T. pityocampa, including generalist avian predators such as the great tit Parus major, have been studied within the moth expansion areas in France (Barbaro et al. 2013). Insectivorous bird communities and moth nest density were sampled on the same 250 m-long linear transects during two consecutive winters in 48 pine forests located along altitudinal (Mont Ventoux) and latitudinal gradients (Aquitaine and Orléans forests) of moth expansion in France. We hypothesized that the numerical reponses of winter bird communities to moth nest density, including foraging tit species flocks, would differ among core (i.e., at lower latitudes and elevations), and expansion areas of the moth distribution (i.e., at higher latitudes and elevations). We recorded foraging bird abundance and richness as the number of individual birds and species observed at least once in foraging activity during 10-min periods at the end of each transect (Brotons and Herrando 2003). Foraging bird assemblages were largely dominated by foraging Paridae tits on both elevation and latitude gradients (respectively 78 % and 55 % of foraging bird species). Dominant foraging species were the great tit and the crested tit Lophophanes cristatus on the latitude gradient and the coal tit Periparus ater on the elevation gradient (Barbaro et al. 2013).
On the elevation gradient (Mont Ventoux), we analysed the responses of foraging bird abundance and richness by Poisson generalized linear mixed models with elevation belts as a random factor to take into account the uncontrolled variation of moth density with elevation (Zuur et al. 2009). We found a significant effect of moth nest density on bird foraging abundance and richness only during the severe winter of 2009 (z = 4.17; P < 0.0005 and z = 4.34; P < 0.0002 for bird abundance and richness respectively; Fig. 7.10a). The significant predictors included in the best models for foraging bird abundance and richness were moth density and dominant tree species after multi-model inference at stand and landscape levels (Barbaro et al. 2013). Foraging bird abundance and richness were also significantly higher in pure Austrian pines (Pinus nigra ssp. nigra), the favourite host tree for T. pityocampa, and lower in mixed pine forests (z = 4.20; P < 0.0003 and z = 2.95; P < 0.003 for bird abundance and richness respectively). There was also a significant winter numerical response of Parus major to moth nest density on the elevation gradient, suggesting that great tits are able to profit from a somewhat abundant, albeit more difficult to handle, food resource in winter (Barbaro et al. 2013; Garcia-Navas et al. 2013). On the latitudinal expansion gradient (Aquitaine and Orléans forests), we found a similar numerical response of foraging bird abundance and richness, but only during an important T. pityocampa outbreak in winter 2010 (z = 3.53 and P < 0.0005 for foraging bird richness; Fig. 7.10b). The separate effect of host pine species (Pinus pinaster, P. nigra and P. sylvestris) on foraging bird abundance and richness along the latitude gradient was not significant.
Poisson generalized linear mixed models linking foraging bird richness to log-transformed nest abundance of pine processionary moth; in (a) winter 2009 on an elevation gradient of moth expansion (Mont Ventoux forest); (b) winter 2010 on a latitudinal gradient of moth expansion (Aquitaine and Orléans forests)
3.3.3 Numerical and Functional Responses of Foraging Bats
To study the numerical and functional responses of forest insectivorous bats to the pine processionary moth, we used an experimental design coupling pheromone traps and ultrasound bat recorders along pine stand edges in south-western France (Charbonnier et al. 2014). We estimated foraging bat activity in response to increasing abundance of mating male moths attracted by pheromone lures along a gradient of pine stand infestation by T. pityocampa. We quantified bat species abundance (numerical response) as the number of echolocation calls recorded per species and bat foraging activity (functional response) as the number of buzz signals indicating effective feeding attempts recorded per species (Fig. 7.7). Pheromone lures were used to simulate male moth aggregations after emergence at pine stand edges used by foraging bats in summer. We used linear mixed models to link the log-transformed abundance of echolocation calls with the nested effect of sampling night within the plot as a random intercept factor (Zuur et al. 2009).
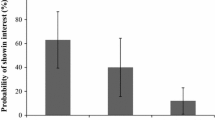
Foraging bats showed both numerical and functional responses to increased abundance of adult pine processionary moths for two generalist species: the Kuhl’s pipistrelle Pipistrellus kuhlii (z = 3.39; P < 0.001 for the numerical response; z = 3.38; P < 0.001 for the functional response; Fig. 7.11a) and the serotine bat Eptesicus serotinus (z = 2.51; P < 0.02; for the numerical response; z = 2.30; P < 0.05 for the functional response; Fig. 7.11b). By contrast, the responses of the moth-feeding bat specialists recorded, Barbastella barbastellus and Plecotus spp., were not significant despite a trend for increased foraging with T. pityocampa abundance. Moth-feeding bat specialist occurred at much lower overall abundance in the study site compared to generalist bats P. kuhlii, P. pipistrellus and E. serotinus, which may explain the lack of significant responses observed for specialist bats compared to generalists, together with distinct prey selection strategies (Charbonnier et al. 2014). Generalist forest bats also exhibited significant functional responses to mating aggregations of male pine processionary moths, since their foraging activities were significantly higher close to moth pheromone lures than in control plots (Charbonnier et al. 2014). Nocturnal activity periods of foraging bats and mating adult moths were also both higher during the first 4 h after sunset, indicating a narrow temporal matching between flying bats and pine processionary moths.
Linear mixed models linking log-transformed foraging activities of generalist bats for; (a) Pipistrellus kuhlii and; (b) Eptesicus serotinus to log-transformed moth abundance monitored by pheromone trapping (Modified from Charbonnier et al. 2014)
3.4 Discussion and Conclusion
Both insectivorous birds and bats are efficient predators able to track prey fluctuations and to aggregate in places where prey density is higher (Crawford and Jennings 1989; Diaz et al. 1998; Barber et al. 2008; Müller et al. 2012). In temperate forests of the northern hemisphere, predatory vertebrates can especially aggregate in highly-defoliated forest areas caused by the collective feeding behaviour of outbreaking forest moths such as Choristoneura fumiferana, C. occidentalis, Ennomos subsignarius, Epirrita autumnata, Operophtera brumata, Lymantria dispar and T. pityocampa (Morris et al. 1958; Haney 1999; Gale et al. 2001; Hogstad 2005; Wilson and Barclay 2006; Pimentel and Nilsson 2007; Barbaro et al. 2013). Such aggregation of predators in high prey density areas are found both in specialist and generalist insectivores and is especially documented in the Coccyzus cuckoos (Gale et al. 2001; Barber et al. 2008) and the Parulinae warblers in North America (Crawford and Jennings 1989; Patten and Burger 1998; Venier and Holmes 2010). The most efficient predators of target lepidopteran pests (spruce budworm Choristoneura fumiferana and elm spanworm Ennomos subsignarius) constitute a species-rich pool of specialist canopy-gleaners including bay-breasted Dendroica castanea, Cape May D. tigrina and Tennessee warblers Vermivora peregrina (Morris et al. 1958; Patten and Burger 1998; Haney 1999; Venier and Holmes 2010). Birds from this key foraging guild can consume up to 84 % of budworm pupae and larvae when prey populations are low, and up to 22 % at higher prey density (Crawford and Jennings 1989). In European forests, numerical responses of forest passerines, including the brambling Fringilla montifringilla, to geometrid moth outbreaks have been demonstrated (Hogstad 2005). However, a similar functional role such as the one played by Parulinae warblers in North America is mostly provided in Europe by Paridae tits, which are resident canopy gleaners able to exploit rapidly a new food resource locally and temporarily abundant, during the breeding season as well as in winter (Diaz et al. 1998; Brotons and Herrando 2003; Velky et al. 2011; Kaunisto et al. 2012; Carrascal et al. 2013). The most significant generalist predator of T. pityocampa outside the breeding season is the great tit Parus major (Gonzalez-Cano 1981; Pimentel and Nilsson 2007; Barbaro and Battisti 2011). Its rapid responses to spatial and temporal fluctuations of food resources is linked to efficient foraging and feeding behaviours allowing to benefit from prey more difficult to handle than usual, such as urticating moth larvae (Gonzalez-Cano 1981; Velky et al. 2011; Garcia-Navas et al. 2013). According to the ideal free distribution, the winter occurrence of foraging great tits is mainly driven by food availability and accessibility (Diaz et al. 1998; Kaunisto et al. 2012; Carrascal et al. 2013). As predicted by optimal foraging theory, overwintering nests of T. pityocampa larvae may constitute large, abundant and aggregated protein-rich prey allowing a temporary specialization, especially during cold periods (Barbaro et al. 2013; Carrascal et al. 2013). The high adaptive plasticity of great tits makes them efficient biocontrol agents in agroecosystems and plantation forests, including for newly occurring or expanding pest insects (Kaunisto et al. 2012). Another generalist predator, the Kuhl’s pipistrelle Pipistrellus kuhlii also exhibited marked numerical and functional responses to adult pine processionary moth density in summer. The Kuhl’s pipistrelle is a Mediterraneo-atlantic bat that experienced a recent expansion of its distribution range with climate warming (Sachanowicz et al. 2006; Rebelo et al. 2010). It may thus be potentially able to track more than other predators the current range expansion of T. pityocampa northwards (Battisti et al. 2005).
Although generalist predators alone can be efficient for pest insect biocontrol (Symondson et al. 2002), the occurrence of the complete vertebrate guild of T. pityocampa predators would provide a biotic insurance through functional complementarity between predators succeeding along the entire moth life cycle, in the absence of marked intraguild competition between moth predators (Philpott et al. 2009; Barbaro and Battisti 2011; see Table 7.1). The predation of pine processionary moth by specialist predators occurs mostly in spring during the critical late-instar larval and pupal stages (Hoyas and Lopez 1998; Battisti et al. 2000). However, specialist bats (barbastelle and long-eared bats) and birds (cuckoos, hoopoe, nightjars) exhibit lower density than generalists due to higher habitat requirements, including resource complementation needs at the landscape scale (Nakamura and Miyazawa 1997; Sierro and Arlettaz 1997; Sierro et al. 2001; Barbaro et al. 2008; Ethier and Fahrig 2011). Promoting silvicultural practices for the highest possible coexistence of generalist and specialist predators within European pine forests at stand and landscape scales is therefore critical to ensure a sustainable management of this key defoliator (Cayuela et al. 2011; Hódar et al. 2012). Such practices would include keeping understorey forest structure compatible with aerial foraging of bats and nightjars on adult moths in summer (Sierro et al. 2001; Jung et al. 2012), favouring stand edge diversity in pine plantations for stopover insectivore migrants potentially feeding on eggs and early-instar larvae during autumn migration (Rodewald and Brittingham 2004), and maintaining short sward structure along pine stand edges for large ground-gleaning insectivores such as the hoopoe (Barbaro et al. 2008; Tagman-Ioset et al. 2012). As a concluding remark, we advocate for considering the pine processionary moth as a keystone species in European pine forests for both forest health and biodiversity rather than an oversimplistic view of a forest pest insect that need to be fully eradicated by non-sustainable practices such as insecticide spraying (Müller et al. 2008; Cayuela et al. 2011).
4 The Expansion of the Pine Processionary Moth in the Southern French Alps and Its Impact on the Populations of the Endangered Spanish Moon Moth
4.1 Introduction
In a number of insect species, the recent warming up has relaxed the thermal barriers previously delimiting the species’ natural range, thus allowing a more or less rapid expansion of this range (Parmesan 2006; Parmesan et al. 1999). Those species are thus entering areas where they were excluded until now. Their arrival is susceptible to affect severely the structure and composition of communities as well as the functioning of ecosystems in the newly colonized areas (Walther et al. 2009). Actually, the presence of an additional organism in an ecosystem can have huge consequences on local species, as it is rather well documented for exotic invasive species introduced into a new continent where they may behave as predators or competitors for the resource, or both, for native species (Gurevitch and Padilla 2004; Hill and Lodge 1999; Kenis et al. 2009). Bøhn et al. (2008) thus listed as possible consequences of such competitive interactions: (i) niche shifts in habitat or diet; (ii) decrease in population density or extinction; (iii) reduced individual growth rate; (iv) reduced food intake; (v) alteration of prey community towards smaller species; and, (vi) altered size structure of prey populations towards smaller individuals.
However, unlike invasive species, the possible impact of expanding populations of native insects on local communities inhabiting the areas they are colonizing with warming up, has been little studied. One of the best documented examples of the effect of climate warming on the release of thermal thresholds constraining species distribution is the pine processionary moth, Thaumetopoea pityocampa (Den. & Schiff.) (Lepidoptera: Notodontidae), a major pine defoliator in southern Europe (Hódar et al. 2003). Since the mid-1990, populations of this winter- developing insect of Mediterranean origin are expanding towards higher latitudes and elevations in Southern and Western Europe (see Roques et al. 2014, Chap. 3, this volume). On the average, the moth progressed northwards by 5.6 km per year in the Paris basin (Robinet et al. 2007, 2012), and its mean upper distribution in elevation in the Italian Alps advanced by 70.1 m per decade (Battisti et al. 2006). Thus, T. pityocampa is entering new bioclimatic and bio-ecological areas where it may affect the resident fauna through eg., novel competitive interactions for pine resources, indirect effects on host quality, indirect cascading effects through shared parasitoids, and virus/disease transmission. The recent penetration of pine processionary moth into some high Alpine valleys of the Southern French Alps offered the possibility of assessing the possible impacts on a remarkable, resident competitor for pine needle resource, the endangered Spanish moon moth, Actias (=Graellsia) isabellae (Graells, 1849) (Lepidoptera: Saturniidae).
4.2 Pine Processionary Moth Expansion Lead to Face New Competitors for Pine Resources
4.2.1 Expansion of Pine Processionary Moth in the Upper Durance Valley
The Upper Durance valley of the Southern French Alps hosts one of the expansion leading edge in altitude of pine processionary moth. The pine processionary moth was historically present in the south of this valley, below 1,000 m elevation, but its presence in the upper areas was limited by the harsh climatic conditions prevailing there. Since the early 2000s, it has entered the sub-alpine mountainous climatic level, colonizing stands of Scots pine (Pinus sylvestris L.) located at 1,200–1,400 m elevation in the Natural park of Queyras (Imbert et al. 2012). At present, the moth has crossed more than 4 km onto the park. The pine processionary moth has thus penetrated the natural range of another moth developing on Scot pines, the Spanish moon moth (Goussard and Roques 2007; Maurel et al. 2013). The natural ranges of the two moth species were contiguous and did not overlap until this moment. In this area, T. pityocampa feed on pine foliage from August on throughout winter and leave the colonized pines by March–April to pupate in the ground (Huchon and Démolin 1971).
4.2.2 A Native Competitor for Pine Foliage, the Spanish Moon Moth, Actias isabellae
The endangered Spanish moon moth, Actias isabellae, is restricted in France to a few upper valleys of the Southern Alps (Goussard and Roques 2007; Maurel et al. 2013). The French Natural park of Queyras, situated in the Guil valley of the Southern French Alps, shelters a large part of the moth populations. This beautiful moth is protected by the Habitats’ Directive and the Bern Convention (revised by Procter and Harding 2005), and included in the French and Spanish official red lists of endangered fauna (Vila et al. 2009).
A. isabellae develops on Scots pine, and probably mountain pine. There is 1 generation per year. The adults emerge, mate and lay eggs in May–June. The hatching larvae feed solitarily on mature needles produced during the previous years but they do not consume the needles of the current year (Goussard and Roques 2007). The larvae pass through five larval stages until August when they finally pupate in the ground where they overwinter (Chefaoui and Lobo 2007).
Thus, the flight and egg-laying period of A. isabellae just follows the departure of T. pityocampa larvae in spring, which leave host pines partially defoliated and showing large white nests. Since 2007, such processionary nests have frequently been observed on pine trees known to regularly host larvae of A. isabellae in the lower part of its range in the Queyras natural park (Imbert et al. 2012). A. isabellae populations usually remain at low density and do not inflict any visible defoliation on Scots pines (Goussard and Roques 2007) in contrast to the defoliation caused by the gregarious larvae of T. pityocampa (Hódar and Zamora 2004).
4.3 Possible Effects of Pine Processionary Moth Expansion on the Choice of Oviposition Sites by Spanish Moon Moth
4.3.1 Pine Processionary Arrival Modifies the Pine Tree Habitat for Spanish Moon Moth
In phytophagous insects, selection by females of a site adequate for egg-laying is crucial for the survival and development of offspring. Often, first-instar larvae are not highly mobile and cannot move to another host if the one on which they have emerged is not adequate (Renwick and Chew 1994). During the search for an oviposition site, the choice of host plant is determined by multiple stimuli. At first, long- distance orientation is governed by visual and/or olfactory signals. Vision has a major role for host plant localization in a number of insect species. Visual cues may include color (e.g., in bark beetles Dendroctonus frontalis Zimmerman; Strom et al. 1999), spectral reflectance and reflectance contrast with the background (e.g., larch cone flies; Roques 1988), but also host shape or silhouette such as in the pine processionary moth (Démolin 1969; see Chap. 5). Volatiles emitted by the host plant also have a major role in the orientation of phytophagous insects and for alimentation and oviposition (Visser 1986) although it appears difficult to get a general pattern in a number of species including pine processionary moth (see Roques et al. 2014, Chap. 5, this volume). However, no data still exist about host selection process in Spanish moon moth, and the possible associated cues. Therefore, the observations could be only empirical at this moment.
The colonization of a pine tree by processionary moths has both direct and indirect consequences. Under temperate and mountainous climates, the gregarious larvae of this winter-developing insect progressively build a large white, silky nest as a protective shelter during the winter period. The final size of this nest can be up to 20 cm in diameter (Démolin 1969). In the Alps, the nest is built by November and it remains on tree, although discolored, several months after the larvae left it by early spring for pupation in the soil. Depending on population density of both insects and hosts, a variable number of nests can be present on the same tree, thus changing drastically its visual aspect. Second, needle consumption by developing larvae can result in a severe defoliation, which can sometimes exceed 90 % of the foliage (Hódar et al. 2003), thus also affecting tree visual appearance. Besides, defoliation by pine processionary larvae results in modifying the chemical composition of the needles (Hódar et al. 2004), and is likely to change as well the profile of volatile emissions by the attacked tree such as it has been observed in other defoliators (Haukioja 1990; Mumm et al. 2003).
4.3.2 In Situ Study of the Response of Spanish Moon Moth to the Arrival of Pine Processionary Moth
The egg-laying behavior of Actias isabellae has not yet been precised. However, in any case, in the areas newly colonized by pine processionary moths females of A. isabellae foraging for pine trees have at present to cope with the presence of white nests and a more or less heavy defoliation due to the processionary larvae which have just left these trees. As soon as the pine processionary moth was first detected in the natural range of A. isabellae in the Queyras valley, the latter species was no more observed on several individual trees where it was frequently recorded before (Goussard, Personal observation). Various factors may be involved, of which annual variations in population density and population displacement in response to climate change – but nothing is yet known about these processes –, but also a response to the habitat disturbance induced by the arrival of pine processionary moth.
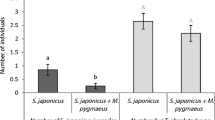
To test whether females of A. isabellae avoid trees previously colonized by pine processionary larvae, we translocated in autumn colonies of second instar larvae of pine processionary moth in an area of the natural range of A. isabellae where processionary moths were still absent (vallon du Fournel, near L’Argentière la Bessée, southern French Alps). In spring 2009, a systematic survey of pine trees in the area using feces traps on the ground allowed to select 40 trees on which A. isabellae was present (for details, see Imbert 2012). During autumn, one to five colonies of pine processionary larvae were grafted on half of these trees, the others being used as a control. The previous presence of pine processionary larvae on a tree did not seem to affect significantly the further occurrence of A. isabellae larvae. Developing larvae of A. isabellae were found again in 2010 on 11 of the 40 surveyed trees, proving that females of Spanish moon moth used at least these trees for egg-laying although mortality factors could have eliminated larvae or eggs laid on other trees. These 11 colonized trees included four ones with no grafted processionary colonies but three of them had one colony and four had five colonies.
Therefore, mated females of A. isabellae apparently lay eggs on pine trees whatever their defoliation status. In phytophagous insects, egg- laying and host choice behavior can help to precise population dynamics. A selective behavior may allow females to avoid an overexploitation of the resource. On the other hand, a lack of selectivity in host choice does not allow the species to cope with an altered food resource and its impact on the offspring development; this could be a reason for the eruptive phases observed in some populations (Tammaru et al. 1995). Mated females of A. isabellae lay approximately 90 eggs within several days (Chefaoui and Lobo 2007). A 15-year survey of their populations in this Alpine area did not reveal any eruptive patterns, or any strong defoliation of the host trees. This could suggest a selective behavior for the egg-laying site. However, under laboratory breeding conditions, mated females can lay eggs on dead or alive branches of any tree species, suggesting a non- selective behavior for the egg- laying site.
In Scot pines, defoliation results in large changes in the physico-chemical composition of needles as well as in the qualitative and quantitative of their volatile emissions (Honkanen et al. 1999; Poykko et al. 2005; Smits and Larsson 1999; Smits et al. 2001). Because hatching larvae of A. isabellae must feed on the tree where the eggs were laid, a non- selective behavior for the oviposition site may be problematic in case of large previous damage by processionary larvae.
4.4 Impact of Previous Defoliation by Pine Processionary Moth on Survival and Development of Actias isabellae
4.4.1 Change in Food Quality Induced by Defoliation Due to Pine Processionary Larvae
Defoliation by an herbivore may induce a defensive response from the attacked plant (Haukioja 1990; Nykanen and Koricheva 2004). This reaction, called ‘Induced Response’, may lead to a modification of the food quality for the defoliators (Smits et al. 2001), and have an impact on their population dynamics (Kaitaniemi et al. 1999; Nykanen and Koricheva 2004; Tscharntke et al. 2001; Williams et al. 2005). Thus, winter defoliation by T. pityocampa larvae is known to affect directly the quality of the juvenile foliage to be produced the following spring (Battisti 1988). Hódar et al. (2004) actually showed a direct relationship between the survival of T. pityocampa larvae on a pine tree in a given year and its defoliation by the previous generations of processionary moths, the more defoliation, the lower the larval survival. Indeed, pine trees generally compensate for defoliation by producing new foliage to restore resources for photosynthesis, but these renewed plant tissues usually show modifications in primary or secondary compounds (Poykko et al. 2005). Hódar et al. (2004) observed that the needles of defoliated pines present higher nitrogen levels and a lower content in fibers, tannins and phenolics than those of undefoliated pines, but they consider it difficult to identify the substances causing a detrimental effects on herbivores. Similarly, Battisti (1988) showed that complete tree defoliation by T. pityocampa significantly modified the physico-chemical characteristics of the subsequent needles in Austrian pines (Pinus nigra), therefore negatively affecting survival and development of the next generation of larvae feeding on the branches that had been previously defoliated. Increased death among the newly-hatched larvae feeding on trees previously defoliated might be an important driver of herbivore populations (Zalucki et al. 2002), and more especially the main cause of the collapse following the eruptive phase in several outbreaks of forest insects (Haukioja 1990; Hódar et al. 2004). Hódar et al. (2004) also showed that the most abundant terpenes of Scots pine foliage in Sierra Nevada Mountains had lower concentrations in trees previously defoliated by T. pityocampa. β-pinene contrastingly increased in pines defoliated the year before but not in these defoliated 2 years before or having two consecutive defoliations. They concluded that terpenes may be more constitutive than inducible in Scots pine. However, the relationships between the chemical profile of a plant and the response of the insects feeding on that plant remains still controversial (Hódar et al. 2004),
4.4.2 Do the Changes in Food Quality Induced by Processionary Larvae Affect Survival and Development of A. isabellae Larvae?
To investigate how the degree of previous defoliation by processionary larvae undergone by host pines might later affect the survival and development of Actias isabellae larvae, manipulative experiments were carried out under semi-natural conditions in a Scots pine nursery at INRA Orléans (Imbert et al. 2012). Sixteen trees from the same clone, and the same age, were manually implanted with processionary colonies during autumn 2009. Four treatments were applied in order to get an increasing rate of defoliation, from control trees without defoliation (0 %), to trees with little defoliation (5–25 %), medium defoliation (25–50 %), and heavy defoliation (more than 50 %). Then, in the following spring groups of 10 larvae Actias isabellae were reared from the first instar until pupae on twigs cut from these 16 test trees in order to assess the survival rate and the development time of each larval instar.
A daily survey revealed that the defoliation rate, whatever its magnitude, did not result in a significant change in the survival rate of Actias isabellae larvae (Fig. 7.12) whereas Hódar et al. (2004) observed an effect of the previous defoliation by pine processionary on the survival of conspecific larvae the year after. However, feeding on foliage of heavily defoliated trees (>50 %) resulted in a significant increase in the development time of A. isabellae larvae and a decrease in relative growth rate compared to feeding on foliage of undefoliated trees (Fig. 7.13a). A longer development time may have an indirect effect on the survival of larvae. The “slow growth, High mortality” hypothesis suggests that herbivores feeding on plant with low nutritional quality are likely to increase the time of their development, and so the window of vulnerability to natural enemies attack and/or climate is enlarged (Benrey and Denno 1997; Williams 1999; Cornelissen and Stiling 2006). However, lower defoliation levels did not result in significant differences in larval performances of A. isabellae compared to control (Imbert et al. 2012).
Mean time of the five larval instars of A. isabellae with regard to the degree of previous defoliation by T. pityocampa of the pines trees on which they are reared. Defoliation groups: C: no defoliation; LD: 5–25 % defoliation; MD: 25–50 %; HD: >50 % defoliation. Letters on the right indicate the differences in total development time according to defoliation groups at αB = 0.008 using multiple pairwise Mann-Whitney test (From Imbert et al. 2012)
Effect of the degree of previous defoliation of Scots pine trees by T. pityocampa on (a) the relative growth rate of larvae of A. isabellae fed with needles from these trees; (b) the dry weight of pupae of A. isabellae issued from these larvae. Defoliation groups: C: no defoliation; LD: 5–25 % defoliation; MD: 25–50 %; HD: >50 % defoliation. Boxes topped with the same letter are not significantly different at αB = 0.008 using multiple pairwise Mann-Whitney tests (From Imbert et al. 2012)
Dry weight of pupae was also significantly decreased when the larvae were fed with foliage of defoliated trees whatever the defoliation degree (Fig. 7.13b), and this may affect imago performances. Pupa weight is considered to be correlated with adult performances and especially the reproductive potential by influencing flight capabilities of males and mating process (Wigglesworth 1972) as well as female fecundity. Defoliation has already been shown to influence negatively the reproductive potential in other species (e.g., larch budmoth, Benz 1974). In females, the egg mass is a part of the adult body and it is related to the female size. In a number of species, smaller females were considered to lay a fewer number of eggs and therefore to present a reduced fecundity (Honek 1993; Pimentel et al. 2011). A. isabellae does not feed during the adult stage and then, the resources to be used during this stage for reproduction directly depend on these obtained during the larval stage (Heisswolf et al. 2009). Therefore, the consumption of needles from trees defoliated by pine processionary moth may reduce the number of eggs carried by females as well as the flight capacities of males. Since high degree of T. pityocampa defoliation is to be expected during the colonization phase, its arrival in the subalpine pine stands may effectively affect the populations of the endangered A. isabellae.
4.5 Are the Parasitoids Arriving with Pine Processionary Moth Susceptible to Affect Actias isabellae Populations?
4.5.1 Apparent Competition Between Pine Processionary Moth and Spanish Moon Moth?
Apparent competition is defined as the reduction of the fitness of a species caused by another species’ natural enemies (Holt 1977). For example in California vineyards the decrease in population density of a native leafhopper, Erythroneura eleganta Osborn, was not directly caused by the introduction of an invasive congeneric species, Erythroneura variabilis Beamer, but by an increase in the parasitism rate following the arrival of the invasive one (Settle and Wilson 1990). Another striking example is the parasitoid complex associated to the invasive gypsy moth, Lymantria dispar in the United States, including more than 60 introduced species (Kenis et al. 2009) of which the dipteran Compsilura concinnata (Meigen), which parasitizes larvae and turned later to be responsible for the extinction of several species of Saturniidae (Boettner et al. 2000). Péré et al. (2010) also showed that apparent competition mediated by shared natural enemies accompanying the spatial invasion in Central and Western Europe of the horse- chestnut leafminer, Cameraria orhidella Deschka & Dimic, affects species richness and abundance of other native leafminers. Such a process can be expected with the generalist parasitoids associated to pine processionary moth which could switch on other species, especially lepidopterans, in the newly colonized areas. Although their rate of expansion is lower than that of the pine processionary moth (see above 7.2.; Imbert 2012; Robinet et al. 2012), the presence of such parasitoids, those affecting moth egg at least, have now been ascertained near the expansion fronts (see 7.1) whereas pupal parasitoids are already present in isolated colonies beyond the front (Robinet et al. 2012). Thus, it can be wondered wether some of these generalist parasitoids can switch on Spanish moon moth.
4.5.2 The Native Parasitoid Complex Associated to Spanish Moon Moth
The natural enemies of Actias isabellae, especially these attacking the larval stages, are quite unknown, in France at least. In Spain, larvae were observed to be attacked by several parasitoids species such as the hymenopterans Pimpla robusta Rondani (Hymenoptera, Ichneumonidae) and Ichneumon microsticus Wsm. (Hymenoptera, Ichneumonidae) and the dipterans Compsilura concinnata (Meigen) (Diptera, Tachinidae), Masicera silvatica (Fallén) (Diptera, Tachinidae) and Drino inconspicua (Meigen) (Diptera, Tachinidae) (Peigler 1996). Tachinid and ichneumonid larval parasitoids have also been observed on other saturniid species (Boettner et al. 2000). Unlike processionary moths, no egg parasitoids have yet been detected. The adults, and principally males looking for females and attracted by their sex pheromones, are predated by bats during the night (Imbert, Personal observation).
4.5.3 Experimental Survey of a Possible Switch of Egg Parasitoids of Pine Processionary Moth on Spanish Moon Moth
Among the numerous parasitoids of T. pityocampa recorded in the Alps (Biliotti 1958), these associated with eggs are more easy to survey, sample and manipulate; an additional interest being that they are known to be capable of affecting largely the host population dynamics. Three of the four species observed in the Southern French Alps are generalists (Ooencyrtus pityocampae. Anastatus bifasciatus and Trichogramma sp.) whilst the last one, Baryscapus servadeii, is specialized on the processionary moth (see 7.2.). O. pityocampae and B. servadeii largely dominate the egg parasitoids in the expanding processionary moth populations found near the expansion front in the Upper Durance valley (see Fig. 7.3.). The generalist Ooencyrtus pityocampae is the first species to hatch, from early June on. This timing perfectly matches the end of the flight period of A. isabellae and, subsequently, the period where its eggs are laid. The eggs of Spanish moon moth largely differ from these of pine processionary moth by both their larger size, their blue- grey color and the much smaller size of the clutch, which corresponds to a tiny pack of ca. 10 eggs. In order to investigate the egg parasitism in A. isabellae under natural conditions and the possible sharing of parasitoids with T. pityocampa, eggs of A. isabellae were artificially offered to parasitoids in three experimental sites of Pinus sylvestris, selected along the Upper Durance valley according to the simultaneous presence or not of both moth species. A first site was located in the core range of A. isabellae where the pine processionary moth was not yet present (06°46′08.7″E; 44°44′15.8′N) whilst the second site located in the subalpine expansion range of processionary moth hosted both species (06°41′52.8″E; 44°40′ 33.8″N), and the third one was located much southern in the core area of the pine processionary moth (06°20′11.3″E; 44°31′38.7″N), where A. isabellae was not present. The last site could be considered as a control site to appreciate the capability of egg parasites to attack A. isabellae egg under high density of pine processionary moth. The eggs used for this experimentation were obtained through manipulative matings realized under natural conditions in the same area of the Alps between females issued from INRA laboratory breedings and wild males naturally attracted by these females. It allowed to expose three series of 20 eggs per site for a total of 60 eggs by site. Each group of 20 eggs was tied to a branch of a different Scots pine tree. The eggs were installed less than 24 h after laying and were left for 10 days on site. Then, the eggs were brought back to the laboratory in order to assess the relative proportion of moth larvae hatching vs. the possible emergence adult parasitoids. Eggs without any hatching were finally dissected to check for dead parasite larvae.
Whatever the site, no parasitism was finally observed on the exposed eggs whilst the percentage of hatching larvae of A. isabellae varied from 56.7 to 68.3 % but did not significantly differ between sites. O. pityocampae was successfully reared in Spain on eggs of A. isabellae eggs but only under controlled conditions, with no simultaneous offering of eggs of another species (Lopez-Sebastian et al. 2004). Even if this generalist parasitoid is present in the natural Alpine range of A. isabellae, it may be prevented to parasitize its eggs because of behavioral and/or physiological incompatibility (Menendez et al. 2008) or it may be unable to identify A. isabellae eggs in the pine forest. Actually, Battisti (1989) showed that O. pityocampae is attracted by the sex pheromone of pine processionnary moth, which allows it to locate the host. However, it has been observed to parasitize eggs of a number of other lepidopteran and hemipteran species of which several also develop on pine trees (e.g. the pine-tree lappet moth, Dendrolimus pini (L.), the pine hawk moth, Sphynx pinastri L.; Noyes 2003), indicating that it is capable of identifying those eggs.
Finally, even if the pine processionary moth has penetrated the natural range of A. isabellae in the Alps, its egg parasitoids do not seem to attack the eggs of the protected moth. However, it is likely that several of the numerous other parasitoid species associated with the larval and pupal life stages of pine processionnary moth (see Battisti et al. 2014, Chap. 2, this volume) have followed the expansion of their host although no precise assessment yet exists. Thus, a thorough study of the parasitoid complex associated to all life stages of A. isabellae seems urgent to be realized in order to both understand its role in the population dynamics of this specie and compare its composition with that of pine processionary moth in the expanding areas.
References
Abrams, P. A., & Ginzburg, L. R. (2000). The nature of predation: Prey dependent, ratio dependent or neither? Trends in Ecology & Evolution, 15, 337–341.
Arnaldo, P. S., & Torres, L. M. (2006). Effect of different hosts on Thaumetopoea pityocampa populations in northeast Portugal. Phytoparasitica, 34, 523–530.
Ashrafi, S., Beck, A., Rutishauser, M., Arlettaz, R., & Bontadina, F. (2011). Trophic niche partitioning of cryptic species of long-eared bats in Switzerland: Implications for conservation. European Journal of Wildlife Research, 57, 843–849.
Augustin, S., Guichard, S., Heitland, W., Freise, J., Svatos, A., & Gilbert, M. (2009a). Monitoring and dispersal of the invading Gracillariidae Cameraria ohridella. Journal of Applied Entomology, 133, 58–66.
Augustin, S., Settele, J., Roques, A., Garcia, J., Lopez-Vaamonde, C., Kenis, M., et al. (2009b). A stowaway species probably arriving from the Balkans, the horse chestnut leafminer, Cameraria ohridella. In J. Settele, L. D. Penev, T. A. Georgiev, R. Grabaum, V. Grobelnik, V. Hammen, S. Klotz, M. Kotarac, & I. Kühn (Eds.), Atlas of biodiversity risk (pp. 160–161). Sofia: Pensoft Publishers.
Barbaro, L., & Battisti, A. (2011). Birds as predators of the pine processionary moth (Lepidoptera: Notodontidae). Biological Control, 56, 107–114.
Barbaro, L., Couzi, L., Bretagnolle, V., Nezan, J., & Vetillard, F. (2008). Multi-scale habitat selection and foraging ecology of the Eurasian hoopoe (Upupa epops) in pine plantations. Biodiversity and Conservation, 17, 1073–1087.
Barbaro, L., Dulaurent, A. M., Payet, K., Blache, S., Vetillard, F., & Battisti, A. (2013). Winter bird numerical responses to a key defoliator in mountain pine forests. Forest Ecology and Management, 296, 90–97.
Barber, N. A., Marquis, R. J., & Tori, W. P. (2008). Invasive prey impacts regional distribution of native predators. Ecology, 89, 2678–2683.
Barbosa, P., Letourneau, D., & Agrawal, A. (2012). Insect outbreaks revisited. Chichester: Wiley, 480 p.
Battisti, A. (1988). Host-plant relationships and population dynamics of the pine processionary caterpillar Thaumetopoea pityocampa (Denis & Schiffermüller). Journal of Applied Entomology, 105, 393–402.
Battisti, A. (1989). Field studies on the behavior of 2 egg parasitoids of the pine processionary moth Thaumetopoea pityocampa. Entomophaga, 34, 29–38.
Battisti, A., Colazza, S., Roversi, P. F., & Tiberi, R. (1988). Alternative hosts of Ooencyrtus pityocampae Mercet (Hymenoptera: Encyrtidae) in Italy. Redia, 71, 321–328.
Battisti, A., Bernardi, M., & Ghiraldo, C. (2000). Predation by the hoopoe on pupae of Thaumetopoea pityocampa and the likely influence on other natural enemies. BioControl, 45, 311–323.
Battisti, A., Stastny, M., Netherer, S., Robinet, C., Schopf, A., Roques, A., et al. (2005). Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecological Applications, 15, 2084–2096.
Battisti, A., Stastny, M., Buffo, E., & Larsson, S. (2006). A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Global Change Biology, 12, 662–671.
Battisti, A., Avcı, M., Avtzis, D., Ben Jamaa, M. L., Berardi, L., Berretima, W., et al. (2014). Natural history of the processionary moths (Thaumetopoea spp.): New insights in relation to climate change. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer (Chapter 2).
Becerra, J. X. (2003). Synchronous coadaptation in an ancient case of herbivory. Proceedings of the National Academy of Sciences, 100, 12804–12807.
Benrey, B., & Denno, R. F. (1997). The slow-growth-high-mortality hypothesis: A test using the cabbage butterfly. Ecology, 78, 987–999.
Benz, G. (1974). Negative Rückkoppelung durch Raum-und Nahrungskonkurrenz sowie zyklischeVeränderung der Nahrungsgrundlage als Regelprinzip in der Populations-dynamik des Grauen Lärchenwicklers, Zeiraphera diniana (Guenée). Zeitschriftfür Angewandte Entomologie, 76, 196–228.
Berggren, Å., Björkman, C., Bylund, H., & Ayres, M. P. (2009). The distribution and abundance of animal populations in a climate of uncertainty. Oikos, 118, 1121–1126.
Biliotti, E. (1958). Les parasites et prédateurs de Thaumetopoea pityocampa Schiff. (Lepidoptera). Entomophaga, 3(1), 23–24.
Boettner, G. H., Elkinton, J. S., & Boettner, C. J. (2000). Effects of a biological control introduction on three nontarget native species of saturniid moths. Conservation Biology, 14, 1798–1806.
Bøhn, T., Amundsen, P. A., & Sparrow, A. (2008). Competitive exclusion after invasion? Biological Invasions, 10, 359–368.
Bretagnolle, V., & Gillis, H. (2010). Predator–prey interactions and climate change. In A. P. Moller, W. Fiedler, & P. Berthold (Eds.), Effects of climate change on birds (pp. 227–248). New York: Oxford University Press.
Brotons, L., & Herrando, S. (2003). Effect of increased food abundance near forest edges on flocking patterns of Coal Tit Parus ater winter groups in mountain coniferous forests. Bird Study, 50, 106–111.
Buckner, C. H. (1967). Avian and mammalian predators of forest insects. Entomophaga, 12, 491–501.
Buffo, E., Battisti, A., Stastny, M., & Larsson, S. (2007). Temperature as a predictor of survival of the pine processionary moth in the Italian Alps. Agricultural and Forest Entomology, 9, 65–72.
Carrascal, L. M., Seoane, J., & Villén-Pérez, S. (2013). Temperature and food constraints in wintering birds: An experimental approach in montane Mediterranean oakwoods. Community Ecology, 13, 221–229.
Cayuela, L., Hódar, J. A., & Zamora, R. (2011). Is insecticide spraying a viable and cost-efficient management practice to control pine processionary moth in Mediterranean woodlands? Forest Ecology and Management, 261, 1732–1737.
Charbonnier, Y., Barbaro, L., Theillout, A., & Jactel, H. (2014). Numerical and functional responses of forest bats to a major insect pest in pine plantations. PLoS ONE, 9, in press.
Chefaoui, R. M., & Lobo, J. M. (2007). Assessing the conservation status of an Iberian moth using pseudo-absences. Journal of Wildlife Management, 71, 2507–2516.
Colautti, R. I., Ricciardi, A., Grigorovich, I. A., & MacIsaac, H. J. (2004). Is invasion success explained by the enemy release hypothesis? Ecology Letters, 7, 721–733.
Cornelissen, T., & Stiling, A. (2006). Does low nutritional quality act as a plant defence? An experimental test of the slow-growth, high-mortality hypothesis. Ecological Entomology, 31, 32–40.
Cornell, H. V., & Hawkins, B. A. (1993). Accumulation of native parasitoid species on introduced herbivores – A comparison of hosts as natives and hosts as invaders. The American Naturalist, 141, 847–865.
Crawford, H. S., & Jennings, D. T. (1989). Predation by birds on spruce budworm Choristoneura fumiferana: Functional, numerical, and total responses. Ecology, 70, 152–163.
Cuadrado, M., & Dominguez, F. (1996). Phenology and breeding success of red-necked nightjar Caprimulgus ruficollis in Southern Spain. Journal für Ornithologie, 137, 249–253.
Démolin, G. (1969). Comportement des adultes de Thaumetopoea pityocampa Schiff. Dispersion spatiale, importance écologique. Annales des Sciences Forestières, 26, 89–102.
Dempster, J. P. (1983). The natural control of butterflies and moths. Biological Reviews, 58, 461–481.
Diaz, M., Illera, J. C., & Atienza, J. C. (1998). Food resource matching by foraging tits Parus spp during spring-summer in a Mediterranean mixed forest: Evidence for an ideal free distribution. Ibis, 140, 654–660.
Dulaurent, A. M., Rossi, J. P., Deborde, C., Moing, A., Menassieu, P., & Jactel, H. (2011). Honeydew feeding increased the longevity of two egg parasitoids of the pine processionary moth. Journal of Applied Entomology, 135, 184–194.
Ethier, K., & Fahrig, L. (2011). Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landscape Ecology, 26, 865–876.
Excoffier, L., Foll, M., & Petit, R. J. (2009). Genetic consequences of range expansions. Annual Review of Ecology, Evolution and Systematics, 40, 481–501.
Gale, G. A., DeCecco, J. A., Marshall, M. R., McClain, W. R., & Cooper, R. J. (2001). Effects of gypsy moth defoliation on forest birds: An assessment using breeding bird census data. Journal of Field Ornithology, 72, 291–304.
Garcia-Navas, V., Ferrer, E. S., & Sanz, J. J. (2013). Prey choice, provisioning behaviour, and effects of early nutrition on nestling phenotype of titmice. Ecoscience, 20, 9–18.
Gebiola, M., Lopez-Vaamonde, C., Nappo, A. G., & Bernardo, U. (2014). Did the parasitoid Pnigalio mediterraneus (Hymenoptera: Eulophidae) track the invasion of the horse chestnut leafminer? Biological invasions, 16, 843–857.
Géri, C. (1980). Application des méthodes d’études démécologiques aux insectes défoliateurs forestiers. Cas de Diprion pini L. (Hyménoptère Diprionidae). Dynamique des populations de la processionnaire du pin Thaumetopoea pityocampa (Lépidoptère Thaumetopoeidae) dans l’île de Corse. Doctorat d’Etat, Université Paris Sud, Orsay, France, 289 p.
Géroudet, P. (1963). Les passereaux II. Des mésanges aux fauvettes. Paris/Neuchâtel: Delachaux et Niestlé, 308 p.
Gill, B. J. (1980). Foods of the shining cuckoo (Chrysococcyx lucidus, Aves: Cuculidae) in New Zealand. New Zealand Journal of Ecology, 3, 138–140.
Glen, D. M. (2004). Birds as predators of lepidopterous larvae. In H. van Hemden & M. Rothschild (Eds.), Insect and bird interactions (pp. 89–106). Andover: Intercept.
Goiti, U., Vecin, P., Garin, I., Saloña, M., & Aihartza, J. R. (2003). Diet and prey selection in Kuhl’s pipistrelle Pipistrellus kuhlii (Chiroptera: Vespertilionidae) in south-western Europe. Acta Theriologica, 48, 457–468.
Gonzalez Cano, J. M. (1981). Predacion de procesionaria del pino por vertebrados en la zona de Mora de Rubielos (Teruel). Boletin de la Estacion Central de Ecologia, 10, 53–77.
Goussard, F., & Roques, A. (2007). Définition d’indicateurs de suivi des populations d’Isabelle de France Graellsia isabellae galliaegloria (Oberthür, 1922). Revue scientifique Bourgogne Nature, 5, 109–112.
Grabenweger, G., Kehrli, P., Zweimueller, I., Augustin, S., Avtzis, N., Bacher, S., et al. (2010). Temporal and spatial variations in the parasitoid complex of the horse chestnut leafminer during its invasion of Europe. Biological Invasions, 12, 2797–2813.
Grobler, B. C., & Lewis, O. T. (2008). Response of native parasitoids to a range-expanding host. Ecological Entomology, 33, 453–463.
Gurevitch, J., & Padilla, D. K. (2004). Are invasive species a major cause of extinctions? Trends in Ecology and Evolution, 19, 470–474.
Halperin, J. (1990). Natural enemies of Thaumetopoea sp. in Israel. Journal of Applied Entomology, 109, 425–435.
Haney, J. C. (1999). Numerical response of birds to an irruption of elm spanworm (Ennomos subsignarius [Hbn.]; Geometridae, Lepidoptera) in old-growth forest of the Appalachian Plateau, USA. Forest Ecology and Management, 120, 203–217.
Hanski, I., Henttonen, H., Korpimaki, E., Oksanen, L., & Turchin, P. (2001). Small-rodent dynamics and predation. Ecology, 82, 1505–1520.
Haukioja, E. (1990). Induction of Defenses in Trees. Annual Review of Entomology, 36, 25–42.
Heisswolf, A., Klemola, T., Andersson, T., & Ruohomaki, K. (2009). Shifting body weight-fecundity relationship in a capital breeder, maternal effects on egg numbers of the autumnal moth under field conditions. Bulletin of Entomological Research, 99, 73–81.
Hernández-López, A., Rougerie, R., Augustin, S., Lees, D. C., Tomov, R., Kenis, M., et al. (2011). Host tracking or cryptic adaptation? Phylogeography of Pediobius saulius (Hymenoptera, Eulophidae), a parasitoid of the highly invasive horse-chestnut leafminer. Evolutionary Applications, 5(3), 256–269.
Hill, A. M., & Lodge, D. M. (1999). Replacement of resident crayfishes by an exotic crayfish: The roles of competition and predation. Ecological Applications, 9, 678–690.
Hódar, J. A., & Zamora, R. (2004). Herbivory and climatic warming, a Mediterranean outbreaking caterpillar attacks a relict, boreal pine species. Biodiversity and Conservation, 13, 493–500.
Hódar, J. A., Castro, J., & Zamora, R. (2003). Pine processionary caterpillar Thaumetopoea pityocampa as a new threat for relict Mediterranean Scots pine forests under climatic warming. Biological Conservation, 110, 123–129.
Hódar, J. A., Zamora, R., Castro, J., & Baraza, E. (2004). Feast and famine, previous defoliation limiting survival of pine processionary caterpillar Thaumetopoea pityocampa in Scots pine Pinus sylvestris. Acta Oecologica, 26, 203–210.
Hódar, J. A., Cayuela, L., & Zamora, R. (2012). Climate change and the incidence of a forest pest in Mediterranean ecosystems, can the North Atlantic Oscillation be used as a predictor? Climatic Change, 113, 699–711.
Hódar, J. A., Torres-Muros, L., & Senhadji, K. (2013). Timing and intensity of katydid predation on egg batches of pine processionary moth, no evidence of population control. Agricultural and Forest Entomology, 15, 204–211.
Hogstad, O. (2005). Numerical and functional responses of breeding passerine species to mass occurrence of geometrid caterpillars in a subalpine birch forest, a 30-year study. Ibis, 147, 77–91.
Holt, R. D. (1977). Predation, apparent competition, and the structure of prey communities. Theoretical Population Biology, 12, 197–229.
Honek, A. (1993). Intraspecific variation in body size and fecundity in insects – A general realtionship. Oikos, 66, 483–492.
Honkanen, T., Haukioja, E., & Kitunen, V. (1999). Responses of Pinus sylvestris branches to simulated herbivory are modified by tree sink/source dynamics and by external resources. Functional Ecology, 13, 126–140.
Hoyas, J., & López, F. (1998). Distribución del críalo según la abundancia de procesionaria del pino. Quercus, 149, 20–22.
Huchon, H., & Démolin, G. (1971). La bioécologie de la processionaire du pin. Dispersion potentielle – Dispersion actuelle. Phytoma, 23, 11–20.
Imbert, C.-E. (2012). Expansion d’un ravageur forestier sous l’effet du réchauffement climatique, la processionnaire du pin affecte-t-elle la biodiversité entomologique dans les zones nouvellement colonisées ? Thèse de doctorat, Université d’Orléans, 196 p.
Imbert, C.-E., Goussard, F., & Roques, A. (2012). Is the expansion of the pine processionary moth, due to global warming, impacting the endangered Spanish moon moth through an induced change in food quality? Integrative Zoology, 7, 147–157.
Jung, K., Kaiser, S., Böhm, S., Nieschulze, J., & Kalko, E. K. V. (2012). Moving in three dimensions, effects of structural complexity on occurrence and activity of insectivorous bats in managed forest stands. Journal of Applied Ecology, 49, 523–531.
Kaitaniemi, P., Ruohomaki, K., Tammaru, T., & Haukioja, E. (1999). Induced resistance of host tree foliage during and after a natural insect outbreak. Journal of Animal Ecology, 68, 382–389.
Kalka, M. B., Smith, A. R., & Kalko, E. K. V. (2008). Bats limit arthropods and herbivory in a tropical forest. Science, 320, 71.
Karp, D. S., & Daily, G. C. (2014). Cascading effects of insectivorous birds and bats in tropical coffee plantations. Ecology, 95, 1065–1074.
Kaunisto, S., Valimaki, P., Kortet, R., Koskimaki, J., Harkonen, S., Kaitala, A., et al. (2012). Avian predation on a parasitic fly of cervids during winter, can host-related cues increase the predation risk? Biological Journal of the Linnean Society, 106, 275–286.
Keane, R. M., & Crawley, M. J. (2002). Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution, 17, 164–170.
Kenis, M., Auger-Rozenberg, M. A., Roques, A., Timms, L., Pere, C., Cock, M., et al. (2009). Ecological effects of invasive alien insects. Biological Invasions, 11, 21–45.
Kerdelhué, C., Battisti, A., Burban, C., Branco, M., Cassel-Lundhagen, A., İpekdal, K., et al. (2014). Genetic diversity and structure at different spatial scales in the processionary moths. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Kervyn, T., & Libois, R. (2008). The diet of the serotine bat, a comparison between rural and urban environments. Belgian Journal of Zoology, 138, 41–49.
Klemola, T., Tanhuanpia, M., Korpimaki, E., & Ruohomaki, K. (2002). Specialist and generalist natural enemies as an explanation for geographical gradients in population cycles of northern herbivores. Oikos, 99, 83–94.
Kohnen, A., Richter, I., & Brandl, R. (2012). No concordant phylogeographies of the rose gall wasp Diplolepis rosae (Hymenoptera, Cynipidae) and two associated parasitoids across Europe. PLoS ONE, 7(10), e47156.
Kunz, T. H., Braun de Torrez, E., Bauer, D., Lobova, T., & Fleming, T. H. (2011). Ecosystem services provided by bats. Annals of the New York Academy of Sciences, 1223, 1–38.
Liu, H., & Stiling, P. (2006). Testing the enemy release hypothesis, a review and meta-analysis. Biological Invasions, 8, 1535–1545.
Lopez-Sebastian, E., Selfa, J., & Ylla, J. (2004). Primeros datos del parasitismo de Ooencyrtus pityocampae (Mercet, 1921) sobre Graellsia isabellae (Graells, 1849) en condiciones de laboratorio. Graellsia, 60, 121–123.
Masutti, L. (1964). Ricerche sui parassiti oofagi della Thaumetopoea pityocampa Schiff. Annali del Centro di Economia Montana delle Venezie, 4, 205–271.
Maurel, N., Andrieux, T., Chanselme, D., Braud, Y., Breton, F., Goussard, F., et al. (2013). Cartographie d’Actias isabellae galliaegloria dans les Alpes françaises à l’aide d’un piège attractif non destructif utilisant une phéromone synthétique (Lep. Saturniidae). Oreina, 23, 15–19.
Menendez, R., Gonzalez-Megias, A., Lewis, O. T., Shaw, M. R., & Thomas, C. D. (2008). Escape from natural enemies during climate-driven range expansion, a case study. Ecological Entomology, 33, 413–421.
Mirchev, P., & Tsankov, G. (2000). Parasitism of egg-batches of pine processionary moth Thaumetopoea pityocampa (Den. and Schiff.) (Lepidoptera, Notodontidae) collected in Portugal. Forest Science, 4, 65–71.
Mirchev, P., Schmidt, G. H., & Tsankov, G. (1998). The egg parasitoids of the pine processionary moth Thaumetopoea pityocampa (Den. and Schiff.) in the Eastern Rhodopes, Bulgaria. Bollettino di Zoologia Agraria e di Bachicoltura, 30, 131–140.
Mirchev, P., Schmidt, G. H., Tsankov, G., & Pllana, S. (1999). Egg parasitoids of the processionary moth Thaumetopoea pityocampa (Den. and Schiff.) collected in Albania. Bollettino di Zoologia Agraria e di Bachicoltura, 31, 152–165.
Mirchev, P., Schmidt, G. H., Tsankov, G., & Avci, M. (2004). Egg parasitoids of Thaumetopoea pityocampa (Den. and Schiff.) (Lep., Thaumetopoeidae) and their impact in SW Turkey. Journal of Applied Entomology, 128, 533–542.
Mitchell, C. E., & Power, A. G. (2003). Release of invasive plants from fungal and viral pathogens. Nature, 421, 625–627.
Morris, R. F., Cheshire, W. F., Miller, C. A., & Mott, D. G. (1958). The numerical response of avian and mammalian predators during a gradation of the spruce budworm. Ecology, 39, 487–494.
Müller, J., Bussler, H., Gossner, M., Rettelbach, T., & Duelli, P. (2008). The European spruce bark beetle Ips typographus in a national park, from pest to keystone species. Biodiversity and Conservation, 17, 2979–3001.
Müller, J., Mehr, M., Bässler, C., Fenton, M., Hothorn, T., Pretzsch, H., et al. (2012). Aggregative response in bats, prey abundance versus habitat. Oecologia, 169, 673–684.
Mumm, R., Schrank, K., Wegener, R., Schulz, S., & Hilker, M. (2003). Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. Journal of Chemical Ecology, 29, 1235–1252.
Nakamura, H., & Miyazawa, Y. (1997). Movements, space use and social organisation of radio-tracked common cuckoos during the breeding season in Japan. Japanese Journal of Ornithology, 46, 23–54.
Nicholls, J. A., Fuentes-Utrilla, P., Hayward, A., Melika, G., Csóka, G., Nieves-Aldrey, J.-L., et al. (2010). Community impacts of anthropogenic disturbance, natural enemies exploit multiple routes in pursuit of invading herbivore hosts. BMC Evolutionary Biology, 10, 322.
Noyes, J. (2003). Universal Chalcidoidea Database. World Wide Web electronic publication. Retrieved November 25, 2013, from http://www.nhm.ac.uk/entomology/chalcidoids/index.html
Nykanen, H., & Koricheva, J. (2004). Damage-induced changes in woody plants and their effects on insect herbivore performance, a meta-analysis. Oikos, 104, 247–268.
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics, 37, 637–669.
Parmesan, C., & Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37–42.
Parmesan, C., Ryrholm, N., Stefanescu, C., Hill, J. K., Thomas, C. D., Descimon, H., et al. (1999). Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature, 399, 579–583.
Patten, M. A., & Burger, J. C. (1998). Spruce budworm outbreaks and the incidence of vagrancy in eastern North American wood-warblers. Canadian Journal of Zoology, 76, 433–439.
Peigler, R. S. (1996). Catalog of parasitoids of Saturniidae of the world. Journal of Research on the Lepidoptera, 33, 1–121.
Péré, C., Augustin, S., Tomov, R., Peng, L.-H., Turlings, T. C. J., & Kenis, M. (2010). Species richness and abundance of native leaf miners are affected by the presence of the invasive horse-chestnut leaf miner. Biological invasions, 12, 1011–1021.
Perez-Contreras, T., & Soler, J. J. (2004). Egg parasitoids select for large clutch sizes and covering layers in pine processionary moths (Thaumetopoea pityocampa). Annales Zoologici Fennici, 41(4), 587–597.
Perez-Contreras, T., Soler, J. J., & Soler, M. (2003). Why do pine processionary caterpillars Thaumetopoea pityocampa (Lepidoptera, Thaumetopoeidae) live in large groups? An experimental study. Annales Zoologici Fennici, 40(6), 505–515.
Philpott, S. M., Soong, O., Lowenstein, J. H., Pulido, A. L., Lopez, D. T., Flynn, D. F. B., et al. (2009). Functional richness and ecosystem services, bird predation on arthropods in tropical agroecosystems. Ecological Applications, 19, 1858–1867.
Pimentel, C., & Nilsson, J.-Å. (2007). Response of great tits Parus major to an irruption of a pine processionary moth Thaumetopoea pityocampa population with a shifted phenology. Ardea, 95, 191–199.
Pimentel, C., Calvão, T., & Ayres, M. P. (2011). Impact of climatic variation on populations of pine processionary moth Thaumetopoea pityocampa in a core area of its distribution. Agricultural and Forest Entomology, 13, 273–281.
Poykko, H., Rautio, P., Hyvarinen, M., Tuomi, J., Markkola, A., Kuikka, K., et al. (2005). Differential performance of two geometrids on previously defoliated Scots pine. Annales Zoologici Fennici, 42, 497–503.
Procter, D., & Harding, P. T. (Eds.). (2005). Red lists for invertebrates: Their application at different spatial scales – Practical issues, pragmatic approaches. Report 367. Peterborough: Joint Nature Conservation Committee, 94 p.
Rebelo, H., Tarroso, P., & Jones, G. (2010). Predicted impact of climate change on European bats in relation to their biogeographic patterns. Global Change Biology, 16, 561–576.
Renwick, J. A. A., & Chew, F. S. (1994). Oviposition behaviour in lepidoptera. Annual Review of Entomology, 39, 377–400.
Robinet, C., Baier, P., Pennerstorfer, J., Schopf, A., & Roques, A. (2007). Modelling the effects of climate change on the potential feeding activity of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Notodontidae) in France. Global Ecology and Biogeography, 16, 460–471.
Robinet, C., Imbert, C.-E., Rousselet, J., Sauvard, D., Garcia, J., Goussard, F., et al. (2012). Human-mediated long-distance jumps of the pine processionary moth in Europe. Biological Invasions, 14, 1557–1569.
Rodewald, P. G., & Brittingham, M. C. (2004). Stopover habitats of landbirds during fall, use of edge-dominated and early-successional forests. Auk, 121, 1040–1055.
Root, T. L., Price, J. T., Hall, K. R., Schneider, S. H., Rosenzweig, C., & Pounds, J. A. (2003). Fingerprints of global warming on wild animals and plants. Nature, 421, 57–60.
Roques, A. (1988). La spécificité des relations entre cônes de conifères et insectes inféodés en Europe occidentale, un exemple d’étude des interactions plantes-insectes. Thèse de Doctorat d’Etat ès Sciences, Université de Pau, 242 pp
Roques, L., Rossi, J. P., Berestycki, H., Rousselet, J., Garnier, J., Roquejoffre, J. M., et al. (2014). Modeling the spatio-temporal dynamics of the pine processionary moth. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Roques, A., Rousselet, J., Avci, M., Avtzis, D. N., Basso, A., Battisti, A., et al. (2014). Climate warming and past and present distribution of the processionary moths (Thaumetopoea spp.) in Europe, Asia Minor and North Africa. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Rousselet, J., Zhao, R., Argal, D., Simonato, M., Battisti, A., Roques, A., et al. (2010). The role of topography in structuring the demographic history of the pine processionary moth, Thaumetopoea pityocampa (Lepidoptera, Notodontidae). Journal of Biogeography, 37, 1478–1490.
Sachanowicz, K., Wower, A., & Bashta, A.-T. (2006). Further range extension of Pipistrellus kuhlii (Kuhl, 1817) in central and eastern Europe. Acta Chiropterologica, 8, 543–548.
Schmidt, G. H., Tanzen, E., & Bellin, S. (1999). Structure of egg-batches of Thaumetopoea pityocampa (Den. and Schiff.) (Lep., Thaumetopoeidae), egg parasitoids and rate of egg parasitism on the Iberian Peninsula. Journal of Applied Entomology-Zeitschrift für Angewandte Entomologie, 123, 449–458.
Schnitzler, H. U., & Kalko, E. K. V. (2001). Echolocation by insect-eating bats. BioScience, 51, 557–569.
Settle, W. H., & Wilson, L. T. (1990). Invasion by the variegated leafhopper and biotic interactions – Parasitism, competition, and apparent competition. Ecology, 71, 1461–1470.
Sherry, T. W. (1990). When are birds dietarily specialized? Distinguishing ecological from evolutionary approaches. Studies in Avian Biology, 13, 337–352.
Sierro, A., & Arlettaz, R. (1997). Barbastelle bats (Barbastella spp.) specialize in the predation of moths, implications for foraging tactics and conservation. Acta Oecologica, 18, 91–106.
Sierro, A., Arlettaz, R., Naef-Daenzer, B., Strebel, S., & Zbinden, N. (2001). Habitat use and foraging ecology of the nightjar Caprimulgus europaeus in the Swiss Alps, towards a conservation scheme. Biological Conservation, 98, 325–331.
Smith, B. T., Escalante, P., Hernandez Banos, B., Navarro-Siguenza, A. G., Rohwer, S., & Klicka, J. (2011). The role of historical and contemporary processes on phylogeographic structure and genetic diversity in the Northern Cardinal, Cardinalis cardinalis. BMC Evolutionary Biology, 11, 136.
Smits, A., & Larsson, S. (1999). Effects of previous defoliation on pine looper larval performance. Agricultural and Forest Entomology, 1, 19–26.
Smits, A., Larsson, S., & Hopkins, R. (2001). Reduced realised fecundity in the pine looper Bupalus piniarius caused by host plant defoliation. Ecological Entomology, 26, 417–424.
Stone, G. N., Lohse, K., Nicholls, J. A., Fuentes-Utrilla, P., Sinclair, F., Schönrogge, K., et al. (2012). Reconstructing community assembly in time and space reveals enemy escape in a Western Palearctic insect community. Current Biology, 22(6), 532–537.
Strom, B. L., Roton, L. M., Goyer, R. A., & Meeker, J. R. (1999). Visual and semiochemical disruption of host finding in the southern pine beetle. Ecological Applications, 9, 1028–1038.
Symondson, W. O. C., Sunderland, K. D., & Greenstone, M. H. (2002). Can generalist predators be effective biocontrol agents? Annual Review of Entomology, 47, 561–594.
Tagmann-Ioset, A., Schaub, M., Reichlin, T. S., Weisshaupt, N., & Arlettaz, R. (2012). Bare ground as a crucial habitat feature for a rare terrestrially foraging farmland bird of Central Europe. Acta Oecologica, 39, 25–32.
Tammaru, T., Kaitaniemi, P., & Ruohomaki, K. (1995). Oviposition choices of Epirrita autumnata (Lepidoptera, Geometridae) in relation to its eruptive population dynamics. Oikos, 74, 296–304.
Tanhuanpää, M., Ruohomaki, K., & Uusipaikka, E. (2001). High larval predation rate in non-outbreaking populations of a geometrid moth. Ecology, 82, 281–289.
Tanzen, E., & Schmidt, G. H. (1995). Identification by meconia of four species of egg parasitoids of Thaumetopoea pityocampa (Den. and Schiff.) (Insecta Lepidoptera Thaumetopoeidae). Bollettino di Zoologia Agraria e di Bachicoltura, 27, 61–70.
Thomson, L. J., Macfadyen, S., & Hoffmann, A. A. (2010). Predicting the effects of climate change on natural enemies of agricultural pests. Biological Control, 52, 296–306.
Tiberi, R. (1990). Egg parasitoids of the pine processionary caterpillar, Thaumetopoea pityocampa (Den and Schiff) (Lep, Thaumetopoeidae) in Italy – Distribution and activity in different areas. Journal of Applied Entomology-Zeitschrift für Angewandte Entomologie, 110, 14–18.
Tsankov, G., & Mirchev, P. (2003). The role of Trichogramma embryophagum (Hartig, 1838) (Hymenoptera, Trichogrammatidae) as a natural regulator of pine processionary moth number (Thaumetopoea pityocampa (Denis et Schiffermüller, 1775) (Lepidoptera, Notodontidae) in Bulgaria. Forest Science, 4, 33–41.
Tsankov, G., Schmidt, G. H., & Mirchev, P. (1995). Impact of parasitoids in egg-batches of Thaumetopoea pityocampa (Den. and Schiff.) in Algeria. Bollettino di Zoologia Agraria e di Bachicoltura, 27, 53–60.
Tsankov, G., Schmidt, G. H., & Mirchev, P. (1996). Parasitism of egg-batches of the pine processionary moth Thaumetopoea pityocampa (Den. and Schiff.) (Lep, Thaumetopoeidae) in various regions of Bulgaria. Journal of Applied Entomology-Zeitschrift für Angewandte Entomologie, 120, 93–105.
Tsankov, G., Schmidt, G. H., & Mirchev, P. (1998). Studies on the egg parasitism in Thaumetopoea pityocampa over a period of four years (1991–1994) at Marikostino/Bulgaria. Anzeiger für Schadlingskunde Pflanzenschutz Umweltschutz, 71, 1–7.
Tsankov, G., Mirchev, P., & Matova, M. (2006). Egg parasitoids, rate of parasitism and structure of egg batches of Thaumetopoea pityocampa (Den. and Schiff.) (Lep., Thaumetopoeidae) from the region of Ochrid (Republic of Macedonia). Silva Balcanica, 7, 77–87.
Tscharntke, T., Thiessen, S., Dolch, R., & Boland, W. (2001). Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochemical Systematics and Ecology, 29, 1025–1047.
Tscharntke, T., Bommarco, R., Clough, Y., Crist, T. O., Kleijn, D., Rand, T. A., et al. (2007). Conservation biological control and enemy diversity on a landscape scale. Biological control, 43, 294–309.
Valverde, J. A. (1971). Notas sobre la biologia reproductora del crialo Clamator glandarius (L.). Ardeola, numero especial, 591–647.
Vaughan, N. (1997). The diets of British bats (Chiroptera). Mammal Review, 27, 77–94.
Velky, M., Kanuch, P., & Kristin, A. (2011). Food composition of wintering great tits (Parus major) habitat and seasonal effects. Folia Zoologica, 60, 228–236.
Venier, L. A., & Holmes, S. B. (2010). A review of the interaction between forest birds and eastern spruce budworm. Environmental Review, 18, 191–207.
Vila, M., Auger-Rozenberg, M. A., Goussard, F., & Lopez-Vaamonde, C. (2009). Effect of non-lethal sampling on life-history traits of the protected moth Graellsia isabelae (Lepidoptera, Saturniidae). Ecological Entomology, 34, 356–362.
Visser, J. H. (1986). Host odor perception in phytophagous insectes. Annual Review of Entomology, 31, 121–144.
Visser, M. E., & Both, C. (2005). Shifts in phenology due to global climate change, the need for a yardstick. Proceedings of the Royal Society of London, Series B: Biological Sciences, 272, 2561–2569.
Walther, G. R., Roques, A., Hulme, P. E., Sykes, M. T., Pyšek, P., Kühn, I., et al. (2009). Alien species in a warmer world, risks and opportunities. Trends in Ecology and Evolution, 24, 686–692.
Waters, D. A. (2003). Bats and moths, what is there left to learn? Physiological Entomology, 28, 237–250.
Whelan, C. J., Wenny, D. G., & Marquis, R. J. (2008). Ecosystem services provided by birds. Annals of the New York Academy of Sciences, 1134, 25–60.
Wigglesworth, V. B. (1972). The principles of insect physiology. London: Chapman & Hall, 827 p.
Williams, I. S. (1999). Slow-growth, high-mortality – A general hypothesis, or is it? Ecological Entomology, 24, 490–495.
Williams, D. T., Straw, N. A., & Day, K. R. (2005). Performance of the green spruce aphid, Elatobium abietinum (Walker) on previously defoliated Sitka spruce. Agricultural and Forest Entomology, 7, 95–105.
Williams-Guillén, K., Perfecto, I., & Vandermeer, J. (2008). Bats limit insects in a neotropical agroforestry system. Science, 320, 70.
Wilson, J. M., & Barclay, R. M. R. (2006). Consumption of caterpillars by bats during an outbreak of western spruce budworm. The American Midland Naturalist, 155, 244–249.
Windmill, J. F. C., Jackson, J. C., Tuck, E. J., & Robert, D. (2006). Keeping up with bats, dynamic auditory tuning in a moth. Current Biology, 16, 2418–2423.
Zalucki, M. P., Clarke, A. R., & Malcolm, S. B. (2002). Ecology and behavior of first instar larval Lepidoptera. Annual Review of Entomology, 47, 361–393.
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., & Smith, G. M. (2009). Mixed effects models and extensions in ecology. New York: Springer, 574 p.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Éditions Quæ
About this chapter
Cite this chapter
Auger-Rozenberg, MA. et al. (2015). Ecological Responses of Parasitoids, Predators and Associated Insect Communities to the Climate-Driven Expansion of the Pine Processionary Moth. In: Roques, A. (eds) Processionary Moths and Climate Change : An Update. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9340-7_7
Download citation
DOI: https://doi.org/10.1007/978-94-017-9340-7_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9339-1
Online ISBN: 978-94-017-9340-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)