Summary
This chapter places two key photoprotective processes in the chloroplast (thermal dissipation of excess excitation energy and removal of reactive oxygen species, ROS, by anti-oxidants) into the context of whole-leaf and whole-plant function in the environment. The emerging evidence for possible trade-offs between effects of altered thermal dissipation and/or anti-oxidation capacity on plant resistance to abiotic stresses (unfavorable physical conditions) versus biotic stresses (pests and pathogens) is summarized. We conclude that more research on this topic is urgently needed, especially for specific crop species and agriculturally relevant environments, including various combinations of multiple abiotic and biotic stresses. As an example of a key redox-signaling pathway impacted by thermal dissipation and/or anti-oxidation capacity, the formation of lipid-peroxidation-derived hormonal messengers of the oxylipin family, with critical roles in plant growth, development, and defenses, is discussed. The available evidence for specific effects of the capacities of thermal dissipation and/or anti-oxidation on sugar loading into foliar phloem conduits, sugar export from leaves, whole-plant growth rate, and plant biotic defenses is reviewed. Lastly, leaf responses to moderate versus massive ROS formation via multiple feedback loops are compared and contrasted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Reactive Oxygen Species
- Reactive Oxygen Species Level
- Reactive Oxygen Species Formation
- Thermal Dissipation
- Excess Excitation Energy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I Introduction
The present volume is dedicated to a key aspect of the suite of photoprotective mechanisms (see Logan et al., Chap. 7) employed by photosynthetic organisms to avoid potential damage encountered during the collection and processing of solar energy. This key photoprotective process is the thermal dissipation of excess excitation energy from the singlet-excited state of chlorophyll a, assessed as the non-photochemical quenching of chlorophyll fluorescence, operating in conjunction with cellular anti-oxidant networks (see Havaux and García-Plazaola, Chap. 26). Assessing the level of employment of these different photoprotective processes in different species under various environmental conditions provides information about how those species cope with stress and excess light, furthering our understanding of each species’ role in the habitats where they thrive. Moreover, a better understanding of the mechanisms of thermal dissipation (and anti-oxidation) promises to provide the tools for identification, breeding, and molecular engineering of crop plants enhanced in various aspect of this component of photoprotection. It is often tacitly assumed that plants with a greater capacity for photoprotective thermal dissipation and/or anti-oxidation would be better protected against losses in productivity caused by unfavorable environmental conditions, thus resulting in plants with higher yields of food and fuels under not-always-perfect growing conditions. Do we, however, know this to be the case? Will crops with augmented photoprotection be higher-yielding crops?
The currently available evidence indeed suggests that losses in plant productivity caused by abiotic stresses, such as drought or unfavorable (excessively low or high) temperatures or soil mineral content, may be counteracted by augmented photoprotection (for abiotic stress tolerance of, e.g., zeaxanthin-deficient and zeaxanthin-overexpressing mutant lines, see Davison et al. 2002; Du et al. 2010; Gao et al. 2010; Wang et al. 2010; Chen et al. 2011). However, net crop productivity is affected not only by plant biomass yield, but is crucially dependent on limiting the staggering losses (over 50 %) to pests and pathogens as biotic stresses (Fletcher et al. 2006). In this chapter, we summarize the emerging evidence that plant resistance to pests and pathogens is affected by factors serving in thermal dissipation and/or anti-oxidation. In fact, it appears from the evidence available to date (as detailed below) that plant biotic stress resistance can be either lowered or increased by augmented anti-oxidation, and can be increased by a decrease in thermal dissipation capacity. Future work in specific crop species and specific environments (pointed out by, e.g., Horton, Chap. 3; Brooks et al., Chap. 13). This future work should also consider differences between defenses against pests versus herbivores as well as defenses against specific biotic agents within each of the two categories.
The call for more research into the potential costs of anti-oxidant overexpression echoes a parallel development in the medical field. For example, Jackson (2008) stated “From the initial view that ROS [reactive oxygen species] were potentially damaging and that prevention of their actions would inevitably be beneficial, we are reaching an understanding that these substances play fundamental roles in metabolism”, and Poljsak and Milisav (2012) elaborated that “Oxidative stress is not necessarily an un-wanted situation, since its consequences are beneficial for many physiological reactions in cells. On the other hand, there are potentially harmful effects of ‘antioxidative stress’”. Furthermore, Villanueva and Kross (2012) stated that, “the normal balance between antioxidants and free radicals in the body is offset when either of these forces prevails… In summary, a hypothesis is presented that ‘antioxidant-induced stress’ results when antioxidants overwhelm the body’s free radicals.”
II Integration of Photoprotection into Whole-Plant Functioning
Strong evidence has accumulated that (i) foliar levels of reactive oxygen species (ROS), and multiple signaling networks responding to cellular redox state (as the balance between oxidants and anti-oxidants), contribute chiefly to the regulation of plant growth and development as well as the orchestration of abiotic and biotic plant defenses (for a review, see, e.g., Munné-Bosch et al. 2013), and that (ii) chloroplast ROS level and redox state is modulated by thermal energy dissipation and anti-oxidant level (Ledford and Niyogi 2005).
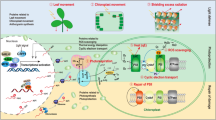
Figure 28.1 places thermal dissipation and anti-oxidation into the context of oxidative signaling networks regulating plant growth and development as well as plant defenses against pathogens and herbivores. When exposed to light levels that are limiting to photosynthesis, plants use as much of the light they absorb as possible in the photochemical pathway leading (via charge separation and photosynthetic electron transport) to sugar production. Plants grown in environments with fluctuating light levels harmlessly dissipate excess excitation energy (light absorbed in excess of what can be utilized photochemically) via photoprotective thermal dissipation of excessive excitation energy, which represents a non-photochemical route leading to non-photochemical quenching (NPQ) of chlorophyll fluorescence (Fig. 28.1). Excess singlet-excited chlorophyll a (1Chl*) is de-excited via thermal dissipation (involving, as summarized in the present volume, various xanthophyll pigments and proteins of the family of light-harvesting proteins; Fig. 28.1; see also, e.g., Büchel, Chap. 11; Brooks et al., Chap. 13; Morosinotto and Bassi, Chap. 14). Remaining 1Chl* (not dissipated via the combination of photochemical and non-photochemical pathways) can convert to triplet-excited chlorophyll (3Chl*) that readily passes its excitation energy to ground-state oxygen (Fig. 28.1). The NPQ-deficient Arabidopsis mutants npq1 (lacking violaxanthin de-epoxidase that catalyzes the conversion of violaxanthin to zeaxanthin; see, e.g., Demmig-Adams et al., Chap. 24) and npq4 (lacking the PsbS protein; see Brooks et al., Chap. 13), both deficient in NPQ (and thermal dissipation), are likely to form elevated levels of 3Chl* under excess light. While light-harvesting proteins possess potent quenchers of 3Chl* (see, e.g., Dall’Osto et al. 2006), any remaining 3Chl* will transfer its energy on to oxygen, forming highly reactive singlet oxygen 1O2* (Fig. 28.1). Tocopherols (vitamin E) as well as zeaxanthin are potent anti-oxidant quenchers of ROS like 1O2* (see Havaux and García-Plazaola, Chap. 26; concerning the terminology of vitamin E versus tocopherols, vitamins are specifically defined as compounds not synthesized in the organism in which they have essential functions, which does not apply to photosynthetic organisms proficient in the synthesis of compounds serving as vitamins in non-photosynthetic organisms. We will, therefore, refer mainly to tocopherols with occasional reference to the fact that these compounds also serve as vitamin E).
Schematic depiction, placing excitation energy conversion during the primary light reactions of photosynthesis in the context of ROS formation and ROS-triggered signaling with an emphasis on lipid-peroxide-derived oxylipin hormones. 1 Chl*: singlet-excited chlorophyll, 3 Chl*: triplet-excited chlorophyll, 1 O 2 *: singlet-excited oxygen, O 2 •−: oxygen radical anion (superoxide), LHC: light-harvesting complex, NPQ: non-photochemical quenching of chlorophyll fluorescence from singlet-excited chlorophyll a, npq1 Arabidopsis: mutant deficient in violaxanthin de-epoxidase catalyzing conversion of violaxanthin to zeaxanthin, npq4 Arabidopsis: mutant deficient in the photosystem II protein PsbS, ROS: reactive oxygen species, vte Arabidopsis: mutants lacking alpha-, beta-, gamma-, and delta-tocopherol as a result of deficiency in either the enzyme tocopherol cyclase (vte1) or homogentisate phytyltransferase (vte2).
Any remaining ROS (not removed by anti-oxidants) can give rise to oxidative signals – for example, lipid-peroxidation-derived oxylipin hormones like jasmonic acid (Fig. 28.2; see also section III below) that regulate plant growth and development as well as plant biotic defense (see also Brooks et al., Chap. 13). It should be noted that ROS-dependent oxylipin production is but one of a multitude of signaling pathways controlling plant function via redox-based signals (Munné-Bosch et al. 2013). For reviews of other redox signaling networks, see Baginsky and Link (2006), Dietz et al. (2006), Foyer et al. (2006), and Mullineaux et al. (2006).
III Lipid-Peroxidation-Derived Hormones as an Example for Redox Modulation of Plant Form and Function
Tocopherol-deficient plants exhibit defects in growth and development (for a review, see Falk and Munné-Bosch 2010), in particular impaired phloem loading, decreased carbohydrate export from leaves, enhanced foliar starch accumulation (Russin et al. 1996; Botha et al. 2000; Provencher et al. 2001; Sattler et al. 2003, 2004, 2006; Hofius et al. 2004; Maeda et al. 2006, 2008), and premature senescence (Abbasi et al. 2009). Falk and Munné-Bosch (2010) pointed out that these impairments in the growth of tocopherol-deficient plants are most obvious when the corresponding wild type plants exhibit a high sink demand (i.e., high carbohydrate consumption by the plant’s growing or carbohydrate-storing sinks); they stated that “the sucrose export phenotype is more clearly observed in tocopherol-deficient plants in species with a higher sink demand, such as potato or maize, and also in other species when grown at low temperature, such as Arabidosis thaliana.” Our own work has shown that Arabidopsis wild type increases the number of phloem cells in its leaf veins when grown under cool versus warm temperature, which likely contributes to a maintenance of carbon export flux and elevated levels of photosynthesis despite increased phloem sap viscosity at lower temperature (Cohu et al. 2013a,b).
The mechanism of growth inhibition in tocopherol-deficient plants apparently involves increased levels of ROS, lipid-peroxidation-derived hormonal messengers (such as jasmonic acid and related oxylipins), and deposition of callose in cells involved in phloem loading (in the leaf’s sugar-loading veins; Amiard et al. 2007). Callose formation has been shown to be associated with lipid peroxidation (Yamamoto et al. 2001), and a closing of phloem-loading routes via callose deposition occurs in plants infected by fungi (Koh et al. 2012), presumably to prevent the spread of the pathogen (Zavaliev et al. 2011). It has been shown that fungi and other pathogens utilize the plant’s phloem conduits to spread themselves throughout the plant (Gilbertson and Lucas 1996; Waigman et al. 2004; Scholthof 2005). While the benefit of an ROS-triggered shutdown of phloem conduits may lie in a greater pathogen resistance, the cost of this protection would be the plant’s diminished ability to export sugars from the photosynthesizing leaves for plant growth. Conversely, upregulation of foliar anti-oxidant levels, and a corresponding suppression of lipid-peroxidation-derived (and other oxidative) signals, has the potential benefit of preventing obstruction of phloem conduits (allowing unimpaired carbon export from source leaves to sinks), while potentially simultaneously rendering plants more vulnerable to succumbing to pathogen attack.
We note that a multitude of oxylipins is formed by either non-enzymatic or enzymatic lipid peroxidation, with both processes being modulated by ROS and anti-oxidants. Enzymatic lipid peroxidation occurs via lipoxygenases (LOX) with a catalytic iron center; ROS oxidize LOX from the inactive form LOX-Fe2+ to the LOX-Fe3+ form active in lipid peroxidation (Maccarrone et al. 1996), while anti-oxidants, such as tocopherols reduce LOX from the LOX-Fe3+ active form to the inactive form LOX-Fe2+ (Maccarrone et al. 1999). Products of both enzymatic and non-enzymatic lipid peroxidation serve as gene regulators in many organisms (see, e.g., Demmig-Adams and Adams 2010; Demmig-Adams et al. 2013).
Figure 28.3 summarizes the available evidence on altered redox state, altered oxidative messenger production, and altered biotic defenses in plants deficient in tocopherols (as key anti-oxidants: see, e.g., Havaux and García-Plazaola, Chap. 26), the PsbS protein (as a PS II protein and member of the light-stress-associated sub-family of light-harvesting proteins: see, e.g., Brooks et al., Chap. 13; Morosinottto and Bassi, Chap. 14), or the xanthophyll zeaxanthin (as a close correlate of thermal dissipation in plants grown in nature; see, e.g., Demmig-Adams et al., Chap. 24). Here we summarize the evidence showing increased ROS levels, increased oxylipin levels, and increased defenses against certain pests or pathogens, respectively, in plants deficient in tocopherols (Fig. 28.3; Vitamin E-deficient, vte), PsbS (Figs. 28.3, 28.4a, and 28.5a; PsbS-deficient, npq4), or zeaxanthin (Figs. 28.3, 28.4b, and 28.5b; Zeaxanthin-deficient, npq1 or npq1 lut2). Increased callose deposition in sugar-loading phloem complexes in tocopherol-deficient plants and in plants deficient in zeaxanthin and lutein has been observed (Fig. 28.5b). Furthermore, PsbS-deficient plants growing under highly variable light conditions in the field exhibit decreased sensitivity to a specific herbivore (Fig. 28.5a; see also Brooks et al., Chap. 13). To date, no information is available on the susceptibility of tocopherol-deficient plants to herbivore attack; future research is needed to address the possibility that the latter plants may have augmented herbivore defense, as seen in PsbS-deficient plants. However, it is clear that the defenses against multiple pests and pathogens involve multiple defense pathways that are regulated by different signaling pathways, and can have opposite effects (Derksen et al. 2013). For example, resistance of a range of pepper varieties against the major pepper insect pest flower thrips was positively correlated with foliar tocopherol content, with insect development apparently being inhibited by foliar tocopherol (Maharijaya et al. 2012). Future research is thus urgently needed to address the role of components of the photoprotection network in plant defense to multiple different biotic challenges (and, e.g., pests versus pathogens) as well as to combinations of biotic and abiotic stresses under actual field conditions (see also, e.g., Horton, Chap. 3; Brooks et al., Chap. 13). The possibility that trade-offs may exist between defenses against herbivores versus pathogens, or against any two specific biotic agents, also needs to be considered.
Summary of the available evidence for a role of the anti-oxidant tocopherol, the photosystem II protein PsbS, and the xanthophyll zeaxanthin in modulating ROS and oxylipin levels as well as in plant biotic defenses. (1) Jänkänpää et al. 2013, (2) Havaux et al. 2005; Sattler et al. 2006; Semchuk et al. 2009, (3) Frenkel et al. 2009, (4) Amiard et al. 2007; Demmig-Adams et al. 2013, (5) Munné-Bosch et al. 2007, (6) Botha et al. 2000; Maeda et al. 2006. Both npq1 and npq1 lut2 exhibited a trend (albeit not significant for npq1) for increased cell wall ingrowths/reinforcements. The double mutant npq1 lut2 exhibited even more pronounced cell wall ingrowths than npq1 and yielded significant differences versus wild type. Both npq1 and npq1 lut2 were transferred from low to moderate (double mutant) or high light (npq1) to trigger cell wall ingrowths. The PsbS-deficient npq4 mutant exhibited significant differences in oxylipin levels under herbivore attack in the field but not in the absence of herbivores. npq1, Arabidopsis mutant deficient in violaxanthin de-epoxidase catalyzing conversion of violaxanthin to zeaxanthin (this mutant was used to demonstrate elevated oxylipin levels in plants transferred from low growth light to high light; Demmig-Adams et al. 2013); npq1 lut2; Arabidopsis mutant deficient in both zeaxanthin (deficient in violaxanthin de-epoxidase catalyzing conversion of violaxanthin to zeaxanthin) and lutein (this mutant was used to demonstrate elevated cell wall ingrowths in phloem cells in plants transferred from low growth light to moderate light; Demmig-Adams et al. 2013); npq4, Arabidopsis mutant deficient in the photosystem II protein PsbS; ROS reactive oxygen species, vte, Arabidopsis mutant deficient in tocopherol synthesis and lacking alpha-, beta-, gamma-, and delta-tocopherol as a result of deficiency in either the tocopherol cyclase (vte1) or homogentisate phytyltransferase (vte2).
Augmented foliar oxylipin concentrations in Arabidopsis mutants deficient in either of two components of the photoprotective system. (a) Difference in jasmonic acid concentration between wildtype Arabidopsis (WT) and the PsbS-deficient npq4 mutant under outdoors/field conditions in the presence of herbivores (Modified after Demmig-Adams et al. 2013; based on original data from Frenkel et al. 2009). (b) Difference in the concentration of the jasmonic acid precursor OPDA (12-oxo-phytodienoic acid) between WT Arabidopsis and the zeaxanthin-deficient npq1 mutant upon transfer from low growth light to high light (modified after Demmig-Adams et al. 2013). *, significantly different at p < 0.05.
(a) Augmented defense against herbivores in the PsbS-deficient npq4 mutant and (b) augmented cell-wall deposition in phloem-loading complexes (as a putative defense against pathogens) in the zeaxanthin- and lutein-deficient double mutant npq1-2 lut2. The percent of plants attacked by herbivores was compared in wild type Arabidopsis (WT) and the PsbS-deficient npq4 mutant under outdoors/field conditions (Modified after Demmig-Adams et al. 2013; based on original data from Frenkel et al. 2009); WT and the zeaxanthin- and lutein-deficient double mutant npq1-2 lut2-1 were compared upon transfer from low growth light to high light (modified after Demmig-Adams et al. 2013). *, significantly different at p < 0.05.
IV Feedback Loops Between Photoprotection and Whole-Plant Function Under Moderately Versus Highly Excessive Light
Redox signaling networks include multiple feedback loops between primary photosynthetic reactions on the one hand, and plant growth, development and defenses on the other hand (Figs. 28.6 and 28.7). The overall outcome of the action of these multiple feedback loops apparently varies depending on how much excessive light is absorbed by a given leaf. Two contrasting scenarios are envisioned in Fig. 28.6 (moderate ROS formation) and Fig. 28.7 (massive ROS formation). Figure 28.6 depicts the putative situation in a leaf consuming/dissipating most of the absorbed light via the combination of photochemistry and thermal dissipation (NPQ), but also forming ROS levels sufficient to trigger production of redox signals that, in turn, fully induce synthesis of factors (i) involved in thermal dissipation (NPQ) and anti-oxidation as well as (ii) biotic defenses. It has, for example, been reported that jasmonic acid triggers de-novo synthesis of the anti-oxidant tocopherols (Sandorf and Holländer-Czytko 2002) and ascorbic acid (Wolucka et al. 2005) as well as of carotenoids (Pérez et al. 1993).
Schematic depiction of the link between light absorption, formation of reactive oxygen species (ROS), redox signaling, and plant responses like biotic defense under moderate ROS formation as well as (positive) feedbacks (green arrows) between redox signals and (i) anti-oxidation or (ii) thermal energy dissipation in the chloroplast (leading to non-photochemical quenching of chlorophyll fluorescence, NPQ).
Schematic depiction of the links among light absorption, formation of reactive oxygen species (ROS), redox signaling, and plant responses under massive ROS formation as well as feedbacks (red arrow and red feedback loops) between redox signals and shut-down of ROS sources via (i) inactivation of the oxygen-evolving complex and photosystem II (PS II) photochemistry and/or (ii) decreased light harvesting as well as (iii) transition from reversible to sustained non-photochemical quenching of chlorophyll fluorescence (NPQ).
Figure 28.7 depicts a putative situation for a leaf experiencing massive ROS formation. Continuing massive ROS formation and, e.g., leaf death (for an overview of senescence and death, see Biswal et al. 2013) can theoretically be prevented by a shutdown of the sources of ROS production. Two major forms of ROS produced in the primary light reactions are electronically excited singlet oxygen, formed in light-harvesting complexes (LHCs), and singly reduced superoxide, formed with electrons ultimately stemming from charge separation in the oxygen-evolving complex (OEC) and photosystem II reaction center (PS II-RC). Consequently, degradation of LHCs and inactivation and/or degradation of OEC and PS II-RC would theoretically serve to shutdown ROS formation (Fig. 28.7). The inactivation and/or net degradation of PS II-RC under highly excessive light indeed involves an inhibition of de-novo synthesis of the D1 protein of the PS II-RC by ROS (e.g., Murata et al. 2007). Future research is needed to address the possibility that inactivation/degradation of PS II-RC and/or OEC does serve to shut down massive ROS formation as proposed in Fig. 28.7 – while simultaneously shutting down photosynthesis (see also, e.g., Murchie and Harbinson, Chap. 25; Morales et al., Chap. 27). Degradation of PS II-RCs and OEC, and photosynthetic shutdown (“photoinhibition”), in overwintering evergreens is associated with maintenance of high Chl (and LHC) levels, and a transition from rapidly reversible NPQ to a sustained form of NPQ locked-in for an entire season during which plant growth is arrested (Adams et al. 1995, 2002, 2004, 2006; Zarter et al. 2006a,b,c).
Adams et al. 2013 (see also Adams et al., Chap. 23) have reviewed the evidence indicating that photoinhibition (as a long-term inactivation of primary photochemistry and photosynthesis) in evergreens, as well as in deciduous perennials, is associated with foliar carbohydrate accumulation, and presumably with either sink limitation or limiting sugar export from leaves. Conversely, species other than evergreens, exposed to mineral stress, typically exhibit pronounced degradation of LHCs and elimination of excess light-harvesting capacity, which is expected to lower ROS formation without requiring sustained NPQ or OEC/PS II-RC shutdown/degradation (see Morales et al., Chap. 27).
Placement of PS II/OEC inactivation into a whole-organism context also begs for re-assessment of the assignment of such inactivation as “damage”, similar to the re-evaluation efforts currently occurring in the medical field,
Reactive oxygen species (ROS) and chronic changes in membrane lipids are not the result of accidental damage. Instead, these changes are the result of a highly evolved, stereotyped, and protein-catalyzed ‘oxidative shielding’ response that all eukaryotes adopt when placed in a chemically or microbially hostile environment…Research efforts need to be redirected. Oxidative shielding is protective and is a misguided target for therapy…An alternative title for this review might be, ‘Oxidative stress or oxidative shielding: can 50 years of research be wrong?’ (Naviaux 2012).
It is important to elucidate whether ROS-triggered responses represent actual damage or a form of prevention, favored by natural selection, of actual damage. Furthermore, accelerated death of pre-existing leaves may actually hasten redeployment of resources towards growth of new leaves better adjusted to altered environmental conditions. Lastly, what is favorable for plant fitness in natural settings may not necessarily be favorable in agricultural settings, and differences among crop species or varieties in the degree of photosynthetic shutdown in response to environmental stress might be exploited in such settings.
V Conclusions
As illustrated by many examples in plant ecology, trade-offs between the benefits and costs of plant adaptations make many specific strategies beneficial only in certain environments but not in others. This principle needs to be further applied to the case of plant photoprotection via thermal dissipation of excess excitation energy and/or anti-oxidation. While plant resistance against losses in productivity due to abiotic stresses may possibly be improved by augmented thermal dissipation/anti-oxidation in certain cases, plant resistance to pests and pathogens can apparently be either lowered or increased by augmented thermal dissipation/anti-oxidation. Future work is urgently needed to address these questions in specific crop species and specific agriculturally relevant environments, including various combinations of abiotic and biotic stresses as well as specific pests and herbivores.
While the benefit of a shutdown of (sugar-exporting) foliar phloem conduits triggered by reactive oxygen species may lie in a greater resistance to, e.g., specific pathogens, the cost to the plant may be a diminished ability to export sugars from photosynthesizing leaves to the plant’s sinks for plant growth (and/or sugar and starch accumulation in fruits, roots, or tubers). We need to address the question of whether upregulation of foliar anti-oxidant levels and of the capacity for thermal dissipation, as well as putative corresponding suppression of oxidative signals, has the potential benefit of preventing any obstruction of phloem conduits, while possibly simultaneously coming with the cost of rendering plants more vulnerable to pathogen attack.
We suggest that research be done to further elucidate specific feedback loops between redox signals and gene regulation of the biosynthesis of factors involved in thermal dissipation and/or anti-oxidation. The possibility also needs to be addressed that inactivation/degradation of PS II reaction centers and the oxygen-evolving complex serves to shut down massive ROS formation under highly excessive light conditions. It should, furthermore, be considered that naturally occurring differences among plant species and varieties/ecotypes in maximal NPQ capacity, antioxidant levels, and lipid peroxidation levels have evolved as a result of trade-offs between the needs for specific abiotic and biotic defenses in specific environments.
Abbreviations
- 1Chl* –:
-
Singlet-excited chlorophyll;
- 1O2* –:
-
Singlet-excited oxygen;
- 3Chl* –:
-
Triplet-excited chlorophyll;
- Chl –:
-
Chlorophyll;
- LHC –:
-
Light-harvesting complex;
- NPQ –:
-
Non-photochemical quenching of chlorophyll fluorescence;
- npq1 –:
-
Non-photochemical quenching mutant #1 of Arabidopsis deficient in violaxanthin de-epoxidase catalyzing conversion of violaxanthin to zeaxanthin;
- npq4 –:
-
Non-photochemical quenching mutant #4 of Arabidopsis deficient in the photosystem II protein PsbS;
- O2 •− –:
-
Oxygen radical anion (superoxide);
- OEC –:
-
Oxygen-evolving complex;
- PS II –:
-
Photosystem II;
- PsbS –:
-
Photosystem II protein S (member of the stress-associated sub-family of light-harvesting proteins);
- RC –:
-
Reaction center;
- ROS –:
-
Reactive oxygen species;
- vte –:
-
Vitamin E-deficient Arabidopsis mutant lacking alpha-, beta-, gamma-, and delta-tocopherol as a result of deficiency in the enzyme tocopherol cyclase (vte1) or homogentisate phytyltransferase (vte2)
References
Abbasi A, Saur A, Hennig P, Tschiersch H, Hajirezaei M, Hofius D, Sonnewald U, Voll LM (2009) Tocopherol deficiency in transgenic tobacco (Nicotiana tabacum L.) plants leads to accelerated senescence. Plant Cell Environ 32:144–157
Adams WW III, Demmig-Adams B, Verhoeven AS, Barker DH (1995) ‘Photoinhibition’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol 22:261–276
Adams WW III, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V (2002) Photosynthesis and photoprotection in overwintering plants. Plant Biol 4:545–557
Adams WW III, Zarter CR, Ebbert V, Demmig-Adams B (2004) Photoprotective strategies of overwintering evergreens. Bioscience 54:41–49
Adams WW III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2006) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht, pp 49–64
Adams WW III, Cohu CM, Muller O, Demmig-Adams B (2013) Foliar phloem infrastructure in support of photosynthesis. Front Plant Sci 4:194. doi:10.3389/fpls.2013.00194
Amiard V, Demmig-Adams B, Mueh KE, Turgeon R, Combs AF, Adams WW III (2007) Role of light and jasmonic acid signaling in regulating foliar phloem cell wall ingrowth development. New Phytol 173:722–731
Baginsky S, Link G (2006) Redox regulation of chloroplast gene expression. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht, pp 269–287
Biswal B, Krupinska K, Biswal UC (eds) (2013) Plastid Development in Leaves During Growth and Senescence. Advances in Photosynthesis Including Bioenergy and Related Processes, Volume 36. Springer, Dordrecht
Botha CEJ, Cross RHM, van Bel AJE, Peter CI (2000) Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath-vascular parenchyma interface. Protoplasma 214:65–72
Chen X, Han H, Jiang P, Nie L, Bao H, Fan P, Lv S, Feng J, Li Y (2011) Transformation of β-lycopene cyclase genes from Salicornia europaea and Arabidopsis conferred salt tolerance in Arabidopsis and tobacco. Plant Cell Physiol 52:909–921
Cohu CM, Muller O, Demmig-Adams B, Adams WW III (2013a) Minor loading vein acclimation for Arabidopsis thaliana ecotypes in response to growth under different temperature and light regimes. Front Plant Sci 4:240. doi:10.3389/fpls.2013.00240
Cohu CM, Muller O, Stewart JJ, Demmig-Adams B, Adams WW III (2013b) Association between minor loading vein architecture and light- and CO2-saturated rates of photosynthetic oxygen evolution among Arabidopsis thaliana ecotypes from different latitudes. Front Plant Sci 4:264. doi:10.3389/fpls.2013.00264
Dall’Osto L, Lico C, Alric J, Guiliano G, Havaux M, Basssi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6:32. doi:10.1186/1471-2229-6-32
Davison PA, Hunter CN, Horton P (2002) Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418:203–206
Demmig-Adams B, Adams WW III (2010) Overview of diet-gene interaction and the example of xanthophylls. Adv Exp Med Biol 698:17–26
Demmig-Adams B, Cohu CM, Amiard V, van Zadelhoff G, Veldink GA, Muller O, Adams WW III (2013) Emerging trade-offs – impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins and biotic defense. New Phytol 197:720–729
Derksen H, Rampitsch C, Daayf F (2013) Signaling cross-talk in plant disease resistance. Plant Sci 207:79–87
Devoto A, Turner JG (2005) Jasmonate-regulated Arabidopsis stress-signaling network. Physiol Plant 123:161–172
Dietz K-J, Stork T, Finkemeier I, Lamkemeyer P, Li W-X, El-Tayeb MA, Michel K-P, Pistorius E, Baier M (2006) The role of peroxieredoxins in oxygenic photosynthesis of cyanbacteria and higher plants: Peroxide detoxification or redox sensing? In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht, pp 303–319
Du H, Wang N, Cui F, Li X, Xiao J, Xiong L (2010) Characterization of the β-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol 154:1304–1318
Falk J, Munné-Bosch S (2010) Tocochromanol functions in plants: antioxidants and beyond. J Exp Bot 61:1549–1566
Fletcher J, Bender C, Budowle B, Cobb WT, Gold SE, Ishimaru CA, Luster D, Melcher U, Murch R, Scherm H, Seem RC, Sherwood JL, Sobral BW, Tolin SA (2006) Plant pathogen forensics: capabilities, needs, and recommendations. Microbiol Mol Biol Rev 70:450–471
Foyer CH, Trebst A, Noctor G (2006) Signaling and integration of defense functions of tocopherol, ascorbate and glutathione. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, Gene Regulation, and Environment.Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht, pp 241–268
Frenkel M, Külheim C, Jänkänpää HJ, Skogström O, Dall’Osto L, Ågren J, Bassi R, Moritz T, Moen J, Jansson S (2009) Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol 9:12
Gao S, Han H, Feng H-L, Zhao S-J, Meng Q-W (2010) Overexpression and suppression of violaxanthin de-epoxidase affects the sensitivity of photosystem II photoinhibition to high light and chilling stress in transgenic tobacco. J Integr Plant Biol 52:332–339
Gilbertson RL, Lucas WJ (1996) How do viruses traffic on the ‘vascular highway’? Trends Plant Sci 1:260–268
Havaux M, Eymery F, Profirova S, Rey P, Dorman P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17:3451–3469
Hofius D, Hajirezael MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U (2004) RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol 135:1256–1268
Jackson MJ (2008) Free radicals generated by contracting muscle: by-products of metabolism or key regulators of muscle function? Free Radic Biol Med 44:132–141
Jänkänpää HJ, Frenkel M, Zulfgarov I, Reichelt M, Krieger-Liszkay A, Mishra Y, Gershenzon J, Moen J, Lee C-H, Jansson S (2013) Non-photochemical quenching capacity in Arabidopsis thaliana affects herbivore behaviour. PLoS One 8:e53232. doi:10.1371/journal.pone.0053232
Koh EJ, Zhou L, Williams DS, Park J, Ding N, Duan YP, Kang BH (2012) Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with “Candidatus Liberibacter asiaticus”. Protoplasma 249:687–697
Ledford HK, Niyogi KK (2005) Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ 28:1037–1045
Maccarrone M, Corasantini MT, Guerrieri P, Nistico G, Finazzi-Agrò A (1996) Nitric oxide-donor compounds inhibit lipoxygenase activity. Biochem Biophys Res Commun 219:128–133
Maccarrone M, Lorenzon T, Guerrieri P, Finazzi-Agrò A (1999) Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur J Biochem 265:27–34
Maeda H, Song W, Sage TL, DellaPenna D (2006) Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 18:2710–2732
Maeda H, Sage TL, Isaac G, Welti R, DellaPenna D (2008) Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell 20:452–470
Maharijaya A, Vosman B, Verstappen F, Steenhuis-Broers G, Mumm R, Purwito A, Visser RGF, Voorrips RE (2012) Resistance factors in pepper inhibit larval development of thrips (Frankliniella occidentalis). Entomol Exp Appl 145:62–71
Mullineaux PM, Karpinski S, Creissen GP (2006) Integration of signaling in antioxidant defenses. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration, Volume 21. Springer, Dordrecht, pp 223–239
Munné-Bosch S, Weiler EW, Alegre L, Muller M, Duchting P, Falk J (2007) α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225:681–691
Munné-Bosch S, Queval G, Foyer CH (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161:5–19
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Naviaux RK (2012) Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 342:608–618
Pérez AG, Sanz C, Richardson DG, Olías JM (1993) Methyl jasmonate vapor promotes β-carotene synthesis and chlorophyll degradation in golden delicious apple peel. J Plant Growth Regulate 12:163–167
Poljsak B, Milisav I (2012) The neglected significance of “antioxidative stress”. Oxidative Med Cell Longev 480895. doi:10.1155/2012/480895
Provencher LM, Miao L, Sinha N, Lucas WJ (2001) Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13:1127–1141
Russin WA, Evert RF, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8:645–658
Sandorf I, Holländer-Czytko H (2002) Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta 216:173–179
Sattler SE, Cajon EB, Coughlin SJ, DellaPenna D (2003) Characterization of tocopherol cyclases from higher plants and cyanobacteria: evolutionary implications for tocopherol synthesis and function. Plant Physiol 132:2184–2195
Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16:1419–1432
Sattler SE, Mene-Saffrane L, Farmer EE, Krischke M, Mueller MJ, DellaPenna D (2006) Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 18:3706–3720
Scholthof HB (2005) Plant virus transport: motions of functional equivalence. Trends Plant Sci 10:376–382
Semchuk NM, Lushchak OV, Falk J, Krupinska K, Lushchak VI (2009) Inactivation of genes, enconding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol Biochem 47:384–390
Villanueva C, Kross RD (2012) Antioxidant-induced stress. Int J Med Sci 13:2091–2109
Waigman E, Ueki S, Trutnyeva K, Citovsky V (2004) The ins and outs of nondestructive cell-to-cell systemic movement of plant viruses. Crit Rev Plant Sci 23:195–250
Wang H, Li B, Feng H-L, Zhang Q-Y, Yang X-H, Meng Q-W (2010) Anti-sense mediated suppression of tomato zeaxanthin epoxidase alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. Photosynthetica 48:409–416
Wolucka BA, Goossens A, Inzé D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56:2527–2538
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Zarter CR, Adams WW III, Ebbert V, Adamska I, Jansson S, Demmig-Adams B (2006a) Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant Cell Environ 29:869–878
Zarter CR, Adams WW III, Ebbert V, Cuthbertson D, Adamska I, Demmig-Adams B (2006b) Winter downregulation of intrinsic photosynthetic capacity coupled with upregulation of Elip-like proteins and persistent energy dissipation in a subalpine forest. New Phytol 172:272–282
Zarter CR, Demmig-Adams B, Ebbert V, Adamska I, Adams WW III (2006c) Photosynthetic capacity and light harvesting efficiency during the winter-to-spring transition in subalpine conifers. New Phytol 172:283–292
Zavaliev R, Ueki S, Epel B, Citovsky V (2011) Biology of callose (beta-1,3-glucan) turnover at plasmodesmata. Protoplasma 248:117–130
Acknowledgments
We wish to thank Drs. Barry Logan and Stefan Jansson for their helpful comments on our manuscript. The work in our laboratory is currently supported by the University of Colorado at Boulder and the National Science Foundation (award number DEB-1022236).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Demmig-Adams, B., Stewart, J.J., Adams, W.W. (2014). Chloroplast Photoprotection and the Trade-Off Between Abiotic and Biotic Defense. In: Demmig-Adams, B., Garab, G., Adams III, W., Govindjee, . (eds) Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration, vol 40. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9032-1_28
Download citation
DOI: https://doi.org/10.1007/978-94-017-9032-1_28
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9031-4
Online ISBN: 978-94-017-9032-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)