Abstract
The synthesis of organic compounds from aqueous carbon dioxide, the aquatic primary production, principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through chemosynthesis, which uses the oxidation or reduction of chemical compounds as its source of energy. Here, we present both pathways and their contribution to the primary production in Lake Kinneret. Phytoplankton density, measured as chlorophyll a (Chl a), and photosynthetic primary productivity (PP), measured as radiocarbon isotope uptake, have been monitored in the lake for more than four decades. The average Chl a areal concentration in the lake is 208 mg m−2 and the PP is 1.66 g C m−2 day−1. Both parameters displayed marked seasonal patterns, with highest values occurring in April and May. From July to December, Chl a and PP values were on average only 24 and 64 %, respectively, of the spring maxima. However, since the mid-1990s, a definite weakening of the seasonal periodicity of Chl a and PP was observed.
Intensive chemosynthetic microbial activity, fueled by H2S oxidation, was measured at the chemoclines during its deepening below the photic zone in late autumn, and close to the sediment–water interface in May when the chemocline starts to form. Averaged depth-integrated chemoautotrophic primary production at the chemocline was 16 and 24 % of the photosynthetic primary production in May and during autumn, respectively. The δ13C of particulate organic matter at the chemocline ranged between − 27 and − 39 ‰, suggesting the involvement of intensive chemosynthesis. Mass and isotopic balance of carbon and H2S suggest that chemosynthetic production contributes between 20 and 30 % of the total primary production in Lake Kinneret annually. This component should be considered when the lake’s carbon budgets and food webs are assessed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Carbon fixation
- Chemoautotrophy
- Chemocline
- Chlorophyll
- H2S oxidation
- Light attenuation
- Light penetration
- Primary production
- Oxic–anoxic interface
- Sediment–water interface
For many years, the primary production in Lake Kinneret was solely assessed from in situ measurement of photosynthetic carbon assimilation . Phytoplankton abundance (in terms of chlorophyll a concentration) and photosynthetic primary production have been systematically measured for more than four decades within the framework of the Lake Kinneret monitoring program. The contribution of chemosynthetic bacteria was only occasionally measured and indicated the role and contribution of chemosynthesis to the overall primary production and to the carbon cycle in the lake. This chapter summarizes the observed trends of intra- and interannual variation in key phytoplankton variables, chlorophyll abundance, photosynthetic primary production rates, as well as the contribution of chemotrophic bacteria to the production.
1 Photosynthetic Primary Production
Yosef Z Yacobi
1.1 A Short Historical Account
The first systematic measurement of primary productivity (PP) in Lake Kinneret was made from June 1956 to July 1957 at a single offshore station located at the southwestern side of the lake (Yashouv and Alhunis 1961). The measurements were based on the oxygen light and dark bottle method. Using the same methodology, Hepher and Langer (1970) extended the work in 1964–1965 to six stations distributed over the lake; they were also the first to systematically measure chlorophyll a (Chl a) as a crude estimate of phytoplankton biomass . Both studies revealed typical seasonality of phytoplankton abundance and PP, with a substantial peak in spring, when the dinoflagellate Peridinium gatunense bloomed. Thus, Chl a and photosynthetic rates reached maximum values before solar input and temperatures reached their annual maxima. In 1969, the 14C-tracer method (Steemann-Neilsen 1952) was introduced by Rohde (1972) and was soon established as the routine method for the assessment of PP in Lake Kinneret. Multiannual summaries of chlorophyll a and primary production dynamics in Lake Kinneret have been published on several occasions (Berman and Pollingher 1974; Pollingher and Berman 1977, 1982; Berman et al. 1992, 1995; Yacobi and Pollingher 1993; Yacobi 2006). The methodologies used for measurement of Chl a (Holm-Hansen et al. 1965) and PP (Steemann-Nielsen 1952) have remained unchanged throughout the entire period, enabling direct comparison of measurements in the multiannual record.
1.2 Light Characteristics
Photosynthetically active radiation (PAR) input has been measured systematically in Lake Kinneret since 1994, using a quantum sensor LI−190 (Licor, Lincoln, NE, USA). The average (± SD) PAR measured at the Lake Kinneret water surface during 1995–2011 was 1,232 (± 472) µμμmol photon m−2 s−1. Maximum values were usually recorded in June and minimum values in December (Fig. 24.1). The early PAR measurements within the water column of the lake were restricted to four wavelength bands (Rohde 1972; Berman 1976a) and 19 wavelength bands (Dubinsky and Berman 1976, 1979). The result of these studies showed: (a) seasonal changes in PAR penetration in the lake and suggested a two-way relationship between light penetration and phytoplankton density (phytoplankton affected light penetration, and limited its own light availability); (b) the dense crop formed by P. gatunense dramatically shifted the spectral properties of the underwater light field, from green being the most penetrating wavelength at the beginning of the bloom towards red prior to its collapse; (c) light utilization efficiency in the water column increased with depth. The integrated value for light utilization efficiency in the trophogenic layer ranged from 0.33 to 4.01 %, with lowest values associated with domination of P. gatunense.
From 1970 until the present, in-water PAR measurements, based on a broadband (400–700 nm) sensor, were used for routine calculation of the light attenuation coefficient, K d. The vertical distribution of particles was mostly fairly uniform and the value of K d varied only slightly with depth in the euphotic zone, except when massive blooms of P. gatunense occurred. However, when P. gatunense dominated the lake phytoplankton, with a dense crop in the uppermost layers of the water column, K d changed conspicuously with depth (Dubinsky and Berman 1979). In order to circumvent the issue of heterogeneous vertical distribution of particles during P. gatunense blooms, a depth interval of 0.5–2.5 m was used to calculate K d (Yacobi 2006) . From 1990 through 2011, the average K d in the 0.5–2.5-m water layer ranged by almost an order of magnitude (Fig. 24.2a, Table 24.1), with the highest values in April and the lowest in July and August (Fig. 24.3). Over the period from 1990 to 2011, there was a nonsignificant trend of decrease in K d (Fig. 24.2a). A more extensive data set based on measurements from 1970 to 2008 also showed a very slight time-dependent increase of K d (Rimmer et al. 2011). Thus, it appears that the average in-water light penetration in Lake Kinneret has remained essentially unchanged over the past four decades.
The slope of the regression of K d versus Chl a concentration yields the value of the partial attenuation attributed to Chl (K c) . For the period 1990 to 2011, K c was 0.0033 mg−1 Chl a m−2. Elimination of entries where Chl a was > 60 mg m−3, i.e., when P. gatunense dominated the phytoplankton community, brought the K c value to 0.007 mg−1 Chl a m−2.
The Secchi depth showed a significant decreasing trend from 1990 to 2011 (Fig. 24.2b), suggesting that in-water light scattering increased in that time interval. By contrast, in the period from 1970 to 1990, no significant trend for the Secchi depth was observed. Increase in scattering may be the result of increase in the concentration of particles or in light scattering per particle. Data on particle concentrations in the lake do not indicate a consistent time-dependent increase since 1998 (Chap. 26). Thus, it appears more likely that the decrease in the Secchi depth reflects changes in the taxonomic composition of the phytoplankton (Chap. 10) and in the size and/or composition of detrital and inorganic particles (Chap. 26).
The reciprocal of Secchi depth and K d were positively correlated for all data points from 1990 to 2011 (r 2 = 0.31, p < 0.001, n = 495). Both optical parameters were moderately correlated with the average Chl a density in the 0–15-m water column or in the uppermost (0–1 m) layer of the lake, with the strongest correlation between Chl a concentration in the uppermost layer and the reciprocal of Secchi depth (r 2 = 0.52, p < 0.001, n = 495). When Chl a values &>20 mg m−3 were excluded, a much weaker correlation resulted (r 2 = 0.06, p < 0.001 n = 393) suggesting that in periods when P. gatunense was not dominant, phytoplankton was not the major factor determining the light climate in the water column. The levels of colored dissolved organic matter in the epilimnion were extremely low; consequently, particulate detritus was probably the major modifier of water optical characteristics.
1.3 Chl a and Primary Production: 1970–2011
Mean (± SD) Chl a in the epilimnion (0 to 15 m) of the lake from 1970 to 2011 was 208 (± 186) mg m−2 and mean (± SD) daily PP 1.67 (± 0.70) g C m−2 day−1 (Table 24.1). Monthly averages of Chl a were positively correlated with microscopically determined phytoplankton wet weight biomass (r 2 = 0.67, p < 0.001, n = 504,). For all sampling dates from 1972 until 2011, Chl a and phytoplankton wet weight biomass were significantly positively correlated with PP, but with relatively low coefficients of correlation (r 2 = 0.19 and 0.16, respectively, p < 0.001, n = 441). Primary production per unit of Chl a changed conspicuously with phytoplankton taxonomic identity (Yacobi and Zohary 2010) as well as temporally, indicating variation in light climate in the water column and probably the availability of nutrients.
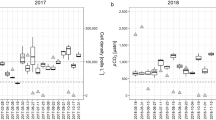
The monthly averages of Chl a (Fig. 24.4a) and PP (Fig. 24.4b) displayed a seasonal pattern of phytoplankton density and photosynthetic activity, with a clear Chl a peak in April and somewhat less prominent peaks of PP in April and May. The high spring peaks were caused by the development of dense populations of P. gatunense, a highly regular phenomenon until the early 1990s (Zohary 2004; Chap. 10). Overall, the monthly averages of Chl a showed greater variability than those of PP, as shown by the ratio between maximum and minimum monthly averages (4.5 and 2.2 for Chl a and PP, respectively). Similarly, a comparison of all measurements showed that integrated Chl a varied more widely than PP (Table 24.1).
The seasonality of Chl a and PP is shown in Fig. 24.5 from 1970 to 2011. Changes in the seasonal pattern occurred after the mid-1990s due to the absence of P. gatunense blooms in some years (1996–1997, 2001–2002, 2005, 2008–2011) but very dense P. gatunense populations in 1994, 1995, 1998, 2003, and 2007, with a ratio of > 10 between the highest and lowest monthly averages. Judging by the running average (Fig. 24.5), it seems that overall, from 1970 to 2011, Chl a concentrations and PP rates have not changed dramatically. On the other hand, the pattern of intra-annual variation of phytoplankton parameters did change.
Lake Kinneret data have often been presented in terms of semiannual averages, representative of the winter–spring (January–June) and the summer–autumn (July–December) conditions (e.g., Berman et al. 1995). Both Chl a concentrations and PP rates were higher in winter–spring than in summer–autumn, although only the former differed significantly. The semiannual averages of Chl a varied within a restricted range from 1970 to 1994 (Fig. 24.6a); since then, a sharp increase occurred in the variability of the semiannual Chl a averages. Comparison of the Chl a averages for the winter–spring season from 1970 to 1994 with the Chl a averages from 1995 to 2011 showed no significant difference. By contrast, in the summer–autumn season, average Chl a concentrations from 1995 to 2011 were significantly higher than from 1970 to 1994. The overall variability of PP declined from 1997 to 2011 (Fig. 24.6b).
1.4 Primary Production at Optimal Depth
Optimal depth is the depth in the water column “at which the light intensity is optimal for photosynthesis” (Kirk 1994). The very definition of optimal depth indicates that it fluctuates diurnally with the solar energy input. The data used for the current analysis were derived from biweekly measurements made from 09.00 to 12.00 as part of the routine PP monitoring program . From 1990 to 2011, mean optimal depth was 1.34 (± 0.94) m with a mean assimilation number (A.N.) of 2.97 (± 1.43) mgC mg Chl a −1 h−1 (n = 518). A.N. values at 1-, 2-, and 3-m depth were comparable, but significantly different from those at 0 m (Table 24.2). The highest A.N. in Lake Kinneret were recorded when cyanobacteria were the most abundant phytoplankton, followed by diatoms and chlorophytes ; the lowest A.N. were calculated when dinoflagellates were the dominant phytoplankton (Yacobi and Zohary 2010). The mean A.N. of samples dominated by P. gatunense, from 1990 to 2011, was 1.43 (± 0.73) mg C mg Chl a −1h−1 (n = 48). The relatively low A.N. values at 0-m depth resulted from the tendency of P. gatunense to concentrate close to the water surface and to reduce the light available near the surface as well as in deeper strata. In addition, P. gatunense dominated when solar input was often below the value enabling maximum photosynthetic activity. Consequently, P. gatunense in Lake Kinneret was seldom photo-inhibited, although it does not lack the genetic potential destined for coping with excessive radiation (Yacobi 2003). The highest A.N. were recorded in August and September and the lowest from March to May (Fig. 24.7), when P. gatunense reached maximum densities. Optimal depth was relatively shallow from December to February and deepest throughout the summer months (Fig. 24.7), corresponding to the intensity of solar input (Fig. 24.1). A.N. varied widely from 1990 to 2011 (Fig. 24.8), although it was fairly stable from 1970 to 1990 (Ostrovsky et al. 2013; Table 24.3).
1.5 Phytoplankton Characteristics Driven by the Physicochemical Environment
The differences in phytoplankton community structure and metabolic activity during the winter–spring and the summer–autumn were basically driven by the changes in thermal structure of the lake, i.e., full mixing at about the beginning of January to March, followed by stratification and, finally, stable stratification in June. The lake layers remain stratified throughout the summer. From September, the thermocline deepens until the overturn in the last third of December or early January. Nutrient supply, either by release from bottom sediments (Nishri et al. 2000) or from inflows from the watershed, is relatively high during winter–spring (Nishri 2010). This may explain the higher PP rates and Chl a concentrations that occurred in months when water temperatures , insolation, and PAR within the water column were low as compared to summer–autumn. When the lake became strongly stratified, part of this nutrient supply was retained within the plankton biomass in the epilimnion . During the stratification period, this store of nutrients was actively recycled within the epilimnion and provided the main nutrient source for phytoplankton in the upper, productive water layers in summer. This was probably the reason for the significant correlations between the average rates of PP in winter–spring and those in the following summer–autumn period (Fig. 24.9a). A positive correlation between the average Chl a in winter–spring and Chl a in the subsequent summer–autumn period was also observed (Fig. 24.9b).
The fluxes of particles and solutes, which strongly affect nutrient availability for phytoplankton, depend on the thermal structure of the lake. The long-term continuous change in the thermal structure of Lake Kinneret reported for the period 1969–2008 was reflected by a decrease in epilimnion thickness of ~ 3 cm year−1 and an average epilimnion temperature increase of ~ 0.028°C year−1 (Rimmer et al. 2011). The increased range of annual water-level fluctuations together with the trend of decreasing lake levels was most probably the trigger for these slight, but detectable modifications in the thermal structure and may have impacted the entire lake ecosystem. Serruya and Pollingher (1977) and Hambright et al. (1997) proposed the following cascade of events for a situation of declining lake levels: (1) a decrease in hypolimnion volume, (2) enhanced nutrient concentrations in the hypolimnion , (3) increased nutrient concentrations in the epilimnion following lake mixing, and (4) changes in phytoplankton population composition and reduction of total algal biomass. As predicted, N and P concentrations in the hypolimnion have indeed increased with the water level lowering (Zohary and Ostrovsky 2011). However, the observed response of phytoplankton after lake overturn and holomixis only partially followed the predicted sequence of events. Changes in phytoplankton community composition have occurred (Zohary 2004), but total phytoplankton biomass , determined as Chl a, did not decline but, on the contrary, increased in summer (Fig. 24.5).
In summary, four decades of monitoring Chl a and PP in Lake Kinneret have showed wide ranges of variability (Fig. 24.5). Seasonal patterns of Chl a and PP that characterized the lake from 1970 until the mid-1990s have changed dramatically. The regular appearance of the late-winter–spring bloom of P. gatunense has become erratic. In years without blooms, maximum values of Chl a and PP were not observed in April or May, but either earlier or in summer, in sharp contrast to the typical pattern found until the mid-1990s (Fig. 24.10).
1.6 Primary Production in the Carbon Balance of Lake Kinneret
The standard 14C method used since 1972 in Lake Kinneret for measurement of PP is based on the uptake of radiolabeled carbon and yields a result between gross primary production (GPP) and net primary production (NPP) . The 14C method may even underestimate NPP (based on oxygen metabolism measurements), if the uptake of the radioisotope is hindered by intracellular or environmental factors (Ostrom et al. 2005; Yacobi et al. 2007). Part of the fixed carbon is readily respired or lost in exudates (Berman 1976b); therefore, the amount of 14C retained in the particulate matter underestimates the total carbon uptake or GPP (Berman and Pollingher 1974; Williams and Robertson 1991; Marra 2009). GPP, however, is a critical parameter in any estimation of ecosystem carbon flux (Häkanson and Peters 1995).
A recent study by Parparov and Yacobi (unpublished) compared 14C to ∆O2 methods for measuring primary production. GPP was measured by ∆O2 concentration in light and dark bottles incubated for 24 h in situ in the lake, together with 14C uptake in separate, parallel samples. A close relationship was found between these two variables (r 2 = 0.44, p &<0.0001, n = 64), with an O2:C molar ratio of ~ 1.5 (Fig. 24.11). Thus, it appears that the PP values obtained by the 14C method that has been routinely used in Lake Kinneret since 1972 are close to NPP (Berman and Pollingher 1974; Figs. 24.12 and 24.13).
Comparison of primary production measurements based on the 14C method (14CP) versus gross primary production (GPP) . GPP is based on the measurement of ∆O2 in light and dark bottles incubated for 24 h in situ from 0 to 5 m in Lake Kinneret. The experiments were carried out from October 2005 to June 2006. The regression equation is given by GPP = 1.48* 14CP + 0.003 (r 2 = 0.44, n = 64, p < 0.001). The line represents a 1:1 ratio
a Photosynthetic and chemosynthetic carbon fixation rates at the time of chemocline formation at Sta. A, June 2003. b Depth profiles of temperature, dissolved oxygen, and sulfide concentrations at the time of sampling. Due to the cold and rainy winter of 2003, the beginning of stratification shifted from May to June
Throughout most of the year, GPP measured by the uptake of 18O2 (supplied to the medium as H2 18O2) was higher by a factor of approximately 2 than PP measured by the 14C method. However, when there were dense crops of P. gatunense in the lake, PP measured with 18O2 was sevenfold higher than PP measured with 14C; far higher than the limits found in other aquatic systems. This extremely high ratio between 18O2 and 14C-based PP estimates was explained as resulting, at least in part, from oxygen uptake by the Mehler reaction and from the recycling of the 14C tracer by dark respiration and the alternative oxidase (AOX) pathway (Luz et al. 2002).
Primary production is the major source for organic carbon in Lake Kinneret, fixing approximately 608 g m−2 year−1, or when extrapolated to the entire lake area, ~ 1.02 × 105 ton C year−1 (based on 14C uptake values). External inputs of organic carbon are much lower; the average annual input from the Jordan River is 1.8 × 103 ton organic C year−1 (Lake Kinneret database). The major sinks of organic carbon, respiration (Berman et al. 2009; Sect. 25.1), and sedimentation (Ostrovsky and Yacobi 2010; Chap. 27) taken together are approximately equal to the organic carbon provided to the system by primary production. Lake Kinneret is thus net autotrophic (Sect. 25.5). Net autotrophy may be rather the rule than the exception for lakes such as Lake Kinneret, located in arid and semiarid zones, where the water supply is limited and seasonally restricted with low levels of soil organic matter-content in the surrounding watershed.
2 Chemosynthetic Primary Production: A Missing Input into the Carbon Cycle
Ora Hadas, Jonathan Erez
2.1 Introduction
All organisms require carbon and energy sources to drive cellular maintenance, growth , biosynthesis, and function. The carbon source can be obtained by the conversion of CO2 to organic carbon (autotrophy) or by consuming fixed organic material (heterotrophy) . Energy can come from light (radiant) and be converted to chemical energy via the process of photosynthesis or from oxidation–reduction processes using inorganic or organic molecules. Organisms that use CO2 as their carbon source and get their energy by oxidizing inorganic reduced chemicals are defined as chemolithoautotrophs or chemoautotrophs (Konhauser 2007).
Chemoautotrophs use inorganic energy sources, such as hydrogen sulfide, elemental sulfur, thiosulfate, tetrathionate, ferrous iron , molecular hydrogen, methane, and ammonia . Most are bacteria or archaea that utilize the chemical energy released when those reduced compounds are oxidized for CO2 fixation. They generally fall into several groups: sulfur oxidizers and reducers, methanogens, nitrifiers, anammox bacteria, and thermoacidophiles.Oxic–anoxic interfaces (chemoclines) are favorable for growth of chemoautotrophic bacteria , which oxidize H2S and other reducing substances to obtain energy for CO2 fixation. The microscale mixing patterns within the chemocline will control the temporal and spatial distribution of the various sulfide-oxidizing, ammonium-oxidizing, and other oxidizing microorganisms.
H2S and NH4 + are the most important electron donors for chemosynthesis in Lake Kinneret. Chemoautotrophic bacteria, which oxidize H2S (Thiobacillus, Thiomicrospira, and Beggiatoa) , require the concomitant presence of both suitable electron acceptors and suitable electron donors. Oxygen is the preferred electron acceptor for all sulfide oxidizers, but nitrate and oxidized iron may serve as alternative acceptors. Furthermore, denitrification processes in the lake may be driven by sulfide-oxidizing bacteria (Sect. 25.3). Ammonium and nitrite (via nitrification), hydrogen gas (utilized by some sulfate-reducing bacteria), and methane (used by methanotrophs) are other reduced compounds used by chemoautotrophs in Lake Kinneret. This chapter describes spatial and temporal variations in chemosynthetic primary production due to H2S oxidation , and its relative importance in the carbon cycle in Lake Kinneret. Ammonium oxidation is addressed in Chap. 22)
2.2 Oxic–Anoxic Interfaces (Chemoclines)
In Lake Kinneret, oxic–anoxic interfaces are found at different locations at different times of the year. In the pelagic water, such interfaces are found within the metalimnic layer, during stable stratification (June–December). A chemocline with a steep gradient of sulfide (H2S), ammonium (NH4), and dissolved oxygen (DO) is formed and creates unique chemical conditions for various groups of microbial communities. During summer, when the oxic–anoxic (O2–H2S) interface is between 14 and 20 m, where some light is still available (~ 0.1 to 1 % of the incident light), the development of photosynthetic bacteria performing anoxygenic photosynthesis is observed, contributing about 1 % to primary production of the lake (Butow and Bergstein-Ben Dan 1992; Sect. 15.2). In October, this thermocline/oxycline deepens below the euphotic zone, excluding photosynthetic but not chemoautotrophic carbon fixation .
Chemoclines are also formed in the deeper part of the water column in late spring when fresh organic matter is supplied to the hypolimnion, benthic boundary layer, and sediments due to degradation of phytoplankton blooms (such as P. gatunense and Mougeotia sp.) . There is a decrease and depletion in oxygen and, due to intensive sulfate-reduction processes in the hypolimnion , H2S is released near to “pockets” and layers of the still oxygenated water column, forming local chemoclines suitable for chemosynthetic activity (Chap. 21). In addition, a chemocline is formed near the bottom sediments, where H2S and ammonium are released to the overlying water in late spring–early summer. Beggiatoa sp. mats are observed at the sediment–water interface during this period all over the lake (Hadas and Pinkas 1995a).
In the littoral and sublittoral zones, where physical processes such as “wave break” and resuspension of the sediments provide a continuous supply of H2S from the sediments to the oxygenated water column, an oxic–anoxic interface exists year-round with intensive chemoautotrophic activity. For details on physical processes and thermocline/chemocline structure, see Chap. 9.
2.3 Methods
Chemosynthetic carbon fixation was measured by the 14C method in parallel light and dark bottles, with and without the addition of 5 μM 3-(3, 4-dichlorophenyl)-1,1-dimethylurea (DCMU), an inhibitor of photosystem II. This approach enabled distinguishing between photosynthetic (in the light) and chemosynthetic (dark) carbon fixation (Hadas et al. 2001).
2.4 Chemosynthesis in the Lower Water Mass and Sediment–Water Interface During the Formation of the Chemocline (May)
In early summer, when the thermocline and chemocline begin to develop, they are at different depths. The thermocline forms by warming of epilimnic surface water while the chemocline starts to develop at the sediment–water interface as the result of anaerobic mineralization processes. Oxic–anoxic interfaces can be found throughout the entire lower water mass , where sulfide and DO coexist at low concentrations, favoring mainly H2S-based bacterial chemosynthesis (Fig. 24.12). Chemosynthetic carbon fixation ranged from 0.42 ± 0.01 to 16.9 ± 1.24 mg C m−3 h−1 at depths of 30 and 40 m in 2003 and 2004, respectively. The low carbon fixation rate measured at 30 m in 2003 was the result of the DO concentrations at this depth (Fig. 24.12 and Chap. 21), conditions which were not favorable for chemosynthetic sulfide oxidation. In May 1995, a 4-m-thick chemocline region, at the benthic boundary layer, at 38–42 m, showed chemosynthetic activity ranging from 9.83 ± 0.15 to 12 ± 0.15 mg C m−3 h−1 (Hadas et al. 2001).
2.5 Chemosynthesis in the Metalimnion in Autumn, the Time of Chemocline Deepening
Chemosynthesis was measured as dark 14CO2 fixation in the metalimnion of Lake Kinneret at Station A (Sta. A), from October, when the chemocline declines below the euphotic zone, until a turnover in December or January. At that time, the chemocline was characterized by steep gradients of H2S and DO and a thin layer of 0.5–2.0 m thickness, where the two coexisted (up to 0.4 mg L−1 of oxygen and 0.2 mg L−1 of H2S). As the thermocline deepened, the temperature gradient was smaller, but the steep gradients of O2 and H2S at the chemocline were maintained or even increased. Measured rates of dark 14CO2 fixation were in the range of 0.4–40 mg C m−3 h−1 with the highest value recorded at 32.5-m depth at Sta. A in December 1996, when the activity was restricted to a 0.5-m-thick chemocline. The thickness and depth of the interface layer in the pelagic waters in the lake varied as a result of internal waves . The amplitude of the thermocline/chemocline tilt due to seiche oscillations can reach 10 m over 24 h, causing mixing and displacement of water layers within the metalimnion (Serruya 1975; Chap. 9). These daily mixing events can enhance the chemosynthetic process by mixing DO and H2S within the chemocline. For example, during 2 days between 15 and 17 December 1996, the chemocline shifted upwards by 8 m (from 32 to 24 m), with chemoautotrophic dark CO2 fixation of 40 and 29 mg C m−3 h−1, respectively. In December 1994, a ~ 2-m-thick chemocline was located at a depth of 32–34 m (Fig. 24.13) in which much higher chemosynthetic carbon fixation was measured (23.4 ± 2.2 mg C m−3 h−1) than photosynthetic carbon fixation (5 ± 0.9 mg C m−3 h−1) at 2 m in the euphotic zone. In water, from 2-m depth, carbon fixation was totally inhibited in the dark or in the presence of DCMU, whereas dark carbon fixation in the sample from the chemocline was entirely due to bacteria and was DCMU-resistant (Hadas et al. 2001).
Photosynthetic primary production in the euphotic zone (0–15 m) from October to December averaged 1,300 ± 686 mg C m−2 day−1 during the years 1992–1996. Chemoautotrophic primary production at the chemocline (below the euphotic zone) over the same period averaged 314 ± 172 mg C m−2 day−1, i.e., ~ 24 % of the photosynthetic primary production during that period. The maximal monthly value for chemosynthesis was observed in December 1992 when it reached 92 % of the photosynthetic production fixation. In May 1992, photosynthetic and chemosynthetic primary production values averaged 3,276 ± 972 and 533 ± 453 mg C m−2 day−1, respectively, i.e., chemosynthetic production was 16 % of photosynthetic production (Table 24.3). Similar values were measured at Sta. A in June 1997. The difference in primary production between the two periods was due to the characteristic yearly bloom of P. gatunense in winter/spring with 7–10 times higher biomass than that of nanophytoplankton in summer/autumn (Berman et al. 1995). It should be stressed that these values were measured during the 1990s based on integrated values of chemosynthesis of 4 m above the sediments. Recent studies indicated that chemosynthesis may also occur at O2 and H2S interfaces within the hypolimnion (Fig. 24.12), and its contribution to the carbon fixation during this period may be much higher, depending on the extent of sulfide and oxygen coexistence, which differs from year to year.
2.6 Bacterial Carbon Fixation at the Shallow Areas of the Lake
During the period of the Peridinium bloom, the littoral zone is characterized by the highest PP and biomass, providing the sediments with fresh organic matter after the degradation and decomposition of the bloom. This supply of decomposed organic matter results in intensive sulfate reduction within the sediments and H2S production (Hadas and Pinkas 1995b; Hadas et al. 2000). Wind and resuspension processes induce mixing at this area, and reduced materials, mostly H2S and NH4, are released from the sediments to meet oxic waters and form chemoclines . The appearance of white filamentous bacterial mats of Beggiatoa on sulfide-rich sediments indicates high organic load and oxygen depletion (Sweerts et al. 1990).
In the euphotic littoral and sublittoral zones, where light reached the chemocline, the continuous supply of sulfide supported bacterial photosynthetic carbon fixation as well. Photosynthetic bacteria contributed 40 % of the bacterial carbon fixation in this region (Hadas et al. 2000). In addition, in the sublittoral of the lake, fish avoided the zone of 1–2 m from the bottom sediments, implying that this layer with reduced compounds (H2S) is toxic (Ostrovsky et al. 1996).
2.7 Chemoautotrophic Activity and Carbon Isotopes
Carbon flow between different trophic levels in marine and freshwater ecosystems can be traced by stable carbon isotope ratios of various components in the food web (e.g., Fry and Sherr 1984; Rau et al. 1990; Kelley et al. 1998; Fry 2006). In Lake Kinneret, two phenomena were observed, i.e., (1) in late autumn–early winter, low standing stocks of phytoplankton and (2) low Chl a concentrations were observed in the water column (Chap. 10). At this time, the zooplankton was characterized by very low δ13C values (ca. − 34 ‰; Zohary et al. 1994). These negative δ13C values did not correspond to those expected if the zooplankton had been feeding on nanoplanktonic algae which have 6–10 ‰ more positive δ13C values. This raised the question of what food source zooplankton were using during this period. Because chemoautotrophic bacteria have highly negative δ13C signatures (Ruby et al. 1987), it was proposed that these bacteria served as the major carbon source for zooplankton during this season, either directly or via the microbial food web.
The δ13C values of total particulate carbon in the chemocline of Lake Kinneret ranged from − 27 to − 39 ‰. Particulate organic matter from water layers in the lake with high bacterial chemosynthetic activity was found to have low δ13C (Zohary et al. 1994), typical of chemosynthetic bacteria (Cavanaugh et al. 1981). The total particulate carbon of pelagic waters in Lake Kinneret had lighter carbon composition as compared to the littoral stations. The relatively higher abundance of photosynthetic bacteria , characterized by heavy carbon composition, may have contributed to the heavier carbon composition in the shallow area of the lake.
There are two main possibilities to explain the low δ13C values displayed by the chemosynthetic bacteria. First, the δ13C of the hypolimnetic inorganic carbon is low, in the range of − 6 to − 8 ‰ (Stiller and Nissenbaum 1999). Second, the pH in these waters is 7.8, and hence the CO2(aq) concentrations are high, well above 100 µM, so that the bacteria can fractionate carbon isotopes at their maximum capacity. With the deepening of the thermocline and oxycline below the euphotic zone in October and during the formation of the chemocline (April–May), there was an increase in both numbers and biovolumes of ciliates in this region. In December 1996, at 32.5-m depth, an almost pure culture of Coleps hirtus was observed (180 Coleps ml−1), corresponding to high dark carbon fixation (~ 40 mg C m−3 h−1) and low δ13C value of − 31.59 ‰, suggesting that Coleps was feeding on chemoautotrophic bacteria . Other protozoa present were the ciliates Cyclidium , Vorticella mayeri, and small flagellates. High numbers of anaerobic or facultative ciliates (Saprodinium dentatum, Plagiopyla) were observed near the bottom sediments in April–May, feeding on sulfur cycle bacteria . Zooplankton may feed on these ciliates (Chaps. 14 and 17) and this way acquires the observed low δ13C isotopic signatures in winter.
2.8 Conclusions
Bacterial chemosynthetic production in Lake Kinneret is significant and should be taken into account in the carbon budgets calculated for the lake as well as in food web carbon fluxes. It should be stressed that this production was energetically derived from organic carbon , which at an earlier stage was fixed by photosynthesis. The use of reduced compounds (sulfide , ammonium, and methane) originating from anaerobic metabolism, which is energetically inefficient, can be partly compensated by utilization of these compounds. In this way, at least part of the photosynthetic energy, potentially convertible to biomass under aerobic conditions, can be gained back by chemosynthesis; otherwise, this energy would be completely lost to the system. The phylogenetic lineages of the microbial populations involved in chemoautotrophic processes in the lake should be elucidated in the future using molecular biology tools.
References
Berman T (1976a) Light penetrance in Lake Kinneret. Hydrobiologia 49:41–48
Berman T (1976b) Release of dissolved organic matter by photosynthesizing algae in Lake Kinneret, Israel. Freshw Biol 6:13–18
Berman T, Pollingher U (1974) Annual and seasonal variations of phytoplankton, chlorophyll and photosynthesis in Lake Kinneret. Limnol Oceanogr 19:31–54
Berman T, Yacobi YZ, Pollingher U (1992) Lake Kinneret phytoplankton: stability and variability during twenty years (1970–1989). Aquat Sci 54:104–127
Berman T, Stone L, Yacobi YZ, Kaplan B, Schlichter M, Nishri A, Pollingher U (1995) Primary production and phytoplankton in Lake Kinneret: a long-term record (1972–1993). Limnol Oceanogr 40:1064–1076
Berman T, Yacobi YZ, Parparov A, Gal G (2009) Estimation of long-term bacterial respiration and growth efficiency in Lake Kinneret. FEMS Microbiol Ecol 71:351–363
Butow B, Bergstein-Ben Dan T (1992) Occurrence of Rhodopseudomonas palustris and Chlorobium phaeobacteroides blooms in Lake Kinneret. Hydrobiologia 232:193–200
Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB (1981) Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: a possible chemosynthetic symbiont. Science 213:340–342
Dubinsky Z, Berman T (1976) Light utilization efficiencies of phytoplankton in Lake Kinneret (Sea of Galilee). Limnol Oceanogr 21:226–230
Dubinsky Z, Berman T (1979) Seasonal changes in the spectral composition of downwelling irradiance in Lake Kinneret (Israel). Limnol Oceanogr 24:652–663
Erez J, Bouevitch A, Kaplan A (1998) Carbon isotope fractionation by photosynthetic aquatic microorganisms: experiments with Synechococcus PCC 7942 and simple carbon flux model. Can J Bot 76:1109–1118
Fry B (2006) Stable isotope ecology. Springer, Heidelberg
Fry B, Sherr E (1984) δ13C measurements as indicators of carbon flow in marine and fresh waters ecosystems. Contrib Mar Sci 27:13–47
Hadas O, Pinkas R (1995a) Sulfate reduction in the hypolimnion and sediments of Lake Kinneret, Israel. Freshw Biol 33:63–72
Hadas O, Pinkas R (1995b) Sulfate reduction processes in sediments at different sites in Lake Kinneret, Israel. Microb Ecol 30:55–66
Hadas O, Rushansky-Malinsky N, Pinkas R, Halicz E, Erez J (2000) High chemoautotrophic primary production across a transect in Lake Kinneret, Israel. Arch Hydrobiol Spec Issues Adv Limnol 55:413–420
Hadas O, Pinkas R, Erez J (2001) High chemoautotrophic primary production in Lake Kinneret, Israel—a neglected link in the C cycle of the lake. Limnol Oceanogr 46:1968–1976
Hakanson L, Peters RH (1995) Predictive limnology: methods for predictive modelling. SPB Academic, Amsterdam
Hambright KD, Zohary T, Eckert W (1997) Potential influence of low water levels on Lake Kinneret: re-appraisal and modification of an early hypothesis. Limnologica 27:149–155
Hepher B, Langer J (1970) On the primary production by phytoplankton in Lake Kinneret (Tiberias). Sea Fish Res Stn Haifa Bull 55:21–61
Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JDH (1965) Fluorometric determination of chlorophyll. J Cons Cons Int Explor Mer 30:3–15
Imberger J (1994) Transport processes in lakes. In: Margalef R (ed) Limnology now, a paradigm of planetary problems. Elsevier, Oxford
Kelley CA, Coffin RB, Cifuentes LA (1998) Stable isotope evidence for alternative bacterial carbon sources in the Gulf of Mexico. Limnol Oceanogr 43:1962–1969
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems. Cambridge University, Cambridge
Konhauser K (2007) Introduction to geomicrobiology. Blackwell, London
Luz B, Barkan E, Sagi Y, Yacobi YZ (2002) Evaluation of community respiratory mechanisms with oxygen isotopes: a case study in Lake Kinneret. Limnol Oceanogr 47:33–42
Marra J (2009) Net and gross productivity: weighing in with 14C. Aquat Microb Ecol 56:123–131
Nishri A (2010) Phosphorus and nitrogen transport mechanisms from the Hula Valley to the Jordan River. Isr J Earth Sci 58:87–98
Nishri A, Imberger J, Eckert W, Ostrovsky I, Geifman Y (2000) The physical regime and the respective biogeochemical processes in the lower water mass of Lake Kinneret. Limnol Oceanogr 45:972–981
Ostrom NE, Carrick HJ, Twiss MR, Pieinsky L (2005) Evaluation of primary production in Lake Erie by multiple proxies. Oecologia 144:115–124
Ostrovsky I, Yacobi YZ (2010) Sedimentation flux in a large subtropical lake: spatio-temporal variations and relation to primary productivity. Limnol Oceanogr 55:1918–1931
Ostrovsky I, Yacobi YZ, Walline P, Kalikhman I (1996) Seiche induced mixing: its impact on lake productivity. Limnol Oceanogr 41:323–332
Ostrovsky I, Rimmer A, Yacobi YZ, Nishri A, Sukenik A, Hadas O, Zohary T (2013) Long-term changes in the Lake Kinneret ecosystem: the effects of climate change and anthropogenic factors. In: Goldman CR, Kumagai M, Robarts RD (eds) Climatic change and global warming of inland waters: impacts and mitigation for ecosystems and societies. Wiley, pp 271–293. ISBN:9781118470596. doi:10.1002/9781118470596.ch16
Pollingher U, Berman T (1977) Quantitative and qualitative changes in the phytoplankton of Lake Kinneret, Israel, 1972–1975. Oikos 29:418–428
Pollingher U, Berman T (1982) Relative contributions of net and nanno phytoplankton to primary production in Lake Kinneret (Israel). Arch Hydrobiol 96:33–46
Rimmer A, Gal G, Opher T, Lechinsky Y, Yacobi YZ (2011) Causes for long-term variations of thermal structure in a warm lake. Limnol Oceanogr 56:974–988
Rau GH, Teyssie JL, Rassoulzadegan F, Fowler SW (1990) 13C/12C and 15N/14N variations among size-fractionated marine particles: implications for their origin and trophic relationships. Mar Ecol Prog Ser 59:33–38
Rohde W (1972) Evaluation of primary production parameters in Lake Kinneret (Israel). Verh Int Ver Limnol 18:93–104
Ruby EG, Jannasch HW, Deuser WG (1987) Fractionation of stable carbon isotopes during chemoautotrophic growth of sulfur-oxidizing bacteria. Appl Environ Microbiol 53:1940–1943
Serruya C (1972) Metalimnic layer in Lake Kinneret, Israel. Hydrobiologia 40:355–359
Serruya S (1975) Wind, water temperature and motion in Lake Kinneret: general pattern. Int Ver Theor Angew Limnol Verh 19:73–87
Serruya C, Pollingher U (1977) Lowering of water level and algal biomass in Lake Kinneret. Hydrobiologia 54:73–80
Steemann-Neilsen E (1952) The use of radioactive carbon (14C) for measuring organic production in the sea. J Cons Cons Int Explor Mer 18:117–140
Stiller M, Nissenbaum A (1999) A stable carbon isotope study of dissolved in organic carbon in hardwater Lake Kinneret (Sea of Galilee). S Afr J Sci 95:166–170
Sweerts JP, De Beer RA, Nielsen LP, Verdouw H, Van Den Heuvel JC, Cohen Y, Capenberg TE (1990) Denitrification of sulfur oxidizing Beggiatoa spp. mats on freshwater sediments. Nature 344:762–763
Williams PJ leB, Robertson JE (1991) Overall planktonic oxygen and carbon dioxide metabolisms: the problem of reconciling observations and calculations of photosynthetic quotients. J Plankton Res 13(Suppl):153–169
Yacobi YZ (2003) Seasonal variation of photosynthetic pigments in the dinoflagellate Peridinium gatunense (Dinophyceae) in Lake Kinneret Israel. Freshw Biol 48:1850–1858
Yacobi YZ (2006) Temporal and vertical variation of chlorophyll a concentration, phytoplankton photosynthetic activity and light attenuation in Lake Kinneret: possibilities and limitations for simulation by remote-sensing. J Plankton Res 28:725–736
Yacobi YZ, Ostrovsky I (2008) Downward flux of organic matter and pigments in Lake Kinneret (Israel): relationships between phytoplankton and the material collected in sediment traps. J Plankton Res 30:1189–1202
Yacobi YZ, Pollingher U (1993) Phytoplankton composition and activity: response to fluctuations in lake volume and turbulence. Verh Int Ver Limnol 25:796–799
Yacobi YZ, Zohary T (2010) Carbon: chlorophyll a ratio, assimilation numbers and turnover times in Lake Kinneret phytoplankton. Hydrobiologia 639:185–196
Yacobi YZ, Perel N, Barkan E, Luz B (2007) Unexpected underestimation of primary productivity by 18O and 14C methods in a lake: implications for slow diffusion of isotope tracers in and out of cells. Limnol Oceanogr 52:329–337
Yashouv A, Alhunis M (1961) The dynamics of biological processes in Lake Tiberias. Bull Res Counc Isr (B) 10:12–35
Zohary T (2004) Changes to the phytoplankton assemblage of Lake Kinneret after decades of a predictable, repetitive pattern. Freshw Biol 49:1355–1371
Zohary T, Ostrovsky I (2011) Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inland Waters 1:47–59
Zohary T, Erez J, Gophen M, Berman-Frank I, Stiller M (1994) Seasonality of stable carbon isotopes within the pelagic food web of Lake Kinneret. Limnol Oceanogr 39:1030–1043
Acknowledgments
This chapter is based on data collected mainly by KLL supporting staff. YZY thanks Ms. Sara Chava and Mr. Semion Kaganovsky for their skilled and devoted work on the lake and in the laboratory. Skippers Mr. Moti Diamant and Mr. Oz Tsabari-Dar were essential and reliable partners in the execution of the lake work. The Israeli Water Commission and the Israeli Ministry of Science (MOS) grant no. 0033-GR01421 via DISUM-BMFT program supported the chemosynthetic production study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Yacobi, Y., Erez, J., Hadas, O. (2014). Primary Production. In: Zohary, T., Sukenik, A., Berman, T., Nishri, A. (eds) Lake Kinneret. Aquatic Ecology Series, vol 6. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8944-8_24
Download citation
DOI: https://doi.org/10.1007/978-94-017-8944-8_24
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8943-1
Online ISBN: 978-94-017-8944-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)