Abstract
Salt stress is one of the major abiotic stresses limiting productivity and quality of agricultural crops. The adverse effects concern germination, plant vigor, and crop yield in arid and semiarid regions. Most crops are salinity sensitive or even hypersensitive and they are described as glycophytes. In contrast, high salinity is tolerated by halophytes, which are present in very small numbers, accounting for approx. only 1 % of the world’s flora. Glycophytes develop some adaptation mechanisms to monitor salt stress and regulate plant physiology and metabolism in order to cope with this stress. Phytohormones are recognized as vital agents in the adaptation process during salt stress. Plant hormones including abscisic acid, salicylic acid, jasmonic acid, ethylene, brassinosteroids, and the others can regulate that cross talk and responses to salt stress. Molecules, such as transcription factors and MAP kinases, are main agents involved in stress signaling pathways and phytohormone cross talk. This chapter provides an explanation of the salt stress mechanism, while salt stress tolerance and the roles of different plant hormones are presented. The effects of endogenous and exogenous phytohormones on adaptation to salt stress are characterized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Plant hormones are small molecules that regulate plant growth and development, as well as responses to changing environmental conditions. By modifying the production, distribution, or signal transduction of these hormones, plants are able to regulate and coordinate both growth and stress tolerance to promote survival and establishment under environmental stresses such as drought, salinity, UV, ozone , and high or low temperature. Hormones are produced in one part of plant and translocated to other parts, where at very low concentrations, they stimulate physiological response (Kaya et al. 2009). It is suggested that phytohormones act as signals to communicate the stress between roots and shoots. This chapter is focused on the involvement of abscisic acid , salicylic acid , jasmonic acid , ethylene , brassinosteroids , nitric oxide, cytokinins , auxins , gibberellins , and strigolactones in plant tolerance to salinity.
4.2 Salinity
Under optimal conditions plants grow and reproduce, but often face a changing environment that may cause unfavorable conditions. In such an environment, plants are considered to be “stressed”; it prevents them from expressing their full ability to reproduce. The consequence of stress in plants can vary from impeded growth to death. Among a wide range of environmental stresses (such as drought, salinity, high and low temperature, UV stress), salt stress is one of the major abiotic stresses limiting the productivity and quality of agricultural crops, with adverse effects on germination, plant vigor, and crop yield, especially in arid and semiarid regions (Koca et al. 2007; Munns and Tester 2008; Parvaiz and Satyawati 2008). These factors are the most problematic in arid and semiarid regions and the area affected by salinity is estimated at as much as 800 million ha (Pessarakli and Szabolcs 2010). Salinity is found both in irrigated and nonirrigated croplands. According to Yadav et al. (2011), salinity is defined as an excess of salts above the level required by plants. This phenomenon occurs due to dissolved salts and their tendency toward excessive accumulation in soil water. Sources of salinity include natural processes, e.g., rock weathering. Also winds and rains carry sea salts such as NaCl, CaCl2, MgCl2, sulfates, and carbonates. These natural processes are long lasting and may span even millions of years. In contrast, human agricultural activity causes salinity much faster. Improper irrigation systems (salt-rich irrigation, insufficient drainage), clear-cutting, and cultivation of annul crops instead of perennial crops, leaching, land clearing, and deforestation are mainly responsible for salinity (Zhang et al. 2006; Yadav et al. 2011).
Soil salinity is determined by the measurement of electrical conductivity (EC) in decisiemens per meter (dS/m). Electrical conductivity below 2 dS/m at a soil depth up to 60 cm is a standard for nonsaline soil. Other criteria are 2–4 dS/m for weak salinity, 4–6 dS/m for moderate salinity, 6–8 dS/m for strong salinity, and below 8 dS/m for very strong salinity (Alberta Agriculture 2001). In general, soil is considered saline when electrical conductivity is 4 dS/m or more. It corresponds to 40 mM NaCl (Cabot et al. 2014).
4.3 Glycophytes and Halophytes
Most crops are salinity sensitive or even hypersensitive and they are described as glycophytes. They develop some adaptation mechanisms to monitor salt stress and regulate plant physiology and metabolism in order to cope with this stress (Sairam and Tyagi 2004). Glycophytes rapidly inhibit the root and shoot growth (Parvaiz and Satyawati 2008). Salinity tolerance depends on the stage of development. For example, one of the main food crops is rice, which is thought to be highly sensitive to salinity. In fact, its seedlings are highly salt sensitive, which greatly affects crops, whereas at the germination stage, rice is relatively resistant (Singh et al. 2008).
In contrast, high salinity is tolerated by halophytes, which constitute natural flora in saline regions (Keshtehgar et al. 2013; Kosova et al. 2013). Adaptation mechanisms are varied, ranging from biochemical reactions to specialized morphologies (Rajaravindran and Natarajan 2012; Shabala et al. 2012). Unfortunately, halophytes, adapted to high salinity (about 200 mM NaCl), are present in very small quantities, accounting for approx. 1 % of the world’s flora (Sairam and Tyagi 2004; Sulian et al. 2012). Natural halophytes grow in sea shallows, salt marshes, salt lakes, and saline desserts. Typical examples are sea grasses and mangroves forests, as well as Salicornia bigelovii (dwarf glasswort), Anemopsis californica (lizard tail), Panicum virgatum (switch grass), Atriplex (saltbush), Attalea speciosa (babassu), Spartina alterniflora (smooth cordgrass), and Tetragonia tetragonioides. At the end of the scale (in terms of salt tolerance), there are euhalophytes. They are adapted to extreme exposure to seawater in the root zone (Flowers and Colmer 2015). Halophytes are studied as models of salt tolerance, but they may also be potentially cultivated as sources of antioxidants such as phenols, flavonoids, ascorbate, reduced/oxidized glutathione, and reactive oxygen species (ROS)-scavenging enzymes. Production of secondary metabolites is induced by salinity in plants from the families Amaranthaceae, Brassicaceae, Plantaginaceae, and Rhizophoraceae. Species Tripolium pannonicum, Plantago coronopus, Lepidium latifolium, and Salicornia europaea seem to be potential functional foods and nutraceuticals (Flowers and Muscolo 2015).
Most halophytes and glycophytes reveal similar survival mechanisms during salt stress. Some halophytes are able to adapt to extreme salinity due to their very special anatomical and morphological characteristics.
4.4 Plant Responses to Salt Stress
Salinity causes salt stress, one of abiotic stresses and the main reason of decreasing crop yields. Excessive salt concentrations in soils affect plants as a result of water stress, ion toxicity, nutritional disorders, oxidative stress, disturbance of metabolic processes, membrane disorganization, reduction of cell division, and genotoxicity (Carillo et al. 2011).
Salt stress affects the integrity of cellular membranes and enzymatic activities and disrupts the photosynthetic apparatus (Jithesh et al. 2006). Inhibition of microtubule polymerization and skewed root growth may also be observed (Dinneny 2015). Roots may be the first place to initiate the protection and adaptation mechanism. Under salt stress, phospholipid signaling results in differential auxin response. As a result roots are curved to avoid soil of the highest salinity. Arabidopsis, tomato, and sorghum roots grow avoiding salinity, even against the gravity axis. Such a phenomenon is called halotropism and it is a response of plant roots in order to reduce their exposure to salinity (Galvan-Ampudia et al. 2013).
The main stressor in saline soil is NaCl. Firstly, it causes osmotic imbalance and changes in K+ and Ca2+ concentrations. As a result, nutritional imbalance is observed and toxic effects of NaCl lead to disorders affecting membranes and enzymes. Then subsequent oxidative stress is manifested, as ROS , such as superoxide radicals (•O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH), are produced, which are scavenged by both enzymatic and nonenzymatic antioxidants (Qureshi et al. 2007; Roychoudury et al. 2008). Excessive Na+ accumulation manifests itself by damage to old leaves. Other negative effects include disturbed root growth, water consumption, nutrient uptake (microelements P, Fe, and Zn), and a negative influence on mycorrhizal fungi (Parvaiz and Satyawati 2008). When salt stress occurs, salt-tolerant plants are able to retain a higher K+ ion concentration and a lower Na+ ion concentration in the cytosol of cells (Li et al. 2014).
Two distinct kinds of effects could be noticed in plants following salt stress, which are categorized as osmotic (physical) and toxic (chemical) (Ellouzi et al. 2014). During plant response to salt stress, two phases are distinguished (Munns and Tester 2008; Parvaiz and Satyawati 2008) and they are presented in Fig. 4.1. The first phase is fast and the growth is inhibited due to the osmotic response to salt outside the roots. Hyperosmotic stress (Gupta and Huang 2014) limits water absorption by roots, while water loss from leaves is increased. Consequently, shoots stop growing. Next stomata become closed for a short time to protect the plant against ion flow. Afterward, stomata are opened for continuing carbon assimilation. The growth of younger leaves is inhibited.
Plant response to salt stress proposed by Munn and Tester (2008)
The second phase is slower and salt concentrations inside plants become toxic. Plant growth is reduced. The response is salt specific (Munns and Tester 2008) and is considered to be hyperionic stress (Gupta and Huang 2014). Toxic ions are accumulated in plant tissues (Gupta and Huang 2014); mainly Na+ ions are accumulated in leaf blades (Munns and Tester 2008). Old leaves are more exposed to ion toxicity. Less resistant plants lose old leaves, demonstrating necrosis, and photosynthesis is reduced, and the carbohydrate supply is limited. To protect and save plants, their older tissues die back (Gupta and Huang 2014). A negative effect of salt stress is connected with the production of ROS , such as singlet oxygen, superoxide, hydroxyl radical, and hydrogen peroxide that cause oxidative damage to proteins, lipids, and DNA (Gupta and Huang 2014).
Physiological and biochemical mechanisms of salt tolerance are complex. One of them is biosynthesis of osmoprotectants. They are simple sugars (fructose, glucose), sugar alcohols (glycerol, inositol, mannitol, pinitol), complex sugars (trehalose, raffinose), quaternary amino acid derivatives (proline , glycine betaine, β-alanine betaine, proline betaine), tertiary amines (1,4,5,6-tetrahydro-2-methyl-4-carboxyl pyrimidine), and sulfonium compounds (choline-o-sulfate, dimethyl sulfonium propionate) (Yokoi et al. 2002). They make membranes more stable and protect enzymes from denaturation (Carillo et al. 2011).
The additional mechanisms are based on the activation of antioxidant enzymes and synthesis of antioxidant compounds, polyamines, and nitric oxide (NO). Also ion uptake, compartmentalization, and transport are essential for ion homeostasis (Gupta and Huang 2014). The excess of salt is transported to vacuoles or separated in older parts of plants. Toxic ion transport is mediated by the Na+/H+ transporter. There are two types of H+ pumps in vacuolar membranes such as vacuolar V-ATPase and vacuolar pyrophosphatase V-PPase, the major H+ pump inside plant cells (Gupta and Huang 2014).
Plants under salt stress conditions use the signaling system, which consists of the perception of stress signal and response for adaptation through gene expression. These genes particularly osmotic stress-responsive genes (OR) are not activated unless plants face stress. Water loss through stomata or cuticles is minimized by osmotic adjustment, which is most important in osmotic homeostasis. Signaling induced by stress includes ionic and osmotic stress signaling, detoxification signaling, and cell division signaling (Aryadeep et al. 2013).
Moreover, salt stress is accompanied by the production of plant hormones such as abscisic acid (ABA). Other phytohormones, which are potentially helpful in improving salt tolerance, include salicylic acid (SA) and brassinosteroids (BRs) (Gupta and Huang 2014).
Investigations providing insight into the mechanisms of plant response to salt stress are crucial, as it could improve salt tolerance and have practical implication in crop improvement.
4.5 Phytohormones
Phytohormones are hormones that are produced by plant cells in low concentrations. They act as plant growth regulators (PGR), while they also regulate development and differentiation of plant cells and tissues (Argueso et al. 2009; Santner and Estelle 2009; Messing et al. 2010). Plant growth regulators include the five classical phytohormones, abscisic acid (ABA), ethylene (ET), cytokinin (CK) , auxin (IAA), and gibberellin (GA) , as well as jasmonic acid (JA), brassinosteroids (BRs), salicylic acid (SA), nitric oxide (NO), and strigolactone (SL). It is also likely that additional growth regulators are yet to be discovered (Srivastava 2002; Peleg and Blumwald 2011). Some of them are recognized as vital agents in the adaptation process during abiotic stress (Khan and Khan 2013).
The evolution process in the plant kingdom brought a variety of mechanisms developed for their survival under environmental stresses, e.g., salt stress. Some molecules, such as transcription factors and MAP kinases, are main agents involved in stress signaling pathways and phytohormone cross talk . Transcription factors (sequence-specific DNA-binding factors) are proteins, which are attached to specific DNA sequences. Mitogen-activated protein kinases (MAP kinases) are protein kinases which are specific to amino acids. MAP kinases play a role in cellular responses to environmental factors, including osmotic and salt stress, and they regulate cell functions. Plant hormones including abscisic acid, salicylic acid, jasmonic acid , ethylene, and brassinosteroids can regulate that cross talk and responses to salt stress (Fujita et al. 2006). The plant signaling cascade is presented schematically in Fig. 4.2 (adopted from Bajguz and Hayat 2009).
4.5.1 Abscisic Acid
4.5.1.1 Biosynthesis
Firstly (in the 1960s), this compound was identified in abscised and fallen leaves and as a compound responsible for seed dormancy. Abscisic acid (5-(1-hydroxy-2,6,6-trimethyl-4-oxocyclohex-2-en-1-y1)-3-methylpenta-2,4-dienoic acid) is a sesquiterpenoid (C15H20O4), with one asymmetric, optically active carbon atom in position 1′ (Fig. 4.3). In plants, it is found as S-ABA, while R-ABA, the mirror-image form, has not been reported in nature (Zaharia et al. 2005). ABA biosynthesis consists of two enzymatic steps starting from C40 carotenoids (e.g., zeaxanthin). The first interconversion is catalyzed by zeaxanthin epoxidase (ABA1). All-trans-violaxanthin is converted to 9-cis-violaxanthin and 9-cis-neoxanthin. These C40 carotenoids are split to C15 aldehyde xanthoxin and C25 apocarotenals by means of violaxanthin de-epoxidase (VDE). Xanthoxin is exported to the cytosol and it is converted to abscisic aldehyde by dehydrogenase/reductase (ABA2). Next abscisic aldehyde is oxidized to abscisic acid by abscisic aldehyde oxidase (AAO) (Christmann et al. 2006).
4.5.1.2 ABA in Physiological Plant Development
Apart from abiotic stresses , abscisic acid (ABA) plays a role during physiological and optimal plant development as well as seed germination. ABA protects from premature growth under adverse conditions, as it affects bud and seed dormancy. Physiological dormancy is described as the mechanism, which inhibits and prevents the formation of radicles. The role of ABA in dormancy is well known and both ABA content and sensitivity are essential (Finkelstein 2013). There is genetic evidence on the role of ABA in seed dormancy in several plant species such as Arabidopsis. ABA mutants which are unable to produce ABA or insensitive to ABA do not exhibit dormancy. In contrast, dormancy is prolonged with excess ABA biosynthesis and accumulation (in ABA-oversensitive species) (Dekkers and Bentsink 2015).
There are complex interactions between ABA and sugar glycolysis. The metabolic pathway of glycolysis converts glucose into pyruvate and free energy is released, which forms the high-energy compounds – ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). In this way carbohydrate metabolism provides essential energy for plant growth and development. The interactions between glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPCp) and ABA signal transduction in Arabidopsis have been investigated. However, the detailed mechanism is still unknown. GAPCp deficiency causes ABA insensitivity and weak ABA signal transduction (Muńoz-Bertomeua et al. 2011). Glyceraldehyde-3-phosphate is the substrate of the precursor of GAPCp, while it is also the precursor of the ABA biosynthetic pathway (methylerythritol phosphate pathway). In plants, glyceraldehyde-3-phosphate dehydrogenase interacts directly with protein kinase (OSAK), which is activated by osmotic stress during salinity (Muńoz-Bertomeua et al. 2011).
4.5.1.3 Participation ABA in Salt Stress
Abscisic acid is a phytohormone which sends an endogenous signal during drought and salt stress. The mode of ABA action depends on its content. Nanomolar concentrations promote the growth of plants, while micromolar concentrations inhibit growth (Dinneny 2015). When plants recover from salt stress, ABA, via the signaling system, enables primary root growth and ABA concentration becomes low. Thus ABA plays a vital role in plant response to unfavorable environmental conditions.
ABA biosynthesis is enhanced with the change in water status that eventually induces the closure of stomata. Thus stomatal closure is mediated by ABA with the change in ion fluxes in guard cells. ABA-activated protein kinase open stomata 1 (OST1) is a regulator of stomatal closure; it activates the anion channel (slow anion channel associated 1, SLAC1) and inhibits the cation channel (KAT1) by phosphorylation. The two channels are regulated by the ABA signaling pathway and Ca2+ (Raghavendra et al. 2010).
The activity of ABA and its protective role during salt stress are connected with increasing the contents of K+, Ca2+, and other osmolytes. ABA-induced signaling may also be responsible for regulating the expression of selected genes in response to salt stress. For instance, ABA regulates the expression of the H+ pump and Na+/H+ antiporter genes, key determiners of salt tolerance (Fukuda and Tanaka 2006).
A concept has been proposed that ABA signaling is based on intracellular messengers. The plasma-membrane-localized perception site, soluble receptor, and ABA-binding proteins are constantly studied. ABA-induced stomatal closure is associated with signaling molecules such as nitric oxide, reactive oxygen species , and cytosolic free calcium. ABA perception and signaling have been studied using genetic approaches. As a result of salt stress, ABA induces osmotic stress-responsive genes (OR). These genes encode the LEA proteins and enzymes. They are involved in the biosynthesis of osmolytes and detoxification. The OR genes also encode ion transporters and regulatory molecules (TFs), protein kinases, and phosphatases. Apart from ABA-dependent responses to salt stress, there are also ABA-independent pathways that are activated in response to salt stress. Both pathways affect each other in a cross-talk network (Aryadeep et al. 2013).
The analysis of ABA/stress-responsive genes revealed that a DNA sequence element consisting of ACGTGGC is essential during ABA regulation (Qureshi et al. 2007). The abscisic acid response element (ABRE) is used to describe transcription factors regulating the expression of ABA/stress-responsive genes. Arabidopsis bZIP proteins are related to ABFs (ABRE-binding factors). Their expression is initiated by ABA and high salt stress (Qureshi et al. 2007).
ABA perception is based on the binding of ABA to ABA receptors, referred to as RCARs/PYR1/PYLs, which stands for regulatory components of ABA receptor/pyrabactin resistance protein1/PYR-like proteins. These binding proteins are found in the chloroplast and nucleus. The binding of ABA to the receptors leads to inactivation of type 2C protein phosphatases, such as ABI1 and ABI2. There are other ABA-binding proteins, designated as ABAR/CHLH/GUN5 and located in the chloroplast and nucleus, GCR2, GTG1, and GTG2 in plasma membranes (Raghavendra et al. 2010).
Abscisic acid plays a key protective role in all halophyte species under salinity conditions. Atriplex leucoclada, Suaeda fruticosa, and Salicornia virginica exhibit higher concentrations of ABA under salt stress as compared to indole acetic acid (IAA) , which plays a major role in regulating plant growth (Bano and Bano 2011). Salinity stress is a major factor inducing carotenogenesis in some species of halotolerant green algae Dunaliella, and it also induces Dunaliella to produce high levels of ABA. There has been little information concerning the mechanism, through which ABA responds to environmental stress in algae (Sarmad et al. 2007).
Concentrations of ABA were studied in salt-tolerant and salt-sensitive tomatoes under salt stress (Amjad et al. 2014). Three sodium salt concentrations (0, 75, 150 mM NaCl) and two potassium salt concentrations were tested (0, 4.5 M KCl) in climatic chamber. It turned out that salt-tolerant species produce more ABA and ethylene in comparison to salt-sensitive species and to control cultivation (0 mM NaCl). When K+ ions were applied (4.5 mM), the phytohormone concentrations were lower. K+ ions compete with Na+ ions under salt stress, resulting in an increased chlorophyll content and stomatal conductance and better plant growth. The chlorophyll content index (CCI) can be a good measure of photosynthetic activity in plants under salt stress. It has been applied as a good indicator of salt tolerance in some species such as cotton, rice, soybean, wheat, quinoa, and radish (Amjad et al. 2014). Summing up, salt stress decreased CCI, xylem sap K+, and K+/Na+ and increased xylem sap Na+ and ABA concentrations which can be considered as a biomarker of salt tolerance.
4.5.1.4 The Effect of Exogenous Abscisic Acid on Adaptation to Salt Stress
The response of two canola cultivars (Brassica napus), Fornex (salt tolerant) and Okamer (salt sensitive) to ABA foliar application, was investigated under salinity stress (0 and 120 mM NaCl). The results showed that shoot dry matter, photosynthetic rate, peroxidase and catalase activity, and shoot K+ concentration increased in the salt-sensitive variety, while Na+ concentration in shoots decreased. However, too high concentration of ABA treatments inhibited growth. The adverse effects of ABA foliar application were observed in the case of the salt-tolerant cultivar (Farhoudi and Saeedipour 2011).
Foliar application of ABA was studied in turfgrass species, creeping bent grass (Agrostis stolonifera) and Kentucky bluegrass (Poa pratensis). Abscisic acid was effective in mitigating physiological damage resulting from drought or salinity for both grass species, but effects were more pronounced in Kentucky bluegrass. The effects of ABA application included the suppression of membrane electrolyte leakage (EL) and membrane lipid peroxidation (expressed as malondialdehyde (MDA) content) and an increase in ascorbate peroxidase (APX), peroxidase (POD), and superoxide dismutase (SOD) activities after 35 days of salinity stress. These results suggest that foliar application of ABA mitigates salinity stress in turfgrass (Yang et al. 2012).
Induced salinity stress and foliar ABA applications were studied in wheat genotypes. High salinity stress (10 dS/m) had a positive effect on proline synthesis of wheat genotypes. The results showed that exposure of plants to both salinity stress and foliar application of ABA resulted in higher levels of proline and even the yield was better (Bakht et al. 2012). Also, exogenous application of ABA alleviated osmotic stress effects on wheat seedlings (Marcinska et al. 2013).
Exogenous application of small molecules, such as ABA (as well as NO, CaCl2, H2S, polyamine, and melatonin), is able to alleviate the adverse effects of salt stress. During warm season application of such small molecules influenced the accumulation of osmoprotectants and antioxidants in Bermuda grass. They enabled the maintenance of cell membrane integrity, increased photosynthesis, and helped to keep ion homeostasis. These actions protected Bermuda grass against salt stresses (Chan Zhulong and Shi Haitao 2015).
Plants react to environmental changes through very complex signaling networks. Phytohormones, which are involved in these processes, can generate positive and negative effects. It is well accepted that salinity induces an increase in ABA content and ABA has been linked to plant susceptibility to bacteria, fungi, and oomycetes (DiLeo et al. 2010). The influence of salt stress on infection of tomato and chrysanthemum roots by Phytophthora spp. was studied. Roots in hydroponic cultures were exposed to NaCl stress. The increase in root ABA contents in tomato was related to stress-induced susceptibility. Exogenous ABA could substitute for salt stress and intensify pathogen colonization. ABA-deficient tomato mutants did not exhibit susceptibility, and it could be reversed by supplementation with exogenous ABA. This phytohormone seems to be an important factor in predisposition to Phytophthora spp. infection induced by salt stress (DiLeo et al. 2010).
Abscisic acid decreased Na+ exclusion in leaves of Phaseolus vulgaris L. under salt stress. Short-term NaCl treatment in beans caused higher contents of leaf ABA, and ABA concentration was released by Na+, not by Cl−. When fluridone (an ABA inhibitor) pretreatment was used, beans under salt stress showed a lower Na+ uptake and a higher leaf Na+ exclusion in comparison to beans without fluridone pretreatment. Na+ uptake was higher and leaf Na+ exclusion was lower when an ethylene inhibitor – aminooxyacetic acid (AOA) – and ABA were applied. Such a non-ion-specific increase in ABA concentration may be a signal of the osmotic component of salt. A higher ABA concentration influences leaf Na+ concentrations due to a lower Na+ exclusion or an increased root-shoot Na+ translocation (Cabot et al. 2009).
4.5.2 Ethylene
4.5.2.1 Ethylene in Plant Development
Ethylene (ET) is a gaseous phytohormone which is involved in various processes in plants, e.g., inhibition of growth and fruit ripening (Hahn and Harter 2009). It is also a modulator of auxins, gibberellins , cytokinins, and ABA when seeds ripen. It is well known that ethylene stimulates dormancy (the primary, secondary, and light-induced dormancy) (Khan et al. 2009). Salinity is one of the factors which causes an increased ethylene production. Excessive content of ethylene leads to premature decay of plants (Siddikee et al. 2012).
4.5.2.2 Biosynthesis
The biosynthesis of ethylene in plants starts from methionine, which is converted to S-adenosyl-methionine (SAM). Next, SAM is converted to 1-aminocyclopropane-1-carboxylic acid (ACC) using ACC synthase (ACS). Finally, ACC is converted to ethylene by ACC oxidase (ACO). All these compounds and enzymes are produced when salt stress occurs. The last step (conversion to ACC) is referred to as the rate-limiting process during ethylene synthesis. Salt stress might be reduced by inhibition of ethylene production by halotolerant bacteria which produce ACC deaminase. Plant growth-promoting rhizobacteria (PGPR) transform ACC into ammonia (Siddikee et al. 2012).
4.5.2.3 Receptors
In the endoplasmic reticulum, five receptors encoded by various ethylene-responsive genes such as ethylene response1 (ETR1), ethylene response2 (ETR2), ethylene resistant1 (ERS1), ethylene resistant2 (ERS2), and ethylene insensitive4 (EIN4) have been found. The main part of the ethylene response pathway is a constitutive triple response1 (CTR1), which is a signaling regulator acting downstream of ethylene receptors (Li et al. 2014). Ethylene insensitive2 (EIN2) is a downstream component of CTR1, while ethylene response factor1 (ERF1) is an upstream component. When there is no ethylene, CTR1 acts with ethylene receptors ETR1 and ERS1 and reduces the ethylene signal response. When ethylene is produced, then CTR1 becomes inactive. Ethylene formation is directed by the mitogen-activated protein kinase (MAPK) cascade. One of the causes for the activation of this cascade is salt stress (Hahn and Harter 2009).
4.5.2.4 Ethylene During Salt Stress
Ethylene is an active agent in ion homeostasis when salt stress occurs. It regulates the H+-ATPase gene expression (Amjad et al. 2014). Also ethylene acts as a downstream signal of cGMP, which stimulates plasma membrane PM H + −ATPase. cGMP (3′,5′-cyclic guanosine monophosphate) is a messenger in response to salt stress and its concentration increases even ten times. The evidence that ethylene participates in signaling is provided by the fact that ethylene-insensitive mutants are more exposed to the effects of salt stress. The mechanism of ethylene signaling under salt stress is still unknown (Li et al. 2014).
Ethylene is one of the molecules of the alternative respiratory pathway (AP). AP is active during responses to different stresses and helps plants to adapt to adverse environmental conditions. AP is the second pathways, additional to the cytochrome pathway (CP). AP may stop the production of reactive oxygen species (ROS) during salt stress, when CP is not efficient. Ethylene and hydrogen peroxide induce AP in plants. It seems that ethylene is needed for AP. There is a hypothesis that ethylene, NO, and H2O2 are signaling agents under salt stress in Arabidopsis. When salt stress occurs, nitric oxide (NO) is produced using nitric oxide synthase (NOS). Also plasma membrane (PM) NADPH oxidase induces H2O2 production. Hydrogen peroxide activates ACC synthase (ACS), and it results from ethylene production as well as AOX activity, AOX1a expression, and pyruvate production. Finally, alternative respiratory pathway (AP) is induced (Wang et al. 2010).
There is an integrating role of ethylene and ABA in tomato plant adaptation to salt stress (Amjad et al. 2014). Ethylene increases the K+/Na+ ratio by increasing plasma membrane H+-ATPase activity. Since elevated levels of ABA and ethylene are associated with salt stress, their levels decreased as plants were supplied with potassium.
4.5.2.5 The Effect of Exogenous Ethylene on Adaptation to Salt Stress
The application of ethylene to some halophyte seeds reduces negative effects of salt stress (Khan et al. 2009).
The influence of ethylene and nitric oxide on germination of Arabidopsis seeds under salt stress was studied, but the detailed nature of their interaction and the effect on germination have not been specified (Yingchao et al. 2013). These molecules modulate seed germination under unfavorable environmental conditions. Application of 1-aminocyclopropane-1-carboxylate (ACC), a precursor of ethylene biosynthesis, helps to mitigate the inhibition of germination when salinity occurs. Aminoisobutyric (AIB) acid is an inhibitor of ethylene biosynthesis and this compound decreases the positive influence of exogenous ethylene. This is the evidence that ethylene, together with nitric oxide, is involved in the protection against salt stress. Ethylene and NO cooperate in the germination process during excessive salinity. It was shown that the precursor of ethylene (ACC) is able to increase NO content (Yingchao et al. 2013).
Ethylene has been studied in terms of alleviation of salt stress-induced inhibition of seed germination in cucumber (Cucumis sativus L.). As it was expected, seed germination was significantly inhibited by salt stress, but this negative effect was reduced by the precursor of ethylene biosynthesis (ACC). On the other hand, ACC did not affect the growth of radicles under salt stress. Also, exogenous glutamate (Glu) was observed as an alleviating agent during the inhibition of seed germination and radicle growth induced by salt stress. The positive effect of l-Glu on seed germination was smaller when two antagonists of ethylene synthesis, aminoethoxyvinylglycine (AVG) and CoCl2, were applied. This fact indicates that ethylene is involved in the suppression of seed germination under salt stress and that l-Glu interacts with ethylene and modulates seed germination under salt stress (Chang Chenshuo et al. 2010).
Ethylene and nitric oxide (NO) were studied in terms of their protective action that modulates ion homeostasis in Arabidopsis calli under salt stress. The ethylene-insensitive mutant was more prone to salt stress than the wild variety. The treatment in the ethylene-insensitive mutant using 100 mM NaCl caused a greater Na+/K+ ratio and a lower plasma membrane H+-ATPase activity in calli in comparison to calli in the wild variety. Under NaCl stress the NO accumulation and ethylene production appeared at the beginning. NO production induced ethylene emission in wild calli. Application of exogenous ACC or sodium nitroprusside (a NO donor) mitigated the negative effects of NaCl only in the ethylene-sensitive variety. The results showed a lower Na+/K+ ratio and a higher plasma membrane H+-ATPase activity in calli of the wild variety, but not in calli of the mutant. Additionally, the expression of PM H+-ATPase genes was induced by ethylene when salt stress started. Summing up, ethylene and NO act together to increase H+-ATPase activity under salt stress. They both promote maintenance of ion homeostasis (Wang Huahua et al. 2009).
4.5.3 Jasmonates
4.5.3.1 Jasmonates in Plant Development
Jasmonates, i.e., jasmonic acid (JA) and its derivatives, e.g., methyl jasmonate (MeJA), play a crucial role in plant development, in biotic and abiotic stresses . Figure 4.4 presents the chemical structure of jasmonic acid. Jasmonic acid (JA) and its conjugates with amino acids, especially with l-isoleucine, are involved in numerous plant responses to almost all types of stresses (drought, salinity, UV, ozone , cold, high temperatures, osmotic stress). They act in arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria (PGPR). Also jasmonates are vital in the development of plant organs, embryos, seedlings, roots, trichomes, tuber, and seed germination. Moreover, gravitropism, leaf movements, and plant deterioration with age are dependent on these compounds (Wasternack and Hause 2013; Wasternack 2014).
4.5.3.2 Biosynthesis of Jasmonate
The main pathway of jasmonate biosynthesis starts from membrane lipids. α-Linolenic acid is released from the membrane by means of phospholipase A1. Linolenic acid is oxygenated to 13-(S)-hydroxy linolenic acid (13-HPOT) by lipoxygenase. 13-HPOT is transformed to 12-oxophytodienoic acid (OPDA) by allene oxide synthase (AOS) and allene oxide cyclase (AOC). OPDA is reduced and follows three steps of α-oxidation and jasmonic acid (JA) is produced. JA is converted to methyl jasmonate by JA carboxyl methyltransferase (JMT) (Dar et al. 2015).
4.5.3.3 Jasmonates During Salt Stress
Jasmonates are active as signaling molecules during signal transduction when abiotic stresses take place (Wasternack 2014). During salt stress, the content of endogenous jasmonates increases (Javid et al. 2011a, b). In tomato cultivars salt stress induced a higher level of JA in the salt-tolerant variety at the beginning of salt stress. In the case of the salt-sensitive variety, JA content was decreased after one day of salt stress (Javid et al. 2011a, b). Similarly, in salt-sensitive rice, the content of JA was higher than in salt-tolerant rice (Javid et al. 2011a, b). Besides the levels of jasmonic acid, the content of its conjugates with l-isoleucine is increased as well (Wasternack 2014). The signaling pathway which involves jasmonates is connected with a specific protein family, known as jasmonate ZIM domain (JAZ) proteins. They are negative regulators of jasmonic acid-induced gene expression. JAZ proteins undergo ubiquitination via the SCF COI1 complex. The abbreviation COI1 stands for coronatine insensitive1 and it is an F-box protein. The abbreviation SCF is a Skp1/Cullin/F-box complex, which is an E3 ubiquitin ligase. The F-box protein is able to indicate specific target proteins, which are proteasomal degraded. The SCF COI1 complex consists of the bond of the ligand for the COI1-JAZ interaction, an enantiomer of jasmonate and (+)-7-iso-l-isoleucine. This enantiomeric form is accumulated under physiological and stress conditions. When JA/JA-Ile content is low, the promoters of JA-responsive genes are not activated by transcription factors (MYC2), because repression takes place. When JAZ proteins undergo proteasomal degradation, transcription factors, such as the MYC or MYB families, are released, and they are linked to promoters of JA-responsive genes. During JA signaling, there is an important SCFCOI1-JAZ co-receptor complex. This complex has a function of the JA receptor, the JAZ protein, and the transcription factors (MYC2) (Wasternack 2014). The mitigation of salt stress by jasmonates was studied in species Glycine max, Oryza sativa, Pisum sativum, Hordeum vulgare, and Iris hexagona (Dar et al. 2015). The JA signaling cooperates with other hormones, e.g., salicylic acid, auxins, or gibberellins via cross talk.
4.5.3.4 The Effect of Exogenous Jasmonates on Adaptation to Salt Stress
It has been observed that exogenous application of jasmonates can help plants to cope with salinity and the effects of salt stress are less severe (Javid et al. 2011a, b). The application of exogenous JA (or an increased level of endogenous JA) is assisted by the synthesis of large amounts of proteins, known as JIPs.
The results of salt stress and the application of JA were studied in the case of two different rice (Oryza sativa L.) cultivars, i.e., Dongjinchalbyeo (DJC, salt tolerant) and Dongjinbyeo (DJ, salt sensitive). As a result of salt stress, their roots were shorter and the concentration of abscisic acid was lower. The ABA concentrations in the salt-tolerant cultivar progressively increased when NaCl amounts increased, whereas in the salt-sensitive cultivar, the ABA concentrations decreased at 80 mM NaCl. In shoots JA concentrations in the salt-tolerant cultivar were lower than in the salt-sensitive cultivar. Post-application of JA (24 and 48 h after NaCl) was more beneficial for the recovery from salt stress than the application at 24 h and 48 h before salt stress or in the middle of salt stress. Also JA treatment caused a decrease of Na uptake in the salt-sensitive cultivar and an increase in Ca and Mg contents. Leaf water potential, leaf photosynthetic rate, and maximum quantum yield of photosystem II (PSII) improved after the JA application. The exogenous JA post-application is helpful in the recovery of rice from salt stress, more effectively in the case of the salt-sensitive cultivar. The influence of JA application on the balance of other endogenous plant hormones has been suggested as well (Kang et al. 2005).
4.5.4 Brassinosteroids
4.5.4.1 Brassinosteroids in Physiological Processes
Brassinosteroids (BRs) are a large group of phytohormones with the structures of polyhydroxysteroids. They are present in most parts of plants as free compounds or bounded up with sugars or fatty acids. About 70 of brassinosteroids have been extracted from plants (Bajguz and Hayat 2009). One of the first discovered representatives is brassinolide, which was extracted from Brassica napus. Its chemical structure is presented in Fig. 4.5.
Brassinosteroids are agents in various physiological processes such as growth, seed germination, rhizogenesis, senescence, and leaf abscission. The effects of brassinosteroids are pleiotropic (Javid et al. 2011a, b). Plant varieties, which are lacking BR biosynthesis and signaling, are scrubby, with short hypocotyls, stems, and dark green leaves, and delayed aging is observed (Chung and Choe 2013).
Besides the participation in plant physiology, e.g., plant development, cell division, and cell elongation in stems or roots, brassinosteroids are active in stress responses (Ahammed et al. 2015; Fariduddin et al. 2013).
4.5.4.2 Brassinosteroids During Salt Stress
The mechanism of the stress response and the regulation of stress-responsive gene expression require further investigations. Probably, brassinosteroids interact and give impulses for other hormones. The cross talk between brassinosteroids and other hormones takes place in plants (Ahammed et al. 2015). The expression of hormone biosynthetic genes and signaling intermediates varies. BRs increase the ethylene content. Also additive effects of BRs were observed with gibberellins and synergistic with auxin on stem segment elongation (Bajguz and Hayat 2009).
For example, abscisic acid (ABA) is a signaling molecule of salt stress, which inhibits the action of brassinosteroids. The mechanism is not fully understood, although it is known that ABA and BRs act antagonistically on their target genes at or after the BIN2 (BR-insensitive 2) step in BR signaling, and as a result plants adapt to salinity conditions. BRs suppress the expression of the ABA-responsive genes to improve ABA-dependent stress tolerance (Chung et al. 2014).
Physiological responses of BRs under salt stress include influences on photosynthesis, the antioxidant system, and ion homeostasis (Fariduddin et al. 2013). First of all, BRs defend the photosynthetic system against any reduction in the activity of photosynthetic enzymes such as RuBisCO. Secondly, photosystem II (PSII) is protected and its yield is increased by BRs.
The application of BRs reduces the excessive levels of ROS and minimizes lipid peroxidation under salt stress. BRs protect plant cells against the accumulation of high concentrations of sodium ions. BRs activate the high-affinity K+ transporters, so the K+/Na+ ratio is higher. Also the uptake of Ca2+ and K+ is increased by BRs; as a result the ratios of Ca2+/Na+ and K+/Na+ are higher as well.
4.5.4.3 The Effect of Exogenous Brassinosteroids on Adaptation to Salt Stress
One of the techniques to reduce salt stress is exogenous application of some plant growth regulators (Ashraf et al. 2010). When brassinosteroids are used, they reduce the effects of salt stress and modify antioxidant enzymes. For example, the activity of catalase in Arachis hypogaea was higher following foliar treatments of 28-homobrassinolide (28-HBL) and 24-epibrassinolide (24-EBL) (Fariduddin et al. 2013). When salt stress occurs, BRs maintain the content of chlorophyll. Also the activity of nitrate reductase is increased by brassinosteroids (Javid et al. 2011a, b). This enzyme is responsible for nitrogen supply in plants. 24-Epibrassinolide (EBR) influences positively the growth and photosynthesis in wheat under salt stress (Fariduddin et al. 2013).
It was observed that BR application increased growth and seed yield of rapeseed, cotton, and rice. Moreover, germination is improved in Eucalyptus camaldulensis and Oryza sativa (Bajguz and Hayat 2009). In general, the application of BRs helps plants to grow at excessive salt concentrations (Javid et al. 2011a, b).
Wheat germ agglutinin (WGA) is a cereal lectin; its content is higher when biotic and abiotic stresses , such as salt stress, occur. The treatment using BRs reduces WGA concentration in roots; thus BRs might serve a protective role (Bajguz and Hayat 2009).
EBR was studied as a potential remedy for salt stress in salt-tolerant and salt-sensitive pea genotypes. EBR treatment significantly increased the leaf water status and production of osmolytes (Shahid et al. 2015).
The results of brassinolide (BL) treatment on cotton growth were analyzed. Cotton roots, both in cv. Sumian 12 (salt sensitive) and cv. Sumian 22 (salt tolerant), were subjected to NaCl stress (200 mM). Too high salinity caused an increase in Na+, proline , and malondialdehyde concentrations, while protein contents in the roots decreased in both varieties. The application of BL alleviated growth inhibition of cotton, reduced the accumulation of Na+, and increased proline concentration. The positive effects of BL were more evident in the salt-sensitive variety. The analysis showed that BL influenced the gene expression in roots of the salt-sensitive variety under salt stress. Summing up, BL applied to cotton roots is able to ameliorate NaCl stress by improving the activity of roots, physiological processes, and gene expression (Shu Hongmei et al. 2015).
There are a variety of papers concerning advantages of BR application in order to ameliorate salt stress (reviewed by Ahammed et al. 2015). The beneficial effects were observed in the case of oilseed rape (Efimova et al. 2014), Cucumis sativus L. (Fariduddin et al. 2014), basmati rice (Sharma et al. 2013), eggplant Solanum melongena L. (Ding et al. 2012), lettuce (Ekinci et al. 2012), and strawberry (Karlidag et al. 2011).
4.5.5 Salicylic Acid
4.5.5.1 Role of Salicylic Acid in Biological Processes
Salicylic acid (SA, 2-hydroxy benzoic acid, Fig. 4.6) – produced by a wide range of prokaryotic and eukaryotic organisms – is a phenolic compound of hormonal nature, consisting of an aromatic ring bearing a hydroxyl group or its functional derivative , which is synthesized by plants (Chen et al. 2009; Seyfferth and Tsuda 2014). SA acts as a key regulator of the signaling network in plants under abiotic and biotic stresses, such as low and high temperature, salts, and oxidative conditions (Gunes et al. 2007; Noreen et al. 2009; Hara et al. 2012; Fu and Dong 2013; Miura and Tada 2014). Indeed, SA exerts stimulatory effects on various physiological processes related to plant growth and development and plays key signaling roles in thermogenesis and disease resistance (Vlot et al. 2009; Robert-Seilaniantz et al. 2011; Pieterse et al. 2012; Chandran et al. 2014). The SA signaling pathway is highly interconnected with other phytohormone signaling, such as jasmonic acid (JA), ethylene (ET), and abscisic acid (ABA) (Robert-Seilaniantz et al. 2011; Pieterse et al. 2012; Derksen et al. 2013). For example, JA and ET signaling negatively regulates SA biosynthesis at the transcriptional level (Chen et al. 2009; Zheng et al. 2012).
Szalai et al. (2005) tested maize plants under NaCl conditions (50 and 100 mM). In leaves and roots collected after the 1st, 3rd, and 7th days of salt treatment, there were no changes in endogenous free and bound salicylic acid levels and in catalase and ascorbate peroxidase activities.
Recently, SA-induced expression has been shown for 59 proteins in cucumber, which were identified for their involvement in various cellular responses and metabolic processes, including antioxidative reactions, cell defense, photosynthesis, carbohydrate metabolism, respiration and energy homeostasis, protein folding, and biosynthesis (Hao et al. 2011).
4.5.5.2 Exogenous Application of Salicylic Acid Under Salinity
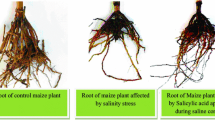
Several studies refer the application of exogenous salicylic acid to improved resistance to salt stress in various types of plants (Szalai et al. 2005; Hussein et al. 2007; Kaydan et al. 2007; Azooz 2009; Noreen et al. 2009; Khan et al. 2010; Shahba et al. 2010; Noreen et al. 2011; Ghafiyehsanj et al. 2013; Ismail 2013; Karlidag et al. 2009; Khan et al. 2015; Singh et al. 2015).
The impact of salinity (140 mM NaCl) and SA application (0.2 mM) on physiological processes in two faba bean genotypes (115 and 125) was tested (Azooz 2009). Salt stress induced high K+/Na+ ratios in shoots and roots with changes in dry weight and tissue water contents, while the SA application alleviated the effect of salinity in both cultivars, and in some cases better results were obtained than in the untreated plants. In a similar study, Khan et al. (2010) examined the role of SA (0.1, 0.5, and 1.0 mM) in tolerance of salinity (50 mM NaCl) in mung bean. The best results manifested in the maximum decrease in the contents of Na+, Cl−, H2O2, and thiobarbituric acid-reactive substances (TBARSs) and in an increase of N, P, K, and Ca contents; activities of antioxidant enzymes, glutathione content, photosynthesis, and yields were recorded in the case of plants treated with 0.5 mM SA.
Hussain and coworkers (2007) investigated the addition of salicylic acid (200 ppm) on growth parameters in maize plants under salinity conditions (2000 and 4000 ppm NaCl). Spraying plants with SA improved all tested parameters, i.e., plant height, the number and area of green leaves, stem diameter, and dry weight of stems, leaves, and the whole plants. In other studies – apart from growth parameters – the level of phenolic compounds was also tested in 2-week-old maize plants growing under salinity conditions (0, 50, 100, 150, and 200 mM NaCl) and treated with salicylic acid (0.5 mM) (Singh et al. 2015). After salt treatment, the results showed reduction in plant dry weight, leaf relative water content and contents of photosynthetic pigments, and an increase in total phenolics . Exogenous application of SA resulted in an increase of growth parameters and decreased phenolic contents. Among all phenolics, ferulic acid was dominant. Also Ismail (2013) investigated the effect of exogenous application of salicylic acid (200 ppm) in maize plants under different salt concentrations (20, 40, 60, 100 mM NaCl) by analyzing such parameters as shoot and root lengths, fresh and dry weights, leaf area, antioxidant enzymatic activities, chlorophylls a and b, total chlorophyll, and chlorophyll stability index. Salinity reduces the abovementioned growth parameters and enzyme contents, while SA treatment improved them. In the case of a low NaCl concentration (20 mM), the toxic effects were completely eliminated by SA application.
The biochemical characteristics of salt-stressed wheat plants after the application of salicylic acid were demonstrated by Ghafiyehsanj et al. (2013) and Kaydan et al. (2007). In the first study, two levels of salinity (75 and 150 mM NaCl) and two SA concentrations (200 and 400 mg/L) were tested. With increasing salinity the protein and insoluble sugar contents and shoot and root weight were reduced, while soluble sugar, proline , and malondialdehyde (MDA) concentrations were increased. After the application of SA, the above parameters were improved, while an exogenous application of SA without salt stress caused no changes in the levels of proline, soluble and insoluble sugars, and MDA. Kaydan et al. (2007) tested the addition of salicylic acid (10−2, 10−4, 10−6 mol/L) under salinity (8 dS/m) and its impact on growth parameters, osmotic potential, and photosynthetic pigments in wheat plants. Exogenous SA increased emergence percentage, osmotic potential, shoot and root dry weight, the K+/Na+ ratio, and the contents of photosynthetic pigments (chlorophylls a and b, carotenoids), thus indicating a positive role of hormone application.
In a study conducted by Shahba et al. (2010), the effect of salicylic acid (0.5, 1.0, and 1.5 mM) on tomato germination, growth, and photosynthetic pigments under different salt stress levels (25, 50, 75, and 100 mM) were investigated. The germination rate decreased with increasing salinity. At low salt concentrations (25 and 50 mM), SA application leads to a decrease in germination percentage.
Studies concerning the effect of salicylic acid on physiological parameters under salinity were also carried out in two sunflower cultivars (Hisun-33 and SF-187) (Noreen et al. 2009, 2011). After foliar application of SA (10, 200, and 300 mM) under salt stress conditions (120 mM), an increase was observed in the levels of pigments (chlorophylls a and b), activities of antioxidant enzymes (superoxide dismutase, catalase, and peroxidase), growth, leaf turgor potential, and leaf and root Ca2+ concentrations. Among applied SA levels, 200 and 300 mM were relatively more effective than the level of 100 mM in improving chlorophyll content, leaf turgor potential, and leaf and root Ca2+ concentrations.
Karlidag and coworkers (2009) assessed the impact of different applications of SA (0.25, 0.50, 1.0 mM) on growth and yield parameters of strawberry under salt stress (35 mM NaCl) and greenhouse conditions. In stressed plants treated with SA, an increase was demonstrated in root and shoot fresh and dry weight, levels of chlorophyll, and all nutrients (N , P, K, Ca, Mg, Zn, Cu, Fe) in leaves and roots, with the highest values at 1.0 mM salicylic acid.
Recent available literature includes studies on less popular plants in the context of NaCl and SA treatment, such as fenugreek (Babar et al. 2014), vetch (Namdari and Baghbani 2013), violet (Hussain et al. 2011), garden cress (Habibi and Abdoli 2013), or basil (Delavari Parizi et al. 2011; Mohammadzadeh et al. 2013).
In their study Babar et al. (2014) demonstrated that foliar application of salicylic acid (100 mg/L) on two cultivars (Deli Kabul and Kasuri) of fenugreek under salt stress conditions (100 mM NaCl) mitigated reduction in growth biomass, gas exchange attributes, and chlorophyll content.
Low seed germination and seedling emergence are the main problems in saline areas. Seed priming treatment with SA (0.5 mM) was tested on the early growth of smooth vetch under different levels (50, 100, 150 mM NaCl) of salt stress (Namdari and Baghbani 2013). After SA application a significant emergence and growth of 18-day-old seedlings were observed, which was associated with lesser oxidative damage. In addition, the activity of ascorbate peroxidase, superoxide dismutase, and glutathione reductase was increased, while catalase activity was not dependent on the SA application. To investigate seed germination rates, the seedling vigor index, and growth parameters of garden cress, three levels of NaCl (50, 100, and 150 mM) and SA (500, 1000, and 1500 μM) were applied (Habibi and Abdoli 2013). Application of different levels of salicylic acid did not significantly increase seedling fresh weight and length. At a low SA concentration (500 μM), the germination percentage was increased, but with the increasing SA concentration, germination percentage was decreased.
The role of exogenous application of salicylic acid (30 mg/L) under salinity conditions (5 dS/m) in violet plants was investigated using three treatments: the control (unstressed plants), NaCl, and NaCl + SA (Hussain et al. 2011). In the combination with NaCl + SA, a strong reduction was observed in the accumulation of Na+, K+, Ca2+, Cl−, glycine betaine, and total soluble sugars, and an increase was recorded in plant and root lengths and plant fresh and dry weights.
Mohammadzadeh et al. (2013) tested the response of four basil cultivars (Shandabad Tabriz, Shiraz, Isfahan, and Sabzevar) to the application of salicylic acid (0.5 mM) under salt stress conditions (50, 100, and 150 mM NaCl) by analyzing morphological parameters. After SA addition all traits (root and stem lengths, stem diameter, the number of branches and leaves, and leaf area) were positively affected, except for internode length and root fresh weight/shoot fresh weight. In turn, Delavari Parizi et al. (2011) investigated the effect of SA (0.01, 0.1, 0.5, 1.0, 1.5, 2.0, and 3.0 MM) and salinity (100 and 200 mM NaCl) on Na+ and K+ contents in basil plants. In plants treated with both levels of NaCl, the level of the Na+ ion increased and that of K+ decreased. Among the SA levels applied, the concentrations of 0.01 and 0.1 mM were selected for further analyses. When basil plants were treated with SA (0.01 and 0.1 mM), Na+ concentration decreased and K+ concentration increased in their experiment.
4.5.6 Cytokinins
4.5.6.1 Cytokinins in Biological Processes
Cytokinins (CKs) are phytohormones, which regulate numerous biological processes, including responses to environmental stresses, via a complex network of CK signaling (Ha et al. 2012). Cytokinins found in plants are adenine derivatives substituted at the N6-position with either an isoprenoid or an aromatic side chain (Fig. 4.7). In both groups, there are small variations in side-chain structure, such as the absence or presence of hydroxyl groups and their stereoisomeric position (Sakakibara 2006; Dolezal et al. 2007). Cytokinins play an important role in the development and growth of both root and shoot systems. Processes regulated by cytokinins include senescence, apical dominance, branching, flowering, and seed germination. These molecules also regulate responses to various stimuli such as water and nutrient availability, light conditions, and infection (Werner and Schmülling 2009).
Cytokinins are major leaf senescence-inhibiting hormones, since senescence is delayed after the exogenous application or the overproduction of CKs in transgenic plants (Cowan et al. 2005; Rivero et al. 2007; Ghanem et al. 2011). Also cytokinins are especially important in regulating cell division and expansion (Kurakawa et al. 2007; Argueso et al. 2009) and delaying senescence (Guo and Gan 2007).
Studies conducted by Javed and coworkers (2011a, b) indicated that an increase in the rice grain yield, 1000-grain weight, and filled-grain percentage are associated with an increase in the contents of starch, sucrose, glucose, and fructose in grain caused by the exogenous application of indole-3-acetic acid and kinetin. Salinity-induced leaf growth inhibition and premature senescence were correlated with decreased root, xylem sap, and leaf bioactive CK concentrations (Albacete et al. 2008; Ghanem et al. 2010). Root-synthesized CKs may regulate shoot responses under salinity, conferring better growth and fruit yield and increasing shoot xylem CK concentrations (Albacete et al. 2009).
4.5.6.2 Exogenous Application of Cytokinins Under Salinity Conditions
The significance of endogenous cytokinin alteration for growth and development of salt-stressed maize and pea plants (50, 100, 150 mM NaCl) was investigated by Atanassova et al. (1996). Zeatin and isopentenyl adenine and their ribosides were measured in roots and leaves under control and salinity conditions. The high concentration of cytokinins may be the decisive factor influencing plants during early formation and flowering at different stress conditions.
Ghanem et al. (2011) tested root-synthesized cytokinins and whether specific root-localized transgenic IPT (a key enzyme for cytokinin biosynthesis) gene expression could substantially improve tomato (Solanum lycopersicum L.) plant growth and yield under salinity (100 mM NaCl). Research showed that a transient root IPT induction increased root, xylem sap, and leaf bioactive cytokinin concentrations two- to threefold without shoot IPT gene expression. In addition, the induced root IPT gene expression increased root-to-shoot CK transport improving salt tolerance by increasing vegetative and fruit growth and also delaying leaf senescence and maintaining stomatal conductance. This approach may be applied in the cultivation of other crops.
4.5.7 Auxins
4.5.7.1 Role Auxins in Biological Processes
Indole-3-acetic acid (IAA, Fig. 4.8) is the most frequently found natural auxin and plays a major role in plant morphogenesis, including tropistic growth, root patterning, vascular tissue differentiation, auxiliary bud formation , and flower organ development (Hamdia and Shaddad 2010, Zhao 2010, Javid et al. 2011a, b; Jung and Park 2011; Mano and Nemoto 2012; Rosquete et al. 2012; Nakhooda et al. 2012; Du et al. 2013; Sauer et al. 2013). IAA is produced in meristematic tissues through tryptophan-dependent and tryptophan-independent biosynthetic pathways (Kazan 2013). Auxin signaling interacts with the signaling pathways of all other known plant hormones and is also proposed to have a role in regulating the communication both within the same plant (root-shoot communication) and between different plants (root-root communication) (Kazan 2013).
4.5.7.2 Exogenous Application of Auxins Under Salinity
Akbari et al. (2007) showed that the application of auxin increased hypocotyl length, seedling fresh and dry weights, and hypocotyl dry weight in three wheat cultivars under salinity conditions. A similar study conducted by Khorasani and coworkers (2015) showed that auxin increased plumule length, seedling fresh and dry weight, and plumule dry weight, but did not influence seed germination percentage or radicle length. Many auxin-signaling genes are responsive to stress responses (Javid et al. 2011a, b; Du et al. 2013).
Auxin also plays a role in seed germination, a crucial stage in the life history of plants, and salt tolerance during germination (Birgit et al. 2005; Akbari et al. 2007). In a study conducted by Jung and Park (2011), transgenic plants overexpressing microRNA160 (miR160) or its target transcription factor gene Auxin Response Factor10 (ARF10) were used to support the contribution of auxin to seed germination. To verify the notion that auxin plays a negative regulatory role in seed germination under high salinity, the transgenic plants overexpressing the YUCCA3 (YUC3) gene, which encodes an auxin biosynthetic enzyme, were generated. It was observed that seed germination of the YUC3-overexpressing plants was more sensitive to high salinity. Based on these studies, it was found that auxin signals are incorporated into the NTM2-mediated salt signal transduction pathway in modulation of seed germination under high salinity.
In a similar study, seeds of three wheat cultivars (Mahdavi, Pishtaz, Shiraz) were used to investigate the effects of different salinity levels (0, −0.6, −1.2 MPa) and auxin concentrations (0, 1, 2 mg L−1) on their germination rate, radical and hypocotyl lengths, seedling fresh and dry weight, and radical and hypocotyl dry weight (Akbari et al. 2007). Positive effects of auxin were shown in the case of some germination traits (hypocotyl length, seedling fresh and dry weight, and hypocotyl dry weight). In addition, the IAA hormone did not influence seed germination percentage.
Khorasani et al. (2015) tested three wheat cultivars (Sepahan, c-84-8, and c-83-1) in terms of the effects of salt stress (0, 4, and 8 dSm-1 NaCl) and auxin (0, 0.5, and 1 ppm) on germination factors. Results showed that an increasing NaCl concentration reduced germination percentage, radicle length, plumule length, seedling fresh and dry weight, and plumule dry weight. In turn, auxin increased plumule length, seedling fresh and dry weight, and plumule dry weight, but did not influence seed germination percentage or radicle length. In a similar study, the effects of different levels of salinity (0, 40, 80, and 120 mM of NaCl) and indole-3-acetic acid on the early growth and germination of wheat seedlings were investigated (Abdoli et al. 2013). Application of IAA at the cell division stage of grain growth caused a significant increase in seedling growth parameters under tested salinity levels. The interaction between the application of IAA and salinity levels significantly affected the final germination percentage.
One method of mitigating the effects of stress is exogenous application of phytohormones (alone or in mixture). A foliar application of growth regulators as a commercial mixture with gibberellins , auxins, and cytokinins (0 mL L−1 – control – and 5 mL L−1 at the beginning of flowering (BF) and 5 mL L−1 at the vegetative stage + the beginning of flowering) on bean under different salt stress levels (0, 1000, and 2000 ppm of NaCl) was used (Torres-Gracia et al. 2009). The analysis showed that salinity tolerance could be induced by the addition of exogenous growth regulators such as gibberellins, auxins, and cytokinins, which inhibited the synthesis of ABA and ethylene, increased fruit size, and delayed leaf senescence. Darwesh (2014) also applied SA (at 400 ppm once a week) and IAA (30 ppm once a week) during two successive seasons on date palm Phoenix dactylifera L. cv. Bartomouda exposed to salt stress at 14000 ppm. The treatments with SA and IAA enhanced most growth parameters, i.e., plant height, leaf number, fresh and dry weight of leaves, and plant tolerance to salinity.
Kim et al. (2006) investigated the effects of soaking in two plant hormones, gibberellic acid (GA3) (10 μM) and indole-3-acetic acid (IAA) (20 μM), on dehulled rice seeds exposed to low NaCl (20 mM) stress. The IAA- and GA3-soaked rice seeds showed relatively high amounts of endogenous IAA under salt stress; in particular, the IAA content increased more markedly in GA3-soaked rice seeds than in IAA-soaked rice seeds.
The influence of salt stress on plant hormones, ABA and auxin (IAA, indole-3-butyric acid – IBA), in two maize cultivars differing in salt resistance (a salt-resistant hybrid and a sensitive hybrid) was tested by Zorb and coworkers (2013). Results showed that the salt-resistant maize significantly increased IBA concentrations in growing leaves and maintained IAA concentration in roots.
4.5.8 Gibberellins
4.5.8.1 Biosynthesis of Gibberellins and Their Role in Biological Processes
Gibberellins (GAs) are a class of phytohormones, commonly known as gibberellic acids, that impact various aspects of plant growth and development (Fleet and Sun 2005; Gupta and Chakrabarty 2013). Gibberellic acid plays a key role in seed germination through coordinating interactions with other growth hormones and external signals. These compounds are also produced by some species of lower plants, fungi, and bacteria. Gibberellic acids are tetracyclic diterpenoid compounds (Fig. 4.9) and stimulate trigger transitions from the meristem to shoot growth, juvenile to adult leaf stage, and vegetative to flowering and grain development along with the interaction of different environmental stress factors (Verelst et al. 2010; Gupta and Chakrabarty 2013; Colebrook et al. 2014). Their names – gibberellin A1, gibberellin A2, and gibberellin A3 – are derived from the fungus Gibberella fujikuroi, from which they have been isolated (Kawaide 2006). GAs are synthesized via the terpenoid pathway requiring three enzymes, terpene synthase, cytochrome P450 monooxygenase, and 2-oxoglutarate-dependent dioxygenases, located, respectively, in plastids, the endomembrane system, and the cytosol (Hedden and Thomas 2012).
4.5.8.2 Exogenous Application of Gibberellins Under Salinity
Tuna and coworkers (2008) showed that foliar application of gibberellin A3 (50 ppm) counteracted some of the adverse effects of NaCl (100 mM) salinity with the accumulation of proline , which maintained membrane permeability and increased macro- and micronutrient levels.
The effect of salinity on rice and the role of GA3 application (150 ppm) were observed in two salt-tolerant rice cultivars (Pokkali and MR219) grown at various salt concentrations (0, 50, 100, 150, and 200 mM) in a greenhouse experiment (Misratia et al. 2013). Results showed the best salinity alleviating the role of GA3 in the case of moderate salinity stress at 50 and 100 mM NaCl.
In a similar study, the role of gibberellic acid was tested on two wheat cultivars (Sohag 3 and Giza 168) in improving their salt stress tolerance (Shaddad et al. 2013). The plants were grown under different salt concentrations (0.0, 50, 100, 150, 200 mM) and then treated with 100 ppm GA3. The hormonal supplementation alleviated salt stress as observed from the increase in protein contents in the different organs, improved growth parameters, contents of photosynthetic pigments, and consequently yields of two wheat cultivars (Sohag 3, sensitive to salinity, and Giza 168 – tolerant).
Ghodrat and Rousta (2012) investigated the role of exogenous gibberellic acid (0, 1.5, 2.5, and 5 mg L−1) in germination and growth of corn under different levels of salt stress (0, 5, 10, 12, and 15 dsm−1). Results showed that priming with low concentrations of GA3 had no effect on seed germination; however, in some concentrations GA3 could increase shoot and root lengths (the most important parameters for salt stress), dry weight, fresh weight, and tissue water content. Based on these experiments, an appropriate concentration of gibberellic acid may be suggested which has the greatest effect on growth parameters.
Low concentrations of gibberellic acid (5 μM) or spermine (50 μM) were used in tests on mung bean (Vigna radiata L. Wilczek) under different NaCl contents (25, 50, and 100 mM) (Ghosh et al. 2015). The combined effect of salinity and low GA3 concentration in the test plants showed a significant alteration (an increase in seedling elongation, biomass production, chlorophyll content, and a decrease in all antioxidant enzymatic activities) and may play an important role in salt uptake.
Maggio et al. (2010) tested effects of gibberellic acid (0 and 100 mg L−1) application on tomato exposed to three levels of salinity (28, 55, 88 mM Na and 55, 111, 177 mM Cl). GA3 treatment reduced stomatal resistance and enhanced plant water use (by 30 %) at low salinity. Results showed that exogenous applications of GA3 may compensate for the salt-induced growth deficiency and consequently facilitate plant adaptation to a saline environment.
The effects of gibberellic acid (5 ml of 10−5 M hormone for each plant) on growth, physiology, and yield of mustard (Brassica juncea L. Czern and Coss) cv. Varuna under salinity conditions (25 and 50 mM NaCl) were studied by Shah (2007). Hormone treatment mitigated the adverse effects of salt stress, with a greater amelioration response in the case of 50 mM NaCl.
In a hydroponic experiment, it is indicated that supplementing exogenous gibberellic acid – GA3 (100 ppm) – plays an important role in enhancing nutrient uptake and in counteracting growth inhibition of sugarcane under salt stress (EC 0 and 9 dSm−1) (Shomeili et al. 2011). In a similar study, exogenous GA3 application in soybean significantly promoted plant length and plant fresh/dry biomass, while it was markedly hindered by NaCl-induced salt stress (Hamayun et al. 2010). Phytohormonal analysis of soybean showed that the level of bioactive gibberellins (GA1 and GA4) and jasmonic acid increased in GA3-treated plants, while the endogenous abscisic acid and salicylic acid contents declined under the same conditions. Also Abdel-Hamid and Mohamed (2014) demonstrated that the application of GA3 (0 and 100 μM) counteracts salinity (0, 100, and 300 mM) by improving membrane permeability and nutrient levels in leaves and also induced physiochemical changes responsible for the induction of salt tolerance in two cultivars of barley. It is noteworthy that exogenous applications of GA3 could be a useful tool in promoting seedling growth and establishment under salt stress conditions.
Seed germination and establishment are the most sensitive stages to abiotic stresses (Patade et al. 2011; Ansari et al. 2012). The effect of salicylic acid (50 ppm) and gibberellin (50 ppm) on enzymatic activity and germination characteristics of wheat seeds under salt stress (at osmotic potentials of 0 (as control), − 4, −8, −12, and −16 bar) was tested by Tabatabaei (2013). Hormone priming improved germination percentage, germination index, normality seedling percentage, and seedling length and also increased catalase and ascorbate peroxidase levels as compared to the untreated seeds.
4.5.9 Nitric Oxide
4.5.9.1 Role of Nitric Oxide in Biological Processes
Nitric oxide (NO) is a short-life bioactive gaseous molecule in plants and plays a central role in a variety of physiological processes including germination, senescence, flowering, ripening of fruits, and response to various abiotic and biotic stresses, such as bacterial disease (Delledonne et al. 1998), drought (Gracia-Mata and Lamatina 2001), high temperature (Hasanuzzaman et al. 2012), salinity (Ruan et al. 2002; Uchida et al. 2002; Ruan et al. 2004a; Zheng et al. 2010; Manai et al. 2014), and UV radiation (Mackerness et al. 2001). Studies demonstrated that NO promoted the accumulation of proline and the probable protective effect of NO against salt-induced oxidative damage to wheat seedlings, but poorly documented how NO regulates proline accumulation and its role in the ABA pathway underlying proline accumulation under salinity conditions.
Ruan et al. (2004b) investigated the relationship of NO and ABA in the process of proline accumulation responding to salt stress. It was shown that NO could activate the synthesis of endogenous ABA and the level of proline was significantly elevated by exogenously supplied ABA in wheat seedling leaves under 150 mmol/L NaCl salt stress after 4 days of treatment.
4.5.9.2 Effect of Exogenous Application of Nitric Oxide Under Salinity
Several studies indicate that the application of exogenous nitric oxide alleviates oxidative stress caused by salt. The protective role of NO treatment was tested in various plants such as tomato, pepper, wheat, barley, rice, maize, soybean, mangrove, or chamomile (Li et al. 2008; Zheng et al. 2010; Fallahi and Khajeh Hosseini 2011; Nalousi et al. 2012; Nasrin et al. 2012; Simaei et al. 2012; Kausar et al. 2013; Ali and Ismail 2014; Chen et al. 2014; Egbichi et al. 2014; Habib and Ashraf 2014; Manai et al. 2014; Kaya et al. 2015; Sanam et al. 2015). In studies conducted by Ali and Ismail (2014), the application of NO (10 μM of sodium nitroprusside) improved tomato fruit quality in the face of salinity (100 mM NaCl) by enhancing the synthesis of health-promoting compounds (phenolic compounds, flavonoids, and alkaloids) in tomato fruits along with significant changes in other quality parameters. In addition, exogenous application of NO improves plant K+ contents, while decreasing Na+ concentration, thereby maintaining the K+/Na+ ratio in plants (Zheng et al. 2009; Chen et al. 2013), and enhances chlorophyll content; activities of CAT, POD, and SOD; and levels of soluble proteins and total free proline in the salt-stressed plants (Kausar et al. 2013).
The protective role of nitric oxide (by spraying with different levels of sodium nitroprusside, SNP – 0.05, 0.10, and 0.15 mM – as the NO source) in wheat plants exposed to salinity (150 mM) was tested by Kausar et al. (2013). The NO treatment of wheat enhanced the activities of antioxidant enzymes (superoxide dismutase, peroxidase, and catalase) and increased levels of proline, chlorophyll, and soluble proteins, showing a protective role against salt-induced oxidative damage. In addition, growth and yield parameters were improved only in unstressed plants.
To investigate the alleviating role of NO in barley under salinity conditions, Li and coworkers (2008) tested three treatments (50 μM SNP, 50 mM NaCl, 50 μM SNP + 50 mM NaCl) compared to the control. Similarly to previous studies, they found that a simultaneous application of SNP and NaCl increased the activities of antioxidant enzymes, protecting plants against salt stress-induced negative changes. Moreover, NO induced an increase of ferritin accumulation to chelate larger numbers of ferrous ions. Sanam et al. (2015) also observed an increase in activities of enzymes and protein contents when two rice cultivars (Khazar and Goohar) were treated with 50 μM SNP + 50 mM NaCl. Habib and Ashraf (2014) also tested plants of four rice cultivars (KS-282, IRRI-6, Shaheen Basmati, and Basmati PB-95) supplemented with NaCl (80 mM) and three levels of NO (0, 0.1, 0.2 mM). Salinity caused a significant increase in leaf water contents and osmotic potentials and a decrease in leaf turgor potential and relative water content. After seed treatment with nitric oxide, leaf osmotic and water potentials and shoot and root Cl− and Na+ concentrations decreased with a simultaneous increase in leaf relative water content, leaf turgor potential K+ and Ca2+ contents, and K+/Na+ ratio in shoots and roots of rice cultivars under salinity conditions.
Soybean, one of the most important plants used as a source of protein and oil in its seeds, is moderately sensitive to salinity. High salt stress significantly reduces seed germination, seedling growth, and yield. An effective action in mitigating the effect of salt stress is provided by nitric oxide application, sometimes in combination with other phytohormones (Egbichi et al. 2014; Simaei et al. 2012). In their study Egbichi et al. (2014) tested six experimental variants: the control, 10 μM 2,2’(hydroxynitrosohydrazono)bis-ethanimine – DETA/NO as a donor of NO – 10 μM DETA, 80 mM NaCl, 10 μM DETA/NO + 80 mM NaCl, and 10 μM DETA + 80 mM NaCl. Results showed that long-term salinity over a 16-day period negatively affected soybean plants, while supplementation with DETA/NO increased shoot, root, and nodule weights, nodule number, as well as the enzymatic activity of ascorbate peroxidase.
Simaei and coworkers (2011; 2012) showed a protective role of NO (100 μM SNP) and SA (100 μM) in soybean under salinity conditions (100 mM NaCl). After the application of phytohormones + SNP, the activities of polyphenol oxidase and phenylalanine ammonia lyase increased, thus reducing the damaging effects of salt stress by enhancing the activity of antioxidative systems.
Kaya et al. (2015) tested the effect of NO + thiourea (TU) treatment (two levels of NO + TU: 3 + 400 mg/L and 6 + 500 mg/L) as seed soaking treatment and foliar application in two maize cultivars (Apex 836 and Dk 5783) under salinity conditions (100 mM NaCl). Seed treatment was more effective than foliar application in terms of improvement in fresh weight. In both types of treatment, N and P contents increased and leaf Na+ contents decreased. Results indicate that exogenous application of NO + TU in maize plants should increase resistance to salinity by improving plant growth.
The role of exogenous application of SNP in ROS production and lipid peroxidation in salt-treated mangrove (as the species with high salinity tolerance) was demonstrated by Chen et al. (2014). In the first stage of the experiment, different levels of SNP (0, 10, 50, 100, 500, and 5000 μM) and NaCl (0, 100, 350, and 500 mM) were tested to select appropriate concentrations. In the second stage, three combinations in three replicates were tested: (1) 100 μM SNP, (2) 350 mM NaCl, and (3) 100 μM SNP + 350 mM NaCl. Results indicated the protective role of NO against oxidative damage in leaves of mangrove by reducing hydrogen peroxide levels, lipid peroxidation, and increase in reduced glutathione and polyphenol contents.
In another study Nalousi et al. (2012) investigated the impact of exogenous SNP as the NO donor (0, 25, 50, and 75 mM SNP) in germination and seedling growth of bell pepper under salinity conditions (0, 4, 6, and 8 ms/cm3 NaCl). As in the previous research, the presence of NO decreased lipid peroxidation in plant leaves and increased the activities of superoxide dismutases and peroxides. In addition, seed germination was promoted at concentrations between 0.2 and 0.8 mM SNP.
Research conducted by Guo et al. (2008) concerned Kosteletzkya virginica – a native species in the southeastern USA – treated with SNP (0.06 mM) and NaCl (100, 200, 300, and 400 mM NaCl) in order to verify their impact on dry weight, antioxidant enzymatic activities, proline accumulation, lipid peroxidation, and distribution of sodium in plants. After SNP application under salinity conditions, the researchers recorded an increase in dry weight, catalase, peroxidase, and superoxide dismutase activities and proline accumulation and a lower ratio of Na+/K+, which might protect plants against oxidative membrane damage and translocation of Na+ from roots to shoots.
Tests with various doses of nitrogen (0, 120, 240, and 360 kg/ha urea ) on wheat (cv. Gascogen) under salinity conditions (0, 121.5, 243, 364.5, and 486 mM NaCl) showed that nitrogen at low levels of salt has a positive or negligible effect on germination and growth factors, while at high salinity levels, its effect was negative (Fallahi and Khajeh Hosseini 2011).
4.5.10 Strigolactones, Their Hormonal Functions, and Ameliorative Role Under Salt Stress Conditions
Strigolactones (SLs, Fig. 4.10) are a small group of sesquiterpene lactones produced from carotenoid precursors mainly in plant roots initially identified as seed germination stimulants for parasitic weeds (Dun et al. 2009; Marzec et al. 2013; Cavar et al. 2014; Van Ha et al. 2014). SLs are also known as a chemical signal between plant roots and arbuscular mycorrhizal fungi (AMF) (Akiyama et al. 2005; Lopez-Raez et al. 2011; Aroca et al. 2013); their hormonal functions likely predate the evolution of the signaling role in the rhizosphere (Delaux et al. 2012). In addition, SLs contribute to a number of crucial processes in plant growth and development, namely, in the shaping of plant architecture, which is of major agronomic importance, as it is closely related to the potential grain yield and adaption to fluctuating environmental conditions. SLs are secondary metabolites derived from a carotenoid precursor (Matusova et al. 2005). To date, more than 15 natural SLs have been characterized from various plant species, and they all share a common four-cycle skeleton (A, B, C, and D), with cycles A and B bearing various substituents and cycles C and D being lactone heterocycles connected by an ether-enol bond (Fig. 4.10) (Liu et al. 2013). Plants produce so small amounts of SLs in the roots and lower part of shoots that they are very difficult to determine (Xie et al. 2010).
Arbuscular mycorrhizal (AM) symbiosis can alleviate salt stress in plants. Aroca and coworkers (2013) investigated the effects of salinity (0, 40, and 80 mM NaCl) on lettuce plant performance and production of strigolactones and assessed its influence on mycorrhizal root colonization under three different salt concentrations. The results show a correlation between strigolactone production, ABA content, AM root colonization, and salinity level, which suggests that AM symbiosis alleviates salt stress by altering the hormonal profiles and affecting physiology in the host plant.
Recent research suggests that genetic modulation of SL content/response could provide a new, potential approach to reduce the negative impact of salt stress on crop productivity (Van Ha et al. 2014).
4.6 Conclusion and Future Prospects
Salt stress is associated with decreases in auxin, cytokinin , gibberellin , and SA in the plant tissues and an increase in ABA and JA . These changes in phytohormone are thought to be an initial process controlling growth reduction due to salinity. Therefore, reduction in plant growth under abiotic stress can be mitigated by exogenous application of plant growth regulators. Different strategies are tested to maximize plant growth under salt stress conditions. One of them is to produce salt-tolerant genotypes of different crops which is much technical and time consuming. In contrast, application of plant growth regulators (plant hormones, minerals, amino acids, quaternary ammonium compounds, polyamines, and vitamins) is economic and effective to improve plant tolerance to salinity. Future studies are to be designed keeping real-world scenario in mind, where more than one biotic and/or abiotic stress may coexist. Understanding complex cross talk between multiple stresses and phytohormones will help to shape future crop management and genotype improvement strategies.
References
Abdel-Hamid AME, Mohamed HI. The effect of the exogenous gibberellic acid on two salt stressed barley cultivars. Eur Sci J. 2014;10(6):228–45.
Abdoli M, Saeidi M, Azhand M, Jalali-Honarmand S, Esfandiari E, Shekari F. The effects of different levels of salinity and indole-3-acetic acid (IAA) on early growth and germination of wheat seedling. J Stress Physiol Biochem. 2013;9(4):329–38.
Ahammed GJ, Xia XJ, Li X, Shi K, Yu JQ, Zhou YH. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sc. 2015;16:462–73.
Akbari G, Sanavy SA, Yousefzadeh S. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.). Pak J Biol Sci. 2007;10:2557–61.
Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–7.
Albacete A, Ghanem ME, Martinez-Andujar C, Acosta M, Sanchez-Bravo J, Martinez V, Lutts S, Dodd IC, Perez-Alfocea F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinised tomato (Solanum lycopersicum L.) plants. J Exp Bot. 2008;59:4119–31.
Albacete A, Martinez-Andujar C, Ghanem ME, Acosta M, Sanchez-Bravo J, Asins MJ, Cuartero J, Dodd IC, Perez-Alfocea F. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence and increased leaf area and crop productivity in salinised tomato. Plant Cell Environ. 2009;32:928–38.
Alberta Agriculture. Salt tolerance of plants. Agri-facts, Agdex 518–517 Edmonton, AB, Canada: Alberta Agriculture, Food and Rural Development. In: Wentz D, editors. 2001.
Ali HEM, Ismail GSM. Tomato fruit quality as influenced by salinity and nitric oxide. Turk J Bot. 2014;38:122–9.
Amjad M, Akhtar J, Anwar-ul-Haq M. Integrating role of ethylene and ABA in tomato plants adaptation to salt stress. Sci Hortic. 2014;172:109–16.
Ansari O, Choghazardi HR, Sharif Zadeh F, Nazarli H. Seed reserve utilization and seedling growth of treated seeds of mountain ray (Secale montanum) as affected by drought stress. Cercetări Agronomiceîn Moldova. 2012;2(150):43–8.
Argueso CT, Ferreira FJ, Kieber JJ. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell Environ. 2009;32:1147–60.
Aroca R, Ruiz-Lozano JM, Zamarreño AM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol. 2013;170(1):47–55.
Aryadeep R, Saikat P, Supratim B. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 2013;32(7):985–1006.
Ashraf M, Akram NA, Arteca RN, Foolad MR. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Critic Rev Plant Sci. 2010;29(3):162–90.
Atanassova L, Pissarska M, Stoyanov I. Cytokinins and growth responses of maize and pea plants to salt stress. Bulg J Plant Physiol. 1996;22(1–2):22–31.
Azooz MM. Salt stress mitigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerance. Int J Agr Biol. 2009;11(4):343–50.
Babar S, Siddiqi EH, Hussain I, Bhatti KH, Rasheed R. Mitigating the effects of salinity by foliar application of salicylic acid in fenugreek. Physiol J. 2014. http://dx.doi.org/10.1155/2014/869058.
Bajguz A, Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Phys Bioch. 2009;47(1):1–8.
Bakht J, Khan MJ, Shafi M. Effect of salinity and ABA application on proline production yield in wheat genotypes. Pak J Bot. 2012;44(3):873–8.
Bano S, Bano A. Physiological and biochemical analysis of the selected halophytes of district Mardan. Pak Int J Biosci Biochem Bioinf. 2011;1:239–43.
Birgit K, Marc AC, Gerhard LM. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281–307.
Cabot C, Sibole JV, Barcelo J. Abscisic acid decreases leaf Na+ exclusion in salt-treated Phaseolus vulgaris L. J Plant Growth Regul. 2009;28(2):87–192.
Cabot C, Sibole JV, Barcelo J. Lessons from crop plants struggling with salinity. Plant Sci. 2014;226:2–13.
Carillo P, Annunziata MG, Pontecorvo G, Fuggi A, Woodrow P. Salinity stress and salt tolerance, abiotic stress in plants – mechanisms and adaptations. In: Shanker A, editors. InTech; 2011. p. 21–38.
Cavar S, Zwanenburg B, Tarkowski P. Strigolactones: occurrence, structure, and biological activity in the rhizosphere. Phytochem Rev. 2014;14:591–711. doi:10.1007/s11101-014-9370-4.
Chan Zhulong, Shi Haitao. Improved abiotic stress tolerance of bermudagrass by exogenous small molecules. Plant Signal Behav. 2015;10(3):e991577.
Chandran D, Rickert J, Huang Y, Steinwand MA, Marr SK, Wilder-Muth MC. Atypical E2F transcriptional repressor DEL1 act sat the intersection of plant growth and immunity by controlling the hormone salicylic acid. Cell Host Microbe. 2014;15:506–13.
Chen Z, Zheng Z, Huang J, Lai Z, Fan B. Biosynthesis of salicylic acid in plants. Plant Sig Behav. 2009;4(6):493–6.
Chen J, Xiong D-Y, Wang W-H, Hu W-J, Simon M, Xiao Q. Nitric oxide mediates root K+/Na + balance in a mangrove plant, Kandelia obovata, by enhancing the expression of AKT1-Type K+ channel and Na+/H+ antiporter under high salinity. PLoS One. 2013;8(8):e71543.
Chen J, Xiao Q, Wang C, Wang WH, Wu FH, Chen J. Nitric oxide alleviates oxidative stress caused by salt in leaves of a mangrove species, Aegiceras corniculatum. Aquat Bot. 2014;117:41–7.
Chenshuo C, Baolan W, Lei S. Alleviation of salt stress-induced inhibition of seed germination in cucumber (Cucumis sativus L.) by ethylene and glutamate. J Plant Physiol. 2010;167(14):1152–6.
Christmann A, Moes D, Himmelbach A. Integration of abscisic acid signalling into plant responses. Plant Biol. 2006;8(3):314–25.
Chung Y, Choe S. The regulation of brassinosteroid biosynthesis in Arabidopsis. Crit Rev Plant Sci. 2013;32(6):396–410.
Chung Y, Kwon SI, Choe S. Antagonistic regulation of Arabidopsis growth by brassinosteroids and abiotic stresses. Mol Cell. 2014;37(11):795–803.
Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin signaling in plant responses to abiotic stress. J Exp Biol. 2014;217:67–75.
Cowan AK, Freeman M, Bjorkman PO, Nicander B, Sitbon F, Tillberg E. Effects of senescence-induced alteration in cytokinin metabolism on source-sink relationships and ontogenic and stress-induced transitions in tobacco. Planta. 2005;221:801–14.
Dar TA, Uddin M, Khan MMA, Hakeesm KR, Jaleel H. Jasmonates counter plant stress: A Review. Environ Exp Bot. 2015;115:49–57.
Darwesh RSS. Exogenous supply of salicylic acid and IAA on morphology and biochemical characteristics of date palm plantlets exposed to salt stress. Middle East J Agr Res. 2014;3(3):549–59.
Dekkers BJW, Bentsink L. Regulation of seed dormancy by abscisic acid and delay of germination 1. Seed Sci Res. 2015;25(2):82–98.
Delaux PM, Xie X, Timme RE, Puech-Pages V, Dunand C, Lecompte E, Delwiche CF, Yoneyama K, Becard G, Sejalon-Delmas N. Origin of strigolactones in the green lineage. New Phytol. 2012;195(4):857–71.
Delavari Parizi M, Manouchehri Kalantari K, Enteshari S, Baghizadeh A. Effect of salicylic acid and salt stress on Na and K content in Ocimum basilicum L. Iran J Plant Physiol. 2011;1(3):133–9.
Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–8.
Derksen H, Rampitsch C, Daayf F. Signaling cross-talk in plant disease resistance. Plant Sci. 2013;207:79–87.
Dinneny JR. Traversing organizational scales in plant salt – stress responses. Curr Opin Plant Biol. 2015;23:70–5.
DiLeo MV, Pye MF, Roubtsova TV. Abscisic acid in salt stress predisposition to phytophthora root and crown rot in tomato and Chrysanthemum. Phytopatol. 2010;100(9):871–9.
Ding HD, Zhu XH, Zhu ZW. Amelioration of salt-induced oxidative stress in eggplant by application of 24-epibrassinolide. Biol Plant. 2012;56(4):767–70.
Dolezal K, Popa I, Hauserova E, Spichal L, Chakrabarty K, Novak O, Krystof V, Voller J, Holub J, Strnad M. Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorg Med Chem. 2007;15:3737–47.
Du H, Liu H, Xiong L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci. 2013;4:397.
Dun EA, Brewer PB, Beveridge CA. Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009;14(7):364–72.
Efimova MV, Savchuk AL, Hasan JAK. Physiological mechanisms of enhancing salt tolerance of oilseed rape plants with brassinosteroids. Russ J Plant Physiol. 2014;61(6):733–43.
Egbichi I, Keyster M, Ludidi N. Effect of exogenous application of nitric oxide on salt stress responses of soybean. South Afr J Bot. 2014;90:131–6.
Ekinci M, Yildirim E, Dursun A. Mitigation of salt stress in lettuce (Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. Hortsci. 2012;47(5):631–6.
Ellouzi H, Ben KH, Hernandez I. A comparative study of the early osmotic, ionic, redox and hormonal signaling response in leaves and roots of two halophytes and a glycophyte to salinity. Planta. 2014;240(6):1299–317.
Fallahi J, Khajeh Hosseini M. Effects of applying various levels of nitrogen on parent plants on the resistance to salinity stress in achieved seeds in Triticum aestivum L. cv. Gaskojen at germination period. J Agr Technol. 2011;7(6):1743–54.
Farhoudi R, Saeedipour S. Effect of exogenous abscisic acid on antioxidant activity and salt tolerance in rapeseed (Brassica napus) cultivars. Res Crops. 2011;12(1):122–30.
Fariduddin Q, Mir BA, Yusuf M. 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica. 2014;52(3):464–74.
Fariduddin Q, Khalil RRAE, MIR BA, Yusuf M, Ahmad A. 24-epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of cucumis sativus under salt and/or copper stress. Environ Monit Assess. 2013;185(9):7845–56.
Finkelstein R. Abscisic acid synthesis and response. The Arabidopsis Book. 2013;11:e0166.
Fleet CM, Sun TP. A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol. 2005;8:77–85.
Flowers TJ, Colmer TD. Plant salt tolerance: adaptations in halophytes. Ann Bot. 2015;115(3):327–31.
Flowers TJ, Muscolo A. Introduction to the special issue: halophytes in a changing world. AoB Plant. 2015;7:1–5.
Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–63.
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–42.
Fukuda A, Tanaka Y. Effects of ABA, auxin, and gibberellin on the expression of genes for vacuolar H+-inorganic pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter in barley. Plant Physiol Bioch. 2006;44(5–6):351–8.
Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA. Halotropism is a response of plant roots to avoid a saline environment. Curr Biol. 2013;23(20):2044–50.
Ghafiyehsanj E, Dilmaghani K, Shoar HH. The effects of salicylic acid on some of biochemical characteristics of wheat (Triticum aestivum L.) under salinity stress. Ann Biol Res. 2013;4(6):242–8.
Ghanem ME, Albacete A, Smigocki AC, Frébort I, Pospísilová H, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Lutts S, Dodd IC, Pérez-Alfocea F. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot. 2010;62(1):125–40.
Ghanem ME, Albacete A, Smigocki AC, Frebort I, Pospisilova H, Martinez-Andujar C, Acosta M, Sanchez-Bravo J, Lutts S, Dodd IC, Perez-Alfocea F. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot. 2011;62(1):125–40.
Ghodrat V, Rousta MJ. Effect of priming with gibberellic acid (GA3) on germination and growth of corn (Zea mays L.) under saline conditions. Intl J Agri Crop Sci. 2012;4(6):289–92.
Ghosh S, Mitra S, Paul A. Physiochemical studies of sodium chloride on mungbean (Vigna radiata L. Wilczek) and its possible recovery with spermine and gibberellic acid. Sci World J. 2015. http://dx.doi.org/10.1155/2015/858016.
Gracia-Mata C, Lamatina L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001;126:1196–204.
Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol. 2007;164:728–36.
Guo YF, Gan SS. Genetic manipulation of leaf senescence. In: Gan S, editor. Senescence processes in plants. Oxford: Blackwell Publishing; 2007. p. 304–22.
Guo Y, Tian Z, Yan D, Zhang J, Qin P. Effects of nitric oxide on salt stress tolerance in Kosteletzkya virginica. Life Sci J. 2008;6(1):67–75.
Gupta R, Chakrabarty SK. Gibberellic acid in plant. Plant Signal Behav. 2013;1,8(9):e25504.
Gupta B, Huang B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Inter J Genom. 2014;article ID 701596:18 pages, doi:10.1155/2014/701596.
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP. Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012;17:172–9.
Habib N, Ashraf M. Effect of exogenously applied nitric oxide on water relation and ionic composition of rice (Oryza sativa L.) plants under salt stress. Pak J Bot. 2014;46(1):111–16.
Habibi A, Abdoli M. Influence of salicylic acid pre-treatment on germination, vigor and growth parameters of garden cress (Lepidium sativum) seedlings under water potential loss at salinity stress. Inter Res J Appl Basic Sci. 2013;4(6):1393–9.
Hahn A, Harter K. Mitogen-activated protein kinase cascades and ethylene: signaling, biosynthesis, or both? Plant Physiol. 2009;149(3):1207–10.
Hamayun H, Khan SA, Khan AL, Shin J-H, Ahmad B, Shin D-H, Lee I-J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J Agric Food Chem. 2010;58(12):7226–32.
Hamdia MA, Shaddad MAK. Salt tolerance of crop plants. J Stress Physiol Biochem. 2010;6(3):64–90.
Hao JH, Donga CJ, Zhanga ZG, Wanga XL, Shang QM. Insights into salicylic acid responses in cucumber (Cucumis sativus L.) cotyledons based on a comparative proteomic analysis. Plant Sci. 2011;187:69–82.
Hara M, Furukawa J, Sato A, Mizoguchi T, Miura K. Abiotic stress and role of salicylic acid in plants. In: Parvaiz A, Prasad MNV, editors. Abiotic stress responses in plants. New York: Springer; 2012. p. 235–51.
Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2012;6:1314–23.
Hedden P, Thomas SG. Gibberellin biosynthesis and its regulation. Biochem J. 2012;15–444(1):11–25.
Hongmei S, Wanchao N, Guo S. Root-applied brassinolide can alleviate the NaCl injuries on cotton. Acta Physiol Plant. 2015;37(4):1–11.
Hussain K, Nawaz K, Majeed A, Ilyas U, Lin F, Ali K, Nisar MF. role of exogenous salicylic acid applications for salt tolerance in violet. Sarhad J Agric. 2011;27(2):171–5.
Hussein MM, Balbaa LK, Gaballah MS. Salicylic acid and salinity effects on growth of maize plants. Res J Agric Biol Sci. 2007;3(4):312–28.
Ismail MA. Alleviation of salinity stress in white corn (Zea mays L.) plant by exogenous application of salicylic acid. Am J Life Sci. 2013;1(6):248–55.
Javid MG, Sorooshzadeh A, Moradi F, Sanavy SAMM, Allahdadi I. The role of phytohormones in alleviating salt stress in crop plants. Austral J Crop Sci. 2011a;5(6):726–34.
Javid MG, Sorooshzadeh A, Sanavy SAMM, Allahdadi I, Moradi F. Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul. 2011b;65:305–13.
Jithesh MN, Prashanth SR, Sivaprakash KR. Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet. 2006;85(3):237–54.
Jung J-H, Park C-M. Auxin modulation of salt stress signaling in Arabidopsis seed germination. Plant Signal Behav. 2011;6(8):1198–200.
Kang DJ, Seo YJ, Lee JD. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J Agron Crop Sci. 2005;191(4):273–82.
Karlidag H, Yildirim E, Turan M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci Agr. 2009;66(2):180–7.
Karlidag H, Yildirim E, Turan M. Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragaria x ananassa). Sci Horticult. 2011;130(1):133–40.
Kausar F, Shahbaz M, Ashraf M. Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turk J Bot. 2013;37(6):1155–65.
Kawaide H. Biochemical and molecular analyses of gibberellin biosynthesis in fungi. Biosci Biotechnol Biochem. 2006;70(3):583–90.
Kaya C, Tuna AL, Yokas I. The role of plant hormones in plants under salinity stress. Book salinity and water stress. 2009;44:45–50.
Kaya C, Sönmez O, Ashraf M, Polat T, Tuna L, Aydemir S. Exogenous application of nitric oxide and thiourea regulates on growth and some key physiological processes in maize (Zea mays L.) plants under saline stress. Soil Water J. 2015;Special Issue:61–6.
Kaydan D, Yagmur M, Okut N. Effects of salicylic acid on the growth and some physiological characters in salt stressed wheat (Triticum aestivum L.). Tarim Bilimleri Dergisi. 2007;13(2):114–19.
Kazan K. Auxin and the integration of environmental signals into plant root development. Ann Bot. 2013;112(9):1655–65.
Keshtehgar A, Rigi K, Vazirimehr MR. Effects of salt stress in crop plants. Int J Agr Crop Sci. 2013;5(23):2863–7.
Khan MIR, Khan NA. Salicylic acid and jasmonates: approaches in abiotic stress tolerance. J Plant Biochem Physiol. 2013;1:e113.
Khan MA, Ansari R, Gul B, Li W. Dormancy and germination responses of halophyte seeds to the application of ethylene. Compt Rendus Biol. 2009;332(9):806–15.
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:462.
Khan N, Syeed S, Masood A, Nazar R, Iqbal N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int J Plant Biol. 2010;1(e1):1–8.
Khorasani FM, Besharat H, Mahmoodzadeh H. Involvement of auxin in the responses of wheat germination to salt stress. Iranian J Plant Physiol. 2015;5(1):1195–201.
Kim SK, Son TK, Park SY, Lee BH, Lee IJ, Kim HY, Lee SC. Influences of gibberellin and auxin on endogenous plant hormone and starch mobilization during rice seed germination under salt stress. J Environ Biol. 2006;27(2):181–6.
Koca M, Bor M, Ozdemir F, Turkan I. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot. 2007;60:344–51.
Kosova K, Vitamvas P, Urban MO. Plant proteome responses to salinity stress – comparison of glycophytes and halophytes. Funct Plant Biol. 2013;40(8–9):775–86.
Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–5.
Li QY, Niu HB, Yin J, Wang MB, Shao HB. Protective role of exogenous nitric oxide against oxidative-stress induced by salt stress in barley (Hordeum vulgare). Colloids Surf B-Biointerf. 2008;65(2):220–5.
Li J, Jia H, Wang J. cGMP and ethylene are involved in maintaining ion homeostasis under salt stress in Arabidopsis roots. Plant Cell Report. 2014;33(3):447–59.
Liu J, Lovisolo C, Schubert A, Cardinale F. Signaling role of strigolactones at the interface between plants, (micro)organisms, and a changing environment. J Plant Interact. 2013;8(1):17–33.
Lopez-Raez JA, Charnikhova T, Fernandez I, Bouwmeester H, Pozo MJ. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J Plant Physiol. 2011;168:294–7.
Mackerness SAH, John CF, Jordan B, Thomas B. Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett. 2001;489:237–42.
Maggio A, Barbieri G, Raimondi G, de Pascale S. Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul. 2010;29:63–72.
Manai J, Gouia H, Corpas FJ. Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J Plant Physiol. 2014;171(12):21028–35.
Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Exp Bot. 2012;63:2853–72.
Marcinska I, Czyczylo-Mysza I, Skrzypek E. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int J Mol Sci. 2013;14(7):13171–93.
Marzec M, Muszynska A, Gruszka D. The role of strigolactones in nutrient-stress responses in plants. Int J Mol Sci. 2013;14:9286–304.
Matusova R, Rani K, Verstappen FW, Franssen MC, Beale MH, Bouwmeester HJ. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139(2):920–34.
Messing SAJ, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzel LM. Structural insights into maize Viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell. 2010;22:2970–80.
Misratia KM, Ismail MR, Oad FC, Hanafi MM, Puteh A. Effect of salinity and alleviating role of gibberellic acid (GA3) for enhancement of rice yield. Inter J Chem Environ Biol Sci. 2013;1(2):330–4.
Miura K, Tada Y. Regulation of water, salinity and cold stress responses by salicylic acid. Plant Sci. 2014;5(4):1–12.
Mohammadzadeh M, Arouee H, Neamati SH, Shoor M. Effect of different levels of salt stress and salicylic acid on morphological characteristics of four mass native basils (Ocimum basilcum). Int J Agr Plant Prod. 2013;4(S):3590–6.
Munns R, Tester M. Mechanisms of salinity tolerance. Ann Rev Plant Biol. 2008;59:651–681. New Delhi: Academic.
Muńoz-Bertomeua J, Djoro AA, Toujania W. Interactions between abscisic acid and plastidial glycolysis in Arabidopsis. Plant Signal Behav. 2011;6(1):157–9.
Khan N, Syeed S, Masood A, Nazar R, Iqbal N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int J Plant Biol. 2010;1(e1):1–8.
Nakhooda M, Watt MP, Mycock D. The properties and interaction of auxins and cytokinins influence rooting of shoot cultures of Eucalyptus. Afr J Biotechnol. 2012;11(100):16568–78.
Nalousi AM, Ahmadiyan S, Hatamzadeh A, Ghasemnezhad M. Protective role of exogenous nitric oxide against oxidative stress induced by salt stress in bell-pepper (Capsicum annum L.). Am Eurasian J Agric Environ Sci. 2012;12(8):1085–90.
Namdari A, Baghbani A. Seed priming with salicylic acid induces salinity tolerance in smooth vetch (Vicia dasycarpa) seedling by enhanced antioxidant activities. Int J Agr Plant Prod. 2013;4(8):1798–805.
Nasrin F, Nasibi F, Rezazadeh R. Comparison the effect of nitric oxide and spermidine pretreatment on alleviation of salt stress in chamomile plant (matricaria regutita L.). J Stress Physiol Biochem. 2012;8:214–23.
Noreen S, Ashraf M, Hussain M. Exogenous application of salicylic acid enhances antioxidative capacity in salt stressed sunflowers (Helianthus Annuus L.) plant. Pak J Bot. 2009;41(1):473–9.
Noreen S, Ashraf M, Akram NA. Does exogenous application of salicylic acid improve growth and some key physiological attributes in sunflower plants subjected to salt stress? J Appl Bot Food Qual. 2011;84(2):169–77.
Parvaiz A, Satyawati S. Salt stress and phyto-biochemical responses of plants - a review. Plant Soil Environ. 2008;54(3):89–99.
Patade VY, Maya K, Zakwan A. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res J Seed Sci. 2011;4(3):125–36.
Peleg Z, Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol. 2011;14:290–5.
Pessarakli M, Szabolcs I. Soil salinity and sodicity as particular plant/crop stress factor. Handbook of plant and crop stress, 3rd ed. Edited by Mohammad Pessarakli, CRC Press; 2010. p. 3–21.
Pieterse CM, VanDer DD, Zamioudis C, Leon-Reyes A, VanWees SC. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521.
Qureshi MI, Qadir S, Zolla L. Proteomics-based dissection of stress-responsive pathways in plants. J Plant Physiol. 2007;164(10):1239–60.
Rajaravindran M, Natarajan S. Effects of salinity stress on growth and biochemical constituents of the halophyte Sesuvium portulacastrum. Inter J Res Biol Sci. 2012;2:18–25.
Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA percepting and signaling. Trends Plant Sci. 2010;15(7):395–401.
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci U S A. 2007;104:19631–6.
Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–43.
Rosquete MR, Barbez E, Kleine-Vehn J. Cellular auxin homeostasis: gatekeeping is housekeeping. Mol Plant. 2012;5:772–86.
Roychoudury A, Basu S, Sarkar SN, Sengupta DN. Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic indica rice cultivars. Plant Cell Rep. 2008;27:1395–410.
Ruan H-H, Shen W-B, Ye M-B, Xu L-L. Protective effects of nitric oxide on salt stress-induced oxidative damages to wheat (Triticum aestivum L.) leaves. Chin Sci Bull. 2002;47:677–81.
Ruan H-H, Shen W-B, Xu L-L. Nitric oxide modulates the activities of plasma membrane ATPase and PPase in wheat seedling roots and promotes the salt tolerance against salt stress. Acta Bot Sin. 2004a;46:415–22.
Ruan H-H, Shen W-B, Xu L-L. Nitric oxide involved in the abscisic acid induced proline accumulation in wheat seedling leaves under salt stress. Acta Bot Sin. 2004b;46:1307–15.
Sairam RK, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–21.
Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol. 2006;57:431–49.
Sanam SA, Zavareh M, Hashempour A. Protective effect of exogenous nitric oxide on alleviation of oxidative damage induced by high salinity in rice (Oryza sativa L.) seedlings. Iran Agric Res. 2015;34(1):63–70.
Santner A, Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009;459:1071–8.
Sarmad J, Shariati M, Haghjou MM. Relationship between endogenous abscisic acid and β-carotene synthesis in unicellular green alga Dunaliella. Am-Eurasian J Agr Environ Sci. 2007;2:559–64.
Sauer M, Robert S, Kleine-Vehn J. Auxin: simply complicated. J Exp Bot. 2013;64:2565–77.
Seyfferth C, Tsuda K. Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Front Plant Sci. 2014;5(697):1–10.
Shabala L, Mackay A, Tian Y, Jacobsen S-E, Zhou D, Shabala S. Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa (Chenopodium quinoa). Physiol Plantarum. 2012;146(1):26–38.
Shaddad MAK, Abd El-Samad HM, Mostafa D. Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Inter J Plant Physiol Biochem. 2013;5(4):50–7.
Shah SH. Effects of salt stress on mustard as affected by gibberellic acid application. Gen Appl Plant Physiol. 2007;33(1-2):97–106.
Shahba Z, Baghizadeh A, Yosefi M. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) germination, growth and photosynthetic pigment under salinity stress (NaCl). J Stress Phys Bioch. 2010;6(3):4–16.
Shahid MA, Balal RM, Pervez MA. Exogenous 24-epibrassinolide elevates the salt tolerance potential of pea (Pisum sativum L.) by improving osmotic adjustment capacity and leaf water relations. J Plant Nutr. 2015;38(7):1050–72.
Sharma I, Ching E, Saini S. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem. 2013;69:17–26.
Shomeili M, Nabipour M, Meskarbashee M, Memari HR. Effects of gibberellic acid on sugarcane plants exposed to salinity under a hydroponic system. Afr J Plant Sci. 2011;5(10):609–16.
Siddikee MA, Chauhan PS, Sa T. Regulation of ethylene biosynthesis under salt stress in red pepper (Capsicum annuum L.) by 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing halotolerant bacteria. J Plant Growth Reg. 2012;31(2):265–72.
Simaei M, Khavari-Nejad RA, Saadatmand S, Bernard F, Fahimi H. Interactive effects of salicylic acid and nitric oxide on soybean plants under NaCl salinity. Rus J Plant Physiol. 2011;58:783–90.
Simaei M, Khavari-Nejad RA, Bernard F. Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am J Plant Sci. 2012;3:1495–503.
Singh AK, Ansari MW, Pareek A. Raising salinity tolerant rice: recent progress and future perspectives. Physiology and molecular biology of plants. Int J Funct Plant Biol. 2008;14(1–2):137–1354.
Singh PK, Shahi SK, Singh AP. Effect of salt stress on physico-chemical changes in maize (Zea mays L.) plants in response to salicylic acid. Indian J Plant Sci. 2015;4(1):2319–3824.
Srivastava LM. Plant growth and development: hormones and environment. Amsterdam: Academic; 2002.
Sulian LV, Jiang P, Chen X, Fan P, Wang X, Li Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol Biochem. 2012;51:47–52.
Szalai G, Páldi E, Janda T. Effect of salt stress on the endogenous salicylic acid content in maize (Zea mays L.) plants. Acta Biol Szegediensis. 2005;49(1–2):47–8.
Tabatabaei SA. The effect of salicylic acid and gibberellin on enzyme activity and germination characteristics of wheat seeds under salinity stress conditions. Inter J Agr Crop Sci. 2013;6(5):236–40.
Torres-Gracia JR, Escalante-Estrada JA, Rodriguez-Gonzalez MT, Ramirez-Ayala C, Martinez-Moreno D. Exogenous application of growth regulators in snap bean under water and salinity stress. J Stress Physiol Biochem. 2009;5(3):13–21.
Tuna AL, Kaya C, Dikilitas M, Higgs D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ Exp Bot. 2008;62(1):1–9.
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002;163:515–23.
Van Ha C, Leyva-Gonzalez M, Osakabe Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Dong NV, Yamaguchi-Shinozaki K, Shinozaki K, Herrera-Estrella L, Tran L-SP. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci U S A. 2014;111(2):851–6.
Verelst W, Skirycz A, Inzé D. Abscisic acid, ethylene and gibberellic acid act at different developmental stages to instruct the adaptation of young leaves to stress. Plant Signal Behav. 2010;5(4):473–5.
Vlot AC, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Ann Rev Phytopathol. 2009;47:177–206.
Wang Huahua, Liang Xiaolei, Wan Qi. Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta. 2009;230(2):293–307.
Wang H, Huang J, Bi Y. Induction of alternative respiratory pathway involves nitric oxide, hydrogen peroxide and ethylene under salt stress. Plant Signal Behav. 2010;5(12):1636–7.
Wasternack C. Action of jasmonates in plant stress responses and development. Appl Asp Biotech Adv. 2014;32(1):31–9.
Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111(6):1021–58.
Werner T, Schmülling T. Cytokinin action in plant development. Curr Opin Plant Biol. 2009;12:527–38.
Xie XN, Yoneyama K, Yoneyama K. The strigolactone story. Annu Rev Phytopathol. 2010;48:93–117.
Yadav S, Mohd I, Aqil A. Causes of salinity and plant manifestations to salt stress: A review. J Environ Biol. 2011;32(5):667–85.
Yang Z, Yu J, Merewitz E. Differential effects of abscisic acid and glycine betaine on physiological responses to drought and salinity stress for two perennial grass species. J Am Soc Sci. 2012;137(2):96–106.
Yingchao L, Lei Y, Matthew P. Ethylene promotes germination of Arabidopsis seed under salinity by decreasing reactive oxygen species: Evidence for the involvement of nitric oxide simulated by sodium nitroprusside. Plant Physiol Bioch. 2013;73:211–18.
Yokoi R, Bressan A, Hasegawa PM. Salt stress tolerance of plants. JIRCAS working report. 2002. p. 25–33.
Zaharia LI, Walker-Simmon MK, Rodríguez CN, Abrams SR. Chemistry of abscisic acid, abscisic acid catabolites and analogs. J Plant Growth Regul. 2005;24:274–84.
Zhang J, Jia W, Yang J, Ismail AM. Role of ABA in integrating plant responses to drought and salt stress. Field Crops Res. 2006;97:111–19.
Zheng CF, Dong JG, Liu FL, Dai TB, Liu WC, Jing Q, Cao WX. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot. 2009;67:222–7.
Zheng C, Jiang D, Dai T, Jing Q, Cao W. Effects nitroprusside, a nitric oxide donor, on carbon and nitrogen metabolism and the activity of the antioxidation system in wheat seedlings under salt stress. Acta Ecol Sinica. 2010;30:1174–83.
Zheng XY, Spivey NW, Zeng W, Liu PP, Fu ZQ, Klessig DF. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibit salicylic acid accumulation. Cell Host Microbe. 2012;11:587–96.
Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49–64.
Zorb C, Geilfus C-M, Muhling KH, Ludwig-Muller J. The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance. J Plant Physiol. 2013;170:220–4.
Acknowledgments
The authors highly acknowledge the technical assistance by Przemysław Kaptur during the preparation of the chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Waśkiewicz, A., Gładysz, O., Goliński, P. (2016). Participation of Phytohormones in Adaptation to Salt Stress. In: Ahammed, G., Yu, JQ. (eds) Plant Hormones under Challenging Environmental Factors. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7758-2_4
Download citation
DOI: https://doi.org/10.1007/978-94-017-7758-2_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7756-8
Online ISBN: 978-94-017-7758-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)