Abstract
Histories of sponges and reefs have been intertwined from the beginning. Paleozoic and Mesozoic sponges generated solid building blocks, and constructed reefs in collaboration with microbes and other encrusting organisms. During the Cenozoic, sponges on reefs have assumed various accessory geological roles, including adhering living corals to the reef frame, protecting solid biogenic carbonate from bioeroders, generating sediment and weakening corals by eroding solid substrate, and consolidating loose rubble to facilitate coral recruitment and reef recovery after physical disturbance. These many influences of sponges on substratum stability, and on coral survival and recruitment, blur distinctions between geological vs. biological roles.
Biological roles of sponges on modern reefs include highly efficient filtering of bacteria-sized plankton from the water column, harboring of hundreds of species of animal and plant symbionts, influencing seawater chemistry in conjunction with their diverse microbial symbionts, and serving as food for charismatic megafauna. Sponges may have been playing these roles for hundreds of millions of years, but the meager fossil record of soft-bodied sponges impedes historical analysis.
Sponges are masters of intrigue. They play roles that cannot be observed directly and then vanish without a trace, thereby thwarting understanding of their roles in the absence of carefully controlled manipulative experiments and time-series observations. Sponges are more heterogeneous than corals in their ecological requirements and vulnerabilities. Serious misinterpretations have resulted from over-generalizing from a few conspicuous species to the thousands of coral-reef sponge species, representing over twenty orders in three classes, and a great variety of body plans and relationships to corals and solid carbonate substrata.
Dynamics of living sponges are difficult to document because most sponges heal after partial mortality and vanish quickly after death. Thus observations of localized increases or overgrowths of corals by a few unusual sponge species have led to recent assertions that sponges are in the process of overwhelming coral reefs. However, a consistent pattern of high mortality in the few long-term census studies done on full assemblages suggests that, perhaps for the first time in their long history, sponges may actually be unable to keep up with changes in the sea. Diminished sponge populations could have profound consequences, many of them negative, for corals and coral reefs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sponges
- Reef-building
- Reef restoration and repair
- Water column filtering
- Positive ecosystem roles of sponges on coral reefs
5.1 Introduction: Sponges and Reefs Have Been Linked from the Beginning
Sponges are daunting creatures, diverse and difficult to identify. Their growth forms are challenging to quantify, and they impede post-mortem analysis by vanishing quickly without a trace . Sponges are also entrancingly beautiful, expressing an unsurpassed diversity of color and form. They are masters of wound healing, regeneration, and mutually beneficial associations. Geological and biological roles of sponges on reefs are inextricably inter-tangled by the many strong influences that sponges have had, and continue to have, on the stability of solid biogenic substrata and the viability of the organisms producing these substrata . Paleozoic and Mesozoic sponges built primary reef framework blocks with their dense skeletons of calcium carbonate or densely interlocking silica spicules (e.g., Hartman 1977; Wood 1990). Most modern sponges play various accessory roles, many of them required for reef building and maintenance, and played only by sponges. These roles include: (1) fortifying the framework with dense solid carbonate; (2) breaking down solid substrate into silt-sized chips and eroding and weakening the skeletons of framework -builders; (3) aiding reef repair by facilitating consolidation of loose rubble and stabilizing it until carbonate secreting organisms can bind it permanently; (4) improving survival of living corals by “gluing” them to the reef frame if their bases are eroded, and protecting their skeletons from excavators; (5) harboring hundreds of symbiont species (microbes, plants, animals) for all or part of their life cycles; (6) maintaining water clarity and possibly also minimizing water-borne pathogens by efficiently filtering and digesting picoplankton ; (7) serving as food for mobile organisms like angelfishes , hawksbill turtles , and nudibranchs ; and, (8) in collaboration with their microbial symbionts , influencing seawater concentrations of dissolved inorganic and organic components (reviews in Rützler 1978; Diaz and Rützler 2001; Wulff 2001; Rützler 2004; Wulff 2006e; Bell 2008; Rützler 2012; Wulff 2012). In addition, aesthetic considerations are not trivial in a world in which conservation can be motivated by recreational enthusiasm, and sponges are star contributors of intriguing colors and shapes on reefs.
5.2 The Nature of Sponges
The structure of sponges is more homogeneous and simple than that of other multicellular heterotrophs (e.g., Simpson 1984). Most modern sponges have relatively soft bodies, with living tissue throughout their three-dimensional forms. The living tissue is pervaded by a supporting skeletal meshwork, as well as a system of canals through which the sponges pump water, from which they very efficiently remove picoplankton and in some cases dissolved organic material. Informal homogeneous construction, along with a high degree of cellular totipotency, allows sponges to heal wounds extremely rapidly, attach to substrata with any portion of their bodies, and accommodate intimate associations with symbionts of every group of organisms without mortally disrupting their own function.
Versatility and lability, in both ecological and evolutionary senses, have contributed to the astonishing persistence and diverse functional roles of the Porifera. Sponges of four different body plans, each with a unique set of relationships with corals and reef substrata , have figured prominently throughout the history of reefs :
-
(1)
free-standing, epibenthic: of every possible growth form , from thin crusts to giant baskets, clusters of tall tubes, and bushes of erect branches. Their skeletons may be fine meshworks of spongin fibers or spicules or, usually, both. The skeletons entirely pervade the body, which is relatively soft and flexible when spongin dominates the skeletal composition and rigid when there is a higher proportion of spicules . The majority of the sponge biomass on many current coral reefs represents this group of sponges , and in the following account this is the group I refer to if no further specification than ‘sponges’ is given.
-
(2)
cryptic: inhabiting crevices and other cryptic spaces within the reef framework . Most have the same set of skeletal properties as the free-standing sponges , and some species have no skeletons at all, or skeletons of calcareous spicules . These occur in either of two growth forms : thinly encrusting the walls of crevices or else entirely filling small internal spaces in the reef framework . Some members of this sciophilic (shade -loving) community also live on exposed surfaces, but many are confined to cryptic spaces and evidently never achieve large sizes (van Soest 2009).
-
(3)
excavating: boring into solid carbonate substrata , and either living entirely within their burrows, or in some cases also forming thin or thick crusts on the substratum surface.
-
(4)
hypercalcified or coralline: tissue confined to the surface of extremely dense, solid and massive carbonate skeletons , often with silica spicules as well. On modern reefs these sponges are also sciophilic.
Curiously, each of these four relationships to reef substrata is expressed by extant species in from 3 to all 21 of the currently recognized orders of marine demosponges, suggesting that this range of possible roles on reefs has ancient roots in this clade. Ecological interactions, ecosystem roles, and vulnerabilities to environmental challenges differ substantially among these four types of sponges , and much confusion relating to sponges on reefs has been caused by generalizing from work on a few species representing one of these four sponge types to all “sponges”.
5.3 Species Diversity of Sponges on Present-Day Reefs
Of the 8553 described sponge species at the time of the most recent review of global sponge diversity (van Soest et al. 2012), about 42 % inhabit realms with coral reefs (i.e., Western, Central, and Eastern Indo-Pacific , Tropical Eastern Pacific , Tropical Atlantic) . The Demospongiae, with 83 % of the extant species, are by far the most speciose of the four classes of sponges ; and the proportional representation of Demospongiae among reef -associated species is even greater. Diversity at high taxonomic levels (e.g., more than twenty orders, representing three of the four classes of the Phylum Porifera: Demospongiae, Homoscleromorpha, and Calcarea ) is reflected in a variation in geological and biological roles of sponges far exceeding that of the relatively homogeneous reef-building corals, most of which are in the single anthozoan order Scleractinia.
Sponges are also more heterogeneous than corals with respect to abiotic factors and ecological interactions that cause them to thrive or perish. Abiotic factors such as chronic rough water, periodic storm -associated wave action, temperature extremes, freshwater, UV light, sunlight in photosynthetically useful wavelengths, water column nutrients and resulting picoplankton abundance and composition, sediment , competition with other sponges , and opportunistic spongivory have all been demonstrated to influence habitat , depth, or latitudinal distribution and abundance of sponges (detailed review in Wulff 2012). Most striking is how differently sponges respond to factors that influence distribution and abundance. One sponge’s nightmare can be another’s paradise.
Species diversity of sponges on present day coral reefs exhibits a similar pattern all over the world. When many sites representing a range of depths and local circumstances are sampled within a local area, 100–300 sponge species are typically reported regardless of the ocean basin, e.g.: 157 species in 102 stations in Jamaica (Lehnert and Fischer 1999), 300 species in 417 stations in the Bahamas (Reed and Pomponi 1997), 96 species at 42 stations on three remote southeastern Caribbean atolls (Zea 2001), 261 species at 103 stations at Ningaloo Reef (Schönberg and Fromont 2012), 150 species at 43 stations in the Dampier Archipelago NW Australia (Fromont et al. 2006), 226 species in 22 stations in SE Queensland (Hooper and Kennedy 2002; Hooper et al. 2002), 150 species at 37 stations in the Spermonde Archipelago, Indonesia (Cleary and de Voogd 2007), and 148 species at 30 stations in the Thousand Islands, Indonesia (de Voogd and Cleary 2008). Another consistent world-wide pattern within local areas is extreme heterogeneity from station to station with respect to which subsets of the regional species pool are represented (e.g., Zea 1994; Hooper and Kennedy 2002; Hooper et al. 2002).
Realm-wide faunas include up to ten or more times as many species of sponges as of corals. Moreover, species diversity is relatively similar among tropical ocean basins rather than dramatically lower in the Tropical Atlantic as it is for corals. Van Soest et al. (2012) listed 945, 975, and 1325 described sponge species in the tropical Western Atlantic, Indian Ocean , and Coral Sea/NE Australia , respectively. Reported diversity patterns still reflect the amount of taxonomically focused study, and new sponge species are being described at a rate of 35–87 per year (van Soest et al. 2012). Biogeographic comparisons of current and past diversity and the detailed tracings of taxonomic patterns through time that have been so informative for corals (e.g., Collins et al. 1996; Budd 2000; Schwartz et al. 2012) are impeded for sponges, with the exception of the coralline sponges, by their extremely poor fossil record . Sponge skeletons disarticulate quickly after death, and silica spicules are subject to dissolution in seawater (Hartman 1977; Rützler and Macintyre 1978; Hartman et al. 1980).
5.4 Geological Roles of Sponges: Reef Frame-Building and Fortifying
Ancient groups of reef builders have led humans in a merry chase with respect to their relationships with extant taxa. In her 1990 review of the history of the study of reef-building sponges , Wood (1990) aptly referred to them as “ancient waifs”. Fossils designated as archaeocyaths , sphinctozoans , stromatoporoids , and chaetetids suggested tantalizing possibilities to generations of paleontologists. For example, between 1826 and 1970, stromatoporoids were placed with Anthozoa , Bryozoa, Hydrozoa, Cyanobacteria, tabulate corals, Foraminifera , Cephalopoda, Plantae, as well as Porifera (see table in Debrenne 1999, after Wood 1987). Discovery of living sponges with dense calcium carbonate skeletons , some with an initially surprising combination of solid carbonate with silica spicules and collagenous fibers, finally allowed definitive assignment of many of these creatures previously known only as fossils to the sponges (Hartman and Goreau 1970; Hartman and Goreau 1975; Vacelet 1970). Canal systems of the living coralline sponges were strongly reminiscent of traces on the surfaces of the skeletons of fossil stromatoporoids . As soon as Hartman and Goreau (1970) had proposed the shift of stromatoporoids to the Porifera, based on their analysis of Ceratoporella from Jamaica (Fig. 5.1) features that had not been previously observed or accorded importance were observed in other fossils. Focused searches for sponge characteristics in fossil material revealed siliceous spicules in some fossils in which they had been assumed to be absent, and astrorhizae were noted to be surface features of fossil chaetetids, providing another link to living examples (review by Wood 1990). The revelation that siliceous spicules in the living relicts can dissolve as they grow helped to further link living examples to fossils lacking spicules (e.g., Stock 2001).
Living hypercalcified sponges . All photos were taken by, and contributed to this paper by courtesy of Philippe Willenz: (a) Ceratoporella nicholsoni (Hickson) a large healthy specimen on a cave wall, Pear Tree Bottom, Jamaica , (b) the same specimen of C. nicholsoni as in photo a, 3 years later; note the virtual lack of growth that is typical of these extraordinarily slow-growing animals, and also the damage on the top; (c) a broken specimen of C. nicholsoni, showing the extreme density of the basal calcareous skeleton and the very thin layer of living tissue; (d) entrance to the Pear Tree Bottom cave, within which live the few species of hypercalcified sponges that are the surviving remnants of a diverse set of species that thrived on open surfaces and built reefs prior to the Cenozoic
Hartman and Goreau’s (1970) elegant discussion of the challenges and joys of relating unusual living organisms to fossils, as well as to other living groups, engaged their readers with questions revolving around what constitutes reliable evidence of clade membership rather than grade (i.e., groups defined by evolutionary relationship vs. groups defined by observable structural similarity). This issue became an important focus of researchers who discovered additional living species and availed themselves of the exciting possibility of learning about ancient groups by detailed study of living representatives. Accumulated details of their biology, skeleton formation, larvae , soft tissue, and spicules have revealed that chaetetid, stromatoporoid, and sphinctozoan are indeed grades rather than clades (e.g., Vacelet et al. 2010; West et al. 2010); and living coralline sponges represent at least five orders of Demospongiae that are represented by non-coralline sponges on modern coral reefs : Clionaida, Merliida, Agelasida, Haplosclerida, and Dictyoceratida; as well as the Class Calcarea , which is far less represented on modern reefs. Curiously sponges of the same grade (i.e., stromatoporoid, sphinctozoan , chaetetid) can be separated by live tissue characteristics into different higher taxa; and skeletons that are readily grouped together as the same grade may exhibit very different microstructure (Willenz and Hartman 1989; Vacelet et al. 2010). Delving into skeletal structure at very fine scales has demonstrated shared pathways in skeletal formation among sponges with different microstructure, a further surprise (e.g., Gilis et al. 2013). Hypercalcified sponges known only from fossils represent additional orders of demosponges, but the lack of matching between grade and clade requires that caution be used in assignment to higher taxa, and a classification based on observable morphological characters must remain in practice for fossil taxa (West et al. 2010). Diversity of living hypercalcified sponges is a small remnant of those that built reefs in Paleozoic and Mesozoic oceans.
5.4.1 Archaeocyatha
Archaeocyaths were the earliest reef -building sponges . These Lower Cambrian builders of sturdy carbonate skeletons have been grouped with cnidarians, algae , sponges, vascular plants, and foraminiferans at various times, assigned their own phylum or kingdom, and finally in the early 1990s grouped back where they had been placed in the 1860s and again in the 1930s – among the sponges (Rowland 2001). Similarities in skeleton formation between the living Vaceletia and the extinct Archaeocyatha help to link them to the demosponges, as do morphological evidence of filter feeding, crypt cells, and style of asexual propagation (Debrenne and Zhuravlev 1994; Debrenne 1999; Reitner et al. 2001). Although the solitary cone shapes of earlier Archaeocyathans were not conducive to formation of solid reef frameworks , later forms were more integrated (Wood et al. 1992). Reef-formation may nevertheless have depended on the collaboration of associated calcimicrobes (calcium secreting micro-organisms) with the archaeocyaths (Debrenne 2007; Kiessling 2009).
This central theme of the need for collaboration between primary framework builders and various groups of accessory reef -binders for successful reef building has persisted ever since this ancient example. Just as for modern reefs , environmental factors, including water movement and depositional setting, as well as temperature , determined where archaeocyath -calcareous depositing cyanobacterial associations resulted in resistant reefs (Gandin and Debrenne 2010). Environmental requirements must be satisfied for both the organisms contributing solid building blocks and those binding the blocks into a reef.
5.4.2 Hypercalcified Sponges
Following the archaeocyath extinction 500 MYA, sponges of stromatoporoid , sphinctozoan , and chaetetid grades built reefs at various times throughout the Paleozoic and Mesozoic , generally in conjunction with microalgae and metazoan taxa capable of growing in encrusting forms (Hartman et al. 1980; Wood 1995, 2011). Hypercalcified sponges suffered substantial extinctions at the end of both the Devonian and the Triassic (e.g., Kiessling et al. 2007). Extracting clues from ancient sponge reefs that might advise us on the long-term prospects for modern reefs becomes more complex the more we learn. Among the factors that must be considered are Mg/Ca concentrations in seawater (e.g., Stanley and Hardie 1998), as well as temperature , nutrients , sediment , and interactions of all of these factors with each other and with a variety of biotic agents (e.g., Wood 1993; Kiessling 2009; Wood 2011; Chaps. 8 and 9).
Correlations of paleoenvironmental conditions with reef development must be interpreted cautiously. For example, Middle Carboniferous reef mound building by chaetetids is known from low light, low sediment habitats , similar to the situations in which present day coralline sponges are found (West and Kershaw 1991). This could be interpreted as evidence that these were always the favored habitats of coralline sponges, or that during this time period they were forced to such sites, or that such sites were simply where preservation and/or subsequent discovery were more likely. In a comprehensive evaluation of taphonomic issues, Wood (2011) gives many examples of how to avoid misinterpretations by focusing on detailed mechanisms and processes of reef-building, and understanding form-function relationships. Historic roles of solid-skeletoned organisms can be problematic enough when all we have is a snapshot in rock. The likelihood of misinterpretation is exacerbated by the possibility that soft-bodied sponges have played roles in reef construction, maintenance, and repair that leave no traces in the finished reef frame.
Extremely slow growth rates of living coralline sponges (e.g., 0.18–0.23 mm/year for Ceratoporella: Willenz and Hartman 1985, 1999; 0.05–0.1 mm/year for Acanthochaetetes: Reitner and Gautret 1996; 0.236 mm/year for Astrosclera: Wörheide 1998) lend credence to the idea that competition from rapidly growing scleractinians may have played a role in restricting reef -building sponges to caves and other cryptic habitats . Changes in reefs that coincided with the blossoming of scleractinian zooxanthellate corals in the middle Jurassic included the creation of caves and other cryptic spaces by the combined foliaceous, branching, and plate-like morphologies of rapidly growing corals needing to collect sunlight (Jackson et al. 1971). These cryptic spaces provided a new habitat in which sediment and competition from organisms that are fueled by sunlight are minimized (Jackson et al. 1971). Although species diversity may now be relatively low, coralline sponges continue to be key fortifiers of the reef frame (Fig. 5.1), working from inside by depositing skeletons that are at least twice as dense and with compressive strength several times as great as those of scleractinian corals (Schumacher and Plewka 1981; Willenz and Hartman 1999; Vacelet et al. 2010). Individual Ceratoporella nicholsoni Hickson can be a meter in diameter and populations can be dense, with as many as 5–12 individuals of greater than 10 cm in diameter per m2 (Lang et al. 1975). Large individuals must be thousands of years old, suggesting a strategy that has favored resistance to physical damage over efficient recovery from damage (Vacelet et al. 2010). The disadvantage to this strategy, i.e., less efficient recovery, is increasingly apparent on modern reefs that are beset by multiple, larger, and more chronic disturbances (e.g., Wulff 2006b).
5.4.3 Reef-Building Sponges with Siliceous Skeletons: Lithistids and Hexactinellids
Hypercalcified sponges were not the only reef -builders; sponges with hard dense skeletons made of elaborate silica spicules called desmas also built reefs (e.g., Hartman et al. 1980). Ordovician reefs containing large proportions of these lithistid sponges, as well as stromatoporoids , depended on crust-producing microbes, and sometimes encrusting bryozoans , to bind the sponges together and fill gaps between them, thus helping to hold the framework together (Adachi et al. 2011). These sponges may have also served as baffles encouraging deposition of sediment , and subsequent lithification . In the Mesozoic , lithistid sponges contributed especially to Jurassic reefs of the Tethys Sea, but lithistids diminished dramatically in the Cretaceous and early Tertiary and became largely confined to deep water (Maldonado et al. 1999). Experimental support for the idea that these reef-builders diminished near the Cretaceous-Tertiary boundary due to depletion of silicon in shallow water as diatoms proliferated comes from studies of recent sponges grown in silica-enhanced seawater. When Maldonado et al. (1999) grew the common Mediterranean encrusting species Crambe crambe in silica-enriched water, it augmented its typical spicule assortment with elaborate spicules similar to those found in fossil deposits. Conversely, the high abundance of lithistids in the Jurassic may have been promoted by higher dissolved silica levels due to volcanic activity in the Triassic (Maldonado et al. 1999). Another possible contributor to the demise of reefs built by siliceous sponges is the extreme post-Jurassic decline of calcimicrobes that both the lithistids and the fused-silica-spicule sponges in the Class Hexactinellida required as collaborators in reef-building (Brunton and Dixon 1994).
Differences among the reef -building sponges in skeletal materials can have far-reaching ramifications for reef accretion . One important difference between lithistid and hypercalcified reef building sponges is the resistance of the lithistids’ silica skeletons to boring organisms. On a geological time scale, the same Triassic volcanism that may have boosted silica for lithistid sponges may have also altered seawater chemistry to the detriment of hypercalcified reef-building sponges, which suffered substantial extinction at the end of the Triassic (e.g., Kiessling et al. 2007; Kiessling 2009; Pandolfi and Kiessling 2014).
5.5 Geological Roles of Sponges: Promoting Reef-Frame Integrity, Increasing Coral Survival, and Facilitating Repair
Geological roles of sponges in building and maintaining reefs shifted profoundly in the Tertiary , after over 490 million years of primary framework building (Wood 1990). Currently, sponges serve chiefly as binders, consolidators, eroders, reinforcers, and protectors of solid carbonate (Table 5.1). Soft-bodied sponges may also have played these accessory roles during the Paleozoic and Mesozoic , but (aside from excavations that are readily attributable to boring sponges) it is hard to know how we would recognize such roles of soft-bodied sponges in the fossil record , given that these sponge roles are so difficult to perceive on modern reefs. Even where sponges have been experimentally demonstrated to significantly affect the success of reef building, their contributions are far from obvious by observation alone.
5.5.1 Increasing Coral Survival by Adhering Living Corals to the Reef and Protecting Exposed Skeletons Against Eroders
Goreau and Hartman (1963, 1966) observed that sponges could adhere living corals securely to the reef frame even after their basal attachments were eroded by excavating organisms, and suggested that association with sponges could therefore increase coral survival. In addition to compensating for the erosion of the bases of the corals by gluing them to the reef, sponge cover of coral skeletons where they lack living coral tissue can simultaneously block further invasion by eroders (Fig. 5.2). Wulff and Buss (1979) confirmed these benefits of sponges to corals by mapping and measuring all of the corals on eight fore-reef patch reefs in the San Blas Islands , Panama , and then removing sponges from half of the patch reefs . Only 6 months later, 40 % of the corals, representing 46 % of the percent cover of living coral tissue, had fallen off the reefs from which sponges were removed, in striking comparison with losses of only 4 % of the coral colonies (3 % of the surface area of live coral tissue) from the control reefs. Thus the observably negative role of boring sponge species can be countered by the positive roles of adhesive and protective coating by epibenthic and cryptic sponges. These results illustrate how easy it is to misinterpret the net effect of an interaction of a sponge and coral. Even when the sponge is actually saving the coral’s life, it may appear to be engaged in aggressive overgrowth . Time-series observations are essential for determining if a sponge is advancing over the coral. Wulff and Buss (1979) framed their report of this mutually beneficial association in terms of carbonate balance in order to underscore how the net effect of sponge-coral interactions on reef building and maintenance may not be surmised correctly by simple observation .
Sponges adhering corals to the reef and protecting exposed solid carbonate from excavators. (a) base of a branching sponge, Aplysina cauliformis (Carter) from which the erect portion was broken during Hurricane Allen, 1980, Jamaica ; the wound healed quickly and regrowth could already be seen after a few weeks; (b) a branch of A. cauliformis, broken during Hurricane Allen, and caught in a pile of coral rubble also generated by the storm . Within a few days the sponge had attached to several pieces of rubble, binding them together; (c) the branching sponge Niphates erecta Duchassaing and Michelotti covering bare coral (Orbicella annularis (Ellis and Sollander)) skeleton and adhering to live portions of the colony; (d) the semi-cryptic sponge Mycale laevis (Carter) protecting bared coral (Porites astreoides Lamarck) skeleton, gluing the coral to the reef, and even providing an increasing substratum over which the coral can grow (Goreau and Hartman 1966)
5.5.2 Rubble Stabilization: A Key Step in Reef Recovery After Physical Damage
Rubble generated by storm waves and other disturbances can be inhospitable to coral recruits , as they are jostled about by chronic water movement and foraging animals. Sponges can solve this instability problem in two ways: (1) sponges living in cryptic spaces under the reef surface can grow upwards into rubble piled upon them, and (2) epibenthic sponges that have been broken by storms can be incorporated into rubble piles as errant fragments (Fig. 5.2). In both cases, its homogeneous 3-dimensional body allows a single sponge to quickly attach (within 2–5 days) to several pieces of rubble, holding them steady against each other until carbonate -secreting organisms, especially encrusting coralline algae , can bind them together permanently (Wulff 1984; Biggs 2013). Without rapid binding by sponges, slower-growing carbonate-secreting binders could not grow from one piece of rubble to the next. The sponges are the “fingers holding the pieces together while the superglue sets” (thank you to D. Hubbard for this analogy).
Experimental exploration of each step of this process on a shallow Caribbean coral reef in Panama revealed that rubble piles with sponges remained elevated above the reef surface, became bound together by encrusting coralline algae within 5 months, and became colonized by coral recruits within 10 months. Rubble piles without sponges remained loose and increasingly collapsed, although each individual piece of rubble became encrusted with coralline algae (Wulff 1984). Small corals on stabilized rubble survived significantly better than small corals on loose rubble (for respectively stabilized and unstabilized rubble: 66 % undamaged vs. 35 % undamaged after 4 months, and 13 % survival vs. 1 % survival after 4 year).
In the tropical eastern Pacific , rubble on the tops of reefs in the Gulf of Chiriqui , Panama , was stabilized as cryptic sponges grew up through the reef frame to bind it, but the absence of exposed sponges on the reefs resulted in aprons of rubble at their bases, each rubble piece thickly coated with many layers of coralline algae (Glynn 1974; Wulff 1997c). The lack of a mechanism for stabilizing rubble against the challenges of episodic storms and chronic disturbance agents such as large foraging triggerfish and sea urchins prevents these rhodoliths from being incorporated into solid reef framework : pieces of loose rubble do not remain still next to each other long enough for encrusting coralline algae to grow from one piece of rubble to another, welding them into a stable structure . A similar dearth of epibenthic sponges in the Galapagos may contribute to extensive rhodolith piles, in which individual pieces of rubble resulting from massive coral mortality during the 1982/83 ENSO event have become encrusted by coralline algae so that they are up to 15 cm in diameter (Halfar and Riegl 2013). No recovery has occurred, and what was once incipient coral framework has remained as a rubble bed with no signs of recovery for over 20 years.
Discrepancies between growth of individual corals and reef accretion remind us of the diversity and complexity of the processes that contribute to reef building , maintenance and repair (e.g., Hubbard 1985, 1988; Hubbard et al. 1998). It is possible that some of these discrepancies may be explained at least partially by differences in abundances of epibenthic and cryptic sponges that are capable of mediating the cycling of loose rubble back to stable substrata suitable for coral recruitment and growth. The coincidence of coral reefs and hurricanes in shallow tropical water suggests that the cycle of rubble generation, consolidation, and recruitment of corals has long been a normal part of scleractinian reef-building. Sponges have likely played key roles in ensuring that it is actually a cycle instead of a one-way path from living corals to rubble. The only other organisms capable of rapidly adhering to multiple rubble pieces are fleshy algae , but their need for light causes them to overgrow the stabilized substrata , impeding coral recruitment.
Goreau and Hartman (1966) and Hartman (1977) pointed out that sponge binding could also aid reef growth by preventing piles of rubble from cascading down steep slopes, sweeping everything in their path into talus piles at the bases of fore-reef walls. Soft-bodied, non-excavating sponges , i.e., the vast majority of sponges, that participate in gluing living corals to the reef frame and mediating rubble consolidation, vanish shortly after they perish because their skeletal frameworks of protein fibers and spicules fall apart (Wulff 2006c, 2008a). Thus they do not appear in fossil reefs ; and even on modern reefs , these roles are invisible unless observed in action or explored by experiments that explicitly compare dynamics with and without sponges.
5.5.3 Improving Reef Restoration by Harnessing the Ability of Sponges to Bind Rubble
Expanding on experiments demonstrating sponge-mediated rubble consolidation (Wulff 1984; Biggs 2013) experimentally showed how sponges can be sustainably used to restore damaged reef sites. Erect branching sponges, the growth form most likely to become broken and included in rubble piles under natural circumstances (e.g., Wulff 2006b; Fig. 5.2), can be harvested sustainably because the branches from which fragments are cut grow back quickly. Once the sponge fragments are inserted into rubble piles, each fragment rapidly reattaches to several pieces of rubble. Using sponges to bind loose rubble into stable structures on which coral recruits are more likely to survive is not only less expensive and more attractive than artificial cements , but sponge-mediated binding is autocatalytic, as the sponge fragments grow and multiply, continually adding to their binding power. Moreover, framework -building coral species recruit significantly more to coral rubble bound with sponges than to cement bound structures, adding another reason to use living sponges in reef restoration (Biggs 2013).
5.6 Geological Roles of Sponges: Bioerosion
Bioeroding sponges have provided mysteries aplenty, and in spite of publications throughout the nineteenth century declaring them to indeed be sponges and also active borers rather than inhabitants of holes made by other creatures, it was not until nearly the twentieth century that these were accepted as facts (see Schönberg 2008 for a comprehensive history). The exact mechanism of boring , in particular the relative importance of chemical dissolution and mechanical removal of chips, is still an active area of research (review by Schönberg 2008). Although the ability to excavate and otherwise whittle down solid biogenic carbonate may seem as astonishing a feat for sponges as generating dense solid carbonate skeletons , the ability to excavate is currently represented in five orders of the class Demospongiae, suggesting the possibility of an ancient origin within the sponges.
Reefs may have been re-shaped by sponges from the start. Excavations that could have been made by sponges have been found in Cambrian archaeocyath reefs and middle Ordovician hard substrata (Kobluk 1981). However, although bioerosion by a variety of macro-organisms was common in Paleozoic tropical biogenic carbonate , the radiation of the group currently responsible for the majority of excavations in reefs, the clionaid sponges (Order Clionaida), was a Mesozoic phenomenon (Tapanila et al. 2004). Cenozoic boring in reef substrata is dominated by sponges (e.g., Perry 2000), and on currently accreting reefs sponges accomplish up to 90 % of the macroscopic boring (e.g., Goreau and Hartman 1963; Perry 1998; Rützler 2012). Although the great majority of sponge species are not capable of excavating corals, and the biomass of excavating species is relatively small, their influence can be dramatic (excellent reviews dispersed over the last few decades include Goreau and Hartman 1963; Hartman 1977; Wilkinson 1983; Rützler 2002; Schönberg 2008).
Abundance of boring sponges and the rate at which they break down solid carbonate varies widely. This variation has been recognized as a possible source of clues about environments for fossil reefs , and the value of sponge borings has been discussed for paleo-reconstruction, e.g., for bathymetric patterns (Bromley 1970; Bromley and d’Alessandro 1984, 1990; see also Chap. 4). Schönberg and Tapanila (2006) matched the morphology of bioerosion by the modern Siphonodictyon paratypicum to the fossil bioerosion trace Entobia devonica for paleoecological interpretation with respect to bathymetry and sedimentation , and their findings largely matched earlier ones, i.e. that Siphonodictyon spp. typically occur in shallow, low energy environments (Reitner and Keupp 1991). Evidence from the late Oligocene suggested that bioeroding sponge distributions were influenced by salinity gradients, just as they are today (Lawrence 1969). In Ordovician -Silurian reefs built by tabulate corals and stromatoporoids , a pattern of higher proportion of specimens bored in off-reef facies could have been caused by the greater competition for space on reefs, which may have diminished the success of boring (Tapanila et al. 2004). A similar pattern in the amount of boring was found in Pleistocene deposits of north Jamaica , where the percent of framework removed by borers was greater in back-reef/lagoonal settings than on the fore-reef . Sponges were responsible for most of the excavations on fore-reefs. Overall 64.7 % of framework carbonate was removed by sponges, and only 8.2 % by bivalves and 25.8 % by a variety of worms (Perry 2000). Pleydell and Jones (1988) reported similar rates for Grand Cayman Oligocene-Miocene bioerosion.
The clearest environmental correlate of sponge bioerosion has been eutrophication, and increased sponge bioerosion with nutrient increases has been detected in different settings and geological times (e.g., Hallock 1988; Edinger and Risk 1997; see also Chap. 4). On modern reefs , excavating sponges have been demonstrated to increase in abundance with increased water column nutrients (e.g., Rose and Risk 1985; Holmes 1997; Ward-Paige et al. 2005; Alcolado 2007). The relationship with nutrients depends on the species, and is not monotonic. Even boring sponges cannot cope with extremely high nutrient levels, and the toxic effects of the resulting eutrophication (Rützler 2002). Chaves-Fonnegra et al. (2007) found increased abundance of Cliona delitrix Pang as they evaluated sites closer and closer to a sewage outfall on San Andrés, Colombia, but this species declined to zero at the site closest to the outfall. As the authors pointed out, negative influence on sponges of the high nutrients at the outfall could have been confounded with increased sediment , a frequent covariant. Negative effects of sediment may also explain increased importance of boring by bivalves and worms relative to sponges within the bay at Discovery Bay, Jamaica , in spite of increased food for sponges in the water column (Macdonald and Perry 2003).
Advance of boring sponges into coral skeletons can be influenced by characteristics of the interacting species and the idiosyncrasies of immediate context, including angle of encounter, coral growth form or species, sponge species (Rützler 2002; Schönberg 2002, 2003; López-Victoria et al. 2006), and even parrotfish bites at the sponge-coral interface (Marquez and Zea 2012). The excavating sponge Siphonodictyon coralliphagum Rützler produces mucus that kills coral tissue, allowing this sponge to penetrate coral tissue and possibly also settle on live coral as a larva (Rützler 2004, 2012); and other boring sponges can undermine polyps in order to make their way into the skeleton (e.g., Chaves-Fonnegra and Zea 2011). The strong preference of the voraciously excavating species Cliona delitrix for massive corals might even, over time, change the composition of the coral community to favor species of foliose and branching corals (Chaves-Fonnegra and Zea 2011).
Rate of spread by excavating sponges is not only enhanced by factors that spur on the sponges, but also by the more sheltered habit of the sponges (Schönberg and Wisshak 2012) and factors such as temperature that stress the corals enough to hinder their ability to fend off the sponges (Rützler 2002). Spread of boring sponges can be slowed or halted by prior encrustation of solid carbonate or by overgrowth of coralline or other macroalgae ; and recruitment and excavation can be prevented by cover of other sponges (e.g., López-Victoria et al. 2006; Chaves-Fonnegra and Zea 2011; González-Rivero et al. 2012; pers. observ. Figs. 5.2 and 5.3).
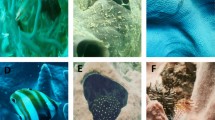
Sponges boring , overgrowing , and protecting coral. (a, b) the boring sponge Cliona caribbaea Carter being overgrown and killed by the encrusting sponge Chondrilla caribensis; (c) the boring sponge C. tenuis Zea and Weil steadily diminishing chances of survival for a coral, in the absence of epibenthic or semi-cryptic sponges ; (d) the readily storm -broken sponge Svenzea zeai (Alvarez et al.) temporarily overgrowing coral (Diploria)
Some excavating clionaids harbor symbiotic zooxanthellae, but this symbiosis does not tend to break down under abnormally high temperatures as readily as in scleractinians . When 84–87 % of the corals on Orpheus Reef, GBR , bleached in 1998, the boring sponge Cliona orientalis Thiele retained its zooxanthellae (Schönberg and Wilkinson 2001), an advantage that may be conferred by the sponge’s ability to move the zooxanthellae deeper into its tissue during stressful events (Schönberg and Suwa 2007) and by their relatively heat-resistant G-clade zooxanthellae (Schönberg and Loh 2005; Schönberg et al. 2008). The abundance of zooxanthellate sponges significantly increased after the 1998 bleaching , which was interpreted to be a result of their ability to survive, remain healthy , and recruit where corals died (Schönberg and Ortiz 2009). It may appear obvious to ascribe aggressive behavior of boring sponges, as well as their consistent increases with water column nutrients , to benefits from symbiotic zooxanthellae (e.g., Fang et al. 2014), but some species of rapidly advancing excavators, e.g., Pione lampa (de Laubenfels), S. coralliphagum, and C. delitrix in the Caribbean , do not have photosynthetic symbionts (Rützler 2002).
As part of an overall carbonate budget for five sites in Bonaire, Perry et al. (2012) calculated that loss rates related to boring sponges ranged from 0.002 to 0.07 kg/CaCO3/m2-year, which is smaller than losses to parrotfishes (0.95–2.75 kg/CaCO3/m2-year) at the same sites (for context, CaCO3 production by corals ranged from 0.20 to 12.07 kg/CaCO3/m2-year). This relationship was different in Jamaica , where fish bioerosion was only 8–20 % of the internal macro-bioerosion , which was dominated by sponges (Mallela and Perry 2007). Water quality and maturity of the community may influence the relative losses to endolith and grazer bioerosion, with bioerosion by sponges often being more important on reefs where they have had time to establish and where nutrient concentrations are higher (Carreiro-Silva and McClanahan 2012). As all of these reports point out, variation is great, even among sites near each other. Perry et al. (2012) remark on the difficulties with interpreting differences when comparing data collected in different ways [they cite 0.2 kg/CaCO3/m2-year in Barbados from Scoffin et al. (1980) and 0.02–1.04 kg/CaCO3/m2-year in French Polynesia from Pari et al. (2002)]. Environmental conditions play a key role, and recently ocean acidification was recognized as a strong driver of sponge bioerosion (Wisshak et al. 2012; Fang et al. 2013; Wisshak et al. 2013; Fang et al. 2014; Wisshak et al. 2014; Stubler et al. 2014), while it simultaneously suppresses coral calcification (Jokiel et al., Chap. 2 this volume).
The amount of solid carbonate eroded into sediment may not be the most important measure of the influence of boring sponges on reef building and maintenance. Although at many sites parrotfishes may reduce more substrate mass to sediment than do boring sponges, the result may reduce coral survival and reef growth far less. Parrotfishes scrape only from the surface, whereas sponges can erode the bases of corals, causing them to topple from the reef frame and perish in the sediment (e.g., Goreau and Hartman 1963; Wulff and Buss 1979). Thus with only a small amount of sponge erosion, entire living coral colonies may be lost (Fig. 5.3). Preventing this aspect of coral death and the loss of large chunks of solid carbonate may be one of the most important roles of epibenthic, semi-cryptic, and cryptic sponges on coral reefs (Wulff and Buss 1979, Fig. 5.2), especially if boring sponges are increasing in abundance , and if they increase their activity as climate change progresses.
5.7 Biological Roles of Sponges: Overgrowth of Living and Dead Coral
Some sponge species have been demonstrated to kill coral tissue by allelochemicals , and a few species have been demonstrated to aggressively overgrow living corals at some sites (recent review in Wulff 2012, pp. 308–312). Still the combined number of species that have been shown to be able to kill corals, or to kill them conspicuously in at least some places is fewer than 0.4 % of the sponge species that have been described in biogeographic realms with coral reefs . Other sponges may kill a small patch of coral tissue to allow the sponge to adhere to the underlying skeleton , but this can bind the corals securely onto the reef frame (Wulff and Buss 1979). As with most examples of mutualism, there is a price to pay for the benefits . In this case, even several cm2 of tissue is a very small price for a tenfold gain in the entire colony’s survival rate. Further expansion of branching, semi-cryptic, or massive sponge species over living coral has been reported only rarely. Time-series observations of interactions that had initially appeared to be overgrowths on reefs in Colombia showed that most sponges did not actually progress over living coral. Only 16 of the 95 sponge species present overgrew coral at all, and only three of these overgrew coral in more than 10 % of contacts (Aerts and van Soest 1997; Aerts 2000).
Cases in which field observations have demonstrated overgrowth of live corals over time usually fall into three categories: (1) sponges that are alien to the reefs on which they are overgrowing corals, e.g., Mycale grandis Gray, an Indonesian and Australian native, in Kaneohe Bay, Hawaii (Coles and Bolick 2007), and Chalinula nematifera (de Laubenfels), an Indo-Pacific native, in the Mexican Pacific (Ávila and Carballo 2008); (2) thinly encrusting sponges that are densely inhabited by cyanobacteria, e.g., Terpios hoshinota Rützler and Muzik, in the Pacific (Rützler and Muzik 1993), and Chondrilla caribensis Rützler et al. (Vicente 1990) in the Caribbean ; and (3) cases in which the corals are particularly stressed (Wulff 2012).
At a particular moment and site, an aggressive sponge species can devastate corals. For example, sponges of an aggressive species may infest half a locale’s corals (Benzoni et al. 2008), cover half the substratum (Reimer et al. 2010), or spread over coral tissue at rates of nearly a mm a day (Bryan 1973). In none of these cases, however, has the aggressive sponge species caused continually increasing devastation. Rather, there is a consistent, curious pattern of infestations being found only at some sites, and being ephemeral at any particular site. The most dramatic example of a sponge that can overgrow corals is the cyanobacteria-packed thinly encrusting sponge T. hoshinota. Since it was first reported in Guam (Bryan 1973), it has been found at far ranging sites across the Pacific including Okinawa , Taiwan , Indonesia and Lizard Island , Australia , but has vanished from some sites where it was once common (e.g., review in Wulff 2012, pp. 309–310; de Voogd et al. 2013). Coral-threatening Mycale grandis in Hawaii has recently diminished in abundance (pers. observ.); and Chalinula nematifera was only found at two of 150 sites in the Mexican Pacific, and has not increased (Ávila and Carballo 2008). Although Chondrilla caribensis quickly covered dead coral skeletons at a central lagoon site on the Belize Barrier Reef where the coral Agaricia tenuifolia had suffered catastrophic mortality due to bleaching (Aronson et al. 2002), at other nearby sites it is extremely rare on coral reefs (Wulff 2012, pp. 310–312). Aronson et al. (2002) pointed out that Chondrilla did not overgrow living coral, but only recruited and grew after coral death. Although the sponge cover would prevent recruitment of corals, this cover also protects solid carbonate from being reduced to sediment by boring organisms. Chondrilla has been observed to overgrow Cliona-infested coral skeletons in Belize, putting the boring sponge out of business on the spot (Fig. 5.3).
The role of stress in spurring overgrowth of living corals by sponges is uncertain, perhaps because stress has been defined in a variety of ways. But just as coral health can influence the advance of boring sponges, coral health can influence overgrowth. Time series observations of T. hoshinota in Okinawa revealed a pattern of devastation to live corals at sites where development had increased turbidity of coastal waters (Rützler and Muzik 1993), and recent experiments have shown that circumstances allowing T. hoshinota to overgrow corals depend on relative health of both the corals and sponges at a particular site (Wang et al. 2012). The threat of a thinly encrusting Clathria species that was killing recently transplanted massive reef corals, Porites lutea Milne Edwards and Haime, at a Gulf of Aden site diminished as the infested corals recovered from the stress of being transplanted (Seguin et al. 2008). Although Aerts and van Soest (1997) found that overgrowth of corals by sponges was not more likely on reefs deemed stressed (evaluated by higher sedimentation rate and poorer water column visibility), they did discover that the thinly encrusting Clathria (Thalysias) venosa (Alcolado), which one-time observations suggested could be a threat, only overgrew living coral if the coral was first experimentally damaged (Aerts 2000).
5.8 Biological Roles of Sponges: Water-Column Influences
As sponges pump water through their internal canals, their uniquely fine-scale filter system (the collars of the choanocytes) captures picoplankton that pass through the coarser filters of other filter -feeding taxa. Reiswig (1971) demonstrated that sponges of three Caribbean species representing different orders could capture an astonishing 96.4 % of the bacteria in the water column . Reiswig’s classic, and still unsurpassed, studies (1971, 1973, 1974) relating sponge feeding, respiration , abundance , and population dynamics, allowed him to estimate that the sponges of the fore-reef at Discovery Bay, Jamaica , could filter the entire water column above them every 24 h. Technological advances have made it possible to add further details. Now we also know that sponges can use dissolved organic matter, as well as filter a suite of minute particles, including procholorophytes, picoeukaryotes, cyanobacteria, and heterotrophic bacteria. The efficiency with which they capture each of these components of the picoplankton or absorb dissolved organic matter is influenced by their species, shape , size, densities of microbial symbionts , and internal morphology , as well as by what is available (e.g., Strimaitis 2012 and reviews in Rützler 2004, 2012; Wulff 2012).
5.8.1 Maintaining Water Clarity
Losses of sponges have unfortunately corroborated Reiswig’s (1974) estimate of the great importance of sponge filtering . After Hurricane Allen in Jamaica (Woodley et al. 1981), pulverized organisms and the bacteria devouring them kept the water column murky as long as sponges that had survived the storm remained shut down. When the sponges resumed their pumping, the water cleared rapidly (pers. observ.). Florida Bay, from which water flows out to the reefs of the Florida Keys, has been devastated by many cyanobacteria blooms since 1982, when the first blooms killed up to 90 % of the sponges (Butler et al. 1995). Peterson et al. (2006) combined estimates of sponge biomass and filtering abilities to figure the cost to the water column of sponge loss, and concluded that reduced filtration of the water column resulting from heavy sponge mortality during the first bloom can entirely explain the subsequent blooms. This conclusion raises the disturbing possibility that the recent loss of 71 % of sponge biomass from a shallow reef in the central Belize Barrier Reef during an extended phytoplankton bloom (Wulff 2013) could allow future incipient phytoplankton blooms to billow forth because badly depleted sponge populations can no longer nip them in the bud.
Zooxanthellae or cyanobacteria contribute in various degrees to the nutrition of some sponge species, with zooxanthellae largely confined to excavating species of the order Clionaida (Rützler 1990; López-Victoria and Zea 2005; Hill et al. 2011; but also see Garson et al. 1999; Scalera-Liaci et al. 1999, for interesting exceptions). Sponges that harbor photosynthetic symbionts are not as consistently reliant on them as are scleractinian corals, and complete shading of photosymbiont-bearing sponges can result in diminished growth , or loss of biomass , or no apparent negative effects at all (e.g., Erwin and Thacker 2007; Freeman and Thacker 2011). The coral-killing Terpios hoshinota is capable of escaping from shading by extending fine threads until they reach sunlit substrata , where they resume growth as a continuous sheet (Soong et al. 2009). Variation in the importance of the photosymbionts may depend in part on symbiont identity (e.g., Thacker 2005; Erwin and Thacker 2007) and also on the ability of the sponge host species to switch between different modes of acquiring food (review in Wulff 2012, pp. 301–303).
5.8.2 Influences on Dissolved Organic and Inorganic Water-Column Components
Sponges can have profound effects on dissolved water-column components, especially carbon , nitrogen , and silicon (review by Maldonado et al. 2012). In collaboration with their microbial symbionts , some sponges can soak up and make use of dissolved organic material (Reiswig 1981; de Goeij et al. 2008; Weisz et al. 2008). Some species of sponges that inhabit cryptic spaces within the reef frame, may acquire a significant portion of their nutrition from dissolved organic matter (DOM) generated by corals or coralline algae rather than relying on sparse picoplankton (van Duyl et al. 2011). Recent reports have suggested that this could be a major force in nutrient cycling on coral reefs (de Goeij et al. 2013), with sponges and their symbionts transforming DOM into sponge biomass , and extremely rapid cell cycling resulting in the shedding of cells that serve as food to organisms incapable of using dissolved organic matter directly. Scaling-up processes identified for a few species at the level of cubic centimeters, to entire communities and the vastly larger water column above a coral reef must be done cautiously, as reefs vary widely in biomass of both cryptic and epibenthic sponges (e.g., Wilkinson 1987; Wilkinson and Cheshire 1990). For example, a conclusion that cryptic sponges account for orders of magnitude more biomass than epibenthic sponges was based on endoscopic observations of a Red Sea reef (Richter et al. 2001) where epibenthic biomass estimates were extremely small (0.8–1.2 % cover, no volume measurements given). On reefs where epibenthic sponges are more evident (e.g., in most Caribbean locations), the assumption that biomass of cryptic sponges is greater is less likely to be applicable.
While it is not yet clear how important these processes are in overall nutrient cycling on coral reefs in general, sponge-mediated nutrient cycling within the reef frame is an intriguing reminder of the possibility that there are other surprising sponge-mediated processes on coral reefs that we have not yet even imagined. This is underscored by the recent discovery that the diet of two species of Caribbean excavating sponges can be mainly dissolved organic carbon (Mueller et al. 2014).
Sponges, in collaboration with their symbiotic microbes, can also greatly influence nitrogen cycling on coral reefs . Transformations in which sponge microbes participate include nitrification, nitrogen fixation, denitrification, and anaerobic ammonium oxidation (e.g., Corredor et al. 1988; Webster and Taylor 2012). This is a rapidly growing area of sponge and microbial research, as new techniques are developed and the potential importance to coral reef ecosystems becomes more apparent (Maldonado et al. 2012).
5.9 Biological Roles of Sponges: Providing Shelter and Food
5.9.1 Animal and Plant Guests of Sponges
How the hundreds of species of echinoderms, worms , molluscs , arthropods , fishes and multicellular algae hosted by sponges , within and on the surfaces of their bodies, might influence coral reef functioning is not clear, but these species certainly bolster biodiversity substantially (Cerrano et al. 2006; Wulff 2006e). Sponges and their symbionts offer opportunities to study community and population ecology in patchy habitats in which the patches (i.e., individual sponges) can be readily manipulated. Among the surprising and fascinating results of studies on sponge inquilines is the discovery of eusocial shrimps in sponges (Duffy 1996). Some symbionts use their hosts only as a shelter or breeding site, but others also consume their host (Wulff 2006e; Schönberg and Wisshak 2012). For obligate symbionts, the loss of their host sponges can be a disaster, leading to a cascade of local extinctions with potentially grave consequences. After a couple of major sponge mortality events on the Belize Barrier Reef (Wulff 2013) in which a total of 74 % of the sponge biomass was lost, eusocial shrimps became extremely difficult to find (J.E. Duffy, pers. comm.), and other inquilines vanished or died when their sponge host died (Fig. 5.4). Economically important spiny lobster populations were negatively influenced when 71 % of the sponges that provided shelter for their juveniles perished in a dense cyanobacterial bloom (Butler et al. 1995). We have barely begun to explore this aspect of coral-reef sponge interactions.
Interactions of coral reef sponges . (a) a recently dead Callyspongia vaginalis (Lamarck) with dying symbiotic zoanthids, and a symbiotic goby that was gone the following day; (b) Verongula rigida (Esper) with one bite removed by an angelfish just before the photo was taken, and a wound healing where bites had been taken 2 days earlier; (c) the easily confounded congeners Tedania ignis (Duchassaing and Michelotti) and T. klausi (Wulff) which differ from each other with respect to vulnerability to starfish predation , disease , and temperature and salinity extremes; (d) Aplysina fulva (Pallas) suffering (skeleton exposed where tissue died a few days earlier, and black or white signs of necrosis where tissue has died more recently; the ochre yellow portions are still alive) in the midst of a dense cyanobacteria bloom in which 71 % of the biomass of the sponge fauna was killed (Wulff 2013)
5.9.2 Consumers of Sponges
Although most epibenthic sponges are well defended against consumption by most of the large mobile predators with which they share habitat , a few spongivores depend on sponges. Angelfishes tend to consume most of the sponge species that they encounter in a “smörgåsbord” fashion, by taking only small amounts of any particular sponge (a mean of 2.8 bites in Wulff’s 1994 study of unmanipulated angelfishes ) before moving on to another sponge that is generally of a different species (Randall and Hartman 1968; Wulff 1994, 2006e, 2012 pp. 313–315; Fig. 5.4). Epibenthic sponges on coral reefs are not severely limited by routine spongivory of this type, because they readily regenerate where they have been bitten, and because only a small amount is ever eaten at one time.
Although angelfishes disproportionately feed on some species (Wulff 1994), they spread their feeding over most of the sponge community . Randall and Hartman (1968) found a total of 70 sponge species in gut contents of four species of angelfishes, Hourigan et al. (1989) observed that three species of angelfishes consumed 23 sponge species, Padilla Verdín et al. (2010) found 24 sponge species in gut contents of two species of angelfishes, and Wulff (1994) observed that angelfishes of three species consumed 64 sponge species on a coral reef in Panama , including 36 of the 39 species in a fully censused 16 m2 plot.
The other large dedicated spongivores on coral reefs are hawksbill turtles , which can devour large quantities of sponge tissue; but they only eat a handful of species in three orders of demosponges (Meylan 1988, 1990; van Dam and Diez 1997; León and Bjorndal 2002). The presence on coral reefs of charismatic mobile species, such as angelfishes , trunkfishes, and hawksbill turtles that routinely consume sponges may depend on diverse, thriving sponge assemblages. Curiously routine spongivory may have less dramatic effects on prey sponge species than opportunistic spongivory (Wulff 2006e).
Opportunistic spongivory can be an important trophic pathway on coral reefs . The conspicuous large Caribbean starfish Oreaster reticulatis may depend on occasional consumption of coral reef sponges that wash off reefs into adjacent seagrass meadows where the starfish reside. Oreaster (adults and juveniles ) maintained in tanks on their usual diet of microalgae fared poorly relative to those fed on coral reef sponges (Scheibling 1979); and populations of Oreaster inhabiting seagrass meadows into which sponges were more frequently washed by storms included a significantly higher proportion of large individuals (Wulff 1995). Oreaster reject sponge species that inhabit seagrass, but readily eat many of the coral reef species that are only available to Oreaster if they are washed off the reef into the seagrass (Wulff 1995). Opportunistic spongivory by herbivorous parrotfishes may also exert control on habitat distribution of sponge species, by preventing some of the species that are typically confined to cryptic spaces within the reef frame and in rubble piles from growing out of their hiding places, as some of these species appear to be relatively undefended against predators . Herbivorous parrotfishes , Sparisoma aurofrenatum (Cuvier and Valenciennes) S. viride (Bonnaterre) and S. chrysopterum (Bloch and Schneider) and to a lesser extent Scarus iserti Bloch battled each other over the opportunity to consume normally cryptic sponges that were exposed when researchers broke open their hiding places within the reef framework or rubble piles (Wulff 1997b). The possibility that sponges constitute an important supplement to their possibly nitrogen -limited diet is suggested by their battles for the sponges, and also by the alacrity with which they responded to exposure of cryptic sponges, veering from their paths and charging straight to the sponges as soon as they were exposed. Similar behavior was observed in the eastern Pacific , where the angelfish Holacanthus passer Valenciennes usually feeds on plankton in the water column above the reef, but responds immediately to the exposure of cryptic sponges when the reef is cracked apart, plummeting to the seafloor and engaging in battles with other fishes (including the parrotfish Scarus ghobban Forsskål and the Moorish idol, Zanclus cornutus (Linneaus) that are also attracted to the exposed sponges (Wulff 1997c).
5.10 Future of Sponges on Coral Reefs: Assessing and Ascribing Causes to Increases and Decreases
Literature concerning coral reef sponge abundance and dynamics, and interactions of corals with sponges , includes some striking discrepancies. Demonstrated dramatic declines of sponges contrast with assertions that sponges are increasing unchecked; reports of experimentally demonstrated extreme benefit to corals by associations with sponges contrast with assertions that sponges constitute one of the chief enemies of corals and reefs . Roots of these discrepancies are embedded in: (1) application of inappropriate methods for assessing and monitoring sponges; (2) lumping together as “sponges” a highly heterogeneous group of animals with a wide range of responses to changing conditions and influences on corals and coral reefs, rather than distinguishing sponge species; and (3) a tendency to generalize from studies on single conspicuous, and often unusual, species to entire regional faunas of many hundreds of species. In the hope of clarifying the pitfalls involved, each of these problems is discussed in detail below.
5.10.1 Inappropriate Methods for Assessing and Monitoring Sponges Yield Data That Are Difficult to Interpret
As sessile animals that can be large and exhibit a diversity of growth forms , sponges superficially appear to be amenable to the same field assessment and monitoring methods that work well for corals. However the tissue in most sponges is not a thin layer over the surface of a solid skeleton , as it is for corals, but fully three-dimensional. Ecosystem roles of sponges, therefore, scale with their volume rather than the surface area of live tissue, and their abundance in the context of population dynamics and vulnerability to local extinction must also be measured by volume (detailed discussions in Rützler 1978; Wulff 2001; Rützler 2004; Wulff 2012). Video transects, that adequately record corals that are oriented to sunlight, fail for sponges that live on vertical surfaces, under corals, and within crevices or embedded in the substratum (eg., Abdo et al. 2004). Point counts, and other percent-cover measures, do not reflect the abundance of most sponges. An encrusting sponge 2 cm in diameter and 1-mm thick, a spherical sponge with 2-cm diameter, and a tube sponge 2 cm across (with a 0.5-cm diameter cavity ) and height of 8 cm, all have the same percent cover (i.e., 3.14 cm2) on a planar projection, but the sphere has 13 times the volume and the tube has 75 times the volume of the encrusting sponge. Number of sponge individuals is rarely informative, as the size of an individual can range over many orders of magnitude (e.g., McMurray et al. 2010; Schönberg and Fromont 2012). A barrel sponge, 1 m in diameter and 1 m tall has 2.5 million times the volume of the encrusting sponge 2 cm across; thus it might filter 2.5 million times as much picoplankton from the water column and provide 2.5 million times as many bites of food to spongivores! Moreover, a physical disturbance or pathogen infection can quickly increase the numbers of individuals by fragmentation , while simultaneously diminishing biomass .
Explicit comparison of sponges of different growth forms on a shallow reef in Caribbean Panama (Wulff 2001) revealed that sponges in the four growth form categories of erect branching, massive , thickly encrusting, and encrusting each contributed about 25 % of the total percent cover , but with respect to volume, the erect branching sponges were 63 % of the total, and the encrusting sponges were a trivial 1.8 %. The one-quarter of the volume that constituted massive sponges was contributed by only 8 % of the individuals.
Sponges differ from corals in another way that diminishes usefulness of data acquired by methods often used for studying corals: most sponges vanish shortly after their death because the skeletal scaffolding (made of protein fibers or protein and spicules ) that supports their living tissue deteriorates quickly when not embedded in tissue (e.g., Wulff 2006c, 2008a). Thus mortality cannot be documented, and is likely to go unnoticed unless individual sponges were monitored before a mortality event. Substantial biomass can also be lost from sponges due to various agents of partial mortality (disease , predators , storms, e.g., Wulff 2006a, 2006b, 2006c, 2008a, 2013). Extreme regeneration ability of many sponges renders partial mortality quickly invisible unless pre-mortality monitoring includes measurements of total volume of each sponge (Wulff 2010, 2013).
Growth of sponges over exposed coral skeletons from which the tissue was previously lost can readily be confused with aggression against living corals in snap-shot observations . Time-series observations of the boundary between live sponge and live coral are the only way in which the two very different processes of aggression against living coral and protection of coral skeletons from excavators (by covering exposed portions) can be distinguished (e.g., Aerts 2000).
In summary, data on sponge population and community dynamics and interactions with corals can be readily misinterpreted, unless the data are gained by the somewhat arduous process of monitoring volume changes of individual sponges over time (Wulff 2001, 2006e, 2012, 2013 pp. 276–281). One-time observations tend to under-estimate sponge mortality and over-estimate negative effects on corals perpetrated by sponges.
5.10.2 Lumping Together Sponges of Diverse Talents, Vulnerabilities, and Relationships with Corals
Different sponge species, even closely related ones, can have dramatically different relationships with corals and reefs (Hartman 1977; Rützler 1978; Wulff 2001; Rützler 2004; Wulff 2006e; Rützler 2012; Wulff 2012; Wulff 2013). Abundance of sponges on present day coral reefs, and the determinants of abundance , must be considered separately for each of the four types of sponges (i.e., epibenthic, cryptic, boring , hypercalcified). Identification to species is key, because sponges that look similar can play very different roles. For example two Caribbean species, Iotrochota birotulata and Desmapsamma anchorata, are both erect brancing forms in the Order Poecilosclerida, but exhibit growth and mortality rates that differ by an order of magnitude (Wulff 2008b). Iotrochota forms mutually beneficial associations with branching sponges of other species that increase growth rates and survival of participating individuals, while Desmapsamma behaves as a parasite on other sponges (Wulff 1997a, 2008b), and also overgrows gorgonians (e.g., McLean and Yoshioka 2008). Species that are of the same genus can react very differently to environmental changes. Four Caribbean species of Ircinia responded to a mass mortality caused by dense phytoplankton in contrasting ways: two species that grow as clusters of mounds lost much biomass but began to recover within months, while two other species (one grows as large spheres and one as thick-walled vases) were entirely eliminated by the phytoplankton bloom (Wulff 2013). Likewise, two conspicuous Caribbean Tedania species were long thought to be a single species because of their similar appearances and spicule complements, but one is immune to starfish predation and can therefore inhabit seagrass meadows from which the other is barred by Oreaster readily consuming it, and the two species differ in susceptibility to disease and to extreme environmental conditions as well (Wulff 2006d, Fig. 5.4). Sponge taxonom y is unquestionably challenging, but failing to distinguish sponge species in field surveys makes no more sense than combining data on parrotfishes and snappers as “fishes ”, or Acropora and Porites as “corals”.
5.10.3 Are “Sponges” Overwhelming Coral Reefs?
Assertions that some coral reefs may be turning into sponge reefs and that sponges are increasingly overwhelming corals have been presented recently from two different viewpoints. Bell et al. (2013) support their assertion by pointing out that Mesozoic reefs of siliceous sponges provide historical precedents for reefs dominated by sponges, that reports on sponge disease are less prevalent than reports on coral disease, and that sponges can be abundant in high nutrient and turbidity settings such as lagoons . In contrast, Pawlik (2011) and Loh and Pawlik (2014) assert that palatable sponges that can outcompete corals are increasing in response to loss of spongivorous fishes by overfishing , a scenario that parallels increases in fleshy algae after herbivores have been overfished. I will discuss whether or not sponges are actually increasing at all in a later section, and for the moment only discuss two of these possible influences on future abundance of coral reef sponges: disease and water column nutrients . For the former it is clear that there are inadequate data for any conclusions, and for the latter there may be sufficient reports from a variety of reef sites to allow some tentative conclusions.
Diseases of corals have caused huge declines (e.g., Miller et al. 2009), and seem likely to continue to be devastating; but fewer reports of sponge diseases than of coral diseases may not necessarily reflect fewer losses of sponges to disease (Rützler 2004, 2012; Webster 2007) because sponge disease is very likely to be underestimated and under-reported. Sponge disease is virtually impossible to document unless it is caught in progress. Sponges that have died from disease tend to deteriorate quickly and vanish (e.g., Cowart et al. 2006; Wulff 2006c), while the skeletons of dead corals remain to proclaim for years afterwards that they existed before a disaster killed them, even if the exact disaster cannot be determined from the skeletons. Evidence for partial mortality due to disease is readily seen months later for corals, in the form of denuded skeleton ; but partial mortality in sponges is entirely effaced within days or weeks as the denuded skeleton deteriorates and the sponge generates a new surface. Monitoring programs that have tracked coral disease for decades tend to not include sponges at all. Even in cases where sponge disease prevalence is reported, an inverse relationship between the speed at which a pathogen can entirely kill a particular sponge, and the probability that the diseased sponge will be observed before it disintegrates and vanishes argues against accurate evaluation of sponge disease in one-time field surveys (Wulff 2006c). Monitoring disease in sponges will have to be done differently (e.g., at greater frequency, and at sites in which every sponge has previously been mapped and measured with respect to volume) than for corals if we are to learn how important sponge disease really is, or is not.
Water column nutrient concentrations, and the consequent productivity and availability of picoplankton (heterotrophic bacteria , cyanobacteria, prochlorophytes, and pico eukaryotes), are factors that appear to influence the abundance of sponges in general on coral reefs . Greater sponge biomass has been related to greater availability of sponge food both within and between regions. Wilkinson and Cheshire (1990) measured much greater sponge biomass on nearshore parts of the Great Barrier Reef , where water column production is high, than on oceanic reefs in the highly oligotrophic waters of the outer Great Barrier Reef . Taking this comparison a step further, making an explicit comparison between oceans, Wilkinson (1987) measured 7.9–570 g of living sponges per m2 on the Great Barrier Reef vs. 367–2458 g of living sponges per m2 on Caribbean coral reefs, which are characterized by greater water column productivity. Transplant experiments have demonstrated higher growth rates with higher water-column picoplankton concentrations between depths on the same reef (sponges of a tube-shaped species grew faster in deep relative to shallow water, Lesser 2006; Trussell et al. 2006). Reef sponges transplanted onto mangrove prop roots, where picoplankton densities were much higher than on the reef, grew 2–3 times as fast as they grew on the coral reef where they normally live (Wulff 2005). The relationship between sponge biomass and growth rates with picoplankton is not monotonic however, and the down-side of dense phytoplankton is that blooms have caused the most striking mass mortalities of sponges that have ever been documented by comparisons of census data from both before and after a mortality event (Butler et al. 1995; Stevely et al. 2011; Wulff 2013).
5.10.4 Data on Sponge Increases and Decreases
More to the point perhaps, than examining scenarios that might explain proliferation of epibenthic sponges over coral reefs , is determining whether or not there are data that demonstrate this proliferation. Although they have opposing ideas about what might cause proliferation of sponges, Pawlik (2011), Bell et al. (2013) and Loh and Pawlik (2014) cite a similar set of papers to support assertions of a phase shift to sponge dominance on coral reefs, including Aronson et al. (2002), Maliao et al. (2008), Norström et al. (2009), McMurray et al. (2010) and Colvard and Edmunds (2011). Because second-hand citations can result in plausible scenarios becoming established facts, it may be useful to examine the data in this set of papers, as not all of the authors claimed that their data demonstrate a general increase in epibenthic sponges. Aronson et al. (2002) discovered that the encrusting sponge Chondrilla caribensis increased from 15 to 43 % cover at an unusual site where the corals had previously suffered catastrophic mortality. This sponge species is virtually absent from other reefs nearby, as well as from most other Caribbean reefs in which full fauna surveys have been made (Wulff 2012 pp. 310–312). It would be interesting to know why it became so abundant so quickly at this site. McMurray et al. (2010) also monitored a single species, the barrel sponge Xestospongia muta, and acknowledged that although numbers of individuals increased at their two sites, total percent cover and volume did not. Mortality of large individuals, which constituted the bulk of the biomass of the populations, and which are susceptible to a fast-moving disease and to hurricane damage, could abruptly diminish abundance . Colvard and Edmunds (2011) monitored sites in the US Virgin Islands for 14 years, with a primary focus on corals. They documented a slight increase in numbers of individuals (0.17/m2 in 1992, 0.21/m2 in 2006) of three sponge species with either erect branching or thinly encrusting forms. Because sponges of these growth forms are readily fragmented by disease or storms into more but smaller individuals by partial mortality, it is possible for an increase in numbers to be linked with decrease in biomass. Maliao et al. (2008) refer to “proliferation of macroalgae and sponges” and include a figure showing how a “phase-shift” has occurred, illustrated by a pair of drawings in which there is apparently three times the amount of sponge mass in the post-phase-shift drawing. This is a puzzling conclusion, given that the data they present are 2.2 % cover of sponges at the start of the study, and 2.2 % at the end, indicating not only very low abundance, but also no sponge increase. Norström et al. (2009) compile data from the publications listed above, and also include studies of boring sponges, but no independent data. In addition to these Caribbean studies, Bell et al. (2013) include a study in which numbers of sponge individuals, most of the species Lamellodysidea herbacea, increased from 60–80 per m2 to 100–120 per m2 at sites in southeast Sulawesi with high sedimentation and turbidity . Without volume or percent cover information it cannot be determined if this represents an increase in sponges or merely fragmentation into more but smaller individuals of the sponges present earlier. Bell et al. (2013) were careful to make the point that, although dramatic increases of Terpios hoshinota have been well documented, these have not been stable. Excavating sponges are a very different story, and increases have been well documented (see Sect. 5.6 and references cited therein). However, even though an impression may be given that sponges are relatively more abundant in places where corals have decreased, with the exception of a few unusual species (refer to Sect. 5.7, and Wulff 2012, pp. 308–312), data have not yet been published to support the assertion that epibenthic sponges in general are proliferating over corals and coral reefs (Table 5.2).
5.10.5 Sponge Dynamics Documented by Full Censuses in Time Series
Mapping , identifying, and measuring the volume of every sponge within a permanently marked plot, again and again at regular intervals, is not fashionable, and often not feasible. This arduous process has only been accomplished at a few sites (see below), but is required if we really want to know whether sponges are increasing or decreasing on coral reefs . As discussed earlier, dead sponges tend to quickly fall apart and vanish so that there is no record that they existed unless they had been previously mapped. Signs of partial mortality are effaced quickly by regeneration, and thus repeated volume measurements are the sole way to know about non-fatal biomass losses.
Three census sites in the Florida Keys have revealed extreme losses of sponges . Stevely et al. (2011) reported losses over just 2 years, at Marathon Key and Long Key of 93 % and 88 % by volume (respectively 69 % and 45 % by number of individuals). The cause was cyanobacteria blooms , which also caused prior losses of 90 % of the sponges in Florida Bay (Butler et al. 1995). At a third site in the Florida Keys, Biggs and Strimaitis (pers. comm.) documented losses of 30 % by volume on a reef influenced by an extended cold snap.
The other two sites where all sponges of all species have been measured in time series were both chosen to represent especially healthy reefs with little human interference. In the San Blas Islands , Panama , in the course of 14 years, 41 % of the volume was lost, and 44 % of the species were lost from a 16 m2 plot (Wulff 2006a). Although the plot was small, it included 1395 individuals representing 39 species at the start, and loss of species could not be explained by simple stochastic loss of rare species from a small plot. Rare species were not disproportionately represented among those that vanished, and the same species that disappeared from the plot were also missing from other reefs in Kuna Yala that were being followed more qualitatively. Disease was observed in many of the species that vanished, but it is not known that this was the cause of all losses. On the Belize Barrier Reef , two mortality events occurred during 6 years of annual censusing, with the second having a more dramatic effect and a clear cause: an extended dense phytoplankton bloom (Wulff 2013). A total of 74 % of the volume and 44 % of the individuals were lost.
These are not many studies on which to base generalizations, but it should be noted that enormous losses have been documented in every case in which individuals of all or most species in an assemblage have been followed over time, even when sites were chosen as especially favorable for corals and sponges . It would seem prudent to at least reevaluate our assumptions about the overgrowth of coral reefs by sponges and to encourage more studies that are adequate to resolve this issue (Table 5.3).
5.11 Summary: What Would Happen to Coral Reefs if Sponges Were Entirely Deleted?
If we plucked all sponges out of present day coral reefs , the changes would be dramatic and varied, given the very different roles played by epibenthic, cryptic, excavating, and hypercalcifying sponges. Possible positive changes include: (a) living corals would no longer be threatened by the few aggressive sponge species and (b) excavations into coral skeletons would decrease unless boring bivalves increased in response to absence of boring sponges. Possible negative changes include: (a) the water column could become clogged with prokaryotic and other picoplankton growing in response to nutrients , with the loss of the only biological filters efficient enough at capturing picoplankton to keep it in check; (b) living corals would lose adhesive to bolster their grips on the reef frame when their bases are eroded, and would be more likely to fall to their deaths in the sediment ; (c) reef repair might cease, and accretion rates could diminish wherever coral rubble remains unstabilized and, therefore unsuitable for successful coral recruitment ; (d) the loss of sponge protection on portions of coral skeletons that are not covered with tissue would allow greater access to boring organisms that remain, such as some bivalves and worms ; (e) hundreds of species of invertebrates, fishes , and microbes that are obligate symbionts of sponges would lose their habitat , possibly resulting in extinction ; (f) obligate spongivores, many of which are attractive mobile fauna , such as angelfishes , hawksbill turtles , and dorid nudibranchs , would lose their prey, and (g) reef frames would be weakened by the loss of reinforcement contributed by skeletons of hypercalcifying sponges that are twice as dense as those of scleractinian corals. Many of these situations have already been documented by controlled experiments or time-series observations . Substantial losses of epibenthic and semi-cryptic sponge species have been documented by all of the few studies in which coral-reef sponge assemblages have been censused in time-series.
We have insufficient data for confident prediction about whether or not sponges will increase or decrease, but two consistent patterns appear to be emerging, both of them related to nutrient levels. Water column nutrient increases may cause increases in sponges in general, and appear to especially spur on boring sponges and the few species that can overgrow living corals. Simultaneously corals may be more susceptible to both boring and overgrowth when they are stressed by water column issues, such as increased sedimentation and diminished light, that are frequently concomitant with higher nutrient levels. Epibenthic and semicryptic sponges (i.e., the only organisms capable of binding coral rubble after physical disturbance and mitigating losses of live corals due to boring sponges) appear to be highly vulnerable to phytoplankton blooms that are caused by especially large increases in water column nutrients . There may be a fine line between increases and complete loss; but it seems all too possible that losses of sponges may accelerate, and that coral reefs deprived of the many positive roles that sponges play will suffer.
References
Abdo D, Burgess S, Coleman G. Osborne K (2004) Surveys of benthic reef communities using underwater video. Long-term monitoring of the Great Barrier Reef. Standard operational procedure 2. 3rd revised edition. Australian Institute of Marine Science, Townsville, 62 p.
Adachi N, Ezaki Y, Liu J (2011) Early Ordovician shift in reef construction from microbial to metazoan reefs. Palaios 26:106–114
Aerts LAM (2000) Dynamics behind stand-off interactions in three reef sponge species and the coral Montastrea cavernosa. Mar Ecol 21:191–204
Aerts LAM, van Soest RWM (1997) Quantification of sponge/coral interactions in a physically stressed reef community, NE Colombia. Mar Ecol Prog Ser 148:125–134
Alcolado PM (2007) Reading the code of coral reef sponge community composition and structure for environmental bio-monitoring: some experiences from Cuba. Porifera Research: Biodiversity, Innovation and Sustainability. Série Livros 28. Museu Nacional, Rio de Janiero. Pp. 3–10
Aronson RB, Precht WF, Toscano MA, Koltes KH (2002) The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar Biol 141:435–447
Ávila E, Carballo JL (2008) A preliminary assessment of the invasiveness of the Indo-Pacific sponge Chalinula nematifera on coral communities from the tropical Eastern Pacific. Biol Invas 11:257–264
Bell JJ (2008) The functional roles of marine sponges. Est Coastal Shelf Sci 79:341–353
Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS (2013) Could some coral reefs become sponge reefs as our climate changes? Global Change Biology 19:2613–2624
Benzoni F, Calcinai B, Eisinger M, Klaus R (2008) Coral disease mimic: sponge attacks Porites lutea in Yemen. Coral Reefs 27:695
Biggs B (2013) Harnessing natural recovery processes to improve restoration outcomes: An experimental assessment of sponge-mediated coral reef restoration. Plos One 8:e64945
Bromley RG (1970) Borings as trace fossils and Entobia cretacea Portlock, as an example. In: Crimes TP, Harper JH (eds) Trace fossils. Geol J Special Issue 3:49–90
Bromley RG, d’Alessandro A (1984) The ichnogenus Entobia from the Miocene, Pliocene and Pleistocene of southern Italy. Riv It Paleont Strat 90:227–296
Bromley RG, d’Alessandro A (1990) Comparative analysis of bioerosion in deep and shallow water, Pliocene to recent, Mediterranean Sea. Ichnos 1:43–49
Brunton FR, Dixon OA (1994) Siliceous sponge-microbe biotic associations and their recurrence through the Phanerozoic as reef mound constructors. Palaios 9: 370–387
Bryan PG (1973) Growth rate, toxicity and distribution of the encrusting sponge Terpios sp.(Hadromerida: Suberitidae) in Guam, Mariana Islands. Micronesica 9:237–242
Budd AF (2000) Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 19:25–35
Butler MJ, Hunt JH, Herrnkind WF, Childress MJ, Bertelsen R, Sharp W, Matthews T, Field JM, Marshall HG (1995) Cascading disturbances in Florida Bay, USA: cyanobacteria blooms, sponge mortality and implications for juvenile spiny lobsters Panulirus argus. Mar Ecol Prog Ser 129:119–125
Carreiro-Silva M, McClanahan TR (2012) Macrobioerosion of dead branching Porites, 4 and 6 years after coral mass mortality. Mar Ecol Prog Ser 458:103–122
Cerrano C, Calcinai B, Pinca S, Bavestrello G (2006) Reef sponges as hosts of biodiversity: cases from North Sulawesi. Proc 10th Int Coral Reef Symp 1:208–213
Chaves-Fonnegra A, Zea S (2011) Coral colonization by the encrusting excavating Caribbean sponge Cliona delitrix. Mar Ecol 32:162–173
Chaves-Fonnegra A, Zea S, Gómez ML (2007) Abundance of the excavating sponge Cliona delitrix in relation to sewage discharge at San Andrés Island, SW Caribbean, Colombia. Bol Invest Mar Cost 36:63–7
Cleary DFR, de Voogd NJ (2007) Environmental associations of sponges in the Spermonde Archipelago, Indonesia. J Marine Biol Assoc UK 87:1669–1676
Coles SL, Bolick H (2007) Invasive introduced sponge Mycale grandis overgrows reef corals in Kaneohe Bay, Oahu, Hawaii. Coral Reefs 26:911
Collins LS, Budd AF, Coates AG (1996) Earliest evolution associated with closure of the Tropical American Seaway. Proc Natl Acad Sci 93:6069–607
Colvard NB, Edmunds PJ (2011) Decadal-scale changes in abundance of non-scleractinian invertebrates on a Caribbean coral reef. J Exp Mar Biol Ecol 397:153–160
Corredor JE, Wilkinson CR, Vicente VP, Morell JM, Otero E (1988) Nitrate release by Caribbean reef sponges. Limnol Oceanogr 33:114–120
Cowart JD, Henkel TP, McMurray SE, Pawlik JR (2006) Sponge orange band (SOB): a pathogenic-like condition of the giant barrel sponge, Xestospongia muta. Coral Reefs 25:513
de Goeij JM, van den Berg H, van Oostveen MM, Epping EHG, van Duyl FC (2008) Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar Ecol Prog Ser 357:139-151
de Goeij JM, Oevelen D van, Vermeigh JJA, Osinga R, Middelbury JJ, de Goeij AFPM, Admiraal W (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108-110
de Voogd NJ, Cleary DFR (2008) An analysis of sponge distribution and diversity at three taxonomic levels in the Thousand Islands/Jakarta Bay reef complex, West Java, Indonesia. Mar Ecol 29:205–215
de Voogd NJ, Cleary DFR, Dekker F (2013) The coral-killing sponge Terpios hoshinota invades Indonesia. Coral Reefs 32:755
Debrenne F (1999) The past of sponges – sponges of the past. Memoirs of the Queensland Museum 44:9–21
Debrenne F (2007) Lower Cambrian archaeocyathan bioconstructions. Comptes Rendus Palevol 6:5–19
Debrenne F, Zhuravlev AY (1994) Archaeocyathan affinities: How deep can we go into the systematic affiliation of an extinct group. In: van Soest RWM, van Kempen TMG, Braekman J-C (eds) Sponges in time and space: biology, chemistry, paleontology. A.A. Balkema, Rotterdam, pp 3-12
Diaz C, Rützler K (2001) Sponges: An essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Duffy JE (1996) Eusociality in a coral-reef shrimp. Nature 381:512–514
Edinger EN, Risk MJ (1997) Sponge borehole size as relative measure of bioerosion and paleoproductivity. Lethaia 29:275–286
Erwin PM, Thacker RW (2007) Incidence and identity of photosynthetic symbionts in Caribbean coral reef sponge assemblages. J Mar Biol Ass UK 87:1683–169
Fang JHK, Athayde MAM, Schönberg CHL, Kline DI, Hoegh-Guldberg O, Dove S (2013) Sponge biomass and bioerosion rates under ocean warming and acidification. Global Change Biol 19:3581–3591
Fang JHK, Schönberg CHL, Athayde MAM, Kline DI, Hoegh-Guldberg O, Dove S (2014) Effects of ocean warming and acidification on the energy budget of an excavating sponge. Global Change Biol 20:1043–1054
Freeman CJ, Thacker RW (2011) Complex interactions between marine sponges and their symbiotic microbial communities. Limnol Oceanogr 56:1577–1586
Fromont J, Vanderklift MA, Kendrick GA (2006) Marine sponges of the Dampier Archipelago, Western Australia: patterns of species distributions, abundance and diversity. Biodiversity and Conservation 15:3731–3750
Gandin A, Debrenne F (2010) Distribution of the archaeocyath-calcimicrobial bioconstructions on the Early Cambrian shelves. Palaeoworld 19:222–241
Garson MJ, Clark RJ, Webb RI, Field KL, Charan RD, McCaffrey EJ (1999) Ecological role of cytotoxic alkaloids: Haliclona n. sp., an unusual sponge/dinoflagellate association. Mem Queensland Mus 44:205–213
Gilis M, Grauby O, Willenz Ph, Dubois P, Heresanu V, Baronnet A (2013) Biomineralization in living hypercalcified sponges: toward a shared mechanism? J Structural Bio 183:441–454
Glynn PW (1974) Rolling stones among the Scleractinia: mobile coralliths in the Gulf of Panamá. Proc 2nd Int Coral Reef Symp 2:183–198
González-Rivero M, Ferrari R, Schönberg CHL, Mumby PJ (2012) Impacts of macroalgal competition and parrotfish predation on growth of a common bioeroding sponge. Mar Ecol Prog Ser 444:133–142
Goreau TF, Hartman WD (1963) Boring sponges as controlling factors in the formation and maintenance of coral reefs. Mechanisms of Hard Tissue Destruction. Amer Assoc Adv Sci 75:25–54
Goreau TF, Hartman WD (1966) Sponge: effect on the form of reef corals. Science 151:343–344
Halfar J, Riegl R (2013) From coral framework to rodolith bed: sedimentary footprint of the 1982/1983 ENSO in the Galápagos. Coral Reefs 32:985
Hallock P (1988) The role of nutrient availability in bioerosion: consequences to carbonate buildups. Palaeogeogr Palaeoclimatol Palaeoecol 63:275–291
Hartman WD (1977) Sponges as reef builders and shapers. Studies in Geology 4:127–134
Hartman WD, Goreau TF (1970) Jamaican coralline sponges: their morphology, ecology, and fossil relatives. In: Fry WC (ed) The biology of the porifera: Zool Soc London Symp 25:205–243
Hartman WD, Goreau TF (1975) A pacific tabulate sponge, living representative of a new order of sclerosponges. Postilla 167:1–21
Hartman, WD, Wendt JW, Wiedenmayer F (1980) Living and fossil sponges. Sedimenta 8:1–274
Hill M, Allenby A, Ramsby B, Schönberg CHL, Hill A (2011) Symbiodinium diversity among host clionaid sponges from Caribbean and Pacific reefs: evidence of heteroplasmy and putative host-specific symbiont lineages. Mol Phylogen Evol 59:81–88
Holmes KE (1997) Eutrophication and its effect on bioeroding sponge communities. Proc. 8th Int Coral Reef Symp 2:1411–1416
Hooper JNA, Kennedy JA (2002a) Small-scale patterns of sponge biodiversity (Porifera) on Sunshine Coast reefs, eastern Australia. Invertebrate Systematics 16:637–653
Hooper JNA, Kennedy JA, Quinn RJ (2002b) Biodiversity “hotspots”, patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges. Biodiversity and Conservation 11: 851–885
Hourigan, TF, Stanton, FG, Motta, PJ, Kelley, CD, Carlson, B (1989) The feeding ecology of three species of Caribbean angelfishes (family Pomacanthidae). Envir Biol Fishes 24:105–116
Hubbard DK (1985) What do we mean by reef growth? Proc 5th Int Coral Reef Congr 6:433–438
Hubbard DK (1988) Controls of modern and fossil reef development: common ground for biological and geological research. Proc 6th Int Coral Reef Symp 1:243–252
Hubbard DK, Burke RB, Gill IP (1998) Where’s the reef? The role of framework in the Holocene. Carbonates and Evaporites 13:3–9
Jackson JBC, Goreau TF, Hartman WD (1971) Recent brachiopod-coralline sponge communities and their paleoecological significance. Science 173:623–625
Kiessling W (2009) Geologic and biologic controls on the evolution of reefs. Ann Rev Ecol Evol Syst 40:173–192
Kiessling W, Aberhan M, Brenneis B, Wagner PJ (2007) Extinction trajectories of benthic organisms across the Triassic – Jurassic boundary. Palaeos 244:201–222
Kobluk DR (1981) Lower Cambrian cavity-dwelling endolithic (boring) sponges. Canadian Journal of Earth Science 18:972–980
Lang JC, Hartman WD, Goreau TF (1975) Sclerosponges: primary framework constructors on the Jamaican deep forereef. J Mar Res 33:223–231
Lawrence D (1969) The use of clionid sponges in palaeoenvironmental analyses. J Palaeontol 43:539–543
Lehnert H, Fischer H (1999) Distribution patterns of sponges and corals down to 107 m off North Jamaica. Mem Queensland Mus 44:307–316
León YM, Bjorndal KA (2002) Selective feeding in the hawksbill turtle, an important predator in coral reef ecosystems. Mar Ecol Prog Ser 245:249–258
Lesser MP (2006) Benthic-pelagic coupling on coral reefs: feeding and growth of Caribbean sponges. J Exp Mar Biol Ecol 328:277–288
Loh T-L, Pawlik JR (2014) Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs. Proc Natl Acad Sci 111:4151–4156
López-Victoria M, Zea S (2005) Current trends in space occupation by encrusting excavating sponges on Colombian coral reefs. Mar Ecol Prog Ser 26:33–41
López-Victoria M, Zea S, Weil E (2006) Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar Ecol Prog Ser 312:113–121
Macdonald IA, Perry CT (2003) Biological degradation of coral framework in a turbid lagoon environment, Discovery Bay, north Jamaica. Coral Reefs 22:523–535
Maldonado M, Carmona MC, Uriz MJ, Cruzado A (1999) Decline in Mesozoic reef-building sponges explained by silicon limitation. Nature 401:785–788
Maldonado M, Ribes M, van Duyl FC (2012) Nutrient fluxes through sponges: biology, budgets, and ecological implications. Adv Mar Bio 62:113–182
Maliao RJ, Turingen RG, Lin J (2008) Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), US. Mar Biol 154:841–853
Mallela J and Perry CT (2007) Calcium carbonate budgets for two coral reefs affected by different terrestrial runoff regimes, Rio Bueno, Jamaica. Coral Reefs 26:129–145
Marquez, J.C., Zea, S. 2012. Parrotfish mediation in coral mortality and bioerosion by the encrusting, excavating sponge Cliona tenuis. Mar Ecol 33:417–426
McLean EL, Yoshioka PM (2008) Substratum effects on the growth and survivorship of the sponge Desmapsamma anchorata. Carib J Sci 44:83–89
McMurray SE, Henkel TP, Pawlik JR (2010) Demographics of increasing populations of the giant barrel sponge Xestospongia muta in the Florida Keys. Ecol 91:560–570
Meylan A (1988) Spongivory in hawksbill turtles: A diet of glass. Science 239:393–395
Meylan A (1990) Nutritional characteristics of sponges in the diet of the hawksbill turtle. Eretmochelys imbricata. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press. Washington D.C. pp 472–477
Miller J, Muller E, Rogers C, Waara R, Atkinson A, Whelan KRT, Patterson M, Witcher B (2009) Coral disease following massive bleaching in 2005 causes 60 % decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 28:925–937.
Mueller B, de Goeij JM, Vermej MJA, Mulders Y, van der Ent E, Ribes M, van Duyl FC (2014) Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). PloS One 9:e90152
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser 376:295–306
Padilla Verdín CJ, Carballo JL, Camacho ML (2010) A qualitative assessment of sponge-feeding organisms from the Mexican Pacific Coast. Open Mar Biol J 4:39–46
Pandolfi J, Kiessling W (2014) Gaining insights from past reefs to inform understanding of coral reef response to global climate change. Curr Opin Environ Sustainability 7:52–58
Pari N, Peyrot-Clausade M, Hutchings PA (2002) Bioerosion of experimental substrates on high islands and atoll lagoons (French Polynesia) during 5 years of exposure. J Exp Mar Bio Ecol 276:109–127
Pawlik JR (2011) The chemical ecology of sponges on Caribbean reefs: Natural products shape natural systems. BioScience 61:888–898
Perry CT (1998) Macroborers within coral framework at Discovery Bay, north Jamaica: species distribution and abundance, and effect on coral preservation. Coral Reefs 17:277–287
Perry CT (2000) Macroboring of Pleistocene coral communities, Falmouth Formation, Jamaica. Palaois 15:483–489
Perry CT, Edinger EN, Kench PS, Murphy GN, Smithers SG, Steneck RS, Mumby PJ (2012) Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and application to the reefs of Bonaire. Coral Reefs 31:853–868.
Peterson BJ, Chester CM, Jochem FJ, Fourqurean JW (2006) Potential role of sponge communities in controlling phytoplankton blooms in Florida Bay. Mar Ecol Prog Ser 328:93–103
Pleydell SM, Jones B (1988) Boring of various faunal elements in the Oligocene-Miocene Bluff Formation of Grand Cayman, British West Indies. J Paleont 62:348–367
Randall JE, Hartman WD (1968) Sponge-feeding fishes of the West Indies. Mar Biol 1:216–225
Reed JK, Pomponi SA (1997) Biodiversity and distribution of deep and shallow water sponges in the Bahamas. Proc 8th Int Coral Reef Symp 2:1687–1692
Reimer JD, Nozawa Y, Hirose E (2010) Domination and disappearance of the black sponge: a quarter century after the initial Terpios outbreak in southern Japan. Zoological Studies 50:394
Reiswig HM (1971) Particle feeding in natural populations of three marine demosponges. Biol Bull 141:568–591
Reiswig HM (1973) Population dynamics of three Jamaican Demospongiae. Bull Mar Sci 23:191–226
Reiswig HM (1974) Water transport, respiration, and energetics of three tropical marine sponges. J Exp Mar Biol Ecol 14:231–249
Reiswig HM (1981) Partial carbon and energy budgets of the bacteriosponge Verongia fistularis (Porifera: Demospongiae) in Barbados. Mar Ecol 2:273–293
Reitner J, Gautret P (1996) Skeletal formation in the modern but ultraconservative Chaetetid sponge Spirastrella (Acanthochaetetes) wellsi (Demospongiae, Porifera). Facies 34:193–208
Reitner J, Keupp H (1991) The fossil record of the haplosclerid excavating sponge Aka de Laubenfels. In: Reitner J, Keupp H (eds) Fossil and recent sponges. Springer Berlin: 102–120
Reitner J, Wörheide G, Lange R, Schumann-Kindel G (2001) Coralline demosponges – a geobiological portrait. Bull of the Tohoku University Museum 1:219–235
Richter C, Wunsch M, Rasheed M, Kötter I, Badran MI (2001) Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature 413:726–730
Rose CS, Risk MJ (1985) Increase in Cliona delitrix infestation of Montastrea cavernosa heads on organically polluted portions of the Grand Cayman fringing reef. Mar Ecol 6:345–363
Rowland SF (2001) Archaeocyaths – A history of phylogenetic interpretation. J Paleon 75:1065–1078
Rützler K (1978) Sponges in coral reefs. In: Stoddart DR, Johannes RE (eds) Coral reefs: research methods. Monographs on Oceanographic Methodology 5, UNESCO, Paris.
Rützler K (1990) Associations between Caribbean sponges and photosynthetic organisms. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press. Washington D.C. pp. 455–466
Rützler K (2002) Impact of crustose clionid sponges on Caribbean coral reefs. Acta Geologica Hispanica 37:61–72
Rützler K (2004) Sponges on coral reefs: A community shaped by competitive cooperation. Boll Mus Ist Biol Univ Genova 68:85–148
Rützler K (2012) The role of sponges in the Mesoamerican Barrier Reef ecosystem, Belize. Adv Mar Bio 61:211–271
Rützler K, Macintyre IG (1978) Siliceous sponge spicules in coral reef sediments. Mar Bio 49:147–159
Rützler K, Muzik K (1993) Terpios hoshinota, a new cyanobacteriosponge threatening Pacific reefs. Sciencias Marinas 57:395–403
Scalera-Liaci L, Sciscioli M, Lepore E, Gaino E (1999) Symbiotic zooxanthellae in Cinachyra tarentina, a non-boring demosponge. Endocytobiosis Cell Res 13:105–114
Scheibling RE (1979) The ecology of Oreaster reticulatus (L.) (Echinodermata: Asteroidea) in the Caribbean. PhD thesis, McGill University, Montreal
Schönberg CHL (2002) Substrate effects on the bioeroding demosponge Cliona orientalis. 1. Bioerosion rates. PSZNI Mar Ecol 23:313–326
Schönberg CHL (2003) Substrate effects on the bioeroding demosponge Cliona orientalis. 2. Substrate colonization and tissue growth. PSZNI Mar Ecol 24:59–74
Schönberg CHL (2008) A history of sponge bioerosion: from past myths and hypotheses to recent approaches. Current Developments in Bioerosion. pp. 165–202
Schönberg CHL, Fromont J (2012) Sponge gardens of Ningaloo Reef (Carnarvon Shelf, Western Australia) are biodiversity hotspots. Hydrobiologia 687:143–161
Schönberg CHL, Loh WKW (2005) Molecular identity of the unique symbiotic dinoflagellates found in the bioeroding demosponge Cliona orientalis Thiele, 1900. Mar Ecol Prog Ser 299:157–166
Schönberg CHL, Ortiz J-C (2009) Is sponge bioerosion increasing? Proc 11th Int Coral Reef Symp, Ft Lauderdale 1:527–530
Schönberg CHL, Suwa R (2007) Why bioeroding sponges may be better hosts for symbiotic dinoflagellates than many corals. In: Custódio MR, Lôbo-Hajdu G, Hajdu, E, Muricy G (eds) Porifera research: biodiversity, innovation and sustainability. Série Livros 28. Museu Nacional, Rio de Janiero pp 569–580
Schönberg CHL, Suwa R, Hidaka M (2008) Sponge and coral zooxanthellae in heat and light: preliminary results of photochemical efficiency monitored with pulse amplitude modulated fluorometry. Mar Ecol 29:247–258
Schönberg CHL, Tapanila L (2006) The bioeroding sponge Aka paratypica, a modern tracemaking analogue for the paleozoic ichnogenus Entobia devonica. Ichnos 13:147–157
Schönberg CHL, Wilkinson CR (2001) Induced colonization of corals by a clionid bioeroding sponge. Coral Reefs 20:69–76
Schönberg CHL, Wisshak M (2012) The perks of being endolithic. Aquat Biol 17:1–5
Schumacher H, Plewka M (1981) Mechanical resistance of reefbuilders through time. Oecologia 49:279–282
Schwartz SA, Budd AF, Carlon DB (2012) Molecules and fossils reveal punctuated diversification in Caribbean “faviid” corals. BMC Evolutionary Biology 12:123
Scoffin T, Stearn C, Boucher D, Frydl P, Hawkins CM, Hunter IG, MacGeachy JK (1980) Calcium carbonate budget of a fringing reef on the west coast of Barbados. I. Erosion, sediments and internal structure. Bull Mar Sci 30:475–508
Seguin, F, Le Brun, O, Hirst, R, Al-Thary, I, Dutrieux, E (2008) Large coral transplantation in Bal Haf (Yemen): an opportunity to save corals during the construction of a liquefied natural gas plant using innovative techniques. Proc 11th Int Coral Reef Symp, Ft Lauderdale 2:1272–1275
Simpson TR (1984) The cell biology of sponges. Springer Berlin, 677 pp
Soong K, Yang S-L, Chen CA (2009) A novel dispersal mechanism of a coral-threatening sponge, Terios hoshinota (Suberitidae, Porifera). Zoological Studies 48:596.
Stanley SM, Hardie LA (1998) Secular oscillations in the carbonate minerology of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Paleogeography, Paleoclimatology, Paleobiology 44:3–19.
Stevely JM, Sweat DE, Bert TM, Sim-Smith C, Kelly M (2011) Sponge mortality at Marathon and Long Key, Florida: Patterns of species response and population recovery. Proc. 53rd Gulf and Caribbean Fisheries Institute 63:384–400
Stock CW (2001) Stromatoporoidea, 1926–2000. J Paleo 75:1079–1089
Strimaitis AM (2012) Filter feeding ecology of erect branching sponges on Caribbean coral reefs. Master’s Thesis, Department of Biological Science, Florida State University
Stubler AD, Furman BT, Peterson BJ (2014) Effects of pCO2 on the interaction between an excavating sponge, Cliona varians, and a hermatypic coral, Porites furcata. Mar Biol 161:1851-1859
Tapanila L, Copper P, Edinger E (2004) Environmental and substrate control on Paleozoic bioerosion in corals and stromatoporoids. Palaios 19:292–306
Thacker RW (2005) Impacts of shading on sponge-cyanobacteria symbioses: A comparison between host-specific and generalist associations. Integr Comp Biol 45:369–376
Trussell GC, Lesser MP, Patterson MR, Genovese SJ (2006) Depth-specific differences in growth of the reef sponge Callyspongia vaginalis: role of bottom-up effects. Mar Ecol Prog Ser 323:149–158
Vacelet J (1970) Les éponges Pharétronides actuelles. Zool Soc Lond Symp 25:189–204
Vacelet J, Willenz Ph, Hartman WD (2010) Living hypercalcified sponges. Treatise Online Number 1, Part E, Revised, Volume 4, Chapter 1
van Dam R, Diez CE (1997) Predation by hawksbill turtles at Mona Island, Puerto Rico. Proc 8th Int Coral Reef Symp 2:1421–1426
van Duyl FC, Moodley L, Nieuwland G, van Ijezerloo L, van Soest RWM, Houtekamer M, Meesters EH, Middelburg JJ (2011) Coral cavity sponges depend on reef-derived food resources: stable isotope and fatty acid constraints. Mar Biol 158:1653–1666
van Soest RWM (2009) New sciophilous sponges from the Caribbean (Porifera: Demospongiae). Zootaxa 2107:1–40
van Soest RWM, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, de Voogd NJ, da Santodomingo N, Vanhoorne B, Kelly M, Hooper JNA (2012) Global biodiversity of sponges (Porifera). PLoS One 7(4):e35105
Vicente VP (1990) Overgrowth activity by the encrusting sponge Chondrilla nucula on a coral reef in Puerto Rico. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington, D.C. pp 436–443
Wang J-T, Chen Y-Y, Meng P-J, Sune Y-H, Hsu C-H, Wei K-Y, Chen CA (2012) Diverse interactions between corals and the coral-killing sponge Terpios hoshinota (Suberitidae: Hadromerida). Zool Stud 51:150–159
Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC (2005) Clionid sponge surveys on the Florida reef tract suggest land-based nutrient inputs. Mar Poll Bull 51:570–579
Webster NS (2007) Sponge disease: a global threat? Envir Microbio 9:1363–1375
Webster NS, Taylor MW (2012) Marine sponges and their microbial symbionts: love and other relationships. Envir Microb 14:335–346
Weisz JB, Lindquist N, Martens CS (2008) Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia 155:367–376
West RR, Kershaw S (1991) Chaetetid habitats. In: Reitner J, Keupp H, eds. Fossil and Recent Sponges. Springer-Verlag Berlin Heidelberg Pp. 445–455
West RR, Vacelet J, Wood RA, Willenz Ph, Hartman WD (2010) Hypercalcified extant and fossil chaetetid-type and post-Devonian stromatoporoid-type Demospongiae: systematic descriptions. Treatise online number 58, part E, revised, volume 4, chapter 4A-B
Wilkinson CR (1983) Role of sponges in coral reef structural processes. In: Barnes DJ (ed) Perspectives on coral reefs. Brian Clouston, Publisher. pp 263–274
Wilkinson CR (1987) Interocean differences in size and nutrition of coral reef sponge populations. Science 236:1654–1657
Wilkinson CR, Cheshire AC (1990) Comparisons of sponge populations across the barrier reefs of Australia and Belize: evidence for higher productivity in the Caribbean. Mar Ecol Prog Ser 67:285–294
Willenz Ph, Hartman WD (1985) Calcification rate of Ceratoporella nicholsoni (Porifera: Sclerospongiae): An in situ study with calcein. Proc 5th Int Coral Reef Congr 5:113–118
Willenz Ph, Hartman WD (1989) Micromorphology and ultrastructure of Caribbean sclerosponges 1. Ceratoporella nicholsoni and Stromatospongia norae (Ceratoporellidae: Porifera). Mar Bio 103:387–401
Willenz Ph, Hartman WD (1999) Growth and regeneration rates of the calcareous skeleton of the Caribbean coralline sponge Ceratoporella nicholsoni: A long term survey. Memoirs of the Queensland Museum 44:675–685
Wisshak M, Schönberg CHL, Form A, Freiwald A (2012) Ocean acidification accelerates reef bioerosion. PLoS One 7:e45124
Wisshak M, Schönberg CHL, Form A, Freiwald A (2013) Effects of ocean acidification and global warming on bioerosion – lessons from a clionaid sponge. Aquatic Biol 19:111–127
Wisshak M, Schönberg CHL, Form A, Freiwald A (2014) Sponge bioerosion accelerated by ocean acidification across species and latitudes? Helgol Mar Res 68:253–262
Wood R (1987) Biology and revised systematics of some late Mesozoic stromatoporoids. Special papers in palaeontology 37:1–89
Wood R (1990) Reef-building sponges. American Scientist 78:224–235
Wood R (1993) Nutrients, predation and the history of reef-building. Palaios 8:526–543
Wood R (1995) The changing biology of reef-building. Palaios 10:517–527
Wood R (2011) Taphonomy of reefs through time. Topics in Geobiology 32:375–409
Wood R, Zhuravlev AY, Debrenne F (1992) Functional biology and ecology of Archaeocyatha. Palaios 7:131–156
Woodley JD, Chornesky EA, Clifford PA, Jackson JBC, Kaufman LS, Lang JC, Pearson MP, Porter JW, Rooney MC, Rylaarsdam KW, Tunnicliffe VJ, Wahle CW, Wulff JL, Curtis ASG, Dallmeyer MD, Jupp BP, Koehl MAR, Neigel J, Sides EM (1981) Hurricane Allen’s impact on Jamaican coral reefs. Science 214:749–755
Wörheide G (1998) The reef cave dwelling ultraconservative coralline demosponge Astrosclera willeyana Lister 1900 from the Indo-Pacific: micromorphology, ultrastructure, biocalcification, isotope record, taxonomy, biogeography, phylogeny. Facies 38:1–88
Wulff JL (1984) Sponge-mediated coral reef growth and rejuvenation. Coral Reefs 3:157–163
Wulff JL (1994) Sponge-feeding by Caribbean angelfishes, trunkfishes, and filefishes. In: van Soest RWM, van Kempen TMG, Braekman J-C (eds) Sponges in time and space: biology, chemistry, paleontology. A.A. Balkema, Rotterdam, pp 265–271
Wulff JL (1995) Sponge-feeding by the Caribbean starfish Oreaster reticulatus. Mar Biol 123:313–325
Wulff JL (1997a) Mutually beneficial associations among species of coral reef sponges. Ecology 78:146–159
Wulff JL (1997b) Parrotfish predation on cryptic sponges of Caribbean coral reefs. Mar Biol 129:41–52
Wulff JL (1997c) Causes and consequences of differences in sponge diversity and abundance between the Caribbean and eastern Pacific at Panama. Proc 8th Int Coral Reef Symp 2:1377–1382
Wulff JL (2001) Assessing and monitoring coral reef sponges: Why and how? Bull Mar Sci 69:831–846
Wulff JL (2005) Trade-offs in resistance to competitors and predators, and their effects on the diversity of tropical marine sponges. J Anim Ecol 74:313–321
Wulff JL (2006a) Rapid diversity and abundance decline in a Caribbean coral reef sponge community. Biol Conserv 127:167–176
Wulff JL (2006b) Resistance vs. recovery: morphological strategies of coral reef sponges. Func Ecol 20:699–708
Wulff JL (2006c) A simple model of growth form-dependent recovery from disease in coral reef sponges, and implications for monitoring. Coral Reefs 25:419–426
Wulff JL (2006d) Sponge systematics by starfish: predators distinguish cryptic sympatric species of Caribbean fire sponges, Tedania ignis and Tedania klausi n. sp. (Demospongiae, Poecilosclerida). Biol Bull 211:83–94
Wulff JL (2006e) Ecological interactions of marine sponges. Canadian J Zool Spec Ser 84:146–166
Wulff JL (2008a) Collaboration among sponge species increases sponge diversity and abundance in a seagrass meadow. Mar Ecol 29:193–204
Wulff JL (2008b) Life history differences among coral reef sponges promote mutualism or exploitation of mutualism by influencing partner fidelity feedback. Amer Nat 171:597–609
Wulff JL (2010) Regeneration of sponges in ecological context: Is regeneration an integral part of life history and morphological strategies? Integr Comp Biol 50:494–505
Wulff J (2012) Ecological interactions and the distribution, abundance, and diversity of sponges. Adv Mar Biol 61:273–344
Wulff J (2013) Recovery of sponges after extreme mortality events: morphological and taxonomic patterns in regeneration versus recruitment. Integr Comp Biol 53:512–523
Wulff JL, Buss LW (1979) Do sponges help hold coral reefs together? Nature 281:474–475
Zea S (1994) Patterns of coral and sponge abundance in stressed coral reefs Santa Marta, Colombian Caribbean. In: van Soest RWM, van Kempen TMG, Braekman J-C (eds) Sponges in time and space: biology, chemistry, paleontology. A.A. Balkema, Rotterdam, p 257–264
Zea S (2001) Patterns of sponge (Porifera, Demospongiae) distribution in remote, oceanic reef complexes of the southwestern Caribbean. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 25:579–592
Dedication and Acknowledgements
This paper is dedicated to Willard D. Hartman. In his many thoughtful papers, and in his teaching and informal interactions as a beloved professor and researcher at Yale University and the Discovery Bay Marine Laboratory in Jamaica , as well as on expeditions and at conferences, Willard modeled the value of integrating processes spanning temporal scales from evolutionary to seconds, and spatial scales from world-wide to those revealed by high-quality electron microscopy. He had the breadth and depth of understanding of invertebrate form, function, ecosystem roles, and evolutionary history that allowed him to quickly connect a living sponge sporting a dense carbonate skeleton to fossils weighted with a motley history of presumed relations. Work of his students and colleagues continues to be inspired by Willard’s kindness, wit, elegance of expression, and his vision of geological and biological aspects of sponges on coral reefs as inseparable.
I am very grateful to Philippe Willenz, Egbert Leigh, Christine Schönberg, and Caroline Rogers for contributing significantly to this chapter with many thoughtful comments. In particular the section on boring sponges was enhanced, and Pacific examples bolstered, by Christine’s energetic application of her expertise. Philippe’s generous contributions of his beautiful photos of, and expertise on, the hypercalcified sponges were essential. Along with Willard Hartman, Klaus Rützler, Michele Sarà, and Clive Wilkinson fired my inspiration for many long-term studies of sponges; and my funding sources for those long-term sponge studies have been primarily the NSF, Smithsonian Tropical Research Institute, and the Caribbean Coral Reef Ecosystem program of the US National Museum of Natural History, SI.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wulff, J. (2016). Sponge Contributions to the Geology and Biology of Reefs: Past, Present, and Future. In: Hubbard, D., Rogers, C., Lipps, J., Stanley, Jr., G. (eds) Coral Reefs at the Crossroads. Coral Reefs of the World, vol 6. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7567-0_5
Download citation
DOI: https://doi.org/10.1007/978-94-017-7567-0_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7565-6
Online ISBN: 978-94-017-7567-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)