Abstract
Members of the seaweed family Fucaceae have been recurrent models in North Atlantic phylogeographic research; numerous studies have been published since 2000, and this review synthesizes their major findings. Fucoid species exhibited diverse responses to glacial–interglacial cycles, but evidence indicates there were a few common refugial areas such as north-western Iberia, the Celtic Sea (Brittany/Ireland) region and the North-west Atlantic. In genetically rich refugial areas, pervasive genetic breaks confirmed presently limited gene flow between adjacent distinct genetic groups. In contrast with the maintenance of sharp genetic breaks, most species experienced extensive migration during post-glacial expansion. Poleward migrations in the North-east Atlantic followed routes along north-western Ireland and the transgressing English Channel. These patterns support the role of density-blocking in maintaining sharp genetic breaks at contact zones, and of long-distance dispersal from range edges in mediating expansion into uninhabited regions. The data also indicate that expansions involve mostly the genetic groups located at range edges rather than the entire species’ gene pool, both poleward during interglacials and toward warmer regions during glacial periods. Fucoid expansions have also been linked to introgressive recombination of genomes at (and beyond) contact zones and to gene surfing leading to present large-scale dominance by alleles that were located at the expanding edge. Phylogeographic approaches have also proven useful to identify and track the sources of introductions linked to marine traffic. The integration of environmental niche models with molecular data have further allowed hindcasting southern distributions during glaciation and predicting the potentially negative effects of future climate warming, including the loss of vulnerable, unique trailing-edge lineages, as species’ ranges are predicted to continue shifting northward. Collectively, these studies have contributed greatly to elucidating the links between past and ongoing climatic shifts, range dynamics and geographical patterns of genetic variability in the North Atlantic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- North-Atlantic intertidal
- Climatic refugia
- Comparative phylogeography
- Fucoid seaweeds
- Genetic diversity
- Latitudinal range shifts
- Marine introductions
- Glacial–interglacial cycles
- Ongoing climate change
- Pathways of range expansion
- Rafting
- Restricted dispersal
1 Introduction
The large climatic shifts experienced across the Quaternary glacial–interglacial cycles (2.5 Ma to 12 ka) have played a major role in shaping the modern distributions and genetic make-ups of extant species (Hofreiter and Stewart 2009). In the Northern Hemisphere, climatic, paleontological and molecular evidence show that glacial advances commonly pressed temperate organisms into periglacial or more southern refugial areas (Stewart et al. 2010), sometimes in the form of small and scattered populations (Stewart and Lister 2001). During interglacials, such as the current Holocene period (12 ka–present), warming climate has eventually allowed subsets of species (and specifically subsets of populations within species) to vastly expand their ranges northward (Petit et al. 2002; Bennett and Provan 2008; Provan 2013), sometimes associated with the loss (Hampe and Petit 2005) or displacement (e.g. Davis and Shaw 2001) of the southern range margins.
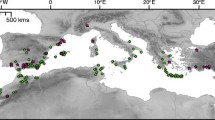
Coastal organisms inhabiting temperate latitudes of the North Atlantic (sensu Spalding et al. 2007) have been particularly impacted by these global climatic shifts. Understanding responses of these species has been limited by the absence and/or inaccessibility of adequate fossil records (particularly from colder periods, when sea levels were lower), but recent phylogeographic studies (typically based on mtDNA and often complemented with nuclear microsatellite loci) have provided new insights. Indeed, a diverse range of taxa including coastal invertebrates (Roman and Palumbi 2004; Kelly et al. 2006; Remerie et al. 2009; Handschumacher et al. 2010), fishes (Wilson and Eigenmann Veraguth 2010; Kettle et al. 2011; Woodall et al. 2011), seagrasses (Coyer et al. 2004a; Olsen et al. 2004; Alberto et al. 2008) and seaweeds (red algae: Provan et al. 2005b; Hu et al. 2010, 2011; Provan and Maggs 2012; brown algae: Assis et al. 2013) have been surveyed in the last decade for phylogeographic patterns in the North Atlantic alone, advancing considerably our understanding of past distributions and (in some cases) demographic histories of species in this region (Maggs et al. 2008). Members of the brown algal family Fucaceae (Fucales, Phaeophyceae, Stramenopiles; Fig. 11.1) and several red algal taxa (Rhodophyceae; see chapter by Li et al. (2016) in this volume) have been particularly fruitful research models in marine phylogeography of the North Atlantic, contributing to elucidating the links between climatic history, range dynamics and their consequences for genetic variability across species ranges in the region. This prominence stems from their ecology, life history and dispersal capacities.
Simplified Bayesian phylogenetic tree of Fucaceae based on 13 coding loci, with branches proportional to divergence time and with Atlantic taxa (and respective mating system) highlighted. Adapted from Cánovas et al. (2011), where detailed reconstruction methods and node age estimates are discussed. Fucus radicans is part of the clade F. vesiculosus N.; Fucus distichus s.l. includes F. gardneri and F. evanescens
Fucoids (here focused on the family Fucaceae) play a fundamental and important ecological role in intertidal habitats, where they form conspicuous ecosystem-structuring assemblages on rocky shores and in some estuarine and salt marsh environments (Lüning 1990; Chapman 1995). Like kelps (Laminariales and ecologically equivalent members of the order Tilopteridales) and the related subtidal fucoid forests (e.g. Cystoseiraceae, Sargassaceae), intertidal fucoid beds provide habitat structure, energy, food and shelter for other organisms, as well as relief against tidal/seasonal variations in thermal and desiccation stress (Schiel and Foster 2006; Christie et al. 2009; Dijkstra et al. 2012). In the North Atlantic, three genera and c. 10 species are present and all but one (F. distichus sensu lato) are endemic to the region (Fig. 11.2). Ascophyllum nodosum and Pelvetia canaliculata are representatives of monotypic genera, whereas Fucus comprises ca. eight species originating in a dynamic radiation (Serrão et al. 1999; Coyer et al. 2006a; Cánovas et al. 2011) that continues in contemporary times [e.g. speciation of Baltic F. radicans in the past 2000–400 years (Pereyra et al. 2009)]. Fucus distichus , comprising multiple regional entities of uncertain taxonomic validity, is the only species naturally occurring in both the Pacific and Atlantic, and is also the species extending deepest into the Arctic (Coyer et al. 2011b). It can be hypothesized that the Atlantic monotypic genera (Ascophyllum, Pelvetia) are the only surviving lineages of past radiations followed by extinctions, similar to the monotypic Pelvetiopsis and Hesperophycus in the Pacific. Lineage 2 of Fucus (Serrão et al. 1999) has greatly diversified along eastern Atlantic shorelines, as has the still poorly studied Fucus distichus sensu lato along the Pacific American coast. It can be hypothesized that the newly available European Atlantic habitats might have favoured diversification after the split from the most recent common ancestor with its sister Pacific lineage (Coyer et al. 2006a; Cánovas et al. 2011).
In sharp contrast with marine organisms that exhibit extensive dispersal by planktonic stages, fucoids are characterized by spatially restricted dispersal. They are sessile and perennial diplonts with direct development; free-living stages are restricted to short-lived gametes liberated in gametangia (antheridia and oogonia) that immediately sink and are preferentially released under low water motion (Serrão et al. 1996). Gametes typically do not disperse more than a few metres from the broadcasting parent (Chapman 1995; Serrão et al. 1997; Dudgeon et al. 2001). Rafting by floating thalli may extend the dispersal ranges by several orders of magnitude, and although in general its relevance for genetic connectivity among established populations is perceived to be modest (Fraser et al. 2009; Neiva et al. 2012b, c; Waters et al. 2013), rafting is nonetheless likely to have played an important role in large-scale range expansions, such as post-glacial northern recolonizations. Short-distance dispersal (SDD) , as a reproductive trait, implies that fucoid populations persist to a significant extent via local recruitment (Pearson and Serrão 2006), making them particularly susceptible to inbreeding and intra-/inter-specific hybridization (Coyer et al. 2002, 2007, 2011a; Engel et al. 2005; Moalic et al. 2011). SDD coupled with coastal patchiness also causes isolation, small effective population size (Coyer et al. 2008) and density-barrier effects (Neiva et al. 2012c), and facilitates genetic bottlenecks , allelic surfing (spread of particular alleles following chance increase or even fixation at the leading edge of expansions) and introgression during range expansions (Neiva et al. 2010). In short, SDD accentuates population structure, providing an historical footprint of change that can be easily traced (e.g. Hoarau et al. 2007; Assis et al. 2014). In addition, the strong high- to low-intertidal zonation at the local scale (habitat replication) and the natural warm temperate to cold temperate gradients of sea surface temperature (SST) (a phylogeographic cline), provide a natural laboratory for studying ecological speciation and the impacts of large-scale climatic changes for species exhibiting similar/contrasting biogeographical affinities (species “replication”) (Lüning 1990; Chapman 1995).
In this review we summarize and synthesize the major findings of phylogeographic studies of Atlantic Fucaceae. We: (1) highlight North Atlantic paleoclimatic history, identify refugia and discuss the principal pathways of post-LGM recolonization that have shaped current distribution patterns; (2) discuss (un)intentional historical introductions of fucoids; and (3) discuss current climate change and modelled dynamics of the projected future ranges of North Atlantic fucoids.
2 Climate-Driven Range Dynamics of North Atlantic Fucoids
2.1 Glaciations Affect SST, Sea level and Habitat Availability
The last of many Quaternary glaciations, the Last Glacial Maximum (LGM, 0.026–0.019 Ma) was characterized by massive ice sheets and permafrost belts covering large parts of what is now temperate Eurasia and North America (Fig. 11.3a). On the Atlantic European side, the Eurasian ice sheet reached as far south as the Brittany coast of France and the global marine regression (sea levels were lower by as much as 130 m; Lambeck et al. 2015) resulted in the complete emersion of many shallow seas, including the North, Celtic and Irish Seas, and also the English Channel. The latter was a land bridge between continental Europe and the British Isles and was crossed by a large paleo-river draining most of the regional rivers (e.g. Seine, Thames and Rhine) as well as large quantities of melting ice produced by the declining Eurasian ice sheet at the onset of the present interglacial (Gibbard and Lautridou 2003; Ménot et al. 2006). The huge shoreline displacements (hundreds of km in some cases) between glacial and interglacial conditions regularly transfigured the coastal geography of this region, and have been associated with major ecological shifts elsewhere (Graham et al. 2003).
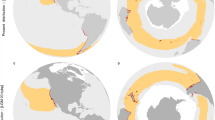
Schematic representation of the North Atlantic during (a) the LGM, and (b) the present, depicting the Laurentide, Greenland and Eurasian ice sheets (stripped areas), landmasses (light grey) and “temperate” oceanographic areas (dark grey). The oceanographic area corresponds to average winter SSTs temperatures below 18 °C and average summer temperatures above 8 °C. Note the broader latitudinal range of temperate conditions along the North-east Atlantic/European coast as compared with the narrow isotherms on the North-west Atlantic/North American coast. Also note the northward shift since the LGM (c. 20 ka ago). R1: Brittany/Ireland refugia; R2: northern Iberia; R3: south-western Iberia/Morocco; R4: North-west Atlantic
On the Atlantic North American side, the Laurentide ice sheet covered almost all of the Canadian shoreline and stretched as far south as Cape Cod (USA), although small coastal regions (present offshore seamounts) remained relatively ice-free (Carlson and Winsor 2012). Because of compressed SST isotherms along the American coast and the absence of rocky coastlines south of Cape Cod, substantial extinction of the pre-glacial biota undoubtedly occurred, differing from the splayed SST isotherms and plentiful rocky shore habitat along the European coastlines (Fig. 11.3). The lower species diversity in the North-west Atlantic is supported by molecular studies on numerous benthic organisms and further suggests recent post-glacial (re)colonizations from Europe by some (but not all) amphi-Atlantic organisms (Wares and Cunningham 2001; Ilves et al. 2010; Waltari and Hickerson 2013). In any case, due to the tempering effect of the Gulf Stream, suitable areas for temperate rocky intertidal species have been latitudinally wider along the North-east versus the North-west Atlantic across both full glacial and interglacial conditions and have migrated northwards between the two periods (Fig. 11.3).
2.2 Southern Glacial Ranges, Post-Glacial Range Shifts and Glacial Pockets
Seaweed species are especially sensitive and responsive to climatic perturbations at their warmer trailing edges (Wernberg et al. 2011a; Bartsch et al. 2012; Duarte et al. 2013; Nicastro et al. 2013; Oppliger et al. 2014). The lack of a fossil record and the absence of the species today make it difficult to confirm the extent of the southern ranges of fucoids during the last glacial period. Given the southern shift of the isotherms, North-west Africa and some of the Atlantic islands (e.g. Canaries, Azores) could have been colonized during past colder periods, although evidence today is only available through hindcasting using ecological niche models (ENMs) for Pelvetia canaliculata (Neiva et al. 2014) and Fucus vesiculosus (Assis et al. 2014) (Fig. 11.4). Likewise, evidence for more extensive northern ranges can also be inferred where land masses protruded from the iceless coastal pockets (e.g. offshore Newfoundland). Southern expansions during cold periods have also been inferred in other coastal seaweeds and invertebrates (Kettle et al. 2011; Provan and Maggs 2012; Waltari and Hickerson 2013) and may well be representative of temperate organisms in general.
Long-term biogeographic distributions of Atlantic fucoid seaweeds are expected to consist of: (1) previously favourable (during glacial periods) but presently unsuitable or increasingly marginal southern areas, where species have become extirpated or currently persist as isolated, trailing edge climatic relicts; (2) more central regions where suitable climatic conditions have allowed a more stable presence across glacial/interglacial cycles (i.e. climatic refugia) ; and (3) previously glaciated/emerged regions that were colonized post-glacially following ice sheet retreat and sea level rise. For low-dispersal marine species such as fucoids, higher regional genetic diversity and uniqueness (as a proxy for long-term persistence and isolation) is expected where glacial and interglacial distributions overlap (Assis et al. 2014). In the North Atlantic, several of these putative long-term climatic refugia have been identified, including distinct areas within the larger paleo-Celtic Sea/Channel area, north-western Iberia, and for a smaller set of species, central-southern Iberia and North-west Africa (Fig. 11.3). Evidence also suggests that Iceland and the Andøya region of northern Norway are and were refugia for the colder adapted F. distichus (Coyer et al. 2011b).
2.3 Glacial Refugia
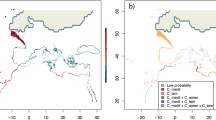
2.3.1 Brittany and South-Western Ireland
One of the earliest suggestions of a refugium for the Fucaceae was the exceptionally high level of microsatellite allelic diversity found in populations of Fucus serratus in the Brittany region of France relative to other areas throughout the North Atlantic (Coyer et al. 2003). Subsequent work indicated that the ancient Hurd Deep (a canyon and river in the present-day English Channel), and an inland sea between Brittany and south-western Ireland were glacial refugia for marine species such as seaweeds (Provan et al. 2005b), invertebrates (Jolly et al. 2006) and fish (Finnegan et al. 2013). In addition to F. serratus, several other fucoids that have high genetic diversity in the Brittany and south-western Ireland periglacial refugia are A. nodosum (Olsen et al. 2010), F. vesiculosus (Coyer et al. 2011a; Assis et al. 2014) and P. canaliculata (Neiva et al. 2014). For these two last species, as well as for F. ceranoides (Neiva et al. 2010) and F. spiralis (Coyer et al. 2011a), these refugia harboured several well-differentiated phylogeographic groups (Figs. 11.5, 11.6 and 11.7). For F. serratus, two phylogeographic groups have contributed to distinct phases (via different routes) of the global post-glacial expansion (Hoarau et al. 2007) (Fig. 11.5a). Patterns are more complex for the other species. For F. vesiculosus, ENMs show that the combined refugia formed the largest area of persistent habitat in the Atlantic. This proxy for long-term size of favourable habitat explained the high diversity of F. vesiculosus in this region (Assis et al. 2014). Brittany was clearly a refugium for A. nodosum, but unlike the other fucoid species, there is no evidence for additional refugia in Iberia (Fig. 11.6). In general, genetic data confirm that the colonization of coastlines north of Brittany and south-western Ireland relied on populations originating in this general periglacial region, with little contribution from Iberia.
Haplotype distribution and haplotype network for the combined IGS–trnW locus of Ascophyllum nodosum. Grey-shaded pie charts indicate that samples were missing from that location. Modified from Olsen et al. (2010)
MtDNA haplotype diversity and inset haplotype network for F. vesiculosus (left) and F. spiralis/F. guiryi (right). Haplotypes within boxes represent haplotypes collected in low and high intertidal zones at a single site, X indicates not collected/not found. Introgression of haplotypes (bottom) for F. spiralis (blue and lilac colours), introgressed F. guiryi (brown, green), pure F. guiryi (“South clade”, yellow, orange, red), and F. vesiculosus (ghosted in grey) also based on microsatellite identification. Modified from Coyer et al. (2011a)
The Celtic Sea/Brittany area is a biogeographical transition zone between the Northern European Seas and Lusitanian provinces within the “temperate Northern Atlantic” realm (Spalding et al. 2007). In this contact zone , higher genetic diversity could also be explained by complex patterns of secondary contact and admixture of populations expanding from distinct refugia (Maggs et al. 2008). The occurrence of tension zones with mosaically distributed hybrid zones has been documented for different Mytilus species in this area (Bierne et al. 2003). For several Fucus species, hybridization has also profoundly shaped patterns of gene diversity in this contact zone . Conflicting nuclear and organellar genomes were observed and explained by organellar sweeps caused by past hybridization and introgression between F. vesiculosus, F. ceranoides and species of the F. spiralis/F. guiryi complex (Engel et al. 2005; Coyer et al. 2006a, 2011a; Neiva et al. 2010; Moalic et al. 2011). For these, patterns of genetic diversity are more complex because post-glacial recolonization did not involve a single expansion from southern refugia and because of inter-specific gene flow (Fig. 11.7).
2.3.2 North-western Iberia
North-western Iberia represents the southern range limit (in some cases already as isolated climatic relicts) for a large number of cold-temperate seaweeds characteristic of present northern European shores. Examples include rhodophytes (e.g. Palmaria palmata), kelps (e.g. Laminaria hyperborea , Saccharina latissima) and fucoids (e.g. Himanthalia elongata , Halidrys siliquosa ), including also the fucoids A. nodosum , P. canaliculata, F. serratus, F. ceranoides and F. spiralis (Fig. 11.2; Bárbara et al. 2005; Araújo et al. 2009). This is also the southern limit of the rocky shore lineage of F. vesiculosus (Ladah et al. 2003); farther south it becomes a distinct genetic lineage found only in estuaries and lagoons. In addition to this southern boundary, Iberian kelps and wrack are physically separated from Brittany by the Bay of Biscay, where a combination of adverse conditions interact to create a shared distributional gap, including exposed shorelines in northern Spain, large stretches of soft substrate in south-western France, and increasing summer SSTs warming towards the innermost part of the gulf (Lüning 1990; Gorostiaga et al. 2004). The scale of this distributional gap varies between species and, for some of them, also in time (Duarte et al. 2013; Fernández 2013).
ENM hindcasting suggests that during the LGM, Iberia was a central climatic optimum for P. canaliculata (Neiva et al. 2014) and F. vesiculosus (Assis et al. 2014), and probably for cold-temperate seaweeds in general (Fig. 11.4). Notwithstanding its present marginality and isolation, north-western Iberian fucoids harbour high levels of genetic endemism and/or diversity: distinct haplotype lineages in F. serratus (Hoarau et al. 2007), F. ceranoides (Neiva et al. 2010) and P. canaliculata (Neiva et al. 2014) (Fig. 11.5), and genetically differentiated groups (based on microsatellites ) in A. nodosum (Olsen et al. 2010) and in F. vesiculosus (Assis et al. 2014) (Fig. 11.8). The endemism of F. ceranoides stands out in this region; concordant mtDNA and microsatellite data show that Iberian populations are grouped hierarchically in small shoreline sectors (<200 km) showing extremely limited admixture, each defined by a distinct mtDNA lineage and many private alleles (Neiva et al. 2012c). Regional haplotypic diversity was striking, particularly within the ranges of the westernmost, Iberian-endemic phylogroups. For instance, more haplotypes were found in eight estuaries in western Galicia than in the entire northern range, where the species is introgressed with F. vesiculosus organelles (Neiva et al. 2010, 2012c). When compared to more central, former periglacial areas, Iberia also differs in terms of microsatellite diversity and intra-regional population differentiation (Neiva et al. 2012b). These genetic data confirm that Iberia still represents a suitable climatic region for F. ceranoides.
Predicted range changes of F. vesiculosus due to near-future (2090–2100) climatic change. Gains and losses are superimposed over four recognized intra-specific genetic clusters (microsatellite data). Dashed lines depict the areas where genetic data is not available. Adapted from Assis et al. (2014) and Vuorinen et al. (2015)
In contrast, Iberian lineages in other fucoids are much less diverse than extra-Iberian phylogroups or Iberian lineages of F. ceranoides (Hoarau et al. 2007; Neiva et al. 2014). In F. serratus, microsatellite data further revealed that the two sampled localities (separated by only 140 km) were highly differentiated from each other and from the remaining extra-Iberian range and were also the least diverse (Coyer et al. 2003), suggesting that extant Iberian populations are the remnants of a larger, and ecologically and geographically more central, glacial range. Contemporary marginality is demonstrated by historical distributional records revealing regular expansions and contractions of F. serratus along the northern coast of Spain during the past century, apparently associated with decadal-scale oscillations in oceanographic conditions (Arrontes 1993; Duarte et al. 2013). Current low levels of genetic diversity and diminished functional responses at the very rear-edge (see Pearson et al. 2009) in otherwise highly differentiated and isolated populations clearly reflect these recurrent extinction–recolonization cycles, and increased genetic drift in small and demographically unstable populations (Coyer et al. 2003).
Only a single local extinction of Iberian P. canaliculata has been documented (Berlengas Island off central Portugal, Neiva et al. 2014). This documentation, coupled with nineteenth century herbarium records describing unattached specimens south of Lisbon suggest that P. canaliculata may have occurred hundreds of kilometres south of its current southern edge. The contemporary genetic depletion of the two unique Iberian lineages indicates bottleneck effects, and the homogeneity of most of the Iberian range suggests re-expansion from a single north-western source except in the genetically distinct Bay of Biscay. Although evidence is missing, warm periods such as the mid-Holocene Climatic Optimum (c. 9–5 ka) may have eliminated much of the pre-existing Iberian variation (Neiva et al. 2014). The same could apply, perhaps to an even larger extent, to A. nodosum , another regionally depleted species whose much differentiated (based on microsatellite data) Iberian populations harbour a single mitochondrial haplotype that is also widespread in and beyond Brittany (Olsen et al. 2010) (Fig. 11.6). North-western Iberia is also the contact zone between the southern species F. guiryi and its sister species F. vesiculosus (open-shore northern lineage) and F. spiralis, both of which find here their southernmost limit. The nuclear genome of F. guiryi is introgressed with these species in this sympatric region (but not further south in allopatry ), as demonstrated by microsatellite evidence (Moalic et al. 2011) and by 13 protein-coding loci (Zardi et al. 2011) (see also Fig. 11.7).
2.3.3 Southern Iberia/Morocco
ENMs suggest that suitable habitat for cold-temperate fucoids (Assis et al. 2014; Neiva et al. 2014) and a range of other coastal organisms (Kettle et al. 2011; Provan and Maggs 2012; Waltari and Hickerson 2013) was present in North-west Africa and the Mediterranean during glacial periods. Discounting the restricted Mediterranean relict F. virsoides , only two fucoids presently extend their distributions beyond the north-western Iberian biogeographic boundary (Fig. 11.2): the stress-tolerant high/mid-shore F. guiryi (Zardi et al. 2011) and a vanishing lineage of F. vesiculosus (Nicastro et al. 2013).
Among all members of the genus, F. vesiculosus has the broadest latitudinal distribution, occurring above the Arctic Circle in the north to warm temperate shores in southern Iberia and Morocco. It also has the widest habitat range, from soft sediments in estuaries and coastal lagoons, to the brackish Baltic Sea , in addition to extensive stands on marine rocky shores. Some of this variability might include cryptic species, as was the case of the recently evolved F. radicans in the Baltic Sea (Pereyra et al. 2009). South of 41°N latitude in the North-east Atlantic (central Portugal), F. vesiculosus is absent from open coastal rocky shores, but is found exclusively in coastal lagoons and estuarine habitats (Ladah et al. 2003), possibly a consequence of the more moderate thermal environment offered by estuarine and lagoon habitats in warmer southern regions (Ladah et al. 2003; Zardi et al. 2013). Concordant with the shift in habitat occupancy, phylogenetic and phylogeographic evidence support, a genetically distinct southern lineage within F. vesiculosus (based on multiple protein-coding nuclear genes Cánovas et al. 2011) and microsatellite markers (Nicastro et al. 2013; Assis et al. 2014). This lineage, coinciding with one climate refugium along central Iberia and north-western Morocco, where the species was hindcast to have persisted during both extreme cold (LGM) and warm (mid-Holocene) periods (Assis et al. 2014), is undergoing a regressive trend (Nicastro et al. 2013).
Phylogenetic analyses using multiple protein-coding regions successfully resolved the phylogenetic position of F. guiryi as a unique species sister to F. spiralis and F. vesiculosus (Cánovas et al. 2011; Coyer et al. 2011a; Zardi et al. 2011) (Fig. 11.1). However, clear phylogenetic resolution is restricted to allopatric southern populations (i.e. Portugal and Morocco), while inclusion of individuals from the sympatric range results in unresolved relationships between the three species. This suggests that genetic boundaries in the northern range of F. guiryi have been blurred by introgression (Zardi et al. 2011) despite limited contemporary gene flow (Billard et al. 2005, 2010; Coyer et al. 2011a). Further support for introgression comes from mitochondrial data, which show distinct and divergent haplotypes in allopatry in the south (previously referred to as F. spiralis South), while northern sympatric populations (previously designated F. spiralis Low) share haplotypes with F. vesiculosus and F. spiralis (previously designated F. spiralis High) (Coyer et al. 2011a) (Fig. 11.7). All these studies indicate that F. guiryi represents a unique genetic lineage in the south (central-southern Iberia, Morocco and the Canary Islands), distinct from the introgressed regions farther north. Although the complex history of inter-specific genetic exchange complicates phylogeographic inferences at more northern latitudes, mtDNA shows that the pure and southern allopatric F. guiryi exhibits differentiated populations, particularly distinct in the Azores (Fig. 11.7). This pattern of high and structured mtDNA diversity reflects the selfing mating system and poor dispersal abilities of F. guiryi, but high diversity also implies a relatively stable existence in the region during glacial and ongoing warmer climatic phases (Coyer et al. 2011a).
2.3.4 Northern Europe
A refugium for F. distichus, a species with colder climatic affinities, may have existed off the coastal island of Andøya, northern Norway where it presently forms a highly resolved microsatellite cluster (Coyer et al. 2011b). As such, this southern refugium may overlap with the northern refugia identified in Norway for a range of terrestrial and freshwater organisms (e.g. Alm and Birks 1991; Brunhoff et al. 2006). Although an Icelandic refugium has been inferred in several floristic and phylogeographic studies of plants, birds and marine invertebrates, some of these data and their interpretation are controversial (Ægisdóttir and Þórhallsdóttir 2004; Ingólfsson 2009) and no evidence of an Icelandic refugium was found for the closely related species F. spiralis and F. vesiculosus (Coyer et al. 2011a). Nevertheless, the presence of high diversity, private alleles and an intergenic spacer (IGS) divergence date of 0.06–0.60 Ma suggest that Iceland is a present-day southern refugium for F. distichus as the species is not found (naturally) in the British Isles or along the southern half of the west Norwegian coast.
2.3.5 Canadian Maritimes
Until phylogeographic data became available in the early 1990s, it was widely believed that the North-west Atlantic could not have been a refugium for rocky shore invertebrates or seaweeds due to a lack of suitable substrate in combination with ice scour and very narrow isotherms for cold-adapted taxa. For example, the presence of A. nodosum and F. vesiculosus along the Atlantic Canadian coast was thought to be the result of post-LGM recolonization from Europe, possibly via Iceland (e.g. Lüning 1990). Determining the presence of F. distichus was more problematic, given its distribution in both the eastern Atlantic and North Pacific. Subsequent phylogeographic studies, however, demonstrated that glacial refugia did exist in the Canadian Maritimes for some marine species (Cunningham and Collins 1998; Wares and Cunningham 2001). Among North-west Atlantic fucoids, A. nodosum (Olsen et al. 2010) (Fig. 11.6) and the cold-adapted F. distichus (Coyer et al. 2011b) (Fig. 11.9) have differentiated lineages with unique alleles in the West Atlantic, supporting a scenario of glacial persistence .
Combined haplotype distribution of mtIGS and COI for F. distichus, showing inset of haplotype network. Atlantic haplotypes are derived from two Pacific regions. Modified from Coyer et al. (2011b)
In F. vesiculosus, distinguishing between in situ survival and post-glacial colonization scenarios has been more challenging. Muhlin and Brawley (2009) found low microsatellite diversity and differentiation in populations spanning Canada to North Carolina, and the most widespread haplotype was identical to a common haplotype in Europe, implying that the populations of F. vesiculosus in the North-west Atlantic originated from European colonizers from the south-western Ireland and/or Brittany refugia (Muhlin and Brawley 2009; Coyer et al. 2011a). Another study combining ENMs and microsatellite data (Assis et al. 2014), however, suggested ample habitat availability during the LGM and also important genetic differentiation , more consistent with long-term persistence of F. vesiculosus in North-west Atlantic (Fig. 11.8). Introgression could explain conflicting mtDNA signatures of a demographic sweep. Indeed, low mtDNA diversity characterizes the entire distribution of F. vesiculosus (Coyer et al. 2011a) and its haplotypes are more similar to F. spiralis and F. guiryi (“F. spiralis south”) than among themselves (Coyer et al. 2011a), a finding in contradiction with nuclear evidence of phylogenetic relationship (Cánovas et al. 2011) (Figs. 11.1 and 11.7). MtDNA evidence suggests that F. spiralis sensu stricto in the West Atlantic was colonized from the East Atlantic (Coyer et al. 2011a). F. serratus did not colonize the West Atlantic by natural means, but was introduced about 150 years ago via human activities (Brawley et al. 2009).
2.4 Patterns of Post-glacial Poleward Expansions in the North-east Atlantic
During the LGM, expansion of the Eurasian ice sheet and the regional westward migration of the coastline would have prevented survival of temperate organisms north of western Ireland (Fig. 11.3a). Present distributions of many fucoids clearly indicate extensive colonization following glacier retreat: F. ceranoides extends at least to Nordland (northern Norway), whereas P. canaliculata and F. serratus reach the White Sea (Russia), and A. nodosum , F. vesiculosus and F. spiralis are present off Russia and southern Greenland (Fig. 11.2). Globally, molecular signatures are consistent with a post-glacial expansion scenario, as evidenced by the lower regional genetic diversity and higher homogeneity of colonized areas versus more southern periglacial regions (Brittany, Channel and western Ireland; Fig. 11.10). In a few species, comprehensive sampling and genetic resolution allowed post-glacial colonization sources and routes along the north-eastern Atlantic to be tentatively inferred by combining paleogeographic reconstitutions and the spatial distribution of widespread northern haplotypes.
In F. serratus, the distribution of cluster 1 (blue in Fig. 11.5a) suggests a first expansion wave originating in the genetically diverse area of south-eastern Ireland and proceeding via north-western Ireland and Scotland to Scandinavia, eventually reaching the White Sea to the north and the Kattegat and the lower Baltic Seas to the south (Hoarau et al. 2007). The latter region could only be colonized after its establishment as a marine environment ca. 7500 years bp (Björck 1995), and its populations apparently descended from a single founder event , as implied by mtDNA (nad11) heteroplasmy unique to the area (Coyer et al. 2004b). The modern distribution of the cluster 2 haplotypes (yellow in Fig. 11.5a) suggest that a second independent wave, originating from the Hurd Deep sea/Brittany region, proceeded northwards and eastwards following the progressive transgression of the Irish Sea and the English Channel (at the time land bridges connecting Ireland and Great Britain and these to continental Europe). Secondary contact between the “Irish” front and descendants of the first wave was eventually established in the central Irish Sea following the fall of the terrestrial bridge connecting Ireland and South-west England (Hoarau et al. 2007). The westward front eventually expanded into the south of the North Sea following the breach of Dover Straits that resulted in the connection of the transgressed Channel and the Southern Bight some time between 8000 and 7500 BP. The absence of samples throughout much of the eastern part of the North Sea makes it difficult to determine where along eastern England/Scotland secondary contact with cluster 1 has been established.
In F. vesiculosus, the greatest demographic expansion occurred well before the LGM, but post-LGM recolonization originated in the south-western Ireland-Brittany area (Coyer et al. 2011a). Two dominant haplotypes were present in this refugium; both expanded north and are presently found in the Shetlands and the Faeroes. One haplotype expanded throughout Scandinavia, the Baltic, and Iceland, and possibly crossed the Atlantic colonizing the Canadian Maritimes and eastern USA [but see Assis et al. 2014; the other spread to France and areas south (Coyer et al. 2011a) (Fig. 11.7)].
In F. ceranoides and in P. canaliculata, the colonization routes are less clear given the existence of only one phylogroup throughout both periglacial and post-glacially colonized regions (Neiva et al. 2012b, 2014) (Fig. 11.5b, c). In both species, Scottish populations (and nearby areas) are fixed for specific alleles (yellow haplotypes), apparently excluding the region as the source of Scandinavian populations. Alternatively, these distinct haplotypes may simply reflect the arrival there of later waves from the Irish Sea (F. ceranoides) or from eastern England (P. canaliculata).
Unlike the previously discussed fucoids, F. distichus is cold-adapted and of North Pacific origin, having a unique distribution along Arctic ice margins, including northern Canada, central Greenland, Iceland, Svalbard, and Novaya Zemlya (Lüning 1990; Adey and Hayek 2005). Tracing post-glacial pathways in the North Atlantic is, therefore, more speculative, as the populations from which recolonization expansions emerged are no longer present in areas likely to have been populated during glacial periods. Nevertheless, ENMs and extant haplotype distributions suggest that F. distichus was restricted to the North-west Atlantic coastal areas between Brittany and central Portugal and in the North-west Atlantic to the Canadian Maritimes (Coyer et al. 2011b) (Fig. 11.9). As F. distichus began a post-glacial poleward recolonization, it could no longer survive in the warming refugia along the southern European coast and consequently, the Icelandic/Faeroes region may now represent a southern interglacial refugium (Coyer et al. 2011b).
3 Long-Distance Range Expansions but Poor or no Gene Flow Across Genetic Breaks
The apparent absence of barriers in the oceans and the speed of ocean currents , are expected to allow marine organisms to be connected over broad spatial scales (Hellberg 2009). Rafting of reproductive individuals is known to play an important role in long-distance dispersal (LDD) of many seaweed species including kelps and fucoids with pneumatocysts (Norton 1992; Coyer et al. 2001; Thiel and Haye 2006; McKenzie and Bellgrove 2008). LDD, therefore, may explain the extensive post-glacial range expansions experienced by Atlantic fucoids, the colonization of remote mid-Atlantic islands such as Iceland and the Azores, the swift spread of some fucoid invaders (Kraan 2008), and fucoid ubiquity despite large habitat discontinuities (e.g. estuarine species or those circumscribed to scattered sheltered stations in otherwise exposed shores).
Paradoxically, Atlantic fucoids maintain sharp genetic breaks (i.e. fixed or nearly fixed haplotypic and allelic differences) throughout their ranges (Fig. 11.5), suggesting that while LDD may be common, successful gene flow is not. Only SDD maintains populations at seemingly small spatial scales. Regionally, genetic discontinuities are seldom spatially coincident between different species, which are often evenly distributed across contact zones (Hoarau et al. 2007; Neiva et al. 2012b, c, 2014). The origin, stability and pervasiveness of (neutral) genetic breaks thus appear to reflect historical contingencies and intrinsically restricted dispersal (stemming from fucoid life history traits), although dispersal barriers (including large habitat discontinuities) may play a role at the regional level.
Taken together, the phylogeographic patterns of Atlantic fucoids indicate that typical dispersal rates (including rafting ) enable colonization of empty habitat patches and range shifts, but are much less effective in neutralizing local to regional founder events or secondary contact, with recurrent genetic drift contributing to population differentiation. The contrasting dispersal and gene flow effects of dispersal into vacant (colonization) and populated (immigration) habitats suggest an important role for density-barriers such as priority-colonization or “founder takes all” effects (De Meester et al. 2002; Tellier et al. 2011; Waters et al. 2013). These are expected when infrequent but successful recruitment occurs into already dense populations and when available habitat patches are saturated (see also Guillemin et al., this book). In newly colonized habitats, exponential population growth ensures a disproportionate contribution of early settlers for the genetic make-up of establishing populations. Conversely, in demographically mature habitats, increased competition and numerical resident/immigrant disparity acts as a demographic buffer against changes in allele frequencies, since new arriving alleles will likely be rare and prone to be lost by genetic drift, rather than magnified by unconstrained growth.
Rare dispersal further constrains the dynamics of the colonization front(s), particularly in narrow intertidal habitats. Several Atlantic fucoids exhibit clear parapatric phylogeographic sectors (Fig. 11.5) thought to have been formed during expansion and secondary contact of differentiated source populations (Hoarau et al. 2007; Neiva et al. 2012b, c, 2014; Assis et al. 2014). In addition, commonly observed poleward reductions in genetic diversity suggest genetic bottlenecks and allelic surfing at species’ leading edges , which contributes to the progressive erosion of refugial gene pools in post-glacially colonized regions (Excoffier and Ray 2008). Fucoid research has further shown that allelic surfing may also facilitate introgression between hybridizing species, as revealed by the massive spread of introgressed F. vesiculosus organellar genomes throughout the central and northern range of F. ceranoides associated with the post-glacial expansion of the latter (Neiva et al. 2010). Other outcomes are possible, such as newly formed hybrid zones between F. serratus and F. distichus (Coyer et al. 2002; Hoarau et al. 2015). In contrast to other fucoid species, the phylogeographic pattern of A. nodosum was typical of long lifespan species with large population size , high fecundity , high within population diversity and weak large-scale differentiation (Olsen et al. 2010).
4 Human Impacts —Species Introductions and Climate Change
4.1 Unintentional Introductions Linked to Maritime Traffic Can Affect Phylogeography
Anthropogenically mediated LDD of coastal species, including Atlantic fucoids, is of increasing concern because it can potentially affect local ecosystem functioning and native biodiversity. Common vectors include shipping, mariculture , aquarium trade and intentional introductions (Miller et al. 2004; Provan et al. 2005a; Hewitt et al. 2007; Kim et al. 2010; Riosmena-Rodríguez et al. 2012). Documented examples of human-mediated introductions of Atlantic fucoids include F. distichus into the Oslofjord (southern Norway) in the mid-1890s (Schueller and Peters 1994), F. serratus to Iceland and the Faroes in the mid-1800s and 1900s respectively (Coyer et al. 2006b) and Nova Scotia (North-west Atlantic) around 1868 (Brawley et al. 2009), and of A. nodosum to San Francisco Bay, first detected in 2001 (Miller et al. 2004). Another putative human-mediated introduction (the unlikely alternative being a trans-Arctic crossing) is that of F. spiralis in the Pacific, first reported by Harvey in 1862 (Norris and Conway 1974), who did not identify the species, but reported it to be similar to the Fucus species from the Canary Islands. Its single haplotype is identical to a dominant haplotype in northern Atlantic populations, indicating a recent origin from an Atlantic source (Coyer et al. 2011a).
Several fucoid introductions have proven successful with both intercalation and expansion beyond the original introduction point. For example, F. distichus rapidly expanded south of its introduction site in Oslofjorden in the mid-1890s, appearing in the Skagerrak Sea in 1924, the central Kattegat Sea in 1933 and in the Kiel Bight (western Baltic) by 1992 (Schueller and Peters 1994). Similarly, the original distribution of F. spiralis from the Aleutian Islands to northern Washington State (Norris and Conway 1974) has expanded to include Oregon (Neiva et al. 2012a) and California (EAS and GAP personal observations 2014). Fucus serratus has expanded over 1500 km along North American shorelines, by both natural and further human-mediated transport (Johnson et al. 2011). As an exception, alien A. nodosum was eradicated from San Francisco Bay in 2002, but the number and size of the individuals removed suggest that it had been present, but undetected for several years (Miller et al. 2004). It was probably introduced as discarded seafood or bait worm packing material, and as it presently continues to appear in seafood restaurants of San Francisco Bay (JLO personal observations), re-establishment is likely.
Molecular techniques have been used to determine the native or introduced status of species, and to determine putative population sources. Microsatellite-based approaches indicated that Icelandic populations of F. serratus originated from the Oslofjorden (southern Norway), an introduction that had to have occurred sometime between 900 AD, when the first Icelandic settlers arrived from Norway, and 1900, when it was first noted in a phycological survey (Coyer et al. 2006b). Despite anthropogenic passages to Iceland from numerous areas in Norway, the UK and Germany during the intervening years, historical records and microsatellite assignment tests show that the most parsimonious source of the introduced population was via logs from the Oslofjorden logging area during the mid-nineteenth century (Coyer et al. 2006b). Further analysis indicated that F. serratus was introduced to the eastern Faeroes Islands from the Reykjavik area, probably via fishing activities, during the late twentieth century (Coyer et al. 2006b).
Human emigration and shipping activities also played a role in the introduction of F. serratus to Nova Scotia, where a similar combination of historical records, microsatellite genotypes and assignment tests revealed two introductions in the mid-nineteenth century. One stemmed from Galway (Ireland) to Pictou and the other from Greenock (Scotland) to Western Cape Breton Island (Brawley et al. 2009). In both cases, the introduction vector was probably ballast rocks; ballasted ships arrived in Nova Scotia with passengers and the ballast was discarded before departing with log cargo (Brawley et al. 2009). Ballast rocks taken from the shallow intertidal at the source inevitably were inhabited by a thriving community of invertebrates and algae, which could easily survive in the dark and damp holds for the weeks-long transatlantic voyages. There has been at least one unsuccessful intentional introduction of F. serratus from Europe to North America. In 1902, the New York Botanical Gardens transplanted several individual F. serratus from Pictou, Nova Scotia to Pelham Bay and Hunters Island near New York City as part of a programme to increase local diversity (Anonymous 1992), but the transplant presumably was unsuccessful. Through unknown means, F. serratus was found at Newburyport, Massachusetts in 1852, but in 1902, was reported to have “long ceased to exist there” (Anonymous 1992).
In addition to potential competitive interactions with the local flora, a further consequence of inadvertent or intentional fucoid introductions may be hybridization with closely related native species. Molecular data revealed signatures of introgressive hybridization between the recently introduced F. serratus and its native sister species F. distichus (F. evanescens in Coyer et al. 2006b) in Iceland, and vice versa in Oslofjorden (Coyer et al. 2002, 2007) that were an order of magnitude higher than between the two species in northern Norway where they have been in sympatry for several thousands of years (Hoarau et al. 2015). The reductions in hybridizations and introgressions with increasing time of sympatry strongly suggest rapid evolution of pre-zygotic reinforcement mechanisms (Hoarau et al. 2015), as shown also when comparing southern allopatric F. guiryi and a contact zone with F. vesiculosus and F. spiralis farther north (Moalic et al. 2011).
4.2 Climate Change and Predicted Loss of Southern Ranges and Associated Endemic Diversity
Species’ responses to climate change range from shifts in phenology and productivity to local extinctions associated with physiological tipping points. Such responses can be a function of adaptation , maladaptation, acclimation history of the individuals and their parents, or even speciation. For example, the embryos of F. vesiculosus descendent from warm-acclimated individuals survive for longer periods when exposed to thermal stress (Li and Brawley 2004). Local adaptation has been reported for southern range populations of F. serratus (Jueterbock et al. 2014), but at the southernmost limit, the populations show no local adaptation, coping instead by restricting their vertical range in the intertidal (Pearson et al. 2009). In another example, involving Laminaria digitata (which reaches its southern limit in southern Brittany) and L. hyperborea (which extends its distribution farther south to northern Spain), reduced genetic diversity in southern Brittany was only observed for L. digitata (Robuchon et al. 2014) and was best explained by local maladaptation through altered sexual reproduction (Oppliger et al. 2014). Under strong selective pressures, fucoid species may even undergo speciation (e.g. F. radicans, Pereyra et al. 2009). Nevertheless, the rapid pace of recent climate change seems to largely outpace the potential of species to acclimate or adapt, typically producing more detrimental effects and leading to extinction of seaweed populations at regional scales (Wernberg et al. 2011b; Duarte et al. 2013; Nicastro et al. 2013).
Local extinctions are probably the most common outcome of climate change, particularly at lower latitude trailing edges , where environmental conditions generally border the thermal tolerance of species and small microhabitat differences can make an enormous difference (e.g. Mota et al. 2015 for F. vesiculosus). For instance, the progressive warming trend (due in part to intense heat waves) reported for the Iberian Peninsula and the Mediterranean Sea (Belkin 2009; Lima and Wethey 2012), contracted southern ranges by hundreds of kilometres for many fucoid species such as F. serratus and H. elongata (Martínez et al. 2012; Duarte et al. 2013), F. vesiculosus (Nicastro et al. 2013), F. virsoides (Mačić 2006) and Cystoseira spp. (Perkol-Finkel and Airoldi 2010). Distinct model-based approaches using contrasting scenarios of greenhouse gas emissions showed that several fucoid species (e.g. F. serratus, F. vesiculosus and A. nodosum) may shift ranges northward as a unit, likely colonizing the Arctic shores in Canada, Greenland and Svalbard within the next 50–75 years, while severely contracting ranges south of 45° N (Jueterbock et al. 2013; Assis et al. 2014) (Figs. 11.8 and 11.11).
Predicted range changes of F. serratus, F. vesiculosus and Ascophyllum nodosum in the coming century based on increasingly severe IPCC model predictions (B1 < A1B < A2) of climate warming. The dotted line delimits North-west from North-east Atlantic. From Jueterbock et al. (2013)
Extinction of canopy-forming seaweeds causes loss of essential habitats, but a major concern is impoverishment of intra-specific diversity (Provan 2013). This is particularly important for the low-dispersing fucoids, which presently harbour high unique diversity at vulnerable lower latitude trailing edges due to long-term persistence in southern climatic refugia (Hoarau et al. 2007; Diekmann and Serrão 2012; Neiva et al. 2012b, c; Provan and Maggs 2012) (Figs. 11.5 and 11.8). In F. vesiculosus, a distinct southern genetic lineage has regressed more than 1000 km (Nicastro et al. 2013) and is predicted to continue towards extinction (Fig. 11.8) (Assis et al. 2014). Because unique genetic lineages are not morphologically distinct, the loss of gene pools may occur unnoticed in many species (i.e. shifting genetic baselines; Assis et al. 2013).
5 General Conclusions
Fucoid seaweeds are the most extensively investigated group of algae in Atlantic phylogeographic research. Their life history, ecology and rapid speciation are well studied making it possible to gain insights into dispersal, population structure and distribution at multiple spatial resolutions. Fucoid species with cold-to-warm temperate affinities vary in responses to climatic shifts that overlap for some species along a few common persistence zones (i.e. refugia ). Most studies found persistent climatic refugia in north-western Iberia, as inferred from the distinct lineages that evolved and persisted. For some, the region has become progressively marginal in the course of the present (warming) interglacial. For F. guiryi , Iberia appears to be an introgressive contact zone , likely associated with expansion from the non-introgressed south (north-western Morocco), another long-term climatic refugium for a reduced set of more warm-tolerant species. The large Irish to Brittany paleocoastline is the most impressive refugium as its large size allowed persistence of populations with high genetic diversity for most fucoid species. The few fucoids (F. distichus, A. nodosum and F. vesiculosus) occurring in the North-west Atlantic (south of the Laurentide ice sheet) exhibit distinct genetic variability, supporting a hypothesis of glacial persistence . The eurythermal F. vesiculosus is noteworthy as the only species having distinct genetic lineages supporting persistence in all these refugia, which is further supported by hindcasting ENMs.
For most fucoids, the scale of Atlantic post-glacial expansions was notable, with several reaching Russia and Greenland. Waves of colonization involved routes along north-western Scotland into Scandinavia, and simultaneously along the transgressing English Channel into the North Sea. These created large geographical areas along the recolonized northern ranges where populations are genetically homogeneous and less diverse. Range expansions have also changed genetic diversity and structure by means of introgressive recombination of genomes at contact zones . Phylogeographic tools have also proven useful to identify cryptic and non-cryptic introductions , which have had some consequences for native biodiversity in terms of introgression and reduced genetic variation.
Collectively, North Atlantic fucoids demonstrate how limited dispersal modes can have contrasting effects at the scales of marine meta-population (connectivity) versus range dynamics (habitat tracking), promoting both sharp genetic divergence between refugial gene pools and large-scale homogeneity along recently colonized areas, depending on the past demographic conditions of populations. Climatic refugia typically harbour differentiated gene pools, but for several species the present highest diversity hotspots are not the southernmost species range limits, but rather in the large, stable Brittany–Celtic Sea refugium. This might result from the past loss of southernmost diversity due to population bottlenecks , drift and local extinctions at species’ rear edges, and is likely to intensify in the coming decades of climatic change.
In summary, Atlantic fucoids are excellent models to investigate the responses of species to global environmental change . Putative climatic refugia occurred across several main regions (broadly North-west Africa, north-western Iberia, Celtic Sea, Grand Banks), but each species survived only in a subset of these regions and their relative size or importance was variable for individual fucoid species with contrasting physiological affinities and ranges. Their many range shifts , including southern contractions and northward recolonizations also provide useful insights about how climate warming has affected and likely will affect rocky intertidal communities. Future scenarios for changing fucoid distributions do not include species extinctions, but do include loss of unique, rear-edge diversity as poleward range expansions are counteracted by southern contractions. Future research challenges should focus on how specific gene pools and genetic structuring (e.g. intertidal zonation) drive adaptation to local conditions. Ecological and functional genomic approaches applied in an already robust phylogeographic framework will open further possibilities to understand the complex dynamics of community genetic/genomic interactions. As the “canary in a mine”, fucoid algae are only a sign of the major changes expected on rocky shores in the coming decades.
References
Adey WH, Hayek LAC. The biogeographic structure of the western North Atlantic rocky intertidal. Cryptogam Algologie. 2005;26:35–66.
Ægisdóttir HH, Þórhallsdóttir ÞE. Theories on migration and history of the North-Atlantic flora: a review. Jokull. 2004;54:1–16.
Alberto F, Massa S, Manent P, Diaz-Almela E, Arnaud-Haond S, Duarte CM, Serrão EA. Genetic differentiation and secondary contact zone in the seagrass Cymodocea nodosa across the Mediterranean-Atlantic transition region. J Biogeogr. 2008;35:1279–94.
Alm T, Birks HH. Late Weichselian flora and vegetation of Andøya, Northern Norway-macrofossil (seed and fruit) evidence from Nedre Æråsvatn. Nord J Bot. 1991;11:465–76.
Anonymous (1992) Increasing New York’s Sea Flora. New York Times.
Araújo R, Bárbara I, Tibaldo M, Berecibar E, Tapia PD, Pereira R, Santos R, Pinto IS, Ba I, Dı P. Checklist of benthic marine algae and cyanobacteria of northern Portugal. Bot Mar. 2009;52:24–46.
Arrontes J. Nature of the distributional boundary of Fucus serratus on the north shore of Spain. Mar Ecol Prog Ser. 1993;93:183–93.
Assis J, Coelho NC, Alberto F, Valero M, Raimondi P, Reed D, Serrão EA. High and distinct range-edge genetic diversity despite local bottlenecks. PLoS ONE. 2013;8(7):e68646.
Assis J, Serrão EA, Claro B, Perrin C, Pearson GA. Climate-driven range shifts explain the distribution of extant gene pools and predict future loss of unique lineages in a marine brown alga. Mol Ecol. 2014;23:2797–810.
Bárbara I, Cremades J, Calvo S, López-Rodriguez MC, Dosil J. Checklist of the benthic marine and brackish Galician algae (NW Spain). Anales del Jardín Botánico de Madrid. 2005;62:69–100.
Bartsch I, Wiencke C, Laepple T. Global seaweed biogeography under a changing climate: the prospected effects of temperature. In: Wiencke C, Bischof K, editors. Seaweed biology. Berlin Heidelberg: Springer; 2012. p. 383–406.
Belkin IM. Rapid warming of large marine ecosystems. Prog Oceanogr. 2009;81:207–13.
Bennett K, Provan J. What do we mean by “refugia”? Quat Sci Rev. 2008;27:2449–55.
Bierne N, Borsa P, Daguin C, Jollivet D, Viard F, Bonhomme F, David P. Introgression patterns in the mosaic hybrid zone between Mytilus edulis and M. galloprovincialis. Mol Ecol. 2003;12:447–61.
Billard E, Daguin C, Pearson GA, Serrão EA, Engel C, Valero M. Genetic isolation between three closely related taxa: Fucus vesiculosus, F. spiralis, and F. ceranoides (Phaophyceae). J Phycol. 2005;41:900–5.
Billard E, Serrão EA, Pearson GA, Destombe C, Valero M. Fucus vesiculosus and spiralis species complex: a nested model of local adaptation at the shore level. Mar Ecol Prog Ser. 2010;405:163–74.
Björck S. A review of the history of the Baltic Sea, 13.0–8.0 ka BP. Quat Int. 1995;27:19–40.
Brawley SH, Coyer JA, Blakeslee AMH, Hoarau G, Johnson LE, Byers JE, Stam WT, Olsen JL. Historical invasions of the intertidal zone of Atlantic North America associated with distinctive patterns of trade and emigration. Proc Nat Acad Sci USA. 2009;106:8239–44.
Brunhoff C, Yoccoz NG, Ims RA, Jaarola M. Glacial survival or late glacial colonization? Phylogeography of the root vole (Microtus oeconomus) in north-west Norway. J Biogeogr. 2006;33:2136–44.
Cánovas FG, Mota CF, Serrão EA, Pearson GA. Driving south: a multi-gene phylogeny of the brown algal family Fucaceae reveals relationships and recent drivers of a marine radiation. BMC Evol Biol. 2011;11:371.
Carlson AE, Winsor K. Northern hemisphere ice-sheet responses to past climate warming. Nat Geosci. 2012;5:607–13.
Chapman ARO. Functional ecology of fucoid algae: twenty-three years of progress. Phycologia. 1995;34:1–32.
Christie H, Norderhaug KM, Fredriksen S. Macrophytes as habitat for fauna. Mar Ecol Prog Ser. 2009;396:221–33.
Coyer JA, Diekmann OE, Serrão EA, Procaccini G, Milchakova N, Pearson GA, Stam WT, Olsen JL. Population genetics of dwarf eelgrass Zostera noltii throughout its biogeographic range. Mar Ecol Prog Ser. 2004a;281:51–62.
Coyer JA, Hoarau G, Costa JF, Hogerdijk B, Serrão EA, Billard E, Valero M, Pearson GA, Olsen JL. Evolution and diversification within the intertidal brown macroalgae Fucus spiralis/F. vesiculosus species complex in the North Atlantic. Mol Phylogenet Evol. 2011a;58:283–96.
Coyer JA, Hoarau G, Oudot-Le Secq MP, Stam WT, Olsen JL. A mtDNA-based phylogeny of the brown algal genus Fucus (Heterokontophyta; Phaeophyta). Mol Phylogenet Evol. 2006a;39:209–22.
Coyer JA, Hoarau G, Van Schaik J, Luijckx P, Olsen JL. Trans-Pacific and trans-Arctic pathways of the intertidal macroalga Fucus distichus L. reveal multiple glacial refugia and colonizations from the North Pacific to the North Atlantic. J Biogeogr. 2011b;38:756–71.
Coyer JA, Hoarau G, Sjøtun K, Olsen JL. Being abundant is not enough: a decrease in effective over eight generations in a Norwegian population of the seaweed Fucus serratus. Biol Lett. 2008;4:755–7.
Coyer JA, Hoarau G, Skage M, Stam WT, Olsen JL. Origin of Fucus serratus (Heterokontophyta; Fucaceae) populations in Iceland and the Faroes: a microsatellite-based assessment. Eur J Phycol. 2006b;41:235–46.
Coyer JA, Hoarau G, Stam WT, Olsen JL. Geographically specific heteroplasmy of mitochondrial DNA in the seaweed Fucus serratus (Heterokontophyta: Phaeophyceae, Fucales). Mol Ecol. 2004b;13:1323–6.
Coyer JA, Hoarau G, Stam WT, Olsen JL. Hybridization and introgression in a mixed population of the intertidal seaweeds Fucus evanescens and F. serratus. J Evol Biol. 2007;20:2322–33.
Coyer JA, Jason Smith G, Andersen RA. Evolution of Macrocystis spp. (Phaeophyceae) as determined by ITS1 and ITS2 sequences. J Phycol. 2001;37:574–85.
Coyer JA, Peters AF, Hoarau G, Stam WT, Olsen JL. Hybridization of the marine seaweeds Fucus serratus and Fucus evanescens (Heterokontophyta: Phaeophyceae) in a 100-year-old zone of secondary contact. Proc R Soc B. 2002;269:1829–34.
Coyer JA, Peters AF, Stam WT, Olsen JL. Post-ice age recolonization and differentiation of Fucus serratus L. (Phaeophyceae; Fucaceae) populations in Northern Europe. Mol Ecol. 2003;12:1817–29.
Cunningham CW, Collins TM. Beyond area relationships: extinction and recolonization in molecular marine biogeography. In: Desalle R, Schierwater B, editors. Molecular approaches to ecology and evolution. Basel: Birkhauser Verlag; 1998. p. 297–321.
Davis MB, Shaw RG. Range shifts and adaptive responses to Quaternary climate change. Science. 2001;292:673–9.
De Meester L, Gómez A, Okamura B, Schwenk K. The monopolization hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecologia. 2002;23:121–35.
Diekmann OE, Serrão EA. Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol Ecol. 2012;21:1647–57.
Dijkstra JA, Boudreau J, Dionne M. Species-specific mediation of temperature and community interactions by multiple foundation species. Oikos. 2012;121:646–54.
Duarte L, Viejo RM, Martínez B, DeCastro M, Gómez-Gesteira M, Gallardo T. Recent and historical range shifts of two canopy-forming seaweeds in North Spain and the link with trends in sea surface temperature. Acta Oecol. 2013;51:1–10.
Dudgeon S, Kübler JE, Wright WA, Vadas RL, Petraitis PS. Natural variability in zygote dispersal of Ascophyllum nodosum at small spatial scales. Funct Ecol. 2001;15:595–604.
Engel CR, Daguin C, Serrão EA. Genetic entities and mating system in hermaphroditic Fucus spiralis and its close dioecious relative F. vesiculosus (Fucaceae, Phaeophyceae). Mol Ecol. 2005;14:2033–46.
Excoffier L, Ray N. Surfing during population expansions promotes genetic revolutions and structuration. Trends Ecol Evol. 2008;23:347–51.
Fernández C. The retreat of large brown seaweeds on the north coast of Spain: the case of Saccorhiza polyschides. Eur J Phycol. 2013;46:352–60.
Finnegan AK, Griffiths AM, King RA, Machado-Schiaffino G, Porcher JP, Garcia-Vazquez E, Bright D, Stevens JR. Use of multiple markers demonstrates a cryptic western refugium and postglacial colonisation routes of Atlantic salmon (Salmo salar L.) in Northwest Europe. Heredity. 2013;111:34–43.
Fraser CI, Nikula R, Spencer HG, Waters JM. Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. Proc Nat Acad Sci USA. 2009;106:3249–53.
Gibbard PL, Lautridou JP. The Quaternary history of the English Channel: an introduction. J Quat Sci. 2003;18:195–9.
Gorostiaga JM, Santolaria A, Secilla A, Casares C, Diez I. Check-list of the Basque coast benthic algae (North of Spain). Anales del Jardín Botánico de Madrid. 2004;61:155–80.
Graham MH, Dayton PK, Erlandson JM. Ice ages and ecological transitions on temperate coasts. Trends Ecol Evol. 2003;18:33–40.
Hampe A, Petit RJ. Conserving biodiversity under climate change: the rear edge matters. Ecol Lett. 2005;8:461–7.
Handschumacher L, Steinarsdóttir MB, Edmands S, Ingólfsson A. Phylogeography of the rock-pool copepod Tigriopus brevicornis (Harpacticoida) in the northern North Atlantic, and its relationship to other species of the genus. Mar Biol. 2010;157:1357–66.
Hellberg ME. Gene flow and isolation among populations of marine animals. Ann Rev Ecol Evol Syst. 2009;40:291–310.
Hewitt C, Campbell M, Schaffelke B. Introductions of seaweeds: accidental transfer pathways and mechanisms. Bot Mar. 2007;50:326–37.
Hoarau G, Coyer JA, Giesbers MCWG, Jueterbock A, Olsen JL. Pre-zygotic isolation in the macroalgal genus Fucus from four contact zones a tale of reinforcement? R Soc Open Sci. 2015;2:140538.
Hoarau G, Coyer JA, Veldsink JH, Stam WT, Olsen JL. Glacial refugia and recolonization pathways in the brown seaweed Fucus serratus. Mol Ecol. 2007;16:3606–16.
Hofreiter M, Stewart J. Ecological change, range fluctuations and population dynamics during the Pleistocene. Curr Biol. 2009;19:R584–94.
Hu ZM, Guiry MD, Critchley AT, Duan DL. Phylogeographic patterns indicate trans-Atlantic migration from Europe to North America in the red seaweed Chondrus crispus (Gigartinales, Rhodophyta). J Phycol. 2010;46:889–900.
Hu ZM, Li W, Li JJ, Duan DL. Post-Pleistocene demographic history of the North Atlantic endemic Irish moss Chondrus crispus: glacial survival, spatial expansion and gene flow. J Evol Biol. 2011;24:505–17.
Ilves KL, Huang W, Wares JP, Hickerson MJ. Colonization and/or mitochondrial selective sweeps across the North Atlantic intertidal assemblage revealed by multi-taxa approximate Bayesian computation. Mol Ecol. 2010;19:4505–19.
Ingólfsson A. A marine refugium in Iceland during the last glacial maximum: fact or fiction? Zool Sci. 2009;38:663–5.
Johnson LE, Brawley SH, Adey WH. Secondary spread of invasive species: historic patterns and underlying mechanisms of the continuing invasion of the European rockweed Fucus serratus in eastern North America. Biol Invasions. 2011;14:79–97.
Jolly MT, Viard F, Gentil F, Thiébaut E, Jollivet D. Comparative phylogeography of two coastal polychaete tubeworms in the Northeast Atlantic supports shared history and vicariant events. Mol Ecol. 2006;15:1841–55.
Jueterbock A, Kollias S, Smolina I, Fernandes JM, Coyer JA, Olsen JL, Hoarau G. Thermal stress resistance of the brown alga Fucus serratus along the North-Atlantic coast: acclimatization potential to climate change. Mar Genomics. 2014;13:27–36.
Jueterbock A, Tyberghein L, Verbruggen H, Coyer JA, Olsen JL, Hoarau G. Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol Evol. 2013;3:1356–73.
Kelly DW, MacIsaac HJ, Heath DD. Vicariance and dispersal effects on and speciation in a widespread estuarine invertebrate. Evolution. 2006;60:257–67.
Kettle AJ, Morales-Muñiz A, Roselló-Izquierdo E, Heinrich D, Vøllestad LA, Rosell E. Refugia of marine fish in the northeast Atlantic during the last glacial maximum: concordant assessment from archaeozoology and palaeotemperature reconstructions. Clim Past. 2011;7:181–201.
Kim SY, Weinberger F, Boo SM. Genetic data hint at a common donor region for invasive atlantic and pacific populations of Gracilaria vermiculophylla (Gracilariales, Rhodophyta). J Phycol. 2010;46:1346–9.
Kraan S. Sargassum muticum (Yendo) Fensholt in Ireland: an invasive species on the move. J Appl Phycol. 2008;20(5):825–32.
Ladah L, Bermudez R, Pearson GA, Serrão EA. Fertilization success and recruitment of dioecious and hermaphroditic fucoid seaweeds with contrasting distributions near their southern limit. Mar Ecol Prog Ser. 2003;262:173–83.
Lambeck K, Rouby H, Purcell A, Sun Y, Sambridge M. Sea level and global ice volumes from the Last Glacial Maximum to the Holocene. PNAS. 2015;111:15296–303.
Li JJ, Hu ZM, Duan DL. Survival in glacial refugia vs. postglacial dispersal in the North Atlantic: the cases of red seaweeds. In: Hu ZM, Fraser CI, editors. Seaweed: adaptation and evolution of seaweeds under environmental change. Springer: Berlin Heidelberg; 2016.
Li R, Brawley SH. Improved survival to heat stress in intertidal embryos simultaneously exposed to hypersalinity and the effect of parental thermal history. Mar Biol. 2004;144:205–13.
Lima FP, Wethey DS. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun. 2012;3:704.
Lüning K (1990) Seaweeds: their environment, biogeography, and ecophysiology. Wiley.
Mačić M. Distribution of seaweed Fucus virsoides J. Agardh in Boka Kotorska Bay (South Adriatic Sea). Ann Ser Hist Nat. 2006;16:1–4.
Maggs CA, Castilho R, Foltz D, Henzler C, Jolly MT, Kelly J, Olsen J, Perez KE, Stam W, Väinölä R, Viard F, Wares J. Evaluating signatures of glacial refugia for north atlantic benthic marine taxa. Ecology. 2008;89:108–22.
Martínez B, Arenas F, Rubal M, Burgués S, Esteban R, García-Plazaola I, Figueroa FL, Pereira R, Saldaña L, Sousa-Pinto I, Trilla a, Viejo RM. Physical factors driving intertidal macroalgae distribution: physiological stress of a dominant fucoid at its southern limit. Oecologia. 2012;170:341–53.
McKenzie PF, Bellgrove A. Dispersal of Hormosira banksii (Phaeophyceae) via detached fragments: reproductive viability and longevity. J Phycol. 2008;44:1108–15.
Ménot G, Bard E, Rostek F, Weijers JWH, Hopmans EC, Schouten S, Damsté JSS. Early reactivation of European rivers during the last deglaciation. Science. 2006;313:1623–5.
Miller AW, Chang AL, Cosentino-Manning N, Ruiz GM. A new record and eradication of the Northern Atlantic alga Ascophyllum nodosum (Phaeophyceae) from San Francisco Bay, California, USA. J Phycol. 2004;40:1028–31.
Moalic Y, Arnaud-Haond S, Perrin C, Pearson GA, Serrão EA. Travelling in time with networks: revealing present day hybridization versus ancestral polymorphism between two species of brown algae, Fucus vesiculosus and F. spiralis. BMC Evol Biol. 2011;11:33.
Mota CF, Engelen AH, Serrão EA, Pearson GA. Some don’t like it hot: microhabitat-dependent thermal and water stresses in a trailing edge population. Funct Ecol. 2015;29:640–9.
Muhlin JF, Brawley SH. Recent versus Relic: discerning the genetic signature of Fucus vesiculosus (Heterokontophyta; Phaeophyceae) in the Northwestern Atlantic. J Phycol. 2009;45:828–37.
Neiva J, Assis J, Fernandes F, Pearson GA, Serrão EA. Species distribution models and mitochondrial DNA phylogeography suggest an extensive biogeographical shift in the high-intertidal seaweed Pelvetia canaliculata. J Biogeogr. 2014;41:1137–48.
Neiva J, Hansen GI, Pearson GA, Van De Vliet MS, Maggs CA, Serrão EA. Fucus cottonii (Fucales, Phaeophyceae) is not a single genetic entity but a convergent salt-marsh morphotype with multiple independent origins. Eur J Phycol. 2012a;47:461–8.
Neiva J, Pearson GA, Valero M, Serrão EA. Surfing the wave on a borrowed board: range expansion and spread of introgressed organellar genomes in the seaweed Fucus ceranoides L. Mol Ecol. 2010;19:4812–22.
Neiva J, Pearson GA, Valero M, Serrão EA. Drifting fronds and drifting alleles: the genetic architecture of the estuarine seaweed Fucus ceranoides L. J Biogeogr. 2012b;39:1167–78.
Neiva J, Pearson GA, Valero M, Serrão EA. Fine-scale genetic breaks driven by historical range dynamics and ongoing density-barrier effects in the estuarine seaweed Fucus ceranoides L. BMC Evol Biol. 2012c;12:78.
Nicastro KR, Zardi GI, Teixeira S, Neiva J, Serrão EA, Pearson GA. Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 2013;11:6.
Norris RE, Conway E. Fucus spiralis L. in the northeast Pacific. Syesis. 1974;7:79–81.
Norton TA. Dispersal by macroalgae. Brit Phycol J. 1992;27:293–301.
Olsen JL, Stam WT, Coyer JA, Reusch TBH, Billingham M, Boström C, Calvert E, Christie H, Granger S, la Lumière R, Milchakova N, Oudot-Le Secq MP, Procaccini G, Sanjabi B, Serrão EA, Veldsink J, Widdicombe S, Wyllie-Echeverria S. North Atlantic phylogeography and large-scale population differentiation of the seagrass Zostera marina L. Mol Ecol. 2004;13:1923–41.
Olsen JL, Zechman FW, Hoarau G, Coyer JA, Stam WT, Valero M, Åberg P. The phylogeographic architecture of the fucoid seaweed Ascophyllum nodosum: an intertidal “marine tree” and survivor of more than one glacial-interglacial cycle. J Biogeogr. 2010;37:842–56.
Oppliger LV, Von Dassow P, Bouchemousse S, Robuchon M, Valero M, Correa JA, Mauger S, Destombe C. Alteration of sexual reproduction and genetic diversity in the kelp species Laminaria digitata at the southern limit of its range. PLoS ONE. 2014;9(7):e102518.
Pearson GA, Lago-Leston A, Mota C. Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J Ecol. 2009;97:450–62.
Pearson GA, Serrão EA. Revisiting synchronous gamete release by fucoid algae in the intertidal zone: fertilization success and beyond? Integr Comp Biol. 2006;46:587–97.
Pereyra RT, Bergström L, Kautsky L, Johannesson K. Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evol Biol. 2009;9:70.
Perkol-Finkel S, Airoldi L. Loss and recovery potential of marine habitats: an experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PLoS ONE. 2010;5(5):e10791.
Petit J, Brewer S, Cheddadi R, Coart E, Cottrell J, Csaikl UM, Van Dam B, Deans JD, Espinel S, Fineschi S, Finkeldey R, Glaz I, Goicoechea PG, Lowe AJ, Svejgaard J, Ko AO, Munro RC, Flemming S, Ma G, Popescu F, Slade D, Tabbener H, De Vries SGM, Ziegenhagen B, De Beaulieu J, Kremer A. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For Ecol Manag. 2002;156:49–74.
Provan J. The effects of past, present and future climate change on range-wide genetic diversity in northern North Atlantic marine species. Front Biogeogr. 2013;5:60–6.
Provan J, Maggs CA. Unique genetic variation at a species’ rear edge is under threat from global climate change. Proc R Soc B. 2012;279:39–47.
Provan J, Murphy S, Maggs CA. Tracking the invasive history of the green alga Codium fragile ssp. tomentosoides. Mol Ecol. 2005a;14:189–94.
Provan J, Wattier RA, Maggs CA. Phylogeographic analysis of the red seaweed Palmaria palmata reveals a Pleistocene marine glacial refugium in the English Channel. Mol Ecol. 2005b;14:793–803.
Remerie T, Vierstraete A, Weekers PHH, Vanfleteren JR, Vanreusel A. Phylogeography of an estuarine mysid, Neomysis integer (Crustacea, Mysida), along the north-east Atlantic coasts. J Biogeogr. 2009;36:39–54.
Riosmena-Rodríguez R, Boo GH, López-Vivas JM, Hernández-Velasco A, Sáenz-Arroyo A, Boo BM. Sargassum filicinum (Fucales, Phaeophyceae) is on the move along the Mexican Pacific coastline. Bot Mar.2012;55:547–551.
Robuchon M, Le Gall L, Mauger S, Valero M. Contrasting genetic diversity patterns in two sister kelp species co-distributed along the coast of Brittany, France. Mol Ecol. 2014;23:2669–85.
Roman J, Palumbi SR. A global invader at home: population structure of the green crab, Carcinus maenas, in Europe. Mol Ecol. 2004;13:2891–8.
Schiel DR, Foster MS. The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Ann Rev Ecol Evol Syst. 2006;37:343–72.
Schueller G, Peters A. Arrival of Fucus evanescens (Phaeophyceae) in Kiel Bight (Western Baltic). Bot Mar. 1994;37:471–477.
Serrão EA, Alice LA, Brawley SH. Evolution of the Fucaceae (Phaeophyceae) inferred from nrDNA-ITS. J Phycol. 1999;35:382–94.
Serrão EA, Kautsky L, Lifvergren T, Brawley SH. Gamete dispersal and mortality in baltic Fucus vesiculosus. Phycologia. 1997;36:101–2.
Serrão EA, Pearson GA, Kautsky L, Brawley SH. Successful external fertilization in turbulent environments. Proc Nat Acad Sci USA. 1996;93:5286–90.
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson MAX, Halpern BS, Jorge MA, Lombana AL, Lourie SA, Martin KD, Manus MC, Molnar J, Recchia CA, Robertson J. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–83.
Stewart JR, Lister AM. Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol. 2001;16:608–13.
Stewart JR, Lister AM, Barnes I, Dalén L. Refugia revisited: individualistic responses of species in space and time. Proc R Soc B. 2010;277:661–71.
Tellier F, Tapia J, Faugeron S, Destombe C, Valero M. The Lessonia nigrescens species complex (Laminariales, Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J Phycol. 2011;47:894–903.
Thiel M, Haye PA. The ecology of reafting in the marine environment. III. Biogeographical and evolutionary consequences. Oceanogr Mar Biol Ann Rev. 2006;44:323–429.
Vuorinen I, Hänninen J, Rajasilta M, Laine P, Eklund J, Montesino-Pouzols F, Corona F, Junker K, Meier MHE, Dippner JW. Scenario simulations of future salinity and ecological consequences in the Baltic Sea and adjacent North Sea areas–implications for environmental monitoring. Ecol Indic. 2015;50:196–205.
Waltari E, Hickerson MJ. Late Pleistocene species distribution modelling of North Atlantic intertidal invertebrates. J Biogeogr. 2013;40:249–60.
Wares JP, Cunningham CW. Phylogeography and historical ecology of the North Atlantic intertidal. Evolution. 2001;55:2455–69.
Waters JM, Fraser CI, Hewitt GM. Founder takes all: density-dependent processes structure biodiversity. Trends Ecol Evol. 2013;28:78–85.
Wernberg T, Russell BD, Moore PJ, Ling SD, Smale DA, Campbell A, Coleman MA, Steinberg PD, Kendrick GA, Connell SD. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J Exp Mar Biol Ecol. 2011a;400:7–16.
Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJ, Poloczanska ES, Connell SD. Seaweed communities in retreat from ocean warming. Curr Biol. 2011b;21:1828–32.
Wilson AB, Eigenmann Veraguth I. The impact of Pleistocene glaciation across the range of a widespread European coastal species. Mol Ecol. 2010;19:4535–53.
Woodall LC, Koldewey HJ, Shaw PW. Historical and contemporary population genetic connectivity of the European short-snouted seahorse Hippocampus hippocampus and implications for management. J Fish Biol. 2011;78:1738–56.
Zardi GI, Nicastro KR, Canovas F, Costa JF, Serrão EA, Pearson GA. Adaptive traits are maintained on steep selective gradients despite gene flow and hybridization in the intertidal zone. PLoS ONE. 2011;6:e19402.
Zardi GI, Nicastro KR, Ferreira Costa J, Serrão EA, Pearson GA. Broad scale agreement between intertidal habitats and adaptive traits on a basis of contrasting population genetic structure. Estu Coast Shelf Sci. 2013;131:140–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Neiva, J. et al. (2016). Climate Oscillations, Range Shifts and Phylogeographic Patterns of North Atlantic Fucaceae. In: Hu, ZM., Fraser, C. (eds) Seaweed Phylogeography. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7534-2_11
Download citation
DOI: https://doi.org/10.1007/978-94-017-7534-2_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7532-8
Online ISBN: 978-94-017-7534-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)