Summary
Photosynthetic organisms must respond to several external stimuli to adjust their photosynthetic performances in order to keep a high capacity of carbon assimilation and avoid photodamage. They do so integrating processes occurring at different time scales starting from very fast light harvesting, down to slow changes of electron flow and protein composition of the photosynthetic apparatus. Central to these regulatory mechanisms is the cytochrome b 6 f complex, which regulates intersystem electron flow via photosynthetic control and modulates light harvesting capacity by acting as a redox sensor to trigger an acclimation process named state transitions. In this chapter we describe how the cytochrome b 6 f complex performs to regulate these different functions in both plants and microalgae, proposing new mechanisms of regulation, and critically discussing how these processes are compatible with the existing literature on the structure and function of this complex.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cyclic Electron Flow
- Ferredoxin NADP Reductase
- Linear Electron Flow
- Rieske Protein
- Photosynthetic Control
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Photosynthesis is an old invention of nature, which, since its onset, has drastically shaped life on earth (Gould et al. 2008). O2 evolution by early oxygenic photosynthetic organisms opened up the possibility of land colonization, while imposing new constraints on photosynthesis, mostly related to the interactions between molecular oxygen produced by water splitting in photosystem (PS) II and excited states of the pigment. This interaction triggers the generation of reactive oxygen species (ROS), which are harmful for the photosynthetic apparatus and biological systems in general. ROS production becomes particularly high when photochemical light conversion (charge separation) is impaired by a decrease in electron flow capacity, leading to a reduction of the electron acceptors for the two photosystems. Thus, the photosynthetic electron transfer chain must be regulated to avoid ROS production. Key regulatory processes are linked to the generation of a proton motive force (a pH gradient, ∆pH) across the thylakoid membrane. Acidification of the thylakoid lumen results in down regulation of light harvesting capacity of PSII via the onset of Non Photochemical Quenching (NPQ), a process which dissipates excess light energy as heat. This mainly occurs in the pigment-containing proteins of PSII, thanks to the ∆pH induced synthesis of specific xanthophylls (e.g. zeaxathin in plants and green algae, diatoxanthin in other microalgae) and the activation of protein sensors (PsbS in plants and LHCSR3 in eukaryotic algae) (Li et al. 2009).
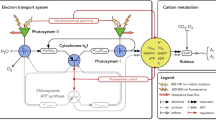
Regulation of the pH gradient across the thylakoid membrane is central to the regulation of photosynthesis. To understand how the ∆pH is controlled, however, we need to consider the (literally) central role of the cytochrome b 6 f complex (Cyt b 6 f). This complex is both crucial in generating ∆pH but is also itself directly affected by that gradient. Lumen acidification modulates the rate of electron flow through the Cyt b 6 f complex (termed “photosynthetic control”, see Eberhard et al. 2008 and Fig. 22.1).
Structure of the cytochrome b6f complex (Stroebel et al. 2003). Left: atomic structure of the complex from Chlamydomonas reinhardtii. Right: Schematic representation of the complex. The low potential and high potential paths are represented in red and blue, respectively. The chlorophyll is shown in green. Catalytic activity of the high potential path is linked to H+ release in the lumen, and consequently regulated by the establishment of a ∆pH across the membranes. The main subunits of the complex are represented: IV, subunit IV; b 6 and f, cytochrome b 6 and f, respectively; ISP Rieske protein containing and Fe-S cluster (square). Closed circles, ellipses and polygons indicate the quinone binding sites, hemes and chlorophyll, respectively. Note that only one monomer of the cytochrome b 6 f dimer is shown.
In what is usually seen as its primary role, in the linear electron flow (LEF) pathway, Cyt b 6 f catalyzes the transfer of electrons originating from PSII, and transferred through the membrane via the plastoquinone (PQ) pool, to a hydrophilic one-electron acceptor protein (plastocyanin (PC) or a c-type cytochrome (Cyt c 6)). The electron transfer activity is coupled to proton translocation across the membrane. Reduced PC or Cyt c 6 (depending on species and growth conditions) is released from Cyt b 6 f and binds to PSI, where it reduces the primary electron donor of this complex, P700. Electrons arriving at the donor side of PSI are transferred to its acceptor side via charge separation and there, either continue their journey towards the Calvin Benson Bassham cycle (CBB) via ferredoxin (Fd), ferredoxin NADP reductase (FNR) and then NADP+, or are employed directly to provide reducing power for other cellular metabolic processes (such as nitrogen and sulfur metabolism, lipid, amino acid, pigment biosynthesis) via Fd oxidation (Eberhard et al. 2008). In addition to this linear pathway, the electrons from PSI can also be transferred back to Cyt b 6 f via cyclic electron flow (CEF) around PSI (Shikanai 2007; Johnson 2011). This process, the significance of which was debated for many years, is now thought to occur in all oxygenic photosynthetic systems. Cyt b 6 f is critical in controlling the relative efficiency of linear and cyclic flow, either indirectly (by setting the rate of plastoquinol (PQH2) oxidation) or directly, e.g. via the formation of a biochemically stable PSI-Cyt b 6 f complex (CEF supercomplex), which is seen in the green alga Chlamydomonas reinhardtii (see Chap. 23 by Minagawa). Finally, Cyt b 6 f plays a specific role, which is not seen in its respiratory counterparts, the cytochrome bc 1 complexes. Being the site of PQH2 oxidation, this complex senses the redox state of the photosynthetic electron transfer chain, and triggers the induction of state transitions, a process regulating the relative light absorption capacity of the two photosystems via the reversible migration of (a part of) the light harvesting complexes between the two photosystems (see Chap. 24 by Rochaix).
II. H+/e− Coupling in the Cytochrome b 6 f Complex and Its Role in Photosynthetic Control
Photosynthetic electron flow is kinetically controlled at the level of PQH2 oxidation at the lumenal side of the Cyt b 6 f complex. Regulation can occur in response to acidification of the thylakoid lumen. This kinetic effect, known as “photosynthetic control,” is the consequence of the tight coupling between electron and proton transfer during oxidation of PQH2 (West and Wiskich 1968). During this reaction, electrons originating from PQH2 are divided between the Rieske Fe-S protein, the first step in the so-called high-potential chain (Fig. 22.1) and a cyclic route that comprises the b hemes (Q cycle; reviewed in Cramer et al. 2011), and possibly the c’ (or c n) heme located on the stromal side of the complex (see Chap. 15 by Zito and Alric). In isolated Cyt b 6 f isolated from Chlamydomonas (Nitschke et al. 1992; Pierre et al. 1995) the Rieske protein has an E m of +290 mV. Cyt f, which oxidizes the Rieske Fe-S cluster, has an E m of +330 mV, and the two Cyt b 6 hemes have E m values of −84 and −158 mV, respectively.
Due to the less positive E m of the cytochrome comprising the b-heme pathway (as compared with Cyt f and the Rieske Fe-S protein), this route is referred to as the low-potential chain. According to the Q cycle mechanism proposed by Mitchell (1975) and modified by Crofts et al. (1983), quinones are oxidized and reduced at two distinct sites in the protein, the Qo and Qi (or Qp and Qn) sites, respectively, which are located on opposite sides of the membrane (Cramer et al. 2011). PQH2 oxidation on the lumenal side is associated with the reduction of both Cyt f and heme b L (Crofts et al. 1983) and the release of protons into the lumen. Oxidation of the b 6 hemes occurs through a two-step reduction of a PQ molecule at the Qi site, possibly involving the recently discovered c’ heme (Cramer et al. 2011; and Chap. 15 by Zito and Alric). The electron transfer sequence is (1) the reduction of Cyt b L by PQH2, a process that is coupled to proton release to the lumen, (2) electron transfer to b H, and then (3) most likely a double electron transfer from these hemes to a PQ molecule bound at the Qi site, a process that is coupled to proton uptake from the stroma. Overall, the Q cycle results in additional proton pumping into the lumen, and therefore increases the H+/e− ratio of photosynthetic electron transfer.
Previous work has suggested that this cycle is constitutively active in physiological conditions (Sacksteder et al. 2000), and can be bypassed only upon drastic modifications of the redox features of the low potential chain (Malnoe et al. 2011). On average, each electron passes twice through the Cyt b 6 f complex, once through the low- and once through the high-potential pathways and transports 2 protons from the stroma to the lumen. The reaction PQH2 + PC+ ↔ PQ + PC + 2H+ is expected to be sensitive to the lumenal H+ concentration and this is simply as a result of a mass transfer effect. In particular, the pH effect would reflect the pH modulation of the formation of hydrogen bonds between the PQH2 and protonatable residues in the Qo site. One of these protonatable residues is His126 of the Rieske protein of C. reinhardtii. The role of His126 in the modulation of the pH effect on the quinol oxidation rate has been first demonstrated in the case of cytochrome bc 1 complexes (reviewed in Berry et al. 2000). Its involvement in the control of Cyt b 6 f activity was confirmed by the pH profile of this complex in Chlamydomonas, where a pK of 6.25–6.5 has been detected (Finazzi 2002). This value is very close to the one previously attributed to His126 (Berry et al. 2000) of the Rieske protein in bacterial systems. The second residue involved in proton release in the lumen during PQH2 oxidation is Glu78 of the PEWY sequence, which is extensively conserved in respiratory and photosynthetic cytochromes (Berry et al. 2000). This residue is located in a key position in the lumenal binding pocket (see e.g. Hasan et al. 2013) and its removal significantly altered the pH dependence of PQH2 oxidation in Chlamydomonas cells (Zito et al. 1998).
Although plausible, a careful examination of the features of PQH2 oxidation in the Cyt b 6 f complex suggests that the simple mass effect explanation invoked previously to explain the photosynthetic control is probably a too simplistic view. Indeed the E m differences between the initial electron donor (PQH2 ∼ 0 mV) and the terminal acceptor (PC) is ∼300 mV. This means that a very large pH gradient would be needed to counteract the driving force for electron transfer under steady state coupled conditions. However, it has been proposed that no such large proton gradient can be built in the thylakoid membranes under physiological conditions (Kramer et al. 2004). Measurements of the kinetics of P700 reduction following a light-dark transition, an indicator of the flux through the Cyt b 6 f complex, indicate that no inhibition of PQH2 oxidation normally occurs in unstressed plants across the range of light intensities experienced in nature (Harbinson and Hedley 1989; Ott et al. 1999). This also implies that the in vivo lumen pH is not acidic enough to inhibit Cyt b 6 f activity, consistent with previous in vivo measurements of the size of the H+ gradient in microalgae (pH gradient of about 1.5 units; Finazzi and Rappaport 1998).
Interestingly, a mutant of Arabidopsis, pgr1, has been shown to have a mutation in the Rieske protein that results in an altered pH sensitivity, with a pK of 6.5–7 (Jahns et al. 2002). This shift results in plants that are deficient in qE (the ∆pH-mediated ‘high-energy’ quenching of PSII), and the electron flux through Cyt b 6 f is inhibited at a pH that is too high to induce significant quenching. This is in principle consistent with the notion that the lumenal pH does not affect the turnover of the Cyt b 6 f complex in vivo, unless its pH sensitivity is modified. On the other hand, Joliot and Johnson (2011) demonstrated that partial uncoupling of the thylakoid membrane, by infiltrating leaves with nigericin, resulted in an inhibition of NPQ, as expected if the ∆pH is inhibited, but also in a net reduction of the electron transport chain. Overall, these observations can be rationalized assuming that the lumen pH is involved in regulating the activity of the Cyt b 6 f complex, although possibly via a different mechanism than a simple H+-mediated mass effect.
As an alternative explanation for the photosynthetic control, one can assume the existence of a pH dependent equilibrium between two kinetic states of the Cyt b 6 f complex (characterized by a fast or a slow electron transfer rate). Under the hypothesis that the equilibration rate between the two states is faster than the electron transfer reaction, the pH dependence of Cyt b 6 f activity can be mimicked in the frame of this very simple model, where only two kinetic states and a pK regulating their equilibration is conceived (Rappaport and Finazzi, unpublished data). The resolution of the structure of the Cyt b 6 f and of its respiratory counterpart (cytochrome bc 1) has provided a structural basis supporting this model. It has been shown that diversion of electrons into the high and low potential branches occurs via a conformational change at the level of the Rieske iron sulfur protein. In the related mitochondrial bc 1 cytochrome complexes, the rotational swing of the Rieske protein extra-membrane domain leads to a cluster movement over 20 Å, which prevents a double electron injection into the high potential chain (for a review see Berry et al. 2000). Evidence for this movement was provided by three-dimensional Cyt bc 1 structures (Xia et al. 1997; Iwata et al. 1998; Zhang et al. 1998), electron paramagnetic resonance spectroscopy (Brugna et al. 2000), and mutagenesis studies (for review, see Darrouzet et al. 2001). Several lines of evidence support the existence of a similar movement in the b 6 f complex. These are two-dimensional crystals (Breyton 2000a), viscosity studies (Heimann et al. 2000), electron transfer from the Rieske ISP to Cyt f in vitro (Soriano et al. 2002), inhibitor binding (Schoepp et al. 1999), mutagenesis studies (de Vitry et al. 2004), and three-dimensional Cyt b 6 f structures (Kurisu et al. 2003; Stroebel et al. 2003). Thus, one may speculate that the fast kinetics would be the one where the ISP and the PQH2 lie close together in the lumenal site, while the slow one would correspond to the ISP protein being close to the Cyt f moiety, thereby preventing proper interaction of PQH2 with its binding site. The pK involved in this regulation would mainly be the one of His126, which is involved in making H–bonds with the quinone.
As mentioned above, the main consequence of the Q cycle is the increase in the H+/e− stoichiometry. A corollary of this model is that, although increased, the stoichiometry of protons pumped into the lumen per electron is fixed, being set by the conformational changes in the lumenal site. This could be a limitation for photosynthesis where the H+ gradient is required not only for photoprotection, but obviously also for ATP synthesis. In oxygenic photosynthesis, carbon assimilation is mainly driven by LEF, which produces NADPH as well as ATP, although probably in a ratio not sufficient to support the formation of glyceraldehyde-3-phosphate, the exported product of the CBB cycle (see Allen 2003 for a discussion). On the other hand, other electron fluxes, e.g. to reduce nitrogen, have different ATP:NADPH requirements. Thus every means available to modulate the H+/e− ratio in a flexible way, depending on demand, would allow plants to better regulate carbon assimilation, other metabolism and photoprotective responses. This possibility was investigated in Chlamydomonas using a genetic approach.
Mutants with an engineered Glu78 residue in subunit IV of Cyt b 6 f were generated and their responses to pH changes were tested (Zito et al. 1998; Finazzi 2002). Spectroscopic analysis revealed that this residue not only controls PQH2 oxidation, as previously reported (Finazzi 2002), but also modulates the number of charges that are transferred through the Cyt b 6 f complex. This suggests that a transfer of protons from the stroma to the lumen during catalysis takes place in the complex itself, and that this is linked to PQH2 oxidation at the lumenal pocket; suggesting the existence of a H+ pumping activity in the complex (Finazzi 2002). This is in agreement with the finding of a relevant isotopic effect on the initial rate of charge transfer within the complex (Deniau and Rappaport 2000).
To account for this observation a model was proposed (Fig. 22.2), which reconciles the Q cycle with experimental observations showing that dicyclohexylcarbodiimide (DCCD), a chemical capable of modifying protonatable residues, induces a significant proton slip during catalysis (Brandt and Trumpower 1994). The model assumes that a channel exists between the lumenal and stromal sites for PQ redox reactions, providing an internal shuttle for protons (Finazzi 2002). This channel would be operational either in the stroma to lumen direction (proton pumping) or in the opposite way (proton slip) depending on the pH of the two compartments and on the redox state of the electron flow cofactors within Cyt b 6 f (i.e. the high and low potential b 6 hemes). The transition from proton slip to proton pumping, observed in the pH 5–6 region, i.e. in the range compatible with the pK of this residue was compatible with the observation that the number of charges transferred through the cytochrome complex during turnover becomes constant in mutants where the Glu78 residue has been changed to a neutral residue, suggesting that this glutamic acid residue would act as a gate, modulating the opening and closing of this channel depending on lumenal pH. Overall the activity of the redox-linked proton could represent a useful mean to help maintain pH homeostasis in the chloroplast lumen by modulating the H+/e− stoichiometry in vivo.
Proposed mechanism for the redox controlled H+ pump in the Cyt b6f complex. PQH2 binding at the lumenal sites occurs via H+ interaction with the H126 and E78 residues. This results in H+ and electron transfer to the Rieske protein (top). The Rieske protein is then oxidized by Cyt f (thick arrow) thanks to conformational changes of its head. In parallel, a movement of the protonated semiquinone allows the transfer of an electron to the b L heme. Oxidation of neutral semiquinone is proposed to take place via the E78 residue (1), which is connected to a proton channel PC1 (2). E78 would modulate the activity of a transmembrane H+ channel (PC2, (2)) gated by two protonatable residues (W and Z). Their protonation would be regulated by electrostatic interactions with the semiquinone (dashed line) and the b 6 hemes. The activity of this channel would affect the H+/e− stoichiometries of PQH2 oxidation, because of oscillation between proton pumping ((3) + (4) + (5)) and proton slip (6). (7) Protonation of the quinone at the stromal side. Dotted lines: proton transfer reactions. Solid lines electron transfer reactions (Modified from Finazzi 2002).
Although appealing, this hypothesis has not yet found a solid structural support. Indeed, no clear proton wire has been detected between the stromal and lumenal sides in the structure of Cyt b 6 f complexes, at variance with the H+ chain that is observed at the level of Cyt f, to channel proton release into the lumen (Cramer et al. 2011).
III. A Redox Kinetic Control of Cytochrome b 6 f?
As discussed above (Sect. II), the effective rate limiting step in the electron transport chain is generally thought to be the oxidation of PQH2 by Cyt b 6 f. However, the factor that limits overall photosynthesis will often be downstream of this, either the supply of CO2 for ribulose-5-phosphate reduction or the recycling of inorganic phosphate. For example, if a leaf is deprived of CO2, either naturally, due to drought induced stomatal closure, or artificially, by lowering the external CO2 supply, demand for both NADPH and ATP declines. In simple terms this should result in a reduction of the whole electron transport chain, through a simple blockage of electron flow. This is not, however, what is seen. If CO2 is abruptly removed, PSII acceptors, including the PQH2 pool, become reduced, but Cyt f and the high potential chain become more oxidized, the opposite of what might be expected (Golding and Johnson 2003; Golding et al. 2005). This observation clearly shows that flux through the high potential chain is regulated at the level of the Cyt b 6 f complex.
As already discussed, a simple effect of pH on the oxidation of PQH2 might explain this observation; however various observations suggest that more is occurring. Importantly, another major pH-dependent process, high-energy state quenching, differs both in terms of kinetics of induction and steady state levels from the down-regulation of electron transport. For example, a wide range of NPQ values can be observed with no change in the flux through Cyt b 6 f and, conversely, Cyt b 6 f can be downregulated without a corresponding increase in NPQ (Ott et al. 1999). This led Johnson (2003) to put forward a model for feedback regulation by the redox poise of the PSI acceptor pool. Initially it was suggested that this may be via thioredoxin; however, later evidence pointed to NADPH redox poise being the crucial factor (Hald et al. 2008). Mutants in which photosynthesis was inhibited by antisense repression of glyceraldehyde-3-phosphate dehydrogenase, which consumes NADPH, showed a strong downregulation of Cyt b 6 f activity and an oxidation of the high potential chain. Meanwhile, mutants repressed in FNR had a reduced electron transport chain. Both showed high levels of pH-dependent fluorescence quenching (a form of NPQ), indicating that the difference could not be explained by lumen pH alone.
Experiments such as these speak against a simple role for lumen pH controlling the flux of electrons through the Cyt b 6 f complex. A notable example would be the Arabidopsis pgr5 mutant, where the control of electron flux through Cyt b 6 f fails to operate (Munekage et al. 2002b; Nandha et al. 2007; Joliot and Johnson 2011). This mutant was first identified as a result of a screen for plants deficient in NPQ (Munekage et al. 2002a); however, in contrast to the pgr1 mutant (Jahns et al. 2002), identified in the same screen, pgr5 has a reduced electron transport chain across all conditions, implying that the PQH2 oxidation is not being down regulated. At low CO2 it does, however, generate NPQ, implying the formation of a large ΔpH (Nandha et al. 2007). This alone is not sufficient to cause an inhibition of PQH2 oxidation. In another study by Joliot and Johnson (2011) it was shown that infiltration of leaves with the ionophore nigericin, which dissipates ∆pH without affecting the potential gradient, did not inhibit PSII electron transport but did prevent both NPQ formation and the downregulation of Cyt b 6 f activity. Results such as these speak in favor of a role of pH in regulating electron flow, but not through a simple photosynthetic control mechanism. Rather, the pH may modulate the redox sensitivity, shifting the E m of redox sensitive components involved in regulation (Fig. 22.3). Johnson (2003) showed that inhibition of the Cyt b 6 f complex by dithiothreitol is pH sensitive, with a shift in E m of −90 mV/pH. Hence, changes in the pH in the thylakoid lumen may increase the sensitivity of the Cyt b 6 f complex to reducing agents, promoting at the same time redox regulation of electron flow, most likely exerted by NADPH.
Feedback control of Cyt b6f. Linear electron transport generates NADPH (black arrows). At the same time, there is a net movement of protons into the thylakoid lumen, generating a ∆pH, which is used to drive ATP synthesis (blue arrows). Under optimal conditions, NADPH and ATP are used in the Calvin-Benson-Basham (CBB) cycle. If the CBB cycle is inhibited, e.g. due to drought, NADPH feeds back to down-regulate the Cyt b 6 f complex, while the ∆pH, which is no longer being dissipated in ATP synthesis, inhibits electron flow either by directly inhibiting PQH2 oxidation or indirectly by modulating the sensitivity to NADPH. See text for further explanation.
IV. Linear Versus Cyclic Electron Flow
Among electron transport processes alternative to LEF, cyclic electron flow (CEF), which only involves PSI, has a major role in allowing plants and algae to control their ATP production via the regulation of the proton gradient, ∆pH. CEF in flowering plants has been the subject of a number of reviews in recent years (e.g. Shikanai 2007; Johnson 2011) and we do not intend here to review in detail the evidence for its occurrence. Rather the focus will be on the regulation of this pathway and the involvement of the Cyt b 6 f complex. The functioning of CEF in plants follows the same mechanism: electrons produced at the PSI acceptor side are oxidized in a way that results in the reduction of PQ, feeding electrons back into the electron transport chain between the two photosystems. That reaction may be catalyzed by either an NAD(P)H dehydrogenase (NDH) complex homologous to Complex I in mitochondrial electron transport (Rumeau et al. 2007; Shikanai 2007), or via an alternative reaction possibly involving Cyt b 6 f. The relative contribution of the two paths is still under debate. Plants deficient in the NDH complex appear to perform well across a wide range of conditions, though there is evidence that they have increased sensitivity to drought stress (Rumeau et al. 2007; Lehtimaki et al. 2010). It seems, however, that NDH is not essential for plant growth. On the other hand, a recently identified mutant with enhanced CEF activity in Arabidopsis shows higher accumulation levels of this complex (Livingston et al. 2010), and it was recently proposed that this complex could mediate an H2O2 driven activation of CEF (Strand et al. 2015).
In addition to the NDH pathway, electron transfer can take place via a pathway often referred to as the FQR (ferredoxin quinone reductase) pathway (Bendall and Manasse 1995). In early studies, the FQR pathway was proposed to involve the Cyt b 6 f complex (Tagawa et al. 1963). However, later studies hypothesized a distinct enzyme for FQR that bypasses Cyt b 6 f and directly interfaces with PQ pool reduction by Fd (Bendall and Manasse 1995). No one, however, was able to identify or purify the FQR specific enzyme until recently where evidence was presented for FQR being a complex of PGR5-PGRL1 (Hertle et al. 2013).
The physical association of the Cyt b 6 f complex with PSI and FNR has also been suggested as a platform for the electron transfer in the FQR pathway (Carrillo and Vallejos 1983; Arnon 1995). Mathematical modeling of the electron transfer (Laisk 1993) and in vivo observation of its high efficiency (Joliot and Joliot 2002) suggest that FQR activity might operate in a complex involving Cyt b 6 f and PSI. Indeed, Cramer and his co-workers reported that the Cyt b 6 f complex co-purified with FNR and was reduced by Fd (Zhang et al. 2001). Physical interactions between PGRL1 and PsaD (subunit of PSI), PetB (Cyt b 6), FNR, and PGR5 were shown by a yeast two-hybrid assay using the corresponding genes from Arabidopsis (DalCorso et al. 2008). This suggests that the formation of a CEF competent supercomplex is likely required to stabilize this pathway in a system where it could otherwise be outcompeted by linear flow.
While no such supercomplex was found in spinach leaves (Breyton et al. 2006) and indeed it has been argued that no such complexes are required (Johnson 2011; Joliot and Johnson 2011), biochemical analysis of thylakoid membranes from Chlamydomonas allowed Minagawa and colleagues (Iwai et al. 2010) to isolate such a complex from State 2-locked cells, where CEF is the prominent electron flow pathway. Using a sucrose density gradient, they purified a super-supercomplex composed of the PSI-LHCI supercomplex with LHCIIs, Cyt b 6 f, FNR, and PGRL1 in a fraction heavier than that of the PSI-LHCI supercomplex alone. Spectroscopic analyses of this super-supercomplex indicated that, upon illumination, reducing equivalents downstream of PSI were transferred to Cyt b 6 f, while the oxidized PSI was re-reduced by reducing equivalents from Cyt b 6 f (Iwai et al. 2010; see Chap. 23 by Minagawa for a further description of this CEF supercomplex).
What is the role of this supercomplex, and why is it observed only in Chlamydomonas? To answer these questions one has to consider that a key point in our understanding of CEF is the question of how the relative fluxes through LEF and CEF are regulated. It is acknowledged that photosynthetic complexes are not evenly distributed in the thylakoid membrane (Albertsson 2001). Functional PSII is localized in the membrane stacks. PSI is distributed between the grana margins (∼70 %) and the stromal lamellae. Cyt b 6 f is evenly distributed throughout the membrane. It has been shown that the diffusion of PQ/PQH2 in the thylakoid membrane is highly restricted, probably due to the very high protein concentrations that characterize this membrane (Tremmel et al. 2003). This means that PQ localized in the granal stacks will primarily act in mediating electron transport between PSII and those Cyt b 6 f complexes localized in the stacked regions. For reasons of access, it is unlikely that electrons will be transferred from Fd to Cyt b 6 f complexes localized in the granal regions. Rather, a separate pool of Cyt b 6 f, with associated PQ will be found in the stromal membranes. The physical distance between the different domains and the limitations on PQ diffusion mean that these different pools could be quite separate, thus promoting independent functioning of the photosynthetic complexes present in the grana and grana margins (LEF) and the stromal lamellae (CEF). On the other hand, thylakoid stacking is less defined in Chlamydomonas and algae in general, and therefore the functional segregation of LEF and CEF based on the physical segregation of the photosynthetic complexes in two distinct domains hardly applies to these algae. It is therefore possible that the super-supercomplex, which localize the mobile electron carriers (PQ, Fd, and PC) within a physically restricted space would ensure the compartmentation of the two pathways, thereby preventing CEF from being outcompeted by LEF activity. By doing this, super-supercomplex formation would play a similar role to that suggested for the segregation of PSI and PSII in the granal and stromal lamellae in plants.
V. Role of Cytochrome b 6 f in State Transitions
In oxygenic photosynthesis, the two photosystems working in series have distinct pigment containing antenna complexes with distinct light absorbance properties. PS I has an enriched absorption capacity in the far-red region, whereas PS II lacks the far-red absorption capacity, but has a higher efficiency in the spectral regions where chlorophyll b absorbs (i.e. around 475 and 650 nm). Thus, in natural environments, where light quality and quantity fluctuate with time (Allen 1992; Bellafiore et al. 2005) unbalanced absorption can take place. This effect can be minimized by a phenomenon known as state transitions. Discovered independently by Murata and Sugahara (1969) and Bonaventura and Myers (1969), this process relies on a redox-triggered phosphorylation of the PSII antenna complexes (LHCII), which leads to a physiological displacement of LHCII from PSII to PSI under conditions where absorption of the former is enhanced (i.e. by enhanced light absorption by chlorophyll b). This state is called State 2. Conversely, over-excitation of PSI (e.g. by far red light) leads to the reassociation of LHCII with PSII (State 1; Allen 1992).
Pharmacological and genetic work has shown that state transitions are triggered by the PQ pool, which acts as a redox regulator of the phosphorylation of LHCII (Wollman 2001), via the activation of an LHCII kinase. However, reports exist claiming that LHCII kinase(s) is/are not only activated by the reduced PQ pool, but also deactivated by the reduced thioredoxin pool that is downstream of PS I in the stroma of the chloroplasts in pumpkin (Rintamaki et al. 2000) and pea (Hou et al. 2002), and in C. reinhardtii (Vink et al. 2004). Why is the PQ pool responsible for such a redox signaling role? Being functionally located between PSII and PSI this redox active molecule can “sense” the relative light harvesting capacity of the two photosystems, being largely reduced when PSII activity overcomes that of PSI, and becoming oxidized when PSI prevails.
The nature of the kinase responsible for LHCII phosphorylation has been first identified in Chlamydomonas thanks to a genetic screening: it is a Ser/Thr kinase (Stt7) present in the chloroplast thylakoid membranes (Depege et al. 2003). Later, it was shown that an ortholog exist in plants (STN7; Bellafiore et al. 2005). The reversible phosphorylation of LHCII observed with thylakoids during state transitions implies that a phosphatase (TAP38/PPH1) is also active, being responsible for the dephosphorylationn of LHCII (Pribil et al. 2010; Shapiguzov et al. 2010).
Signal transduction from PQH2 to the Stt7/STN7 kinase is performed by the Cyt b 6 f complex, as shown by several independent pieces of evidence. Wollman and Lemaire (1988) found that C. reinhardtii mutants lacking Cyt b 6 f were unable to undergo a State 2 transition, indicating an essential role of this complex in the kinase activation. Later, spectroscopic (Vener et al. 1997) and genetic approaches (Zito et al. 1999) revealed that the committed step in kinase activation is the binding of PQH2 to the lumenal quinone-binding pocket (Qo site) of Cyt b 6 f. While a consensus exists on the nature of the partners involved in the signaling from light to the kinase, the mechanism leading to the activation of Stt7/STN7 by reduced PQ is still under debate.
Based on a pharmacological analysis of kinase activation (Finazzi et al. 2001) a model has been built for this activation (Finazzi 2005), which involves several steps (Fig. 22.4). The signal from PQH2 would be transduced by an “active” Cyt b 6 f complex, capable of switching between a docking conformation, which activates the kinase, and a releasing one, where the active kinase becomes capable of interacting with LHCII. Docking would involve conformational changes at the level of the Rieske protein and results in the phosphorylation of the Cyt b 6 f-associated subunit PetO (Hamel et al. 2000; Finazzi et al. 2001; Wollman 2001). This step would precede phosphorylaytion of LHCII, which likely requires the release of the Stt7 kinase by a subsequent conformational change. This is suggested by the observation that while the PQ analog tridecylstigmatellin blocks Cyt b 6 f catalysis and LHCII phosphorylation by preventing the movement of the Rieske protein head (Zhang et al. 1998; Breyton 2000b; Finazzi et al. 2001), this chemical does not affect the phosphorylation of PetO (Wollman 2001).
Hypothetical mechanism of Stt7 activation by the cytochrome b6f complex. The model conceives that upon binding of the PQH2 molecule (red dot) to the lumenal Qo site of Cyt b 6 f, conformational changes in the lumenal part of the Rieske protein (blue) would take place (transition form “a” to “b”). This would allow docking of the kinase (spheres and sticks) on the “lipid exposed” part of the complex, in close vicinity to the chlorophyll ring (c), leading to kinase activation (represented as boxes and sticks) and phosphorylation of the Cyt b 6 f associated subunit PetO (black loops). Release of the active kinase after dissociation of the quinone from its binding pocket (d) would trigger LHCII trimer-kinase interactions, as required for antenna complex phosphorylation. The site of PQH2 and kinase docking on Cyt b 6 f, as well as the site of inhibition by stigmatellin are shown.
How are the conformational changes occurring in the lumenal region of the complex transduced to the stromal site where the active moiety of the kinase is located? Analysis of the Stt7 kinase has suggested that this protein contains a putative transmembrane helix. This helix might, therefore, be directly involved in sensing PQH2 binding to the Qo site, as already suggested (Vener et al. 1997). However, the existence of a direct signaling within the cytochrome complex is also possible, and supported by phenotypic analysis of Cyt b 6 f mutants of Chlamydomonas. In particular, a mutant was generated (Zito et al. 2002) to fuse a small Cyt b 6 f subunit (PetL) with subunit IV to “transform” the b 6 f complex, which contains seven helices in its core subunits (Cyt b 6 and subunit IV), into a bc 1-type complex (which contains eight helices in its unique core complex subunit, Cyt b). At variance with expectations, this mutation did not lead to an altered electron flow, or PQH2 binding to the lumenal Qo site of the modified b 6 f complex, but led to a complete abolition of state transitions.
Based on these results, it was proposed that docking of the kinase, which follows the structural changes induced by the PQH2-Cyt b 6 f interaction, might take place on the cytochrome portion located in the proximity of the C terminus of subunit IV (where the PetL subunit was fused). This being the case, the mutant phenotype could be explained assuming that because of the presence of an additional helix close to its docking site, the formation of the kinase-Cyt b 6 f complex was prevented, even if the cytochrome complex was in its “active” state. Interestingly, the 3D structure of Cyt b 6 f from Chlamydomonas, (Stroebel et al. 2003), has revealed the presence of a chlorophyll molecule, which is exposed to the lipid phase through its tetrapyrrole ring, being sandwiched between helices F and G of subunit IV (see also Chap. 9 by Cramer and Hasan). Its phytol chain goes deeply into the complex structure ending up in the lumenal, PQH2 binding pocket. Thus it is conceivable that the chlorophyll could “sense” the binding of PQH2 to the Cyt b 6 f complex via the phytol chain and transduce this information to the kinase docking site, via the chlorophyll ring (Finazzi 2005).
The possible involvement of the chlorophyll ring in kinase activation was later confirmed by the analysis of mutants affecting the environment of the chlorophyll ring in the complex, again in Chlamydomonas (de Lavalette et al. 2008). This study showed that in one mutant strain where the chlorine ring was likely displaced from its original site because of steric hindrance, state transitions were largely compromised, although PQH2 binding to the Qo site was not significantly affected. Based on these results, a scenario can be proposed to account for the mechanism of Stt7 activation by the Cyt b 6 f complex (Fig. 22.4). However, further work will be needed to further confirm this model at the experimental level.
VI. Conclusions
The role of the cytochrome b 6 f complex in electron flow is a topic that has been investigated for many years combining functional and genetic approaches in both plants and microalgae. The resolution of the structure of this complex has led to major advances in this field, bridging the functional mechanism to the precise structural changes within the complex. Biochemical analyses have since then been instrumental in defining a further level of regulation, which involves a different interaction of the cytochrome with other photosynthetic complexes, as exemplified by the isolation of the PSI-Cyt b 6 f supercomplex from Chlamydomonas. However, despite these major achievements, big questions are still open concerning both the intimate electron transfer mechanisms (the proton electron stoichiometry during catalysis, the obligatory occurrence of the Q cycle during turnover, proton versus redox regulation of the activity, and the possible existence of a signal transduction pathway within the complex allowing the activation of the kinase responsible for state transitions by reduced plastoquinone molecules). Major multidisciplinary efforts will be needed in the future to cast light on these fundamental aspects of bioenergetics.
Abbreviations
- CBB:
-
Calvin Benson Bassham cycle
- CEF:
-
Cyclic electron flow
- Cyt:
-
b 6 f the cytochrome b 6 f complex
- Cyt:
-
Cytochrome
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin-NADP+ oxidoreductase
- FQR:
-
Ferredoxin quinone reductase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- LEF:
-
Linear electron flow
- NDH:
-
NAD(P)H dehydrogenase
- NPQ:
-
Non Photochemical Quenching
- P700 :
-
Primary electron donor to PSI
- PC:
-
Plastocyanin
- PQ:
-
Plastoquinone
- PQH2 :
-
Plastoquinol
- PS:
-
Photosystem
- ROS:
-
Reactive oxygen species
References
Albertsson P-A (2001) A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci 6:349–354
Allen JF (1992) Protein-phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Allen JF (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8:15–19
Arnon DI (1995) Divergent pathways of photosynthetic electron transfer: the autonomous oxygenic and anoxygenic photosystems. Photosynth Res 46:47–71
Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433:892–895
Bendall DS, Manasse RS (1995) Cyclic photophosphorylation and electron-transport. Biochim Biophys Acta 1229:23–38
Berry EA, Guergova-Kuras M, Huang LS, Crofts AR (2000) Structure and function of cytochrome bc complexes. Annu Rev Biochem 69:1005–1075
Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189:366–383
Brandt U, Trumpower B (1994) The protonmotive Q-cycle in mitochondria and bacteria. Crit Rev Biochem Mol Biol 29:165–197
Breyton C (2000a) The cytochrome b(6)f complex: structural studies and comparison with the bc(1) complex. Biochim Biophys Acta 1459:467–474
Breyton C (2000b) Conformational changes in the cytochrome b6f complex induced by inhibitor binding. J Biol Chem 275:13195–13201
Breyton C, Nandha B, Johnson GN, Joliot P, Finazzi G (2006) Redox modulation of cyclic electron flow around photosystem I in C3 plants. Biochemistry 45:13465–13475
Brugna M, Rodgers S, Schricker A, Montoya G, Kazmeier M, Nitschke W, Sinning I (2000) A spectroscopic method for observing the domain movement of the Rieske iron-sulfur protein. Proc Natl Acad Sci USA 97:2069–2074
Carrillo N, Vallejos RH (1983) The light-dependent modulation of photosynthetic electron-transport. Trends Biochem Sci 8:52–56
Cramer WA, Hasan SS, Yamashita E (2011) The Q cycle of cytochrome bc complexes: a structure perspective. Biochim Biophys Acta 1807:788–802
Crofts AR, Meinahrdt SW, Jones KR, Snozzi M (1983) The role of the quinone pool in the cyclic electron-transfer chain of Rhodopseudomonas spaeroides. A modified Q-cycle mechanism. Biochim Biophys Acta 723:202–218
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Nemann DS, Finazzi G, Joliot P, …, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285
Darrouzet E, Moser CC, Dutton PL, Daldal F (2001) Large scale domain movement in cytochrome bc(1): a new device for electron transfer in proteins. Trends Biochem Sci 26:445–451
de Lavalette AL, Finazzi G, Zito G (2008) b(6)f-Associated chlorophyll: structural and dynamic contribution to the different cytochrome functions. Biochemistry 47:5259–5265
de Vitry C, Ouyang YX, Finazzi G, Wollman FA, Kallas T (2004) The chloroplast Rieske iron-sulfur protein – at the crossroad of electron transport and signal transduction. J Biol Chem 279:44621–44627
Deniau C, Rappaport F (2000) New insights on the proton pump associated with cytochrome b(6)f turnovers from the study of H/D substitution effects on the electrogenicity and electron transfer reactions. Biochemistry 39:3304–3310
Depege N, Bellafiore S, Rochaix JD (2003) Rote of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299:1572–1575
Eberhard S, Finazzi G, Wollman F-A (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515
Finazzi G (2002) Redox-coupled proton pumping activity in cytochrome b(6)f, as evidenced by the pH dependence of electron transfer in whole cells of Chlamydomonas reinhardtii. Biochemistry 41:7475–7482
Finazzi G (2005) The central role of the green alga Chlamydomonas reinhardtii in revealing the mechanism of state transitions. J Exp Bot 56:383–388
Finazzi G, Rappaport F (1998) In vivo characterization of the electrochemical proton gradient generated in darkness in green algae and its kinetic effects on cytochrome b(6)f turnover. Biochemistry 37:9999–10005
Finazzi G, Zito F, Barbagallo RP, Wollman FA (2001) Contrasted effects of inhibitors of cytochrome b(6)f complex on state transitions in Chlamydomonas reinhardtii – the role of Q(o) site occupancy in LHCII kinase activation. J Biol Chem 276:9770–9774
Golding AJ, Johnson GN (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218:107–114
Golding AJ, Joliot P, Johnson GN (2005) Equilibration between cytochrome f and P700 in intact leaves. Biochim Biophys Acta Bioenerg 1706:105–109
Gould SB, Waller RR, McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59:491–517
Hald S, Nandha B, Gallois P, Johnson GN (2008) Feedback regulation of photosynthetic electron transport by NADP(H) redox poise. Biochim Biophys Acta 1777:433–440
Hamel P, Olive J, Pierre Y, Wollman FA, de Vitry C (2000) A new subunit of cytochrome b(6)f complex undergoes reversible phosphorylation upon state transition. J Biol Chem 275:17072–17079
Harbinson J, Hedley CL (1989) The kinetics of P-700+ reduction in leaves – a novel in situ probe of thylakoid functioning. Plant Cell Environ 12:357–369
Hasan SS, Yamashita E, Baniulis D, Cramer WA (2013) Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b(6)f complex. Proc Natl Acad Sci USA 110:4297–4302
Heimann S, Ponamarev MV, Cramer WA (2000) Movement of the Rieske iron-sulfur protein in the p-slide bulk aqueous phase: effect of lumenal viscosity on redox reactions of the cytochrome b(6)f complex. Biochemistry 39:2692–2699
Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49:511–523
Hou CX, Pursiheimo S, Rintamaki E, Aro EM (2002) Environmental and metabolic control of LHCII protein phosphorylation: revealing the mechanisms for dual regulation of the LHCII kinase. Plant Cell Environ 25:1515–1525
Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464:1210–U1134
Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, …, Jap BK (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc(1) complex. Science 281:64–71
Jahns P, Graf M, Munekage Y, Shikanai T (2002) Single point mutation in the Rieske iron-sulfur subunit of cytochrome b(6)/f leads to an altered pH dependence of plastoquinol oxidation in Arabidopsis. FEBS Lett 519:99–102
Johnson GN (2003) Thiol regulation of the thylakoid electron transport chain – a missing link in the regulation of photosynthesis? Biochemistry 42:3040–3044
Johnson GN (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807:384–389
Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108:13317–13322
Joliot P, Joliot A (2002) Cyclic electron transport in plant leaf. Proc Natl Acad Sci USA 99:10209–10214
Kramer DM, Avenson TJ, Edwards G (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9:339–348
Kurisu G, Zhang HM, Smith JL, Cramer WA (2003) Structure of the cytochrome b(6)f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Laisk A (1993) Mathematical modelling of free-pool and channelled electron transport in photosynthesis: evidence for a functional supercomplex around photosystem 1. Proc R Soc Lond Ser B Biol Sci 251:243–251
Lehtimaki N, Lintala M, Allahverdiyeva Y, Aro EM, Mulo P (2010) Drought stress-induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer. J Plant Physiol 167:1018–1022
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Livingston AK, Cruz JA, Kohzuma K, Dhingra A, Kramer DM (2010) An Arabidopsis mutant with high cyclic electron flow around photosystem I (hcef) involving the NADPH dehydrogenase complex. Plant Cell 22:221–233
Malnoe A, Wollman F-A, de Vitry C, Rappaport F (2011) Photosynthetic growth despite a broken Q-cycle. Nat Commun 2:301
Mitchell P (1975) The protonmotive Q cycle: a general formulation. FEBS Lett 59:137–199
Munekage Y, Hojo M, Endo T, Shikanai T (2002a) Arabidopsis pgr5 is defective in cyclic electron flow around photosystem I. Plant Cell Physiol 43:S23–S23
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002b) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371
Murata N, Sugahara K (1969) Control of excitation transfer in photosynthesis. III. Light-induced decrease of chlorophyll a fluorescence related to photophosphorylation system in spinach chloroplasts. Biochim Biophys Acta 189:182–189
Nandha B, Finazzi G, Joliot P, Hald S, Johnson GN (2007) The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim Biophys Acta 1762:1252–1259
Nitschke W, Joliot P, Liebl U, Rutherford AW, Hauska G, Muller A, Riedel A (1992) The ph-dependence of the redox midpoint potential of the 2Fe2S cluster from cytochrome-B6f complex (the Rieske center). Biochim Biophys Acta 1102:266–268
Ott T, Clarke J, Birks K, Johnson G (1999) Regulation of the photosynthetic electron transport chain. Planta 209:250–258
Pierre Y, Breyton C, Kramer D, Popot JL (1995) Purification and characterization of the cytochrome B(6)F complex from Chlamydomonas-reinhardtii. J Biol Chem 270:29342–29349
Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D (2010) Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol 8:e1000288
Rintamaki E, Martinsuo P, Pursiheimo SAro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97:11644–11649
Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30:1041–1051
Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM (2000) The proton to electron stoichiometry of steady-state photosynthesis in living plants: a proton -pumping Q cycle is continuously engaged. Proc Natl Acad Sci USA 97:14283–14288
Schoepp B, Brugna M, Riedel A, Nitschke W, Kramer DM (1999) The Q(o)-site inhibitor DBMIB favours the proximal position of the chloroplast Rieske protein and induces a pK-shift of the redox-linked proton. FEBS Lett 450:245–250
Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix J-D, Vener AV, Goldschmidt-Clermont M (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci USA 107:4782–4787
Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58:199
Soriano GM, Ponamarev MV, Carrell CJ, Xia D, Smith JL, Cramer WA (1999) Comparison of the cytochrome bc(1) complex with the anticipated structure of the cytochrome b(6)f complex: De plus ca change de plus c’est la meme chose. J Bioenerg Biomembr 31:201–213
Soriano GM, Guo L-W, de Vitry C, Kallas T, Cramer WA (2002) Electron transfer from the Rieske Iron-Sulfur Protein (ISP) to cytochrome f in vitro: is a guided trajectory of the ISP necessary for competent docking? J Biol Chem 277:41865–41871
Strand DD, Livingston AK, Satoh-Cruz M, Froehlich JE, Maurino VG, Kramer DM (2015) Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci 112:5539–5544
Stroebel D, Choquet Y, Popot JL, Picot D (2003) An atypical haem in the cytochrome b(6)f complex. Nature 426:413–418
Tagawa K, Tsujimoto HY, Arnon DI (1963) Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci USA 49:567–572
Tremmel IG, Kirchhoff H, Weis E, Farquhar GD (2003) Dependence of plastoquinol diffusion on the shape, size, and density of integral thylakoid proteins. Biochim Biophys Acta 1607:97–109
Vener AV, VanKan PJM, Rich PR, Ohad I, Andersson B (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc Natl Acad Sci USA 94:1585–1590
Vink M, Zer H, Alumot N, Gaathon A, Niyogi K, Herrmann RG, Andersson B, Ohad I (2004) Light-modulated exposure of the light-harvesting complex II (LHCII) to protein kinase(s) and state transition in Chlamydomonas reinhardtii xanthophyll mutants. Biochemistry 43:7824–7833
West KR, Wiskich JT (1968) Photosynthetic control by isolated pea chloroplasts. Biochem J 109:527–532
Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20:3623–3630
Wollman FA, Lemaire C (1988) Studies on kinase-controlled state transitions in photosystem II and b6f mutants from Chlamydomonas reinhardtii which lack quinone-binding proteins. Biochim Biophys Acta 933:85–94
Xia D, Yu CA, Kim H, Xian JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J (1997) Crystal structure of the cytochrome bc(1) complex from bovine heart mitochondria (vol 277, pg 60, 1997). Science 278:2037–2037
Zhang ZL, Huang LS, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, …, Kim SH (1998) Electron transfer by domain movement in stockbroker bc(1). Nature 392:677–684
Zhang HM, Whitelegge JP, Cramer WA (2001) Ferredoxin: NADP(+) oxidoreductase is a subunit of the chloroplast cytochrome b(6)f complex. J Biol Chem 276:38159–38165
Zito F, Finazzi G, Joliot P, Wollman FA (1998) Glu78, from the conserved PEWY sequence of subunit IV, has a key function in cytochrome b(6)f turnover. Biochemistry 37:10395–10403
Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA (1999) The Qo site of cytochrome b(6)f complexes controls the activation of the LHCII kinase. EMBO J 18:2961–2969
Zito F, Vinh J, Popot JL, Finazzi G (2002) Chimeric fusions of subunit IV and PetL in the b6f complex of Chlamydomonas reinhardtii – structural implications and consequences on state transitions. J Biol Chem 277:12446–12455
Acknowledgments
We would like to thank Dr. Cecile Breyton for her help in preparing Fig. 22.1. GF acknowledges the Marie Curie Initial Training Network Accliphot [FP7-PEPOPLE-2012-ITN, grant agreement number 316427], the French National Agency “DiaDomOil” [ANR-NT09_567009], a grant from the region Rhone Alpes and the Centre National de la Recherche Scientifique program “Defi PF-ENRS13-1 Milli_Oil”. JM was supported by JSPS and NEDO through KAKENHI (26251033) and a project for the strategic development of next-generation bioenergy utilization technology (P07015). GNJ acknowledges support from the UK Biotechnology and Biological Sciences Research Council (BB/C508877/1) and the Royal Society (ref: 24718)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Finazzi, G., Minagawa, J., Johnson, G.N. (2016). The Cytochrome b 6 f Complex: A Regulatory Hub Controlling Electron Flow and the Dynamics of Photosynthesis?. In: Cramer, W., Kallas, T. (eds) Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling. Advances in Photosynthesis and Respiration, vol 41. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7481-9_22
Download citation
DOI: https://doi.org/10.1007/978-94-017-7481-9_22
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7479-6
Online ISBN: 978-94-017-7481-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)