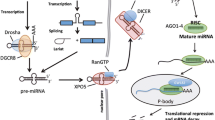
Abstract
Prostate cancer is a heterogeneous disease for which the molecular mechanisms are still not fully elucidated. Prostate cancer research has traditionally focused on genomic and epigenetic alterations affecting the proteome, but over the last decade non-coding RNAs, especially microRNAs, have been recognized to play a key role in prostate cancer progression. A considerable number of individual microRNAs have been found to be deregulated in prostate cancer and their biological significance elucidated in functional studies. This review will delineate the current advances regarding the involvement of microRNAs and their targets in prostate cancer biology as well as their potential usage in the clinical management of the disease. The main focus will be on microRNAs contributing to initiation and progression of prostate cancer, including androgen signalling, cellular plasticity, stem cells biology and metastatic processes. To conclude, implications on potential future microRNA-based therapeutics based on the recent advances regarding the interplay between microRNAs and their targets are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Annually, close to 900,000 new prostate cancer cases are diagnosed worldwide making it the second most frequently diagnosed cancer in men, mainly affecting elderly men. Even though the incident is much higher in developed regions , the mortality is similar in developed and developing regions, and constitutes the sixth leading cause of cancer related deaths in men (Ferlay et al. 2010). Prostate cancer is a multifocal and heterogeneous disease, making characterisation challenging (Arora et al. 2004). An in depth examination of the small non-coding RNA (ncRNA) content in prostatic tissues using next generation sequencing identified microRNA (miRNA), small nucleolar RNA (snoRNA), small nuclear RNA (snRNA), ribosomal RNA (rRNA), transfer RNA (tRNA) and fragments of large ncRNA (Martens-Uzunova et al. 2012). The most abundant class of ncRNA detected in the prostate was miRNAs, constituting 95 % of the RNA pool. The first systematic profiling of miRNAs in prostate cancer was presented by Porkka et al. in 2007, and since then several screening studies, and a multitude of individual expression analyses, have been published using different methodology (Volinia et al. 2006; Ambs et al. 2008; Ozen et al. 2008; Lu et al. 2005; Prueitt et al. 2008; Mattie et al. 2006; Tong et al. 2009; Martens-Uzunova et al. 2012; Szczyrba et al. 2010; Wach et al. 2012; Schubert et al. 2013; Porkka et al. 2007). The collective effort towards elucidating the function and mode of action of miRNAs in prostate cancer is increasing, and the impact of individual miRNAs on prostate cancer initiation and progression has been studied from different biological aspects ( summarised in Table 8.1). The consensus is that ncRNAs may represent novel therapeutic targets and biomarkers for prostate cancer, but more basic research and clinical studies are needed. In this review, the main biological processes reported to be regulated by miRNAs will be outlined. This includes regulation of androgen signalling and the androgen receptor (AR), the transition to castration resistant prostate cancer (CRPC), cellular plasticity, stem cells and metastases. The best characterised miRNAs regulating these processes in the prostate, or miRNAs suggested to have therapeutic potential, will be presented together with ncRNAs used for diagnostic purposes.
8.2 Androgen Receptor
Androgen signalling through the AR is vital for the development and maintenance of the prostate, as well as governing the initiation and progression of prostate cancer . It has been shown that miRNAs are mediators of androgen action in the prostate and the existence of feedback loops between miRNAs, AR, and AR co-repressors, has been suggested (Narayanan et al. 2010). The AR seem to be able to act through different mechanisms in addition to the AR being recruited to the promoter of target genes, there also seem to exist a three-step pathway including miRNA activation, co-repressor suppression and DNA interaction. In LNCaP cells treated with siRNA against Dicer the induction of androgen-regulated prostate specific antigen (PSA) upon androgen stimulation was abolished, and tissue-specific knockout of Dicer in mouse models significantly reduced the activity of AR resulting in androgen insensitivity syndrome (Narayanan et al. 2010). The AR interaction with Dicer seems to be dependent on the conformational change brought upon by ligand interaction, indicating that the interaction between miRNAs and AR is signal specific. So far only a relatively modest number of miRNAs has been found to be regulated by androgens, compared to the large number of androgen-regulated mRNAs (Jalava et al. 2012; Hagman et al. 2013b; Ostling et al. 2011). In contrast, the regulation of AR seems to be complex. In a functional miRNA library screen using reverse phase protein array 71 unique miRNAs was found to affect AR levels in human prostate cancer cells (Ostling et al. 2011). Fifteen miRNAs down regulating AR, including miR-34a and miR-34c , was further confirmed to decrease androgen-induced proliferation. There are also several examples of indirect regulation of the AR activity e.g. the androgen-regulated miR-27a regulates the AR co-repressor prohibitin, and miR-21 and AR are involved in a positive feedback loop possibly through the phosphatase and tensin homolog (PTEN) (Fletcher et al. 2012; Mishra et al. 2014). Together this corroborates that miRNAs are important regulators of the AR and androgen signalling in normal prostate development, as well as in prostate cancer progression (summarized in Fig. 8.1).
8.2.1 miR-34 Family
The miR-34 family are known as master regulators of tumour suppression. The family is comprised of mir-34a located at chromosome 1p36, and mir-34b and -c clustered at chromosome 11q23. In prostate cancer, all miR-34 family members have been shown to be down regulated, and the expression of miR-34c correlates with the tumour grade, occurrence of metastases, and overall survival (Hagman et al. 2010; Kong et al. 2012). This down regulation has been linked to methylation of the CpG islands in the promoter of mir-34a and mir-34b/c, loss of heterozygosity, and also direct regulation by the cellular tumour antigen p53 in response to DNA damage, hypoxia, and oncogenic stress, or by an alternative ATM-dependent pathway involving the p38-MAPK/MK2 pathway (Cannell et al. 2010; Toyota et al. 2008; Dahiya et al. 1997; Corney et al. 2007). Reconstituted levels of miR-34 can induce changes in proliferation, apoptosis, EMT and migration, and invasiveness of prostate cancer cells in vitro (Fig. 8.2). The family members have overlapping, but not identical targets. In prostate cancer cells, miR-34a has been shown to regulate AR, CD44, and NOTCH (Liu et al. 2011a; Ostling et al. 2011; Kashat et al. 2012), miR-34b regulates RAC-alpha serine/threonine-protein kinase (AKT) and the proto-oncogene protein MYC (Majid et al. 2013; Benassi et al. 2012), and miR-34c regulates AR, the apoptosis regulator BCL2, transcription factor E2F3, hepatocyte growth factor receptor cMET, and MYC (Hagman et al. 2010, 2013a; Ostling et al. 2011; Benassi et al. 2012). The miR-34 family has also been shown to be involved in epithelial-to-mesenchymal transition (EMT), caught up in a double negative feedback loop with Zinc finger protein SNAI1, and a similar feedback loop with the target ZEB1 (Siemens et al. 2011).
It has also been suggested that miR-34 acts as a barrier for somatic cell transition to stem/progenitor cells. Knockout mice of mir-34a-c show increased number of induced pluripotent stem cells and reprogramming efficiency without compromising self-renewal (Choi et al. 2011). In contrast, miR-34a also has been reported to decreased self-renewal capacity of prostate cancer cells (Kashat et al. 2012). It is possible the miR-34 family members individually have different functions that are modulated when the whole family is altered together. One hypothesis is that the expression of miR-34 family members is one of the mechanisms that keep the normal prostatic stem cells in control, but when the levels decrease upon prostate cancer initiation, the stem cell population is activated. Reintroduction of miR-34a or -b has been shown to significantly decrease androgen-independent prostate xenograft tumour growth in nude mice (Majid et al. 2013; Yamamura et al. 2012). In addition, miR-34a has been shown to reduce prostate cancer metastasis and increase the lifespan of xenografted mice (Liu et al. 2011a). Surprisingly, mir-34a-c knockout mice do not show increased effect on induced or spontaneous tumourigenesis (Concepcion et al. 2012). It is possible that this is due to the entire family being altered or that their function is compensated by other systems activated by the feedback loops the family is involved in .
8.3 CRPC
While confined to the prostate gland, the cancer is curable by either prostatectomy or radiation therapy . As the tumour progresses, it develops the abilities to invade surrounding tissue, induce angiogenesis, and metastasize. Androgen deprivation therapy, either chemical or surgical castration, is the gold standard treatment for advanced prostate cancer. Androgen depletion induces apoptosis of prostate cancer cells resulting in tumour regression, but this is followed by subsequent progression to castration resistance. To survive and resume growth in an androgen depleted surrounding, the cells must either adapt the AR pathway or induce alternative survival and growth pathways. Mechanisms underlying adaptation of the AR can be increased expression of AR, increased local production of androgens, hypersensitivity or constitutively active, truncated forms of the AR, promiscuity and/or ligand independent activation through kinase cross-talk. To bypass the AR pathway the epithelial cells might also be able to switch to autocrine production of growth factors such as epidermal growth factor (EGF), insulin-like growth factor (IGF1), hepatocyte growth factor (HGF), keratinocyte growth factor (KGF) or interleukin 6 (IL-6) (Jenster 1999). It has also been reported that individual miRNAs can promote androgen independent growth e.g. miR-21 (Ribas et al. 2009).
8.3.1 miR-21
The miRNA mir-21 is an oncomiR located at 17q23.1, which is amplified in prostate cancer (Kasahara et al. 2002). The expression of miR-21 is induced by androgens through binding of the AR complex to its promoter region and is highly upregulated in CRPC (Jalava et al. 2012; Ribas et al. 2009). It has been reported that miR-21 promotes androgen resistance, but it is active in both androgen-dependent and -independent prostate cancer, and has been shown to stimulate prostate cancer xenograft growth in mouse models in both a ligand-dependent and -independent manner (Ribas et al. 2009). However, others report that ectopic expression of miR-21 only has a limited effect on cellular proliferation and invasiveness of prostate cancer cells, and contrary to in other cancer settings, miR-21 does not regulate the tumour suppressors PTEN and programmed cell death protein 4 (PDCD4) in prostate cancer cells (Folini et al. 2010). Nevertheless, it has been suggested that miR-21 has an effect on differentiation of prostate cancer cells as expression of miR-21 decreases B-cell translocation gene 2 (Btg2) levels, instigating the expression of luminal markers and a switch from epithelial to a mesenchymal phenotype in prostate cancer cells (Coppola et al. 2013). Hypoxia increases the expression of miR-21 and overexpression of miR-21 increases the levels of hypoxia-inducible factor 1-alpha (HIF1α) and vascular endothelial growth factor (VEGF), as well as AKT and extracellular signal-regulated kinase (ERK) through targeting of Pten leading to increased tumour angiogenesis (Liu et al. 2011b; Bao et al. 2012). Another target of miR-21 is Reck, a known regulator of tumour cell invasion (Reis et al. 2012). In conclusion, miR-21 might contribute to the tumourigenesis by affecting several pathways leading to increased proliferation, EMT, angiogenesis and invasion. In addition, serum and plasma levels of miR-21 have been shown to be associated with aggressive prostate cancer, including castration resistant growth and metastases (Shen et al. 2012; Zhang et al. 2011; Yaman Agaoglu et al. 2011).
8.4 EMT and Cellular Plasticity
The transition from an epithelial to a mesenchymal phenotyp e plays a key role in prostate cancer progression. Tumour cells undergo EMT to be able to disseminate, yet the resulting metastases exhibit epithelial phenotypes. Hence, after seeding at the secondary sight, the cell has to undergo the reversal of EMT, i.e. mesenchymal-to-epithelial transition (MET ), in order to proliferate and establish macrometastases. A finding supporting this plasticity scenario was reported in a murine prostate model in which 24 transcripts were compared in GFP-tagged PC3 primary tumour cells, circulating tumour cells (CTCs) and metastases. The primary and metastatic signatures were close to identical, whereas the CTCs signature stood out, for example Bcl2 expression was increased in CTCs compared to the primary and metastatic tumour cells (Helzer et al. 2009). Disseminated metastatic cells can remain dormant for a variable period of time before forming secondary tumour sites. These secondary tumours may then shed novel metastatic cells to the blood stream resulting in multiple metastatic sites even if the primary tumour has been removed. It seems that the cells must exhibit phenotypic plasticity, a transient EMT-MET process, in response to the changing microenvironment as they invade through the basement membrane, enter and exit the circulation, and survive and grow at distant locations. Transcription factors , including SNAI1, SNAI2, TWIST and ZEB1/2, have been shown to regulate epithelial-mesenchymal plasticity. But recently, miRNAs have also been shown to be crucial regulators of EMT and cancer cell invasion. In fact, several studies suggest that cellular plasticity is governed by reciprocal feedback loops between miRNAs and their EMT-inducing targets (Fig. 8.3); e.g. the miR-200 family and ZEB1/2 (Burk et al. 2008; Bracken et al. 2008), miR-203 and SNAI1/2 (Qu et al. 2013b) and the miR-34 family and SNAI1 (Siemens et al. 2011). It is possible that these and similar feedback loops regulate the reversible phenotypic switch that allows tumour cells to exhibit EMT/MET plasticity in response to the microenvironment.
8.4.1 miR-200
The miR-200 family members have been described to act as tumour suppressors. As mentioned, miR-200 can repress expression of ZEB1 and ZEB2 transcription factors through direct targeting, leading to enhanced E-cadherin expression and inhibition of EMT. Conversely, ZEB1 and ZEB2 repress miR-200 expression by binding to the promoter of the mir-200 encoding gene cluster, forming a double negative feedback loop controlling expressions of both during EMT. Snai2 is another target of miR-200, and conversely SNAI2 is a direct repressor of miR-200 expression (Liu et al. 2013). In benign prostate cells, SNAI2 was found to be important for EMT initiation, while ZEB in cooperation with the miR-200 family opposed the reversal of the EMT (Slabakova et al. 2011). By preventing EMT, miR-200 prevents invasion and distant metastasis. Depletion of SNAI2 inhibits EMT during tumourigenesis, whereas reintroduction of miR-200 inhibited both EMT and tumourigenesis in human and mouse model systems (Liu et al. 2013). In concordance with these findings, decreased miR-200 expression has been associated with the acquisition of cancer stem cell traits and tumour-initiating capacity in other cancer settings. For example, the miR-200 family members also target Notch pathway components, such as Jag1, Notch1, mastermind-like gene 2 (Mam2) and Mam3 (Brabletz et al. 2011; Kong et al. 2009). Further, it has been suggested that miR-200 plays a key role in linking the characteristics of cancer stem-like cells with EMT-like cells in prostate cancer. Cells with the EMT phenotype display stem-like cell features and decreased expression of miR-200, but re-introduction of miR-200 lead to reversal of EMT, reduced prostasphere formation, and expression of Notch1 and Lin28b (Kong et al. 2009). It is possible that miR-200 is essential for cellular plasticity of cancer stem cells and hence of driving cancer progression towards metastasis. The levels of this potent molecule is tightly regulated by multiple reciprocal feedback loops with their targets and this might explain why miR-200 has not been found to be deregulated when identifying general expression patterns in larger prostate cancer cohorts (Porkka et al. 2007; Martens-Uzunova et al. 2012). However, in a study identifying the miRNA profile of primary prostate cancers using deep sequencing, miR-200c was found to be the most common transcript representing approximately 10 % of all miRNAs in pooled prostate cancer tissue (Szczyrba et al. 2010).
8.5 Stem Cells
The prostate contains a subpopulation of cells that do not depend directly on androgens for their survival; the prostate epithelial stem cells. Prostate stem cells are proposed to be present in the basal cell layer and, when dividing, give rise to another stem cell and a daughter progenitor cell/transit amplifying cell, which after a few divisions differentiate into end stage secretory luminal cells. They have the capacity to make the tumour recur from a single cell (Leong et al. 2008). Androgen deprivation therapy leads to expansion of the existing population of stem/progenitor cells (Lee et al. 2013). miRNAs, e.g. miR-145, have been suggested to play a central role in stem cell biology and regulate vital features such as self-renewal, pluripotency and differentiation. Further, exposure to increasing concentrations of chemotherapy has been shown to correlate to cancer stem cell-like traits and induction of EMT through downregulation of miRNAs such as miR-205 (Puhr et al. 2012).
8.5.1 miR-145
The intergenic MIR145 is located on chromosome 5q32, and is co-transcribed with MIR143. The level of miR-145 is consistently reported to be decreased in prostate cancer (Larne et al. 2013; Wach et al. 2012; Ozen et al. 2008). A possible mechanism for the down regulation of miR-145 is methylation of the promoter as has been reported for the prostate cancer cell lines PC3, DU145 and LNCaP (Suh et al. 2011). miR-145 has also been suggested to be transcriptionally activated by p53, which frequently is mutated in advanced prostate cancer cells, and repressed by IL6, which is commonly up regulated in prostate cancer (Sachdeva et al. 2009; Suh et al. 2011; Zaman et al. 2010). Several reports also indicate that miR-145 is further decreased in metastatic prostate cancer, especially bone metastases compared to localised prostate cancer (Leite et al. 2013; Peng et al. 2011). This agrees well with the described tumour suppressive functions of miR-145 in prostate cancer. It has been shown that miR-145 target oncogenic pathways such as C-myc (Sachdeva et al. 2009) and Ras (Kent et al. 2010), and is involved in the regulation of EMT and invasion (Peng et al. 2011; Guo et al. 2013). miR-145 also inhibits tumour growth and bone metastases of PC3 cells by repressing cancer stem cell properties in vivo, and in cooperation with miR-143, mir-145 supress prostatic tumour sphere formation and stemness markers in PC3 cells (Huang et al. 2012). Further, miR-145 inhibit stem cell renewal and pluripotency by targeting OCT4, SOX2 and Krueppel-like factor 4, and the reciprocal inhibition of miR-145 and OCT4 is believed to establish an irreversible switch priming cells to enter the differentiation program (Jain et al. 2012; Xu et al. 2009; Hu et al. 2012).
8.6 Metastases
Metastatic disease is the major cause of cancer-related deaths in men with prostate cancer, the 5 year survival rate is only 32 % compared to almost 100 % in localised early stages (Jemal et al. 2008). The development of metastases is a complex and dynamic process, involving detachment of the tumour cells from the primary site, entering and surviving in the bloodstream, migrating to distant locations where they extravasate and establish secondary tumours. Only a small fraction of the tumour cells in circulation give rise to distant metastasis, it has been suggested that cellular plasticity and transient acquisition of stem cell characteristics is necessary. The prostate cancer metastases are predominantly detectable in bone; autopsies reveal the presence of bone metastases in ∼90 % of men with spread prostate cancer (Bubendorf et al. 2000). In bone, the prostate cancer induces the formation of lesions that are primarily osteoblastic in nature, causing the patient to experience severe bone pain and skeletal fragility . The prostate cancer cells at the metastatic site might still respond to androgen ablation but will also at the distant sites transform to CRPC. It has been shown that androgen deprivation therapy induces EMT and expansion of the existing population of stem cells, as a consequence the transition to CRPC is associated with increased incidence of metastases (Sun et al. 2012; Lee et al. 2013). While it is clear that the tumour microenvironment play a crucial role in determining the lethal phenotype of cancer cells, the molecular events associated with metastasis, homing to bone and colonization, invasion and survival at the secondary site are not well understood. It has been shown that miRNA expression in the primary tumour correlates to metastatic disease and also that a certain miRNA signature, the miRNA index quote (miQ), is an independent predictor of metastases events occurring 0.5–10 years after the removal of the primary tumour (Larne et al. 2013).
8.6.1 miR-205
The miR-205 encoding gene is located within the gene LOC642587 of unknown function at chromosome 1q32, and has been shown to have decreased expression in prostate cancer (Majid et al. 2010; Gandellini et al. 2009). The miR-205 expression is regulated by p53, p63 and epigenetic silencing (Piovan et al. 2012; Wiklund et al. 2011; Hulf et al. 2013). In addition, the miR-205 expression is mainly localized to the basal epithelial cells, but as these cells disappear or differentiate during prostate cancer progression, this conceivably result in a loss of miR-205 expression (Gandellini et al. 2012; Zhang et al. 2010; Hagman et al. 2013b). It is reasonably a combination of these regulatory events that are responsible for the decrease of miR-205 levels corresponding to prostate cancer progression that is described in several independent studies (Majid et al. 2010; Gandellini et al. 2009; Hagman et al. 2013b). The expression of miR-205 is also inversely correlated to occurrence of metastases, castration resistant and shortened overall survival (Hagman et al. 2013b). In concordance with this, there are several reports indicating that miR-205 act as a tumour suppressor in prostate cancer cells. miR-205 has been shown to directly regulate PKC-epsilon resulting in an effect on migration and invasiveness of prostate cancer cells (Wu et al. 2009). The expression of miR-205 decreases 100-fold when cells undergo EMT, but also contribute to EMT by targeting ZEB1/2, the transcriptional repressors of E-cadherin, this also contribute to enhanced migration (Gregory et al. 2008; Tucci et al. 2012). In addition, miR-205 has been shown to directly target the AR, a finding that was corroborated in a patient cohort were miR-205 expression inversely correlated to AR immunostaining in malignant prostate cells and to serum levels of the androgen regulated PSA (Hagman et al. 2013b). During prostate cancer progression, miR-205 levels decrease and this seem to result in activated AR signalling and increased migratory potential.
8.6.2 miR-15a/16
The first miRNA encoding genes identified to be frequently deleted in cancer was MIR15A and −16–1 located at chromosome 13q14 (Calin et al. 2002). They have also been found to be homozygously deleted in a subset of prostate cancers and to correlate with tumour progression (Porkka et al. 2011; Dong et al. 2001; Hyytinen et al. 1999). Loss of miR-15a/16 induce cellular proliferation in prostate cancer cells and restoration of miR-15a/16 result in growth arrest, apoptosis, and regression of prostate tumours in xenograft models (Cimmino et al. 2005; Bonci et al. 2008). These miRNAs promote apoptosis by targeting among others Bcl2 (Bonci et al. 2008). Prostate luminal stem cells express Bcl2 (Ceder et al. 2008) and it is plausible that miR-16 has an impact on the sensitivity to apoptosis in these cells. miR-15/16 have also been found to be down regulated in fibroblasts surrounding prostate tumours, and to repress fibroblast growth factor 2 (FGF2) and its receptor, which act on both stromal and tumour cells to enhance cancer cell survival, proliferation and migration (Musumeci et al. 2011). In a xenograph bone metastasis model, injection of miR-16 via the tail vein significantly inhib ited the growth of prostate tumours in bone (Takeshita et al. 2010).
8.7 ncRNAs with Diagnostic Potential
A special characteristic of prostate cancer is that the latent form of the disease is very common; microscopic lesions are found in more than 50 % of 70–80 year old men (Gronberg 2003). However, most cases will never experience cancer symptoms during their lifetime. The current clinical practice for diagnosis and decision making of prostate cancer involve digital rectal examination, serum PSA and subsequent biopsies for histopathological staging and Gleason scoring . Each of these methods has its shortcomings and today a momentous problem is over diagnosis and treatment of patients with indolent prostate cancer. The management of prostate cancer would benefit from better tools for detection, prognosis and treatment response . The miRNAs are technically suitable as biomarkers as they are deregulated in prostate cancer, easy to detect and found to be stable in plasma, serum, fresh frozen, and formalin fixed paraffin embedded tissues. Many studies highlight the diagnostic and prognostic potential of individual miRNAs, however, no single miRNA has been consistently validated or implemented as a biomarker in clinical management of prostate cancer.
8.7.1 miRNAs Signatures
Lately several studies focusing on different miRNA signatures have been published. By extensive microarray analyses Martens-Uzunova et al. derived a miRNA diagnostic classifier that distinguishes prostate cancer from benign specimens; this classifier contains 54 miRNAs and gives an area under the curve of 0.95. The same team also constructed a prognostic signature consisting of 25 miRNAs that was able to independently predict postoperative outcome (Martens-Uzunova et al. 2012). Even smaller number of prostate derived miRNAs has successfully been combined into quotes e.g. the miQ; ((miR-96 × miR-183)/(miR-145 × miR-221)), was found to successfully predict diagnosis with high accuracy in several independent cohorts, and also has prognostic power to predict aggressiveness of tumours, metastatic status, and overall survival (Larne et al. 2013). The advantage using a quote is increased discrimination, no need for house-keepings, and most important it may be an advantage considering the heterogeneity of the disease. There have also been preliminary data of non-invasive miRNA signatures that have prognostic properties e.g. miR-141 + miR151-3p + miR-16, that could discriminating between metastatic CRPC and localized prostate cancer in a cohort of 50 men (Watahiki et al. 2013). However, recent reports highlight the need for caution in the interpretation of the cancer-specificity of circulation miRNAs. It has been reported that 58 % of the published circulating miRNA biomarkers are highly expressed in hematopoietic cells, e.g. miR-16 is expressed in red blood cells, and that the levels of miR-21 and miR-141 in circulating are unaltered by radical prostatectomy raising question s of th e origin of these miRNAs (Pritchard et al. 2012; Egidi et al. 2013).
8.7.2 PCA3
The only ncRNA that has made it all the way into clinical practice is the long ncRNA , prostate cancer antigen 3 (PCA3 ) . PCA3 is only expressed in the prostate, and it is highly overexpressed in 95 % of prostate cancer cells. Although the mechanisms of action are still unknown, PCA3 has shown to be a useful prostate cancer biomarker. It can be detected by PCR in urine obtained after digital rectal examination (Bussemakers et al. 1999; Hessels et al. 2003). The urinary based test PCA3-to-PSA transcript has been approved for use in men suspected to have prostate cancer due to digital rectal examination and PSA but the first prostate biopsies is negative, as it has been shown to be useful to predict the presence of malignancy in this setting, and can thus reduce the number of unnecessary prostate biopsy (Haese et al. 2008; Marks et al. 2007). The PCA3 score h as also been shown to predict tumour volume, which might help in selecting prostate cancer patients for active surveillance (Ploussard et al. 2011).
8.8 Conclusions and Future Perspective
A decade ago small ncRNAs was first discovered to be involved in carcinogenesis (Chan et al. 2005; Calin et al. 2002). Now they are at the centre of attention and suggested to be suitable in the clinical management for most cancer forms. The miRNAs are promising biomarkers as they are deregulated, stable in both tissue and body fluids and easy to detect. As the technology will become even cheaper and more accessible the knowledge of the role of the ncRNAs and their potential applications will increase. It is conceivable that the miRNA signatures in circulating tumour cells or cancer-cell secreted microvesicles can enable personalized and patient tailored treatments for prostate cancer patients.
Individual miRNAs have evolved to coordinate the regulation of groups of molecules involved in essential cellular functions in the prostate. Prostate cancer progression is driven by not one but an array of genetic mutations and epigenetic alterations, individual miRNAs might provide a strategy to target systems rather than one molecule at a time in a fine-tuned manner. During the multi-step cascade of transient processes the tumour cells have to go through to enable e.g. metastatic spread, it is reasonable to speculate that reversible epigenetic modification is more likely to be regulating the balance between these events rather than fixed genetic alterations. In this context, it is interesting to note that the majority of miRNAs discussed in this review are epigenetically regulated. This also points to the very interesting interference option to reverse these processes by targeting the miRNAs.
However, it has lately become apparent that the miRNAs interact with their targets in more intricate manners than initially recognized. It has been discovered that many miRNAs are involved in both positive and negative feedback loops with their targets, hypothetically enhancing the robustness of gene regulation by creating a homeostasis between miRNAs and their targets. The tumour suppressor miR-34a is involved in a positive feedback loop with p53; miR-34a inhibits the expression of NAD-dependent protein deacetylase sirtuin-1 (SIRT1) that activates p53 (Yamakuchi and Lowenstein 2009). Loss or mutation of p53 is rare in primary prostate cancer but frequent in advanced cases. It is possible that reintroduction of miR-34a in the prostate cells with disturbed p53 function would lead to the expected tumour inhibition, but in adjacent cells with WT p53 it could potentially give negative long term effects of increased mutation rates leading to accelerated tumourigenesis. Then there is the heterogeneity of the 3′UTRs of the targets to take into considerations. It has lately been shown that over 50 % of all genes have alternative polyadenylation signals, and shorter 3′UTRs would lead to altered miRNA susceptibility in a specific setting (Tian et al. 2005). However, with increase knowledge about these changes, it is possible that this could be an advantage when designing specific gene targeting. Another factor complicating future miRNA based therapeutics might be target competition (Poliseno et al. 2010). Each miRNA targets several genes, as the levels of one of the targeted transcripts are increased in malignant tissues the available level of the inhibiting miRNA are decreased, and the repressive effect this is having on other transcripts might be relieved through target competition. This highlights the importance of targeted expression in the right setting and also the importance of selection of eligible patients. More in depth pre-clinical studies are needed to investigate the interplay between miRNAs and their targets in prostate cancer cells and the long term effects of manipulation of these delicate networks, as well as improved strategies for deregulation of the miRNAs in a prostate specific manner.
References
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RM, Croce CM (2008) Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res 68:6162–6170
Amir S, Ma AH, Shi XB, Xue L, Kung HJ, Devere White RW (2013) Oncomir miR-125b suppresses p14(ARF) to modulate p53-dependent and p53-independent apoptosis in prostate cancer. PLoS One 8:e61064
Arora R, Koch MO, Eble JN, Ulbright TM, Li L, Cheng L (2004) Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer 100:2362–2366
Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y, Banerjee S, Padhye S, Sarkar FH (2012) Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One 7:e43726
Benassi B, Flavin R, Marchionni L, Zanata S, Pan Y, Chowdhury D, Marani M, Strano S, Muti P, Blandino G, Loda M (2012) MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer Disc 2:236–247
Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D’urso L, Pagliuca A, Biffoni M, Labbaye C, Bartucci M, Muto G, Peschle C, DE Maria R (2008) The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14:1271–1277
Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T (2011) The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J 30:770–782
Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 68:7846–7854
Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31:578–583
Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9:582–589
Bussemakers MJ, Van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, Debruyne FM, Ru N, Isaacs WB (1999) DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 59:5975–5979
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM (2002) Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 99:15524–15529
Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, Heery DM, Gaestel M, Eilers M, Willis AE, Bushell M (2010) p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci U S A 107:5375–5380
Ceder JA, Jansson L, Ehrnstrom RA, Ronnstrand L, Abrahamsson PA (2008) The characterization of epithelial and stromal subsets of candidate stem/progenitor cells in the human adult prostate. Eur Urol 53:524–531
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033
Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, Ozturk A, Hicks GG, Hannon GJ, He L (2011) miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol 13:1353–1360
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102:13944–13949
Clape C, Fritz V, Henriquet C, Apparailly F, Fernandez PL, Iborra F, Avances C, Villalba M, Culine S, Fajas L (2009) miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS One 4:e7542
Concepcion CP, Han YC, Mu P, Bonetti C, Yao E, D’andrea A, Vidigal JA, Maughan WP, Ogrodowski P, Ventura A (2012) Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet 8:e1002797
Coppola V, Musumeci M, Patrizii M, Cannistraci A, Addario A, Maugeri-Sacca M, Biffoni M, Francescangeli F, Cordenonsi M, Piccolo S, Memeo L, Pagliuca A, Muto G, Zeuner A, DE Maria R, Bonci D (2013) BTG2 loss and miR-21 upregulation contribute to prostate cell transformation by inducing luminal markers expression and epithelial-mesenchymal transition. Oncogene 32:1843–1853
Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY (2007) MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res 67:8433–8438
Dahiya R, Mccarville J, Lee C, Hu W, Kaur G, Carroll P, Deng G (1997) Deletion of chromosome 11p15, p12, q22, q23-24 loci in human prostate cancer. Int J Cancer 72:283–288
Dong JT, Boyd JC, Frierson HF Jr (2001) Loss of heterozygosity at 13q14 and 13q21 in high grade, high stage prostate cancer. Prostate 49:166–171
Egidi MG, Cochetti G, Serva MR, Guelfi G, Zampini D, Mechelli L, Mearini E (2013) Circulating microRNAs and Kallikreins before and after radical prostatectomy: are they really prostate cancer markers? Biomed Res Int 2013:241780
Fan X, Chen X, Deng W, Zhong G, Cai Q, Lin T (2013) Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer 13:61
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL (2012) Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum Mol Genet 21:3112–3127
Folini M, Gandellini P, Longoni N, Profumo V, Callari M, Pennati M, Colecchia M, Supino R, Veneroni S, Salvioni R, Valdagni R, Daidone MG, Zaffaroni N (2010) miR-21: an oncomir on strike in prostate cancer. Mol Cancer 9:12
Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafre SA, Farace MG (2007) miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 282:23716–23724
Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta P, Valdagni R, Daidone MG, Zaffaroni N (2009) miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res 69:2287–2295
Gandellini P, Profumo V, Casamichele A, Fenderico N, Borrelli S, Petrovich G, Santilli G, Callari M, Colecchia M, Pozzi S, De Cesare M, Folini M, Valdagni R, Mantovani R, Zaffaroni N (2012) MiR-205 regulates basement membrane deposition in human prostate: implications for cancer development. Cell Death Differ 19(11):1750–1760
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10:593–601
Gronberg H (2003) Prostate cancer epidemiology. Lancet 361:859–864
Guo W, Ren D, Chen X, Tu X, Huang S, Wang M, Song L, Zou X, Peng X (2013) HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem 114:1606–1615
Haese A, DE LA Taille A, VAN Poppel H, Marberger M, Stenzl A, Mulders PF, Huland H, Abbou CC, Remzi M, Tinzl M, Feyerabend S, Stillebroer AB, VAN Gils MP, Schalken JA (2008) Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 54:1081–1088
Hagman Z, Larne O, Edsjo A, Bjartell A, Ehrnstrom RA, Ulmert D, Lilja H, Ceder Y (2010) miR-34c is downregulated in prostate cancer and exerts tumor suppressive functions. Int J Cancer 127:2768–2776
Hagman Z, Haflidadottir BS, Ansari M, Persson M, Bjartell A, Edsjo A, Ceder Y (2013a) The tumour suppressor miR-34c targets MET in prostate cancer cells. Br J Cancer 109(5):1271–1278
Hagman Z, Haflidadottir BS, Ceder JA, Larne O, Bjartell A, Lilja H, Edsjo A, Ceder Y (2013b) miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br J Cancer 108(8):1668–1676
Hart M, Wach S, Nolte E, Szczyrba J, Menon R, Taubert H, Hartmann A, Stoehr R, Wieland W, Grasser FA, Wullich B (2013) The proto-oncogene ERG is a target of microRNA miR-145 in prostate cancer. FEBS J 280:2105–2116
Helzer KT, Barnes HE, Day L, Harvey J, Billings PR, Forsyth A (2009) Circulating tumor cells are transcriptionally similar to the primary tumor in a murine prostate model. Cancer Res 69:7860–7866
Hessels D, Klein Gunnewiek JM, Van Oort I, Karthaus HF, Van Leenders GJ, Van Balken B, Kiemeney LA, Witjes JA, Schalken JA (2003) DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol 44:8–15; discussion 15–6
Hu J, Guo H, Li H, Liu Y, Liu J, Chen L, Zhang J, Zhang N (2012) MiR-145 regulates epithelial to mesenchymal transition of breast cancer cells by targeting Oct4. PLoS One 7:e45965
Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X (2012) miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep 28:1831–1837
Hudson RS, Yi M, Esposito D, Glynn SA, Starks AM, Yang Y, Schetter AJ, Watkins SK, Hurwitz AA, Dorsey TH, Stephens RM, Croce CM, Ambs S (2013) MicroRNA-106b-25 cluster expression is associated with early disease recurrence and targets caspase-7 and focal adhesion in human prostate cancer. Oncogene 32:4139–4147
Hulf T, Sibbritt T, Wiklund ED, Patterson K, Song JZ, Stirzaker C, Qu W, Nair S, Horvath LG, Armstrong NJ, Kench JG, Sutherland RL, Clark SJ (2013) Epigenetic-induced repression of microRNA-205 is associated with MED1 activation and a poorer prognosis in localized prostate cancer. Oncogene 32:2891–2899
Hyytinen ER, Frierson HF Jr, Boyd JC, Chung LW, Dong JT (1999) Three distinct regions of allelic loss at 13q14, 13q21-22, and 13q33 in prostate cancer. Genes Chromosomes Cancer 25:108–14c
Jain AK, Allton K, Iacovino M, Mahen E, Milczarek RJ, Zwaka TP, Kyba M, Barton MC (2012) p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol 10:e1001268
Jalava SE, Urbanucci A, Latonen L, Waltering KK, Sahu B, Janne OA, Seppala J, Lahdesmaki H, Tammela TLJ, Visakorpi T (2012) Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 31:4460–4471
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Jenster G (1999) The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol 26:407–421
Kasahara K, Taguchi T, Yamasaki I, Kamada M, Yuri K, Shuin T (2002) Detection of genetic alterations in advanced prostate cancer by comparative genomic hybridization. Cancer Genet Cytogenet 137:59–63
Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH (2012) Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res 4:432–442
Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT (2010) Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev 24:2754–2759
Kojima S, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M, Seki N (2012) Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer 106:405–413
Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH (2009) miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells 27:1712–1721
Kong D, Heath E, Chen W, Cher M, Powell I, Heilbrun L, Li Y, Ali S, Sethi S, Hassan O, Hwang C, Gupta N, Chitale D, Sakr WA, Menon M, Sarkar FH (2012) Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment. Am J Transl Res 4:14–23
Larne O, Martens-Uzunova E, Hagman Z, Edsjo A, Lippolis G, Den Berg MS, Bjartell A, Jenster G, Ceder Y (2013) MiQ-a novel microRNA based diagnostic and prognostic tool for prostate cancer. Int J Cancer 132(12):2867–2875
Lee SO, Ma Z, Yeh CR, Luo J, Lin TH, Lai KP, Yamashita S, Liang L, Tian J, Li L, Jiang Q, Huang CK, Niu Y, Yeh S, Chang C (2013) New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells. J Mol Cell Biol 5:14–26
Leite KR, Tomiyama A, Reis ST, Sousa-Canavez JM, Sanudo A, Camara-Lopes LH, Srougi M (2013) MicroRNA expression profiles in the progression of prostate cancer-from high-grade prostate intraepithelial neoplasia to metastasis. Urol Oncol 31(6):796–801
Leong KG, Wang BE, Johnson L, Gao WQ (2008) Generation of a prostate from a single adult stem cell. Nature 456:804–808
Lin PC, Chiu YL, Banerjee S, Park K, Mosquera JM, Giannopoulou E, Alves P, Tewari AK, Gerstein MB, Beltran H, Melnick AM, Elemento O, Demichelis F, Rubin MA (2013) Epigenetic repression of miR-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res 73:1232–1244
Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG (2011a) The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 17:211–215
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH (2011b) MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One 6:e19139
Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K (2013) MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 32:296–306
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838
Majid S, Dar AA, Saini S, Yamamura S, Hirata H, Tanaka Y, Deng G, Dahiya R (2010) MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer 116:5637–5649
Majid S, Dar AA, Saini S, Shahryari V, Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, Deng G, Dahiya R (2013) miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin Cancer Res 19:73–84
Marks LS, Fradet Y, Deras IL, Blase A, Mathis J, Aubin SM, Cancio AT, Desaulniers M, Ellis WJ, Rittenhouse H, Groskopf J (2007) PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 69:532–535
Martens-Uzunova ES, Jalava SE, Dits NF, VAN Leenders GJLH, Moller S, Trapman J, Bangma CH, Litman T, Visakorpi T, Jenster G (2012) Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 31:978–991
Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C (2006) Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer 5:24
Mishra S, Deng JJ, Gowda PS, Rao MK, Lin CL, Chen CL, Huang T, Sun LZ (2014) Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogenesis 33(31):4097–4106
Musumeci M, Coppola V, Addario A, Patrizii M, Maugeri-Sacca M, Memeo L, Colarossi C, Francescangeli F, Biffoni M, Collura D, Giacobbe A, D’urso L, Falchi M, Venneri MA, Muto G, DE Maria R, Bonci D (2011) Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 30:4231–4242
Narayanan R, Jiang J, Gusev Y, Jones A, Kearbey JD, Miller DD, Schmittgen TD, Dalton JT (2010) MicroRNAs are mediators of androgen action in prostate and muscle. PLoS One 5:e13637
Ostling P, Leivonen SK, Aakula A, Kohonen P, Makela R, Hagman Z, Edsjo A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perala M, Kallioniemi O (2011) Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res 71:1956–1967
Ozen M, Creighton CJ, Ozdemir M, Ittmann M (2008) Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 27:1788–1793
Peng X, Guo W, Liu T, Wang X, Tu X, Xiong D, Chen S, Lai Y, Du H, Chen G, Liu G, Tang Y, Huang S, Zou X (2011) Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS One 6:e20341
Piovan C, Palmieri D, DI Leva G, Braccioli L, Casalini P, Nuovo G, Tortoreto M, Sasso M, Plantamura I, Triulzi T, Taccioli C, Tagliabue E, Iorio MV, Croce CM (2012) Oncosuppressive role of p53-induced miR-205 in triple negative breast cancer. Mol Oncol 6:458–472
Ploussard G, Durand X, Xylinas E, Moutereau S, Radulescu C, Forgue A, Nicolaiew N, Terry S, Allory Y, Loric S, Salomon L, Vacherot F, DE LA Taille A (2011) Prostate cancer antigen 3 score accurately predicts tumour volume and might help in selecting prostate cancer patients for active surveillance. Eur Urol 59:422–429
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038
Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T (2007) MicroRNA expression profiling in prostate cancer. Cancer Res 67:6130–6135
Porkka KP, Ogg EL, Saramaki OR, Vessella RL, Pukkila H, Lahdesmaki H, VAN Weerden WM, Wolf M, Kallioniemi OP, Jenster G, Visakorpi T (2011) The miR-15a-miR-16-1 locus is homozygously deleted in a subset of prostate cancers. Genes Chromosomes Cancer 50:499–509
Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M (2012) Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res 5:492–497
Prueitt RL, Yi M, Hudson RS, Wallace TA, Howe TM, Yfantis HG, Lee DH, Stephens RM, Liu CG, Calin GA, Croce CM, Ambs S (2008) Expression of microRNAs and protein-coding genes associated with perineural invasion in prostate cancer. Prostate 68(11):1152–1164
Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z (2012) Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol 181:2188–2201
Qu F, Cui X, Hong Y, Wang J, Li Y, Chen L, Liu Y, Gao Y, Xu D, Wang Q (2013a) MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem 377:121–130
Qu Y, Li WC, Hellem MR, Rostad K, Popa M, Mccormack E, Oyan AM, Kalland KH, Ke XS (2013b) MiR-182 and miR-203 induce mesenchymal to epithelial transition and self-sufficiency of growth signals via repressing SNAI2 in prostate cells. Int J Cancer 133:544–555
Reis ST, Pontes-Junior J, Antunes AA, Dall’Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR, Nesrallah AJ, Piantino C, Srougi M, Leite KR (2012) miR-21 may acts as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancer. BMC Urol 12:14
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang S, Zou X, Peng X (2013) Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR145. Int J Oncol 42:1473–1481
Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R, Mendell JT, Lupold SE (2009) miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 69:7165–7169
Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY (2009) p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A 106:3207–3212
Schubert M, Spahn M, Kneitz S, Scholz CJ, Joniau S, Stroebel P, Riedmiller H, Kneitz B (2013) Distinct microRNA expression profile in prostate cancer patients with early clinical failure and the impact of let-7 as prognostic marker in high-risk prostate cancer. PLoS One 8:e65064
Shen J, Hruby GW, Mckiernan JM, Gurvich I, Lipsky MJ, Benson MC, Santella RM (2012) Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. Prostate 72:1469–1477
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, Devere White RW (2007) An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci U S A 104:19983–19988
Shi XB, Xue L, Ma AH, Tepper CG, Kung HJ, White RW (2011) miR-125b promotes growth of prostate cancer xenograft tumor through targeting pro-apoptotic genes. Prostate 71:538–549
Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, Kung HJ, Devere White RW (2013) Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene 32:4130–4138
Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, Hermeking H (2011) miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 10:4256–4271
Slabakova E, Pernicova Z, Slavickova E, Starsichova A, Kozubik A, Soucek K (2011) TGF-beta1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/slug. Prostate 71:1332–1343
Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R (2011) MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis 32:772–778
Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, Settleman J, Johnson L (2012) Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res 72:527–536
Sun T, Wang X, He HH, Sweeney CJ, Liu SX, Brown M, Balk S, Lee GS, Kantoff PW (2014) MiR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogenesis 33(21):2790–2800
Szczyrba J, Loprich E, Wach S, Jung V, Unteregger G, Barth S, Grobholz R, Wieland W, Stohr R, Hartmann A, Wullich B, Grasser F (2010) The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol Cancer Res 8:529–538
Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, Kawamata M, Kelnar K, Bader AG, Brown D, Ochiya T (2010) Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther 18:181–187
Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin C, Zhang W (2012) microRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol Rep 27:1967–1975
Tian B, Hu J, Zhang H, Lutz CS (2005) A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res 33:201–212
Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S, Senzer N, Eklund AC, Han J, Nemunaitis J (2009) MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther 16(3):206–216
Toyota M, Suzuki H, Sasaki Y, Maruyama R, Imai K, Shinomura Y, Tokino T (2008) Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res 68:4123–4132
Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, Muller PA, Dotsch V, Kehrloesser S, Sayan BS, Giaccone G, Lowe SW, Takahashi N, Vandenabeele P, Knight RA, Levine AJ, Melino G (2012) Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A 109:15312–15317
Vallejo DM, Caparros E, Dominguez M (2011) Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J 30:756–769
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, IORIO M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103:2257–2261
Wach S, Nolte E, Szczyrba J, Stohr R, Hartmann A, Orntoft T, Dyrskjot L, Eltze E, Wieland W, Keck B, Ekici AB, Grasser F, Wullich B (2012) MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int J Cancer 130:611–621
Wang L, Tang H, Thayanithy V, Subramanian S, Oberg AL, Cunningham JM, Cerhan JR, Steer CJ, Thibodeau SN (2009) Gene networks and microRNAs implicated in aggressive prostate cancer. Cancer Res 69:9490–9497
Watahiki A, Macfarlane RJ, Gleave ME, Crea F, Wang Y, Helgason CD, Chi KN (2013) Plasma miRNAs as biomarkers to identify patients with castration-resistant metastatic prostate cancer. Int J Mol Sci 14:7757–7770
Wiklund ED, Bramsen JB, Hulf T, Dyrskjot L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, Peter ME, Orntoft TF, Kjems J, Clark SJ (2011) Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer J Int Cancer 128:1327–1334
Wu H, Zhu S, Mo YY (2009) Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res 19:439–448
Wu D, Huang P, Wang L, Zhou Y, Pan H, Qu P (2013) MicroRNA-143 inhibits cell migration and invasion by targeting matrix metalloproteinase 13 in prostate cancer. Mol Med Rep 8(2):626–630
Xiao J, Gong AY, Eischeid AN, Chen D, Deng C, Young CY, Chen XM (2012) miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate 72:1514–1522
Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS (2009) MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137:647–658
Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, Hua L, Wang X (2011) miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem 350:207–213
Yamakuchi M, Lowenstein CJ (2009) MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle 8:712–715
Yamamura S, Saini S, Majid S, Hirata H, Ueno K, Deng G, Dahiya R (2012) MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS One 7:e29722
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, Gezer U (2011) Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol: J Int Soc Oncol Dev Biol Med 32:583–588
Yang X, Bemis L, Su LJ, Gao D, Flaig TW (2012) miR-125b regulation of androgen receptor signaling via modulation of the receptor complex co-repressor NCOR2. BioRes Open Access 1:55–62
Zaman MS, Chen Y, Deng G, Shahryari V, Suh SO, Saini S, Majid S, Liu J, Khatri G, Tanaka Y, Dahiya R (2010) The functional significance of microRNA-145 in prostate cancer. Br J Cancer 103:256–264
Zhang L, Zhao W, Valdez JM, Creighton CJ, Xin L (2010) Low-density Taqman miRNA array reveals miRNAs differentially expressed in prostatic stem cells and luminal cells. Prostate 70:297–304
Zhang HL, Yang LF, Zhu Y, Yao XD, Zhang SL, Dai B, Zhu YP, Shen YJ, Shi GH, Ye DW (2011) Serum miRNA-21: elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate 71:326–331
Zheng C, Yinghao S, Li J (2012) MiR-221 expression affects invasion potential of human prostate carcinoma cell lines by targeting DVL2. Med Oncol 29:815–822
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Ceder, Y. (2016). Non-coding RNAs in Prostate Cancer: From Discovery to Clinical Applications. In: Wilhelm, D., Bernard, P. (eds) Non-coding RNA and the Reproductive System. Advances in Experimental Medicine and Biology, vol 886. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7417-8_8
Download citation
DOI: https://doi.org/10.1007/978-94-017-7417-8_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7415-4
Online ISBN: 978-94-017-7417-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)