Abstract
Correct sexual development is arguably the most important trait in an organism’s life history since it is directly related to its genetic fitness. The developing gonad houses the germ cells, the only legacy we pass on to subsequent generations. Given the pivotal importance of correct reproductive function, it is confounding that disorders of sex development (DSDs) are among the most common congenital abnormalities in humans (Lee et al. J Pediatr Urol 8(6):611–615, 2012). Urogenital development is a highly complex process involving coordinated interactions between molecular and hormonal pathways in a tightly regulated order. The controls that regulate some of the key events in this process are beginning to be unraveled. This chapter provides an overview of our understanding of urogenital development from the gonads to the urogenital ducts and external genitalia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Development of the Indifferent Gonadal Ridge
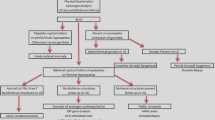
The gonadal ridge first appears as a bulge of intermediate mesoderm on the ventromedial surface of the intermediate embryonic kidney, the mesonephros, at around 10.5 days post coitum (dpc) in mouse. At this stage, the gonad is identical in structure between males and females and is comprised largely of somatic cells with germ cells migrating in from surrounding tissues. The somatic cells will contribute to the supporting, interstitial and steroid-producing cell lineages, while the germ cells will form the gametes (Merchant-Larios et al. 1993). As development proceeds, the epithelium and underlying mesenchyme of the gonad and mesonephros proliferates, and both organs increase in size. The mesonephros contains the mesonephric and paramesonephric ducts that facilitate fluid movement during kidney development but will later form aspects of the male and female reproductive tracts respectively. Both ducts exist as paired structures that sit adjacent to the gonads (Fig. 1.1). Several homeobox genes including Lhx1, Lhx9 and Emx2 have been implicated in the early patterning of the gonadal ridge and mesonephros ducts (Svingen and Koopman 2007). Mutations in the Wilms’ tumour 1 gene (Wt1) cause the gonads to fail to progress past the indifferent stage as well as defects in mesonephric development and kidney tumours (Kreidberg et al. 1993). Interestingly, loss of Wt1 has been shown to alter the expression of several long noncoding RNAs and their gene targets during tumorigenesis (Hubertus et al. 2011). Mutations in the gene encoding steroidogenic factor 1 (Sf1) and M33 also cause a block in early gonadal development (Luo et al. 1994; Shinoda et al. 1995; Katoh-Fukui et al. 1998).
Sexual differentiation of the urogenital system. The early embryo has a bipotential urogenital system (top diagram) that can proceed towards a female (left) or male (right) fate. Female urogenital development occurs in the absence of testis development and subsequent absence of AMH and testosterone. Under these conditions the Wolffian/mesonephric duct will fail to proliferate while the Müllerian/paramesonephric duct will develop in to the oviduct, uterus and upper portion of the vagina (pink). The lower region of the vagina (black) is derived from the urogenital sinus. Male urogenital development occurs in the presence of a testis and the subsequent production of AMH and testosterone. AMH actively drives the regression of the paramesonephric ducts while testosterone promotes the differentiation of the Wolffian ducts to form the epididymis, vas deferens, seminal vesicle, prostate and bulbourethral glands (blue). The penile urethra forms from the fusion of tissue from the urogenital sinus and urorectal septum
Following the formation of the sexually indifferent bipotential gonad, molecular factors will initiate its progression towards one of two separate developmental fates; testis or ovary . The developing gonad then produces the hormones and factors necessary to facilitate the coordinated development of the associated ductal systems and development of the appropriate external genitalia and other secondary sexual characteristics. An outline of the key events involved in each of these processes in males and females will be discussed below.
1.2 Sex Determination
In therian mammals, the decision to follow a male or female fate is held largely in the hands of the Y-linked Sry gene (Gubbay et al. 1990; Lovell-Badge and Robertson 1990; Koopman et al. 1991; Harry et al. 1995). Sry is the master trigger for testis differentiation, initiating Sertoli cell differentiation (Koopman et al. 1990; Rossi et al. 1993). Recently it was shown that Sry expression from the Y-chromosome is epigenetically regulated by the autosomal histone H3K9 demethylase JMJD1A (Kuroki et al. 2013). Sry expression triggers the direct upregulation of the critical testis gene Sox9 where it acts as a transcriptional activator of the testis differentiation pathway (Sekido and Lovell-Badge 2008). Interestingly, SOX9 is initially present in the indifferent gonad of both XX and XY foetuses. In males it is rapidly upregulated and translocated to the nucleus, while in females, in the absence of the Y chromosome and Sry, Sox9 transcription is not upregulated, the protein remains cytoplasmic and the gonad will proceed towards an ovarian developmental fate (Malki et al. 2005; Pask et al. 2010). The upregulation of Sox9 is arguably the most critical step in the initiation of a testis (Qin et al. 2004), since loss of Sox9 in XY gonads results in ovarian development (Barrionuevo et al. 2006), while ectopic expression of Sox9 in XX gonads can induce testis formation. Thus, SOX9 is both necessary and sufficient for testicular development (Bishop et al. 2000; Vidal et al. 2001; Qin and Bishop 2005).
1.3 Testicular Development
Following the upregulation of Sry and Sox9 in the bipotential gonad, the first morphological signs of testis development are the appearance of male pattern vasculature , including a prominent coelomic vessel in eutherian mammals, and the formation of testicular cords. SRY also triggers proliferation of the coelomic epithelium, a feature that is required for testis development but not ovarian differentiation (Schmahl and Capel 2003). All these features contribute to a greatly increased abundance of cellular proliferation within the XY gonad, a key hallmark of male development (Schmahl et al. 2000).
The rapid growth of the testis is further facilitated by cell immigration (Capel et al. 1999). Sry triggers the movement of mesonephric cells, which invade the developing testis and were believed to contribute to the Leydig, peritubular myoid and endothelial cell lineages (Fig. 1.2) (Martineau et al. 1997). However, recent research has shown that these cells exclusively comprise of migrating endothelial cells, which help to establish testis-specific vasculature, including the development of the characteristic coelomic vessel on the outermost surface of the testis . The formation of side branches from the coelomic vessel delineates the position and division of the developing testis cords (Coveney et al. 2008).
Cell lineages of the developing gonads . The indifferent or bipotential gonad (left) contains a mixture of somatic (orange) and germ (blue) cells, with the latter migrating in from surrounding tissues. In males (top row), SRY triggers the development of Sertoli cells (orange) that organize themselves into cords surrounding groups of germ cells (prespematogenic testis). The cord is surrounded by peritubular myoid cells (grey), which together with Sertoli cells provide structural integrity to the cord through the deposition of the basal lamina. Between the cords, the steroidogenic Leydig cells (green) secrete testosterone. In the spermatogenic testis, mitotic spermatogonial stem cells, located at the outer edge of the cord (blue), provide for continuous spermatogenesis. In females (bottom row), the absence of SRY causes the supporting cell lineage (orange) to follow a granulosa cell fate. These cells eventually surround individual germ cells (blue) to form primordial follicles. The steroidogenic cell lineage (green) become theca cells which surround the granulosa cells. Following ovulation, the granulosa and theca cells luteinize to form the steroidogenic corpus luteum
Testis cords are initially composed of three cell types: germ cells which will form spermatogonia; Sertoli cells which enclose the germ cells, and peritubular myoid (PM) cells which surround and contribute to the structural integrity of the testis cords. A basement membrane is deposited around the testis cord forming a distinct barrier. Located between the cords are interstitial cells, which include Leydig and endothelial cells (Fig. 1.2).
Sertoli cells differentiate early in testicular development and are critical mediators of testis patterning. While SRY is the primary trigger for Sertoli cell development, it is not necessary. Sertoli cells can develop from XX bearing cells lacking the Y-chromosome (and Sry) altogether in mouse XY – XX chimeric gonads (Palmer and Burgoyne 1991). Prostaglandin D2 (PGD2) , a paracrine signal secreted by Sertoli cells is able to recruit neighbouring supporting cell precursors to a Sertoli fate (Wilhelm et al. 2005). As such, Sertoli cells both direct and help reinforce an overall testis phenotype from the developing gonad.
Once Sertoli cells are specified, the formation of testicular cords involves their aggregation around clusters of immigrant germ cells (Kanai et al. 1991, 1992). Sertoli cells form tight junctions with one another building a continuous barrier around each cord, followed by another peripheral layer of flattened PM cells (Pelliniemi and Frojdman 2001; Frojdman et al. 1989). A basement membrane , which forms the blood-testis barrier, is deposited on the basal side of the Sertoli cell through interactions with the adjacent PM cells (Richardson et al. 1995; Tung and Fritz 1993). The basement membrane (basal lamina) is an essential component for male fertility and the structural integrity of the testis. Structurally, it is comprised of laminin (Kleinman et al. 1993), collagen (Paulsson 1992) and heparin sulphate proteoglycans (Timpl 1993). Similar structural components are also found in the ovary but are fragmented and do not arrange into continuous basement membranes.
The germ cells , which will later form the sperm, are critical for male fertility but, interestingly, are dispensable for testicular differentiation. In germ cell deficient mice, testicular cords form normally (Merchant 1975). However, the converse does not old true and Sertoli cell development and cord formation are necessary for triggering male germ cell development and spermatogenesis. The decision for a germ cell to follow a male or female fate is not intrinsically determined by its chromosomal complement XX or XY, but instead is determined extrinsically by the identity of the supporting cells which surround it (Palmer and Burgoyne 1991; Burgoyne et al. 1988). In both males and females the gonad is flooded with retinoic acid (RA) , which triggers germ cell entry into meiosis, a female developmental fate during foetal life. However, in males, the meiosis-inducing signal from RA is rapidly catabolized to prevent it from reaching the germ cells . This is mediated by the CYP26B1 enzyme , produced in large quantities by the Sertoli cells (Bowles et al. 2006; Koubova et al. 2006). In the absence of an appropriately timed meiotic entry signal from RA, germ cells in a testis instead arrest in mitosis, preserving their proliferative potential in the mature gonad.
Leydig cells are the major steroidogenic cell lineage of the testis that reside between the testicular cords, and are primarily associated with blood vessels. Two different types of Leydig cells have been identified. The foetal Leydig cells are derived in part from mesonephric cells, while adult Leydig cells form much later in development and have different embryological origins (Payne et al. 1996). Both types, however, serve to produce testosterone, responsible for the coordinated masculinization and virilisation of the reproductive system (Payne et al. 1996).
1.4 Male Urogenital Tract Differentiation
The early embryo has both Wolffian (mesonephric) and Müllerian (paramesonephric) ducts contained within the mesonephros (Fig. 1.1). Males must promote the differentiation of the Wolffian duct (Josso 1970a, b), while actively driving the removal of the Müllerian ducts. The maintenance and elaboration of the Wolffian ducts is driven by testosterone produced initially by the fetal Leydig cell population. Under the influence of androgens , the Wolffian duct will undergo regionally restricted differentiation to form the epididymis, vas deferens and seminal vesicles (Fig. 1.1). The differential development of regions of the Wolffian duct is controlled in part by homeobox genes and growth-factors that show restricted domains of expression throughout the tubule, each acting downstream of androgen signalling (Hannema and Hughes 2007).
The removal of the Müllerian ducts is critical for normal testicular descent to occur in males. This is mediated by Müllerian inhibiting substance (MIS) , also known as anti-Müllerian hormone (AMH) , one of the first proteins produced by the Sertoli cells in the early gonad. AMH actively drives the degeneration of the Müllerian ducts (Behringer et al. 1994; Behringer 1994) through binding to the AMH receptor, AMHR2, and inducing apoptosis (Kobayashi et al. 2011). Mutations in either AMH or AMHR2 cause persistent Müllerian duct syndrome (PMDS) in human. If left untreated, PMDS can dramatically affect male fertility and most often requires surgical intervention to retrieve abdominal testes and remove the Müllerian derivatives, which interfere with the development of the vas deferens and epididymis (Ju et al. 2013).
The development of the prostate and male external genitalia is driven by conversion of foetal testosterone to the more potent androgen, dihydrotestosterone (DHT), by the enzyme 5α-reductase (Yamada et al. 2006). The prostate forms from the internal (pelvic) urethral tissue of the urogenital sinus. Androgen stimulation causes prostatic buds to form from the urethra, which then undergo elongation and further branching morphogenesis to form the basis of the adult prostate (Keil et al. 2012). Although the adult mouse and human prostate have dissimilar morphologies, their embryological origin and function are the same (Allgeier et al. 2010). Continued epithelial budding of the urogenital sinus under the influence of androgens also gives rise to the bulbourethral glands , a male accessory organ that produces a viscous fluid to aid in ejaculation, caudal to the prostate and adjacent to the penile urethra (Fig. 1.1) (Allgeier et al. 2010).
Not unlike the indifferent gonad, the external genitalia initially form as bipotential anlagen identical in structure between males and females. This structure, located anterior to the cloaca is known as the genital tubercle (Fig. 1.3). Under the influence of androgens, namely DHT, the genital tubercle shows increased cellular proliferation and outgrowth in males. The urethra becomes internalized in the forming penis by the invasion of the urorectal septum (URS) into the phallus. The URS originates from within the cloaca and grows distally into the phallus, resulting in the urethral meatus terminating at the tip of the penis (Perriton et al. 2002; Cohn 2004). Androgens also drive the fusion of the labio-scrotal bulges, which form the male scrotum into which the testes will later descend.
Sexual differentiation of the external genitalia . The external genitalia initially form the sexually indifferent genital tubercle . This structure will develop into the male and female external genitalia, dependent on the presence or absence of testosterone respectively. In the presence of androgen (namely testosterone and DHT), the genital tubercle will under go outgrowth and elongation. The glans (red) will form the glans penis and the urethral folds (pink) will fuse and form the penile shaft and prepuce eventually covering the glans penis. The labio-scrotal bulges (blue) will fuse at the midline to form a continuous scrotum into which the testes will descend. The perineum also undergoes active proliferation in males resulting in a distally located anus (yellow) which septates from the cloaca early in development. This leads to a greater anogenital distance (distance between the anus and genital opening) in males compared to females. In the absence of androgens, the glans (red) forms the clitoris and proliferates only marginally compared to males. The urethral folds (pink) and labio-scrotal bulges (blue) fail to fuse at the midline and go on to form the labia minora and labia majora respectively. The anus (yellow) septates from the cloaca early in development like in males, but fails to migrate as far distally in the absence of androgens, leading to reduced anogenital distance in females
Thus, the development of the male sexual phenotype is a very active process, driven by the rapid development of the testis, which immediately begins to output androgen and AMH , which together act to masculinize the reproductive system. This process is characterized by increased cellular proliferation throughout the male urogenital system, but particularly in the gonads and external genitalia . This is in contrast to several aspects of female urogenital development, which is, at least at the outset, a less active process.
1.5 Ovarian Development
The indifferent gonad will proceed towards an ovarian fate in the absence of the Y-chromosome and/or the upregulation of the Sry gene. Unlike the rapid growth and gross morphological remodelling that happens in a very short time window in the early developing testis, ovarian development starts at a more modest pace. The first gross morphological sign of ovarian development is the distinction between the cortex and medulla and the formation of germ cell nests in the outer cortex which will be the site of oogenesis (Wilhelm et al. 2007). In all mammals, this morphological event occurs at a later developmental time point to the formation of cords in the testis.
The ovary is made up of the same cast of cell lineages as the testis albeit arranged differently to support oogenesis (Fig. 1.2). The germ cells are surrounded by granulosa cells, the female equivalent of the Sertoli cells, which will nurture germ cell development. As development progresses each individual germ cell becomes surrounded by its own layer of granulosa cells forming the primordial follicle (Brennan and Capel 2004). The female equivalent of the Leydig cells are the steroidogenic theca cells, which form around the primordial follicles and provide the hormones necessary for follicle growth and oocyte maturation . The theca cells differentiate from the ovarian stroma in response to signals secreted from the growing follicle (Magoffin 2005). Once a follicle has ovulated, the theca and granulosa cells luteinize to form the highly steroidogenic corpus luteum, which produces progesterone to maintain pregnancy should the ovulated oocyte become fertilized (Henkes et al. 2003).
Early vascularization of the ovary is also very different to that seen in the testis. The ovary forms an extensive network of small capillaries that initially surround each of the germ cell nests and later the individual primordial follicles (Bullejos et al. 2002). These vessels are thought to ensure delivery of growth factors to nurture follicular development and, following ovulation, they export hormones out of the corpus luteum.
Female germ cell proliferation only takes place during early development, in contrast to the continuous proliferation seen in the male germ line. This results in a finite number of follicles being present in the mature ovary. Interestingly, in addition to their finite numbers, the female germ cells also undergo large amounts of apoptosis in early development resulting in an even more limited number of primordial follicles (Borum 1961). This is thought to be a selection process that results in the retention of only the fittest germ cells (Morita et al. 1999). It is still unknown if this represents those germ cells that are intrinsically more fit and/or physically located in a better position within the developing ovary.
The number of ovarian germ cells becomes finite once the cells have entered meiotic arrest in the early foetus. Entry into meiotic arrest is triggered by RA that floods the developing gonads of both males and females. RA triggers the expression of Stra8, which in turn directs germ cell entry into meiosis (Koubova et al. 2006; Bowles et al. 2006).
Interestingly, formation and maintenance of the somatic ovarian environment is dependent, at least in part, on the presence of meiotic germ cells. This is in contrast to the testis where Sertoli cells and testicular cords will form in the complete absence of germ cells (Couse et al. 1999; Hashimoto et al. 1990). In the ovary, the absence of germ cells causes granulosa cell to transdifferentiate into Sertoli-like cells and arrange into cords, reminiscent of a testis-like morphology (Couse et al. 1999). These Sertoli-like cells upregulate key male genes such as Sox9 as if they were in a testis (Britt et al. 2004). Thus, there appears to be vital communications between the germ and somatic cell lineages in the developing ovary that are critical for its correct patterning and maintenance.
Oestrogen is also a critical factor for maintaining ovarian fate. In the oestrogen receptor deficient or aromatase deficient mice (ERKO and ArKO respectively), early ovarian differentiation appears to proceed normal, however, shortly after birth the germ cells are lost. As seen in the developing germ cell-deficient ovary (described above) the granulosa cells transdifferentiate to Sertoli-like cells, form cords and upregulate Sox9 (Britt et al. 2002). Upon administration of oestrogen back to ArKO mice, the ovarian histology is restored and Sox9 is downregulated (Britt et al. 2004). Furthermore, exogenous oestrogen administration to the bipotential gonad in marsupial mammals can drive complete ovarian development from XY gonads (Pask et al. 2010). Together, these findings suggest that ovarian somatic cell fate is malleable and can transdifferentiate towards a Sertoli cell fate in the absence of oestrogen or meiotic germ cells.
1.6 Female Urogenital Tract Differentiation
Without the formation of a testis there is no early production of testosterone or AMH . Testosterone and its more potent metabolite DHT are required for the persistence and elaboration of the Wolffian (mesonephric) ducts, and in their absence in females, the ducts passively regress. Conversely, in the absence of AMH, the Müllerian (paramesonephric) ducts persist and elaborate to form the fallopian tubes, uterus, cervix and upper portion of the vagina (Fig. 1.1). Because development of the female urogenital system happens in the absence of both testosterone and AMH it is often referred to as the passive or default pathway . However, development of the Müllerian ducts is still an active process that requires precise regional development that is controlled, in part, by Hox genes (Du and Taylor 2004).
Similarly, in the absence of testosterone and DHT in the circulation of early female embryos, the external genitalia become feminized. Rather than this being an active process, the feminization results from failure of the external genitalia to become masculinized. In the absence of testosterone the embryonic phallus elongates only marginally and becomes the clitoris. The urethral folds and genital swellings remain separated (unlike in males where these fuse to form the penis and scrotum) and become the labia minora and labia majora respectively (Fig. 1.3). These structures proliferate as the embryo grows and eventually surround the clitoris to form the female external genitalia . The urogenital sinus remains open and contributes to the formation of the lower vagina (Yamada 2005).
Another sexually dimorphic feature and a defining characteristic of mammals is the development of the mammary glands . The underlying mammary line (milk duct) forms around the time when gonads first appear in the early embryo. The mammary placodes form along the mammary lines and bud inwards from an invagination of the epithelium. These mammary buds then undergo branching morphogenesis in association with the mammary fat pad and will eventually give rise to the nipples. As development progresses oestrogen production from the ovary triggers the mammary ducts to continue to expand through branching morphogenesis to invade the underlying mammary fat pad (Hens and Wysolmerski 2005). During pregnancy and partuition the mammary ducts undergo further dynamic rounds of cell division and differentiation to prepare for milk production and let-down. Luminal cells within mammary aveoli formed from the branching ducts are the sites of milk production. Milk is channelled through the ductal system to the nipple through myoepithelial contractions (Daniel and Smith 1999). Following weaning of the young the mammary gland undergoes significant rounds of apoptosis (involution) to remodel to the pre-pregnancy state. A large number of dynamically expressed non-coding RNAs have been identified during specific stages of mammary development and involution, however their precise roles in these processes are yet to be defined (Hennighausen and Robinson 2005; Siegel and Muller 2010).
Interestingly , several genes subject to genomic imprinting are expressed in the mammary gland that are either lncRNAs themselves or regulated in part by lncRNAs (Adriaenssens et al. 1999; Stringer et al. 2012b). Genomic imprinting is hypothesized to have evolved to help regulate nutrient provisioning between mother and offspring. As a result, many imprinted genes are expressed in the invasive placenta of eutherian mammals (Angiolini et al. 2006). In contrast, marsupial mammals have a relatively short gestation, with a substantially less invasive placenta that appears to be less reliant on imprinted gene expression (Renfree et al. 2008). However, the short gestation is traded for a long and sophisticated period of lactation during which the bulk of maternal provision to the young occurs (Tyndale-Biscoe and Renfree 1987). Thus, in marsupials , the primary site of maternal-young nutrient exchange occurs through the mammary gland (Renfree et al. 2013). Consistent with a role for imprinted genes in nutrient provision, several imprinted genes known to be regulated by long non-coding RNAs, have been identified in the marsupial mammary gland (Stringer et al. 2012a, b). Thus it appears that long non-coding RNAs may play a highly conserved and fundamental role in the regulation of this sexually dimorphic organ.
1.7 Summary
Development of the gonad and associated urogenital and secondary sexual characteristics is a highly dynamic, tightly regulated and critical for species fitness. However, despite its importance, the process is also highly fallible. The following chapters will describe recent advances in our understanding of the control of the molecular pathways underpinning reproductive development and the role that non-coding RNAs play in this complex and critical process.
References
Adriaenssens E, Lottin S, Dugimont T, Fauquette W, Coll J, Dupouy JP, Boilly B, Curgy JJ (1999) Steroid hormones modulate H19 gene expression in both mammary gland and uterus. Oncogene 18(31):4460–4473. doi:10.1038/sj.onc.1202819
Allgeier SH, Lin TM, Moore RW, Vezina CM, Abler LL, Peterson RE (2010) Androgenic regulation of ventral epithelial bud number and pattern in mouse urogenital sinus. Dev Dyn 239(2):373–385. doi:10.1002/dvdy.22169
Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M (2006) Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta 27(Suppl A):S98–S102, doi:S0143-4004(06)00003-8 [pii]
Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G (2006) Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod 74(1):195–201
Behringer RR (1994) The in vivo roles of mullerian-inhibiting substance. Curr Top Dev Biol 29:171–187
Behringer RR, Finegold MJ, Cate RL (1994) Mullerian-inhibiting substance function during mammalian sexual development. Cell 79(3):415–425, doi:0092-8674(94)90251-8 [pii]
Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA (2000) A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet 26(4):490–494
Borum K (1961) Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res 24:495–507
Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P (2006) Retinoid signaling determines germ cell fate in mice. Science 312(5773):596–600, doi:1125691 [pii]
Brennan J, Capel B (2004) One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5(7):509–521. doi:10.1038/nrg1381
Britt KL, Kerr J, O’Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK (2002) Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J 16(11):1389–1397. doi:10.1096/fj.01-0992com
Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK (2004) Estrogen actions on follicle formation and early follicle development. Biol Reprod 71(5):1712–1723. doi:10.1095/biolreprod.104.028175
Bullejos M, Bowles J, Koopman P (2002) Extensive vascularization of developing mouse ovaries revealed by caveolin-1 expression. Dev Dyn 225(1):95–99. doi:10.1002/dvdy.10128
Burgoyne PS, Buehr M, McLaren A (1988) XY follicle cells in ovaries of XX – XY female mouse chimaeras. Development 104(4):683–688
Capel B, Albrecht KH, Washburn LL, Eicher EM (1999) Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev 84(1–2):127–131
Cohn MJ (2004) Developmental genetics of the external genitalia. Adv Exp Med Biol 545:149–157
Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS (1999) Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science 286(5448):2328–2331, doi:8111 [pii]
Coveney D, Cool J, Oliver T, Capel B (2008) Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A 105(20):7212–7217, doi:0707674105 [pii]
Daniel CW, Smith GH (1999) The mammary gland: a model for development. J Mammary Gland Biol Neoplasia 4(1):3–8
Du H, Taylor HS (2004) Molecular regulation of mullerian development by Hox genes. Ann N Y Acad Sci 1034:152–165, doi:1034/1/152 [pii]
Frojdman K, Paranko J, Kuopio T, Pelliniemi LJ (1989) Structural proteins in sexual differentiation of embryonic gonads. Int J Dev Biol 33(1):99–103
Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346(6281):245–250. doi:10.1038/346245a0
Hannema SE, Hughes IA (2007) Regulation of Wolffian duct development. Horm Res 67(3):142–151, doi:96644 [pii]
Harry JL, Koopman P, Brennan FE, Graves JA, Renfree MB (1995) Widespread expression of the testis-determining gene SRY in a marsupial. Nat Genet 11(3):347–349. doi:10.1038/ng1195-347
Hashimoto N, Kubokawa R, Yamazaki K, Noguchi M, Kato Y (1990) Germ cell deficiency causes testis cord differentiation in reconstituted mouse fetal ovaries. J Exp Zool 253(1):61–70. doi:10.1002/jez.1402530109
Henkes LE, Davis JS, Rueda BR (2003) Mutant mouse models and their contribution to our knowledge of corpus luteum development, function and regression. Reprod Biol Endocrinol 1:87. doi:10.1186/1477-7827-1-87
Hennighausen L, Robinson GW (2005) Information networks in the mammary gland. Nat Rev Mol Cell Biol 6(9):715–725. doi:10.1038/nrm1714
Hens JR, Wysolmerski JJ (2005) Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res 7(5):220–224, doi:bcr1306 [pii]
Hubertus J, Lacher M, Rottenkolber M, Muller-Hocker J, Berger M, Stehr M, von Schweinitz D, Kappler R (2011) Altered expression of imprinted genes in Wilms tumors. Oncol Rep 25(3):817–823. doi:10.3892/or.2010.1113
Josso N (1970a) Action of human testis on rat fetus Muller’s duct in organ culture. C R Acad Sci Hebd Seances Acad Sci D 271(23):2149–2152
Josso N (1970b) Action of testosterone on the Wolffian duct of rat fetus in organ culture. Arch Anat Microsc Morphol Exp 59(1):37–49
Ju X, Li Z, Zhang C, Qin C, Shao P, Li J, Li P, Cao Q, Zhang W, Wang Z, Yin C (2013) Clinical aspects and molecular genetics of persistent mullerian duct syndrome associated with transverse testicular ectopia: report of three cases. Urol Int 90(1):83–86, doi:000339599 [pii]
Kanai Y, Hayashi Y, Kawakami H, Takata K, Kurohmaru M, Hirano H, Nishida T (1991) Effect of tunicamycin, an inhibitor of protein glycosylation, on testicular cord organization in fetal mouse gonadal explants in vitro. Anat Rec 230(2):199–208. doi:10.1002/ar.1092300207
Kanai Y, Kawakami H, Takata K, Kurohmaru M, Hirano H, Hayashi Y (1992) Involvement of actin filaments in mouse testicular cord organization in vivo and in vitro. Biol Reprod 46(2):233–245
Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, Higashinakagawa T (1998) Male-to-female sex reversal in M33 mutant mice. Nature 393(6686):688–692. doi:10.1038/31482
Keil KP, Mehta V, Abler LL, Joshi PS, Schmitz CT, Vezina CM (2012) Visualization and quantification of mouse prostate development by in situ hybridization. Differentiation 84(3):232–239, doi:S0301-4681(12)00106-5 [pii]
Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS (1993) The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm 47:162–186
Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, Behringer RR (2011) beta-Catenin is essential for Mullerian duct regression during male sexual differentiation. Development 138(10):1967–1975, doi:dev.056143 [pii]
Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R (1990) Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348(6300):450–452
Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351(6322):117–121. doi:10.1038/351117a0
Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 103(8):2474–2479, doi:0510813103 [pii]
Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R (1993) WT-1 is required for early kidney development. Cell 74(4):679–691, doi:0092-8674(93)90515-R [pii]
Kuroki S, Matoba S, Akiyoshi M, Matsumura Y, Miyachi H, Mise N, Abe K, Ogura A, Wilhelm D, Koopman P, Nozaki M, Kanai Y, Shinkai Y, Tachibana M (2013) Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science 341(6150):1106–1109, doi:341/6150/1106 [pii]
Lee P, Schober J, Nordenstrom A, Hoebeke P, Houk C, Looijenga L, Manzoni G, Reiner W, Woodhouse C (2012) Review of recent outcome data of disorders of sex development (DSD): emphasis on surgical and sexual outcomes. J Pediatr Urol 8(6):611–615, doi:S1477-5131(12)00253-7 [pii]
Lovell-Badge R, Robertson E (1990) XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109(3):635–646
Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77(4):481–490, doi:0092-8674(94)90211-9 [pii]
Magoffin DA (2005) Ovarian theca cell. Int J Biochem Cell Biol 37(7):1344–1349, doi:S1357-2725(05)00057-9 [pii]
Malki S, Berta P, Poulat F, Boizet-Bonhoure B (2005) Cytoplasmic retention of the sex-determining factor SOX9 via the microtubule network. Exp Cell Res 309(2):468–475, doi:S0014-4827(05)00326-5 [pii]
Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B (1997) Male-specific cell migration into the developing gonad. Curr Biol 7(12):958–968, doi:S0960-9822(06)00415-5 [pii]
Merchant H (1975) Rat gonadal and ovarioan organogenesis with and without germ cells. An ultrastructural study. Dev Biol 44(1):1–21
Merchant-Larios H, Moreno-Mendoza N, Buehr M (1993) The role of the mesonephros in cell differentiation and morphogenesis of the mouse fetal testis. Int J Dev Biol 37(3):407–415
Morita Y, Manganaro TF, Tao XJ, Martimbeau S, Donahoe PK, Tilly JL (1999) Requirement for phosphatidylinositol-3′-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology 140(2):941–949
Palmer SJ, Burgoyne PS (1991) In situ analysis of fetal, prepuberal and adult XX – XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 112(1):265–268
Pask AJ, Calatayud NE, Shaw G, Wood WM, Renfree MB (2010) Oestrogen blocks the nuclear entry of SOX9 in the developing gonad of a marsupial mammal. BMC Biol 8(1):113, doi:1741-7007-8-113 [pii]
Paulsson M (1992) Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol 27(1–2):93–127. doi:10.3109/10409239209082560
Payne AH, Hardy MP, Russell LD (1996) The Leydig cell. Cache River Press, Vienna
Pelliniemi LJ, Frojdman K (2001) Structural and regulatory macromolecules in sex differentiation of gonads. J Exp Zool 290(5):523–528. doi:10.1002/jez.1096
Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ (2002) Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol 247(1):26–46. doi:10.1006/dbio.2002.0668
Qin Y, Bishop CE (2005) Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum Mol Genet 14(9):1221–1229
Qin Y, Kong LK, Poirier C, Truong C, Overbeek PA, Bishop CE (2004) Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum Mol Genet 13(12):1213–1218. doi:10.1093/hmg/ddh141
Renfree MB, Ager EI, Shaw G, Pask AJ (2008) Genomic imprinting in marsupial placentation. Reproduction 136(5):523–531, doi:REP-08-0264 [pii]
Renfree MB, Suzuki S, Kaneko-Ishino T (2013) The origin and evolution of genomic imprinting and viviparity in mammals. Philos Trans R Soc Lond B Biol Sci 368(1609):20120151, doi:rstb.2012.0151 [pii]
Richardson LL, Kleinman HK, Dym M (1995) Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod 52(2):320–330
Rossi P, Dolci S, Albanesi C, Grimaldi P, Geremia R (1993) Direct evidence that the mouse sex-determining gene Sry is expressed in the somatic cells of male fetal gonads and in the germ cell line in the adult testis. Mol Reprod Dev 34(4):369–373
Schmahl J, Capel B (2003) Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol 258(2):264–276
Schmahl J, Eicher EM, Washburn LL, Capel B (2000) Sry induces cell proliferation in the mouse gonad. Development 127(1):65–73
Sekido R, Lovell-Badge R (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453(7197):930–934, doi:nature06944 [pii]
Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O et al (1995) Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204(1):22–29. doi:10.1002/aja.1002040104
Siegel PM, Muller WJ (2010) Transcription factor regulatory networks in mammary epithelial development and tumorigenesis. Oncogene 29(19):2753–2759, doi:onc201043 [pii]
Stringer JM, Suzuki S, Pask AJ, Shaw G, Renfree MB (2012a) Promoter-specific expression and imprint status of marsupial IGF2. PLoS One 7(7):e41690. doi:10.1371/journal.pone.0041690
Stringer JM, Suzuki S, Pask AJ, Shaw G, Renfree MB (2012b) Selected imprinting of INS in the marsupial. Epigenetics Chromatin 5(1):14, doi:1756-8935-5-14 [pii]
Svingen T, Koopman P (2007) Involvement of homeobox genes in mammalian sexual development. Sex Dev 1(1):12–23, doi:96235 [pii]
Timpl R (1993) Proteoglycans of basement membranes. Experientia 49(5):417–428
Tung PS, Fritz IB (1993) Interactions of Sertoli cells with laminin are essential to maintain integrity of the cytoskeleton and barrier functions of cells in culture in the two-chambered assembly. J Cell Physiol 156(1):1–11. doi:10.1002/jcp.1041560102
Tyndale-Biscoe CH, Renfree MB (1987) Reproductive physiology of marsupials, Monographs on marsupial biology. Cambridge University Press, Cambridge/New York
Vidal VP, Chaboissier MC, de Rooij DG, Schedl A (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28(3):216–217
Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P (2005) Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol 287(1):111–124
Wilhelm D, Palmer S, Koopman P (2007) Sex determination and gonadal development in mammals. Physiol Rev 87(1):1–28. doi:10.1152/physrev.00009.2006, 87/1/1 [pii]
Yamada G (2005) Reproductive/urogenital organ development and molecular genetic cascades: glamorous developmental processes of bodies. J Biochem 137(6):665–669. doi:10.1093/jb/mvi085, 137/6/665 [pii]
Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y (2006) Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn 235(7):1738–1752. doi:10.1002/dvdy.20807
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Pask, A. (2016). The Reproductive System. In: Wilhelm, D., Bernard, P. (eds) Non-coding RNA and the Reproductive System. Advances in Experimental Medicine and Biology, vol 886. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7417-8_1
Download citation
DOI: https://doi.org/10.1007/978-94-017-7417-8_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7415-4
Online ISBN: 978-94-017-7417-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)