Abstract
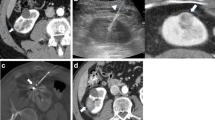
Radiofrequency thermal ablation (RFA) can be performed under the guidance of ultrasound (Fig. 5.1a), computed tomography (CT, Fig. 5.1b), and magnetic resonance imaging (MRI). Ultrasound-guided therapy is the most effectual modality of percutaneous RFA because of adequate targeting capability. However, RFA-generated echogenic cloud of steam during procedure can obscure the target and induce the repositioning of ablation probe and result in overlapping burns difficult. The application of ultrasound contrast available in Europe and Asia is very helpful for ablation operation. Although CT provides the improved depiction of both curative target and RAF ablation probe, working on a gantry is time consuming and less suitable for complex access angles. It is difficult to fit the ablation probes with the gantry, particularly in the treatment of shallow tumors. CT and ultrasound can be used simultaneously to combine the advantages of both of these techniques. Alternatively, image fusion software can be used to superimpose the superior depiction of diagnostic CT and MRI datasets on real-time ultrasound scans to allow the ultrasound-guided probe being placed into sonically occult tumors [1]. MRI guidance, if available, can afford the added advantage of real-time thermal monitoring of the ablation zone.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bile Duct Injury
- Ablation Zone
- Barcelona Clinic Liver Cancer
- Radiofrequency Thermal Ablation
- Magnetic Resonance Imaging Dataset
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Modalities of Imaging-Guided Radiofrequency Thermal Ablation (RFA)
Radiofrequency thermal ablation (RFA) can be performed under the guidance of ultrasound (Fig. 5.1a), computed tomography (CT, Fig. 5.1b), and magnetic resonance imaging (MRI). Ultrasound-guided therapy is the most effectual modality of percutaneous RFA because of adequate targeting capability. However, RFA-generated echogenic cloud of steam during procedure can obscure the target and induce the repositioning of ablation probe and result in overlapping burns difficult. The application of ultrasound contrast available in Europe and Asia is very helpful for ablation operation. Although CT provides the improved depiction of both curative target and RAF ablation probe, working on a gantry is time consuming and less suitable for complex access angles. It is difficult to fit the ablation probes with the gantry, particularly in the treatment of shallow tumors. CT and ultrasound can be used simultaneously to combine the advantages of both of these techniques. Alternatively, image fusion software can be used to superimpose the superior depiction of diagnostic CT and MRI datasets on real-time ultrasound scans to allow the ultrasound-guided probe being placed into sonically occult tumors [1]. MRI guidance, if available, can afford the added advantage of real-time thermal monitoring of the ablation zone.
5.2 Patient Evaluation
The joint expertise of an interdisciplinary oncology team involving hepatology, radiation therapy, medical oncology, oncologic surgery, transplant surgery, pathology, radiology, and interventional oncology is critical in the formulation of treatment protocol for patients with malignant tumors. Given the wide range of participants involved, multidisciplinary meetings are useful to triage challenging and complex patients and establish a two-way referral pattern. A typical clinical evaluation includes the history of present illness (HPI), review of systems, medical and surgical experience, performance status, family and social conditions, allergies, medications, physical examination, laboratory tests, imaging comments, and intention of therapeutic options and prognosis, etc.
The documents on previous radiation therapy and chemotherapy, overall course of the disease, and its progression should be particularly noticed in HPL. Performance status is an indicator of entire practical functional level and the self-care ability of patients, which serves as an index of patients’ well being and is one of the most powerful indexes of prognosis for overall survival rate. The Eastern Cooperative Oncology Group Scale of Performance Status is a 6-point scale ranging from 0 to 5. Point 0 represents that the patient has normal activities without limitation, and point 5 indicates the death of a patient [2]. Evaluation of health-related quality of life in patients is somewhat similar to the estimation of performance status; however, performance status seems more directly focused on the ability of patients to perform daily life activities (eating, bathing, dressing) and other activities (driving, shopping, paying bills) [3]. The laboratory tests should include a complete blood count, creatinine concentration, prothrombin time, and/or international normalized ratio. Additional tests such as a liver function panel, relevant tumor markers such as α-fetoprotein, carcinoembryonic antigen, chromogranin A, and CA199 should be assayed for identifying specific undetermined malignancy. Personal review of cross-sectional imaging is critical during the process of evaluation; simply reading the radiological report is a far way to achieve satisfaction. Baseline imaging should be taken optimally within a month prior to therapy, which will be used for comparisons of tumor curative response more accurately later. Careful evaluation of tumor number, size, morphology, adjacent structures, and extrahepatic metastases will benefit the selection of treatment modality and feasibility.
The prognosis of patient is closely associated with tumor staging. The Child-Turcotte-Pugh (CTP) score is implemented for assessment of liver disease, which is not a tumor staging system but rather a measure of hepatic reserve. The CTP score is derived from a point system based on serum levels of albumin, total bilirubin, prothrombin time, the presence of ascites, and hepatic encephalopathy. While the CTP score increases, surgical risk increases as well and the life expectancy decreases correspondingly. The HCC staging systems are composed of the Okuda staging system, the Cancer of the Liver Italian Program classification (CLIP), the Barcelona Clinic Liver Cancer Staging classification (BCLC), the United Network for Organ Sharing (UNOS TNM), the Chinese University Prognostic Index, the Liver Cancer Study Group of Japan, and the American Joint Committee on Cancer/ International Union Against Cancer. No single staging system has been proven better than the other. Nevertheless, the BCLC and CLIP staging systems of HCC appear to be the more preferred systems for interventional oncologists. Currently, a variety of transplant centers use the UNOS staging in clinical practice [4].
After a comprehensive evaluation, all therapeutic options should be considered when formulating a treatment regimen. Orthotopic liver transplant is a curative option for a part of HCC patients. Surgical resection of HCC is often not an option due to inadequate functional liver reserve (FLR) of cirrhosis [4]. Interventional oncologists play a key role in the management of patients who are not eligible or who are unwilling to accept surgery. Based on the tumor size and tumor number, ablation may be the sole choice of operation; alternatively, it may be combined with other regional and systemic therapies. The location of tumors will affect the feasibility and safety of percutaneous ablation when close to great vessels versus a regular surgical approach (Figs. 5.2, 5.3, 5.4, and 5.5). Such patients are a rich source of two-way referrals with surgical oncology.
5.3 Indications and Contraindications of RFA
The Milan criteria (solitary HCC <5 cm in diameter, multiple HCCs ≤3 in number and each tumor < 3 cm in diameter) have been accepted as the indications of RFA in many institutes. The limitation of tumor numbers in patients with multiple HCCs depends on several clinical considerations, including the general performance of the patients, the capacity of tolerance to surgical procedure, the skill and experience of the operator, and the whole processing time. Therefore, the number of tumors treatable in one session should be determined based on the individual situation although “no greater than three” appears to be the most widely accepted criterion.
With respect to the maximal tumor diameter, there are several issues that are required to be taken into consideration. Apparently, RFA has limited capability to completely ablate the tumors with “large size.” However, what is the definition of “large size” is undetermined [5, 6]. Some investigators suggested that the borderline of HCC <2 cm in diameter was considered “large size,” which was used as a criterion for patients who received RFA at a very early tumor stage in the Barcelona Clinic Liver Cancer (BCLC) staging system and showed excellent long-term outcomes after RFA treatment. From the histopathological perspective, HCC <2 cm in diameter is known to have a greater well-differentiated area and fewer microsatellite lesions (3 % of the cases), which are usually within 5 mm of the tumor with less portal microinvasion [7]. The treatment guidelines published by the BCLC group in 2012 recommended that ablation prior to small HCCs resection is a curative option to patients, except liver transplantation [8], which is a major revision from the previous version. Given these facts, we propose that RFA can be used as a first-line therapy for patients with a small HCC <2 cm in diameter, even if the tumor resection is a feasible surgical option.
In view of the inadequacy of RFA devices available currently and the disadvantages of overlapping ablation techniques and the ablative margin, the patients with HCC sized ≥5.0 cm in diameter should not be treated with RFA alone [8]. For them, other therapeutic options or combination therapy should be considered.
Nonetheless, what about the patients who are in gray zone between these situations? It is evident that the larger tumor size is commonly accompanied by a higher incidence of local tumor progression (LTP) after RFA, whereas a larger ablation zone would increase the risks of complications. Therefore, the decision of whether to take RFA for HCCs patients in gray zone should be made only after serious evaluation of hepatic functional reserve of the patients and the availability of other therapeutic modalities. This is even more important if we take into account that extending the tumor size to 3 cm in diameter increases the chance of spreading satellite nodules to 19 % [9]. RFA should be avoided in cases of impending portal venous invasion or in patients with risk factors for possible diffuse recurrence [10]. When tumor resection is infeasible and RFA is replaced for HCC patients in gray zone, skill to enlarge the ablation area or combination therapy such as TACE should be adopted. The survival benefits of combination therapy of RFA plus TACE superior to RFA alone in the treatment of HCCs sized at 3–5 cm in diameter were demonstrated in a randomized controlled trial (RCT) [11]. A consensus-based clinical practice manual [12] of the Japanese Society of Hepatology recommends to taking RFA combined with TACE in the management of HCC with size larger than 3 cm.
As for hepatic functions, it should be minimally kept at the level which is able to tolerate the loss of hepatic parenchyma induced by ablation in order to benefit from the tumor removal in terms of survival gain. Thus, patients with Child-Pugh class C are not usually indicated for RFA. Contraindications of percutaneous RFA include uncorrectable coagulopathy, liver failure, unadjustable proximity to critical structures, and extrahepatic metastases (except small, slow-growing lung metastases or minimal adenopathy) [13]. Mortality of HCC during the treatment ranged from 0.1 % to 0.5%, and the incidences of major complication were from 2.2 % to 3.1%. The most common causes of death were sepsis, hepatic failure, colon perforation, and portal vein thrombosis except for several patients who died of cardiac or pericardial injury. Tumors in precarious locations should be referred for laparoscopic or open ablation. The most common complications were intraperitoneal bleeding hepatic abscess, bile duct injury, hepatic decompensation, and grounding pad burns. Tract ablation or embolization may help to prevent bleeding. Patients with prior biliary stents or surgery are at increased risk for liver abscess and should receive prophylactic antibiotics [14]. Ablation zones should be kept at 1 cm away from the liver hilum to avoid major bile duct injury. The grounding pads should be checked periodically during the procedure, especially during prolonged ablations at high wattage to avoid skin burns. Tumor seeding metastasis occurred in 0.2–0.6 % of patients and was of particular concern in patients who were candidates for liver transplantation [15].
5.4 Percutaneous RFA Procedure
Percutaneous ablation is usually conducted in an outpatient clinic under intravenous moderate sedation or monitored anesthesia care. Ablation with heat is very painful and requires deep sedation to induce the patients with spontaneous breathing but no voice response. An oxygen mask is often required to maintain adequate oxygen saturation. Pay attention to proper neck and jaw position of the patient to maintain an unobstructed airway. Electrocardiogram, blood pressure, pulse, and pulse oximetry should be monitored throughout the procedure. After careful review of the diagnostic images, preliminary scanning is performed to choose an appropriate site for probe entry based on the conditions of trajectory to the target, probe length, nontarget structures, and physical constraints of the CT or MRI gantry. When RFA operation is close to the bowel, abdominal wall, or diaphragm, infusion of 500–1000 mL 5 % dextrose to develop artificial ascites is necessary to protect against thermal injury of nontarget tissues and decrease intra- and post-operative pain [16]. The type and number of probes and the number of ablations depend on the operative goal to achieve a total ablation zone 5–10 mm larger than the tumor entity. This additional circumferential margin of ablation is beneficial to treat the microscopic satellite lesions and mitigates local recurrence. If the tumor diameter is equal to or larger than the ablative size of a single ablation, it requires multiple overlapping burns. Geometric modeling assuming spherical target and ablation zones implicate that eight perfectly overlapped ablations would be needed to initialize an ablative coverage of 1 cm larger than tumor size. An impossibility in clinical practice accounts for the high local failure rate as tumor size larger than 3 cm in diameter. Another technical consideration is perfusion of mediated cooling via adjacent veins (the “heat-sink” effect). Veins even as small as 3 mm have sufficient flow to produce convective cooling in ablation area. Placement of a balloon occlusion catheter into the hepatic vein or inferior vena cava during operation may help to mitigate the burning effect [17]. RFA operation is monitored by automated systems within the generator that provide the feedback of time, temperature, and/or impedance with management resolution specified by an individual manufacturer. The most thermal systems allow to perform ablation procedure along with the needle track to decrease the risk of bleeding and tumor seeding metastasis. Triple-phase enhanced CT or MRI may be applied at the end of the procedure to evaluate the completion of ablation and identify the intense inflammatory reaction to RFA resulting in substantial perilesional enhancement and abnormal perfusion patterns in surrounding liver. The ablation zone shows a nonenhancing area. Peripheral, irregular nodular areas of enhancement near the ablation area are considered of residual tumor.
5.4.1 Skill for Larger Ablation Zones
A conventional RF electrode is able to provoke a coagulation necrosis area less than 1.6 cm in diameter maximally [18]. This function will be reduced by tissue vaporization and/or carbonization, acting as an insulator of electrical currents during performance of RFA. In order to avoid this inherent restriction, several adaptations have been accepted for RF devices available currently, including expandable multi-tined designs, internal cooling by chilled saline, clustered design, pulsing of RF energy, and concomitant saline infusion into the tissue. These techniques contribute to enlarging the RFA zone to 3–4 cm. The application of multiple overlapping ablations or multiple electrodes simultaneously can further increase the area of ablation [18]. Taken together into consideration, HCC sized up to 4–5 cm in diameter can now be treated potentially by RAF technique as well, at least in theory.
5.4.2 Skill for Accurate Targeting
Another crucial factor to confine the application of RFA is the complexity of accurate targeting in certain situations. US is the most common device for guidance of RFA. US-guided visibility of HCC is hindered by a complicated tumor location with a poor acoustic window, which is more likely to present in a cirrhotic liver, or by the small size tumor in a background of macronodular cirrhosis. In the study of Kim et al. [19], US-guided percutaneous RFA was unfeasible in 33.1 % HCC patients mainly ascribed to undetectable tumor. Currently, several skills have been proposed for overcoming this disadvantage. The technique of infusion fluid into the pleural or peritoneal cavity, namely, artificial pleural effusion or artificial ascites, is useful to clear the acoustic window. Minami et al. [20] reported the effectiveness of artificial pleural effusion in achieving a high rate of complete necrosis of HCC (96.4 %). Rhim et al. [21] reported a similar rate of complete treatment (96.0 %) using artificial ascites skill. The artificial ascites is known to be particularly useful in minimizing or preventing the risk of collateral thermal injury by displacing the bowel loop or the diaphragm away from the RFA zone [22]
Fusion imaging is also a useful tool to overcome poor conspicuity of tumors on conventional US imaging. With this method, volumetric CT or MRI data obtained previously are reformatted on a real-time basis in sync with a B-mode US image with the help of an electromagnetic field generator and a sensor attached to the US transducer. Using this technique, Lee et al. [23] reported a technical success rate of 100 % in the treatment of tiny HCCs (mean diameter, 1.0 cm) in patients who had poor conspicuity in US examination. Song et al. [24] applied this method for HCCs patients who were completely non-visible in planning US examinations and showed that 53.3 % of the patients could be treated by RFA. Contrast-enhanced US examination is also helpful for delineating poorly visible HCC. The old generation of contrast agents (e.g., Levovist [Bayer-Schering Phama, Berlin, Germany] and SonoVue [Bracco, Milan Italy]) could only describe hypervascular HCC at the vascular phase. However, the novel microbubble agent Sonazoid (GE Healthcare, Milwaukee, WI) can additionally depict the tumor at post-vascular phase or Kupffer phase (i.e., 10–15 min after administration) as an echo-void lesion on a background of echorich parenchyma, which is much more sustainable. Consequently, RFA is easier for operation technically. Masuzaki et al. [25] reported a significantly increased detection rate of HCC with Sonazoid-enhanced US than those with conventional US (93.2 % vs. 83.5 %, p = 0.04). Combination of fusion imaging with Sonazoid-enhanced US is able to further improve the detection rate of tumors. Min et al. [26] reported that 92 % of fusion imaging in inconspicuous small HCCs might be feasible for RFA after additional Sonazoid enhancement US examination.
5.5 Complications of RFA
There have been substantially more data in terms of the complications occurring in liver ablation than those occurring in any other sites. Overall, the incidence of major complications (2.2–5.7 %) and the mortality (0–1.4 %) were lower in thermal ablation of liver malignancies [27–33]. In contrast, the mortality and major complication rates of microwave ablation (MWA) were reported as 0–0.4 % and 2.6–4.6 %, respectively. In recent studies, the types and incidences of complications caused by HCC RFA and MWA were similar and comparable in clinical setting [28, 34]. The causes of death involved intestinal perforation, portal vein thrombosis, liver failure, septic shock, and massive hepatic hemorrhage [31, 32, 35]. The major complications included hemorrhage, liver failure, injuries to bowel and biliary tree, infections such as abscesses and peritonitis, vascular thrombosis, hepatic infarction, pleural complications (pneumothorax, hemothorax, large effusion drainage), biliary strictures, bilomas, cholecystitis, bronchobiliary fistulas, arteriovenous fistula leading to rapid tumor dissemination, skin burns, and tumor seeding metastasis.
References
Iida H, Aihara T, Ikuta S, et al. Comparative study of percutaneous radiofrequency ablation and hepatic resection for small, poorly differentiated hepatocellular carcinomas. Hepatol Res. 2014;44:E156–62.
Sorensen JB, Klee M, Palshof T, et al. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773–5.
Gomaa AI, Khan SA, Leen EL, et al. Diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2009;15:1301–14.
Gervais DA, Goldberg SN, Brown DB, et al. Society of interventional radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20:S342–7.
Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–9.
Wang JH, Wang CC, Hung CH, et al. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–8.
Livraghi T. Single HCC smaller than 2 cm: surgery or ablation: interventional oncologist’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:425–9.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55.
Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–7.
Lee HY, Rhim H, Lee MW, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23:190–7.
Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–60.
Kudo M, Okanoue T. Japan Society of H: Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72(1):2–15.
Woo S, Lee JM, Yoon JH, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268:589–600.
Elias D, Di Pietroantonio D, Gachot B, et al. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30:823–7.
Stigliano R, Marelli L, Yu D, et al. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437–47.
Hinshaw JL, Laeseke PF, Winter 3rd TC, et al. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol. 2006;186:S306–10.
de Baere T, Bessoud B, Dromain C, et al. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002;178:53–9.
Rhim H, Goldberg SN, Dodd GD 3rd, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;(21 Spec No):S17–35; discussion S36–9.
Kim JE, Kim YS, Rhim H, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011;79:e80–4.
Minami Y, Kudo M, Kawasaki T, et al. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003;38:1066–70.
Rhim H, Lim HK, Kim YS, et al. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190:91–8.
Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–40.
Lee MW, Rhim H, Cha DI, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012;198:1438–44.
Song KD, Lee MW, Rhim H, et al. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol. 2013;201:1141–7.
Masuzaki R, Shiina S, Tateishi R, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759–64.
Min JH, Lim HK, Lim S, et al. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol. 2014;20:61–70.
Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933–40.
Ding J, Jing X, Liu J, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68:608–15.
Buscarini E, Buscarini L. Radiofrequency thermal ablation with expandable needle of focal liver malignancies: complication report. Eur Radiol. 2004;14:31–7.
Nemcek AA. Complications of radiofrequency ablation of neoplasms. Semin Intervent Radiol. 2006;23:177–87.
Howenstein MJ, Sato KT. Complications of radiofrequency ablation of hepatic, pulmonary, and renal neoplasms. Semin Intervent Radiol. 2010;27:285–95.
Takaki H, Yamakado K, Nakatsuka A, et al. Frequency of and risk factors for complications after liver radiofrequency ablation under CT fluoroscopic guidance in 1500 sessions: single-center experience. AJR Am J Roentgenol. 2013;200:658–64.
Koda M, Murawaki Y, Hirooka Y, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42:1058–64.
Bertot LC, Sato M, Tateishi R, et al. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–96.
de Baere T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Zhou, Z., Chen, M. (2016). Percutaneous Radiofrequency Thermal Ablation. In: Chen, M., Zhang, Y., Lau, W. (eds) Radiofrequency Ablation for Small Hepatocellular Carcinoma. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7258-7_5
Download citation
DOI: https://doi.org/10.1007/978-94-017-7258-7_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7257-0
Online ISBN: 978-94-017-7258-7
eBook Packages: MedicineMedicine (R0)