Abstract
Much of the historical development of ecological thought has revolved around disturbance and the responses of organisms, species and assemblages. Coral reefs have figured prominently in this intellectual development. Historically, observed coral populations and communities have been understood as displaying the net balance (though rarely reaching an equilibrium) between disruptive forces and those leading to recovery of coral abundance and composition. The fact of drastic coral decline over the past few decades implies a shift in this balance toward greater influence of disturbance and/or lesser effectiveness of recovery. This chapter examines the likelihood that both expanding disturbances (in identity, scale, intensity, and/or frequency) and impaired recovery processes (resulting at least partially from expanding chronic disturbances) are likely contributors to this shift. The contemplation of progressively more radical management interventions to combat expanding disturbance and faltering recovery invokes a need for targeted research to clarify and minimize risks while maximizing benefits of such intervention strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The concepts and theory of disturbance and recovery are seminal in the field of community ecology and much of this theory is based on coral reefs as a model system. These concepts came to the forefront in the effort to explain the origin and maintenance of species diversity in communities. That is, how do so many species, especially species that appear to make their living in a similar manner (i.e., have similar niches) persist in equilibrium? Several pivotal works in the 1970s revealed that periodic repeated disturbances at different scales generally preclude equilibrium, climax conditions in marine benthic communities (including coral reefs) where space and light are the primary limiting resources (Dayton 1971; Sutherland and Karlson 1977; Connell 1978). By preventing an equilibrium climax community, disturbance is understood to allow the maintenance of high species diversity by precluding competitive exclusion (Connell 1978).
Disturbance, by definition (Box 11.1), is bad for the status quo but not uniformly bad. The effects of a given disturbance event will depend not only on the nature of the disturbance but also on the previous experience of the present assemblage (Hughes and Connell 1999; Mumby et al. 2011). In addition, the variable nature of biotic populations and species implies that the experience and therefore the effects of disturbance will vary between species and among individuals within a species according to their tolerances. These tolerances clearly change over time as genetic or physiological adaptation may adjust the tolerance of individuals within a species and changing species composition may adjust the tolerance of the extant community. Such shifts in species and community tolerance can happen rapidly, especially within the context of rapid environmental changes, and are crucial interactors with disturbance and recovery (discussed more completely in Chap. 7).
Box 11.1
For the purposes of this review, disturbance will be referenced as “killing, displacement, or damaging of one or more individuals (or colonies) that directly or indirectly creates an opportunity for new individuals (or colonies) to become established.” (Sousa 1984, p. 356).
The consequence, however, of disturbance being bad for the status quo is that it creates opportunities for newcomers, both species and individuals. The effective utilization of such opportunities requires successful recruitment and growth of the newcomers. For corals, successful recruitment is determined by a complex interplay of fecundity , fertilization , connectivity , settlement and post-settlement survival (Ritson-Williams et al. 2009). Clearly, the community observed at a given point of space and time represents the balance of loss to recent disturbances and the relative progress of newcomers in recruitment and growth, which will be referred to as recovery . In the classical conception of a coral reef as a stable system with high coral cover and high community diversity , a favorable balance of disturbance and recovery gave the ‘illusion’ of equilibrium.
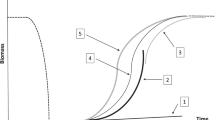
In the past two to three decades, as coral reef ecosystems and corals in particular have begun and proceeded quite far down a course of decline, this balance of disturbance and recovery processes appears to be shifting toward dominance by the former. The progression of ecological theory during this period has been dominated by the growing recognition and discussion of tipping points, alternate stable states, and resilience . Recovery processes may fail to return the community to a pre-disturbance state (i.e. failure of resilience) either because recruitment and growth of newcomers are failing or because the disturbances are too frequent, intense, and/or diverse for recovery to run its course. The result may be an alternate stable state if punctuated acute disturbance s are most important, or a slow, steady decline if chronic disturbance s dominate (Hughes et al. 2012). The trajectory of reefs at Discovery Bay, Jamaica (Fig. 11.1) provide the archetypal example of lost resilience in the face of both acute (hurricane ) and chronic (impaired grazing due to overfishing and collapse of grazing urchin populations) disturbances (Hughes 1994).
Coral cover response to sequential disturbances in the archetypal example of Discovery Bay, Jamaica . The severe acute disturbance of Hurricane Allen (1) precipitated significant coral mortality , after which some recovery was evident, and at a pace that could have returned coral cover (not necessarily community or population structures) to the pre-Allen baseline in less than 15 years (dotted lines). However, the acute mass mortality of Diadema antillarum (2) resulted in massive macroalgal proliferation (a chronic disturbance ) which curtailed coral recovery. A following hurricane , Gilbert (3) continued coral mortality. Subsequent recovery of D. antillarum (at least in small patchy sites) has been reported to have decreased macroalgal cover and greatly increased coral juvenile density in this region (Edmunds and Carpenter 2001) (Redrawn from Hughes 1994)
This chapter will evaluate trends in both disturbance and recovery processes in the radically changing coral reef environments of the ‘Anthropocene’.
11.2 Disturbances: Types and Impacts
Many different typologies of coral reef disturbance have been discussed in previous works. Several of these differentiations are losing meaning in an era wherein human influence on otherwise ‘natural’ or ‘abiotic ’ systems (e.g., climate or ocean carbonate equilibrium) is becoming predominant (Box 11.2). The dichotomy of acute vs. chronic disturbance will be maintained in the current discussion as it retains strong influence on both the recovery processes and on the potential evolutionary responses that result. It should be noted that various disturbance types may occur as both chronic and as acute events, and hence interact and compound each other in complex ways.
Box 11.2: Typologies of Disturbance
Natural vs. Anthropogenic: As denoted by the concept of an ‘Anthropocene’ age, nothing is truly free of anthropogenic influence. We have increasing understanding that ‘natural’ processes determining carbonate chemistry, storms , disease , etc. are all affected by the rapidly changing atmospheric/CO2/ climate system. Most everything is BOTH
Biotic vs. Abiotic: Similarly, changes in fundamental ocean chemistry and climate interact with the biology of organisms in ways that substantially blur this distinction. For example, increased river runoff from extreme rainfall events has been convincingly linked with destructive outbreaks of corallivores (Fabricius et al. 2010).
Global vs. Local: Climate and chemistry-based disturbances are clearly global in origin, but the experience of them is also local. This distinction may be important from a patch dynamics or metapopulation perspective, but several important threats (especially disease and often bleaching ) appear to operate at multiple scales
Chronic vs. Acute: This is a highly relevant dichotomy as these likely have distinct consequences for recovery and evolutionary outcomes. Both types are probably increasing (e.g., extreme thermal bleaching events AND nutrient loading are likely increasing in many reefs). However, as the frequency of acute disturbance s increases, as expected for thermal bleaching over the next two decades, it also blurs to a chronic disturbance .
Various modeling studies have projected effects of various disturbance types or frequencies on coral assemblages. For example the model of Wakeford et al. (2008), based on observed coral mortality , recruitment , growth, and competitive outcomes in a reef patch at Lizard Island between 1981 and 2003, indicates that the actual assemblage was maintained when mortality was concentrated in acute disturbance events (cyclones and/or predation outbreaks) up until 1997. After this date, the observed coral assemblage was only emulated in the model by incorporating additional chronic mortality factors into the model. These authors suggest a fundamental shift in the disturbance regime and/or resilience capacity of this reef during the 1990s decade.
11.2.1 Acute Physical Disturbance
Tropical storms and cyclones are the quintessential physical disturbance, though various degrees of water motion (from swell to tsunamis) and/or substrate disturbance (e.g., earthquakes or ship groundings) may have similar disturbance effects (i.e. killing coral and/or smashing substrate). Their effects can be dramatic but patchy, with the more severe events also causing additional physiographic or geological changes (Stoddart 1963; Connell et al. 1997). Events causing damage to underlying substrate or flow regimes further challenge the recovery potential and time frame. However, over historical times, recovery from storm damage to coral communities was presumed, and many aspects of coral life history, dynamics and genetic structure were attributed to the influence of storm events and their variable effects in time and space (e.g., Hunter 1993; Hughes and Connell 1999; Highsmith et al. 1980; Foster et al. 2013). Tabular or branching coral species show greater susceptibility to storm damage than encrusting or mounding corals. Depth and other habitat characteristics as well as recent history strongly influence the amount of coral damage. For example, Hughes and Connell (1999) delineate the sequence of storm events and their relative impacts in different habitat types in two long term data sets. The effects of repeated storm impacts in both Heron Island, Australia, and the north coast of Jamaica , varied strongly according to the habitat type, the relative dominance of vulnerable (branching or tabular) coral species, and, relatedly, the duration and nature of recovery from the previous disturbance. In both study regions, vast changes in coral composition as well as reduced abundance resulted from storm disturbance when tabular (generally Acropora spp.) corals dominated and little change in composition (and much less change in abundance) was wrought by storms when massive corals comprised the bulk of the community (Hughes and Connell 1999).
Gardner et al. (2005) conducted a large meta-analysis of hurricane effects on Caribbean coral reefs from 1980 to 2001. They estimated that Caribbean reefs affected by a hurricane during this period lost an average of 6 % coral cover per annum in comparison to 2 % per annum for sites not affected by storm damage, a significant difference largely attributable to an average 17 % loss in coral cover in the year following a hurricane impact. Variation in these rates of loss showed positive correlation to storm strength and duration since the previous storm impact. Gardner et al. (2005) also noted a greater difference between the fate of hurricane impacted vs. not-impacted sites in the 1980s decade in comparison to the 1990s, when all sites showed similar rates of decline suggesting non-hurricane factors came to dominate coral trends. On Australia’s Great Barrier Reef, comprehensive monitoring of 214 sites between 1985 and 2012 yielded estimates of average mortality of 1.63 % coral cover per year to cyclones, which was marginally higher than the mortality estimates for predation (1.42 % per year) and substantially higher than that for bleaching (0.34 % per year) (De’ath et al. 2012).
In more recent times, storm -induced coral disturbance has often resulted in follow-on mortality rather than rapid recovery . Evidence is mounting of the linkage of physical disturbance with subsequent coral mortality from disease and/or predation, at least in the Caribbean (Brandt et al. 2013; Knowlton et al. 1981; Bruckner and Bruckner 1997) though the mechanisms behind this interaction, particularly for disease, are not well documented. It is clear, however, that continuing coral mortality following a storm, including mortality of potential fragment propagules, greatly hinders recovery (Williams et al. 2008a). Additional ensuing threats impairing post-storm recovery include the dispersal and propagation of the excavating sponge, Cliona tennis (Lopez-Victoria and Zea 2004) and increased impact from generalist coral predators that may concentrate on reduced or injured populations of preferred prey (Knowlton et al. 1990; Bright 2009)
While the effects of storm disturbances may be increasing over time, there is also the question of whether these disturbance events, themselves, are increasing in frequency or intensity. Additional heat in the ocean/atmosphere system may be expected to drive more tropical cyclones and, indeed, Grinsted et al. (2012) report a significant correlation of large Atlantic cyclone occurrence with warm years and a significant increasing frequency of such events since 1923. A significant increase in the number and proportion of strong tropical cyclones, but not frequency of all cyclones, was detected across all ocean basins over a 35 year period of warming seas (Webster et al. 2005; Emanuel 2005). Meanwhile, Mumby et al. (2011) conducted a spatio-temporal analysis of hurricane occurrence in the Caribbean and showed that Atlantic storms from 1901 to 2010 have been temporally clustered in certain areas, yielding lesser overall reef damage/decline than if storms were randomly distributed. However, the specter of more damaging storms in the future, combined with apparently growing interactions of physical disturbance with other sources of mortality suggests worsening ultimate effects.
11.2.2 Acute Thermal Stress and Mass Bleaching Events
Thermally induced mass bleaching events have become the hallmark of global warming effects on coral reefs, but regional-scale bleaching/mortality events can also result from other environmental stressors such as cold temperatures (Lirman et al. 2011) or low salinity runoff events (Haapkylä et al. 2013). Coral bleaching, the breakdown of symbiosis between the coral host and its zooxanthellae endosymbionts resulting in lost energetic subsidy to the host, can occur following a range of physiological stresses. Comprehensive reviews of bleaching causes and consequences have been provided by Brown (1997) and Baker et al. (2008). Mass bleaching events can be predicted on the basis of cumulative thermal stress measured as Degree Heating Weeks (DHW) over the long term monthly maximum. Generally, coral bleaching risk is considered to be elevated at doses above 4 DHW, i.e. 1 °C for 4 weeks or 4 °C for 1 week (Liu et al. 2006). Recent trends in thermal stress dosage have been increasing at large regional scales with considerable variation at individual sites. Figure 11.2 shows annual maximum DHW averaged over the Caribbean region with larger peaks evident over time corresponding to severe mass bleaching events (Eakin et al. 2010). Similar increasing regional trends in mean temperature or thermal stress dosage have been documented in the Coral Triangle (Penaflor et al. 2009), the Arabian Gulf (Riegl et al. 2011), and globally (Lough 2000). Increasing temperature trends are expected to continue with the frequency of mass bleaching disturbances predicted to increase over the coming decades, possibly to annual or nearly so (e.g., Donner et al. 2005) with dire consequences for coral populations and coral reefs (McClanahan et al. 2007; Hoegh-Guldberg 1999).
Cumulative warm thermal stress in Degree Heating Weeks (DHW) over two decades averaged over the Caribbean basin (0.5° map pixels containing coral reefs, bounded by 35°N, 55°W, and the coast of the Americas). Severe regional mass bleaching events occurred in 1995, 1998, and 2005 (Source: Eakin et al. 2010)
The first recognized global-scale mass bleaching events were associated with El Niño warming events in 1982–1983 and 1997–1998. Additional basin-scale events were documented in 2005 in the Atlantic/Caribbean (Eakin et al. 2010) and 2010 throughout Southeast Asia and Arabian Gulf regions (Riegl et al. 2011; Guest et al. 2012). Cumulative thermal stress events as well as corresponding bleaching events in the Caribbean region appear to be intensifying over time (Fig. 11.2; McWilliams et al. 2005).
Differential thermal bleaching susceptibilities among taxa (Marshall and Baird 2000; McClanahan et al. 2007; McField 1999) and among members within a species either based on factors such as symbiont type, latitude or habitat (Ulstrup et al. 2006), and prior thermal history (Guest et al. 2012; Middlebrook et al. 2008; Thompson and Van Woesik 2009) clearly influence the patterns of bleaching and resultant mortality . Generally, colonies or taxa previously exposed to moderately fluctuating or moderately extreme temperatures appear to show greater thermal thresholds and lesser severity of bleaching.
The direct mortality caused by bleaching is generally poorly quantified, but represents the most severe aspect of this disturbance. Meanwhile, bleaching itself represents disturbance as ‘damage’ (Box 11.1) which can manifest as increased disease susceptibility (discussed below) or impaired reproduction. Thermal stress and bleaching of parent colonies have been shown to limit subsequent reproductive success, primarily via reduced fecundity (Szmant and Gassman 1990; Baird and Marshall 2002) and possibly reduced fertilization (Omori et al. 2001). Baird and Marshall (2002) is one of the few studies to quantify both mortality and reproductive impacts by following the fate of bleached colonies of four species after the 1998 bleaching event. Two Acropora species (hyacinthus and millepora) showed greater whole colony mortality than Porites lobata and Platygyra daedalea. These two Acropora spp. also varied in their subsequent reproductive impairment with only 45 % of bleached A. hyacinthus colonies gravid the following year, compared with 88 % for A. millepora. Hence, bleaching susceptibility, associated mortality, and subsequent reproductive impairment are all known to vary greatly among species.
Like physical disturbance, thermal stress and bleaching are often associated with follow-on mortality from coral disease . For example, bleached corals in the US Virgin Islands suffered over 60 % mortality several months after the 2005 bleaching event (Miller et al. 2009). Individual bleached colonies also show greater likelihood of manifesting disease mortality (Brandt and McManus 2009; Muller et al. 2008). Ritchie (2006) describes a possible mechanism for increased disease susceptibility of bleached corals in that the usual antibiotic activity of mucus -associated microbes was found to be absent in mucus from bleached colonies.
11.2.3 Acute Disease Outbreaks
The profound effect of acute diseases (those causing rapid tissue mortality ) on coral assemblages and coral reef communities, especially in the Caribbean basin, in the past three decades is difficult to overstate, yielding massive changes in the overall makeup of coral communities. Beginning in the early-mid 1980s and with progressive extent and effect through the next two decades (Sutherland et al. 2004) disease has ravaged coral populations including the major reef-builders, Acropora spp (Aronson and Precht 2001; Gardner et al. 2003) and later Montastraea (now Orbicella ) spp. (Miller et al. 2009; Bruckner 2012). Impacts of disease outbreaks in the Indo-Pacific region have been less severe, but added scrutiny of coral disease phenomena throughout the Indo-Pacific basin in recent years has revealed that coral disease affects most regions at increasing prevalence and in greater varieties with time (Raymundo et al. 2008). Coral disease impacts have been reported even in the most remote Pacific reefs (Williams et al. 2008b; Aeby 2005). Outbreaks occur both in concert with other acute (discussed above) and chronic disturbance s such as nutrient or sewage pollution (Kaczmarsky and Richardson 2011), but can also occur in apparent isolation (Richardson et al. 1998; Nugues 2002; Roff et al. 2011; Miller and Williams 2006; Williams et al. 2008b).
The apparent disease -induced range-wide mass mortality of grazing urchins , Diadema antillarum, also in the early 1980s, is the other disease event of profound importance in the recent history of Caribbean reefs. The loss of grazing capacity on most reefs was a major contributor to described lack of recovery on these reefs (Fig. 11.1; Lessios 1988).
11.2.4 Acute Predator Outbreaks
The most influential predator on Indo-Pacific corals, the crown-of-thorns seastar (COTS; Acanthaster planci) undergoes dramatic population outbreaks which kill large amounts of coral. COTS display strong and consistent preferences among different coral prey, yielding highly selective mortality of Acroporids and other tabular growth forms in affected reefs with potentially strong effects on coral community structure. COTS outbreaks are common, regional scale acute disturbance s across the Indo Pacific region (Birkeland and Lucas 1990; De’ath and Moran 1998), though their relative influence may be under-appreciated in regions with less rigorous monitoring (Baird et al. 2013). Across Australia’s entire Great Barrier Reef, it is estimated that COTS are responsible for an annual decline of 1.43 % of total coral cover, second only to cyclone mortality and over four times higher than mortality attributed to bleaching (De’ath et al. 2012). Recent studies provide strong evidence for the early hypothesis (Birkeland 1982) that local environmental factors, specifically nutrient loads driving planktonic productivity fostering high larval recruitment , are most responsible for outbreaks (Fabricius et al. 2010; Brodie et al. 2005), as opposed to autogenous genetic factors in ‘rogue’ populations which might lead them to outbreak (Timmers et al. 2012). There is also some evidence that tropic cascades related to fishing may also enhance COTS outbreaks (Sweatman 2008). These factors point out the strong anthropogenic influence even in this ‘natural, biotic’ mechanism of acute disturbance.
While corallivores can be influential in some Caribbean reefs, they do not seem to undergo the outbreak dynamics associated with COTS or, to a lesser extent, Pacific corallivorous gastropods, Drupella spp. (Turner 1994). Outbreaks of Drupella snails have been reported to impose up to 75 % coral mortality but such reports are rare relative to COTS. Largely ecologically analogous corallivorous snails in the Caribbean, Coralliophila abbreviata, can impose substantial coral mortality, but acute predation seems to be more related to coral population declines (Knowlton et al. 1990), rather than corallivore population increases. Fish can certainly also disturb corals, either by predation or territorial activities (Chap. 10) but these are generally of a chronic nature causing relatively small amounts of mortality but potentially enhancing other disturbances and causing some reproductive impairment (e.g., Rotjan et al. 2006; Rotjan and Lewis 2009)
11.2.5 Chronic Disturbances
While certain sorts of chronic stressors may cause direct mortality (e.g., severe sedimentation), most often, their disturbance effects are manifest as sub-lethal damage. From our definition of disturbance (Box 11.1), this means that the opportunity provided for other organisms may be indirect (e.g., via lower production of offspring) or slower to manifest via delayed mortality. Such indirect effects of chronic disturbance , particularly on reproduction and recruitment , greatly influence the recovery side of our balance as discussed in the next section.
It is also axiomatic that any acute disturbance can also be experienced in background levels as a chronic effect. Wave damage, predation, disease , and to some extent, bleaching can all cause small scale disturbance/mortality which, due to its ubiquitous patchiness over time and space, should recover seamlessly. On the other hand, increasing frequencies of acute disturbances , such as predicted annual occurrence for thermal mass bleaching, may yield a dire situation of ‘constant acute’ disturbance, which perhaps should still be distinguished in effect from chronic disturbance .
11.2.5.1 Water Quality Decline
Anthropogenic, land-based inputs to the coastal ocean broadly include sewage and runoff as the major routes of introduction, but introduce a host of constituent stressors including sediment , nutrients, pharmaceuticals, pesticides and other toxicants, microbes, etc. Direct causal evidence of coral mortality or damage is difficult to determine in open ocean environments, but it is clear that this range of factors, especially in combination, have a negative effect on corals (Fabricius 2005). Evidence for combined interaction of poor water quality (or a surrogate of nearby human population density) with coral impairment and disease is increasing (Haapkylä et al. 2011; Kline et al. 2006; Downs et al. 2005; Aeby et al. 2011).
As Fabricius (2005) states ‘In most cases where terrestrial runoff causes reef degradation , disturbances other than eutrophication were the proximate causes of coral mortality , and runoff effects only became obvious when hard corals failed to reestablish after such disturbances’ (p. 134). Reproductive impairment, both via direct stress on the parent colonies and on larvae themselves, may be the most influential effects of poor water quality (Richmond 1997). Increased intensity of land use and coastal development in many regions has clearly resulted in decreasing water quality over time (McKergow et al. 2005), including the introduction of completely novel substances such as pharmaceuticals (Richardson et al. 2005). Most sources of water quality decline such as sewage, agriculture, and other land-use changes are directly related to human population densities, especially in coastal areas, but also inland. As such, these chronic disturbance s are expected to increase over time with human population abundance and consumption levels. However, land-based pollution is also a factor that can be managed effectively and so has the potential to decline in well-managed localities.
11.2.5.2 Fishing and Trophic Disruption
A nigh-ubiquitous disturbance that affects coral reef ecosystems is the trophic disruption that results from artificial removal of biomass by fishing. While corals are directly removed in some locations, local protection and management are most often able to curb this direct disturbance and so the effects of fishing on corals are largely indirect, and hence, more difficult to quantify. For example areas where grazers are intensively removed are more prone to ‘phase shift s’ to persistent seaweed dominated reefs when acute disturbance s cause coral mortality (Jackson et al. 2001; Hughes et al. 2007; Hughes 1994). Conversely, various modeling studies have described how maintaining high levels of grazing can mitigate other disturbances’ (e.g., hurricanes or bleaching mortality) effects on coral decline (Edwards et al. 2010; Mumby et al. 2007). Similarly, some evidence suggests that more diverse fish trophic webs in no-take reserves can reduce the spread of coral diseases, likely via reduced transmission by coral-feeding butterflyfishes (Raymundo et al. 2009). The intensive, long standing, and ubiquitous changes in coral reef food webs from overfishing have been posited to have wrought such profound alterations of trophic structure that microbial and organic carbon dynamics have been disrupted (Jackson et al. 2001) which can also induce coral mortality and disease (Smith et al. 2006; Kline et al. 2006). Although manageable, fishing pressure is also likely to increase with human population and is thus likely to grow in the foreseeable future.
11.2.5.3 Carbonate Chemistry Changes
Ocean acidification is the term used to describe the process of absorption of excess CO2 from the atmosphere into the ocean yielding alterations in the carbonate chemistry equilibrium of ocean waters. The resulting reduced pH and saturation levels of aragonite (the carbonate mineral form of which scleractinians build their skeletons) are expected to impair coral calcification (Cohen and Holcomb 2009), possibly their resistance to physical disturbance (Chap. 4), and their reproduction (though likely via indirect mechanisms such as increased fertilization limitation (Albright et al. 2010), altered settlement cues (Doropoulos et al. 2012), and slowed post-settlement growth rates (De Putron et al. 2010; Suwa et al. 2010; Nakamura et al. 2011). Modelling projections (illustrated in Fig. 11.3) clearly show the anticipated increase in severity and geographic extent of this stressor over the remainder of the twenty-first century.
Projected global patterns of aragonite saturation state (Ω) showing expected progressive expansion and intensity of chronic ocean acidification disturbance throughout tropical seas. Simplistically, calcification requires greater energetic investment by corals under lower levels of Ω (From Hoegh-Guldberg et al. (2007). Reprinted with permission from AAAS)
11.2.6 Ecosystem Effects of Disturbance
The dramatic disturbance-induced coral declines of recent decades beg the question of how coral reef ecosystems are affected. Certain obligate corallivores or coral-dwellers (Stella et al. 2011) clearly suffer when coral is disturbed. Live coral cover, itself, does significantly enhance reef fish communities (Coker et al. 2012), though the loss of physical structure probably has more profound effects and the maintenance of the skeletal structures of even dead coral contribute greatly to habitat value and ecosystem function of reef habitats (Graham et al. 2006; Wilson et al. 2006). Indeed, the more novel sources of disturbance mortality (bleaching and disease ) largely leave skeletal structures in place. However, without living coral to maintain/replace this structure, erosive forces will inevitably whittle it away (Chap. 4). Graham et al. (2008) in a meta-analysis of reef change across nine regions of the Indian Ocean showed a strong correlation of coral cover loss (the primary driver being the 1998 mass bleaching) and loss of structural complexity within 7 years of the acute disturbance . Large scale meta-analyses for Caribbean reefs indicate a long term decline in the architectural complexity of Caribbean reefs (Alvarez-Filip et al. 2009) and simultaneous declines in fish communities that appear to be better explained by habitat degradation than direct fishing pressure per se (Paddack et al. 2009). Combined with poorer cementation/accretion and reduced coral growth rates under declining carbonate saturation environments and potentially increased dissolution and bioerosion rates due to acidification (Andersson and Gledhill 2013), the structural degradation of coral reefs and hence degradation of carrying capacity for associated species is likely to accelerate under increasing CO2 futures.
11.3 Recovery Processes
Recovery of reef ecosystems in the modern ecological literature has substantial overlap with the term ‘reef resilience ’, the speed or effectiveness with which a given reef returns to a pre-disturbance state. There is broad consensus that fundamental ecological processes such as grazing, or other complex interactions affecting coral recruitment and growth are the primary determinants of reef resilience. In the past few decades, the literature is replete with examples of lack of recovery , particularly on Caribbean but also Indo-Pacific reefs. As early as 1997, a synthesis of reef recovery studies indicated a more or less complete lack of recovery on disturbed West Atlantic reefs (Connell 1997). In addition to the intensifying cycles of disturbance described above, the proliferation of macroalgae and lack of coral recruitment, particularly for reef-building species, are associated with poor recovery. The fact of coral reef degradation throughout most all regions of the world implies that the processes of coral recovery lag the combined effects of disturbances. The previous section described many types of disturbance effects, many of which are understood to be increasing individually, and are most certainly increasing in combination. Whereas acute disturbance s often occur very rapidly (e.g., days to weeks), are obvious, and relatively easy to quantify, the processes whereby corals recover are slow (e.g., decades) and important stages (e.g., coral larvae and post-settlers) are difficult or impossible to observe directly. This means that our general understanding of recovery processes and potential trends are much more poorly understood.
Graham et al. (2011) review ecological studies of reef recovery from the 1960s to 2009, analyzing five categories of predictors for reef recovery (in this case, measured as coral cover); disturbance characteristics, reef characteristics, reef connectivity , ecological characteristics and anthropogenic influences. Interestingly, they document an exponential increase in the number of published recovery studies in the latest decade as well as in the diversity of instigating acute disturbance types. Their analyses of reef studies with positive recovery trajectories indicate that geographic region (lowest in the Eastern Pacific, followed by the Caribbean), management status of the reef (lowest in fully protected MPAs, possibly an artifact of prior reef condition rather than an effect of management itself), and the post-disturbance coral cover (faster recovery from 6 % to 10 % cover than <5 % or >10 %) were the important factors in influencing rate of coral recovery, which had an overall mean of 3.56 % per year (95 % CI = 2.89–4.43). The type of disturbance and the physiographic reef type or zone had limited influence on recovery rate, as did the adjacent human population density. These characteristics are derived from a subset of resilient reefs, however, and it would likely be instructive to perform a similar analysis comparing similar characteristics with instances where reefs failed to recover over similar time scales. Indeed, Ateweberhan et al. (2013) contrast recovery of coral cover after the 1998 bleaching event between the remote, nearly pristine Chagos archipelago (less than a decade) and the Seychelles with higher human disturbance (minimal recovery observed over ~15 years) despite similar latitude, pre-disturbance coral cover, and disturbance related declines.
The importance of biogeographic variation in reef recovery processes is highlighted by Roff and Mumby (2012) who discuss evidence and a range of explanatory hypotheses for the apparent lesser recovery potential of Caribbean versus Indo-Pacific reefs. The semi-enclosed Caribbean basin has experienced more intensive human occupation and influence, including intensive fisheries extraction disrupting trophic structure over centuries (Wing and Wing 2001; Jackson 1997). However, the lower Caribbean species diversity in guilds of both fast growing corals and reef herbivores yields a lower functional redundancy. Hence, the sequence of acute disturbance s in the Caribbean which have rendered both fast-growing acroporid corals and the important grazing urchins functionally extinct in this region, has yielded a basic loss in the recovery capacity of these reefs. Roff and Mumby (2012) also compile evidence from a range of studies showing a dramatically higher rate of seaweed recruitment and productivity under experimental conditions of reduced grazing in the Caribbean versus Indo-Pacific. Despite this inherent robustness of Indo-Pacific reef resilience , the repeated patterns of coral decline across subregions (Bruno and Selig 2007; De’ath et al. 2012; Riegl et al. 2012) point to at least a similar trend of increasing imbalance with the pace of recovery lagging the pace of cumulative disturbance.
Of course conclusions about the progress or trends in coral recovery will depend also on the currency one uses. While the most data are available on percent cover, recovering population structure of long-lived organisms will necessarily take a very long time (see Fig. 11.1). More importantly, there are numerous examples that occasions of apparent rapid recovery of coral cover to a pre-disturbance level may mask fundamental changes in species composition, with sensitive species (often Acropora spp.) being largely replaced by more tolerant ones (Berumen and Pratchett 2006; Burt et al. 2008; Hughes and Connell 1999).
11.4 Coral Replenishment
The most basic processes of coral recovery involve the recruitment of new propagules and the re-growth of remnant tissue left from partial mortality on colonies. If the pattern and scale of disturbance allows for this latter mechanism to be effective, recovery can indeed be very rapid (one to a few years) (Diaz-Pulido et al. 2009) or greatly accelerated (Gilmour et al. 2013). The intensity and scale of many reef disturbances dictates that recovery will depend on the influx of new population members, likely larval recruits, highlighting the importance and influence of larval supply from upstream populations. Many, if not most, disturbances also have direct effects on coral reproduction and recruitment success, further impairing coral recovery and widening the potential imbalance in disturbance and recovery processes in coral reef systems. Climate change-related disturbances likely affect coral replenishment by impairing coral larval production (fecundity and fertilization ), dispersal , settlement success and juvenile growth (summarized in Birkeland et al. 2013). Early life phases of corals commonly show direct susceptibility to warm temperature stress including reduced fertilization for some species, developmental abnormalities and reduced larval survivorship, and reduced settlement success (Randall and Szmant 2009; Polato et al. 2010; Negri and Hoogenboom 2011; Negri et al. 2007). Trophic disturbance of reef systems (via fish extraction or mass mortality events) generally impedes coral replenishment by degradation of settlement habitat, especially via proliferation of benthic macroalgae (Kuffner et al. 2006; Birrell et al. 2008). Disturbance from water quality degradation and sedimentation can impede coral replenishment both via larval supply (fecundity, larval survivorship), and via degradation of settlement habitat (Fabricius 2005; Birrell et al. 2005).
At some scales, both chronic and acute disturbance s inherently impair coral replenishment by reducing the production of propagules via both mortality and physiological stress (described in the disturbance sections above and illustrated in Fig. 11.4). Evidence for stock-recruitment relationships in coral populations is often obscured by multiple stressors and the potentially wide scale of dispersal . However, a positive stock-recruitment relationship of acroporid corals driven largely by fecundity , rather than abundance, was shown across broad scales of the Great Barrier Reef in the late 1990s (Hughes et al. 2000). Because corals are sessile, mass mortalities from acute disturbances likely increase fertilization limitation by decreasing adult colony density (Birkeland et al. 2013). Chronic disturbances such as trophic disruption, declining water quality, ocean acidification , and rising mean temperatures also exert physiological stress and impair reproductive success across a range of early life stages. Such feedbacks are influential in instances of lack of coral recovery and suggest a reinforcing downward spiral in coral status (Fig. 11.4).
There is ample empirical evidence of reduced coral recruitment or juvenile success in chronically disturbed sites (e.g., Salinas-de-León et al. 2013; Wittenberg and Hunte 1992). There is also evidence of a temporal trend (generally decadal) of recruitment failure and/or declining success of juvenile corals (Guzner et al. 2012; Edmunds 2007; Vermeij et al. 2011; Hughes and Tanner 2000). While the cause of such declines is no doubt multifaceted, it bodes poorly for coral recovery in a regime of increasing disturbances. However, there are also many cases, including some exceptional cases in the Caribbean region, where effective coral recovery from disturbance has been documented (Manfrino et al. 2013; Graham et al. 2011). Low levels of local human influence are often cited to account for recent examples of rapid coral recovery (Manfrino et al. 2013; Ateweberhan et al. 2013; Gilmour et al. 2013).
11.5 Implications
From the realization that coral disturbance regimes are worsening while recovery capacities are waning, it follows that management strategies might beneficially focus on both of these processes. The management strategy coined ‘reef resilience ’ stresses this point. The recognition of complex interactions of disturbances and other factors that impair recovery invokes a need for management focus on both reducing local disturbances and managing for conditions that may enhance recruitment and recovery of corals. For example, land use and watershed management to reduce nutrient and/or toxicant loading can bolster organismal tolerance and increase thermal thresholds which apply to both bleaching disturbances (Wooldridge and Done 2009; Carilli et al. 2010) and coral larval success (Negri and Hoogenboom 2011). Other examples of management strategies to enhance replenishment most commonly include management actions to maintain herbivory as a necessary factor for coral recruitment as well as designing reserve networks to enhance connectivity and larval supply (Hughes et al. 2005; Nystrom et al. 2008; Mumby et al. 2012; McClanahan et al. 2012). However, these ‘managing-for-resilience’ strategies are still based on the premise that if local, anthropogenic disturbance regimes (but see Box 11.2) are appropriately managed, the recovery processes of coral communities are still capable of maintaining coral reefs within a range of states that sustain ecosystem services (i.e. corals are still resilient).
There is also a growing suspicion that resilience of coral species and assemblages is truly being lost in the onslaught of co-occurring disturbances of increasing frequency and intensity (Figs. 11.4 and 11.5). Loss of resilience suggests a need for more proactive measures to regain balance of disturbance and recovery and maintain biodiversity and ecosystem services provided by coral reefs. Parallel with the resilience strategies, proactive strategies can also be categorized as those mitigating disturbance impacts and those manipulating recovery. For example, interventions to increase the tolerance of organisms via selection for genes involved in increased environmental tolerance (Lundgren et al. 2013) or active manipulation of microbial (Teplitski and Ritchie 2009; Atad et al. 2012) or dinoflagellate symbionts (but see Coffroth et al. 2010) could enhance resistance to disturbances such as warm temperatures, pathogens, or toxicant exposure. Meanwhile most other proactive strategies relate to more fundamental manipulations of recovery and recruitment processes, including culture/restocking and ‘gardening’ for depleted populations of both corals and key grazers such as Diadema antillarum (Rinkevich 2008; Office of National Marine Sanctuaries 2011), or ‘assisted migration ’ interventions to aid corals in the colonization of new habitats such as thermal refugia at higher latitudes (Riegl et al. 2011; Hoegh-Guldberg et al. 2008). While such strategies may seem far-fetched or radical at the current time, the rate of environmental change dictates a prompt research agenda to validate strategies that might be successful and ensure they can be implemented in ways that minimize risk of unintended consequences when and if coral reef status reaches a point that they seem prudent or necessary, if no less radical.
Example of multiple acute and chronic disturbance s at the colony scale. This bleached colony of Orbicella faveolata also displays (a) recent partial mortality from active disease , (b) old, unrecovered partial mortality from a prior unknown source, and (c) active macroalgal encroachment along colony margin. A normally pigmented coral of a hardier species, Porites astreoides (d) is visible in the background for reference (Photo by M. Miller)
References
Aeby GS (2005) Outbreak of coral disease in the Northwestern Hawaiian Islands. Coral Reefs 24(3):481
Aeby GS, Williams GJ, Franklin EC, Haapkyla J, Harvell CD, Neale S, Page CA, Raymundo L, Vargas-Ángel B, Willis BL, Work TM, Davy SK (2011) Growth anomalies on the coral genera Acropora and Porites are strongly associated with host density and human population size across the Indo-Pacific. PLoS One 6(2), e16887. doi:10.1371/journal.pone.0016887
Albright RA, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral, Acropora palmata. Proc Natl Acad Sci 107:20400–20404. doi:10.1073/pnas.1007273107
Alvarez-Filip L, Dulvy NKJA, Gill JA, Côte IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc R Soc B Biol Sci 276:3019–3025
Andersson AJ, Gledhill D (2013) Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Ann Rev Mar Sci 5:321–348
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460(1–3):25–38
Atad I, Zvuloni A, Loya Y, Rosenberg E (2012) Phage therapy of the white plague-like disease of Favia favus in the Red Sea. Coral Reefs 31:665–670
Ateweberhan M, Feary DA, Keshavmurthy S, Chen A, Schleyer MH, Sheppard CR (2013) Climate change impacts on coral reefs: synergies with local effects, possibilities for acclimation, and management implications. Mar Pollut Bull 74(2):526–539
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baird AH, Pratchett MS, Hoey AS, Herdiana Y, Campbell SJ (2013) Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 32(3):803–812. doi:10.1007/s00338-013-1025-1
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80(4):435–471. doi:10.1016/j.ecss.2008.09.003
Berumen ML, Pratchett MS (2006) Recovery without resilience: persistent disturbance and long-term shifts in the structure of fish and coral communities at Tiahura Reef, Moorea. Coral Reefs 25(4):647–653
Birkeland C (1982) Terrestrial runoff as a cause of outbreaks of Acanthaster planci (Echinodermata: Asteroidea). Mar Biol 69:175–185
Birkeland C, Lucas JS (1990) Acanthaster planci: major management problem of coral reefs. CRC Press, Boca Raton
Birkeland C, Miller M, Piniak G, Eakin C, Weijerman M, McElhany P, Dunlap M, Brainard RE (2013) Safety in numbers? Abundance may not safeguard corals from increasing carbon dioxide. BioScience 63:967–974
Birrell CR, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. Mar Pollut Bull 51:408–415
Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol: Annu Rev 46:25–63
Brandt ME, McManus JW (2009) Disease incidence is related to bleaching extent in reef-building corals. Ecology 90(10):2859–2867
Brandt ME, Smith TB, Correa AMS, Vega-Thurber R (2013) Disturbance induced coral fragmentation as a driver of a coral disease outbreak. PLoS One 8(2), e57164
Bright AJ (2009) The effect of swell-generated physical damage on disease prevalence and asexual reproduction in the coral, Acropora palmata (Lamarck). University of the Virgin Islands, St. Thomas, USVI
Brodie J, Fabricius K, De’ath G, Okaji K (2005) Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Pollut Bull 51(1):266–278
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs 16:S129–S138
Bruckner AW (2012) Factors contributing to the regional decline of Montastraea annularis (complex). Proceedings of the 12th International Coral Reef Symposium 11B:9–13
Bruckner AW, Bruckner RJ (1997) Outbreak of coral disease in Puerto Rico. Coral Reefs 16:260
Bruno JF, Selig ER (2007) Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One 2(8), e711
Burt J, Bartholomew A, Usseglio P (2008) Recovery of corals a decade after a bleaching event in Dubai, United Arab Emirates. Mar Biol 154(1):27–36
Carilli JE, Norris RD, Black BA, Walsh SM, McField M (2010) Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob Chang Biol 16:1247–1257
Coffroth MA, Poland DM, Petrou EL, Brazeau DA, Holmberg JC (2010) Environmental symbiont acquisition may not be the solution to warming seas for reef-building corals. PLoS One 5(10), e13258. doi:10.1371/journal.pone.0013258
Cohen AL, Holcomb M (2009) Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22:118–127
Coker DJ, Graham NAJ, Pratchett MS (2012) Interactive effects of live coral and structural complexity on the recruitment of reef fishes. Coral Reefs 31(4):919–927. doi:10.1007/s00338-012-0920-1
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 1999:1302–1310
Connell JH (1997) Disturbance and recovery of coral assemblages. Coral Reefs 16:S101–S113
Connell JH, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr 67(4):461–488
Dayton PK (1971) Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41(4):351–389
De Putron S, McCorkle D, Cohen A, Dillon A (2010) The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals. Coral Reefs 30:321–328. doi:10.1007/s00338-010-0697-z
De’ath G, Moran PJ (1998) Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L.) on the Great Barrier Reef: 2: feeding preferences. J Exp Mar Biol Ecol 220(1):107–126
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci 109(44):17995–17999. doi:10.1073/pnas.1208909109
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Weeks S, Evans RD, Williamson DH, Hoegh-Guldberg O (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS One 4(4):9. doi:10.1371/journal.pone.0005239
Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh Guldberg O (2005) Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Chang Biol 11(12):2251–2265
Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby PJ (2012) Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol Lett 15:338–346. doi:10.1111/j.1461-0248.2012.01743.x
Downs C, Fauth J, Robinson C, Curry R, Lanzendorf B, Halas J, Halas J, Woodley C (2005) Cellular diagnostics and coral health: declining coral health in the Florida Keys. Mar Pollut Bull 51(5-7):558–569
Eakin CM, Morgan JA, Heron SF, Smith TB, Liu G, Alvarez-Filip L, Baca B, Bartels E, Bastidas C, Bouchon C, Brandt M, Bruckner AW, Bunkley-Williams L, Cameron A, Causey BD, Chiappone M, Christensen TRL, Crabbe MJC, Day O, de la Guardia E, Díaz-Pulido G, DiResta D, Gil-Agudelo DL, Gilliam DS, Ginsburg RN, Gore S, Guzmán HM, Hendee JC, Hernández-Delgado EA, Husain E, Jeffrey CFG, Jones RJ, Jordán-Dahlgren E, Kaufman LS, Kline DI, Kramer PA, Lang JC, Lirman D, Mallela J, Manfrino C, Maréchal J-P, Marks K, Mihaly J, Miller WJ, Mueller EM, Muller EM, Orozco Toro CA, Oxenford HA, Ponce-Taylor D, Quinn N, Ritchie KB, Rodríguez S, Ramírez AR, Romano S, Samhouri JF, Sánchez JA, Schmahl GP, Shank BV, Skirving WJ, Steiner SCC, Villamizar E, Walsh SM, Walter C, Weil E, Williams EH, Roberson KW, Yusuf Y (2010) Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One 5(11), e13969. doi:10.1371/journal.pone.0013969
Edmunds PJ (2007) Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals. Mar Ecol Prog Ser 341:1–13
Edmunds PJ, Carpenter RC (2001) Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc Natl Acad Sci 98(9):5067–5071
Edwards HJ, Elliott IA, Eakin CM, Irikawa A, Madin JS, McField M, Morgan JA, van Woesik R, Mumby PJ (2010) How much time can herbivore protection buy for coral reefs under realistic regimes of hurricanes and coral bleaching? Glob Chang Biol. doi:10.1111/j.1365-2486.2010.02366.x
Emanuel K (2005) Increasing destructiveness of tropical cyclones over the past 30 years. Nature 436:686–688
Fabricius K (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Fabricius K, Okaji K, De’ath G (2010) Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29(3):593–605
Foster NL, Baums IB, Sanchez JA, Paris CB, Chollett I, Agudelo CL, Vermeij MJA, Mumby PJ (2013) Hurricane-driven patterns of clonality in an ecosystem engineer: the Caribbean coral Montastraea annularis. PLoS One 8(1), e53283. doi:10.1371/journal.pone.0053283
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2005) Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long-term decline. Ecology 86(1):174–184
Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS (2013) Recovery of an isolated coral reef system following severe disturbance. Science 340(6128):69–71
Graham NA, Wilson SK, Jennings S, Polunin NV, Bijoux JP, Robinson J (2006) Dynamic fragility of oceanic coral reef ecosystems. Proc Natl Acad Sci 103(22):8425–8429
Graham NAJ, McClanahan TR, MacNeil MA, Wilson SK, Polunin NVC, Jennings S, Chabanet P, Clark S, Spalding MD, Letourneur Y, Bigot L, Galzin R, Ohman MC, Garpe KC, Edwards AJ, Sheppard CRC (2008) Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS One 3(8):9. doi:10.1371/journal.pone.0003039
Graham NAJ, Nash KL, Kool JT (2011) Coral reef recovery dynamics in a changing world. Coral Reefs 30(2):283–294. doi:10.1007/s00338-010-0717-z
Grinsted A, Moore JC, Jevrejeva S (2012) Homogeneous record of Atlantic hurricane surge threat since 1923. Proc Natl Acad Sci 109:19601–19605. doi:10.1073/pnas.1209542109
Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One 7(3), e33353. doi:10.1371/journal.pone.0033353
Guzner B, Novoplansky A, Chadwick N (2012) Population dynamics of the reef-building coral Acropora hemprichii as an indicator of reef condition. Mar Ecol Prog Ser 333:143–150
Haapkylä J, Unsworth RKF, Flavell M, Bourne DG, Schaffelke B, Willis BL (2011) Seasonal rainfall and runoff promote coral disease on an inshore reef. PLoS One 6(2), e16893. doi:10.1371/journal.pone.0016893
Haapkylä J, Melbourne-Thomas J, Flavell M, Willis BL (2013) Disease outbreaks, bleaching and a cyclone drive changes in coral assemblages on an inshore reef of the Great Barrier Reef. Coral Reefs 32(3):815–824. doi:10.1007/s00338-013-1029-x
Highsmith RC, Riggs AC, D’Antonio CM (1980) Survival of hurricane-generated coral fragments and a disturbance model of reef calcification/growth rates. Oecologia 46(3):322–329
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50(8):839–866
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP, Thomas CD (2008) Assisted colonization and rapid climate change. Science 321(5887):345–346. doi:10.1126/science.1157897
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265(5178):1547–1551
Hughes TP, Connell JH (1999) Multiple stressors on coral reefs: a long-term perspective. Limnol Oceanogr 44(3):932–940
Hughes TP, Tanner JE (2000) Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81(8):2250–2263
Hughes TP, Baird AH, Dinsdale EA, Motschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81(8):2241–2249
Hughes TP, Bellwood DR, Folke C, Steneck RS, Wilson J (2005) New paradigms for supporting the resilience of marine ecosystems. Trends Ecol Evol 20(7):380–386
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17(4):360–365. doi:10.1016/j.cub.2006.12.049
Hughes TP, Linares C, Dakos V, van de Leemput IA, van Nes EH (2012) Living dangerously on borrowed time during slow, unrecognized regime shifts. Trends Ecol Evol 28:149–155
Hunter CL (1993) Genotypic variation and clonal structure in coral populations with different disturbance histories. Evolution 47:1213–1228
Jackson JBC (1997) Reefs since Columbus. Coral Reefs 16:S23–S32
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293(5530):629–638
Kaczmarsky L, Richardson L (2011) Do elevated nutrients and organic carbon on Philippine reefs increase the prevalence of coral disease? Coral Reefs 30(1):253–257
Kline DI, Kuntz NM, Breitbart M, Knowlton N, Rohwer F (2006) Role of elevated organic carbon levels and microbial activity in coral mortality. Mar Ecol Prog Ser 314:119–125
Knowlton N, Lang JC, Rooney MC, Clifford P (1981) Evidence for delayed mortality in hurricane-damaged Jamaican staghorn corals. Nature 294(5838):251–252
Knowlton N, Lang JC, Keller BD (1990) Case study of natural population collapse: post-hurricane predation on Jamaican staghorn corals. Smithson Contrib Mar Sci 31:1–25
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Lessios HA (1988) Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annu Rev Ecol Syst 19:371–393
Lirman D, Schopmeyer S, Manzello D, Gramer LJ, Precht WF, Muller-Karger F, Banks K, Barnes B, Bartels E, Bourque A, Byrne J, Donahue S, Duquesnel J, Fisher L, Gilliam D, Hendee J, Johnson M, Maxwell K, McDevitt E, Monty J, Rueda D, Ruzicka R, Thanner S (2011) Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida Reef Tract and reversed previous survivorship patterns. PLoS One 6(8), e23047. doi:10.1371/journal.pone.0023047
Liu G, Strong AE, Skirving WJ, Arzayus LF (2006) Overview of NOAA Coral Reef Watch Program’s near-real-time satellite global coral bleaching monitoring activities. Proceedings of the 10th International Coral Reef Symposium Okinawa:1783–1793
Lopez-Victoria M, Zea S (2004) Storm-mediated coral colonization by an excavating Caribbean sponge. Climate Res 26(3):251–256
Lough JM (2000) 1997–98: unprecedented thermal stress to coral reefs? Geophys Res Lett 27(23):3901–3904. doi:10.1029/2000gl011715
Lundgren P, Vera J, Peplow L, Manel S, van oppen M (2013) Genotype – environment correlations in corals from the Great Barrier Reef. BMC Genet 14:9
Manfrino C, Jacoby CA, Camp E, Frazer TK (2013) A positive trajectory for corals at Little Cayman Island. PLoS One 8(10), e75432. doi:10.1371/journal.pone.0075432
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19(2):155–163
McClanahan TR, Ateweberhan M, Graham NAJ, Wilson SK, Sebastian CR, Guillaume MMM, Bruggemann JH (2007) Western Indian Ocean coral communities: bleaching responses and susceptibility to extinction. Mar Ecol Prog Ser 337:1–13
McClanahan TR, Donner SD, Maynard JA, MacNeil MA, Graham NA, Maina J, Baker AC, Beger M, Campbell SJ, Darling ES (2012) Prioritizing key resilience indicators to support coral reef management in a changing climate. PLoS One 7(8), e42884
McField MD (1999) Coral response during and after mass bleaching in Belize. Bull Mar Sci 64(1):155–172
McKergow LA, Prosser IP, Hughes AO, Brodie J (2005) Sources of sediment to the Great Barrier Reef world heritage area. Mar Pollut Bull 51(1):200–211
McWilliams JP, Cote IM, Gill JA, Sutherland WJ, Watkinson AR (2005) Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology 86(8):2055–2060
Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211(7):1050–1056. doi:10.1242/jeb.013284
Miller MW, Williams DE (2006) Coral disease outbreak at Navassa, a remote Caribbean island. Coral Reefs 26:97–101
Miller J, Muller E, Rogers C, Waara R, Atkinson A, Whelan KRT, Patterson M, Witcher B (2009) Coral disease following massive bleaching in 2005 causes 60 % decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 28:925–937
Muller EM, Rogers CS, Spitzack AS, van Woesik R (2008) Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs 27:191–195
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450(7166):98–101. doi:10.1038/nature06252
Mumby PJ, Vitolo R, Stephenson DB (2011) Temporal clustering of tropical cyclones and its ecosystem impacts. Proc Natl Acad Sci 108(43):17626–17630
Mumby PJ, Bejarano S, Golbuu Y, Steneck RS, Arnold SN, van Woesik R, Friedlander AM (2012) Empirical relationships among resilience indicators on Micronesian reefs. Coral Reefs:1–14. doi:10.1007/s00338-012-0966-0
Nakamura M, Ohki S, Suzuki A, Sakai K (2011) Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PLoS One 6(1), e14521. doi:10.1371/journal.pone.0014521
Negri AP, Hoogenboom MO (2011) Water contamination reduces the tolerance of coral larvae to thermal stress. PLoS One 6(5):e19703. doi:10.1371/journal.pone.0019703
Negri AP, Marshall PA, Heyward AJ (2007) Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species. Coral Reefs 26(4):759–763. doi:10.1007/s00338-007-0258-2
Nugues MM (2002) Impact of a coral disease outbreak on coral communities in St. Lucia: what and how much has been lost? Mar Ecol Prog Ser 229:61–71
Nystrom M, Graham NAJ, Lokrantz J, Norstrom AV (2008) Capturing the cornerstones of coral reef resilience: linking theory to practice. Coral Reefs 27(4):795–809. doi:10.1007/s00338-008-0426-z
Office of National Marine Sanctuaries (2011) Florida Keys National Marine Sanctuary Condition Report 2011. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries, Silver Spring, MD, 105 pp
Omori M, Fukami H, Kobinata H, Hatta M (2001) Significant drop of fertilization of Acropora corals in 1999: an after-effect of heavy coral bleaching? Limnol Oceanogr 46(3):704–706
Paddack MJ, Reynolds JD, Aguilar C, Appeldoorn RS, Beets J, Burkett E, Chittaro PM, Clarke K, Esteves R, Fonseca AC, Forrester GE, Friedlander AM, García-Sais J, González-Sansón G, Jordan LKB, McClellan DB, Miller MW, Molloy PP, Mumby PJ, Nagelkerken I, Nemeth M, Navas-Camacho R, Pitt J, Polunin NVC, Reyes-Nivia MC, Robertson DR, Rodríguez-Ramírez A, Salas E, Smith SR, Spieler RE, Steele MA, Williams ID, Wormald CL, Watkinson AR, Côté IM (2009) Recent region-wide declines in Caribbean reef fish abundance. Curr Biol 19:590–595
Penaflor EL, Skirving WJ, Strong AE, Heron SF, David LT (2009) Sea-surface temperature and thermal stress in the Coral Triangle over the past two decades. Coral Reefs 28(4):841–850. doi:10.1007/s00338-009-0522-8
Polato N, Voolstra CR, Schnetzer J, DeSalvo MK, Randall CJ, Szmant AM, Medina M, Baums IB (2010) Location-specific responses to thermal stress in larvae of the reef-building coral Montastraea faveolata. PLoS One 5, e11221
Randall C, Szmant A (2009) Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816). Biol Bull 217:269–282
Raymundo L, Couch C, Harvell CD (eds) (2008) Coral disease handbook: guidelines for assessment, monitoring & management. Coral Reef Targeted Research & Capacity Building for Management, St. Lucia. www.gefcoral.org
Raymundo LJ, Halford AR, Maypa AP, Kerr AM (2009) Functionally diverse reef-fish communities ameliorate coral disease. Proc Natl Acad Sci 106:17067–17070
Richardson LL, Goldberg WM, Kuta KG, Aronson RB, Smith GW, Ritchie KB, Halas JC, Feingold JS, Miller SL (1998) Florida’s mystery coral-killer identified. Nature 392(6676):557–558
Richardson BJ, Lam PK, Martin M (2005) Emerging chemicals of concern: pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar Pollut Bull 50(9):913–920
Richmond RH (1997) Reproduction and recruitment in corals: critical links in the persistence of reefs. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 175–197
Riegl BM, Purkis SJ, Al-Cibahy AS, Abdel-Moati MA, Hoegh-Guldberg O (2011) Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS One 6(9), e24802. doi:10.1371/journal.pone.0024802
Riegl BM, Bruckner AW, Rowlands GP, Purkis SJ, Renaud P (2012) Red Sea coral reef trajectories over two decades suggest increasing community homogenization and decline in coral size. PLoS One 7(5), e38396. doi:10.1371/journal.pone.0038396
Rinkevich B (2008) Management of coral reefs: we have gone wrong when neglecting active reef restoration. Mar Pollut Bull 56:1821–1824
Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Ritson-Williams R, Arnold SN, Fogarty ND, Steneck RS, Vermeij MJA, Paul VJ (2009) New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci 38:437–457
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27(7):404–413
Roff G, Kvennefors ECE, Fine M, Ortiz J, Davy JE, Hoegh-Guldberg O (2011) The ecology of ‘Acroporid White Syndrome’, a coral disease from the southern Great Barrier Reef. PLoS One 6(12), e26829. doi:10.1371/journal.pone.0026829
Rotjan RD, Lewis SM (2009) Predators selectively graze reproductive structures in a clonal marine organism. Mar Biol 156(4):569–577
Rotjan RD, Dimond JL, Thornhill DJ, Leichter JJ, Helmuth B, Kemp DW, Lewis SM (2006) Chronic parrotfish grazing impedes coral recovery after bleaching. Coral Reefs 25(3):361–368. doi:10.1007/s00338-006-0120-y
Salinas-de-León P, Dryden C, Smith D, Bell J (2013) Temporal and spatial variability in coral recruitment on two Indonesian coral reefs: consistently lower recruitment to a degraded reef. Mar Biol 160(1):97–105
Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL (2006) Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol Lett 9:835–845
Sousa WP (1984) The role of disturbance in natural communities. Annu Rev Ecol Syst 15:353–391
Stella JS, Munday PL, Jones GP (2011) Effects of coral bleaching on the obligate coral-dwelling crab Trapezia cymodoce. Coral Reefs 30(3):719–727. doi:10.1007/s00338-011-0748-0
Stoddart DR (1963) Effects of Hurricane Hattie on the British Honduras Reefs and Cays, October 30–31, 1961. Atoll Res Bull 95:1–142
Sutherland JP, Karlson RH (1977) Development and stability of the fouling community at Beaufort, North Carolina. Ecol Monogr 47:425–446
Sutherland KP, Porter JW, Torres C (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser 266:273–302
Suwa R, Nakamura M, Morita M, Shimada K, Iguchi A, Sakai K, Suzuki A (2010) Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora). Fish Sci 76(1):93–99
Sweatman H (2008) No-take reserves protect coral reefs from predatory starfish. Curr Biol 18(14):R598–R599. doi:10.1016/j.cub.2008.05.033
Szmant A, Gassman N (1990) The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastraea annularis. Coral Reefs 8(4):217–224
Teplitski M, Ritchie K (2009) How feasible is the biological control of coral diseases? Trends Ecol Evol 24:378–385
Thompson D, Van Woesik R (2009) Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc R Soc B Biol Sci 276(1669):2893–2901
Timmers MA, Bird CE, Skillings DJ, Smouse PE, Toonen RJ (2012) There’s no place like home: Crown-of-Thorns outbreaks in the central Pacific are regionally derived and independent events. PLoS One 7(2), e31159. doi:10.1371/journal.pone.0031159
Turner SJ (1994) The biology and population outbreaks of the corallivorous gastropod Drupella on Indo-Pacific reefs. Oceanogr Mar Biol Annu Rev 32:461–530
Ulstrup KE, Berkelmans R, Ralph PJ, van Oppen MJH (2006) Variation in bleaching sensitivity of two coral species across a latitudinal gradient on the Great Barrier Reef: the role of zooxanthellae. Mar Ecol Prog Ser 314:135–148
Vermeij MJ, Bakker J, Nvd H, Bak RP (2011) Juvenile coral abundance has decreased by more than 50 % in only three decades on a small Caribbean island. Diversity 3(3):296–307
Wakeford M, Done TJ, Johnson CR (2008) Decadal trends in a coral community and evidence of changed disturbance regime. Coral Reefs 27:1–13
Webster PJ, Holland GJ, Curry JA, Chang H-R (2005) Changes in tropical cyclone number, duration, and intensity in a warming environment. Science 309:1844–1846
Williams DE, Miller MW, Kramer KL (2008a) Recruitment failure in Florida Keys Acropora palmata, a threatened Caribbean coral. Coral Reefs 27:697–705
Williams GJ, Aeby GS, Davy SK (2008b) Coral disease at Palmyra Atoll, a remote reef system in the Central Pacific. Coral Reefs 27(1):207–207. doi:10.1007/s00338-007-0314-y
Wilson SK, Graham NA, Pratchett MS, Jones GP, Polunin NV (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Chang Biol 12(11):2220–2234
Wing SR, Wing ES (2001) Prehistoric fisheries in the Caribbean. Coral Reefs 20(1):1–8
Wittenberg M, Hunte W (1992) Effects of eutrophication and sedimentation on juvenile corals. 1. Abundance, mortality and community structure. Mar Biol 116(1):131–138
Wooldridge SA, Done TJ (2009) Improved water quality can ameliorate effects of climate change on corals. Ecol Appl 19(6):1492–1499
Acknowledgements
Sincere thanks to C Birkeland and BE Brown for their input and guidance in shaping this contribution. Review and comments by DE Williams fostered improvements in the manuscript and are greatly appreciated. Opinions expressed are my own and do not represent official positions of NOAA nor the Southeast Fisheries Science Center.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Miller, M.W. (2015). Coral Disturbance and Recovery in a Changing World. In: Birkeland, C. (eds) Coral Reefs in the Anthropocene. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7249-5_11
Download citation
DOI: https://doi.org/10.1007/978-94-017-7249-5_11
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7248-8
Online ISBN: 978-94-017-7249-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)