Abstract
This chapter reports the findings of a Working Group on how atmospheric nitrogen (N) deposition affects both terrestrial and freshwater biodiversity. Regional and global scale impacts on biodiversity are addressed, together with potential indicators. Key conclusions are that: the rates of loss in biodiversity are greatest at the lowest and initial stages of N deposition increase; changes in species compositions are related to the relative amounts of N, carbon (C) and phosphorus (P) in the plant soil system; enhanced N inputs have implications for C cycling; N deposition is known to be having adverse effects on European and North American vegetation composition; very little is known about tropical ecosystem responses, while tropical ecosystems are major biodiversity hotspots and are increasingly recipients of very high N deposition rates; N deposition alters forest fungi and mycorrhyzal relations with plants; the rapid response of forest fungi and arthropods makes them good indicators of change; predictive tools (models) that address ecosystem scale processes are necessary to address complex drivers and responses, including the integration of N deposition, climate change and land use effects; criteria can be identified for projecting sensitivity of terrestrial and aquatic ecosystems to N deposition. Future research and policy-relevant recommendations are identified.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Reactive nitrogen (Nr), by virtue of being an essential nutrient for life, exerts a major influence on ecosystem structure and function. Human activities now convert more atmospheric nitrogen (N) to reactive plant-available forms than all natural sources combined and this has resulted in N becoming abundant in many ecosystems where it has been historically scarce. Inputs of Nr to the Earth’s ecosystems have increased 20-fold since 1860 (Galloway et al. 2008). Rockström et al. (2009) conclude that the human interference with the global N cycle has already crossed a threshold leading to an unacceptable level of environmental change. Interestingly, these authors posit the threshold for the rate of biodiversity loss (defined as shifts in species composition, loss of individual species, and reduced richness) has also been crossed, and it is most likely that human impacts on N cycling and biodiversity loss are, in part, connected.
Nitrogen has the potential to be transported through the atmosphere and deposited many kilometers from its source area, and atmospheric N deposition has become a major force of ecological change in areas both adjacent to and remote from sources of emissions (Sutton et al. 2011). Large areas of industrial nations, and increasingly large areas of developing nations, now receive Nr from atmospheric deposition in amounts that are orders of magnitude greater than from naturally fixed N.
The following report summarizes the results of a Working Group on the consequences of atmospheric N deposition on biodiversity. We describe a continuum of effects of atmospheric N deposition on species and ecosystems from low, initial increases in N deposition on natural undisturbed areas to high levels of atmospheric N to historically altered and managed landscapes. We summarize known consequences of N for biodiversity, point out information gaps, and make suggestions for research and ways that policy could be altered to protect it. Our conclusions are as striking for what we do not know as for what we know and can document. Records of global N deposition and biodiversity response are sparse, and many regions of the world have little or no information on historic deposition, current deposition, biotic inventories, or susceptibility of native species to N. Much of what we understand about N deposition impacts on biodiversity has been extrapolated from N fertilization experiments, with limited studies along N deposition gradients.
2 Ecosystems, Communities and Organisms at Risk from Nitrogen Deposition
Terrestrial and aquatic ecosystems can respond rapidly to increased N from atmospheric deposition if they are N-poor initially, or have or are surrounded by soils of low to moderate buffering capacity . If nutrient poor, N contributes to eutrophication, and if soils have low buffering capacity, they are susceptible to acidification from strong acid anions, including nitrate. Oligotrophic freshwater ecosystems may be eutrophied or acidified by nitrate or ammonium deposition to surrounding soils with poor buffering capacity (Baron et al. 2000; Rabalais 2002). Evidence from monitoring, paleoecological reconstructions, and experiments show that oligotrophic arctic, alpine, temperate grasslands, heathlands , deserts, Mediterranean vegetation, and lakes are sensitive to slight increases in N availability (Gordon et al. 2001; Wolfe et al. 2001; Fenn et al. 2003; Bowman et al. 2006; Rao et al. 2010; Bobbink et al. 2010; Dias et al. 2014, Chap. 27, this volume). Experimental and observational results indicate the loss of species is nonlinear; the greatest decline in species richness occurs with slight increases of N above a natural, or experimental control deposition rate, and there is less change in diversity at higher depositions (Bobbink et al. 2010; Emmett 2007; Dise et al. 2011). The biodiversity of undisturbed landscapes with background levels of N deposition is, therefore, theoretically at high risk from N deposition .
Certain types of terrestrial organisms will be more susceptible to increased N deposition by virtue of their low stature, higher N accumulation rates, or specialized structures or hosts; these include bryophytes, oligotrophic lichens , insectivorous plants, those with N-fixing nodules and mycorrhizal fungi (Gordon et al. 2001; Bobbink et al. 2010). Specialist herbivorous insects on susceptible plants will be vulnerable (Throop and Lerdau 2004). While N impacts can be predicted for taxa or functional groups based on knowledge of the generalized response to N of some members of the groups, and these are summarized in Bobbink et al. (2010), there is a dearth of information on many particular species or guilds. While some research has addressed the biodiversity of free-living microorganisms in response to N deposition, this literature body has not been synthesized, yet these organisms constitute most of the biodiversity of all ecosystems .
3 Mechanisms by Which Nitrogen Deposition Affects Biodiversity
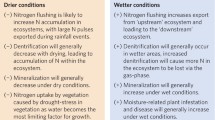
Atmospheric N deposition alters species composition, richness, and productivity in terrestrial and freshwater environments via many pathways (Fig. 49.1). The physical mechanisms, such as eutrophication-induced competition for light and soil acidification, can be described as “bottom-up” processes. Species losses can also be hypothesized to occur from “top-down” ecological processes including increased herbivory and insect outbreaks. Different species are likely to be differentially sensitive to different processes, but there is some evidence that both processes are relevant (e.g. Hautier et al. 2009).
3.1 Physical Mechanisms
Eutrophication is a cascade of events that is triggered by relief from N limitation in individual plants . Rates of native biodiversity loss due to eutrophication will be greatest at the lowest and initial stages of N increases in the most oligotrophic environments (Baron et al. 2000; Bowman et al. 2006; Emmett 2007). Nitrogen deposition continues to reduce plant diversity at higher deposition amounts (Clark and Tilman 2008). Mechanisms include soil acidification which lowers pH, depletes soils of nutrient base cations, and allows toxic metals such as aluminum to become soluble (Bowman et al. 2008). Direct toxicity from gases, acidic aerosols and reduced N (ammonia and ammonium) in heavily polluted industrial or agricultural areas can lead to plant and animal mortality (Riddel et al. 2008; Kronzucker et al. 2003; Wallace et al. 2007; Bobbink et al. 2010). Soil fauna are negatively affected by N deposition through changes in soil acidity (Xu et al. 2009).
Eutrophication reduces the heterogeneous distribution of soil N and gives nitrophilous plants with high maximum growth rates a competitive advantage over others; faster growing and large species outcompete slower growing and smaller stature neighbours for light, water, and other nutrients (Egerton-Warburton and Allen 2000; Feest 2006; Gilliam 2006; Hautier et al. 2009; Bobbink et al. 2010; Dise et al. 2011). Grasses, sedges, and ruderal species, some of which may have invasive characteristics, are successful when N is abundant (Clark and Tilman 2008).
Elevated N availability also disrupts the symbiotic relation between soil mycorrhizae and their host plants, which has evolved to provide N or phosphorus (P) to plants when the availability is low in exchange for a supply of carbon (C) from plants. The loss of symbiosis leads to reduced fungal associations and increased parasitism (Johnson et al. 2003). Where N was previously limiting to growth, increased primary productivity occurs with N deposition or experimental N additions, but productivity occurs at the expense of biodiversity (Wolfe et al. 2001; Hautier et al. 2009). Reductions in richness have been observed in response to N addition experiments and across continental gradients from low to high atmospheric N deposition in North American, European, and tropical Asian ecosystems (Gordon et al. 2001; Stevens et al. 2010; Clark and Tilman 2008; Bobbink et al. 2010; Lu et al. 2010). The decline in richness is attributed to both eutrophication and acidification in temperate and tropical systems (Stevens et al. 2010; Lu et al. 2010).
There are many studies that document increases in biomass and shifts in dominant taxa when oligotrophic waters became enriched with N, but surprisingly few studies of the effect of N additions on algal species richness (Wolfe et al. 2001; Nydick et al. 2004; Bergström and Jansson 2006; Lewis and Wurtsbaugh 2008; Elser et al. 2009). An increase in eutrophic algae has the potential to alter food webs and community structure. The concentrations of N and P drive productivity, but the stoichiometric relation of N:P determines the abundance or dominance of individual taxa (Rabalais 2002). High N, low P phytoplankton make poor food for P-rich zooplankton that consume them. Alterations in zooplankton size and abundance in turn affect higher trophic levels, such as fish (Elser et al. 2009).
3.2 Ecological Mechanisms
The effects of changing plant and fungal mycorrhizal communities can propagate through the food web to influence animal behavior and abundance. Fungi can be important foods for small mammals, and their absence can affect both plant and mammal fitness (Myers et al. 2000). For herbivorous insects, the N concentration of host plants strongly controls processes such as growth, survivorship, population levels and outbreak frequency (Throop and Lerdau 2004). High N accumulation in plants can promote large insect outbreaks that have the potential to damage their host plants, thereby affecting ecosystem structure and function (Kerslake et al. 1998). Changes in plant community composition may affect herbivore community composition. Some insects are host-specific and may decline along with their host plant. Invasions of Mediterranean annual grasses under N deposition in California have reduced densities of the host plant of the rare Bay Checkerspot butterfly in California, and caused local extirpations (Weiss 1999). Nitrogen deposition favors plants with N-based defenses, such as members of the Solanaceae (Throop and Lerdau 2004). Few herbivores are able to consume solanaceous plants, thus a shift toward plants with N-based defenses will cause a shift in the herbivore community.
4 Mechanisms by Which Nitrogen Deposition Affects Ecosystem Function
Alteration of food webs is one important way that N deposition affects how ecosystems operate and the services they provide to society. Nitrogen deposition affects decomposition rates, substrate quality, and can enhance terrestrial C sequestration in some circumstances (Knorr et al. 2005; Hobbie 2008; Goodale et al. 2009). Increased N availability can also increase N2O emissions from soils and freshwaters and lower CH4 uptake (Goodale et al. 2009; Sutton et al. 2011). The occurrence of N saturation in terrestrial systems adversely affects downstream water quality. When excess N accumulates in freshwater and estuarine environments (from raised primary productivity) this promotes the development of noxious algal blooms , decreased water clarity, decreased levels of dissolved oxygen, and lower diversity (Rabalais 2002; Rockström et al. 2009). The role of N in water eutrophication depends on its relative availability with respect to other elements, such as C, P and silica (Billen and Garnier 2007). The anthropogenic increase of N and P in lakes, reservoirs, rivers and coastal waters is the main cause of eutrophication (Sutton et al. 2011).The ultimate consequence of reduced biodiversity is a more homogeneous biotic environment, and recent studies document homogenization and loss of structural diversity, in part due to N deposition (Bobrovsky 2010; Britton et al. 2009; Keith et al. 2009).
5 Interactions of Nitrogen Deposition with Other Human-Induced Stresses
Nitrogen deposition adds an additional stress to many, if not all, ecosystems already affected by climate change, land use, other air pollutants, invasive species, and habitat fragmentation. In arctic and montane systems warming is compounding the effects of even slight increases in atmospheric N deposition for both terrestrial and aquatic habitats (Wolfe et al. 2006; Baron et al. 2009). Nitrogen oxide and sulphur dioxide emissions are the major precursors of acid rain; their deposition has brought about dramatic changes to soil base cation availability and is, or may be, responsible for long-term trends in forest vegetation structure, songbird breeding success, and food web dynamics in lakes of temperate Europe and North America (Bowman et al. 2006; Long et al. 2009; Hames et al. 2002; Jeziorski et al. 2008).
In Californian Mediterranean-type and desert ecosystems, N deposition contributes to a positive feedback between loss of native plants, increases in annual grass invaders, and the frequency of fire that transforms native communities, perhaps permanently (Talluto and Suding 2008; Allen et al. 2009; Rao et al. 2010); in semi-arid European ecosystems, plant community responsiveness to N addition could be mediated by forbs (Ochoa-Hueso and Manrique 2010). A decline in tropical forest floor diversity appears to be due to N deposition-induced soil acidification alone (Lu et al. 2010), but in many areas of the tropics undergoing rapid land use change and industrialization there is the potential for interaction of N deposition with other stress factors.
6 Visualizing the Connectivity Between Nitrogen Deposition and Biodiversity
Bobbink et al. (2010) map the overlay of current and potential future N deposition with the Global 200 Ecoregions (Olson and Dinerstein 2002) that represent areas of distinct assemblages of natural communities and species, and are recognized as priority conservation areas for protecting a broad diversity of the Earth’s ecosystems. Areas of concern include much of Southeast Asia, Mexico and Central America, southern Brazil and Central Africa. Such maps are particularly effective at illustrating regions where investigation is greatly needed to document current species composition and atmospheric deposition, and where monitoring for trends in cause and effect needs to be conducted. Additional visual portrayal is needed for at least two resolutions of scale. General global maps depicting biomes of known concern are important for illustrating the wide extent of N deposition effects (e.g., Fig. 49.2) . Refinements of maps like this to portray finer-scale, or more targeted resolution of specific areas of concern or species of concern will be a useful tool for identifying specific areas for either research or conservation.
Phoenix et al. (2006) noted that analyses of the impacts of N deposition on biodiversity have occurred mainly on terrestrial landscapes of northern Europe and North America. What is lacking is a global-scale description, and analysis of the role of N deposition in ‘biodiversity hotspots’ (defined by high levels of plant species endemism, as well as threat; Myers et al. 2000). The average deposition rate across hotspots was 50 % greater than the global terrestrial average in the mid-1990’s and could more than double by 2050. A comparison of patterns of global N deposition with the recently defined set of freshwater ecoregions of the world (Abell et al. 2008) is also required.
Further, it is essential to compare patterns of N deposition with patterns of species irreplaceability (high endemism) and vulnerability (high threat). This can be done relatively easily, at a regional or global scale, for some taxonomic groups. Regional species distribution and conservation status information is available for many types of animals (e.g. IUCN 2009, www.iucnredlist.org; NatureServe 2009, www.natureserve.org/explorer/). These data provide the capacity to compare distributions of range-restricted or threatened species overlain with current or projected future N deposition maps, providing powerful visual images of where to conduct research on biodiversity and N deposition, or where critical loads for N deposition have been exceeded. Similar distribution maps for fungi, plants, insects, and algae, which are not available, will be needed for this approach to add substantially to the effort already found in Bobbink et al. (2010). Much of this spatial information is available via public databases and portals such as the Integrated Biodiversity Assessment Tool (http://www.ibatforbusiness.org).
7 Conclusions and Recommendations
Specific conclusions were reached regarding the state of the knowledge of regional and global impacts on biodiversity, as well as potential indicators of these impacts:
-
There is a spectrum of responses to N deposition, for example, rates of loss in biodiversity are greatest at the lowest and initial stages of N deposition increase;
-
Changes in terrestrial species compositions are related to the relative amounts of N, C and P in the plant soil system;
-
Enhanced N inputs have implications for both the freshwater C cycle and global C cycle;
-
Nitrogen deposition is having a negative effect on European and North American vegetation composition;
-
While very little is known about tropical ecosystem responses, tropical ecosystems are major biodiversity hotspots and are increasingly recipients of very high N deposition rates;
-
Nitrogen deposition dramatically alters forest fungi and mycorrhyzal relations with plants; the rapid response of forest fungi and arthropods makes them good indicators of change which should receive more attention;
-
Nitrogen deposition increases sensitivity to climate change and other stresses;
-
Climate change, air pollution, and land use must be addressed as integrated problems;
-
Predictive tools (models) that address ecosystem scale processes are necessary to address complex drivers and responses;
-
Criteria can be identified for projecting sensitivity of terrestrial and aquatic ecosystems to N deposition:
-
Nutrient poor conditions (flora adapted to low nutrients and low to moderate buffering capacity) ;
-
Highly weathered soils (high Ca/Al ratio in soil);
-
Sensitive sites typically have one of more of these characteristics: a high proportion of N-fixers, potential for increases in ruderal species, insectivorous plants, high bryophyte cover (often sites with communities of high potential significance); seasonally dry climates.
-
Research recommendations that follow from the scientific evidence to date are to:
-
Expand empirical information about, and theoretical underpinnings of, ecosystem and food web responses to increasing N deposition. Identify which changes are important and potentially irreversible. Investigate responses to the reduction of N deposition. Can recovery occur?
-
Conduct assessments of the distribution, ecology, conservation status, threats, and risk of extinction for species of different taxonomic groups, especially freshwater species and many terrestrial invertebrates, in order to make meaningful comparisons and models of the relationship between species composition in ecosystems, N deposition, and resultant compositional change of the species diversity over time.
-
Conduct an assessment of understudied regions of the world, such as the tropics and developing regions of Asia. Which ecosystems, communities, and organisms are vulnerable to atmospheric N deposition? What are current deposition amounts and trends? What risks do future N deposition amounts pose to natural areas and their species?
-
Quantify the connections between N deposition effects on biodiversity and subsequent change in ecosystem services.
-
Develop and apply more modeling capability related to N deposition effects on ecosystems/biodiversity, particularly for aquatic systems, non-temperate forest or alpine ecosystems .
Based upon the scientific evidence to date, the policy implications are to :
-
Protect highly vulnerable ecosystems that are still unaffected by N deposition impacts;
-
Where current or future N deposition overlaps with areas of high biodiversity conservation value (in terms of numbers of endemics- ecoregions etc), conduct inventories of species and implement monitoring of changes over time;
-
Consider N deposition effects in conjunction with other human-caused drivers of climate change and land and water use;
-
Build on existing knowledge to define connections between biodiversity and ecosystem services;
-
There are different pathways by which N deposition affects biodiversity, which policy making should consider including:
-
Competitive advantage of some species over others;
-
Enhancement of invasive species over natives (which in some places can alter disturbance cycles such as fire return frequency);
-
Nutrient imbalance propagated up the food chain;
-
Acidification through loss of ANC or base cations.
-
Overall, the workshop recommended that:
-
The CBD continue to recognize that excessive N affects biodiversity;
-
The LRTAP Convention continues to recognize that loss of biodiversity is an important adverse effect of N transport and deposition;
-
A combined approach to the problem by both Conventions might be a productive means to address the impacts of N deposition on biodiversity.
References
Abell, R., Thieme, M. L., Revenga, C., Bryer, M., Kottelat, M., Bogutskaya, N., Coad, B., Mandrak, N., Balderas, S. C, Bussing, W., Stiassny, M. L. J., Skelton, P., Allen, G. R., Unmack, P., Naseka, A., Ng, R., Sindorf, N., Robertson, J., Armijo, E., Higgins, J. V., Heibel, T. J., Wikramanayake, E., Olson, D., López, H. L., Re is, R. E., Lundberg, J. G., Sabaj Pérez, M. H., & Petry, P. (2008). Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience, 58, 403–414.
Allen, E. B., Rao, L. E., Steers, R. J., Bytnerowicz, A., & Fenn, M. E. (2009). Impacts of atmospheric nitrogen deposition on vegetation and soils in Joshua Tree National Park. In R. H. Webb, L. F. Fenstermaker, J. S. Heaton, D. L. Hughson, E. V. McDonald, & D. M. Miller (Eds.), The Mojave Desert: Ecosystem processes and sustainability (pp. 78–100). Las Vegas: University of Nevada Press.
Baron, J. S., Rueth, H. M., Wolfe, A. P., Nydick, K. R., Allstott, E. J., Minear, J. T., & Moraska, B. (2000). Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems, 3, 352–368.
Baron, J. S., Schmidt, T. M., & Hartman, M. D. (2009). Climate-induced changes in high elevation stream nitrate dynamics. Global Change Biology, 15, 1777–1789.
Bergström, A., & Jansson, M. (2006). Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Global Change Biology, 12, 635–643.
Billen, G., & Garnier, J. (2007). River basin nutrient delivery to the coastal sea: Assessing its potential to sustain new production of nonsiliceous algae. Marine Chemistry, 106, 148–160.
Bobbink, R., Hicks, K., Galloway, J., Spranger, T., Alkemade, R., Ashmore, M., Bustamante, M., Cinderby, S., Davidson, E., Dentener, F., Emmett, B., Erisman, J.-W., Fenn, M., Gilliam, F., Nordin, A., Pardo, L., & de Vries, W. (2010). Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecological Applications, 20, 30–59.
Bobrovsky, M. V. (2010). Lesnye pochvy Evropejskoj Rossii: bioticheskie i antropogennye faktory formirovaniya [Forest soil in European Russia: biotic and anthropogenic factors in soil forming process].Moscow: KMK (in Russian).
Bowman, W. D., Gartner, J. R., Holland, K., & Wiedermann, M. (2006). Nitrogen critical loads for alpine vegetation and terrestrial ecosystem response: are we there yet? Ecological Applications, 16, 1183–1193.
Bowman, W. D., Cleveland, C. C., Halada, L., Hresko, J., & Baron, J. S. (2008). Negative impact of nitrogen deposition on soil buffering capacity. Nature Geoscience, 1, 767–770.
Britton, A. J., Beale, C. M., Towers, W., & Hewison, R. L. (2009). Biodiversity gains and losses: evidence for homogenisation of Scottish alpine vegetation. Biological Conservation, 142, 1728–1739.
Clark, C. M., & Tilman, D. (2008). Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature, 451, 712–715.
Dias, T., Chaves, S., Tenreiro, R., Martins-Loução, M. A., Sheppard, L., & Cruz, C. (2014). Effects of increased nitrogen availability in Mediterranean ecosystems: A case study in a Natura 2000 site in Portugal. In M. A. Sutton, K. E. Mason, L. J Sheppard, H. Sverdrup, R. Haeuber, & W. K. Hicks (Eds.), Nitrogen deposition, critical loads and biodiversity (Proceedings of the International Nitrogen Initiative workshop, linking experts of the Convention on Long-range Transboundary Air Pollution and the Convention on Biological Diversity). (Chap. 27; this volume). Springer.
Dise, N. B., Ashmore, M., Belyazid, S., Bleeker, A., Bobbink, R., de Vries, W., Erisman, J. W., Spranger, T., Stevens, C. J., & van den Berg, L. (2011). Nitrogen as a threat to European terrestrial biodiversity. In M. A. Sutton, C. M. Howard, J. W. Erisman, G. Billen, A. Bleeker, P. Grennfelt, H. van Grinsven, & B. Grizzetti (Eds.), The European nitrogen assessment (Chap. 20). Cambridge University Press.
Egerton-Warburton, L., & Allen, E. B. (2000). Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecological Applications, 10, 484–496.
Elser, J. J., Andersen, T., Baron, J. S., Bergstrom, A.-K., Jansson, M., Kyle, M., Nydick, K. R., Steger, L., & Hessen, D. O. (2009). Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science, 326, 835–837.
Emmett, B. A. (2007). Nitrogen saturation of terrestrial ecosystems: Some recent findings and their implications for our conceptual framework. Water, Air, & Soil Pollution. Focus, 7, 99–109.
Feest, A. (2006). Establishing baseline indices for the quality of the biodiversity of restored habitats using a standardized sampling process. Restoration Ecology, 14, 112–122.
Fenn, M. E., Baron, J. S., Allen, E. B., Rueth, H. M., Nydick, K. R., Geiser, L., Bowman, W. D., Sickman, J. O., Meixner, T., Johnson, D. W., & Neitlich, P. (2003). Ecological effects of nitrogen deposition in the western United States. Bioscience, 53, 404–420.
Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., Martinelli, L. A., Seitzinger, S. P., & Sutton, M. A. (2008). Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science, 320, 889–892.
Gilliam, F. S. (2006). Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. Journal of Ecology, 94, 1176–1191.
Goodale, C., Thomas, R. Q., Melvin, A. M., Weiss, M. S., Adams, M. B., Baron, J. S., Emmett, B., Evans, C., Fernandez, I., Gundersen, P., Kulmatski, A., Lovett, G., McNulty, S., Moldan, F., Ollinger, S., & Schleppi, P. (2009). Nitrogen deposition and forest carbon sequestration: A quantitative synthesis from plot to global scales. American Geophysical Union, Fall Meeting 2009, abstract #B23G–01.
Gordon, C., Wynn, J. M., & Woodin, S. J. (2001). Impacts of increased nitrogen supply on high Arctic heath: The importance of bryophytes and phosphorus availability. New Phytologist, 149, 461–471.
Hames, R. S., Rosenberg, K. V., Lowe, J. D., Barker, S. E., & Dhondt, A. A. (2002). Adverse effects of acid rain on the distribution of the Wood Thrush Hylocichla mustelina in North America. Proceedings of the National Academy of Sciences of the United States of America, 99, 11235–11240.
Hautier, Y., Niklaus, P. A., & Hector, A. (2009). Competition for light causes plant biodiversity loss after eutrophication. Science, 324, 636–638.
Hobbie, S. E. (2008). Nitrogen effects on decomposition: A five-year experiment in eight temperate sites. Ecology, 89, 2633–2644.
Jeziorski, A., Yan, N. D., Paterson, A. M., DeSellas, A. M., Turner, M. A., Jeffries, D. S., Keller, B., Weeber, R. C., McNicol, D. K., Palmer, M. E., McIver, K., Arseneau, K., Ginn, B. K., Cumming, B. F., & Smol, J. P. (2008). The widespread threat of calcium decline in fresh waters. Science, 322, 1374–1377.
Johnson, N. C., Rowland, D. L., Corkidi, L., Egerton-Warburton, L. M., & Allen, E. B. (2003). Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology, 84, 1895–1908.
Keith, S. A., Newton, A. C., Morecroft, M. D., Bealey, C. E., & Bullock, J. M. (2009). Taxonomic homogenization of woodland plant communities over 70 years. Proceedings of the Royal Society B, 276, 3539–3544.
Kerslake, J. E., Woodin, S. J., & Hartley, S. E. (1998). Effects of CO2 and nitrogen enrichment on a plant-insect interaction: The quality of Calluna vulgaris as a host for Opheroptera brumata. New Phytologist, 140, 43–53.
Knorr, M., Frey, S. D., & Curtis, P. S. (2005). Nitrogen additions and litter decomposition: A meta-analysis. Ecology, 86, 3252–3257.
Kronzucker, H. J., Siddiqi, M. Y., Glass, A. D. M., & Britto, D. T. (2003). Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiologia Plantarum, 117, 164–170.
Lewis, W. M. J., & Wurtsbaugh, W. A. (2008). Control of lacustrine phytoplankton by nutrients: Erosion of the phosphorus paradigm. International Review of Hydrobiology, 93, 446–465.
Long, R. P., Horsley, S. B., Hallett, R. A., & Bailey, S. W. (2009). Sugar maple growth in relation to nutrition and stress in the northeastern United States. Ecological Applications, 19, 1454–1466.
Lu, X., Mo, J., Gilliam, F. S., Zhou, G., & Fang, Y. (2010). Effects of experimental nitrogen deposition on plant diversity in an old-growth tropical forest. Global Change Biology, 16(10), 2688–2700.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858.
Nydick, K. R., Lafrancois, B. M., Baron, J. S., & Johnson, B. M. (2004). Nitrogen regulation of algal biomass, productivity, and composition in shallow mountain lakes, Snowy Range, Wyoming, USA. Canadian Journal of Fisheries and Aquatic Sciences, 61, 1256–1268.
Ochoa-Hueso, R., & Manrique, E. (2010). Nitrogen fertilization and water supply affect germination and plant establishment of the soil seed bank present in a semi-arid Mediterranean scrubland. Plant Ecology, 210, 263–273.
Olson, D. M., & Dinerstein, E. (2002). The Global 200: Priority ecoregions for global conservation. Annals of the Missouri Botanical Garden, 89, 199–224.
Phoenix, G. K., Hicks, W. K., Cinderby, S., Kuylenstierna, S. C. I., Stock, W. D., Dentener, F. J., Giller, K. E., Austin, A. T., Lefroy, R. D. B., Gimeno, B. S., Ashmore, M. R., & Ineson, P. (2006). Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Global Change Biology, 12, 470–476.
Rabalais, N. N. (2002). Nitrogen in aquatic ecosystems. Ambio, 31, 102–112.
Rao, L. E., Allen, E. B., & Meixner, T. (2010). Risk-based determination of critical nitrogen deposition loads for fire spread in southern California deserts. Ecological Applications, 20, 1320–1335.
Riddell, J., Nash, T. H., III, & Padgett, P. (2008). The effect of HNO3 gas on the lichen Ramalina menziesii. flora - morphology, distribution. Functional Ecology of Plants, 203, 47–54.
Rockström, J., Steffen, W., Noone, K., Persson, A., Chapin, F. S., Lambin, E. F., Lenton, T. M., Scheffer, M., Folke, C., Schellnhuber, H. J., Nykvist, B., de Wit, C. A., Hughes, T., van der Leeuw, S., Rodhe, H., Sorlin, S., Snyder, P. K., Costanza, R., Svedin, U., Falkenmark, M., Karlberg, L., Corell, R. W., Fabry, V. J., Hansen, J., Walker, B., Liverman, D., Richardson, K., Crutzen, P., & Foley, J. A. (2009). A safe operating space for humanity. Nature, 461, 472–475.
Stevens, C. J., Thompson, K., Grime, J. P., Long, C. J., & Gowing, D. J. G. (2010). Acidification as opposed to eutrophication is the main cause of declines in species richness seen in calcifuge grasslands impacted by nitrogen deposition. Functional Ecology, 24, 478–484.
Sutton, M. A., Howard, C. M., Erisman, J. W., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H. Grizzetti, B. (Eds.). (2011). The European nitrogen assessment. Cambridge University Press.
Talluto, M. V., & Suding, K. N. (2008). Historical change in coastal sage scrub in southern California in relation to fire frequency and air pollution. Landscape Ecology, 23, 803–815.
Throop, H. L., & Lerdau, M. L. (2004). Effects of nitrogen deposition on insect herbivory: Implications for community and ecosystem processes. Ecosystems, 7, 109–133.
Wallace, Z. P., Lovett, G. M., Hart, J. E., & Machona, B. (2007). Effects of nitrogen saturation on tree growth and death in a mixed-oak forest. Forest Ecology and Management, 243, 210–218.
Weiss, S. B. (1999). Cars, cows, and Checkerspot butterflies: Nitrogen deposition and management of nutrient-poor grasslands for a threatened species. Conservation Biology, 13, 1476–1486.
Wolfe, A. P., Baron, J. S., & Cornett, R. J. (2001). Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). Journal of Paleolimnology, 25, 1–7.
Wolfe, A., Cooke, C., & Hobbs, W. (2006). Are Current Rates of Atsmospheric Nitrogen Deposition Influencing Lakes in the Eastern Canadian Arctic? Arctic, Antarctic, and Alpine Research, 38, 465–476.
Xu, G. L., Schleppi, P., Li, M. H., & Fu, S. L. (2009). Negative responses of Collembola in a forest soil (Alptal, Switzerland) under experimentally increased N deposition. Environmental Pollution, 157, 2030–2036.
Acknowledgments
The authors thank the COST 729 and NinE programmes of the European Science Foundation (ESF), the Packard Foundation, INI and many other organizations for travel support to attend the workshop.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Baron, J. et al. (2014). The Effects of Atmospheric Nitrogen Deposition on Terrestrial and Freshwater Biodiversity. In: Sutton, M., Mason, K., Sheppard, L., Sverdrup, H., Haeuber, R., Hicks, W. (eds) Nitrogen Deposition, Critical Loads and Biodiversity. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7939-6_49
Download citation
DOI: https://doi.org/10.1007/978-94-007-7939-6_49
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7938-9
Online ISBN: 978-94-007-7939-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)