Abstract
We describe the principles of estimating glomerular filtration rate (GFR) with an emphasis on the role of creatinine and cystatin C in the calculation of estimated GFR (eGFR). We present a list of selected equations for eGFR calculation together with a critical evaluation of their role in the diagnostics, classification, and monitoring of kidney function.
History: The first part briefly describes the history of GFR assessment.
Pathophysiology: The second part deals with the physiology of GFR, the Starling equation, and the regulation of GFR.
Creatinine, cystatin C, and urea: The third part describes the pathophysiology, analytical details on the measurements, interferences, reference intervals, and important features of creatinine, cystatin C, and urea, together with data on intraindividual and interindividual variability of these compounds, desirable accuracy, and precision as well as data on real analytical quality, indexes of individuality, and reference change values.
Estimation of GFR: The fourth part describes the principle of renal clearance, creatinine clearance and the Cockcroft and Gault equation, eGFR based on cystatin C, urea clearance and the mean of urea and creatinine clearance, the MDRD formula, the CKD-EPI equations, the Lund-Malmö equations, and equations used in children. Comparison of inulin clearance with other equations for eGFR is also given.
Interpretation: The fifth part deals with the weaker aspects and sources of errors that may occur when using creatinine and cystatin C in the estimation of GFR. The relation of age and body surface area to GFR is also described.
Clinical use of eGFR: The sixth part describes the importance of eGFR in the classification of chronic kidney disease, diabetes mellitus, and acute kidney injury, with an emphasis on recommendations in recent guidelines.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Key Facts
Glomerular Filtration Rate: Key Facts
-
Doctors require information on two principal kidney properties: function of glomeruli and function of tubuli.
-
Glomerular filtration rate (GFR) is a measure of the filtration ability of the kidney.
-
Glomerular filtration rate can be measured, or – much more frequently – estimated (eGFR).
-
Doctors use eGFR for diagnostics and staging of kidney diseases according to internationally accepted guidelines.
Assessment of Glomerular Filtration Rate: Key Facts
-
The gold standard for direct measurement of GFR is inulin clearance. Other slightly less precise but acceptable methods also include iohexol or iothalamate clearance.
-
Direct measurement of GFR is impractical and time consuming; therefore, GFR is estimated from the concentration of serum biomarkers.
-
Two serum biomarkers are used: creatinine and cystatin C, where the higher the creatinine or cystatin C, the lower the GFR.
-
Laboratories provide physicians with information on eGFR by means of calculations based on serum creatinine and/or serum cystatin C.
-
Reference ranges for estimated GFR (eGFR) are not specified, because of the necessity to evaluate kidney function in the context of the clinical condition of the patient; however, values lower than 90 mL/min per 1.73 m2 (1.5 mL/s per 1.73 m2) are considered decreased.
Creatinine and Cystatin C in the Assessment of GFR: Key Facts
-
Creatinine and cystatin C are endogenous compounds eliminated by the kidneys, mainly via glomerular filtration. Both are widely used in medical laboratories.
-
There are a number of internationally accepted equations for eGFR calculation.
-
Creatinine and cystatin C have some disadvantages: creatinine is influenced by a high-protein diet, muscle mass changes, and muscle catabolism; tubular secretion of creatinine can occur; some methods interfere with body substances and drugs; cystatin C is influenced by corticosteroids, in diabetes mellitus and diseases of the thyroid gland.
Definitions
CKD-EPI equations
The recommended equations for estimating glomerular filtration rate, based on serum creatinine (the 2009 CKD-EPI creatinine equation), serum cystatin C (the 2012 CKD-EPI cystatin C equation), or both (the 2012 CKD-EPI cystatin C and creatinine equations).
Cockcroft-Gault equation
Not recommended. An obsolete formula for estimating glomerular filtration rate, based on sex, weight, and serum creatinine.
Creatinine
A metabolic product of muscle creatine, produced by the body at a constant rate. Elimination is influenced by kidney glomerular function, and increased concentration in blood is found in patients with decreased glomerular filtration rate.
Creatinine clearance
Not recommended. A method of estimating glomerular filtration rate, based on the measurement of creatinine concentration in serum and urine together with the collection of urine during a specified time interval. Creatinine clearance has been replaced by more modern equations based on serum concentrations of creatinine and/or cystatin C.
Cystatin C
A protein inhibitor of cysteine proteases, produced by all nucleated cells at a constant rate. Elimination is influenced by kidney filtration, and increased concentration in blood is found in patients with decreased glomerular filtration rate. In contrast to creatinine, a very low amount of cystatin C is excreted by the kidney, because of tubular reabsorption of this compound.
Glomerular filtration rate
The volume of plasma ultrafiltrate (primary urine) generated by the kidneys per unit of time. Values are expressed in ml/min (or ml/s) per standardized body surface area (1.73 m2).
MDRD equation
Not recommended. A formula that was used to estimate glomerular filtration rate between 1999 and 2009.
Urea
A metabolic product of body proteins, synthesized by the liver. Urea represents the main route of nitrogen elimination from the body. Increased concentration in the blood occurs during hypercatabolism of proteins or kidney failure.
Introduction
Estimation of glomerular filtration rate (GFR) is a basic method of assessing kidney function. Decreased GFR, present for more than 3 months, is one of the important diagnostic criteria for chronic kidney disease (CKD). Furthermore, CKD is classified based on the GFR category (KDIGO 2012 Guideline 2013). Gold-standard methods (renal or plasma inulin clearances) are precise, but time consuming. Creatinine has been used as a surrogate marker in GFR assessment for years, but physicians are more interested in function (GFR) than concentration (creatinine in plasma). Therefore, the reliable conversion of plasma biomarker concentration to eGFR is used for practical reasons. Among the available biomarkers, creatinine and cystatin C are mostly recommended to calculate eGFR , provided they are traceable to international calibration and suitable equations are used. Due to the plethora of eGFR equations, it is necessary to develop practical guidelines for international application and support their use in clinical practice.
Historical Background
Max Eduard Jaffé published his paper on the estimation of creatinine in 1886 (Jaffé 1886). Many modifications appeared because of the interference of what became known as Jaffé-positive substances. However, the Jaffé reaction with alkaline picrate is used even today, and enzymatic determination of creatinine is only slowly becoming the main method for the determination of creatinine in routine clinical laboratories. Inulin clearance was described in 1935 by Shannon and Smith (Shannon and Smith 1935), and the recent KDIGO 2012 Guideline (KDIGO 2012 Guideline 2013) recognizes inulin clearance as a gold standard of GFR assessment. The Cockcroft and Gault equation was published in 1976 (Cockcroft and Gault 1976). This equation was derived from samples of 249 patients aged 18–92. The MDRD equation was published by Andrew Levey (Levey et al.1999), and the same author published the CKD-EPI equation in 2009 (Levey et al. 2009). The best and most recent recommendation describing the use of creatinine, cystatin C, and estimations of GFR was published in 2013 in the “KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.”
Physiology of Glomerular Filtration Rate
Approximately 80 % of plasma volume entering afferent renal arterioles passes to efferent arterioles, and the remaining 20 % of plasma volume is filtered. Filtration is illustrated by the Starling equation.
The general Starling equation describes the rate of fluid through the capillary wall:
where
-
JV = rate of fluid
-
KF = capillary filtration constant
-
A = area for fluid exchange
-
PC and PT = capillary and tissue hydrostatic pressure
-
πC and πT = capillary and tissue oncotic pressure
-
δ = coefficient describing possible transfer of proteins
A simplified equation describing net filtration pressure (or net driving force, NDF) in the glomeruli is as follows:
where
-
PC = hydrostatic pressure of plasma (blood pressure)
-
PT = fluid pressure in the Bowman’s capsule
-
πC = oncotic pressure of plasma proteins
-
πT = oncotic pressure of plasma ultrafiltrate in the Bowman’s capsule
Filtration appears where NDF > 0. Filtration is influenced by the filtration area (e.g., 50 % in patients with one kidney), the quality of the glomerular membrane (changed in acidemia or in kidney disease), and the ability of various proteins to enter the Bowman’s capsule (molecular weight, shape of the molecule, isoelectric point of the protein). Approximately 120 mL/min (~170 L per day) of ultrafiltrate is generated every day, while about 1 mL/min (~1.5 L per day) of urine is excreted daily. This means that 99 % of the ultrafiltrate is reabsorbed (i.e., the fractional excretion of water is about 1 %). Glomerular filtration rate (GFR) is regulated by myogenic stretch and tubuloglomerular feedback. Tubuloglomerular feedback represents the autoregulation system, where GFR is regulated according to tubular urine flow. The aim of the system is to decrease loss of water and ions in situations with increased glomerular filtration rate. In case of increased GFR, the delivery of the chloride ion to the thick ascending limb of the loop of Henle is increased, which results in the constriction of afferent arterioles and the decrease of GFR. Sympathetic regulation, angiotensin II, prostaglandins, and natriuretic peptides contribute to the regulation of GFR.
Creatinine, Cystatin C, and Urea
Creatinine
Pathophysiology
Muscle creatine is converted to creatinine by a spontaneous, nonenzymatic reaction at a constant rate; however, ingested meat is a source of creatine and hence of creatinine. Creatinine is eliminated by glomerular filtration, but proximal secretion of creatinine exists; proximal secretion of creatinine increases with decreasing GFR. Also, a small amount of creatinine is reabsorbed by tubuli.
Analytical Remarks
International calibration is based on NIST SRM 967 (human liquid serum, traceable to the ID-MS (isotope dilution mass spectrometry) reference method). Two groups of methods are used throughout the world: the group of Jaffé methods with alkaline picrate and the group of enzymatic methods. Two key enzymes are used: creatinine deaminase (creatinine iminohydrolase, EC 3.5.4.21) and creatininase (creatinine amidohydrolase, EC 3.5.2.10). Other methods, e.g., HPLC, high-performance liquid chromatography, are not used routinely. The reference method is ID-MS.
Interferences
The Jaffé reaction is specific not only to creatinine, but many compounds – known as “Jaffé-positive chromogens” – can falsely increase concentration of creatinine, when measured in plasma or serum. Among them, glucose, protein, fatty acids, acetone, acetoacetate, pyruvate, and cephalosporins are the most important compounds. The measurement in urine is less influenced by these compounds. Other substances can falsely decrease creatinine concentration, e.g., ascorbic acid and bilirubin. Enzymatic methods can be influenced by bilirubin, hemoglobin, monoclonal immunoglobulin M, and some drugs, e.g., dobutamine.
Reference Intervals
Reference intervals are based on the abovementioned international calibration. Reference intervals for plasma (serum) creatinine are always different for both sexes due to the difference in muscle mass. Reference intervals for plasma (serum) are 64–104 μmol/L (0.72–1.18 mg/dL) in men and 49–90 μmol/L (0.55–1.02 mg/dL) in women.
Output of Creatinine
In order to predict the output of creatinine, a great number of equations for estimating glomerular filtration rate (eGFR), e.g., the Cockcroft-Gault equation, are based on the patient’s weight. Therefore, this equation fails in individuals with fluid retention or obesity. Output of creatinine calculated per kilogram of the body weight is expected to be constant in adults without fluid retention, excessive amount of body fat, or muscle atrophies. Figure 1 shows body mass index (BMI) 16–22 kg/m2, where creatinine output calculated per kilogram of the body weight is practically independent of BMI. Output of creatinine ranges from 0.15 to 0.18 mmol/day/kg of the body weight (median 0.17 mmol/day/kg of body weight, 10th percentile 0.13, 90th percentile 0.20 mmol/day/kg of body weight).
The relationship between BMI and creatinine output over 24 h calculated per kilogram of the total body weight in adolescents (Based on data published by Remer 2002 ). As can be seen from the Figure, urinary output (dU) of creatinine (recalculated per kg of the body weight) is constant over a wide range of body mass index (BMI). Two outliers were excluded. Circle = boys, square = girls
If a constant amount of creatinine is excreted from one kilogram of body mass (except for individuals with muscle atrophies, fluid retention, or excessive obesity), we may then check the output of creatinine in adults in comparison to body mass. Table 1 indicates the reference limits of daily creatinine excretion in the urine according to ARUP’s Laboratory Test Directory (http://ltd.aruplab.com/Tests/Pub/0020473).
If values are recalculated based on body weight, i.e., we want to calculate the body weight in relation to the amount of excreted creatinine (a coefficient of 0.17 mmol of creatinine based on one kilogram of the total body weight per day was used), unrealistic values are obtained in some cases. Maximum creatinine output is derived from the maximum possible GFR (150 mL/min, i.e., 2.5 mL/s). When diuresis is 1,500 mL/day, plasmatic concentration of creatinine is 90 μmol/L, and concentration of creatinine in the urine is 13 mmol/L, then creatinine clearance is 2.508 mL/s and creatinine output is 19.5 mmol/day (Schück, 2012, personal communication). Nevertheless, the maximum possible output of creatinine does not represent the reference limit. Therefore, we recommend the following reference ranges for creatinine output in the urine over 24 h:
Men | 10–16 mmol/day (1.13–1.81 g/day) |
Women | 8–14 mmol/day (0.90–1.58 g/day) |
Note: If the coefficient of 0.17 mmol/day per kilogram of the body weight is used, the corresponding weights are 59–94 kg in men and 47–82 kg in women.
Ratios of Urinary Compounds to Creatinine
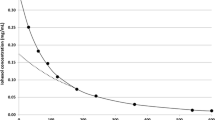
Ratios of urinary compounds to creatinine are becoming increasingly popular as tools suitable for evaluating substances eliminated through the urine, based on the principle that the concentration of creatinine reflects diuresis (Fig. 2). It is obvious that the reliability of the estimation of diuresis from creatinine concentration decreases with decreasing creatinine concentration.
The relationship between diuresis and concentration of creatinine in the urine. Creatinine is excreted at a constant rate. Therefore, concentration of creatinine in the urine decreases with increasing volume of urine. However, kidney function influences the balance between serum and urine creatinine. Higher concentrations of creatinine in a lower volume of urine have an acceptable low error estimate. On the other hand, lower concentrations of creatinine in the urine may represent different volumes of urine, and the estimation of urinary volume is imprecise. For example, concentration of creatinine of 20 mmol/L means that the volume of urine is between approximately 500 and 1,000 mL/day. In contrast, concentration of creatinine of about 5 mmol/L means that the volume of urine is between 1,200 and 3,500 mL/day for different kidney functions. Nevertheless, ratios of urinary compounds to creatinine are better estimates for interpretation purposes than concentrations of these compounds without any correction
The most used ratios are PCR (protein-to-creatinine ratio) and ACR (albumin-to-creatinine ratio). As can be seen from Fig. 2, lower concentrations of urinary creatinine are rather poor predictors of urine volume. On the other hand, interpreting ratios is practical and much better than evaluation of concentration alone. However, timed urine samples can provide the physician with more accurate protein and albumin excretion values.
Cystatin C
Pathophysiology
Cystatin C (cystatin 3, gamma-trace protein) is a member of cystatin superfamily II, gen CST3, locus 20p11.2. Cystatin C is a basic (isoelectric point, pI, 9.3) protein composed of a single polypeptide chain of 120 amino acid residues with a molecular weight of 13.4 kDa (13,359 g/mol). Cystatin C is synthesized at a constant rate by all nucleated cells. The synthesis is not influenced by inflammation, catabolism, or diet. Measurable concentrations of cystatin C are found in the plasma (about 1 mg/L), urine (0.2 mg/L), cerebrospinal fluid (3–14 mg/L), seminal plasma (40–60 mg/L), milk (2 mg/L), saliva (0.4–5 mg/L), tears (1 mg/L), and amniotic (1 mg/L) and synovial fluid (2 mg/L). With regard to molecular weight and pI, cystatin C is freely filtered and completely reabsorbed and degraded in the proximal tubuli. Therefore, the plasma concentration of cystatin C indirectly reflects glomerular filtration rate, and increased urine concentration reflects tubular injury.
Analytical Remarks
International calibration is based on CRM ERM-DA471/IFCC. Immunoassay is a dominant principle of cystatin C measurement.
The Use of Cystatin C in Nephrology
The measurement of cystatin C is recommended in situations where eGFR based on serum creatinine is less accurate and also for confirmation of CKD in adult patients with mildly to moderately decreased eGFR (based on creatinine), i.e., 45–59 mL/min per 1.73 m2 (G3a) (KDIGO 2012 Guideline 2013). As cited from KDIGO 2012 Guideline: “1.4.3.2: We suggest using additional tests (such as cystatin C or a clearance measurement) for confirmatory testing in specific circumstances where eGFR based on serum creatinine is less accurate. (2B).”
Cystatin C, Cardiovascular Mortality, and Morbidity
Shlipak (Shlipak et al. 2005) published a relation between increased concentrations of cystatin and the annual rate of death due to all causes and also death due to cardiovascular causes. While the relation between cystatin C and the rate of death was almost exponential (the higher the level of cystatin C, the higher the hazard ratio), association of creatinine categories and mortality revealed a J-shaped curve. One important result was that within each quintile of creatinine concentration, higher quintiles of cystatin C were associated with increased risk of mortality due to all causes. Cystatin C was further assessed as a cardiovascular risk factor (Taglieri et al. 2009; Woitas et al. 2013) and is classified as a cardiorenal biomarker (Chowdhury et al. 2013).
Reference Intervals
Reference intervals based on the abovementioned international calibration are 0.31–0.99 mg/L in men and 0.4–0.99 mg/L in women.
Urea
Pathophysiology
Urea is an end product of protein catabolism, synthesized in the liver. About 90 % of urea is excreted by the kidneys. Free filtration in the glomeruli is followed by several stages of passive tubular transport, which results in the reentering of the plasma compartment. Clearance of urea is lower than true GFR. Impaired kidney function, dehydration, increased protein catabolism (including catabolism of protein in gastrointestinal bleeding), a high-protein diet, administration of cortisol, and obstruction of the urinary tract are the main reasons for increased urea concentration in plasma. Creatinine rises more slowly than urea in prerenal failure or in cases of decreased renal blood flow.
Analytical Remarks
Older nonenzymatic methods of urea determination are used in exceptional cases, and the majority of laboratories use the enzymatic method with urease (EC 3.5.1.5) and glutamate dehydrogenase (EC 1.4.1.3). International calibration is based on NIST SRM 912a.
Reference Intervals
Increased concentrations of urea are found in older populations and in men. Different reference values can be found in the literature. Reference intervals in men up to 50 years of age are 3.2–7.4 mmol/L and 3.0–9.2 mmol/L in men above 50 years of age. The intervals are 2.5–6.7 mmol/L in women up to 50 years of age and 3.5–7.2 mmol/L in women above 50 years of age.
Output of Urea
Output of urea is influenced by protein catabolism. The normal concentration of urea in the urine is 250–433 mmol/L. If daily volume of urine is about 1,350 mL/day, daily production of urea is calculated at 340–585 mmol/day.
Biological and Analytical Variability of GFR Measures and Related Tests
Intraindividual (variability within a subject) and interindividual (variability between subjects) biological variabilities are important characteristics required for accurate interpretation of laboratory tests. Desirable precision, maximum tolerable bias, and total allowable error are calculated according to biological variability, and they define the requirements for laboratory tests. The index of individuality is calculated as a ratio of intraindividual biological variability and analytical variability. Reasonable analytical variability is expressed as a coefficient of variability (CVa, given in %) and calculated from intermediate precision (long-term analytical variability assessed over a period of several weeks) in the clinical laboratory. The reference change value (RCV, or critical difference, given in %) is calculated from intraindividual variability (CVi) and analytical variability (CVa) as
and expresses changes (in %) in the concentration of the analyte, which represents a significant departure from the basal value. The absolute RCV is calculated for the selected basal value. Required parameters for accurate interpretation of laboratory tests are given in Table 2.
Estimation Methods of GFR
The Principle of Renal Clearance
Renal clearance is defined as “the volume of plasma from which the substance is completely cleared by the kidneys per unit of time” (Burtis 2006). For a substance with specific properties (stable rate of synthesis, stable plasma concentration, freely filtered, no influence of renal tubuli in terms of reabsorption, secretion, synthesis, or catabolism), the amount of the filtered substance is the same as the amount of the excreted substance:
where GFR is the glomerular filtration rate,
PSubst and USubst are plasma and urine concentrations of that substance, respectively, and V is the volume of urine per unit of time.
Then we have
The problem consists in the properties of an “ideal” substance: creatinine is far from ideal due to proximal tubular secretion; inulin, 51Cr-EDTA, and iohexol display extrarenal clearance; the use of 51Cr-EDTA, 125I-iothalamate, and 99mTc-DTPA is connected with the risk of ionizing radiation; other markers are neither sensitive nor specific for the assessment of GFR (beta-2-microglobulin, alpha-1-microglobulin, urea, and retinol-binding protein).
Creatinine Clearance and the Historical Cockcroft and Gault Equation
Creatinine is supposed to be filtrated freely by the glomeruli and excreted in the urine without any tubular secretion or reabsorption. Unfortunately, this is not the case since creatinine is secreted in the proximal tubuli and this secretion increases with decreasing GFR. Renal clearance of creatinine (CCr) is calculated by the following simple equation:
where UCreat is the concentration of creatinine in the urine, V is the volume of urine per unit of time, and SCreat is the plasma (serum) concentration of creatinine. Volume is expressed in mL, time in minutes (SI units: in seconds), and concentrations of creatinine must be in the same units (both urine and plasma in mg/dL, or both urine and plasma in mmol/L). The product (UCreat * V) in Eq. 1 represents the rate of creatinine elimination in the urine, e.g., in g/day (SI: mmol/day). Creatinine is supposed to be synthesized at a constant rate, where the amount of creatinine produced daily is a function of body muscle mass. Because of the correlation between the muscle mass and body weight, one can assume that the rate of creatinine elimination can be deduced from
where X represents the amount of creatinine excreted per day. For example, here is the Cockcroft and Gault formula
where Age is in years, Weight is in kg, SCreat is in μmol/L, factor F is 1.0 for men and 0.85 for women, and eGFR is in mL/s.
Cockcroft and Gault derived this formula in 1976 based on data from 249 healthy volunteers. This formula is not recommended any more. However, there have been instances where this formula has been used among clinical pharmacists; also, some pharmacokinetic programs still use the obsolete equation. A similar principle is used in other equations, where the estimation of creatinine output is calculated from body measurements (height in children, weight in adults, etc.).
eGFR Based on Cystatin C
There are many equations based on the assumption that cystatin C is produced at a constant rate:
where X is an estimate of the daily output of cystatin C.
However, certain extrarenal clearance of cystatin C exists, and therefore, new equations are based on the regression between cystatin C and the “gold” standard.
In 2005, Grubb derived a cystatin C-based equation for use in adults and children (Grubb 2005):
or with gender factor
Results are in mL/min per 1.73 m2.
A review of other cystatin C-based equations in children was published by Andersen (Andersen et al. 2009) and Filler (Filler et al. 2012).
The CKD-EPI formula based on cystatin C was published in 2013 (KDIGO 2012 Guideline 2013). It should be noted, however, that Grubb published a new cystatin C-based equation in 2014:
This equation disregards sex and race and can be used as an assay-independent calculation (Grubb 2014).
Urea Clearance
When compared with GFR measured by inulin clearance, urea clearance underestimates GFR, while creatinine clearance overestimates GFR. Therefore, an equation was derived as a mean of urea and creatinine clearance (listed in the European Best Practice Guidelines for Haemodialysis (ERA-EDTA 2002)).
Mean of Urea Clearance and Creatinine Clearance
This equation is based on the assumption that urea clearance underestimates “true” GFR and creatinine clearance (due to the proximal secretion of creatinine, increased with decreasing GFR) overestimates GFR:
-
V = volume of urine in mL per time
-
t = time in minutes or seconds
-
UUrea and UCreat = urinary concentrations of urea and creatinine in mmol/L
-
SUrea nad SCreat = serum concentrations of urea and creatinine in mmol/L,
-
BSA = body surface area in m2
MDRD Formula
MDRD (Modification of Diet in Renal Diseases) was published by Levey (Levey et al. 1999). Three equations were derived for chronic kidney diseases, the first based on age, gender, and serum creatinine (and race); the second on age, gender, serum creatinine, and serum urea (and race); and the third on age, gender, serum creatinine, serum urea, and serum albumin (and race). The equations were derived from a sample of 1,070 patients and validated using a sample of 558 patients with chronic kidney disease. Renal clearance of 125I-iothalamate was selected as the “gold” standard; creatinine was measured using a kinetic alkaline picrate method. The first equation was enhanced by improvements in international creatinine standardization: the “new” MDRD formula (called the “175 MDRD equation”) replaced previous versions (Levey et al. 2007):
where SCreat is in mg/dL, Age is in years, and F is 1.0 for men and 0.742 for women. Another factor is used for race (1,210 if African American). eGFR (MDRD) is in mL/min per 1.73 m2.
For the SI unit, the MDRD equation is
where SCreat is in μmol/L, Age is in years, and the other factor as above. eGFR (MDRD) is in mL/s per 1.73 m2.
CKD-EPI Equations
The CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation was published by Levey (Levey et al. 2009), and the authors recommend using this equation instead of the older MDRD equation. The 2009 CKD-EPI is based on creatinine in serum, derived from a sample of 8,254 participants and validated using a data set of 3,896 participants. Iothalamate clearance was selected as the “gold” standard. The KDIGO CKD 2012 Guideline strictly recommends using CKD-EPI equations. The use of other creatinine-based equations is possible only when improved accuracy is proved, compared with the 2009 CKD-EPI creatinine equation. When recording eGFR, the equation used should be specified (KDIGO 2012 Guideline 2013).
There are three recently recommended CKD-EPI equations:
-
2009 CKD-EPI creatinine-based equation
-
2012 CKD-EPI cystatin C-based equation
-
2012 CKD-EPI cystatin C- and creatinine-based equation
These equations are described in Table 3.
Lund-Malmö Equations
These equations were derived from a population of Swedish Caucasians (derivation set from Lund, N = 436; validation set from Malmö, N = 414); clearance of iohexol was used as the “gold” standard. The entire cohort of 850 patients was within an age interval of 26–85 years; plasma creatinine was 45–545 μmol/L, and iohexol clearance was 9–121 mL/min per 1.73 m2 (Björk et al. 2007). All equations (Table 4) used creatinine, age in years, and sex; adjustment to lean body mass can be applied. Lean body mass (LBM) can be calculated according to James (see Björk et al. 2007) as follows:
where Weight is in kg, Height in cm, and LBM is in kg.
One of the advantages of the Lund-Malmö equations lies in the fact that the equation estimates GFR using a broad age range and predicts GFR in patients with “normal” serum creatinine. A further advantage is its derivation from a European population. Björk published a revised version of the Lund-Malmö equations in 2011 (Björk et al. 2011). Nyman published a better performance of the revised Lund-Malmö eGFR than MDRD or CKD-EPI in a population of 2,847 adults (Nyman et al. 2014). However, CKD-EPI is now the standard method of GFR estimation.
Estimation of GFR in Children
The Schwartz formula (Schwartz et al. 1976) has been used for years, based on serum creatinine and body height:
where Height is in meters and SCreat is in mg/dL.
This equation was recommended in the KDIGO 2012 Guideline as the “bedside” Schwartz formula.
In 2009, Schwartz published a new formula based on height, serum creatinine, cystatin C, and BUN (Schwartz et al. 2009):
where Height is in meters, SCrea in mg/dL, SCystC in mg/L, and BUN in mg/dL.
Schwartz used plasma iohexol clearance as the “gold” standard. Other equation is
where Height is in meters, SCreat and BUN are in mg/dL, and results are in mL/min per 1.73 m2. This equation was recommended in the 2012 KDIGO CKD (KDIGO 2012 Guideline 2013).
However, Nyman tested the use of the Lund-Malmö equation in children and found this equation performed well in 85 pediatric Caucasian patients, aged 0.3–17 years. The Lund-Malmö equation performed better when not corrected for lean body mass (Nyman et al. 2008).
Example: Male, white, 50 years, 24-h collection of urine (1,440 min.), volume of collected urine 1,500 mL/day. Weight 75 kg, height 180 cm. Measured and calculated values are given in Table 5.
The use of an exogenous filtration marker is recommended in situations where more accurate GFR values are needed to make better treatment decisions. Inulin clearance is recognized as the “gold” standard for GFR assessment (KDIGO 2012 Guideline 2013). There are two principles of inulin clearance measurement: “renal” clearance of inulin (continuous infusion of inulin with measurement of plasma and urine inulin concentrations under steady-state conditions, where timed collection of urine is necessary) and “plasma” clearance of inulin (measurement of several plasma inulin concentrations after an intravenous bolus of inulin). There are several models of “plasma” clearance of inulin (simple exponential analysis, area under curve calculation, the Jung model using early and late plasma concentrations, the biexponential model, etc.).
Table 6 shows our own experience with plasma inulin clearance, based on the Jung model (Jung et al. 1991). Results of other equations are compared.
Interpretation of eGFR
Specific Problems with Cystatin C Measurement and Interpretation
Sources of errors in GFR estimation using creatinine and cystatin C (KDIGO 2012 Guideline, modified) are shown in Table 7. Synthesis, tubular processes, and extrarenal elimination are listed as “non-GFR variables that differ from derivation sets of patients” in KDIGO 2012 Guideline.
GFR and Age
GFR decreases with age; however, values below 60 mL/min per 1.73 m2 (1 mL/s per 1.73 m2) are rather unusual in individuals without renal pathology. Mathew (Mathew 2007) used data from Sikaris (personal communication) to elucidate this relationship. More than 97.5 % of the mixed population (more than 300,000 examinations in a large private pathology, exclusive of creatinine outliers for each decade) have an eGFR above 60 mL/min per 1.73 m2 (1 mL/s per 1.73 m2) at ages ≤57 years, and 80 % of the mixed population have an eGFR above 60 mL/min per 1.73 m2 (1 mL/s per 1.73 m2) at ages ≤80 years. Similar data are available in the KDIGO 2012 Guideline, where inulin clearance is above 60 mL/min per 1.73 m2 in older healthy men and women.
Glomerular Filtration Rate and Body Surface Area
Results of all “new” equations for eGFR are always expressed in standardized format, i.e., in mL/min per 1.73 m2 (or mL/s per 1.73 m2). However, for drug dosing it is necessary to recalculate the standardized results here to reflect the actual filtration of the patient (in mL/min or mL/s).
The following simple equation is used:
where BSA (in m2) is calculated according to the DuBois and DuBois formula (DuBois and DuBois 1916)
where Weight is in kg and Height is in cm. GFR is then in mL/min or mL/s.
Specific Clinical Situations
Chronic Kidney Disease (CKD)
“Chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function, present for more than 3 months, with implications for health and CKD is classified based on cause, GFR category, and albuminuria category (CGA)” (KDIGO 2012 Guideline 2013). This completely new definition reflects the need for both a more “clinical” definition and a more precise “laboratory” classification. GFR categories together with recommended terms are listed in Table 8.
Lower values of eGFR correlate with worse prognoses: the age-standardized rate of hospitalization, rate of death, and rate of cardiovascular events (per 100 person-years) increase almost exponentially with decreasing eGFR (Levey et al. 2006).
Diabetes Mellitus
Pathophysiology and the Development of Diabetic Nephropathy
In type 1 diabetes mellitus (T1DM), five stages of diabetic nephropathy can be distinguished:
-
(a)
Early hypertrophy and hyperfunction, lasting anywhere from months to years. At this stage, eGFR is increased of about 20–40 %, and hyperfiltration is supposed to be a risk factor.
-
(b)
Clinically latent stage of renal impairment, where eGFR can be increased, but the basal membrane is thickened and mesangial expansion can be found.
-
(c)
Incipient nephropathy, with positive albuminuria; eGFR is sometimes increased, but a thickened basal membrane and mesangial expansion is more common.
-
(d)
Manifest nephropathy with proteinuria and decreased eGFR; GFR decreases at a rate of 1.2 mL/min per year (0.2 mL/s per year).
-
(e)
Chronic kidney disease with severely decreased GFR or kidney failure.
Only 25 % of patients with type 2 diabetes (T2DM) mellitus have similar renal impairment, as is common in T1DM, frequently as a result of decompensated diabetes mellitus. About 40 % of patients with T2DM display slight or moderate histological findings with borderline renal function. About 35 % display tubulointersticial changes or glomerulosclerosis.
Guidelines
Recently American Diabetes Association Standards of Medical Care in Diabetes (ADA 2016) describes consequences of decreased GFR in the following chapters: Cardiovascular Disease and Risk Management and Microvascular Complications and Foot Care. Estimated GFR should be used as a screening tool at least once a year in patients with type 1 diabetes mellitus lasting five or more years and in all patients with type 2 diabetes mellitus. Serum creatinine with MDRD formula or preferably CKD-EPI formula is recommended for eGFR assessment of diabetes mellitus. The five stages of CKD are defined in the 2016 ADA Standards of Medical Care in Diabetes (Table 9). In contrast to the KDIGO 2012 Guideline, stage 3a and 3b are not distinguished here.
Both eGFR and albuminuria (and serum/plasma potassium) are measured together. Screening based on albuminuria alone is not sufficient. It should be stressed, however, that albuminuria is measured repeatedly due to its high biological variability. The new term “increased urinary albumin excretion” can be used only if two of three urine specimens collected within a 3- to 6-month period contain ≥30 mg/g creatinine (≥3.0 g/mol creatinine). The use of the term “microalbuminuria” (30–299 mg/g creatinine, 3–29.9 g/mol creatinine) should no longer be used; similarly, the term “macroalbuminuria” (or “clinical albuminuria”, >299 mg/g creatinine or 30 g/mol creatinine) is also inappropriate.
The ADA Standards (ADA 2016) recommends actions for the management of CKD. Patients in stages 4 and 5 should always be referred to a nephrologist, as well as patients with eGFR 45–60 mL/min per 1.73 m2 (0.75–1.0 mL/s per 1.73 m2) and suspect of nondiabetic kidney disease. More frequent monitoring of an expanded set of laboratory tests is recommended in patients with eGFR 30–44 mL/min per 1.73 m2 (0.5–0.74 mL/s per 1.73 m2).
Estimated GFR (or creatinine measurement) should also be monitored (together with potassium levels in serum/plasma) in diabetic patients using ACE inhibitors, angiotensin receptor blockers, loop diuretics, hydrochlorothiazide, or chlorthalidone. Hydrochlorothiazide and chlorthalidone should be avoided with eGFR levels under 30 mL/min per 1.73 m2 (0.5 mL/s per 1.73 m2).
AKI and Intensive Care
Definition and Staging of Acute Kidney Injury
Acute kidney injury (AKI) is present when
-
Serum creatinine increases by 26.5 μmol/L or more (0.3 mg/dL and more) within 48 h, or
-
Serum creatinine increases by at least 50 % above the baseline (at least 1.5 times above the baseline) within 7 days, or
-
The volume of urine is lower than 0.5 mL/kg per hour for 6 h (oliguria).
There are two staging systems – the older RIFLE system (Risk, Injury, Failure, Loss of function, End-stage kidney disease) and the newer AKIN system (Acute Kidney Injury Network) (Ronco 2013). Comparison of the RIFLE and AKIN systems is given in Table 10. Both systems can be used, but a fraction of the patients classified in the respective stages is not equal.
Several methods for GFR estimation are discussed among intensivists with strong emphasis on frequent monitoring of plasma (serum) creatinine; however, creatinine clearance based on a 2-h collection of urine is also admitted, and iohexol or iothalamate is classified as acceptable and realistic by the ADQI group. GFR can be used for staging of acute kidney injury as part of the RIFLE (but not the AKIN) classification.
Monitoring of creatinine concentration is an essential tool for GFR assessment in intensive care. Plasma (serum) creatinine is not a precise predictor of GFR under acute circumstances. On the other hand, dynamic changes in creatinine concentrations are similar to the dynamic changes in GFR. In cases of unknown basal creatinine concentration, it is possible to assess creatinine concentration using the CKD-EPI equation. This principle was recommended for the MDRD equation by Bellomo et al. (2004). They used “basal” GFR of 75 mL/min per 1.73 m2 (1.25 mL/s per 1.73 m2). Table 11 is based on the 2009 CKD-EPI creatinine equation with “basal” GFR of 75 mL/min per 1.73 m2 (1.25 mL/s per 1.73 m2).
Potential Applications to Prognosis, Other Diseases, or Conditions
The relation between eGFR and prognosis is given in the KDIGO 2012 Guideline for CKD. Four variables should be taken into account: cause of CKD, GFR category (G1–G5), albuminuria category (A1–A3), and complications (other risk factors, comorbidities). eGFR is used to diagnose and monitor other diseases and conditions: increased GFR can be expected during pregnancy, with a high-protein diet and in conditions where there is increased output of osmotically active substances (osmotic diuresis, e.g., after mannitol, or in hyperglycemic situations). For extrarenal reasons, decreased eGFR can occur during and after cases of dehydration, e.g., hemorrhagic shock.
Summary
-
Estimated glomerular filtration rate (eGFR) is used for the diagnostics, staging, and monitoring of kidney function.
-
Estimation is based on serum creatinine and/or cystatin C concentration.
-
Methods of determining creatinine and cystatin C should be traceable to international calibrators; enzymatic determination of creatinine is preferred to the Jaffé (alkaline picrate) method.
-
The gold-standard method for GFR assessment (inulin clearance) is preferred to other methods (125I-iothalamate, iohexol).
-
The CKD-EPI equations (Chronic Kidney Disease – Epidemiology Collaboration) are recent equations for calculating eGFR.
-
Three CKD-EPI equations are recommended: the 2009 CKD-EPI creatinine, 2012 CKD-EPI cystatin C, and 2012 CKD-EPI cystatin C and creatinine equations, respectively.
-
The MDRD equation or Cockcroft-Gault formula should not be used for eGFR assessment.
-
The KDIGO 2012 Guidelines (both for chronic kidney disease and acute kidney injury) and ADQI recommendations (Acute Dialysis Quality Initiative) provide suggestions for health care that positively impact on patient benefit and safety.
Abbreviations
- ACR:
-
Albumin-to-creatinine ratio
- ADA:
-
American Diabetes Association
- ADQI:
-
Acute Dialysis Quality Initiative
- AKI:
-
Acute kidney injury
- AKIN:
-
Acute Kidney Injury Network
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- BUN:
-
Blood urea nitrogen
- CCr:
-
Clearance of creatinine
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- eGFR:
-
Estimated glomerular filtration rate
- ESRD:
-
End-stage renal disease
- GFR:
-
Glomerular filtration rate
- HPLC:
-
High-performance liquid chromatography
- ID-MS:
-
Isotope dilution mass spectrometry
- IFCC:
-
International Federation of Clinical Chemistry and Laboratory Medicine
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- LBM:
-
Lean body mass
- MDRD:
-
Modification of Diet in Renal Diseases
- NDF:
-
Net driving force (net filtration pressure)
- NIST SRM:
-
National Institute of Standards and Technology, Standard Reference Material
- PCR:
-
Protein-to-creatinine ratio
- RCV:
-
Reference change value, critical difference
- RIFLE:
-
Risk, Injury, Failure, Loss of function, End-stage kidney disease
- RRT:
-
Renal replacement therapy
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
References
American Diabetes Association (ADA). Standards of medical care in diabetes-2016. Diabetes Care. 2016;39 Suppl 1:S1–112.
Andersen TB, Eskild-Jensen A, Frøkiaer J, et al. Measuring glomerular filtration rate in children; can cystatin C replace established methods? Rev Pediatr Nephrol. 2009;24:929–41.
ARUP’s Laboratory Test Directory. http://ltd.aruplab.com/Tests/Pub/0020473. Accessed February 2016.
Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–12.
Björk J, Bäck SE, Sterner G, et al. Prediction of relative glomerular filtration rate in adults: new improved equations based on Swedish Caucasians and standardized plasma-creatinine assays. Scand J Clin Lab Invest. 2007;67:678–95.
Björk J, Grubb A, Sterner G, et al. Revised equations for estimating glomerular filtration rate based on the Lund-Malmö study cohort. Scand J Clin Lab Invest. 2011;71:232–9.
Burtis CA, Ashwood ER, Bruns DE. Tietz texbook of clinical chemistry and molecular diagnostics. 4th ed. St. Louis: Elsevier Saunders; 2006. p. 818.
Chowdhury P, Kehl D, Choudhary R, et al. The use of biomarkers in the patient with heart failure. Curr Cardiol Rep. 2013;15:372.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71.
European Renal Association-European Dialysis, Transplant Association (ERA-EDTA). European best practice guidelines for haemodialysis (part 1). Nephrol Dial Transplant. 2002;17 Suppl 7:7–9.
Filler G, Huang SH, Yasin A. The usefulness of cystatin C and related formulae in pediatrics. Clin Chem Lab Med. 2012;50:2081–91.
Grubb A, Nyman U, Björk J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–31.
Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 2014;60:974–86.
Jaffé M. Über den Niederschlag, welchen Pikrinsäure in normalen Harn erzeugt und über eine neue Reaction des Kreatinins. Z Physiol Chem. 1886;10:391–400.
Jung K, Henke W, Schulze BD, et al. Single-injection inulin clearance using only one blood sample as a suitable procedure to measure glomerular filtration rate. Nephron. 1991;59:694–5.
KDIGO, CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–70.
Levey AS, Stevens LA, Hostetter T. Automatic reporting of estimated glomerular filtration rate-just what the doctor ordered. Clin Chem. 2006;52:2188–93.
Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Mathew TH, Johnson DW, Jones GR, et al. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: revised recommendations. Med J Aust. 2007;187:459–63.
Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of a initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
Nyman U, Björk J, Lindström V, et al. The Lund-Malmö creatinine-based glomerular filtration rate prediction equation for adults also performs well in children. Scand J Clin Lab Invest. 2008;68:568–76.
Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52:815–24.
Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75:561–9.
Ronco C. ADQI consensus on AKI biomarkers and cardiorenal syndromes. Contrib Nephrol Basel Karger. 2013;182:5–12.
Schwartz GJ, Haycock GB, Edelmann Jr CM, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–63.
Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37.
Shannon JA, Smith HW. The excretion of inulin, xylose and urea by normal and phlorizinized man. J Clin Invest. 1935;14:393.
Shlipak M, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–60.
Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009;55:1932–43.
Woitas RP, Kleber ME, Meinitzer A, et al. Cystatin C is independently associated with total and cardiovascular mortality in individuals undergoing coronary angiography. The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis. 2013;229:541–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Jabor, A., Franeková, J., Hošková, L. (2016). Estimation of Glomerular Filtration Rate. In: Patel, V., Preedy, V. (eds) Biomarkers in Kidney Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7699-9_33
Download citation
DOI: https://doi.org/10.1007/978-94-007-7699-9_33
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7698-2
Online ISBN: 978-94-007-7699-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences