Abstract
Despite the hardness and strength characteristic, the bone is not a static tissue, but is constantly changing and constantly repairing. This process is called bone remodeling. In this cyclical process, the oldest bone tissue is removed and replaced with another new tissue. There are two types of cells responsible for the bone turnover: osteoclasts and osteoblasts. The different phases of bone remodeling are controlled by numerous local and systemic factors. The alteration of balance between these factors can lead to different types of bone disorders. In the recent years, the bone turnover markers underwent extensive development. Pathological bone resorption is a cause of significant morbidity in diseases affecting the skeleton, such as rheumatoid arthritis, osteoporosis, periodontitis, and cancer metastasis. Biochemical monitoring of bone metabolism depends upon the measurement of enzymes and proteins released during bone formation and of degradation products formed during bone resorption. The mammalian chitinases can be considered new bone resorption markers. These molecules belong to the families 18 glycosyl hydrolase (GH) superfamily. Although, these enzymes have been widely implicated in a variety of diseases involving immune dysfunction, their biologic role in bone resorption is poorly understood.
Herein we will focus on what is known in the role chitinase family in bone disease development, as well as the potential of some of the engaged molecules as prognostic or diagnostic markers and their perspective in developing new therapeutic strategies against bone disease.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Key Facts
-
1.
The chitinases (18 glycosyl hydrolase (GH18) superfamily) are able to hydrolyze the glycosidic bond, a covalent bond that connect a sugar (carbohydrate) molecule to another sugar or molecule (chitin, chitosan, and peptidoglycan).
-
2.
The chitin is a polymer of N-acetylglucosamine, a sugar derivate (glucose), present in the exoskeleton of arthropods, in fungi and cell walls.
-
3.
The GH18 family includes both catalytically active and nonhydrolytic proteins that function as carbohydrate binding modules (CBM).
-
4.
The chitinases are pleiotropic molecules distributed in the human body. The main chitinases is chitotriosidase (CHIT1).
-
5.
The innate immunity cells (monocyte, macrophages, dendritic cells, microglia) defend the host from infection by other organisms.
-
6.
The monocyte derived cells produce different chitinases (CHIT1, CHI3L1, CHI3L2, CHIA, and CHID1).
-
7.
The chitinases are related to various disease
-
8.
The CHIT1activity is a prognostic marker for Gaucher disease, sarcoidosis, and acute stroke.
-
9.
The chitinases are polymorphic proteins. The CHIT1 polymorphism (24-bp duplication in exon 10) inhibits its hydrolase activity.
-
10.
The heterozygosis for a 24-bp duplication in the CHIT1 gene has a protective effect in human longevity.
Definition of Words and Terms
- Bone tissue:
-
is the main representative connective tissue of the body. It is by a remarkable hardness and resistance and composed of cells dispersed in an abundant extracellular matrix, formed of fibers and amorphous substance of glycoproteins.
- Calcium:
-
is an alkaline earth metal tender. In the human body is present about one kilogram of calcium, of which 99% is fixed in the bones and the rest circulating free in the blood. Vitamin D is needed to absorb calcium from food.
- Calcium phosphate thin film disks (CPhoDs):
-
consists of submicron synthetic calcium phosphate thin films coated onto various culture vessels. This system has been used as an alternative method for compound screening for direct assessment of osteoclast and osteoblast activity in vitro.
- Carbohydrate-binding domain:
-
is a common region between the chitinase with chitinolytic activity and chitinase-like proteins (CLPs) and is involved in the recognition or binding of chitin subunits.
- Chitinases:
-
The chitinase are glycolytic enzymes that destroy chitin. When pathogens colonize or attack various organisms (such as plants), they defend themselves by secreting enzymes capable of destroying chitin. In the human, the chitinases play a role in the immunity.
- Chitosan:
-
is a linear polysaccharide composed by d-glucosamine and N-acetyl-d-glucosamine, linked via β (1–4) bonds. It is obtained by treating chitin, generally obtained from the exoskeleton of crustaceans (crabs, shrimp, etc.) with a basic aqueous solution.
- Collagen:
-
is the main protein of connective tissue in animals. It is the most abundant protein in mammals (about 25% of the total protein mass), in human represents approximately 6% of body weight.
- Dentin disk:
-
is slide of animal tusk (ivory) the most frequently used in vitro model for the osteoclast resorption assay.
- Hydroxyapatite crystals:
-
is produced and absorbed by body tissues. It is one of the main components of the bone lying in the form of calcium salts: CaCO3 (calcium carbonate), Ca3 (PO4) 2 (calcium phosphate) and CaF2 (calcium fluoride).
- Osteoblasts:
-
cells that process the bone extracellular matrix. Their function is to produce the organic matrix of bone tissue, consisting of fibers of type I collagen, proteoglycans and glycoproteins.
- Osteoclasts:
-
are very large cells, multinucleated (syncytia) and lysosomes rich. The osteoclasts are places to the bone matrix and have the function to reabsorb the bone by exocytosis enzymes and alkaline pH.
- Vitamin D:
-
a group of fat-soluble prohormones consisting of 5 different vitamins: vitamin D1, D2, D3, D4, and D5. The two most important forms of vitamin D are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). The cholecalciferol (D3) is synthesized in animals, while the ergocalciferol (D2) from vegetable origin.
Introduction
In the last few years, the bone degeneration has become an increasingly treated topic by the scientific community. To live better is important to know the function of the human body and the problems behind it. The most frequent disorders and diseases, in particular in older people, are related to the bones and joints. They play a key role in the body supporting and the movements allowing. In order to understand the mechanisms that regulate the bone degeneration processes and the main molecules involved, it is necessary firstly understand the bone tissue structure.
The skeleton that supports the human body consists of 206 bones that are held together by 68 joints. That apparatus has three main functions: supporting the body, protecting internal organs and soft tissues of the body, and allowing the muscle movement. The bones are composed of two portions: organic and inorganic. When the skeleton is affected by varying severity diseases, motor skills and balance, it may be compromised and can also occur difficulty in grasping objects. Bone is a metabolically active tissue, in which the processes of formation and resorption are continued throughout life (le Noble and le Noble 2014; Martin 2014).
The bone tissue, the most predominant connective tissue in the human body, is composed of specialized cells and an extracellular matrix comprising an organic component (35%, with the 90% of type I collagen) and an inorganic component (65%, composed of Calcium (Ca) and phosphate (P) in the form of hydroxyapatite crystals) (Buckwalter et al. 1996). There are three principal types of cells involved in bone tissue homeostasis:
-
Osteoblasts . These cells origin from mesenchymal stem cells and synthesize the cross-linked collagen and several specialized proteins in much smaller quantities (osteocalcin and osteopontin), which comprise the organic matrix of bone. Moreover, these cells produce Ca and P minerals that are deposited into the organic matrix forming a very strong and dense mineralized tissue. In addition, they are equipped with receptors for parathyroid hormone (Arnett 2003).
-
Osteoclasts . These cells belong to the monocytes /macrophages family. They are derived from bone marrow. The mononuclear precursors self-fusion originates giant multinucleate cells having several nuclei. They are rich in mitochondria and lysosomes (just to prove their catabolic activity) and have a large number of receptors for the calcitonin. Designate to active bone resorption, the osteoclast forms a “ruffled border” that opposes the surface of the bone tissue. Among their lysosomal enzymes, it is important to include their acid phosphatase. This permits osteoclasts characterization by their staining for high expression of tartrate-resistant acid phosphatase (TRAP) and cathepsin K (Holtrop and King 1977).
-
Osteocyte: the most abundant cell in the bone tissue. They are osteoblasts that, after processing the bone substance, remain imprisoned in the calcified matrix of gaps inside bone cavities shaped lenticular dug in the slats. Osteocytes remain connected between them through extensions that extend to the haversian canal that allows the nourishment. Former osteoblast in case of trauma or bone fracture can resume its synthetic activity (because freed) transforming then in osteoblast.
Regarding the organic component, the type I collagen represents about 90% of the organic matrix of bone. It is a triple helix structure made from three chains, rich in proline and hydroxyproline. The collagen is synthesized as a precursor to having a long series of carboxy and amminoterminal AA. These extensions are then cleaved during the secretion and formation process (Viguet-Carrin et al. 2006).
As regards the inorganic component, the mineral phase of calcium and phosphorus ions are under the control of three different hormones (Raisz 1988):
Parathyroid hormone (PTH) regulates the level of calcium ion in extracellular fluids in three ways: (*) increases bone resorption, (**) increases the renal tubules reabsorption, (***) increases the intestinal reabsorption and the vitamin D transformation in its active form, the calcitriol (1,25 (OH) 2D3).
Calcitriol is a powerful stimulator of calcium and phosphorus absorption in the intestine. In normal conditions, the calcitriol promotes bone growth and bone formation by providing adequate mineral levels. However, if the dietary intake is insufficient, calcitriol acts directly on the skeleton by promoting the Ca and P reabsorption.
Calcitonin inhibits the Ca reabsorption from the bones, thereby reducing the serum Ca concentration; it decreases Ca fecal excretion and exercises, similar to PTH, a phosphaturia effect.
The different phases of bone remodeling are controlled by numerous local and systemic factors. The systemic factors include the calcium metabolism (parathyroid hormone, calcitonin, vitamin D), sex hormones, and glucocorticoids (Jilka et al. 1998). These possess a modulating activity on the growth and differentiation of osteoblasts and osteoclasts. Local factors include the growth factors, cytokines, prostaglandins, and leukotrienes (de Vernejoul 1996). The role of these factors is very complex because none of these acts independently, but several of them works together.
The alteration of this balance between growth factors, hormones, cytokines, minerals, and specialized cells can lead to different types of bone disorders.
Bone Disease
Bone diseases are disorders and conditions that cause abnormal development and/or impairment in normal bone development. This can result in weakened bones, inflamed joints, and pain. In these conditions, bones naturally lose density after the age of 20 due to the aging process; however, some the bone diseases result in excessive loss of bone strength and density. Nutrient deficiencies, including lack vitamin D or C deficiency, hormonal imbalances, and cell abnormalities can also cause bone disorders in both children and adults. There are many kinds of bone problems:
-
Low bone density and osteoporosis
-
Osteogenesis imperfecta
-
Paget’s disease
-
Bone disease can make bones easy to break
-
Bones can also develop cancer and infections
-
Other bone diseases are caused by poor nutrition, genetic factors or problems with the rate of bone growth or rebuilding
Bone remodeling is exposed to environmental, mechanical injury (Chen et al. 2010). Alterations at different phases of this process result in bone disease onset (Kular et al. 2012). Older people are predisposed to bone tissue atrophy as a result of decreased levels of physical exercise and stationary lifestyle (Kaneko et al. 2014). Bone and cartilage cells are susceptible to mechanical signals and respond through mechano-transduction pathways modifying bone remodeling in proportion to the nature of the mechanical signals received. Different circumstances affecting bone and cartilage biology deregulate bone homeostasis and function. Thus, a constant solicitation of mechanical signals that origin high tension or irregular cargo distribution results in increased bone synthesis (Robling et al. 2002). In contrast, static load reduces bone synthesis and increases bone resorption (Ehrlich and Lanyon 2002). The most frequent disorder where decreased bone mass is a result of hormonal status or disuse is osteoporosis. Other common conditions where bone architecture is subverted are Paget’s disease, osteogenesis imperfecta, osteopetrosis, and osteosarcoma. Bone health can also be affected by changes in cartilage biology. Osteoarthritis (OA) is a disease involving both cartilage and bone tissue. Other conditions affecting the cartilage, but could be possibly the results of altered bone remodeling are achondroplasia, costochondritis, spinal disk herniation, relapsing polychondritis, chondromas, and chondrosarcomas. Additionally, altered remodeling process can be the result of pathological factors like distorted mechano-responsiveness of osteocyte, osteoblast, osteoclast or chondrocyte cells, deregulated mechano-transduction pathways, or altered bone matrix mechanical properties. One or a combination of these factors could induce pathological bone remodeling and consequent disease progression (Cox et al. 2011). Osteolysis is a hallmark of various and etiologically different diseases of bone and joint, which is mediated by osteoclasts and osteoblasts (Sims and Gooi 2008). It is known that osteoclast plays a role in pathological bone resorption. The osteoclasts’ activity is controlled by local factors produced in the bone microenvironment. In addition, osteoclasts are autocrine/paracrine, intracrine regulatory cells able to produce factors such as IL-6, annexin II, TGF-beta, and OIP-1/hSca, which influence their own formation and activity (Yavropoulou and Yovos 2008). A critical initiating event in osteolysis is the activation of pro-inflammatory cytokine signaling within periprosthetic macrophages, which, in turn, leads to an imbalance in the levels of the key osteoclastogenesis regulators RANKL and OPG (Zaidi 2007). Recent investigation indicates that protein groups called chitinases are involved in different processes such as tissue remodeling and injury and osteoclastogenesis (Lee et al. 2011).
Bone Turnovers Markers
In the past decade, the field of bone turnover markers achieved considerable advancement. Biochemical monitoring of bone metabolism depends upon the measurement of enzymes and proteins released during bone formation and of degradation products produced during bone resorption. Various biochemical markers are now available that allow a specific and sensitive assessment of the rate of bone formation and bone resorption (Pagani et al. 2005). Although these markers are not yet recommended for use in diagnosis of osteoporosis, they appear to be useful for the individual monitoring of osteoporotic patients treated with antiresorptive agents. The bone markers constitute a new and potentially important clinical tool for the diagnosis and monitoring of bone metabolism, being not invasive, not expensive, and giving answers much faster than the classic diagnostic tool. The bone mineral density employs 2 or 3 years to show significant changes, for example, in response to an antiresorptive therapy. In contrast, the bone markers indicate the variations of bone metabolism in minor time: i.e., from 1 to 3 months for markers of resorption, and 6–9 months for formation markers. The bone markers reflect the pathophysiological mechanisms of bone disease. They generally increase in patients with diseases in which bone turnover increases as Paget’s disease, hyperparathyroidism, and hypothyroidism and conversely decrease in patients with slower turnover, such as hypothyroidism or hypopituitarism.
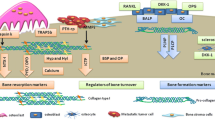
It is possible to distinguish two types of bone remodeling markers (Fig. 1).
The Bone Formation Markers
The principal bone formation markers are:
-
Alkaline phosphatase: has been clinically available for several years as a marker for bone metabolism. Various tissues, such as liver, bone, intestine, spleen, kidney, and placenta, secrete serum alkaline phosphatase. In adults with normal liver function, approximately 50% of the total alkaline phosphatase activity arises from the liver and 50% from the bone. The development of monoclonal antibodies directed to the bone-specific isoform of alkaline phosphatase has improved specificity and sensitivity.
-
Serum osteocalcin: Osteocalcin is synthesized by mature osteoblasts, odontoblasts, and hypertrophic chondrocytes. Serum osteocalcin is considered a specific marker of osteoblast function. Its levels correlate with the bone formation rate. Nevertheless, the molecule is rapidly degraded in the serum, and intact structure and fragmented segments coexist in the serum. Notwithstanding the use of these markers, the resulting heterogeneity of the osteocalcin fragments in the serum clues some limitations. In general, serum levels are elevated in patients with diseases characterized by a high bone turnover rate, and the serum levels reflect the expected changes in bone formation following surgical and therapeutic intervention. Serum osteocalcin levels fluctuate significantly during the menstrual cycle, with the highest levels observed during the luteal phase (Gundberg et al. 1985).
-
Procollagen type 1: Procollagen type 1 contains N- and C-terminal extensions, which are removed by specific proteases during conversion of procollagen to collagen. The extensions are the C- and N-terminal pro-peptides of procollagen type 1 (P1CP and P1NP). Measurement of P1NP appears to be a more sensitive marker of bone formation rate in osteoporosis. These assays are being developed for clinical use.
The Bone Resorption Markers
The principal bone resorption markers are:
-
The acid phosphatase 5, tartrate resistant (TRAP): Under normal condition, TRAP is highly expressed by osteoclasts and in activated macrophages. In osteoclasts, TRAP is localized in the ruffled border area, in the lysosomes, and in the Golgi vesicles (Ljusberg et al. 2005). Plasma levels are altered in various bone diseases. It is easily degradable in the frozen samples. This condition has limited their use in clinical analytical.
-
Collagen-derived assays: Hydroxyproline is a component of the bone collagen. During degradation of bone, it is released into the serum and is detectable in the urine in free and bound forms. Nowadays, serum hydroxyproline is considered a nonspecific marker of bone turnover. It originates from the degradation of newly synthesized collagens, from collagens of tissues other than bone, and from the diet. From a practical standpoint, a major drawback of urinary hydroxyproline is the necessity for dietary restrictions on gelatine intake before applying the test. Therefore, urinary hydroxyproline analysis has been replaced by assays that are more specific.
-
Cross-link assays: The pyridinium compounds, PYD and DPD, are formed during the extracellular maturation of fibrillar collagens and are released upon the degradation of mature collagens. The PYD and DPD production are significantly elevated in diseases with an increased bone turnover. Paget’s disease, the bone disease secondary to carcinoma, primary hyperparathyroidism, renal osteodystrophy, osteomalacia, hyperthyroidism, hypercalcemia, and osteoporosis by immobilization are related to the PYD and DPD levels. The PYD-to-DPD ratio in urine is similar to the ratio of these two cross-links in bone, which suggests that both of the cross-links are derived predominantly from bone. PYD and DPD are present in urine as free moieties (40%) or peptide bound (60%). Free forms can be detected by direct immunoassays (free DPD, Pyrilinks-D).
-
Peptide assays: Several groups have developed assays based on specific antibodies raised against isolated collagen peptides containing cross-links. These fragments detected by radioimmunoassay technique are available for C-telopeptide of type 1 collagen (CTX, CrossLaps) and cross-linked N-terminal telopeptide of type 1 collagen by ELISA technique (NTX, Osteomark). The monoclonal antibody used for NTX assay is directed against the urinary pool of collagen cross-links derived from a patient with Paget disease. Only β-isomer of CTX is measured in the serum CrossLaps assay, while α- and β-isomers of CTX are measured in the urine CrossLaps assay. These assays have shown detectable reaction with urine from healthy individuals, as well as large increases associated with elevated turnover. It has been shown that the NTX constitutes an effective and sensitive marker in early changes in the bone resorption that occur in physiological conditions, such as the onset of menopause. In osteoporotic patients in late menopause, it was frequently observed an increase level of 171% compared to the average premenopausal.
-
Noncollagenous markers: Few noncollagenous proteins or glycoproteins have sufficient specificity for bone to be considered potential markers. Bone sialoprotein (BSP) is thought to be involved in the mineralization of newly deposited bone matrix and/or the calcification of extra skeletal tissues. BSP is a highly acidic protein with strong affinity for hydroxylapatite crystals. BSP may be a sensitive marker of bone turnover, and clinical data suggest that its serum levels predominantly reflect processes related to bone resorption. The discovery that the type 5b Isotype is specific for bone osteoclasts has facilitated an antibody capture activity assay for tartrate-resistant acid phosphatase 5b as a bone resorption marker but is still under development. Another protein group that can be considered markers of resorption bone is the Chitinases (CHIT1 and CHI3L1).
Chitinase a New Potential Noncollagenous Markers of Bone Disease
Characteristics of Human Chitinase Family
Chitinases are classified into families 18 and 19 of glycosyl hydrolase (GH) superfamily based on their amino acid sequence similarities (Henrissat and Bairoch 1993). The glycoside hydrolase 18 is widely expressed in different organisms such as archaea, prokaryotes, and eukaryotes. Some of these enzymes are capable of hydrolyzing the chitin, a β-1, 4-linked N-acetyl-d-glucosamine (GlcNAc) oligosaccharide. Their structure has revealed a common ancestor gene from which they are subsequently divided (Robertus et al. 1998). Chitin is the common element among all the GH18. Chitin is a major structural component of the fungal cell walls, insect exoskeletons, and shellfish and in parasitic nematodes, but is not present in vertebrates. Belonging to the human chitinases family are eight members. Seven of the eight human GH18 family members are located on chromosome 1, including chitotriosidase (CHIT1, 1q32.1) (Renkema et al. 1995; Boot et al. 1995), acidic mammalian chitinase (AMCase, CHIA, 1p13.2) (Boot et al. 2001), chitinase 3-like protein 1 (CHI3L1, YKL-40, GP-39, 1q32.1) (Johansen et al. 1992; Rehli et al. 1997), chitinase 3-like protein 2 (CHI3L2, YKL-39, 1q13.3) (Hu et al. 1996), oviductin (OVGP1, 1p13.2) (Arias et al. 1994), di-N acetylchitobiase (CTBS, 1p22) (Fisher and Aronson 1992), and chitinase acidic pseudogene 2 (CHIAP2, 1p13.2) (Bussink et al. 2007). Located on chromosome 11 in position p15.5, there is the chitinase domain containing 1, also know stabilin-1 (CHID1, SI-CLP) (Kzhyshkowska et al. 2006) (Fig. 2). The human major histocompatibility complex paralogon is located on chromosome 1, close the human GH18 gene family. This position related to the modulation of chitinase-like chitotriosidase, CHI3L1 and CHIA in the immune responses (Welch et al. 2002) confer GH18 gene family a role in innate and adaptive immunity (Funkhouser and Aronson 2007). There are only three chitinases with the ability to hydrolyze the chitin: CHIA, CHIT1, and CTBS. The CHIA functions mainly as an exo-chitinase, while CHIT1 as an endo-chitinase. As regards CTBS, it is a lysosomal enzyme with lower sequence homology to CHIA and CHIT1. It hydrolyses the reducing end of GlcNAc from the chitooligosaccharides following the ordered degradation of asparagine-linked glycoproteins by lysosomal glycosyl-asparaginase. The rest of chitinases lack the catalytic glutamic acid residue and are collectively termed chitinase-like proteins or chitinase-like lectins (chitolectins) as they retain lectin-binding capabilities. The main representatives among chitolectins are YKL-40 and YKL-39. They bind strongly to chitin and chitooligosaccharides due to the presence of a hydrophobic substrate binding cleft (Fusetti et al. 2003). Most likely YKL-40 and YKL-39 may play a role in tissue remodeling and in cancer development. As regards human OVGP1, it is secreted by oviductal epithelium and is a much larger protein (~120 kDa) than other chitinases (Rapisarda et al. 1993). OVGP1 may play a role during the early embryonic development (Buhi 2002). CHID1 is a highly conserved GH18 family member of unknown function. It is known to interact with the protein STAB1(stabilin 1), a type 1 transmembrane endocytic receptor involved in angiogenesis, lymphocyte homing, cell adhesion, trafficking between early/sorting endosomes and the trans-Golgi network in human sinusoidal endothelial cells, and macrophages (Kzhyshkowska et al. 2004). Finally, CHIAP2 is a chitinase-like pseudo gene that does not produce a functional protein. It is a novel chitolectin member and does not align well with other human members of the GH18 family. It is located in the proximity of CHIA and OVGP1 genes, and the role in health and in disease is still unknown (Table 1).
Chromosome and network chitinases. (a) Schematic representation of Chr1 and Chr11. Seven of the eight human GH18 family members are located on chromosome 1(CTBS, CHI3L2, OVGP1, CHIAP2, CHIA, CHI3L1, and CHIT1) instead CHID1 is located in chromosome 11. (b) Differentially expressed genes are depicted: links have been predicted using STRING (http://string.embl.de/). Predicted interactions are depicted according to the type of available evidence. The interactions (see color labels) include direct (physical) and indirect (functional) associations; they are derived from four sources: genomic context, high-throughput experiments, conserved co-expression, and previous knowledge from literature (doi: 10.1371/journal.ppat.1000781.g002)
Herein we will focus on what is known in the role chitinase family in bone disease development, as well as the potential of some of the engaged molecules as prognostic or diagnostic markers and their perspective in developing new therapeutic strategies against bone disease.
Carbohydrate-Binding Domain
Carbohydrate-binding module family 18 (CBM18) (or chitin binding 1 or chitin recognition protein), which binds N-acetylglucosamine, is a common region between the chitinase with chitinolytic activity and chitinase-like-proteins (CLPs) (Henrissat and Davies 1997). This domain may be present in one or more copies and is involved in the recognition or binding of chitin subunits. The CBM of both CHIT1 and CHI3L1 could be a critical region for the interaction with bone target molecules (Fig. 3). The finding that CHIT1 and CHI3L1 are crucial in the osteoclastogenesis and in the osteolysis suggests that their CBM could be also involved in the tumor metastasis of osteolytic lesions. This observation is consistent with the evidence showing that CHI3L1 inhibition restrains tumor growth and metastasis by its own CBM (Chen et al. 2011). Increased concentrations of CHI3L1 have been detected also in serum of patients with rheumatoid arthritis (RA). It has been suggested that neutrophil-released CHI3L1 acts as an autoantigen in RA. Local release of CHI3L1 in the arthritic joint is followed by a secondary increase of CHI3L1 concentration in serum. In contrast to healthy individuals, who show strong bias in the regulatory response to CHI3L1, 50% of patients with RA exhibit polarization towards Th1 phenotype (van Bilsen et al. 2004). At the same time, CHI3L1 is able to suppress the TNFα and IL-1-induced secretion of matrix metalloproteases and IL-8 in both human skin fibroblasts and articular chondrocytes. In contrast, in RA the serum levels of CHI3L1 positively correlated with serum levels of IL-6. Increased levels of CHI3L1 in serum reflect the degree of the synovial inflammation, and joint destruction in patients with RA and CHI3L1 promotes proliferation of human synovial cells (Johansen 2006).
Chitinases and Osteolysis
As above mentioned, the chitinases are a very heterogeneous protein group. Most of the human chitinases have been associated with a wide variety of diseases, and they can be potentially used as diagnostic and prognostic markers for those diseases, but their role remains obscure. In the last decade, it has been focusing attention to the physiological and pathological role of any of the human GH18 chitinases in disease conditions. It has been shown that CHIT1 and CHI3L1 were modulated during the osteoclasts differentiation derived from human monocyte/macrophages and play a role in the bone resorption (Di Rosa et al. 2014b) (Fig. 4).
Intracellular distribution of CHIT1 and CHI3L1in mature osteoclasts. In order to obtain mature osteoclasts, the monocyte was treated with conditioned medium (RANKL and GMCSF) for 21 days and used for immunofluorescent microscopy examination. Most of the cells in immunostaining for CHIT1 and CHI3L1 show a characteristic distribution pattern, the signal being localized in the cytoplasm. Blue represents DAPI nuclear counterstain. Merge of green and red is shown in blue and pink; merge of blue and yellow is shown in red and blue, and green. It is important to note the number of nuclei present per cell. The white arrows indicate the greater levels of concentration in the cells contact proximity. Scale bars equal 10 μm
This discovery indicates that these molecules could play a crucial role in the bone degeneration processes.
The Role of CHIT1 in Osteolytic Process
In the 1995, Boot and colleagues discovered and characterized the most representative of GH18 chitinases, named chitotriosidase. This enzyme has been widely implicated in a variety of diseases involving immune dysfunction (Nair et al. 2005). Recently, CHIT1 has come under increasing scrutiny due to their excess secretion into the serum or overexpressed in tissues, which are chronically inflamed (Lee et al. 2011). The CHIT1 gene is localized on chromosome 1q31-q32 and consists of 12 exons and spans approximately 20 kb of genomic DNA (Boot et al. 1998) and is expressed in prevalence in mature macrophages, but a recent publication showed the presence in osteoclast (Fig. 5). Interestingly, the 71-bp exon 11 can be alternatively spliced. This exon is usually skipped in the splicing process, generating the predominant mRNA species encoding the 50-kDa protein stored in the granules of neutrophilic granulocyte progenitors. Exon 11 introduces a premature stop codon; the alternatively spliced mRNA encodes a 40-kDa CHIT1 that is almost identical to the 39-kDa isoform generated by proteolytic processing of the 50-kDa CHIT1 (Renkema et al. 1997). The N terminal of both isoforms is identical as disclosed by the cloned CHIT1 cDNA. CHIT1 is an enzymatically active chitinase that shows transglycosylation activity toward chitin (Aguilera et al. 2003) and is the major chitinase measured in disease states (Malaguarnera 2006). CHIT1 has been included as one of the secreted biomarkers for Gaucher’s disease (Hollak et al. 1994). The elevation of CHIT1 in these patients may reflect a particular state of activation of macrophages (Boven et al. 2004). In a healthy population, CHIT1 activity is very low and originates in the circulating polymorphonuclear cell. Conversely, during the development of acute/chronic inflammatory disorders, the enzymatic activity of CHIT1 increases significantly (Malaguarnera 2006). A conspicuous number of evidence indicates that CHIT1 possesses an active role in disease states where inflammatory responses overcome (Di Rosa et al. 2005). It has been reported that CHIT1 is strongly correlated with Gaucher’s disease symptoms and is used to monitor the efficacy of therapy (Pacheco and Uribe 2013). Cellular alteration in Gaucher’s disease produced a pro-inflammatory milieu leading to bone destruction through enhancement of monocyte differentiation to osteoclasts and the improvement of osteoclasts resorption activity (Mucci et al. 2012). Therefore, it was hypothesized that CHIT1 play a crucial role in the disruption of bone homeostatic balance in Gaucher’s disease, implying dysfunction of osteoclasts, osteoblasts, and mesenchymal cells (Campeau et al. 2009). A study showed that in periprosthetic soft tissue from patients with osteolysis the expression of alternative macrophage activation markers (CHIT1, CCL18) was increased in comparison to osteoarthritis controls (Koulouvaris et al. 2008), indicating a correlation between CHIT1 and osteolytic lesions (Koulouvaris et al. 2008). Nowadays, only little evidence shows that CHIT1 may play a role in the osteolytic process. As previously mentioned, there are two enzymatically active isoforms of CHIT1, respectively of 39 kDa and 50 kDa (Kawada et al. 2007). The C-terminal domain of 50-kDa CHIT1 mediates a strong binding of this enzyme to chitin, enabling it not only to cleave chitotriose but also hydrolyzes colloidal chitin to yield chitobiose, a feature that is not shown by the 39-kDa isoform. This isoform, formed during the posttranslational process, is expressed and stored in intracellular lysosomes, since lack of the domino C-terminal chitin binding represents the truncated part of the whole protein (Renkema et al. 1995), whereas the 50 kDa moiety is the predominantly secreted isoform. The recent investigation, determining which of these subunits were responsible for the bone resorption, was observed that the 50 kDa subunit seems to be the ruling isoform competent to digest the bone matrix by osteoclast. The involvement of the CHIT1 in the osteolytic process was confirmed by the reduced level of CHIT1 activity in osteoclasts placed on dentine disk and after the treatment with carboxymethyl chitosan molecules (Fig. 5).
CHIT1 related to osteoclast activity. (a, b) During the osteoclast differentiation, the CHIT1 expression and activity are increased. (c) During the osteoclast dentin disk digestion, the activity of CHIT1 is reduced also under the treatment with chitosan. Data are expressed as mean ± SD of at least three independent experiments. *P < 0.01,**P < 0.001, ***P < 0.0001 compared to monocytes untreated (a and b) and OCs (osteoclasts) untreated (c and d)
In addition, the silencing of CHIT1 provided a strong validation that CHIT1 activity is essential in the osteolytic process (Fig. 6). This recent evidence strongly supported an evolving concept regarding the roles of CHIT1 in osteolysis (Norberg et al. 2010). The high expression of CHIT1 observed in osteoclasts could have a detrimental role in the osteolytic processes occurring in the conditions where bone architecture is subverted. Additional studies demonstrated that CHIT1 is expressed in an osteoarthritic rat cartilage model. These findings suggested that patients with elevated serum levels of CHIT1 may have a more increased osteolytic activity and a faster progression of the disease (Di Rosa et al. 2014a). Indeed, silencing CHIT1 with siRNA resulted in a significant decrease in bone resorption activity and transfection with CHIT1 or siRNA and cotransfection with both decreased the levels of the pro-differentiative marker MMP9 (Di Rosa et al. 2014b) (Fig. 6).
Osteoclast digestion reduction by CHIT1 and CHI3L1 silencing. Resorption pits that appeared clear on the disk surface demonstrate the ability of osteoclasts (21 days of culture) to digest the bone matrix; (b) the treatment with the CHIT1 siRNA shows a reduction in the digestion of CPhoDs; (c) the treatment with the CHI3L1 siRNA shows a reduction in the digestion
Therefore, patients with elevated serum levels of CHIT1 may have a more increased osteolytic activity and a faster progression of degenerative skeletal diseases.
Chitinase-3-Like-1 as an Inflammation Marker in Osteolysis Process
CHI3L1 protein also called YLK-40, based on its three N-terminal amino acids: tyrosine (Y), lysine (K), and leucine (L), is a 40 kDa mammalian glycoprotein which is a heparin, chitin, and collagen binding member of the mammalian chitinase-like proteins by CBM (Fig. 3). Unlike CHIA and CHIT1, CHI3L1 binds chitin polymers but lacks the active site residues necessary for cleavage. CHI3L1 has been the best investigated human chitinase-like protein regarding its biological activity and association with various disorders. Biological activities of CHI3L1 embrace regulation of cell proliferation, adhesion, migration, and activation. CHI3L1 is produced by a variety of cells, including neutrophils, monocytes /macrophages, and osteoclasts (Fig. 4) (Johansen 2006). It has been shown that CHI3L1 silencing by siRNA in mature osteoclast reduces the ability of these cells to digest the matrix (dentin disk or calcium phosphate thin film disks) (Norberg et al. 2010) (Fig. 6). CHI3L1 stimulates production of inflammatory mediators (e.g., CCL2, CXCL2, MMP-9) and has been proposed as a pro-inflammatory biomarker. Induction of CHI3L1 has been reported in patients suffering from a surprisingly vast array of diseases, including a number of autoimmune disorders. In addition, elevated plasma levels of CHI3L1 have been found in rheumatoid arthritis and inflammatory-related illnesses in humans (Vaananen et al. 2014). In 2007, Pozzuoli and colleague showed that CHI3L1 was released by intervertebral disk culture. In this condition, CHI3L1 may contribute to the pathophysiology of discal degeneration and inflammation as confirmed by its relationships with COX-2 and NO in disk tissue culture (Pozzuoli et al. 2007). The expression of CHI3L1 has been reported to be significantly associated with migration of human macrophages (Kawada et al. 2012). Immune cells, including tissue macrophages (MØs) that are activated locally, have been considered as major CHI3L1 producers. CHI3L1 is involved in the modulation of the extracellular matrix affecting cell adhesion and migration during the tissue remodeling processes that take place in fibrogenesis (Johansen et al. 2006). In addition, CHI3L1 promotes the proliferation and antagonizes catabolic or degradative processes during the inflammatory response of connective tissues (Ling and Recklies 2004). The ability of CHI3L1 to regulate cell proliferation, adhesion, migration, and activation, as well as to regulate extracellular matrix assembly, correlates well with elevated level of CHI3L1 in the sites of chronic inflammation and active connective tissue turnover. CHI3L1 had been linked to tissue remodeling (Mucci et al. 2012), joint injury (Di Rosa et al. 2014a), and significantly elevated levels of CHI3L1 protein have been detected in serum and synovial fluid from OA patients (Recklies et al. 2002). It has been reported that miR-24 participates in osteogenic differentiation by targeting and regulating Tcf-1 expression in osteoblastic cells (Zhao et al. 2015). In the light of these results, Tao JIN and colleagues showed that miR-24 suppresses the expression of CHI3L1 in osteomyelitis caused by Staphylococcus aureus (Jin et al. 2015). A number of evidence showing that CHI3L1 stimulates proliferation of connective tissue cells, modulates expression levels of chemokines and metalloproteases in inflammatory fibroblasts, and enhances chemotaxis of endothelial cells (Recklies et al. 2005) strongly indicate that CHI3L1 plays crucial role in stromal cells not only in inflammatory conditions. Additionally, in vitro studies demonstrated that CHI3L1 is secreted by osteosarcoma (Johansen et al. 1993). The findings show a correlation between CHI3L1 expression and the development of primary and metastatic tumors that further support the idea that CHI3L1 plays a role in the development and progression of a variety of malignancies.
Chitinase-Like Protein and Bone Disease
To date, there is no evidence that the remaining chitinase is related to bone degeneration. It is also true that CHI3L2, CHID1, and CHIA are closely related to inflammatory processes that may be related to bone degeneration.
Chitinase Versus Chitosan for Bone Tissue Engineering
In recent years, many achievements have been obtained in organ transplantation, surgical reconstruction and the use of artificial protheses to treat the loss or failure of an organ or bone tissue. Chitosan is a new promising natural substance used in biomaterials research that can be applied in tissue engineering. This polymer can be easily combined with other biomaterials in order to obtain a greater tolerance in transplantation. It is obtained in a rapid and economic method from chitin, which forms a major component of crustacean exoskeleton. Thanks to its high tolerance, to an intrinsic antibacterial nature, biocompatibility, biodegradability, and the ability to be folded into various geometric forms such as porous structures, it is an excellent candidate for tissue engineering applications. Chito-oligomers are derivatives from chitosan and chitin. The application of chito-oligomers is highly used to antitumor activity and inhibition of angiogenesis (Xiong et al. 2009). This evidence may be related to the role played by the chitosan to chitinases. It seems reasonable to assume that the chitinases bind the chitosan and these molecules reduce the ability to improve the inflammation, which is the leading cause of the osteolytic process. It has been shown that osteoclasts treated with carboxymethyl chitosan during the dentin disk and CPhoD digestion reduce substrate digestion and CHIT1 activity (Fig. 5 C/D) (Norberg et al. 2010). This result suggests that the action undertaken by CHIT1 in the digestion process of the CPhoDs or dentin disks can be mediated by the binding between its own CBM and dentin disk or CPhoD substratum. Most likely, a similar effect could be also ascribed to CHI3L1. In 2009, Nam KS and colleagues showed that treatment of human breast cancer cells with increasing concentrations of chitosan oligosaccharides led to a concentration-dependent decrease in cell migration and reduced the amounts of secreted MMP-9 (Nam and Shon 2009). The antimetastatic property of chitosan oligosaccharides mediated by CHI3L1 binding was further confirmed by experimental animal data that reveal a decreased tumor dissemination in response to administration of chitosan nanoparticles, which may capitalize on the conserved binding to CHI3L1 (Hamilton et al. 2015). In the light of this, the use of Chitosan scaffold as materials for artificial bone and bone regeneration in tissue engineering could be a solution for reducing the degenerative processes mediated by chitinase.
Potential Application to Prognosis, Other Disease, or Conditions
Most of the human GH18 chitinases, except for those identified recently, have been linked to a variety of diseases, and they can potentially be used as diagnostic and prognostic markers for various diseases (Table 2). Nevertheless, causal relationships between any of these chitinases and corresponding diseases have not been established. Numerous studies have confirmed the correlation between CHIT1, CHI3L1, and osteolytic disease. The presence of polymorphism in CHIT1 and CHI3L1 makes it even more interesting from a clinical point of view. It has been that heterozygous for a 24-bp duplication in the CHIT1 gene could have a protective effect in human longevity (Malaguarnera et al. 2010). Furthermore, the CHI3L1 polymorphism is associated with asthma and with sarcoidosis (Kruit et al. 2007). Studies are still needed to validate the efficacy of evaluating CHIT1 and CHI3L1 as an early diagnostic test for osteolytic disease and its prognostic potential in the identification of bone degeneration.
Summary Points
-
1.
This chapter focuses on role of chitinases in bone disease.
-
2.
The bone tissue is characterized by a process called the bone remodeling (bone resorption and bone formation).
-
3.
The two main cells involved in these process are the osteoblasts (bone formation) and osteoclasts (bone resorption).
-
4.
Bone disorders show an underlying balance alteration in bone remodeling factors.
-
5.
There are many diseases related to bone resorption as rheumatoid arthritis, osteoporosis, periodontitis, and cancer metastasis. All of these diseases are related to the human chitinases expression.
-
6.
The mammalian chitinases can be considered new bone resorption markers.
-
7.
Chitosan is a natural molecule able to reduce chitinases’ activity and function.
-
8.
Chitinases regulation may be a new strategy for bone degenerative processes.
Abbreviations
- BSP:
-
Bone sialoprotein
- Ca:
-
Calcium
- CBM18:
-
Carbohydrate-binding module family 18
- CCL2:
-
Chemokine (C-C motif) ligand 2
- CHI3L1:
-
Chitinase 3 like 1
- CHI3L2:
-
Chitinase 3 like 2
- CHIA:
-
Chitinase, acidic
- CHID1:
-
Chitinase domain containing 1
- CHIT1:
-
Chitotriosidase
- CLPs:
-
Chitinase-like-proteins
- CPhoDs:
-
Calcium phosphate thin film disks
- CTX and NTX:
-
C-telopeptide of type 1 collagen and cross-linked N-terminal telopeptide of type 1 collagen
- CXCL2:
-
Chemokine (C-X-C motif) ligand 2
- DDs:
-
Dentin disks
- GH:
-
Glycosyl hydrolase
- GlcNAc:
-
β-1,4-linked N-acetyl-d-glucosamine
- GMCSF:
-
Granulocyte-macrophage colony-stimulating factor
- IL-6:
-
Interleukin 6
- MMP9:
-
Matrix metallopeptidase 9
- MØs:
-
Macrophages
- OA:
-
Osteoarthritis
- OC:
-
Osteoclast
- OVGP1:
-
Oviductin
- P:
-
Phosphate
- P1CP and P1NP:
-
C- and N-terminal pro-peptides of procollagen type 1
- PDB:
-
Protein data bank
- PTH:
-
Parathyroid hormone
- RA:
-
Rheumatoid arthritis
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- siRNA:
-
Small interfering RNA
- Th1:
-
Lymphocyte T helper 1
- TNFα:
-
Tumor necrosis factor alpha
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Aguilera B, Ghauharali-Van Der Vlugt K, Helmond MT, Out JM, Donker-Koopman WE, Groener JE, Boot RG, Renkema GH, Van Der Marel GA, Van Boom JH, Overkleeft HS, Aerts JM. Transglycosidase activity of chitotriosidase: improved enzymatic assay for the human macrophage chitinase. J Biol Chem. 2003;278:40911–6.
Arias EB, Verhage HG, Jaffe RC. Complementary deoxyribonucleic acid cloning and molecular characterization of an estrogen-dependent human oviductal glycoprotein. Biol Reprod. 1994;51:685–94.
Arnett T. Regulation of bone cell function by acid–base balance. Proc Nutr Soc. 2003;62:511–20.
Boot RG, Renkema GH, Strijland A, Van Zonneveld AJ, Aerts JM. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem. 1995;270:26252–6.
Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, De Meulemeester TM, Mannens MM, Aerts JM. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998;273:25680–5.
Boot RG, Blommaart EF, Swart E, Ghauharali-Van Der Vlugt K, Bijl N, Moe C, Place A, Aerts JM. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276:6770–8.
Boven LA, Van Meurs M, Boot RG, Mehta A, Boon L, Aerts JM, Laman JD. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am J Clin Pathol. 2004;122:359–69.
Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. II: formation, form, modeling, remodeling, and regulation of cell function. Instr Course Lect. 1996;45:387–99.
Buhi WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction. 2002;123:355–62.
Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177:959–70.
Campeau PM, Rafei M, Boivin MN, Sun Y, Grabowski GA, Galipeau J. Characterization of Gaucher disease bone marrow mesenchymal stromal cells reveals an altered inflammatory secretome. Blood. 2009;114:3181–90.
Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff’s law: mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43:108–18.
Chen CC, Llado V, Eurich K, Tran HT, Mizoguchi E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011;140:268–75.
Cox LG, Van Rietbergen B, Van Donkelaar CC, Ito K. Analysis of bone architecture sensitivity for changes in mechanical loading, cellular activity, mechanotransduction, and tissue properties. Biomech Model Mechanobiol. 2011;10:701–12.
de Vernejoul MC. Dynamics of bone remodelling: biochemical and pathophysiological basis. Eur J Clin Chem Clin Biochem. 1996;34:729–34.
Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-gamma, interleukin-10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clin Chem Lab Med. 2005;43:499–502.
Di Rosa M, Szychlinska MA, Tibullo D, Malaguarnera L, Musumeci G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur J Histochem. 2014a;58:2423.
Di Rosa M, Tibullo D, Vecchio M, Nunnari G, Saccone S, Di Raimondo F, Malaguarnera L. Determination of chitinases family during osteoclastogenesis. Bone. 2014b;61:55–63.
Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700.
Fisher KJ, Aronson Jr NN. Cloning and expression of the cDNA sequence encoding the lysosomal glycosidase di-N-acetylchitobiase. J Biol Chem. 1992;267:19607–16.
Funkhouser JD, Aronson Jr NN. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:96.
Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–60.
Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab. 1985;60:736–9.
Hamilton G, Rath B, Burghuber O. Chitinase-3-like-1/YKL-40 as marker of circulating tumor cells. Transl Lung Cancer Res. 2015;4:287–91.
Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1993;293(Pt 3):781–8.
Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol. 1997;7:637–44.
Hollak CE, Van Weely S, Van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–92.
Holtrop ME, King GJ. The ultrastructure of the osteoclast and its functional implications. Clin Orthop Relat Res. 1977;123:177–96.
Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996;271:19415–20.
Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998;101:1942–50.
Jin T, Lu Y, He QX, Wang H, Li BF, Zhu LY, Xu QY. The role of microRNA, miR-24, and its target CHI3L1 in osteomyelitis caused by Staphylococcus aureus. J Cell Biochem. 2015;116:2804–13.
Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209.
Johansen JS, Williamson MK, Rice JS, Price PA. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res. 1992;7:501–12.
Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32:949–55.
Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194–202.
Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A, Ikeda K. Integrin alphav in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447:352–7.
Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007;56:21–7.
Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–23.
Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–16.
Kruit A, Grutters JC, Ruven HJ, Van Moorsel CC, Van Den Bosch JM. A CHI3L1 gene polymorphism is associated with serum levels of YKL-40, a novel sarcoidosis marker. Respir Med. 2007;101:1563–71.
Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45:863–73.
Kzhyshkowska J, Gratchev A, Martens JH, Pervushina O, Mamidi S, Johansson S, Schledzewski K, Hansen B, He X, Tang J, Nakayama K, Goerdt S. Stabilin-1 localizes to endosomes and the trans-Golgi network in human macrophages and interacts with GGA adaptors. J Leukoc Biol. 2004;76:1151–61.
Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, Haus G, Utikal J, Schledzewski K, Scholtze J, Goerdt S. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–8.
Le Noble F, Le Noble J. Bone biology: vessels of rejuvenation. Nature. 2014;507:313–4.
Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501.
Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J. 2004;380:651–9.
Ljusberg J, Wang Y, Lang P, Norgard M, Dodds R, Hultenby K, Ek-Rylander B, Andersson G. Proteolytic excision of a repressive loop domain in tartrate-resistant acid phosphatase by cathepsin K in osteoclasts. J Biol Chem. 2005;280:28370–81.
Malaguarnera L. Chitotriosidase: the yin and yang. Cell Mol Life Sci. 2006;63:3018–29.
Malaguarnera L, Ohazuruike LN, Tsianaka C, Antic T, Di Rosa M, Malaguarnera M. Human chitotriosidase polymorphism is associated with human longevity in Mediterranean nonagenarians and centenarians. J Hum Genet. 2010;55:8–12.
Martin TJ. Bone biology and anabolic therapies for bone: current status and future prospects. J Bone Metab. 2014;21:8–20.
Mucci JM, Scian R, De Francesco PN, Garcia FS, Ceci R, Fossati CA, Delpino MV, Rozenfeld PA. Induction of osteoclastogenesis in an in vitro model of Gaucher disease is mediated by T cells via TNF-alpha. Gene. 2012;509:51–9.
Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–94.
Nam KS, Shon YH. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J Microbiol Biotechnol. 2009;19:629–33.
Norberg AL, Karlsen V, Hoell IA, Bakke I, Eijsink VG, Sorlie M. Determination of substrate binding energies in individual subsites of a family 18 chitinase. FEBS Lett. 2010;584:4581–5.
Pacheco N, Uribe A. Enzymatic analysis of biomarkers for the monitoring of Gaucher patients in Colombia. Gene. 2013;521:129–35.
Pagani F, Francucci CM, Moro L. Markers of bone turnover: biochemical and clinical perspectives. J Endocrinol Invest. 2005;28:8–13.
Pozzuoli A, Valvason C, Bernardi D, Plebani M, Fabris Monterumici D, Candiotto S, Aldegheri R, Punzi L. YKL-40 in human lumbar herniated disc and its relationships with nitric oxide and cyclooxygenase-2. Clin Exp Rheumatol. 2007;25:453–6.
Raisz LG. Hormonal regulation of bone growth and remodelling. Ciba Found Symp. 1988;136:226–38.
Rapisarda JJ, Mavrogianis PA, O’day-Bowman MB, Fazleabas AT, Verhage HG. Immunological characterization and immunocytochemical localization of an oviduct-specific glycoprotein in the human. J Clin Endocrinol Metab. 1993;76:1483–8.
Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–26.
Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 2005;280:41213–21.
Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5.
Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem. 1995;270:2198–202.
Renkema GH, Boot RG, Strijland A, Donker-Koopman WE, Van Den Berg M, Muijsers AO, Aerts JM. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur J Biochem. 1997;244:279–85.
Robertus JD, Monzingo AF, Marcotte EM, Hart PJ. Structural analysis shows five glycohydrolase families diverged from a common ancestor. J Exp Zool. 1998;282:127–32.
Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54.
Sims NA, Gooi JH. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–51.
Vaananen T, Koskinen A, Paukkeri EL, Hamalainen M, Moilanen T, Moilanen E, Vuolteenaho K. YKL-40 as a novel factor associated with inflammation and catabolic mechanisms in osteoarthritic joints. Mediators Inflamm. 2014;2014:215140.
Van Bilsen JH, Van Dongen H, Lard LR, Van Der Voort EI, Elferink DG, Bakker AM, Miltenburg AM, Huizinga TW, De Vries RR, Toes RE. Functional regulatory immune responses against human cartilage glycoprotein-39 in health vs. proinflammatory responses in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:17180–5.
Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–36.
Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem. 2002;277:42821–9.
Xiong C, Wu H, Wei P, Pan M, Tuo Y, Kusakabe I, Du Y. Potent angiogenic inhibition effects of deacetylated chitohexaose separated from chitooligosaccharides and its mechanism of action in vitro. Carbohydr Res. 2009;344:1975–83.
Yavropoulou MP, Yovos JG. Osteoclastogenesis – current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–16.
Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801.
Zhao W, Wu C, Dong Y, Ma Y, Jin Y, Ji Y. MicroRNA-24 regulates osteogenic differentiation via targeting T-Cell factor-1. Int J Mol Sci. 2015;16:11699–712.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Di Rosa, M., Malaguarnera, L. (2017). Chitinases as Biomarkers in Bone Studies. In: Patel, V., Preedy, V. (eds) Biomarkers in Bone Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7693-7_11
Download citation
DOI: https://doi.org/10.1007/978-94-007-7693-7_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7692-0
Online ISBN: 978-94-007-7693-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences