Abstract
Intracranial lipomas are uncommon benign mesenchymal tumors. They are usually found near the midline and the interhemispheric fissure is the most common location. Different malformations of the central nervous system are associated with lipomas specially the agenesis of the corpus callosum. Although asymptomatic, they can sometimes trigger neurological symptoms, specifically epileptic seizures. The radiological diagnostic clue of lipomas is a well delineated lobulated extra axial fatty mass. On computed tomography lipomas demarcate areas of marked hypodensity and on magnetic resonance they appear as hyperintense lesions on T1-weighted sequences and hypointense on T2-weighted images. The majority of lipomas are incidental findings and do not cause life threatening symptoms. Surgical management proves to be challenging due to the high vascularity and adherence to the lesion to the surrounding parenchyma and should be only pursued in cases of hydrocephalus or in sylvian fissure lipomas when epilepsy can not be controlled with medication.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Lipomas of the central nervous system (CNS) are very rare lesions. They were originally considered to be neoplasms of mesodermal origin and today lipomas are known to be a type of benign, slow-growing, congenital hamartomatous condition rather than a true neoplasm. Since the original description of these lesions in 1856 by Rokitansky, ~200 cases have been reported (Loddenkemper et al. 2006). These masses originate from a developmental disorder in mesodermal germ plaque beneath leptomeninxs and neural tissue during the early phase of pregnancy and they are frequently associated with maldevelopment of various nervous system structures.
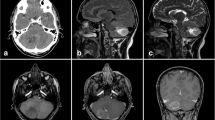
Lipomas of the CNS can be located inside the cranium (intracranial lipomas) or inside the spinal canal. According to the largest dataset of intracranial lipomas performed by Truwit and Barkowich (1990) they are usually found in the medial line in the interhemispheric fissure (40–50 %), the suprasellar region (15–20 %), the pineal region (25 %) and in other uncommon regions like the lateral cerebral fissure (5 %). Interhemispheric fissure lipomas are often placed over the corpus callosum (Figs. 23.1 and 23.2) and may extend into the lateral ventricles or choroid plexus. Intracranial lipomas in the suprasellar region usually attach to the infundibulum or hypothalamus (Fig. 23.3) and those in the pineal region usually attach to the quadrigeminal lamina. Lipomas can also be detected within the subarachnoid cisterns including the ambiens and interpeduncular cisterns, cerebellopontine angle and occasionally in the yugular foramen or foramen magnum. Spinal intradural lipomas are frequently found in the lumbosacral area as components of spinal dysraphism, whereas lipomas of the cervical and thoracic cord are quite rare (Vila Mengual et al. 2009). In particular, cervical location with intracranial extension is extremely rare (Şanh et al. 2010).
(a) Axial T1-weighted cranial magnetic resonance (MR) imaging showing the hyperintense appearance of a giant interhemispheric lipoma; (b) Axial MR T2-weighted images showing the same lesion with reduced density; (c) Coronal MR fat saturation pulse sequence of the same lipoma, agenesis of the corpus callosum can be appreciated
Epidemiology
Intracranial lipomas are usually silent and this fact explains the difficulty of a proper statistical assessment, therefore no robust data concerning the prevalence of intracranial lipomas are available. The first descriptions of intracranial lipomas were achieved mainly through incidental findings at autopsy (Jeffers et al. 2009). The expanded use of neuroimaging techniques has allowed increasing the diagnosis of lipomas. Intracranial lipoma accounts for 0.46–1 % of all intracranial tumors (Donati et al. 1992). Prevalence of intracranial lipomas detected on cranial MRI in the patient population of a hospital was 0.045 % (17/38,000) (Kemmling et al. 2008). Spinal intradural lipoma is a rare condition and occurs in approximately 1 % of all primary spinal cord tumors (Vila Mengual et al. 2009). Lipomas of the spinal cord are frequently associated with spina bifida and are more commonly located in lumbosacral region. The thoracic spinal cord is the second most common region of involvement followed by the cervicothoracic and cervical regions. Intradural lipomas of the spinal cord with intracranial extension are very rare (Şanh et al. 2010).
Pathogenesis
Truwit and Barkowich (1990) have reviewed the numerous theories regarding the pathogenesis of intracranial lipomas. The precise etiopathology of intracranial lipomas has been a topic of discussion. Theories regarding the hystogenesis of these lesions include: (1) hypertrophy from the pre-existing fatty tissue of the meninges, (2) metaplasia of meningeal connective tissue, (3) heterotopic malformation of dermal origin, (4) tumor like malformation derived from the primitive meninx and (5) fatty degeneration of proliferated glia. Today intracranial lipomas are accepted to be the result of meningeal maldifferentiation and they are genetically linked to other midline defects due to the improper closure of the neural tube. The presence of lipomas is explained by the abnormal persistence of the meninx primitive, a mesenchymal derivative of the neural crest that is usually reabsorbed in an orderly fashion during embryogenesis giving rise to the subarachnoid cisterns. When the meninx primitive does not regress into subarachnoid space and maldifferentiates into adipose tissue a lipoma originates. This theory explains the cisternal locations of lipomas and the intralesional locations of blood vessels and cranial nerves.
The malformative mechanism of lipomas is supported by its association with midline malformations of the cns in up to 40 % of cases (Gómez-Gonsálvez et al. 2003). They are mainly associated with maldevelopment of the corpus callosum in the form of agenesis/dysgenesis. Other anomalies of the cns associated with lipomas are the absence of septum pellucidum, cranium bifidum, spina bifida, encephalocele, myelomeningocele and hypoplasia of cerebellar vermis.
There are also reports of cortical abnormalities associated with cerebral lipomas such as architectural disorganization and focal penetration of fibroadipose tissue into the brain parenchyma. As the formation of a lipoma takes part of a complex malformation that involves sulcus formation and cortical development within its vicinity, it may interfere with the growing of cortical tissue during the ongoing formation of the sylvian fissure, resulting in cortical dysplasia of the vicinity (Kakita et al. 2005).
In rare occasions intracranial lipoma is associated with subcutaneous lipoma. In this case different communication patterns can be observed between intracranial and extracranial component of the lipoma. They may have no connection (Tubbs et al. 2007), may connect to each other by a fibrous lipomatous stalk (Yamashita et al. 2005) or may have direct continuity with each other through cranium bifidum (Sethi et al. 2008). A case of intraextracranial lipoma associated with sagittal sinus fenestration, absent straight sinus and falcine sinus has been described.
The development of a lipoma may also involve vascular abnormalities and a variety of vascular abnormalities have been described in association with intracranial lipoma including dilatation, tortuosity or narrowing of feedings arteries and veins, engulfment of the cerebral arteries, arteriovenous malformations and aneurysms and malformations of venous sinus (Saatci et al. 2000). Hypervascularization has been observed adjacent to and within a lipoma located in the Sylvian fissure (Kakita et al. 2005).
Two groups of interhemispheric lipomas have been described (Yildiz et al. 2006). Tubulonodular type is characterized by nodular lesions smaller than 2 cm. They are a result of a more severe insult that occurs at an early embryonic stage and interferes with the normal development of the corpus callosum (Fig. 23.1). It is located anteriorly with the epicentre in the genu in 83 % of cases and associated with a high incidence of cranial defects, frontal masses and encephaloceles. Curvilinear lipomas are thin and located posteriorly around the splenium (Fig. 23.2). This type is generally associated with a normal corpus callosum and has a low incidence of associated anomalies.
Pathology
Macroscopically lipomas vary in size from subcentimeter nodules to large masses. They have a bright yellow appearance and a soft, lobulated and fibrous tissue consistency not easily fragmentably. Microscopic examination after hematoxylin and eosin staining reveals mature adipose tissue surrounded by a fibrous capsule with varied amounts of collagen and blood vessels (Fig. 23.3). The capsule and surrounding parenchyma frequently contain calcifications (Feldman et al. 2001). An exceptional myelomatous change in a lipoma has been described by Suri et al. (2008). These authors consider that metaplastic differentiation in the lipoma gave origin to myelolipoma which contain hematopoietic elements including erytrocytes, myeloid cells, megakaryocytes and focal lymphoid aggregate formation.
Clinical Manifestations
Although the prevalence of symptomatic lipomas remains controversial, epilepsy, headache, psychomotor retardation and cranial nerve paralysis may occur (Venkatesh et al. 2003; Yilmaz et al. 2006). Most intracranial lipomas are considered to be asymptomatic and frequently they are an incidental finding in patients undergoing a cranial computerized tomography (CT) or magnetic resonance (MR) after a cranial trauma (Lin et al. 2009).
Epilepsy is the most common symptom associated with lipomas. A 5 % of cranial lipomas present with epilepsy (Gómez-Gonsálvez et al. 2003). Epileptic seizures have been noted in a large proportion of patients with sylvian lipoma presumably due to irritation of the mesiotemporal cortex or to the variety of associated neocortical abnormalities (Saatci et al. 2000; Feldman et al. 2001; Vela-Yebra et al. 2002; Yildiz et al. 2006). However the association of interhemispheric lipomas and epilepsy remains controversial (Martínez-Lapiscina et al. 2010). Some authors have suggested that the symptomatic nature of corpus callosum lipomas is dependent on the interruption of the callosal fibers, which are replaced by the neoplasm. Such disconnection is responsible for each hemisphere to develop epileptic discharges. However, few case reports reported clinical and electroencephalographic characteristics congruent with cranial MR or CT which could allow attributing the etiology of epilepsy to the lipoma. Loddenkemper et al. (2006) reviewed 3,500 epilepsy patients for the presence of intracranial lipomas; only five cases were found and epilepsy could be linked to the lipoma in only a single patient. Therefore, the authors suggested that intracranial lipomas were incidental findings in this population. Yilmaz et al. (2006) reported a prevalence of epilepsy of 20 % in an adult case series and Gómez-Gonsálvez et al. (2003) reported a prevalence of 5 % in a similar pediatric case series.
Patients with lipomas may present with symptoms that depend on its location. Lipomas located in the quadrigeminal cistern often present with diplopia secondary to brainstem compression and with signs of intracranial pressure due to hydrocephalus secondary to obstruction of the cerebrospinal fluid pathway through the sylvian aqueduct (Truwit and Barkovich 1990). Tubbs et al. (2007) reported an unusual case of a giant lipoma that due to its size compressed the foramina of Monro resulting in hydrocephalus. Cerebellopontine angle lipomas may present with vestibulocochlear dysfunction such as hearing loss, tinnitus, dizziness and vertigo or with hemifacial spasm by compressing the seventh cranial nerve (Romero-Blanco and Monteiro-Santos 2004). Fandiño et al. (2005) reported a case of calcarin fissure lipoma presenting with cuadrantanopsia.
Patients with intradural spinal lipomas present with severe neurological dysfunction as the result of spinal cord compression and produce a progressive paraparesis o tetraparesis depending on the spinal level involved by the lipoma (Şanh et al. 2010).
Radiologic Characteristics
Jabot et al. (2009) have reviewed the neuroimaging appearance of intracranial lipomas. The best diagnostic clue of intracranial lipomas is a well delineated lobulated extra axial fatty mass. On cranial CT lipomas demarcate areas of marked hypodensity, which do not show enhancement after administration of intravenous contrast. They usually have a Houmsfield unit range between −50 UH and −100 UH. Calcification is often present in interhemispheric lipomas most commonly within the fibrous capsule surrounding the lesion. The calcification may be curvilinear, extending around the periphery of the lipoma or it may be nodular within the center of the lesion. Nearly one-half of the suprasellar and interpeduncular lipomas ossify. The magnetic resonance (MR) appearance of the lipoma is that of a homogeneous hyperintense lesion on T1-weighted sequences and hypointense on T2-weighted images. On fat saturation pulse sequences lipomas are isointense to gray matter. Vascular imaging may show arterial abnormalities.
Radiologic differential diagnosis should be established with dermoid tumor and teratomas. Dermoids tumors are radiologically similar as they tend to occur adjacent to midline, but they appear round or lobulated on CT and usually have a slight mass effect and calcification foci with no contrast enhancement or surrounding edema. Dermoids and lipomas display measurably different densities on CT imaging. A dermoid will demonstrate a Houmsfield unit range from −20 to −40 HU. In addition dermoids are more heterogeneous. They have high signal intensity on T1-weighted images due to their lipid content and a heterogeneous signal on T2-weighted images due to the mixed composition of the tumor. Teratomas develop in the same location as lipomas and may have a more heterogeneous appearance with more foci of contrast enhancement. Small intracranial lipomas close to a cerebral artery are hyperintense on time-to-flight MR angiography and could be mistaken with a partially thrombosed aneurysm. A defining characteristic of lipomas on time-to-flight MR angiography results from the out-of-phase India ink artifact. This dark fringe in the periphery of the lesion is characteristic and helps to avoid potential diagnostic pitfalls (Kemmling et al. 2008). In some cases of head trauma injuries, low-density attenuation image of lipoma on brain CT has been misdiagnosed as pneumocranium (Lin et al. 2009).
Treatment and Prognosis
Surgical intervention is generally unnecessary for stable or asymptomatic intracranial lipomas because they grow very slowly, do not involve mass effect on brain tissue and malignant differentiation has never been reported. Surgery should be considered if the lipoma causes compressive effect and in cases of hydrocephalus (Spallone et al. 2004). In cases in which the lipoma causes hydrocephalus, placement of a ventricular shunt will provide adequate treatment. The majority of lipomas do not cause life threatening symptoms and epilepsy can be controlled with medication. In most occasions the risk of surgical intervention outweighs the potential benefit. Radical removal of sylvian fissure lipomas is very difficult if not impossible as well as very dangerous (Feldman et al. 2001). If surgery is necessary, partial resection was recommended by most authors because of the deep location of the lesion, its strong adherence to the sylvian cortex as well as the intricate involvement of the median cerebral artery or its branches. Attempts at radical excision increase the risk of brain injury but some authors reported that some sylvian fissure lipomas could be removed totally without complication and that symptomatic improvement may result (Chao et al. 2008).
Arresting the progression of symptoms is the principal surgical goal for intradural spinal lipomas of any location. The tendency of most neurosurgeons is to be conservative as complete surgical removal is difficult without the risk of great morbidity. Some authors perform a subtotal removal of the tumor with a decompressive laminectomy (Vila Mengual et al. 2009; Şanh et al. 2010).
References
Chao S-C, Shen C-C, Cheng W-Y (2008) Microsurgical removal of sylvian fissure lipoma with pterion keyhole approach-case report and review of the literature. Surg Neurol 70:85–90
Donati F, Vassella F, Kaiser G, Blumberg A (1992) Intracranial lipomas. Neuropediatrics 23:32–38
Fandiño J, Bermúdez J, Arán E (2005) Quadrigeminal cistern and calcarine fissure lipoma: case report and review of the literature. Neurocirugía 16:173–176
Feldman RP, Marcovici A, LaSala PA (2001) Intracranial lipoma of the sylvian fissure. Case report and review of the literature. J Neurosurg 94:515–519
Gómez-Gonsálvez FA, Menor-Serrano F, Téllez de Meneses-Lorenzo M, Aleu Pérez-Gramunt M, Sala-Sánchez AG, Rubio-Soriano A, Carbonell-Nadal J, Mulas F (2003) Intracranial lipomas in pediatrics: a retrospective study of 20 patients. Rev Neurol 37:515–521
Jabot G, Stoquart-Elsankari S, Saliou G, Toussaint P, Deramond H, Lehmann P (2009) Intracranial lipomas: clinical appearance on neuroimaging and clinical significance. J Neurol 256:851–855
Jeffers SK, Bourne TD, Lopes MBS (2009) A 58 year old woman with a corpus callosum nodule at autopsy. Brain Pathol 19:743–744
Kakita A, Inenaga C, Kameyama S, Masuda H, Ueno T, Honma J, Takahashi H, Shimohata M (2005) Cerebral lipoma and the underlying cortex of the temporal lobe: pathological features associated with the malformation. Acta Neuropathol 109:339–345
Kemmling A, Noelte I, Gerigk L, Singer S, Groden C, Scharf J (2008) A diagnostic pitfall for intracranial aneurysms in time-of-flight MR angiography: small intracranial lipomas. Am J Roentgenol 190:W62–W67
Lin Y-F, Hsi S-C, Chen Y-Q, Long W-Z (2009) Interhemispheric lipoma masquerading as pneumocranium in a patient with head injury. Am J Emerg Med 27:516.e1–516.e3
Loddenkemper T, Morris HH III, Diehl B, Lachhwani DK (2006) Intracranial lipomas and epilepsy. J Neurol 253:590–593
Martínez-Lapiscina EH, Moreno García MP, Bujanda Alegría M (2010) Epileptic seizure of lipoma of corpus callosum: cause or incidental finding. Neurologia 25:331–332
Rokitansky C (1856) Lehrbuch der Pathologischen Anatomie. Braumuller, Vienna, pp 468–478
Romero-Blanco M, Monteiro-Santos E (2004) Cerebellopontine angle lipoma: a case report. Rev Neurol 39:238–240
Saatci I, Aslan C, Renda Y, Besim A (2000) Parietal lipoma associated with cortical dysplasia and abnormal vasculature: case report and review of the literature. Am J Neuroradiol 21:1718–1721
Şanh AM, Türkoğlu E, Kahveci R, Şekerci Z (2010) Intradural lipoma of the cervicothoracic spinal cord with intracranial extension. Child Nerv Syst 26:847–852
Sethi PK, Sethi NK, Torgovnick J, Arsura E (2008) Neuroimage: giant intracranial lipoma with extracranial extension. Eur Neurol 60:49–50
Spallone A, Pitskhelauri DI (2004) Lipomas of the pineal region. Surg Neurol 62:52–59
Suri V, Sharma MC, Suri A, Karak AK, Garg A, Sarkar C, Jain D (2008) Myelolipomatous change in an interhemispheric lipoma associated with corpus callosum agenesis: case report. Neurosurgery 62:E745
Truwit CL, Barkovich AJ (1990) Pathogenesis of intracranial lipomas: an MR study in 42 patients. Am J Roentgenol 155:855–864
Tubbs RS, Louis RG Jr, Loukas M, Shoja MM, Blount JP (2007) Giant intracranial lipoma. Folia Neuropathol 45:247–249
Vela-Yebra R, Pastor-Pons E, Altuzarra-Corral A, García Del Moral Garrido R, Hervás-Natividad R, Sánchez-Álvarez JC (2002) Lipoma of the cerebral convexity and refractory focal epilepsy. Rev Neurol 34:742–745
Venkatesh SK, Phadke RV, Kumar S, Mishra UK (2003) MR appearance of interpeduncular lipoma. Singapore Med J 44:39–41
Vila Mengual M, Miranda Lloret P, López González A, Simal JA, Alvarez Garijo JA (2009) Spinal cord lipoma without dysraphism in the infancy that extends intracranially. Case report and review of the literature. Surg Neurol 71:613–615
Yamashita S, Kunishio K, Tamiya T, Nakamura T, Ogawa D, Igawa HH, Kuroda Y, Nagao S (2005) Parietal lipomeningocele. Case report. Neurol Med Chi (Tokyo) 45:112–115
Yildiz H, Hakyemez B, Koroglu M, Yesildag A, Baykal B (2006) Intracranial lipomas: importance of localization. J Neurol 48:1–7
Yilmaz N, Unal O, Kiymaz N, Yilmaz C, Etlik O (2006) Intracranial lipomas – a clinical study. Clin Neurol Neurosurg 108:363–368
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Aguirre, M.E.E., de Lapiscina, E.H.M. (2014). Lipoma: An Overview. In: Hayat, M. (eds) Tumors of the Central Nervous System, Volume 13. Tumors of the Central Nervous System, vol 13. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7602-9_23
Download citation
DOI: https://doi.org/10.1007/978-94-007-7602-9_23
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7601-2
Online ISBN: 978-94-007-7602-9
eBook Packages: MedicineMedicine (R0)