Abstract
Climate change could potentially become one of the most important influences on forest ecosystem function and diversity due to its profound effect on many biotic processes. Additionally, climate change could interact with other anthropogenically driven agents of forest alteration, such as non-native invasive species. Although their arrival is primarily facilitated by global trade and travel, climate and changes to climate have affected and will likely continue to affect rates of invasive species establishment, range expansion, and impact to native ecosystems. In this chapter, we attempt to synthesize broadly the interaction between climate change and non-native insect invasions in temperate forest ecosystems. We highlight four primary effects: changes in distributional ranges, outbreak frequency and intensity, seasonality and voltinism, and trophic interactions. A paucity of data for some processes necessitated the use of exemplar native species in native ranges, and their extrapolation to non-native species. Future studies should give greater attention to the complexity associated with these interacting forces of change in forest ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Climate change and invasions by non-native species each constitute major threats to forest ecosystems worldwide (Dale et al. 2001), yet attempts to synthesize broadly their interacting impacts are limited (Engel et al. 2011; Hellmann et al. 2008; Walther et al. 2009). The inherent complexity of each and the potential confounding and compounding effects when considered jointly have hampered analysis of actual and potential effects on forest ecosystems. Another consideration is that the paramount stage of biological invasions, the arrival stage, is predominately a result not of climate change but of increasing global trade through which new species ‘hitchhike’ on products, nursery stock, packing materials, and in ship hulls and ballast water (Hulme et al. 2008; Lockwood et al. 2007). Consumer demand for many foreign manufactured goods is also not generally dependent upon or influenced by climate, at least not in the short-term. In fact, one could argue that due to changing climates, there could be a greater emphasis on reducing carbon footprints associated with foreign imports with a consequent increase in the desire to purchase locally available products that in turn could conceivably reduce the volume of imports and species that are introduced with these imports.
In the absence of an environmental revolution and major shifts in economic patterns and in governmental policy, however, the arrival of new species will likely continue to increase as long as global trade increases. Once a new species arrives to a novel habitat, the other stages of the invasion process, establishment, spread, and impact, could be directly and indirectly influenced positively or negatively by climate and changes in climate. In this chapter, we first briefly describe the causes and dynamics of biological invasions and climate change independently. We then focus on how these forces interact to affect forest ecosystems by mediating range shifts, altering population outbreak frequency and intensity, changing seasonality and voltinism, and decoupling interactions between and among trophic levels. In some cases, we rely on examples from native species in native ranges, and then extrapolate these observations to non-native species in non-native habitats. We lastly consider the economic ramifications of the interaction between biological invasions and climate change.
2 Dynamics of Biological Invasions
Biological invasions consist of four distinct stages: arrival, establishment, spread, and impacts (Lockwood et al. 2007). The arrival stage is defined as the movement of a species from an area in which it is established to a novel habitat. Although the interchange of biota among biogeographic regions is integral to the history of life (Crosby 1986; di Castri 1989), arrival rates of new species have increased dramatically in recent decades due to increases in global trade and travel (Aukema et al. 2010). Establishment after arrival is a critical transition, and it is believed that most arriving species fail to establish (Simberloff and Gibbons 2004). One of the most important factors influencing the establishment of newly arrived species is propagule pressure: the number of individuals in the arriving founder population, the number of independent introductions, or a combination of both (Lockwood et al. 2007). For example, low-density populations tend to be subject to environmental and demographic stochasticity, and Allee effects (positive density-dependence, Stephens et al. 1999). Allee effects can arise when individuals in sparse populations encounter difficulties finding suitable mates, satiating natural enemies, and overcoming host defense mechanisms, all of which can exacerbate the challenges that small founder populations already face during the establishment phase (Liebhold and Tobin 2008; Taylor and Hastings 2005; Tobin et al. 2011). Other factors influencing establishment success include the level of genetic diversity in founder populations, availability of resources, degree of habitat disturbance, and presence or absence of competitors, mutualists, and natural enemies (Lockwood et al. 2007). Another factor that is important to consider in the context of this chapter is climate suitability (Beaumont et al. 2009; Hayes and Barry 2008; Thuiller et al. 2005), and thus shifting climatic patterns could have critical consequences for the establishment success of invaders.
Following successful establishment, a species often begins to expand its geographic range, often through a process known as stratified dispersal in which local growth and diffusive spread is coupled with long-distance population ‘jumps’ (Hengeveld 1989). These population jumps are often facilitated through anthropogenic (e.g., Gilbert et al. 2004), hydrological (e.g., Davidson et al. 2005), and atmospheric transport mechanisms (e.g., Isard et al. 2005), and can greatly accelerate the rates of spread of invading species (Shigesada and Kawasaki 1997). Long-range dispersal can also serially initialize new invasions, which are effectively subject to the same constraints that affect the establishment success of any newly-arrived species in a non-native environment (Liebhold and Tobin 2008; Taylor et al. 2004).
The fourth stage of the invasion process is the ecological and economic impacts of non-native species (Lockwood et al. 2007). Impacts due to invasive species can vary dramatically among species, and within a species depending upon the region being invaded. A recent analysis on non-native insects in forests within the continental United States has suggested that only a minority of introduced and established insects (≈14 %) since 1860 have caused major damage (Aukema et al. 2010), yet this minority of species can still account for several billion USD in costs (Aukema et al. 2011; Holmes et al. 2009; Pimentel et al. 2005). The costs to the United States of the non-native emerald ash borer, Agrilus planipennis Fairmaire, which are primarily associated with the treatment, removal, and replacement of ash, Fraxinus spp., is predicted to be 10.7 billion USD alone (Kovacs et al. 2010). It is also likely that the impacts and consequent costs due to non-native species will be affected by climate and changes in those regimes.
3 Climate Change
Recent increases in the concentrations of atmospheric greenhouse gases, most notably carbon dioxide, methane, and nitrous oxide, have led to a corresponding change in local and global climates. Global mean surface temperatures have increased by 0.3-0.6 °C over the last century (Mann et al. 1998), and global temperatures are projected to continue to increase by the end of the next century (Intergovernmental Panel on Climate Change 2007). The projected increase in global surface temperatures is thought to range from 1 °C under a low (B1) greenhouse gas emission scenario, which assumes substantial mitigation and reductions in greenhouse gas emissions, to 6 °C under a high (A1fi) greenhouse gas emission scenario, which assumes temperatures under the current conditions and without any mitigating strategies (Hayhoe et al. 2007; Kunkel et al. 2008).
The increase in global surface temperatures can also result in a number of cascading effects. For example, as temperatures warm, there could be changes in hydrological cycles leading to increases or decreases in precipitation patterns, such that some places could be more prone to drought while others more prone to flooding. Changes in climatic variability, including increases in storm event frequency and intensity, could also be the result of recent warming trends (Rosenzweig et al. 2001). More subtle changes, such as the diminishment of winter snow pack, could also have important ecological ramifications for both insects and trees. Consequently, climate change has the potential to destabilize many ecosystem functions, and cause major changes to the dynamics of individual species and to those communities in which they reside.
Insect species are particularly sensitive to climate change because many of their physiological processes are temperature-dependent. Insects could respond to changing climates by going extinct, moving into areas with a more tolerable weather regime, or adapting in situ. Climate change can affect insect species differentially depending upon the latitude at which they live; for example, temperate insects that evolved under strong seasonality could inherently have greater phenotypic plasticity for withstanding the pressures of climate change relative to tropical species already near their thermal tolerances, and thus could be more capable of adapting to warming trends (Deutsch et al. 2008). Such changes could include accelerated developmental rates with a consequent change in the timing of year-to-year phenological events, and/or in the number of generations per year. Species abundance can be directly affected by temperature, resulting in population density extremes, from extinction to outbreaks. The distributional ranges of insects can also be affected by the removal of biogeographic boundaries formed by climatic factors.
4 Ecological Interactions Between Forest Insect Invasions and Climate Change
We now turn our attention to the effects, both documented and those projected to occur, of climate change on insect invasions in forest ecosystems. Although non-native species across a diversity of taxa have been introduced to novel areas (Pimentel 2002; Simberloff and Rejmánek 2011), forest insects, such as xylophagous species, represent a particularly important group of invaders because of their propensity for importation in solid wood packaging material, wood dunnage, and wood pallets (Bartell and Nair 2003; Brockerhoff et al. 2006a; Liebhold et al. 1995; Wingfield et al. 2010), all of which are important components in global trade. Forest insects can also be introduced through imports of infested timber and forest products, as well as on infested live plants (Brasier 2008; Liebhold et al. 2012; Reichard and White 2001). In recent decades, a number of economically damaging, non-native forest insect species have been or were likely introduced through global trade routes, such as A. planipennis (Poland and McCullough 2006) and Anoplophora glabripennis (Motschulsky) (Smith et al. 2009) in North America, Dendroctonus valens LeConte in China (Yan et al. 2005), and Sirex noctilio F. in Australasia, South Africa, South America and most recently, North America (Slippers et al. 2012a).
4.1 Changes in Geographic Ranges
There exists a rich literature around bioclimatic envelope modeling documenting how the distribution and abundance of forest insects are restricted to (or released from) suitable habitat(s) by various climatic factors in time and space (e.g., Hlasny and Turcani 2009; Jonsson et al. 2011; Logan et al. 2003; Robinet et al. 2007; Williams and Liebhold 1995a). Changes in climate compared to historic norms have relaxed these boundaries, allowing for rapid range expansion in some species, and range retraction in others. In this section, we will discuss three types of changes in the geographic ranges of forest insects: expansions in latitudinal range, expansions in geographic range, and range retractions. We restrict our treatment to natural dispersal, even though human-assisted transport may be a confounding factor in many range expansions (Brockerhoff et al. 2006b) that could be detected post hoc within the genetic structure of subpopulations (Kerdelhué et al. 2009).
In montane ecosystems, range expansions frequently become first apparent in altitudinal changes rather than latitudinal changes. Simulation studies have shown that the mountain pine beetle, Dendroctonus ponderosae Hopkins, which is native to North America, responds to increases in temperature first by increasing its altitudinal range before becoming more apparent at more northern locations in western Canada (Sambaraju et al. 2012). In the Greater Yellowstone Ecosystem of the western United States, D. ponderosae has had a long association with lodgepole pine forests with infrequent outbreaks at climatically-inhospitable high elevations. Recently, however, it has exhibited behavior outside of its observed range of natural variability, and threatens to decimate high-elevation five needle pines (Logan et al. 2010; Logan and Powell 2001), a host species that does not appear to have a co-evolved relationship with this insect. These pronounced shifts in elevation are not restricted to bark beetles. In recent years, for example, the winter moth, Operophtera brumata (L.), has defoliated stands of mountain birch at elevations up to the tree line in coastal areas of northern Norway (Hagen et al. 2007). Historically, O. brumata outbreaks have occurred at lower elevations due to bioclimatic and/or competitive effects with a sympatric species, the autumn moth Epirrita autumnata (Borkhausen) (Ammunet et al. 2010). The gypsy moth, Lymantria dispar (L.), also a defoliator, is expected to continue a progression into regions of higher elevations in Europe as it doubles its range compared to 50 years ago (Hlasny and Turcani 2009).
Latitudinal range shifts are perhaps more dramatic than altitudinal shifts, as humans may notice defoliated or killed trees more quickly in regions where such activity was historically unapparent. There exist a number of examples of lepidopteran defoliators in Europe where this phenomenon has occurred over the past decade. While O. brumata has expanded into higher elevations and moved northeast across Norway, the more cold-hardy E. autumnata has exhibited a concomitant increase into colder, continental areas (Jepsen et al. 2008). Recent phenological changes in budburst have facilitated a rapid northward range expansion of another geometrid, the scarce umber moth, Agriopis aurantiaria Hübner, in the same region (Jepsen et al. 2011). In France and Portugal, higher elevation and northward latitudinal incursions have been documented for the pine processionary moth, Thaumetopoea pityocampa (Denis and Schiffermüller), a frequent defoliator of pine and cedar (Arnaldo et al. 2011; Battisti et al. 2005). In North America, a warming climate could facilitate the expansion of L. dispar into areas where current overwintering temperatures are too cold to permit survival (Régnière et al. 2009). However, a constraint in the southern geographic range of L. dispar is the lack of sufficient cooling periods to terminate diapause (Gray 2004); thus, although warming temperatures could allow for northern expansion of L. dispar, there could a concomitant restriction in its potential and realized southern range.
Latitudinal range expansion has not been limited to Coleoptera and Lepidoptera. For example, damage from the poplar woolly adelgid, Phloemyzus passerinii (Signoret), was recently recorded for the first time in northern France (Rouault et al. 2006). The European pine sawfly, Neodiprion sertifer (Geoffroy), is currently limited in its range by the degree of freeze tolerance of its eggs, is also expected to move northwards over the next decades (Veteli et al. 2005). Lethal mortality to overwintering life stages is frequently cited as a key delimiter for range margins (Bale and Hayward 2010), which, once ameliorated, could permit rapid expansion in a number of species.
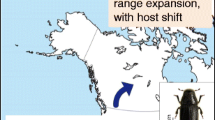
A notable example of climatic release permitting range expansion has been the recent outbreak of D. ponderosae in western Canada (Aukema et al. 2006). Mountain pine beetle has been historically limited in range by a −40 °C thermal isocline, restricting its Canadian range to a line primarily west of the Rocky Mountains (Safranyik et al. 1975). Recently, this insect breached the historic geoclimatic divide and is now reproducing in lodgepole pine forests of northwestern Alberta (de la Giroday et al. 2011, 2012; Robertson et al. 2009). This is of grave concern because range expansions of this magnitude could permit access to new hosts and new habitat corridors. For example, D. ponderosae is now reproducing in an area where lodgepole pine hybridizes with jack pine (Cerezke 1995; Cullingham et al. 2011), which could permit further range expansion eastward through the boreal forest of North America. This insect seems well adapted to jack pine, and exhibits elevated reproductive rates in evolutionarily naïve hosts (Cudmore et al. 2010).
Of course, climatic suitability across the extent of a new range is never assured, and different models could have contradicting outcomes. Projections of habitat suitability for D. ponderosae across the boreal forest of Canada through global simulation models show a wide range of projections, from high to low climatic suitability, depending upon the scenario applied (Safranyik et al. 2010). Projections of climatic suitability for the same insect in the Rocky Mountain region of the western United States, however, are more uniform, predicting decreases in suitable habitat by up to 50 % by the year 2050. In contrast, projections for the same regions for a different insect, the western pine beetle, Dendroctonus brevicomis LeConte, indicate increases or decreases in suitable habitat, depending on the scenario (Evangelista et al. 2011). Potential range retractions are not restricted to bark beetles. Lymantria dispar and the nun moth, Lymantria monacha (L.), are projected to lose up to 900 km from their southern European ranges as temperatures warm (Vanhanen et al. 2007). Many projections of climate warming demonstrate either rapidly altered ranges of the host trees (Refehldt et al. 2006) or maladaptive seasonal phenology that disrupts insect development and host procurement (Williams and Liebhold 1995a).
Climatically-mediated range shifts could also lead to changes in physiological and morphological traits of insects. Indeed, latitudinal clines in physiological, reproductive, and morphological traits of insects are well known (Blanckenhorn and Demont 2004). For example, wing size tends to be larger at higher latitudes in Drosophila subobscura Collin (Gilchrist et al. 2001) and genetic change in this species appears to be tracking climate change (Balanya et al. 2006). Furthermore, invasive populations of this species formed a latitudinal gradient in wing size very similar to that in native populations in only 20 years, which suggests that some invasive species could evolve rapidly in response to elevated temperatures. A number of widespread native forest Lepidoptera exhibit similar adult size across their latitudinal range but express reductions in fecundity and (or) larger offspring with increasing latitude (Ayres and Scriber 1994; Harvey 1983; Parry et al. 2001). A latitudinal shift in a number of fitness parameters is evident in introduced populations of the fall webworm, Hyphantrea cunea (Drury), in Japan (Gomi 2007; see Sect. 4.3). Because such latitudinal clines appear to be common in many species, selection could be expected to form clines in invasive species as their distributional range expands.
4.2 Changes in Outbreak Frequency and Intensity
The study of forest insect outbreaks has a long and storied history, and many of the species that have been the focal point of these studies have broadly served as model systems for studying the conceptual and mechanistic processes of insect population dynamics (e.g., Barbosa and Schultz 1987). Several forest insect species undergo regular cycles in population density, from innocuous to outbreak and back. Apart from being inherently fascinating, forest insect outbreaks often cause considerable economic and ecological damage by affecting nutrient cycles, animal and plant populations (Frost and Hunter 2004; Payette et al. 2000; Work and McCullough 2000), and human uses of forests for timber and recreation (Coyle et al. 2005). Another aspect of many forest insect outbreaks is that they can often be spatially synchronized (Haynes et al. 2009; Johnson et al. 2005; Peltonen et al. 2002), in which the congruence in the temporal variation of abundance among geographically distinct populations results in outbreaks over a large spatial scale (Bjørnstad et al. 1999). Geographically widespread outbreaks can be particularly important for a number of reasons. First, spatially synchronous outbreaks could dilute regulating effects of natural enemies that could otherwise provide local control (Royama 1984). Second, they can reduce the ecological landscape’s ability for buffering because most areas within an ecosystem experience simultaneous disturbance (Lovett et al. 2002). Lastly, they can overwhelm the budgetary and logistical efforts available for protection of economic assets or ecological functions through suppression programs (Tobin et al. 2012).
Many non-native species are innocuous in their native ranges where they evolved in concert with host tree defensives and the regulatory effects of natural enemies, but can be problematic when introduced into naïve environment and/or to naïve host species (Liebhold et al. 1995; Hu et al. 2009). Such a contrast is apparent when comparing the insect borers A. planipennis and Agrilus anxius Gory. The latter species is native to North America where it is relatively innocuous to North American birch species unless coupled with severe stress such as drought. In contrast, A. anxius causes significant mortality in non-native birch species planted in North America, whether trees were stressed or not (Nielsen 1989). Similarly, A. planipennis rarely causes mortality in ash in its native range in Asia, while all North American ash species appear to be extremely susceptible (Poland and McCullough 2006). Non-native species often lack natural enemies in a new area, and consequently, the ‘enemy release’ hypothesis has been suggested to play a role in the ability of species to invade novel habitats and reach outbreaking densities (Keane and Crawley 2002; Torchin et al. 2003).
The effect of climate change could result in changes to both the outbreak intensity and the periodicity of forest insect outbreaks (Logan et al. 2003; Volney and Fleming 2000). Trends in climate warming are thought to have had direct effect on the development, intensity, and geographic extent of outbreaks of D. ponderosae Hopkins, in North America (Kurz et al. 2008). Although this species is native to western North America, it is now achieving outbreak densities in northern British Columbian forests where it had never previously been found, at least not over the last few centuries of direct observation (see Sect. 4.1). Similarly, outbreaks of other eruptive bark beetle species such as the southern pine beetle, Dendroctonus frontalis Zimmermann, the Mexican pine beetle, Dendroctonus mexicanus (Hopkins), and the European spruce bark beetle, Ips typographus (L.), are expected to exhibit outbreaks of increased magnitude as temperature and precipitation regimes change (Kausrud et al. 2012; Waring et al. 2009).
In Fennoscandia, climate warming is thought to have shifted the geographic distribution of outbreaks of O. brumata (Hagen et al. 2007). Although this species is native to Fennoscandia, it, like D. ponderosae in North America, is believed to have crossed altitudinal barriers that were previously impassable due to climate that was historically unfavorable to its winter survival. In addition to the expanse of areas experiencing outbreaks, the duration of outbreaks is thought to have increased due to climate warming (Jepsen et al. 2008). Examples of the interplay between climate change and outbreaks by non-native species are less documented, likely due to the fact that invasions by non-native forest insects are a more recent phenomenon (Aukema et al. 2010), relative to the time scale that native species have existed in their native ranges. Indeed, many important invasive species are still in the active range expansion phase of their colonization, making it impossible to partition climatic influences separately from other processes driving spread. However, the change in outbreak dynamics in native species is likely not unique and will likely result in related changes in the outbreak dynamics of non-native species.
Not all forest insects necessarily benefit from recent trends in climate change, adding complexity to our efforts to understand expected patterns. For example, regular outbreaks have been recorded for the larch budmoth, Zeiraphera diniana Guénée, in the European Alps (Bjørnstad et al. 2002). A recent dendrological reconstruction of these outbreaks has suggested that this cyclical behavior had been occurring for at least 1,173 years and during previous climatic events, such as periods of warming during the Middle Ages and cooling during the Little Ice Age (Esper et al. 2007). However, since 1981, Z. diniana outbreaks have been conspicuously absent with the supposition that recent trends in climatic warming have upset the balance of a system that previously had exhibited remarkable stability (Esper et al. 2007). However, because the absence of Z. diniana outbreaks is thought to be due to climate-mediated disruption of the stability of this system, non-native species, which are agents of disturbance in themselves, may or may not be less prone to collapse in forest ecosystems that are also experiencing disturbance due to changing climates.
4.3 Changes in Seasonality and Voltinism
Increasing temperatures will have direct consequences for insects including alterations to life cycle duration (developmental rate) and changes in voltinism (the number of generations per year). While most insects are capable of increased growth rates at elevated temperatures, a key factor is during which part of a particular insect’s life history that the temperature change occurs. Thus, generalizations concerning the response of insect growth rate and development to global climate change must be tempered with knowledge that a species may behave idiosyncratically with respect to temperature.
At the level of individual insect species, a major determinant of the response to climatic shifts is the type of life-cycle and the developmental strategy employed. Danks (2006) suggested that insect development could be viewed as either an active default, where it proceeds until some reliable environmental cue signals it to stop, or a passive default, where development stops at a preset point irrespective of current environmental conditions and does not resume again until an appropriate cue is received. A good example of this dichotomy would be a multivoltine species that produces additional generations as long as diapause-inducing cues are absent, while an univoltine species would develop faster in its single generation, but would still be constrained by an obligate diapause to one generation annually. Insects using an active developmental default will likely receive greater benefits from warming temperatures than those with passive default development systems. Because many multivoltine insect species use photoperiodic cues to initiate diapause, which do not change in response to changing climates (Tauber and Tauber 1976), sufficient increases in temperature prior to the onset of diapause-inducing photoperiods could be a key determinant to the number of generations possible per year under future climate scenarios (Chen et al. 2011; Tobin et al. 2008).
Many geographically widespread insects exhibit latitudinal gradients in voltinism (Wolda 1988); thus, shifting temperatures should slide the boundaries between voltinism states in predictable directions. Voltinism may be relatively plastic, and in some species governed by photoperiod, temperature, and host plants, whereas in others it is fixed (Tauber and Tauber 1976). For those species with flexible voltinism, warming may be advantageous, permitting faster growth and additional generations annually (Bale et al. 2002; Tobin et al. 2008). In Europe, extensive data sets encompassing hundreds or even thousands of species of Lepidoptera have allowed comparisons among different time periods. For butterflies and moths with the capacity for multivoltinism, there have been significant increases in the frequency of species exhibiting bi- or multivoltine life cycles, with much of this increase occurring in the last two decades (Altermatt 2010; Pöyry et al. 2011).
Increased voltinism could promote faster population growth because more offspring are being produced per seasonal time period, thus increasing the likelihood of outbreaks of pest species or elevating non-pests or minor pests to a more economically important stature (Steinbauer et al. 2004; van Asch and Visser 2007). In forested ecosystems, changes in the voltinism of Lepidoptera and of Coleoptera (particularly scolytid bark beetles), are of concern as these groups contain some of the most economically damaging forest pests. A number of native bark beetle species in both Europe and North America have shifted lifecycles by adding annual generations, in an apparent response to moderating temperatures at higher latitudes or altitudes (Berg et al. 2006; Jonsson et al. 2009; Werner et al. 2006). With respect to the spruce beetle, Dendroctonus rufipennis (Kirby), this change was associated with devastating outbreaks in Alaska’s Kenai Peninsula (Sherriff et al. 2011).
Despite the apparent high frequency of shifts in voltinism in native insects in many temperate zones globally, documentation of such shifts in invasive forest insects has thus far been rare. It is not known if the apparent rarity in forest insects is an artifact or represents real patterns. There are several possibilities for this absence. First, some invasive species could have a fixed voltinism, such as L. dispar, which is exclusively univoltine. Another possibility is that simply too little is known about the biology of many invasive species. Additionally, invasive species could lack sufficient genetic diversity or plasticity to respond to climate shifts, owing to small founder population size and the relatively short interval of observation. Successful species could have also been introduced with, and indeed may owe their success to, the expression of an appropriate voltinism for a given region.
One well-known case of shifting voltinism concerns H. cunea, a relatively benign defoliating lepidopteran accidentally introduced from North America to Europe and Asia where it has become a major pest. In Japan, the founding population was bivoltine, but within 50 years of introduction and coupled with a southward spread, trivoltine populations became the norm in warmer areas (Gomi and Takeda 1996). The shift to a trivoltine life-cycle was associated with a subtle but biologically significant change in sensitivity to photoperiod (Gomi 2007).
Multivoltinism appears to be rare in eruptive folivores, as most appear to be constrained to the nutritionally superior, but inherently risky (see Sect. 4.4) early season foliage of woody plants (Hunter 1991, 1995). For invasive folivorous species with obligate diapause, such as many univoltine spring-feeders, increasing temperatures could provide respite from natural enemies because development will accelerate through the vulnerable larval period, a function of escape from the trap of slow-growth and high mortality (Benrey and Denno 1997; Zalucki et al. 2002). This, however, makes the assumption that natural enemies will not respond similarly to elevated temperatures, or that they will not quickly adapt to a seasonal shift in prey abundance. Some, but not all, studies have suggested that L. dispar outbreaks are correlated with warmer spring temperatures in the year of, and the year prior to, defoliation (Elkinton and Liebhold 1990), although the mechanism underlying the pattern is not known. Communities of forest Lepidoptera irrespective of taxonomic affinity, especially those that are spring-feeders, exhibit concordant population dynamics, suggesting commonality of either positive or negative responses to a significant environmental driver like meteorological conditions (Raimondo et al. 2004; Stange et al. 2011).
A major concern from an invasive species perspective could be the response of wood borers. Unlike folivorous insects, many wood and cambium feeders have considerable plasticity with respect to voltinism and are constrained mainly by the combination of wood as a nutritionally poor resource and the relatively low temperatures in temperate and boreal forests. Increased voltinism observed in variety of native bark beetles (e.g., Faccoli 2009) could be a harbinger of what to expect in this particular guild. Voltinism in A. glabripennis is a function of latitude in China with southern populations requiring only a single year to complete development (Hu et al. 2009), while populations introduced into North American are variable, with both semivoltine or univoltine emergence recorded. Another invasive cerambycid, the brown spruce long-horned beetle, Tetropium fuscum (Fabricius), currently has a univoltine lifecycle but has been recorded as bivoltine in parts of its native range. In China, D. valens has already devastated vast tracts of Chinese red pine. In its native range in the southern United States, this species has up to three generations per year, but only one and perhaps a partial second generation has been recorded in China (Sun et al. 2004), suggesting that this insect could become an even greater threat in its introduced range under warming temperatures. These examples highlight the flexible voltinism apparent in many wood feeding insects and thus a high propensity to benefit from climatic warming.
4.4 Decoupling Species Interactions
One of the first and probably best-documented effects of anthropogenic driven climate change has been a phenological shift in the seasonal occurrence of a diverse array of organisms. Phenology, the seasonally influenced timing of developmental processes (e.g., Visser et al. 2010), is strongly correlated with temperature regime for many organisms including plants, insects, and vertebrates (Parmesan 2006; Root et al. 2003). In temperate regions, a large number of species have shifted seasonal biological activities such as onset of bud break, flowering time, emergence, or migrating earlier or maintaining activity later in the season as a response to recent changes to the onset of spring and the increasing length of the growing seasons, respectively. For example, the spring phenology of European Lepidoptera has advanced significantly over the past four decades (Altermatt 2010; Roy and Sparks 2000; Stefanescu et al. 2003) as it has or will for other insects (Hassall et al. 2007; Logan et al. 2003; Masters et al. 1998); these changes are apparently correlated with an increase in degree-day availability early in the season (Parmesan 2006).
Although changes in the phenology of individual species are well-described (Menzel et al. 2006; Robinet and Roques 2010), less attention has been paid to climatically driven mismatches to the trophic relationships of interacting species, despite predictions about the important negative consequences of asynchrony and its resultant decoupling (Donnelly et al. 2011; Singer and Parmesan 2010). Climatically driven decoupling is expected when synchrony between species is disrupted in time or space (Stenseth and Mysterud 2002). Decoupling can be viewed from either a temporal or spatial perspective. Spatially, rapid range expansion by a species could decouple relationships between predator and prey (Menendez et al. 2008; see Sect. 4.1), whereas temporally, differential response to shifting temperatures could lead to a phenological decoupling of a species relationship, be it plant-herbivore, predator-prey, or tritrophic interactions.
Whether or not a system will become phenologically decoupled depends on the response of the participant species to climatic drivers. For example, no net change could occur if the interacting species respond similarly to the same environmental cues or to different environmental cues in a way that is highly correlated. However, decoupling might be expected where species are responding to specific cues that become less correlated as temperatures and/or seasonality change. For example, a photoperiodic response by one species could lead to a divergent phenology if an interacting species responds primarily to degree-day accumulations. In tri-trophic relationships, elucidating the effects of climatic shift will be difficult and the relative changes in responses by an herbivorous insect, its host plant, and its natural enemies could be neutral, negative, or positive depending on the degree of decoupling and the nature of the decoupled mechanism(s).
The potential for climatically driven phenological decoupling of herbivorous insects and their host plants has long been recognized (Buse and Good 1996; Dewar and Watt 1992; Harrington et al. 1999), but has been investigated extensively in only a few systems. The importance of phenological synchrony of insect herbivores with host plants varies between and among species, functional feeding guild, and the seasonal activity period of a species with effects likely to be neutral or negative, as a positive effect seems implausible. The same mechanisms that drive asynchrony and decoupling, however, could allow insects to utilize hosts that were previously outside of their phenological range as climatic change differentially alters seasonal timing of tree and herbivore (e.g., Jepsen et al. 2011).
Sensitivity to phenological change is likely to be greatest for spring feeding species (Forkner et al. 2008), but could also affect other seasonal guilds depending on the nature and magnitude of change. Increased voltinism (see Sect. 4.3) may push some phenologically insensitive species into more vulnerable early or late season envelopes. Species whose activity (i.e., egg hatch, larval emergence) is timed to bud burst of host trees may be susceptible to even relatively small alterations to synchrony. These species often have a narrow window of opportunity to maximize growth because they are constrained by starvation if they emerge too early, and by declining nutritional value and increasing secondary phytochemical concentration of maturing leaves should development be delayed until later in spring (Ayres and MacLean 1987; Feeny 1970; Hunter 1993; Jones and Despland 2006; Martel and Kause 2002; Parry et al. 1998). While the effects of phenological asynchrony are best known for Lepidoptera, negative consequences have been shown in many other insect herbivores including Homoptera, Diptera, Coleoptera, and Hymenoptera (Dixon 1976; Fox et al. 1997; Martel et al. 2001; Yukawa and Akimoto 2006).
We know of no study that has specifically addressed phenological decoupling of an insect-plant interaction in the context of biological invasions, but it seems unlikely that introduced species would differ substantially from native species. Although direct research is lacking, extrapolation is possible from a few well-studied native insect-plant interactions. One exemplar insect, O. brumata, could be particularly instructive in elucidating consequences of climate change on phenological decoupling as it is well-studied in its native range, and is currently invading North America.
The winter moth is a univoltine spring feeder native to Great Britain and Europe, but was accidentally introduced to Nova Scotia (1940s), British Columbia (1960s), and more recently Massachusetts (2000s) and other New England states (Elkinton et al. 2010; Roland and Embree 1995). Flightless females ascend host trees such as oaks in the fall, and oviposit on branches and twigs in the canopy. Eggs hatch in the following spring in close proximity to bud break. The fitness consequences of synchrony with bud break are significant (van Asch and Visser 2007) as the newly hatched larvae have a limited ability to survive starvation if emergence is early but suffer from the declining value of maturing foliage if emergence is late.
Winter moth has been extensively studied, especially in Great Britain (e.g., Varley et al. 1973) and the existence of several long term data sets allows insight to the effects of climatic change on O. brumata with pendunculate oak. In the Netherlands, the onset of winter moth egg hatch and bud break of this oak species have advanced considerably over a quarter century (Visser and Holleman 2001). Egg hatch, however, has advanced more than bud break, decreasing synchrony by 2–14 days depending on the year. While late hatch decreases fitness through reductions in fecundity, a shift of 5 days too early can result in mortality of 90 % or more, suggesting that an increasingly premature hatch relative to bud break is non-trivial. Winter moth has sufficient genetic variability that selection should act to push hatch time closer to bud break (van Asch et al. 2010), although this does not appear to have happened naturally thus far. Although the effects of climatic change on O. brumata phenology and synchronicity with host plants have not been studied in North America where it is invasive, its extensive use of multiple tree genera in the northeastern United States could buffer it from any deleterious consequences of climatic change.
Considerably less is known about the effects of climate change on phenology in other invasive forest insects, even for those that have been extensively studied. For example, while various models (Régnière et al. 2009; Williams and Liebhold 1995b; see Sect. 4.1) have suggested that the geographic range of L. dispar in North America will expand northward under various warming projections, the potential for asynchrony with host tree species has not been explored. The responses of trees to warming at higher latitudes may differ from the temperature response of L. dispar egg hatch, thus increasing the risk of phenological mismatch. Although L. dispar is sensitive to tree phenology (Hunter 1993; Hunter and Elkinton 2000; Stoyenoff et al. 1994), it has life-history attributes that could mitigate many of the most deleterious effects of asynchrony. Similar to O. brumata, L. dispar larvae feed on a wide-variety of woody plants, which ensures that at least spatially, some hosts will be available to neonates. Furthermore, the temporal distribution of egg hatch, both within a single egg mass and among egg masses in a population, spans extended periods (Gray et al. 2007; Hunter 1993), which also increases the likelihood that the highly mobile larvae will encounter phenologically suitable hosts.
The phenological relationship between insects and plants do not occur in a vacuum; rather, it is a template upon which other environmental factors also enhance or attenuate the effects of asynchrony. Temperature and climatic shifts also occur in concert with rising levels of CO2, which could increase or decrease quality of plant tissue for herbivores depending on species and functional feeding guild (Cornellisen 2011; Stiling and Cornelissen 2007). Other environmental feedbacks and covariates associated with climate change could further confound any analysis. Based on limited studies to date, it seems unlikely that phenological asynchrony will be of significant long-term consequence for many native insect herbivores. Even less confidence can be attached to predictions about non-native species. As many forest insect invasions are initiated from genetically-limited founder populations from only portions of their native range, it is unclear if responses to climatic shifts will differ from that seen in populations of native species. However, many successful invasive species are habitat or host generalists and may express considerable phenotypic plasticity while other species, despite apparently limited genetic diversity, have nonetheless rapidly adapted to climatic variability in recipient regions (Gomi et al. 2009).
The potential for climatic disruption or alteration of coupled species relationships also applies to higher trophic levels, which often exert considerable top-down regulation on herbivore populations. The relative synchrony between natural enemies and their prey could be maintained under climatic change if the organisms respond similarly to the same variable or to variables that remain highly correlated. The decoupling of such relationships could occur as divergent responses to the same or to correlated variables, although few predator-prey interactions and even fewer multi-trophic systems have been examined in detail. A recent study documented considerable species turnover in samples of subarctic parasitoid communities when compared to historical data sets from the same localities, with patterns suggesting a link to climate warming (Fernández-Triana et al. 2011). In a different study, a meta-analytical approach suggested that an amplification of climatic variability was negatively correlated with parasitism of tree-feeding Lepidoptera, particularly for specialist hymenopteran parasitoids, which were disproportionately affected relative to tachinid parasitoids with broader host ranges (Stireman et al. 2005). Thus, at least in the short-term, the influence of parasitoids on the population dynamics of their prey could be reduced, which has important ramifications for outbreak species whether native or introduced. However, selection has favored herbivore life-history strategies that maximize temporal enemy-free or enemy-reduced space, and differential responses to climate variables could also force greater overlap between some herbivores and their natural enemies (Hance et al. 2007).
There is evidence of decoupled predator-prey relationships due to climatic shifts in a number of insectivorous birds in Europe. The fitness of these birds is greatest when the timing of reproduction corresponds with a peak in biomass of primarily lepidopteran caterpillars (Both et al. 2006; Visser et al. 1998, 2006). Warmer springs have shifted this peak earlier, and higher temperatures have compressed the period of abundance as larvae complete their development more rapidly. Although reproductive activity of birds has also advanced, it has not done so at the same rate as caterpillar biomass peak, and this relationship appears to becoming increasingly asynchronous. The implications for invasive forest insects are unclear, but since birds are important predators of many herbivorous insects, especially in low-density insect populations (Holmes et al. 1979; Marquis and Whelan 1994; Parry et al. 1997), a diminution of their capacities would likely benefit both native and non-native insects alike. However, there remains much uncertainty in the degree and importance of divergence of bird and insect prey phenology due to climate change; for example, a long time series data set in England suggests that the great tit, an important predator of O. brumata larvae, has been able to track the shift in spring phenology of its prey item over time (Charmantier et al. 2008).
The success of many invasive insects in forests owes at least in part to an incomplete or missing natural enemy component, and thus the potential effects of climate change on this trophic level could be largely moot for some species. However, for invasive species held in check by classical biological control introductions, climatic shifts potentially could alter these important interactions, leading to a resurgence of previously suppressed populations or hamper efforts to develop new biological control programs. Indeed, it has long been recognized that classic biological control could be vulnerable to climate change (Cannon 1998) because the interactions between introduced enemies and their non-native prey could be inherently more susceptible to decoupling than those interactions involving native species. Many introductions of control agents are initiated with relatively low genetic diversity, which potentially limits the adaptive response to changing climate. Second, specific biotypes of natural enemies, particularly parasitoids, are often selected to match current climatic conditions in a given region (Robertson et al. 2009); these may or may not be suitable for future climatic envelopes. Conversely, some biological control organisms that are currently climatically limited in parts of an invader’s range may become more effective under warming scenarios (Siegert et al. 2009; Zalucki and van Klinken 2006).
Many of the predictions concerning the decoupling of insect herbivore-host or predator-prey interactions are overly general or simplistic because we lack the necessary knowledge to make these predictions in all but a few systems. The effects of climate change on decoupling interactions involving non-native species are even more difficult to generalize as the relationships are often novel and are occurring in environments different from those where the species evolved. For example, in North America, the non-native tachinid Cyzenis albicans (Fallén) is regarded as the most effective regulator of invasive O. brumata populations, but this parasitoid is a trivial source of mortality in native populations (Roland and Embree 1995; Varley et al. 1973). Thus, it may be difficult to generalize the effects of climate from donor to recipient regions, even for well-studied systems.
5 Economic Ramifications of Invasions in the Face of Climate Change
Despite the challenges associated with predicting the ecological consequences of climate-mediated effects on biological invasions, it is arguably even more difficult to quantify the economic costs due to all of these interacting forces. After all, reliable estimates of the economic costs due to specifically non-native forest insects alone are largely lacking (Aukema et al. 2011). Even though these costs are challenging to estimate, they are not always difficult to envision. For example, increases in the availability of suitable habitat due to changing climates facilitating invasions into new areas could in turn increase the costs associated with its management (Cannon 1998; Kiritani 2006). Similarly, increases in abundance, and outbreak intensity and frequency due to climate warming is likely to lead to increased management costs (Hellmann et al. 2008; Rosenzweig et al. 2001; Waring et al. 2009). Costs could also include the increase in the energy footprint of food and fiber production systems due to this increased need for pest control measures (Gandhi and Herms 2010; Pimentel 2002).
Other potential consequences, however, can be complex and involve a cascading array of effects across one or more trophic levels. One such effect of climate change, and specifically the role of increased concentrations of carbon dioxide and ozone in the atmosphere, is the potential change in host plant nutritional quality. For example, plants grown under high levels of carbon dioxide can cause changes in the carbon-to-nitrogen ratio of plant tissues (Hamilton et al. 2005); consequently, herbivores feeding on such plants could eat more leaf matter to compensate for the reduced nutritional quality of their host plants (Coviella and Trumble 1999; Dermody et al. 2008; but see Kopper and Lindroth 2003). Increases in herbivory due to changes in concentrations of atmospheric gases, coupled with increases in herbivore abundance, insect developmental rates, and voltinism owing to increases in surface temperatures (Bale et al. 2002; Chen et al. 2011; Tobin et al. 2008; Yamamura and Kiritani 1998), could have dramatic implications to pest management practices and the costs required to achieve pest control. Furthermore, a need to increase pest control tactics, specifically the use of chemical insecticides, could also intensify the inimical effects to non-target species (Pimentel et al. 1980) as well as select for resistance in the target species (Roush and McKenzie 1987).
Because of the potential for climate change to decouple interactions between natural enemies and their prey (Simon et al. 2002; Stireman et al. 2005), the use of biological control as a management tactic against non-native forest pests could be rendered less effective. In particular, classical biological control has received renewed interest in combating non-native insect pests (Hajek et al. 2007; Hajek and Tobin 2010; Hoddle 2004), and increased scrutiny is given to the specificity of introduced agents to avoid the historical blunders from the import of generalist natural enemies (Elkinton et al. 2006; Simberloff and Stiling 1996; Strong and Pemberton 2000). Because of the need for specificity in selecting a natural enemy for introduction, changes in climate – even if subtle – could influence aspects of these interspecific interactions, and the suitable range of one species could be affected by climate differently than the other. For example, the parasitic nematode Deladenus siricidicola is an effective biological control agent of the wood wasp S. noctilio in Argentina (Corley et al. 2007) and Australia (Neumann and Minko 1981). As a nematode, it is likely more sensitive to changes in moisture conditions, which are predicted to be affected by changes in climate (Rosenzweig et al. 2001), than its insect host. Indeed, the observed geographic variation in the effectiveness of D. siricidicola as a biological control agent of S. noctilio could be due to variation in climate among regions (Slippers et al. 2012b). Although additional and specific forest insect examples are still rare, climate and projections in climate will likely need to be considered when evaluating the short- and long-term efficacy of an introduced natural enemy (Zalucki and van Klinken 2006).
6 Conclusions
In addition to many of the “known unknowns” described above, a final consideration in the context of climate change and its effect on forest insect invasions is the proverbial “unknown unknowns”. The dynamics of forest insects and their interactions with associated pathogens and natural enemies, together with interactions with host species, can be difficult to predict when species are introduced to a new area. Indeed, although many biological and ecological aspects are often highlighted as important when considering the invasion potential of a species and in formulating risk assessments (Liebhold and Tobin 2008; Lockwood et al. 2007; Worner and Gevrey 2006), developing a general paradigm of species invasiveness with broad application has proved challenging (Hulme 2003; Lonsdale 1999; Rejmánek and Richardson 1996). Coupling the uncertainty of biological invasions with the complexity of climate change and its variable effect on individual species and to those communities in which they interact complicates this challenge even further. Innocuous species today could quite possibly become quite invasive under future climatic conditions, whether in their native range, an introduced habitat, or both. Greater attention should be given to this complexity through examinations of landscape-level climatic changes and its combined effect on ecosystem inhabitants.
References
Altermatt F (2010) Climatic warming increases voltinism in European butterflies and moths. Proc R Soc Biol Sci Ser B 277:1281–1287
Ammunet T, Heisswolf A, Klemola N, Klemola T (2010) Expansion of the winter moth outbreak range: no restrictive effects of competition with the resident autumnal moth. Ecol Entomol 35:45–52
Arnaldo PS, Oliveira I, Santos J, Leite S (2011) Climate change and forest plagues: the case of the pine processionary moth in Northeastern Portugal. For Syst 20:508–515
Aukema BH, Carroll AL, Zhu J, Raffa KF, Sickley TA, Taylor SW (2006) Landscape level analysis of mountain pine beetle in British Columbia, Canada: spatiotemporal development and spatial synchrony within the present outbreak. Ecography 29:427–441
Aukema JE, McCullough DG, Von Holle B, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental US. Bioscience 60:886–897
Aukema JE, Leung B, Kovacs K, Chivers C, Britton KO, Englin J, Franke SJ, Haight RG, Holmes TP, Liebhold AM, McCullough DG, Von Holle B (2011) Economic impacts of non-native forest insects in the continental United States. PLoS One 6:e24587
Ayres MP, MacLean SF (1987) Development of birch leaves and the growth energetics of Epirrita autumnata (Geometridae). Ecology 68:558–568
Ayres MP, Scriber JM (1994) Local adaptation to regional climates in Papilio canadensis (Lepidoptera: Papilionidae). Ecol Monogr 64:465–482
Balanya J, Oller JM, Huey RB, Gilchrist GW, Serra L (2006) Global genetic tracks global climate warming in Drosophila subobscura. Science 313:1773–1775
Bale JS, Hayward SAL (2010) Insect overwintering in a changing climate. J Exp Biol 213:980–994
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol 8:1–16
Barbosa P, Schultz JC (eds) (1987) Insect outbreaks. Academic Press, San Diego
Bartell SM, Nair SK (2003) Establishment risks for invasive species. Risk Anal 24:833–845
Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, Roques A, Larsson S (2005) Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol Appl 15:2084–2096
Beaumont LJ, Gallagher RV, Thuiller W, Downey P, Leishman MR, Hughes L (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15:409–420
Benrey B, Denno RF (1997) The slow-growth-high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78:987–999
Berg EE, Henry JD, Fastie CL, DeVoider AD, Matsuoka SM (2006) Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: relationship to summer temperatures and regional differences in disturbance. For Ecol Manag 227:219–232
Bjørnstad O, Ims RA, Lambin X (1999) Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol Evol 11:427–431
Bjørnstad ON, Peltonen M, Liebhold AM, Baltensweiler W (2002) Waves of larch budmoth outbreaks in the European alps. Science 298:1020–1023
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in long distance migratory bird. Nature 441:81–83
Brasier CM (2008) The biosecurity threat to the UK and global environment from international trade in plants. Plant Path 57:792–808
Brockerhoff EG, Bain J, Kimberley M, Knížek M (2006a) Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Can J For Res 36:289–298
Brockerhoff EG, Liebhold AM, Jactel H (2006b) The ecology of forest insect invasions and advances in their management. Can J For Res 36:263–268
Buse A, Good JEG (1996) Synchronization of larval emergence in winter moth (Operophtera brumata L.) and budburst in pedunculate oak (Quercus robur L.) under simulated climate change. Ecol Entomol 21:335–343
Cannon RJC (1998) The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Glob Chang Biol 4:785–796
Cerezke HF (1995) Egg gallery, brood production, and adult characteristics of mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera, Scolytidae), in three pine hosts. Can Entomol 127:955–965
Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC (2008) Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320:800–803
Chen S, Fleischer SJ, Tobin PC, Saunders MC (2011) Projecting insect voltinism under high and low greenhouse gas emission conditions. Environ Entomol 40:505–515
Corley JC, Villacide JM, Bruzzone OA (2007) Spatial dynamics of a Sirex noctilio woodwasp population within a pine plantation in Patagonia, Argentina. Entomol Exp Appl 125:231–236
Cornellisen T (2011) Climate change and its effects on terrestrial insects and herbivory patterns. Neotrop Entomol 40:155–163
Coviella CE, Trumble JT (1999) Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Conserv Biol 13:700–712
Coyle DR, Nebeker TE, Hart ER, Mattson WJ (2005) Biology and management of insect pests in North American intensively managed hardwood forest systems. Annu Rev Entomol 50:1–29
Crosby AW (1986) Ecological imperialism: the biological expansion of Europe, 900–1900. Cambridge University Press, Cambridge, UK
Cudmore TJ, Björklund N, Carroll AL, Lindgren BS (2010) Climate change and range expansion of an aggressive bark beetle: evidence of higher beetle reproduction in naïve host tree populations. J Appl Ecol 47:1036–1043
Cullingham CI, Cooke JEK, Dang S, Davis CS, Cooke BJ, Coltman DW (2011) Mountain pine beetle host-range expansion threatens the boreal forest. Mol Ecol 20:2157–2171
Dale VH, Joyce LA, Mcnulty S, Neilson RP, Ayres MP, Flannigan MD, Hanson PJ, Irland LC, Lugo AE, Peterson CJ, Simberloff D, Swanson FJ, Stocks BJ, Wotton BM (2001) Climate change and forest disturbances. Bioscience 51:723–734
Danks HV (2006) Insect adapatations to cold and changing environments. Can Entomol 138:1–23
Davidson J, Wickland AC, Patterson HA, Falk KR, Rizzo DM (2005) Transmission of Phytophthora ramorum in mixed-evergreen forest in California. Phytopathology 95:587–596
de la Giroday H-MC, Carroll AL, Lindgren BS, Aukema BH (2011) Incoming! Association of landscape features with dispersing mountain pine beetle populations during a range expansion event in western Canada. Landsc Ecol 26:1097–1110
de la Giroday H-MC, Carroll AL, Aukema BH (2012) Breach of the northern Rocky Mountain geoclimatic barrier: initiation of range expansion by the mountain pine beetle. J Biogeogr 39:1112–1123
Dermody O, O’Neill B, Zangerl A, Berenbaum M, DeLucia EH (2008) Effects of elevated CO2 and O3 on leaf damage and insect abundance in a soybean agroecosystem. Arthropod Plant Interact 2:125–135
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci U S A 105:6668–6672
Dewar RC, Watt AD (1992) Predicted changes in the synchrony of larval emergence and budburst under climatic warming. Oecologia 89:557–559
di Castri F (1989) History of biological invasions with special emphasis on the old world. In: Drake JA, Mooney HA, di Castri F et al (eds) Biological invasions: a global perspective. Wiley, New York, pp 1–30
Dixon AFG (1976) Timing of egg hatch and viability of the Sycamore aphid, Drepanosiphum platanoides (Schr.), at bud burst of Sycamore, Acer platanus L. J Anim Ecol 45:593–603
Donnelly A, Caffarra A, O’Neill BF (2011) A review of climate-driven mismatches between interdependent phenophases in terrestrial and aquatic ecosystems. Int J Biometeorol 55:805–817
Elkinton JS, Liebhold AM (1990) Population dynamics of gypsy moth in North America. Annu Rev Entomol 35:571–596
Elkinton JS, Parry D, Boettner GH (2006) Implicating an introduced generalist parasitoid in the invasive browntail moth’s enigmatic demise. Ecology 87:2664–2672
Elkinton JS, Boettner GH, Sremac M, Gwiazdowski R, Hunkins RR, Callahan J, Scheufele SB, Donahue CP, Porter AH, Khrimian A, Whited BM, Campbell NK (2010) Survey for winter moth (Lepidoptera: Geometridae) in northeastern North America with pheromone-baited traps and hybridization with the native Bruce spanworm (Lepidoptera: Geometridae). Ann Entomol Soc Am 103:135–145
Engel K, Tollrian R, Jeschke JM (2011) Integrating biological invasions, climate change and phenotypic plasticity. Comm Integr Biol 4:247–250
Esper J, Büntgen U, Frank DC, Nievergelt D, Liebhold A (2007) 1200 years of regular outbreaks in alpine insects. Proc R Soc Biol Sci Ser B 274:671–679
Evangelista PH, Kumar S, Stohlgren TJ, Young NE (2011) Assessing forest vulnerability and the potential distribution of pine beetles under current and future climate scenarios in the Interior West of the US. For Ecol Manag 262:307–316
Faccoli M (2009) Effect of weather on Ips typographus (Coleoptera Curculionidae) phenology, voltinism, and associated spruce mortality in the southeastern Alps. Environ Entomol 38:307–316
Feeny PP (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Fernández-Triana J, Smith MA, Boudreault C, Goulet H, Hebert PDN, Smith AC, Roughley R (2011) A poorly known high-latitude parasitoid wasp community: unexpected diversity and dramatic changes through time. PLoS One 6:e23719
Forkner RE, Marquis RJ, Lill JT, Corff JL (2008) Timing is everything? Phenological synchrony and population variability in leaf-chewing herbivores of Quercus. Ecol Entomol 33:276–285
Fox CW, Waddell KJ, Groeters FR, Mousseau TA (1997) Variation in budbreak phenology affects the distribution of a leaf-mining beetle (Brachys tessellatus) on turkey oak (Quercus laevis). Ecoscience 4:480–489
Frost CJ, Hunter MD (2004) Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 85:3335–3347
Gandhi JKJ, Herms DA (2010) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12:389–405
Gilbert M, Grégoire J-C, Freise JF, Heitland W (2004) Long-distance dispersal and human population density allow the prediction of invasive patterns in the horse chestnut leafminer Cameraria ohridella. J Anim Ecol 73:459–468
Gilchrist GW, Huey RB, Balanya J, Pascual M, Serra L (2001) A time series of evolution in action: a latitudinal cline in wing size in South American Drosophila subobscura. Evolution 58:768–780
Gomi T (2007) Seasonal adaptations of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) following its invasion of Japan. Ecol Res 22:855–861
Gomi T, Takeda M (1996) Changes in life-history traits in the Fall Webworm within half a century of introduction to Japan. Funct Ecol 10:384–389
Gomi T, Adachi K, Shimizu A, Tanimoto K, Kawabata E, Takeda M (2009) Northerly shift in voltinism watershed in Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) along the Japan Sea coast: evidence of global warming? Appl Entomol Zool 44:357–362
Gray DR (2004) The gypsy moth life stage model: landscape-wide estimates of gypsy moth establishment using a multi-generational phenology model. Ecol Model 176:155–171
Gray DR, Tanner JA, Logan JA, Munson AS (2007) Using sterile gypsy moth eggs as a survey and experimental tool in the field: a comparison of hatching patterns. Ann Entomol Soc Am 100:439–443
Hagen SB, Jepsen JU, Ims RA, Yoccoz NG (2007) Shifting altitudinal distribution of outbreak zones of winter moth Operophtera brumata in sub-arctic birch forest: a response to recent climate warming? Ecography 30:299–307
Hajek AE, Tobin PC (2010) Micro-managing arthropod invasions: eradication and control of invasive arthropods with microbes. Biol Invasions 12:2895–2912
Hajek AE, McManus ML, Delalibera I Jr (2007) A review of introductions of pathogens and nematodes for classical biological control of insects and mites. Biol Control 41:1–13
Hamilton JG, Dermody O, Aldea M, Zangerl AR, Rogers A, Berenbaum MR, DeLucia EH (2005) Anthropogenic changes in tropospheric composition increase susceptibility of soybean to insect herbivory. Environ Entomol 34:479–485
Hance T, Van Baaren J, Vernon P, Boivin G (2007) Impact of extreme temperatures on parasitoids in a climate change perspective. Annu Rev Entomol 52:107–126
Harrington R, Woiwood I, Sparks T (1999) Climate change and trophic interactions. Trends Ecol Evol 14:146–150
Harvey GT (1983) A geographic cline in egg weights in Choristoneura fumiferana (Lepidoptera: Tortricidae) and its significance to population dynamics. Can Entomol 115:1103–1108
Hassall C, Thompson DJ, French GC, Harvey IF (2007) Historical changes in the phenology of British Odonata are related to climate. Glob Chang Biol 13:933–941
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Hayhoe K, Wake CP, Huntington TG, Luo L, Schwartz M, Sheffield J, Wood E, Anderson B, Bradbury J, DeGaetano A, Troy T, Wolfe D (2007) Past and future changes in climate and hydrological indicators in the U.S. Northeast. Clim Dynam 28:381–407
Haynes KJ, Liebhold AM, Fearer TM, Wang G, Norman GW, Johnson DM (2009) Spatial synchrony propagates through a forest food web via consumer–resource interactions. Ecology 90:2974–2983
Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22:534–543
Hengeveld R (1989) Dynamics of biological invasions. Chapman and Hall, London
Hlasny T, Turcani M (2009) Insect pests as climate change driven disturbances in forest ecosystems. In: Strelcová K, Matyas C, Kleidon A et al (eds) Bioclimatology and natural hazards. Springer, Dordrecht, pp 165–177
Hoddle MS (2004) Restoring balance: using exotic species to control invasive exotic species. Conserv Biol 18:38–49
Holmes RT, Schultz JC, Nothnagle P (1979) Bird predation on forest insects: an exclosure experiment. Science 206:462–463
Holmes TP, Aukema JE, Von Holle B, Liebhold A, Sills E (2009) Economic impacts of invasive species in forests past, present, and future. Ann NY Acad Sci 1162:18–38
Hu J, Angeli S, Schuetz S, Luo Y, Hajek AE (2009) Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agr For Entomol 11:359–375
Hulme PE (2003) Biological invasions: winning the science battles but losing the conservation war? Oryx 37:178–193
Hulme PE, Bacher S, Kenis M, Klotz S, Kühn I, Minchin D, Nentwig W, Olenin S, Panov V, Pergl J, Pyšek P, Roques A, Sol D, Solarz W, Vilà M (2008) Grasping at the routes of biological invasions: a framework for integrating pathways into policy. J Appl Ecol 45:403–414
Hunter AF (1991) Traits that distinguish outbreaking and non-outbreaking Macrolepidoptera feeding on northern hardwood trees. Oikos 60:275–282
Hunter AF (1993) Gypsy moth population sizes and the window of opportunity in the spring. Oikos 68:531–538
Hunter AF (1995) Ecology, life-history, and phylogeny of outbreak and non-outbreak species. In: Cappuccino N, Price PW (eds) Population dynamics: new approaches and synthesis. Academic Press, New York, pp 41–64
Hunter AF, Elkinton JS (2000) Effects of synchrony with host plant on populations of a spring-feeding Lepidopteran. Ecology 81:1248–1261
Intergovernmental Panel on Climate Change (2007) The physical science basis. Working group I. Contribution to the fourth assessment report of the IPCC. Cambridge University Press, Cambridge, UK
Isard SA, Gage SH, Comtois P, Russo JM (2005) Principles of the atmospheric pathway for invasive species applied to soybean rust. Bioscience 55:851–861
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77:257–264
Jepsen JU, Kapari L, Hagen SB, Schott T, Vindstad OPL, Nilssen AC, Ims RA (2011) Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Glob Chang Biol 17:2071–2083
Johnson DM, Liebhold AM, Bjørnstad ON, McManus ML (2005) Circumpolar variation in periodicity and synchrony among gypsy moth populations. J Anim Ecol 74:882–892
Jones BC, Despland E (2006) Effects of synchronization with host plant phenology occur early in the larval development of a spring folivore. Can J Zool 84:628–633
Jonsson AM, Appelberg G, Harding S, Barring L (2009) Spatio-temporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Glob Chang Biol 15:486–499
Jonsson AM, Harding S, Krokene P, Lange H, Lindelow A, Okland B, Ravn HP, Schroeder LM (2011) Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Clim Chang 109:695–718
Kausrud K, Økland B, Skarpaas O, Grégoire J-C, Erbilgin N, Stenseth NC (2012) Population dynamics in changing environments: the case of an eruptive forest pest species. Biol Rev 87:34–51
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kerdelhué C, Zane L, Simonato M, Salvato P, Rousselet J, Roques A, Battisti A (2009) Quaternary history and contemporary patterns in a currently expanding species. BMC Evol Biol 9:220
Kiritani K (2006) Predicting impacts of global warming on population dynamics and distribution of arthropods in Japan. Popul Ecol 48:5–12
Kopper BJ, Lindroth RL (2003) Effects of elevated carbon dioxide and ozone on the phytochemistry of aspen and performance of an herbivore. Oecologia 134:95–103
Kovacs KF, Haight RF, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578
Kunkel KE, Huang H-C, Liang X-Z, Lin J-T, Wuebbles D, Tao Z, Williams A, Caughey M, Zhu J, Hayhoe K (2008) Sensitivity of future ozone concentrations in the northeast USA to regional climate change. Mitig Adapt Strateg Glob Chang 13:5–6
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408
Liebhold AM, MacDonald WL, Bergdahl D, Mastro V (1995) Invasion by exotic forest pests: a threat to forest ecosystems. For Sci Monogr 30:1–49
Liebhold AM, Brockerhoff EG, Garrett LJ, Parke JL, Britton KO (2012) Live plant imports: the major pathway for forest insect and pathogen invasions of the United States. Front Ecol Environ 10:135–143
Lockwood JL, Hoopes M, Marchetti M (2007) Invasion ecology. Blackwell Publishing Ltd., Malden
Logan JA, Powell JA (2001) Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). Am Entomol 47:160–173
Logan JA, Régnière J, Powell JA (2003) Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ 1:130–137
Logan JA, Macfarlane WW, Willcox L (2010) Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the Greater Yellowstone Ecosystem. Ecol Appl 20:895–902
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1536
Lovett GM, Christenson LM, Groffman PM, Jones CG, Hart JE, Mitchell MJ (2002) Insect defoliation and nitrogen cycling in forests. Bioscience 52:335–341
Mann ME, Bradley RS, Hughes MK (1998) Global-scale temperature patterns and climate forcing over the past six centuries. Nature 392:779–787
Marquis RJ, Whelan CJ (1994) Insectivorous birds increase growth of white oak through consumption of leaf-chewing insects. Ecology 75:2007–2014
Martel J, Kause A (2002) The phenological window of opportunity for early-season birch sawflies. Ecol Entomol 27:302–307
Martel J, Hanhimaki S, Kause A, Haukioja E (2001) Diversity of birch sawfly responses to seasonally atypical diets. Entomol Exp Appl 100:301–309
Masters GJ, Brown VK, Clarke IP, Whittaker JB, Hollier JA (1998) Direct and indirect effects of climate change on insect herbivores: Auchenorrhyncha (Homoptera). Ecol Entomol 23:45–52
Menendez R, Gonzalez-Meglias A, Lewis OT, Shaw MR, Thomas CD (2008) Escape from natural enemies during climate-driven range expansion: a case study. Ecol Entomol 33:413–421
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavská OG, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Måge F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remišová V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski F-E, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976
Neumann FG, Minko G (1981) The Sirex woodwasp in Australian radiata pine plantations. Aust For 44:46–63
Nielsen DG (1989) Exploiting natural resistance as a management tactic for landscape plants. Fla Entomol 72:413–418
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parry D, Spence JR, Volney WJA (1997) The response of natural enemies to experimentally increased populations of forest tent caterpillar. Ecol Entomol 22:97–108
Parry D, Spence JR, Volney WJA (1998) Bud break phenology and natural enemies mediate survival of early instar forest tent caterpillar (Lepidoptera: Lasiocampidae). Environ Entomol 27:1368–1374
Parry D, Goyer RA, Lenhard GJ (2001) Macrogeographic clines in fecundity, reproductive allocation, and offspring size of the forest tent caterpillar Malacosoma disstria. Ecol Entomol 26:281–291
Payette S, Bhiry N, Delwaide A, Simard M (2000) Origin of the lichen woodland at its southern range limit in eastern Canada: the catastrophic impact of insect defoliators and fire on the spruce-moss forest. Can J For Res 30:288–305
Peltonen M, Liebhold AM, Bjørnstad ON, Williams DW (2002) Spatial synchrony in forest insect outbreaks: roles of regional stochasticity and dispersal. Ecology 83:3120–3129
Pimentel D (ed) (2002) Biological invasions. Economic and environmental costs of alien plant, animal, and microbe species. CRC Press, Boca Raton
Pimentel D, Andow D, Dyson-Hudson R, Gallahan D, Jacobson S, Irish M, Kroop S, Moss A, Schreiner I, Shepard M, Thompson T, Vinzant B (1980) Environmental and social costs of pesticides: a preliminary assessment. Oikos 34:126–140
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien invasive species in the United States. Ecol Econ 52:273–288
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J For 104:118–124
Pöyry J, Leinonen R, Söderman G, Nieminen M, Heikkinen RK (2011) Climate-induced increase of moth multivoltinism in boreal regions. Glob Ecol Biogeogr 20:289–298
Raimondo S, Liebhold AM, Strazanac J, Butler L (2004) Population synchrony within and among Lepidoptera species in relation to weather, phylogeny, and larval phenology. Ecol Entomol 29:96–105
Régnière J, Nealis V, Porter K (2009) Climate suitability and management of the gypsy moth invasion into Canada. Biol Invasions 11:135–148
Rehfeldt GE, Crookston NL, Warwell MV, Evans JS (2006) Empirical analyses of plant-climate relationships for the western United States. Int J Plant Sci 167:1123–1150
Reichard SH, White P (2001) Horticulture as a pathway of invasive plant introductions in the United States. Bioscience 51:103–113
Rejmánek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661
Robertson C, Nelson TA, Jelinski DE, Wulder MA, Boots B (2009) Spatial-temporal analysis of species range expansion: the case of the mountain pine beetle, Dendroctonus ponderosae. J Biogeogr 36:1446–1458
Robinet C, Roques A (2010) Direct impacts of recent climate warming on insect populations. Integr Zool 5:132–142
Robinet C, Baier P, Pennerstorfer J, Schopf A, Roques A (2007) Modelling the effects of climate change on the potential feeding activity of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Notodontidae) in France. Glob Ecol Biogeogr 16:460–471
Roland J, Embree DG (1995) Biological control of winter moth. Annu Rev Entomol 40:475–492
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on animals and plants. Nature 421:57–60
Rosenzweig C, Iglesius A, Yang XB, Epstein PR, Chivian E (2001) Climate change and extreme weather events: implications for food production, plant diseases, and pests. Glob Chang Hum Health 2:90–104
Rouault G, Candau J-N, Lieutier F, Nageleisen L-M, Martin J-C, Warzée N (2006) Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann For Sci 63:613–624
Roush RT, McKenzie JA (1987) Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol 32:361–380
Roy DB, Sparks T (2000) Phenology of British butterflies and climate change. Glob Chang Biol 6:407–416
Royama T (1984) Population dynamics of the spruce budworm, Choristoneura fumiferana. Ecol Monogr 54:429–492
Safranyik L, Shrimpton DM, Whitney HS (1975) An interpretation of the interaction between lodgepole pine, the mountain pine beetle and its associated blue stain fungi in Western Canada. In: Baumgartner DM (ed) Management of lodgepole pine ecosystems symposium proceedings. Washington State University Cooperative Extension Service, Pullman, pp 406–428
Safranyik L, Carroll AL, Régnière J, Langor DW, Riel WG, Shore TL, Peter B, Cooke BJ, Nealis VG, Taylor SW (2010) Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can Entomol 142:415–442
Sambaraju K, Carroll AL, Zhu J, Stahl K, Moore RD, Aukema BH (2012) Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 35:211–223
Sherriff RL, Berg EE, Miller AE (2011) Climate variability and spruce beetle (Dendroctonus rufipennis) outbreaks in south-central and southwest Alaska. Ecology 92:1459–1470
Shigesada N, Kawasaki K (1997) Biological invasions: theory and practice. Oxford University Press, New York
Siegert NW, McCullough DG, Venette RC, Hajek AE, Andresen JA (2009) Assessing the climatic potential for epizootics of the gypsy moth fungal pathogen Entomophaga maimaiga in the North Central United States. Can J For Res 39:1958–1970
Simberloff D, Gibbons L (2004) Now you see them, now you don’t! Population crashes of established introduced species. Biol Invasions 6:161–172
Simberloff D, Rejmánek M (eds) (2011) Encyclopedia of biological invasions. University of California Press, Berkeley
Simberloff D, Stiling P (1996) How risky is biological control? Ecology 77:1965–1974
Simon RB, Thomas CD, Bale JS (2002) The influence of thermal ecology on the distribution of three nymphalid butterflies. J Appl Ecol 39:43–55
Singer MC, Parmesan C (2010) Phenological asynchrony between herbivorous insects and their hosts: signal of climate change or pre-existing adaptive strategy? Philos Trans R Soc Lond B Biol Sci 365:3161–3176
Slippers B, de Groot P, Wingfield MJ (eds) (2012a) The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, Dordrecht
Slippers B, Hurley BP, Mlonyeni XO, de Groot P, Wingfield MJ (2012b) Factors affecting the efficacy of Deladenus siricidicola in biological control systems. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, Dordrecht, pp 119–133
Smith MT, Turgeon JT, De Groot P, Gasman B (2009) Asian longhorned beetle Anoplophora glabripennis (Motschulsky): lessons learned and opportunities to improve the process of eradication and management. Am Entomol 55:21–25
Stange EE, Ayres MP, Bess JA (2011) Concordant population dynamics of Lepidoptera herbivores in a forest ecosystem. Ecography 34:772–779
Stefanescu C, Penuelas J, Filella I (2003) Effects of climatic change on the phenology of butterflies in the northwest Mediterranean Basin. Glob Chang Biol 9:1494–1506
Steinbauer MJ, Kriticos DJ, Lukacs Z, Clarke AR (2004) Modeling a forest lepidopteran: phenological plasticity determines voltinism, which influences population dynamics. For Ecol Manag 198:117–131
Stenseth NC, Mysterud A (2002) Climate, changing phenology, and other life history and traits: nonlinearity and match- mismatch to the environment. Proc Natl Acad Sci U S A 99:13379–13381
Stephens PA, Sutherland WJ, Freckleton RP (1999) What is the Allee effect? Oikos 87:185–190
Stiling P, Cornelissen T (2007) How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2 mediated changes on plant chemistry and herbivore performance. Glob Chang Biol 13:1823–1842
Stireman JO, Dyer LA, Janzen DH, Singer MS, Lill JT, Marquis RJ, Ricklefs RE, Gentry GL, Hallwachs W, Coley PD, Barone JA, Greeney HF, Connahs H, Barbosa P, Morais HC, Diniz IR (2005) Climatic unpredictability and parasitism of caterpillars: implications of global warming. Proc Natl Acad Sci U S A 102:17384–17387
Stoyenoff JL, Witter JA, Montgomery ME, Chilcote CA (1994) Effects of host switching on gypsy moth (Lymantria dispar (L.)) under field conditions. Oecologia 97:143–157
Strong DR, Pemberton RW (2000) Biological control of invading species—risk and reform. Science 288:1969–1970
Sun JH, Miao ZW, Zhang Z, Zhang ZN, Gillette NE (2004) Red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Scolytidae), response to host semiochemicals in China. Environ Entomol 33:206–212
Tauber MJ, Tauber CA (1976) Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu Rev Entomol 21:81–107
Taylor CM, Hastings A (2005) Allee effects in biological invasions. Ecol Lett 8:895–908
Taylor CM, Davis HG, Civille JC, Grevstad FS, Hastings A (2004) Consequences of an Allee effect on the invasion of a Pacific estuary by Spartina alterniflora. Ecology 85:3254–3266
Thuiller W, Richardson DM, Pyšek P, Midgley GF, Hughes GO, Rouget M (2005) Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Chang Biol 11:2234–2250
Tobin PC, Nagarkatti S, Loeb G, Saunders MC (2008) Historical and projected interactions between climate change and voltinism in a multivoltine insect species. Glob Chang Biol 14:951–957
Tobin PC, Berec L, Liebhold AM (2011) Exploiting Allee effects for managing biological invasions. Ecol Lett 14:615–624
Tobin PC, Bai BB, Eggen DA, Leonard DS (2012) The ecology, geopolitics, and economics of managing Lymantria dispar (L.) in the United States. Int J Pest Manag 58:195–210
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630
van Asch M, Visser ME (2007) Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu Rev Entomol 52:37–55
van Asch M, Julkunen-Tiito R, Visser ME (2010) Maternal effects in an insect herbivore as a mechanism to adapt to host plant phenology. Funct Ecol 24:1103–1109
Vanhanen H, Veleli TO, Paivinen S, Kellomaki S, Niemela P (2007) Climate change and range shifts in two insect defoliators: Gypsy moth and nun moth − a model study. Silva Fenn 41:621–638
Varley GC, Gradwell GR, Hassell MP (1973) Insect population ecology. An analytical approach. Blackwell Scientific, Oxford, UK
Veteli TO, Lahtinen A, Repo T, Niemela P, Varama M (2005) Geographic variation in winter freezing susceptibility in the eggs of the European pine sawfly (Neodiprion sertifer). Agr Forest Entomol 7:115–120
Visser ME, Holleman LJM (2001) Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc R Soc Biol Sci Ser B 268:289–294
Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc R Soc Biol Sci Ser B 265:1867–1870
Visser ME, Holleman LJM, Gienapp P (2006) Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:164–172
Visser ME, Caro SP, van Oers K, Schaper SV, Helm B (2010) Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos Trans R Soc Lond B Biol Sci 365:3113–3127
Volney WJA, Fleming RA (2000) Climate change and impacts of boreal forest insects. Agric Ecosyst Environ 82:283–294
Walther G-R, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarošík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Waring KM, Reboletti DM, Mork LA, Huang CH, Hofstetter RW, Garcia AM, Fule PZ, Davis TS (2009) Modeling the impacts of two bark beetle species under a warming climate in the Southwestern USA: ecological and economic consequences. Environ Manag 44:824–835
Werner RA, Holsten EH, Matsuoka SM, Burnside RE (2006) Spruce beetles and forest ecosystems in south-central Alaska: a review of 30 years of research. For Ecol Manag 227:195–206
Williams DW, Liebhold AM (1995a) Herbivorous insects and global change: potential changes in the spatial distribution of forest defoliator outbreaks. J Biogeogr 22:665–671
Williams DW, Liebhold AM (1995b) Influence of weather on the synchrony of gypsy moth (Lepidoptera: Lymantriidae) outbreaks in New England. Environ Entomol 24:987–995
Wingfield MJ, Bernard Slippers B, Wingfield BD (2010) Novel associations between pathogens, insects and tree species threaten world forests. N Z J For Sci 40:S95–S103
Wolda K (1988) Insect seasonality: why? Annu Rev Ecol Syst 19:1–18
Work TT, McCullough DG (2000) Lepidopteran communities in two forest ecosystems during the first gypsy moth outbreaks in northern Michigan. Environ Entomol 29:884–900
Worner SP, Gevrey M (2006) Modelling global insect pest species assemblages to determine risk of invasion. J Appl Ecol 43:858–867
Yamamura K, Kiritani K (1998) A simple method to estimate the potential increase in the number of generations under global warming in temperate zones. Appl Entomol Zool 33:289–298
Yan Z, Sun J, Don O, Zhang Z (2005) The red turpentine beetle, Dendroctonus valens LeConte (Scolytidae): an exotic invasive pest of pine in China. Biodivers Conserv 14:1735–1760
Yukawa J, Akimoto K (2006) Influence of synchronization between adult emergence and host plant phenology on the population density of Pseudasphondylia neolitseae (Diptera: Cecidomyiidae) inducing leaf galls on Neolitsea sericea (Lauraceae). Popul Ecol 48:13–21
Zalucki MP, van Klinken RD (2006) Predicting population dynamics of weed biological control agents: science or gazing into crystal balls? Aust J Entomol 45:331–344
Zalucki MP, Clarke AR, Malcolm SB (2002) Ecology and behavior of first instar larval Lepidoptera. Annu Rev Entomol 47:361–393
Acknowledgments
We are grateful to Andrew Liebhold and Trevor Fenning for helpful comments on an earlier draft of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Tobin, P.C., Parry, D., Aukema, B.H. (2014). The Influence of Climate Change on Insect Invasions in Temperate Forest Ecosystems. In: Fenning, T. (eds) Challenges and Opportunities for the World's Forests in the 21st Century. Forestry Sciences, vol 81. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7076-8_12
Download citation
DOI: https://doi.org/10.1007/978-94-007-7076-8_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7075-1
Online ISBN: 978-94-007-7076-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)