Abstract
Brain tumors are among the leading causes of tumor-related deaths globally; hence considerable research effort is being expended to improve the patient outcome. There are now multiple imaging techniques available for the diagnosis and management of brain tumors in clinical practice, as well as different contrast agents. All these coupled with amino acid tracers newly available in positron emission tomography can offer more accurate tumor diagnosis. The evaluation of tumors using multiple imaging modalities is now one of the trends in neuroradiology, where computed tomography (CT), magnetic resonance imaging (MRI) and molecular imaging all play a vital role in the brain tumor assessment.
In this chapter, we will cover the clinical applications of computed tomography, magnetic resonance imaging with and without gadolinium contrast agents including perfusion weighted imaging, amide proton transfer imaging and magnetic resonance spectroscopy, and positron emission tomography (PET) for the evaluation of brain tumors. The advantages and limitations of each modality, as well as how they perform with respect to certain specific clinical questions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Brain Tumor
- Single Photon Emission Compute Tomography
- Arterial Spin Label
- Radiation Necrosis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Brain tumors are among the leading causes of tumor related deaths globally, with 10–15 out of every 100,000 people diagnosed in Europe and USA alone every year (Essig, 2003). There are a number of imaging techniques used in diagnosis and therapy for brain tumors, such as Computed Tomography (CT), Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET). Variety of MRI sequences and contrast agents have enhanced the range of diagnostic imaging to a new level. Furthermore, the addition of amino acids tracers for use in PET has taken this imaging modality to a molecular level which enables the radiologist to target and measure tumor progression with much greater accuracy.
CT along with MRI (perfusion MRI and dynamic contrast enhanced (DCE) MRI) now play a pivotal role in brain tumor assessment. In this chapter, the latest brain tumor assessment protocols using CT, MRI with and without gadolinium contrast and PET imaging will be discussed and compared. This includes the more recent developments in the field such as amino acid tracer for PET, amide proton transfer imaging and MR spectroscopy. These new imaging methods have shown promising results in research and are now being used concurrently with the conventional imaging techniques to improve the evaluation of brain tumors.
Role of Diagnostic Imaging in Brain Tumor Assessment
Brain tumors can be categorized into primary or secondary tumors based on its originating tissue and intraaxial or extraaxial depending on its origin of growth. The most common primary intraaxial tumors are neuroepithelial tumors including astrocytoma, oligodendroglioma, glioblastoma multiforme and mixed glioma, whereas the most common primary extraaxial tumors are meningioma, pituitary adenoma, acoustic neuroma, schwannoma and caraniopharyngioma. Glioblastoma multiforme is the most common primary intraaxial brain tumor while meningioma is the most common extraaxial tumor accounting for 20 % of all brain tumors. Secondary metastatic brain lesions from systemic cancers are the most common brain tumors in general.
From the time when a patient comes to a radiologist with an intracranial mass (suspected as intracranial tumor) to the management and the post therapeutic prognosis, imaging plays an important role at each step. Although there are many different stages in diagnosis and management of intracranial tumor, they are often closely integrated with each other in practice and imaging can be of great use in some of the following steps (Kandel and Schavinsky, 1972; Mehndiratta and Giesel, 2011):
-
1.
Detection and confirmation of the structural abnormality.
-
2.
Localization and assessment of the extent of the pathological structure (lesion).
-
3.
Grading the tumor: as neoplastic or non-neoplastic mass, if is neoplastic then further classify it as either malignant or benign.
-
4.
Staging of the tumor: lymphatic and vascular spread or spread to other organs (Metastases).
-
5.
Looking for involvement of any vital brain areas which might be of immediate concern for therapeutic planning.
-
6.
Facilitate surgical planning (if required) by reviewing non-invasively the vital structures around tumor.
-
7.
Intraoperative support during the surgical procedure.
-
8.
Prognostic monitoring and follow up.
CT is often the first imaging technique used in brain tumor assessment because of its wide availability, low cost and minimally invasiveness. It is also considered to be a very good screening tool for the detection of a supratentorial abnormality, but MRI with more sophisticated sequences are required to provide accurate anatomical distinction for surgical planning. There are various structural features which are of key interest to a radiologist for the evaluation of brain tumor (Mehndiratta and Giesel, 2011):
-
1.
Signal contrast of tumor with respect to the normal brain parenchyma in vicinity.
-
2.
Degree of contrast enhancement.
-
3.
Tumor structure, margins, size and extent.
-
4.
Associated perifocal edema.
-
5.
Tumor signs (compression syndrome, midline shift etc.).
-
6.
Tumor vascularity, especially the main vessel supplying the tumor and its course.
MR imaging can provide critical information for accurate diagnosis and surgical intervention beyond that is available from CT. Contrast enhanced MR imaging using gadolinium (Gd) contrast agents can depict blood brain barrier (BBB) disruption and if there has been an increase in the extracellular-extravascular space (EES). MR angiography can reveal the blood vessels in the vicinity of tumor which need to be protected during any surgical procedure, as well as the main artery supplying the tumor mass which have to be ligated before surgical removal of the tumor. Simple contrast enhanced morphological imaging is limited in its accuracy for predicting tumor aggressiveness, hence DCE and DSC imaging, which can provide additional information such as hemodynamics and neo-angeiogenic status along with lesion morphology, are often performed. T1w (T1-weighted) and T2* perfusion imaging for a follow up scan are also important as they can differentiate between tumor recurrence and radiation necrosis (Giesel et al., 2010; Mehndiratta and Giesel, 2011) (discussed later in detail).
Table 1.1 summarizes some of the key advantages and limitations of CT, MR and PET imaging in brain tumor assessment (Mehndiratta and Giesel, 2011).
Computed Tomography Imaging in Brain Tumor Assessment
Computed tomography is the mathematical reconstruction of a cross-sectional image of the body from measurement of x-ray transmission through a thin slice of patient tissue. A narrow well-collimated beam of x-rays is generated from one side of the patient and as the beam passes through patients body it fans out. During the course of its transit the x-ray beam is attenuated by absorption and scatter through body tissue. A sensitive x-ray detector array on the opposite side of the patient measures the x-ray transmission. These measurements are repeated systematically from multiple directions as x-ray tube is pulsed while the gantry rotates 360º around the patient. CT values are assigned to each voxel in the image by a back-projection algorithm based on the attenuation of the measured x-ray beam. These values are measured in Hounsfield Units (HU), named after Sir Godfrey N. Hounsfield; the inventor of CT. HU is not an absolute scale but is proportional to the difference in x-ray attenuation of the tissue and of water. Thus, water is normally assigned as a reference having 0 HU, with the HU scale ranging from −1,024 HU for air to +3,000 HU for dense bone. Soft tissue like brain has HU in the range of +20 to +50 HU.
The key advantages of CT compared with MR are rapid image acquisition, superior bone details, and demonstration of calcifications in soft tissue. CT scan is usually limited to the axial plane; however, images may be reformatted in sagittal, coronal or oblique planes and also as three-dimensional images. If image acquisition is anisotropic, the 3D reformatting is usually not very good and thus axial plane images are normally used to perform the assessment. Helical CT, Multi Detector CT (MDCT) and flat panel Volumetric CT (fpVCT) are some of the CT acquisition types which differ in either dimensions of the x-ray detector or the patient table motion during image acquisition.
CT is usually the first imaging to be performed on a patient presented with signs and symptoms of any central nervous system disorder because of its wider availability and low cost. CT is considered to be a very good screening tool for the detection of any supratentorial abnormality or extraaxial brain tumors. Intracranial space occupying lesion presenting with a mass effect can be easily picked by a midline shift in the axial slice of brain CT. CT is the imaging of choice for bone abnormalities like destruction, skull erosion, permeation or hyperostosis. Beside that, CT is used for detection of calcification within an intracranial space occupying lesion. A big unilateral hemorrhage can be identified by CT easily but not subtle bilateral hemorrhage which can be missed sometimes as it easily blend with the brain soft tissue. Calcification within an intracranial lesion might indicate a slow growing mass whereas hemorrhage within an intracranial mass is a sign of malignancy.
CT does not have much role in brain tumor imaging assessment other than the ones mentioned above but is usually the first line of diagnostic imaging in all cases. PET-CT is very common nowadays and could be performed with a hybrid machine. The benefit of this simultaneous PET-CT acquisition is that co-registration of images is not required in the post processing and CT could provide morphological information while acquiring the PET imaging data.
Multi-energy CT (MECT) is a new innovation in the CT technology. MECT at present is performed with two x-ray energies. The methodology is commonly referred to as Dual Energy CT (DECT). DECT employ two X-ray sources and two detector arrays fixed orthogonal to each other equipped for simultaneous acquisition operated at different energy levels for atomic characterization, whereas in traditional CT there is only one x-ray source and one detector array.
DECT is based on the principle that x-ray attenuation is energy dependant. X-ray absorption depends on the atomic number and the density but is independent of its chemical bonding. The peak tube voltage (kVp) determines the energy spectrum of the x-ray beam. Therefore, a change in kVp leads to an alteration of average photon energy. Since the beam attenuation caused by different materials is energy dependent, one can differentiate the different tissue types based on their attenuation characteristics at two different energy levels. For example, iodine has high attenuation at low kVp which changes to half its value at high photon energies. Unlike iodine, the attenuation of calcium changes much less when the kVp of the tube is changed. The strength of DECT is its post processing algorithms that can analyze the acquired data sets simultaneously to extract the material specific differences in attenuation.
Recently, DECT has been proposed (Mehndiratta et al., 2011) as a new tool for the characterization of intracranial neoplastic disease (primary or metastatic) in three essential ways:
-
1.
The internal density and composition of the tumor can be readily studied using CT. For example, DECT is very sensitive in detecting as well as quantitatively measuring internal calcifications, necrosis, foci of haemorrhage, and fatty deposits.
-
2.
The presence and extent of bone erosion or skull base involvement is better depicted by CT than MRI.
-
3.
The presence of neo-vascularity can be evaluated by DECT perfusion studies.
Brain tumor under assessment can be imaged with one iodine contrast enhanced DECT scan (Mehndiratta et al., 2011). The contrast enhanced dual energy images can thus generate an iodine map of the tumor in which the iodine load might be related to the tumor vascularity and hence its malignant potentials (Pharmacokinetic analysis of malignant pleural mesothelioma-initial results of tumor microcirculation and its correlation to microvessel density (CD-34), Acad Radiol. 2008 May; 15(5):563–70. doi: 10.1016/j.acra.2007.12.014). Iodine mapping of brain might also be used in grading the tumor or radiotherapy planning. In addition the contrast enhanced DECT imaging scanning might be beneficial in the follow up scans after chemo-radio-therapy, where the recurrent or remnant tumor might show iodine uptake but not at the region of radiation necrosis due to lack of vascularity after the treatment. The DECT application in brain tumor assessment is yet in its neonatal phase and need to be validated for its clinical utility.
Magnetic Resonance Imaging in Brain Tumor Assessment
Magnetic Resonance Imaging (MRI) is a powerful imaging tool that is based on the resonance phenomenon of body protons with an externally applied magnetic field. Because of each atomic nucleus spin motion there is a induced local magnetic moment (μ). When nuclei are exposed to an external magnetic field (B0, measured in Tesla), the interaction with local magnetic moment causes the nuclei to precess. The frequency at which precession occurs is called the Larmor frequency (ω0), defined by Larmor equation, ω0 = B0 · γ, where γ is the gyro-magnetic ratio (measured in mega Hertz per Tesla) which is an atom specific property defined at a particular magnetic field strength (e.g., for 1H, γ/2π = 42.57 MHz/T) (Pooley, 2005). Free magnetic moments of protons are randomly oriented in body in the absence of an external magnetic source. When the external source is present, all the protons will align along the direction of the magnetic field, B0. This equilibrium can be disturbed by an application of an electromagnetic gradient in the form of radio frequency (RF) pulse having radiation energy (Erf). The radiation energy is atom specific, Erf = ħ · ω0; where ħ = h/2π, h being the Planck’s constant (Pooley, 2005). The RF pulse causes the net magnetization to flip by a certain angle (called flip angle), and thus produces two magnetization vector components, longitudinal and transverse. Due to the resonant irradiation, the spin system takes up additional energy which can be dissipated to surrounding by corresponding relaxation (dephasing) of spinning protons.
When the RF energy is switched off, the net magnetization vector tends to realign with the axis of B0 through the process of T1 recovery, during which longitudinal magnetization recovers. Simultaneously the transverse magnetization decays through the process called T2 decay. Different body tissues have specific T1, T2 and T2* values. As the transverse magnetization precesses in axis of the receivers coil, it induces a current in that coil which is read as the MR signal. In MR imaging, differences in T1, T2 (T2*) and proton density in various tissues create differences in tissue contrast on images (Pooley, 2005). Repetition time (TR), echo time (TE) an flip angle are some commonly used MR sequence parameters to alter the tissue contrast of an image.
MRI is an important diagnostic technique in the evaluation of intracranial tumors. It is highly sensitive to the pathological changes in the brain parenchyma water content, as demonstrated by abnormal high or low signal intensity on T2- or T1-weighted images, respectively. Compared to other imaging modalities, MRI allows more accurate determination of lesion location, extent and subtle mass effects or atrophy especially those along the cerebral convexities. MRI is also very effective in depicting subacute and chronic hemorrhages, permitting more accurate distinction between vascular structure and adjacent parenchyma. When compared to CT which is superior in depicting the presence of calcifications and bone anomalies, MRI is better in soft tissue contrast that is for differentiating between tumor and perifocal edema, for defining the extent of the tumor and showing the relationship of the tumor to critical adjacent structures. Heavily T2-weighted sequences are most sensitive for the detection of tumor and edema extent (Mehndiratta and Giesel, 2011), while T1-weighted sequences following contrast enhancement generally provide better localization of the tumor nidus and improved diagnostic information relating to tumor grade, BBB breakdown, hemorrhage, edema and tissue necrosis (Mehndiratta and Giesel, 2011). Contrast enhanced T1-weighted images can elaborate small focal lesions such as metastases, minimal tumor recurrence and ependymal or leptomeningeal tumor spread because of improved signal contrast. Proton density images are more useful in distinguishing tumor and edema from adjacent cerebrospinal fluid, which may have a similar appearance as high-signal zone on heavily T2-weightd image. All these information are essential for surgical planning purposes.
It has been shown in the literature that imaging findings in MR studies correlate well with the histological grading of cerebral gliomas (Mehndiratta and Giesel, 2011). Generally, a tumor with sharp margins, homogenous in signal intensity and with little contrast enhancement is a low grade glioma. Tumors with indistinct margins which are inhomogeneous in signal and demonstrate intense, irregular contrast enhancement tend to be high-grade gliomas. These are some generalizations, but for diagnostic and therapeutic purposes, imaging findings and contrast-enhancing patterns must be considered all together. Additionally, each case is unique and tumor presentation varies from case to case due to high population variability. Some of the low grade Astrocytomas are primarily infiltrating and histologically benign, but they might demonstrate a poor margination with the surrounding brain, where some of the rapidly growing malignant gliomas may show sharp margins (Mehndiratta and Giesel, 2011).
Dean et al., (1990) found that the degree of mass effect and incidence of cyst formation or necrosis are statistically significant positive predictors of tumor grade. A central non-enhancing area within a highly enhanced tumor mass usually suggests the area is necrosis as a result of the rapidly growing tumor that outstrips the vascular supply. This is a common presentation of malignant behavior and might suggest the diagnosis of Glioblastoma multiforme. Similarly, hemorrhage within a tumor also favors the diagnosis of a malignancy mostly associated with Glioblastoma or secondary metastases. An incredibly large zone of edema surrounding a contrast enhancing intra-axial tumor is also a sign of malignancy which contribute to the mass effect associated with these tumors. A notable exception to this is meningioma, which is a benign tumor often associated with high amount of adjacent edema and mass effect (Mehndiratta and Giesel, 2011). This can be easily distinguished from malignant gliomas by its extra-axial location. Low grade gliomas tend to present with an infiltrating pattern which might appear as edema on imaging but it lacks the contrast enhancement and mass effect which are the key factors associated only with cerebral edema. A slow growing neoplasm can show calcifications; this is a classical feature of oligodendroglioma and ganglioglioma. Occasionally, calcification might also be observed in tumors like Astrocytoma and ependymoma.
There are few limitations associated with the present MR techniques including:
-
1.
There exists a considerable overlap among different intracranial tumors for respective morphological characteristics. This makes the histological diagnostic confirmation almost mandatory for definitive therapy planning.
-
2.
An MR image is a spatial map of the difference in water proton relaxivity characteristics (T1 or T2 relaxation). Though significant intracranial pathological anomalies alter these characteristics, which make their effect visible on an image, but sometimes the changes might be very subtle compared to the surrounding normal tissue. Also, with infiltrative gliomas and small tumors the visible changes are minimal, frequently they are asymptomatic and thus go undiagnosed before they present with a large mass effect distorting the normal anatomy.
-
3.
The well-defined enhancing tumor nidus is easily detectable on MR image, but infiltrating tumor or isolated tumor cells might extend far beyond tumor nidus into the surrounding edematous zone. These abnormalities could be easily overlooked by inexperienced eyes.
-
4.
Large amount of calcification could blur the MR signal, making classification of the tumor almost impossible using MR imaging.
-
5.
Artifacts might degrade the diagnostic image quality so much that normal tissue could be misinterpreted as a pathological mass. Motion artifacts are known for introducing ‘bright’ and ‘dark’ zones in the normal brain which might be misdiagnosed as a lesion.
-
6.
Image spatial resolution is very sensitive to magnetic field inhomogeneity. Poor shimming could alter magnetic field inhomogeneity and distort an image.
Brain Tumor Imaging Using Contrast Enhanced MRI
Contrast enhanced MR neuroimaging (CE-MRI) is widely used in the evaluation of primary and secondary brain tumors. Conventional MRI is very sensitive in tumor detection and delineation which is important for diagnosis and tumor grading, whereas CE-MRI enables the radiologist to localize the vital surrounding structure around the tumor to facilitate appropriate intervention. Contrast agents help to localize surrounding vessels and nerves to aid surgical and radio-therapeutic planning. CE-MRI is also used during post-intervention to monitor the treatment response. An early identification of treatment failure could facilitate alternative therapeutic measures, hence improve patient outcome.
A common imaging protocols for brain tumor evaluation is: (1) T1 weighted, (2) T2 weighted and (3) T2* perfusion imaging sequence followed by T1 post contrast image acquisition. T1 and T2 weighted imaging give good anatomical and morphological information whereas contrast-enhanced images provide well defined tumor margins. The T1w image is also useful for selecting a region of interest (ROI) including the tumor for further perfusion analysis. DSC-MRI could provide information on tumor vascularity and hot spots (areas of high vascularity) which might be potentially useful for tumor grading and therapeutic planning (Essig, 2003).
Given the usefulness of Gd based contrast agents in MRI imaging for brain tumors, it is important to note that excessive use of Gd contrast can cause nephrogenic systemic fibrosis (NHS) particularly in the case of patients with compromised kidney function (Broome et al., 2007). Recent research has shown that contrast agents like gadobenate, gadoxetate and gadofosveset have a transient protein-binding capability that is capable of doubling or more the R1 and R2 relaxivity (1/T1 and 1/T2) (Giesel et al., 2010), enabling the use of a lower dosage of this kind of agents to achieve equivalent image contrast in the cohort of patients with compromised kidney function.
The dosage of gadolinium contrast agent for CE imaging is a topic of considerable research interest, but for clinical practice a dose of 0.1 mmol/kg bodyweight is considered to be safe (Essig, 2003). Many studies have demonstrated the diagnostic benefit of double and triple dose (0.2–0.3 mmol/kg bodyweight) in MR imaging (Giesel et al., 2010; Sze et al., 1998) but recent concerns of NSF has limited the higher Gd contrast dosage protocols to only research studies (Broome et al., 2007). Even though a higher dosage is very sensitive in determining the extent of primary tumor spread, it increases the cost of the MR examination and might produce a higher false positive rate (Giesel et al., 2010; Sze et al., 1998). Most of the currently available Gd-contrast are 0.5 % molarity except gadobutrol (Gadovist®) which is the only high molarity (1 %) contrast agent (Giesel et al., 2010) available for clinical use. Gadobutrol has been demonstrated to be highly sensitive to tumor activity (Giesel et al., 2009) (Fig. 1.1), which could potentially enable intensity-modulated radiotherapy to be used more efficiently based on the underlying tumor activity. Essig et al., (2006) compared single and double dose of gadobutrol and gadobenate dimeglumine at 1.5 T in a healthy control volunteer study where it was found that a double dose of contrast could give a large peak signal drop but there was no statistical difference between agents or dose for its clinical utility. As gadobutrol is double the molarity of other contrast agents, it could be given as half the volume of injection to deliver the same Gd- dose, which has been demonstrated to have a better bolus profile (Giesel et al., 2009). Nevertheless, the study of Essig et al., (2006) showed that the bolus width achieved by gadobutrol and gadobenate dimeglumine were sharp and comparable but the reduced injection time of gadobutrol did not produce any added benefit in perfusion quantification.
(a) T1-weighted; (b) T2-weighted MRI image of patient with tumor in the right frontal lobe; (c) T2* weighted parametric CBV map with Gd-DTPA contrast; (d) T2* weighted parametric CBV map with Gadobutrol contrast. Gadobutrol is shown to be more sensitive to tumor activity (arrow heads) and show more number of hyperactive lesions in tumor with high blood volume
Diagnostic accuracy could be improved by the use of high relaxivity Gd- contrast agents (Giesel et al., 2010) without actually increasing the Gd- dose. Dosing studies have demonstrated the beneficial effects of gadobenate dimeglumine in improving the sensitivity for brain tumor detection, but an incremental triple dose did not have any advantage even for high relaxivity contrast agents (Giesel et al., 2010; Mehndiratta and Giesel, 2011). Later significant evidence supporting improved diagnostic performance of high relaxivity contrast agents came from intra-individual crossover studies directly comparing gadobenate dimeglumine with gadopentetate dimeglumine (Colosimo et al., 2004; Kuhn et al., 2007; Maravilla et al., 2006; Rumboldt et al., 2009), gadoterate meglumine (Colosimo et al., 2001, 2004), gadodiamide (Colosimo et al., 2001; Rowley et al., 2008) and gadofosveset trisodium (Giesel et al., 2010). The first such crossover study, performed in 2001, demonstrated that in patients with metastatic central nervous system disease, the sensitivity for lesion detection with gadobenate dimeglumine (93–100 %) was much higher than that with an equal dose of a comparable agent (65–73 %), like gadopentetate dimeglumine (N = 13), gadodiamide (N = 4), or gadoterate meglumine (N = 5) (Colosimo et al., 2001). In addition, tissue contrast in the main lesion-to-normal brain parenchyma was consistently greater for gadobenate dimeglumine (143 %) than for an equal dose comparable agent (127 %) as compared to unenhanced images (Colosimo et al., 2001). Since then a total of five large population randomized crossover studies have been performed, demonstrating the benefits and limitations of gadobenate dimeglumine compared to non-protein binding Gd-contrast agents (Colosimo et al., 2004; Maravilla et al., 2006; Rowley et al., 2008; Rumboldt et al., 2009). Recently, a specific study on cohort of intra-axial brain tumors (N = 158) has demonstrated the significant (p < 0.01) diagnostic improvement using gadobenate dimeglumine both qualitatively and quantitatively (Kuhn et al., 2007).
T1 weighted (T1w) CE-MRI is important in brain tumor imaging as the BBB within tumor is often found to be damaged leading to leakage of the contrast into the extravascular space. T1w contrast enhancement is often due to BBB damage associated with either neovascular angiogenesis or capillary damage in region of active tumor growth. Angiogenesis is an important marker of active tumor growth and might correlate with tumor aggressiveness (Mehndiratta and Giesel, 2011). As angiogenesis increases microvascular blood volume, perfusion imaging will show a higher CBV (cerebral blood volume) and CBF (cerebral blood flow) that gives a unique insight into tumor physiology and could be used as a metric to monitor therapeutic response. PET has been used in the past for similar measurements but both these techniques are very much dependent on a perfect bolus injection of contrast and require computationally expensive post-processing algorithms.
MRI for the Microvascular Assessment of Brain Tumor
With the raising concern of Gd-contrast related complication in the patients, research efforts have been directed toward non-invasive methodologies for vascular imaging using MRI. The idea is to either magnetically label the blood itself (as in Arterial Spin Labeling, ASL) or to null the blood signal and use it as a negative contrast, measuring blood volume indirectly (Vascular Space Occupancy, VASO) by vascular imaging.
Recently, a new T1 weighted sequence has been developed called Vascular Space Occupancy (VASO) that offers a non-invasive method for detecting CBV (Donahue et al., 2008). The VASO technique uses the difference in the T1 relaxivity of tissue and blood to nullify the intravascular blood signal and thus providing a measure of intravascular volume indirectly by measuring the remaining signal from the tissue. The technique has been used to measure CBV changes during neuronal activity and more recently the methods have been developed to quantify blood volume at rest, but these have not yet been extensively validated for tumor imaging. Studies have also demonstrated that VASO and MPRAGE (Magnetization Prepared Rapid Gradient Echo) MRI could provide soft tissue contrast complementary to Gd-T1w and FLAIR (fluid attenuation inversion recovery) MRI for tumor imaging (Donahue et al., 2008). FLAIR, VASO and MPRAGE are all inversion-based MRI sequences. However, they each offer a range of T1w contrasts, a collective analysis of which provides information not otherwise evident from each alone (Donahue et al., 2008).
Another non-invasive methodology for microvascular imaging is Arterial Spin Labeling (ASL). In ASL, the magnetically labeled water in blood is used as an endogenous contrast to directly measure blood flow and blood volume in the tissue of interest. At the level of the supplying artery for the tissue, a 180° radiofrequency pulse is applied to invert the magnetization of the water protons in the arterial blood. Once this magnetically labeled blood reaches the tissue an image of the tissue is acquired. A second image is subsequently acquired without the application of the inversion pulse (thus in the absence of labeled blood). Upon the subtraction of the two acquired datasets, the difference image provide a measure of blood supply to the tissue. Further images may be acquired varying the delay between labeling and imaging, this time series data can be analyzed in similar manner of perfusion MRI methods. The information provided by ASL is similar to that of DSC MRI, but the method is completely non-invasive (do not require exogenous contrast agents). Additionally, since it is water in blood that is acting as the contrast agent ASL provides a more direct measure of perfusion, the delivery to the tissue, as would be expected from positron labeled water PET. ASL has been used in stroke and other vascular pathologies but it remains to be tested and validated for brain tumor assessment.
Brain Tumor Imaging Using Amide Proton Transfer (APT) Contrast
Although the MR imaging protocols discussed above are the standard of care for assessing the brain tumors before, during and after therapy, it still has limited diagnostic specificity in identifying tumor grade or separating the mass of solid tumor from the surrounding edema. For example, hyperintensities on T2w and FLAIR images may be caused by either solid tumor mass or peritumoral edema. Surgical resection normally targets the bulk of the T1w contrast enhancing tumor with or without a margin clearance of the surrounding tissue with abnormal T2w or FLAIR signal. The motivation is to minimize the resection of the brain tissue that is affected primarily by edema because these tissues may recover to normal function after the surgical treatment of the nearby tumor (Zhou et al., 2008). Therefore, new imaging approaches which can precisely delineate the volume of tumor tissues for resection or can identify the ideal region for biopsy that is able to aid treatment recommendations are still highly desirable.
APT imaging is a new non-invasive cellular and molecular imaging technique for MRI. Through the process of chemical exchange saturation transfer (CEST), APT imaging provides an indirect way to detect endogenous mobile proteins and peptides which would otherwise be undetectable due to their low concentration. During an APT imaging acquisition, radiofrequency irradiation is applied to saturate (decrease the signal) the amide protons of the proteins and peptides that resonance at 3.5 ppm downfield from the water resonance. The saturated amide protons then interact with the unsaturated water protons via chemical exchange, leading to a transfer of magnetization and subsequently a decrease in the measured water intensity at the resonance frequency of amide protons. This chemical exchange led reduction in the intensity of measured water signal allows the detection of these low concentration proteins (milli-molar range). Quantitative in vivo MRI measurement has found that these mobile protein concentrations are generally higher in brain tumors than normal white matter and it increases with the tumor grade (Howe et al., 2003).
The APT ratio (APTR) is a common metric used in the APT imaging to differentiate the tumor from healthy tissues. It is an asymmetric analysis with respect to the water signal and is calculated by normalizing the difference between the measured intensity after saturation at ±3.5 ppm with the unsaturated signal (in the absence of radiofrequency irradiation) (van Zijl and Yadav, 2011). The calculated APTR of viable tumor tissue has been found to be positive and statistically higher than necrotic tissue, surrounding peripheral edema and healthy tissues (Wen et al., 2010). This allows a clear differentiation of solid tumor masses from peritumoral edema which was previously indistinguishable on T2w and FLAIR. In addition, the higher APTR of the active tumor core facilitates the identification of the highly malignant zone of tumor for biopsy. This is useful clinically because some high-grade gliomas are known to demonstrate no contrast enhancement in CE-MRI (Wen et al., 2010).
Although T1w and T2* perfusion imaging together can differentiate the tumor recurrence and radiation necrosis, the latter is an invasive approach which requires the administration of contrast agents. APT imaging on the other hand is a completely non-invasive technique and could assess the tumor response to therapy based on its unique capability of detecting the endogenous mobile proteins and peptides that are markers of functionally active tissue. After radiation therapy, viable glioma would show a hyperintensity on an APT image whereas hypointensity or isointensity would be observed in the necrotic region. This difference is probably caused by the absence of mobile cytosolic proteins and peptides in the necrosis region due to the loss of cytoplasm after cell depolarization (Zhou et al., 2011).
Although APT imaging can be used for detecting brain tumor activity without the need of an exogenous contrast agent and can potentially distinguish the heterogeneity of high grade tumors through the change of concentration of the endogenous mobile proteins, the obtained APTR for the brain at 3 T is small, only about 2–4 % of the water intensity (van Zijl and Yadav, 2011). Current research is, therefore, focused on more optimal strategies for acquisition of the data that in particular may correct for various artifacts including field inhomgeneities and the effects of patient movement. Artifacts are often observed near the surface of brain and ventricles on APT images due to patient movement during the scan (Wen et al., 2010). APT imaging is still a relatively new method for brain tumor imaging and has only been used on a small cohort of patients with high grade gliomas, but its unique capabilities can potentially provide valuable complimentary information to the existing brain tumor diagnostic imaging.
Brain Tumor Imaging Using MR Spectroscopy (MRS)
MR spectroscopy can provide information on tumor biochemical composition which is complimentary to other imaging modalities. Standard proton MRS (1H-MRS) is the most widely explored MRS technique with its application in tumor neuroimaging (Howe and Opstad, 2003).
Brain tumor spectroscopy can be performed in a single- or multi voxel form. The most widely used methods of volume selection are (i) Stimulated Echo Acquisition Mode (STEAM) and (ii) Point-Resolved Spectroscopy Sequence (PRESS). STEAM works best at short echo times (TE) which makes it highly susceptible to motion artifacts. Theoretically for a similar TE, PRESS could achieve almost double the signal to noise ratio than STEAM and would comparatively be less sensitive to motion artifacts (Castillo et al., 1996), but PRESS requires high computational power with sophisticated algorithm design for the analysis. With the advancement of computational analysis nowadays, PRESS is gaining wider acceptance in clinical routine.
Single voxel MRS has been applied for characterizing the metabolic signatures of brain tumors as it has high signal to noise ratio, shorter acquisition time and is easier to be processed. Single voxel MRS faces the limitation of poor spatial resolution and hence lacks the regional mapping of metabolic variations in the tissue. Furthermore, poor spatial resolution makes the single voxel MRS results highly susceptible to partial volume error. Brain tumors are highly heterogeneous in metabolic activity and aggressiveness, hence single voxel MRS is not an optimal solution to perform or assist tumor grading. Multivoxel MRS may be more suitable to study the brain tumor metabolic heterogeneity which is particularly important for planning focal treatments of tumor such as radiation and surgical resection and for following response to therapy.
For the most commonly used clinical MRS sequences, the spectrum is sensitive to five metabolites: Choline (Cho), N-Acetyl Aspartate (NAA), Creatine (Cr), Lactate and Lipid (Law et al., 2002; Shino et al., 1999; Usenius et al., 1994). The NAA peak is exclusive to neuronal cell, Creatine is a marker of energy synthesis and Choline reflects cell membrane turnover in MRS of brain. Lactate reflects the presence of anaerobic metabolism which is not a common finding in healthy tissue but is frequently found elevated in necrotic or infarcted brain tumor (Usenius et al., 1994). Primary brain tumors are notorious for showing a specific pattern in the elevation of Choline and loss of NAA peaks (Usenius et al., 1994).
MRS could aid in detailed evaluation of brain tumor and its grading along with morphological MRI. An elevated Cho/Cr and Cho/NAA ratio has been found to be associated to high grade gliomas (Law et al., 2002). MRS is very sensitive in differentiation of tumor recurrence and necrosis where both appear to be contrast enhancing on T1w MRI. MRS also play a useful role in assessing the therapeutic response as the Cho peak would decrease and lactate or lipids may increase over time during the treatment course (Shino et al., 1999). This is particularly important for early detection of treatment failure so that it can be modified prior to a significant progression of the disease.
In addition, MRS is equally sensitive in differentiation of non-enhancing tumor from edema or other causes of T2 prolongation. Presently, MR spectroscopy is being optimized by different research groups in assessing the treatment response of primary brain tumors or metastases. MR spectroscopy can noninvasively differentiate a solitary metastasis and high-grade gliomas, when used alongside with perfusion MR imaging (Law et al., 2002). The study showed that measurement of Cho and mean CBV in the peri-enhancing region can be used as a good marker for differentiating solitary metastases from high grade glioma, where elevated levels of Cho and mean CBV surrounding a peripherally enhancing mass are more likely to be associated with an infiltrating glioma.
Positron Emission Tomography and Molecular Imaging of Brain Tumors
Positron emission tomography is a form of molecular imaging that requires an injection of a radioactive tracer into the blood stream. Radionuclide tracers are prepared using a cyclotron device and a wide variety of molecules can be labeled by this means, including metabolically active substances. Tracers are often characterized by their half-life, which refers to the length of time it takes for the radioactivity of a radioisotope to decrease by a factor of two. Some of the radionuclides have a relatively short half-life and often tend to be used for medical diagnostic purposes because they do not remain radioactive for long following administration and hence result in a relatively low radiation dose. But isotopes with a relatively long half-life have been used in the past for therapeutic applications in medicine too. Table 1.2 shows the half-life of some commonly used radioisotopes.
The radionuclide is generally administered to the patient in the form of a radiopharmaceutical agent or radiotracer. This follows some physiological pathway to accumulate for a short period of time in some specific organ of the body (Table 1.3). A good example is 99mTc-tin colloid which following intravenous injection accumulates mainly in the liver. The radiopharmaceutical emits gamma-rays and the images are obtained by mapping the distribution of an administered radiopharmaceutical within the body. The radiation is emitted from within the patient and subsequently detected in the imaging device, unlike CT which requires an external X-ray source. Specific organ function is determined by the biological behaviour of the radiopharmaceutical. Conventional imaging achieved using a gamma camera (used to image gamma radiation emitted by radioisotopes) is referred to as Planar Imaging. A more recent variant is Single Photon Emission Computed Tomography (SPECT) which produce axial slice imaging through the body. SPECT uses a gamma camera to record images at a series of angles around the patient, in a similar manner to CT acquisitions, with the resultant data being processed using Filtered Back Projection Iterative Reconstruction algorithms. The more advanced version of the molecular imaging is Positron Emission Tomography (PET) that is also an axial projection acquisition based technique. PET exploits the positron annihilation process where two 0.51 MeV back-to-back gamma-rays are produced. When these gamma-rays are detected, their origin will lie on a line joining two of the detectors on the ring of detectors which encircles the patient. More recently, the limited anatomical definition of radionuclide imaging has been addressed to some degree by the development of hybrid imaging techniques in which radionuclide imaging devices are combined with computed tomography in a single imaging system. The resulting images display the functional data obtained from the radionuclide distribution (in color), overlaid on the anatomical information from CT (in grey scale). As the two image data sets are acquired almost simultaneously using the same imaging device, the two data sets can be co-registered very accurately (Fig. 1.2). Not only do these hybrid systems allow abnormalities seen on radionuclide images to be assigned to the precise anatomical structures, they also enable the morphological appearances of disease processes depicted by CT to be assimilated into the interpretation of the findings on radionuclide images. For example, such combined interpretation can aid the distinction of malignant and inflammatory causes of uptake of the positron emitting radiopharmaceutical 18F-fluorodeoxyglucose (FDG). Further advantages of hybrid systems include the use of the CT data to correct radionuclide images for artifacts resulting from attenuation of photons within the body and the ability to incorporate radionuclide image data into CT-based radiotherapy planning systems.
A brain tumor seen in T1w post contrast (a), FLAIR (b) and T2w (c) axial plane. It could either be a primary or a secondary metastasis tumor of the brain. In sagittal plane of T1w post contrast MRI (d) the tumor showed growth from meninges (arrow). In the PET-CT (e, f) using Ga-68 DOTATOC, the tumor showed positive uptake confirming it as a meningioma shown here in sagittal and axial plane. Meningioma has a high uptake of Ga-68 DOTATOC because of high expression of somatostatin-receptors subtype 2. The sagittal slice (e) also shows high uptake in pituitary (arrow head) which is a normal finding as pituitary also has high expression of SSR2
PET is a metabolic imaging technique that is capable of differentiating benign and malignant tumors more accurately. PET has been used extensively in staging of brain tumors as it can produce a visual mapping of biochemical changes caused by the metabolic activity of the brain tumor.
Fluorodeoxyglucose PET (FDG-PET) produces high background intensity in normal gray matter but still has been shown to provide useful information in management of patient with brain tumors. PET can provide information on global location of high activity tumor zone, hence can be used to guide appropriate biopsy sampling. Besides providing information on the metabolic activity, a PET image represents the tumor aggressiveness thereby enhancing the ability to provide a prognostic indication. PET can also help in the differentiation of post-surgery tumor recurrence and radiation necrosis. In this scenario, the aggressive tumor responds with a bright signal because of its high uptake of FDG whereas radiation necrosis might show no uptake. In addition, FDG-PET has been used in differentiating lymphoma from infectious toxoplasmosis in patients with acquired immune deficiency syndrome with almost 100 % accuracy, and is the investigation of choice in that settings (Basu and Alavi, 2009).
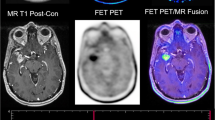
Gallium-68 DOTATOC (DOTA-[Tyr3]-Octreotide) is one specific radiopharmaceutical which is highly sensitive to somatostatin-receptors subtype 2 (SSR2) that is expressed in neuroendocrine tumors and in the brain tumor like meningioma (Fig. 1.2). Other than the FDG and Ga-68 DOTATOC, research has been done in amino acid tracers for PET imaging of brain tumors (Fig. 1.3). The novel tracers which have been employed include positron labeled methionine, thymidine, tyrosine, choline and fluoromisonidazole (Basu and Alavi, 2009). One of the key advantages of these new radiotracers over FDG is a significant reduction in background activity from gray matter which allows the detection of small tumors with high precision (Basu and Alavi, 2009). The emphasis has also being placed on investigating amino acid tracers which are sensitive to recurrent tumor particularly the low-grade ones because they are difficult to be detected. O-(2-[18F]fluoroethyl)-L-tyrosine (FET) is a novel amino acid PET tracer (Fig. 1.3) that has been shown to have better differentiation capabilities for tumor tissue from inflammation. 18F-FET is being investigated for monitoring post therapeutics of Squamous Cell Carcinoma (SCC) because the tumor tissue reaction can now be specifically detected by FET, in which the surrounding inflammatory tissue will not show any uptake (Pauleit et al., 2006).
Patient with a high grade Glioma tumor showing T1w post contrast (a) enhancing margins with the central area of necrosis in the tumor, T2w image (b) confirming the findings. The combined PET-CT using FET tracer (c) shows the tumor clearly with infiltrations to the cerebral cortex and infiltration to the contralateral hemisphere. The bottom row shows the PET image in axial (d), coronal (e) and sagittal (f) plane confirming infiltration to contralateral hemisphere in the coronal plane (arrow)
Hypoxia imaging tracers (such as fluoromisonidazole or more recently EF5) have a promising future in radiotherapy planning and the prediction of treatment response (Basu and Alavi, 2009) because these tracers could play an important role in directing and monitoring targeted hypoxic therapy for tumors with hypoxia. New PET radio-tracers have thus shown a great potential to image important aspects of tumor activity which were only speculative in the past.
Future of Brain Tumor Imaging
Brain tumor imaging has progressed a long way from radiography to the advanced multi-modality imaging of CT, MRI and PET today. In MRI, high relaxivity contrast agents can now provide vital information on tumor vascular supply and high field strength scanners offering better SNR are becoming the norm. In future it remains to be validated how brain tumor diagnostics can be improved with the combination of better contrast agents on high field strength MRI. Another advancement in brain tumor imaging is greater exploitation of multi-modality hybrid imaging, including their coregistration.
Multi-modality imaging is gaining more focus for diagnostic imaging nowadays. This approach might be computationally challenging and expensive but the clinical benefit of it is immensely high. Healthcare providers have shown great interest in using multi-modality information simultaneously for the benefit of patient care. The time in patient journey for diagnostic imaging is very much shortened by using a hybrid multi-modality imaging when compared to using them independently. This is particularly important in trauma and medical emergencies, where an accurate and quick diagnostic technique could aid appropriate management that might be lifesaving. The diagnostic efficiency has also shown to be improved by implementation of such hybrid devices. PET-CT is a very valuable addition to this hybrid imaging technology. It has been used widely for tumor diagnostics as CT could provide simultaneous morphological information that can be coupled with functional information from the PET. PET-MRI is now feasible and has come into clinical practice recently. MR is very good in producing distinctive contrast of soft tissue in the brain and now coupled with functional imaging provided by PET, they have shown promising future prospects. Further advancement in combining PET-MR image acquisition at same time would advance the brain tumor imaging to a new platform of diagnostics.
The newly available CT technique – Multi-Energy CT (MECT), serves on multi-energy x-ray spectrum. It is capable with material characterization of the tissue and is also a promising tool for high resolution anatomical and tumor activity imaging (Mehndiratta et al., 2011). More works need to be done to reveal its real potential in brain tumor assessment.
ASL and VASO imaging are other non-invasive MRI techniques for neurovascular imaging. Both these are being used for stroke and other neurovascular disorders, it will be interesting to know how well it can blend into brain tumor diagnosis and management.
Novel amino acid PET tracers have recently begun to be used for brain tumor diagnosis, together with the development of the exciting PET tracers such as hypoxia imaging tracer, PET will be one of the important imaging tools for brain tumor management in the future.
In conclusion, brain tumor imaging typically requires a high resolution anatomical imaging with computed tomography or magnetic resonance imaging and functional tumor activity with PET. Clinicians rely on both anatomical and functional information to design an appropriate treatment planning for the patient. The future of brain tumor imaging lies in developing optimal image registration and segmentation strategies for the multi-modal imaging as well as novel PET tracers. The development would also be useful for intensity-modulated radiotherapy, and is likely to have important clinical and research applications in radiotherapy planning for patients with brain tumors.
References
Basu S, Alavi A (2009) Molecular imaging (PET) of brain tumors. Neuroimaging Clin N Am 19(4):625–646
Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA (2007) Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 188(2):586–592
Castillo M, Kwock L, Mukherji SK (1996) Clinical applications of proton MR spectroscopy. AJR Am J Roentgenol 17(1):1–15
Colosimo C, Ruscalleda J, Korves M, La Ferla R, Wool C, Pianezzola P, Kirchin MA (2001) Detection of intracranial metastases: a multicenter, intrapatient comparison of gadobenate dimeglumine-enhanced MRI with routinely used contrast agents at equal dosage. Invest Radiol 36(2):72–81
Colosimo C, Knopp MV, Barreau X, Gérardin E, Kirchin MA, Guézénoc F, Lodemann KP (2004) A comparison of Gd-BOPTA and Gd-DOTA for contrast-enhanced MRI of intracranial tumours. Neuroradiology 46(8):655–665
Dean BL, Drayer BP, Bird CR, Flom RA, Hodak JA, Coons SW, Carey RG (1990) Gliomas: classification with MR imaging. Radiology 174(2):411–415
Donahue MJ, Blakeley JO, Zhou J, Pomper MG, Laterra J, van Zijl PCM (2008) Evaluation of human brain tumor heterogeneity using multiple T1-based MRI signal weighting approaches. Magn Reson Med 59(2):336–344
Essig M (2003) Clinical experience with MultiHance in CNS imaging. Eur Radiol 13(Suppl 3):N3–N10
Essig M, Lodemann K-P, Le-Huu M, Brüning R, Kirchin M, Reith W (2006) Intraindividual comparison of gadobenate dimeglumine and gadobutrol for cerebral magnetic resonance perfusion imaging at 1.5 T. Invest Radiol 41(3):256–263
Giesel FL, Mehndiratta A, Risse F, Rius M, Zechmann CM, von Tengg-Kobligk H, Gerigk L, Kauczor HU, Politi M, Essig M, Griffiths PD, Wilkinson ID (2009) Intraindividual comparison between gadopentetate dimeglumine and gadobutrol for magnetic resonance perfusion in normal brain and intracranial tumors at 3 Tesla. Acta Radiol 50(5):521–530
Giesel FL, Mehndiratta A, Essig M (2010) High-relaxivity contrast-enhanced magnetic resonance neuroimaging: a review. Eur Radiol 20(10):2461–2474
Howe FA, Opstad KS (2003) 1H MR spectroscopy of brain tumours and masses. NMR Biomed 16(3):123–131
Howe FA, Barton SJ, Cudlip SA, Stubbs M, Saunders DE, Murphy M, Wilkins P, Opstad KS, Doyle VL, McLean MA, Bell BA, Griffiths JR (2003) Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 49(2):223–232
Kandel EI, Schavinsky YV (1972) Stereotaxic apparatus and operations in Russia in the 19th century. J Neurosurg 37(4):407–411
Kuhn MJ, Picozzi P, Maldjian JA, Schmalfuss IM, Maravilla KR, Bowen BC, Wippold FJ, Runge VM, Knopp MV, Wolansky LJ, Gustafsson L, Essig M, Anzalone N (2007) Evaluation of intraaxial enhancing brain tumors on magnetic resonance imaging: intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for visualization and assessment, and implications for surgical intervention. J Neurosurg 106(4):557–566
Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW (2002) High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 222(3):715–721
Maravilla KR, Maldjian JA, Schmalfuss IM, Kuhn MJ, Bowen BC, Wippold FJ, Runge VM, Knopp MV, Kremer S (2006) Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology 240(2):389–400
Mehndiratta A, Giesel FL (2011) Brain tumor imaging. In: Abujamra AL (ed) Diagnostic techniques and surgical management of brain tumors. InTech, ISBN 978-953-307-589-1
Mehndiratta A, Kapal JM, Prabu A (2011) Iodine mapping in brain tumor imaging using dual-energy computed tomography. Med Hypotheses 76(5):764
Pauleit D, Zimmermann A, Stoffels G, Bauer D, Risse J, Flüss MO, Hamacher K, Coenen HH, Langen KJ (2006) 18F-FET PET compared with 18F-FDG PET and CT in patients with head and neck cancer. J Nucl Med 47(2):256–261
Pooley RA (2005) AAPM/RSNA physics tutorial for residents: fundamental physics of MR imaging. Radiographics 25(4):1087–1099
Rowley HA, Scialfa G, Gao PY, Maldjian JA, Hassell D, Kuhn MJ, Wippold FJ, Gallucci MB, Bowen BC, Schmalfuss IM, Ruscalleda J, Bastianello S, Colosimo C (2008) Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol 29(9):1684–1691
Rumboldt Z, Rowley HA, Steinberg F, Maldjian JA, Ruscalleda J, Gustafsson L, Bastianello S (2009) Multicenter, double-blind, randomized, intra-individual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine in MRI of brain tumors at 3 tesla. J Magn Reson Imaging 29(4):760–767
Shino A, Nakasu S, Matsuda M, Handa J, Morikawa S, Inubushi T (1999) Noninvasive evaluation of the malignant potential of intracranial meningiomas performed using proton magnetic resonance spectroscopy. J Neurosurg 91(6):928–934
Sze G, Johnson C, Kawamura Y, Goldberg SN, Lange R, Friedland RJ, Wolf RJ (1998) Comparison of single- and triple-dose contrast material in the MR screening of brain metastases. AJNR Am J Neuroradiol 19(5):821–828
Usenius JP, Kauppinen RA, Vainio PA, Hernesniemi JA, Vapalahti MP, Paljärvi LA, Soimakallio S (1994) Quantitative metabolite patterns of human brain tumors: detection by 1H NMR spectroscopy in vivo and in vitro. J Comput Assist Tomogr 18(5):705–713
van Zijl PCM, Yadav NN (2011) Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 65(4):927–948
Wen Z, Hu S, Huang F, Wang X, Guo L, Quan X, Wang S, Zhou J (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 51(2):616–622
Zhou J, Blakeley JO, Hua J, Kim M, Laterra J, Pomper MG, Van Zijl PCM (2008) Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 60(4):842–849, Prometheus Books
Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PCM (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17(1):130–134
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Mehndiratta, A., Tee, Y.K., Payne, S.J., Chappell, M.A., Giesel, F.L. (2014). An Introduction to Brain Tumor Imaging. In: Hayat, M. (eds) Tumors of the Central Nervous System, Volume 11. Tumors of the Central Nervous System, vol 11. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7037-9_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-7037-9_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7036-2
Online ISBN: 978-94-007-7037-9
eBook Packages: MedicineMedicine (R0)