Summary
Soil biocrusts are assemblages of cyanobacteria, lichens, and mosses ubiquitous to arid and semi-arid (dryland) systems that offer an array of ecosystem services. Soil crust mosses are taxonomically diverse, account for up to 30 % of crust cover, and offer large contributions to crust biogeochemical functionality, yet remain the least understood component of the community. Because of selective pressures of their growth environment, such species are highly desiccation tolerant, with the ability to withstand the loss of most cellular water for extended periods of time, during which metabolism is suspended. Biocrust mosses can also tolerate larger ranges of temperature, light, and cellular water content than mesic species, yet still remain sensitive to certain aspects of environmental alteration. For one, changes in precipitation regime are likely to heavily influence survival in dryland mosses. Rainfall, occurring as discrete periods of hydration in dryland systems, causes mosses to undergo wet-dry cycles that result in either a positive or a negative carbon balance. Carbon balance can be used as a measure of performance during individual rainfall events, and is a metric for long-term viability. Recent work suggests rainfall event magnitude plays a large role in carbon balance, as does the frequency and seasonality with which events fall. Biocrust mosses are stimulated by elevated CO2, yet may not acclimate photosynthetically to long-term enrichment. Interestingly, elevated CO2 may favor stress tolerance at the expense of growth in biocrust moss, particularly at high temperatures. Finally, despite low annual growth rates, nitrogen appears to place physiological limitations on reproductive biology of biocrust mosses. High levels of nitrogen deposition, however, have been shown to cause toxicity, competitive exclusion by vascular plants, and can reduce cyanosymbioses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Soil biocrusts, also known as cryptobiotic crusts or microbiotic crusts, are assemblages of organisms living amongst soil particles within the top few centimeters of soil and at the soil surface. Crusts are composed primarily of cyanobacteria, lichen, and moss (Rosentreter et al. 2007), and these components exist in various proportions depending on microclimate and disturbance regime of the soil environment. In dryland ecosystems, crusts increase the water holding capacity of soils, reduce erosion (Belnap 2003; Belnap et al. 2006), and influence seedling establishment of grasses and shrubs (Clair and Johansen 1993). Biocrusts also influence elemental cycling in drylands (Housman et al. 2006), as they (a) contain a large proportion of photosynthesizing organisms and supply organic carbon to underlying soil; (b) contain free-living and lichenized cyanobacteria that fix atmospheric nitrogen (N2) into a biologically available form (NH4 +) (Evans and Belnap 1999); and (c) secrete compounds that increase phosphorous availability within soil (Harper and Pendleton 1993). Biocrusts occur in all dryland regions of the world and on every continent, including polar regions, and have been observed on nearly all soil types (Belnap et al. 2001). Crusts are found in the spaces between and under vascular plants, and in some regions where persistence of biocrust communities can be disproportionately favored (hot deserts or cool/cold drylands) can occupy 70 % or more of the living ground cover (Belnap 1995; Fig. 16.1a).
(a) Moss in an intact biocrust from the Colorado Plateau in the Western United States; (b) the common biocrust species Syntrichia caninervis at ~6x magnification in the desiccated state, and (c) in the hydrated state; (d) dry S. caninervis shoots exhibiting normal (left) and reduced (right) pigmentation as a result of rapid wet-dry cycles (Photo credit: Lloyd Stark); and (e) architecture and new growth of an emerging S. caninervis shoot (Photo credit: Lloyd Stark).
Although biocrusts have received significant attention in recent research in dryland ecosystems, bryophytes remain the poorest understood component of the community. In drylands where soil-dwelling mosses can persist, moss can account for 2 % to over 30 % cover in crusts (Thompson et al. 2005), often depending on crust maturity, and are a taxonomically diverse functional group (Brinda et al. 2007). Many common crust moss genera (e.g. Syntrichia, Tortula, Pterogonium, Crossodium, Didymodon) belong to the family Pottiaceae, and are typically slow-growing acrocarps with annual (normally short mosses <0.5 mm in height) as well as perennial (0.5 – several cm in height) life history strategies (Rosentreter et al. 2007).
Evidence suggests that while mosses offer significant contributions to overall biocrust function, they can be sensitive to environmental alterations, from discrete soil disturbance events to direct and subtle aspects of climate change. Moss removal from a biocrust has negative consequences for structure and composition of the crust as well as nutrient cycling in soils below (Reed et al. 2012). Therefore, understanding the physiological determinants of performance and survival of biocrust mosses is important for understanding dryland ecosystem ecology in general. Biocrust mosses possess a suite of adaptations to cope with environmental variability in dryland systems, and the physiological ecology of dryland mosses has received recent research interest, mainly in terms of the response of these organisms to environmental change but also to the ecological role of moss as part of crust communities. This chapter will review the current knowledge of biocrust moss ecophysiology in dryland systems, and will concentrate on water relations, temperature and light tolerance, nutrient status, and responses to elevated CO2 with respect to photosynthetic performance, reproduction, biomass accumulation, and stress tolerance.
II. Desiccation Tolerance, Precipitation Pulses, and Carbon Balance
The growth environment of dryland biocrust mosses is often characterized by extended dry periods interspersed with small precipitation events, resulting in intermittent pulses of resource availability. This, along with high temperatures and exposed soil microclimates results in rapid drying and prolonged periods of desiccation. Because of these factors, there is thought to have been strong evolutionary pressure and selection for a desiccation tolerant strategy among biocrust species (Oliver et al. 2000a, b, 2005; Proctor et al. 2007).
All mosses are poikilohydric organisms where the water content of cells is in equilibrium with the environment and the control of tissue water content is passive. Moss gametophytes are often exposed to a range of environmental water availabilities, and as a consequence must possess the ability to withstand an extreme range of cellular water contents. In drylands, cell water content can often be very low for extended periods. Most, though not all, dryland mosses therefore display some degree of desiccation tolerance. Although cellular water potentials at full turgor are typically between −1.0 and −2.0 MPa, many mosses remain photosynthetically active over water potentials ranging from −0.5 to −10.0 MPa (Dilks and Proctor 1979; Proctor 2008). The limits of tolerance for most mosses are cellular water potentials of −20 to −40 MPa (Proctor and Pence 2002; Oliver et al. 2005; Proctor et al. 2007); the degree of this tolerance depends largely on the growth environments that species are adapted. Mosses with the highest degrees of desiccation tolerance often represent the endpoints of these spectra. While highly desiccation-tolerant mosses occur in dry microclimates (on substrates such as exposed rock, sand, or bark) in all biomes, the diversity and abundance of such species is highest in dryland ecosystems.
Desiccation tolerance represents a strategy to suspend metabolism during dry intervals, and restrict physiological activity to periods of sufficient hydration. During desiccated periods, shoot tissues lose virtually all liquid water and can dry to 5–10 % dry mass and water potentials of −100 MPa in the most desiccation tolerant species (Proctor et al. 2007; Fig. 16.1b). A significant degree of molecular packaging mechanisms involving sugars (Smirnoff 1992) as well as proteins (Buitink et al. 2002; Oliver et al. 2005) are likely involved in the protection of macromolecules in the absence of water as well as maintenance of spatial relationships in cells. Mechanisms of antioxidant production to neutralize reactive oxygen species as well as photoprotection to dissipate excess light (Marschall 2004) also appear to be important as cells transition to the desiccated state. Because it influences the degree to which tissues are preserved and energy is invested in preservation, the rate at which tissues dry is an important determinant of survival in the desiccated state as well as recovery potential for many species, and in general drying speed is inversely correlated with future performance (Oliver et al. 2000a, b, 2005). When compared to shorter dry times, numerous measures of regeneration potential such as cell ultrastructure, pigmentation, electrolyte efflux, and photosynthetic performance have all been shown to be higher in mosses exposed to longer drying periods (Schonbeck and Bewley 1981a, b; Oliver and Bewley 1984; reviewed in Oliver et al. 2000a, b). This relationship is due to the ability of cells to initiate and complete preservation procedures for macromolecules and organelles prior to entering the desiccated state. In general, the more preserved tissues are while desiccated, the higher their recovery potential is given sufficient hydration.
Among mosses, dryland species display the highest levels of desiccation tolerance recorded, both in terms of the length of time a shoot can remain desiccated before full physiological recovery as well as the lowest relative water content (RWC) from which tissues can recover. Dryland biocrust species can survive in the desiccated state (e.g., 5–10 % dry mass in Syntrichia) for months and recover full photosynthetic function within <24 h (Tucker et al. 1975; Oliver et al. 1993; Proctor and Pence 2002). In the extreme, mosses have been observed to remain desiccated for >100 days in the Mojave Desert (Stark 2005). In the desiccated state, shoots can withstand significantly more variability in temperature and light levels, including those that can damage tissues when hydrated. Indeed, because of the stressful environments commonly occupied by biocrusts, many dryland crust mosses may actually require periods of desiccation for survival under temperature and light extremes.
From the perspective of a moss, when rainfall does occur in dryland systems it occurs as a pulse between two desiccation periods. This results in a wet-dry cycle and a characteristic response with periods of net carbon loss (where carbon loss through respiration is greater than carbon gain through photosynthetic fixation) and net carbon gain (when fixation outweighs respiration) (Fig. 16.2). Respiration recovers more rapidly than photosynthesis (due to a lag time in reinstatement of the Calvin cycle), thus the onset of hydration is characterized by a period of net carbon loss as respiratory energy is used to reinstate metabolism, repair membranes, and reconfigure and reorganize cellular components (Hinshiri and Proctor 1971; Tuba et al. 1994; Proctor et al. 2007). Because the photosynthetic apparatus remains intact during desiccation, recovery of photosynthetic function in biocrust mosses is often rapid (only slightly lagging behind respiration), and net carbon fixation can be reached in 10–30 min following hydration in some desiccation tolerant species (e.g. Tortula (Syntrichia) spp.; Bewley 1979; Tuba et al. 1996; Proctor and Smirnoff 2000; Reed et al. 2012; Li et al. 2010). Once photosynthesis outpaces respiration, a phase of net carbon gain occurs, the length of which is determined by the duration of tissue hydration. As tissues dry, photosynthetic rates diminish, and as energy is used to repackage cellular contents for another bout of desiccation, the cycle often ends with another brief phase of net carbon loss (Mishler and Oliver 2009; Reed et al. 2012). Depending on the relative magnitude of the periods of carbon gain and loss over the course of the wet-dry cycle, dryland mosses exhibit either an overall carbon balance that is positive or negative. The carbon balance of biocrust mosses during discrete events, when compounded over long periods, is a strong determinant of performance, growth, and long-term survival. Interestingly, this phenomenon appears to apply to mesic mosses as well and has been shown to be the case in tropical bryophytes exposed to discrete hydration events (see Chap. 15).
Characteristic pattern of net carbon fixation in desert moss over the course of a wet-dry cycle (of length D) initiated by a rainfall event: an initial period of net carbon loss when respiratory costs are high (A) followed by a period of net carbon gains when tissues are hydrated and photosynthesis is higher than respiration (B; length varies as a function of D) and a period of subsequent carbon loss as photosynthesis ceases and tissues prepare for desiccation again (C). Following an individual rainfall event, carbon balance is equivalent to gains from net carbon fixation (area under B) minus carbon loss from respiration (areas under A + C) (Adapted from Coe et al. 2012a).
III. Water Relations
In spite of passive water regulation over the gametophyte surface, cell water relations of mosses are nearly identically to that of other dryland plants with respect to physiological parameters such as water potential (ψw) and its relationship to RWC. As is true in mosses from other environments, the RWC of dryland biocrust species is a strong determinant of photosynthetic performance (also see Chap. 5). Maximum rates of net carbon fixation are typically reached between 40 and 70 % RWC (Tuba et al. 1996; K.K. Coe, unpublished) as photosynthesis is inhibited at higher water contents by limited CO2 diffusion and at lower water contents by low intracellular ψw. However, many dryland mosses possess a greater range of suitable hydration levels for photosynthesis and can withstand cell water potentials (when not physiologically active) well below those of species from mesic environments. Some dryland species (e.g. S. ruralis) can withstand cellular drying to a ψw of <−100 MPa when desiccated and photosynthesis and respiration have been detected in tissues with ψw as low as −10 and −20 MPa, respectively (Dilks and Proctor 1979). Sustained physiological functioning at such water potentials possibly owes to higher cell wall thickness relative to lumen, high cell wall extensibility, and/or low RWC at full turgor (Proctor et al. 1998; Proctor and Tuba 2002).
Dryland mosses possess a suite of morphological and physiological adaptations to maximize photosynthetic performance under dry conditions as well as to take advantage of water when it does become available. At the macro-scale, biocrust species almost always grow within the laminar boundary layer directly above the soil surface, thus minimizing convective water loss. In dryland mosses, cuticular waxes deposited on leaf surfaces play a role in restricting water loss (Xu et al. 2009a, b), as do hair points at the ends of leaves (e.g. Grimmia, Syntrichia) that serve to extend the height of the boundary layer and increase albedo (Valko 2003; Bowker et al. 2010). Shoots of biocrust mosses are also typically short and densely packed in a characteristic clonal cushion or carpet habit, which behaves as a smooth object on the soil surface when exposed to wind (Proctor 2000), and maximizes extracellular water storage between adjacent shoots in capillary spaces.
Capillary water is an essential component of the physiological ecology of dryland mosses for several reasons. First, although some water does move through cells and cell walls, most water conduction is external and water held or moving within capillary spaces between shoots, in sheathing leaf bases and in rhizoid tomenta acts as a direct source of water for the plant. Second, capillary water can be a major component of the water associated with a shoot, can outweigh symplastic water by a factor of five or more (Proctor 2008), and can thus act as a primary source of water storage for moss. Indeed, water stored in this manner is directly related to the length of time shoots remain wet and photosynthetically active and can often assist dryland moss species in bridging gaps in water availability by extending the hydrated period provided by a rain event. Finally, capillary water content can vary widely without influencing the water status of plant cells in a turgid state, but when it is exhausted, cells dry rapidly. One hypothesized function of capillary water in Tortula ruralis is to maintain cells at full turgor until a cytological switch initiates rapid desiccation, whereby cells spend little time at intermediate water contents thought to impose damage (Gaff 1997; Proctor 2000). This hypothesis has not been fully explored in different species, however, and may conflict with the widely demonstrated relationship between drying speed and recovery potential (see section “Desiccation tolerance, precipitation pulses, and carbon balance” of this chapter).
Mosses must reconcile maximizing water conduction and storage and the need for gas exchange between the atmosphere and the interior of the leaf for photosynthesis. When shoots possess external water, exchange of CO2 must occur through films of water where diffusion resistance for gas is four orders of magnitude higher than through air. Typical adaptations to minimize such trade-offs include specialized leaves that perform either water conduction or gas exchange or the use of the outer surfaces of stem-sheathing leaves for gas exchange while employing inner surfaces to provide a capillary channel around the stem (Proctor 2008). Some additional adaptations are present in dryland genera and often involve the division of water conducting and gas exchange surfaces in the same structure or section of leaf on microscopic scales. For example, the genus Syntrichia (Tortula) is characterized by specialized papillae or mammillae extruding from the cellular surface (Fig. 16.3) that serve two simultaneous functions: first, to create a continuous network of interstices for water adhesion and transport in microcapillary spaces between papillae, and, second, to allow sustained photosynthesis at high water contents in the tips of papillae (also shown to contain proportionally higher chloroplast densities) even when the rest of cells are covered with a sheet of water that impedes CO2 diffusion (Tucker et al. 1975; Proctor 1979; Xu et al. 2009a, b).
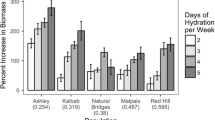
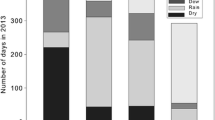
Irrespective of adaptations to limit water loss in dryland mosses, overall environmental water availability (primarily through alterations in precipitation regime) is likely to influence performance and survival. This is because such alterations result in changes in carbon balance following rainfall events, and biocrust mosses are dependent on the maintenance of positive carbon balances from discrete rainfall events for biomass accumulation and long-term viability. Much of the recent research on this topic has been conducted in western North America, where climate models predict changes in mean annual rainfall as well as alterations in intra-annual precipitation parameters including individual event magnitude, timing (frequency) at which events fall, and the time of year precipitation occurs (Lioubimtseva 2004; Meehl et al. 2007; Seager et al. 2010). Rainfall event magnitude is one of the largest determinants of carbon balance in the common biocrust species Syntrichia caninervis, because increased rainfall amounts result in shoots that remain hydrated (thus remain in the phase of net carbon fixation; Fig. 16.2) longer, and small events result in limited gains from carbon fixation that cannot compensate for respiratory costs during the event (Barker et al. 2005; Coe et al. 2012a; Reed et al. 2012). Recovery of the cell cycle as well as reassembly of cytoskeletal elements can take >24 h in Syntrichia, thus brief periods of hydration may not be sufficient for full recovery of physiological function. Further, several studies indicate a rainfall event size threshold for carbon balance in dryland mosses of 2–3 mm, below which moss will, on average, enter carbon deficit (Fig. 16.1d; Barker et al. 2005; Stark 2005; Coe et al. 2012a). Given that >70 % of rainfall events in drylands are <5 mm effective size (Sala and Lauenroth 1982; Loik et al. 2004; Reynolds et al. 2004) and within that, the majority are less than half of that (Huxman et al. 2004), it is probable that small carbon deficits are quite common and dryland mosses are reliant on the presence of larger events for net carbon gain. The frequency with which events fall also influences carbon balance, and Coe et al. (2012a) suggest that increasing the dry interval between events reduces carbon balance during subsequent events. This likely results from increased cost of recovery during hydration following periods of increased desiccation intensity (Hinshiri and Proctor 1971; Dilks and Proctor 1974; Proctor and Pence 2002; Proctor 2003).
Most dryland biocrust species depend on precipitation during the cooler months of the year for annual growth because ambient temperature and humidity permit water from rainfall events to remain available for sustained periods. During the warmer months, meteorological conditions restrict water availability for biocrust species, and mosses appear to exhibit changes in physiological state over the course of the year that influence response to rainfall. For example, when measured under identical laboratory conditions, mosses collected in the winter display higher responsiveness to rainfall and higher average carbon balances when given same event size that caused significantly smaller carbon balances (and sometimes loss) in mosses collected in the summer (Coe et al. 2012a). Several climate models for North American drylands suggest winter rainfall will be reduced, while summer will exhibit more frequent small events and/or an increase in monsoonal systems (Castro et al. 2010; Meehl et al. 2007). The consequences of such changes could be severe, and it has been suggested that (a) more frequent <2 mm events during the summer may cause rapid moss decline and reduced nitrogen availability to crust organisms (Reed et al. 2012) and (b) an increase in monsoonal activity could have negative consequences for sex expression and associations with nitrogen fixing cyanobacteria in Syntrichia (Stark et al. 2011a, b). Finally, reductions in winter rainfall will be detrimental to many biocrust mosses that rely on cumulative carbon gains during these times of year to compensate for carbon losses during warmer, unfavorable periods.
IV. Temperature Relations
In dryland systems, air temperatures have wide diurnal and annual fluctuations. Biocrust mosses grow in close proximity to the soil surface, and shoot temperatures can exceed air temperature by 19 °C or more (Hearnshaw and Proctor 1982). Under these conditions, selective pressures to tolerate such extremes are high, and as a consequence, the range of temperatures under which dryland biocrust mosses can survive greatly exceeds that of species from other biomes. Survival in the desiccated state in these species has been noted in temperatures ranging from <0 °C (for months) up to 100 °C (for minutes) (Hearnshaw and Proctor 1982) and dry biocrust mosses on sun-exposed substrates can frequently reach tissue temperatures >60 °C (Proctor 2008). The ability to withstand high temperatures in the desiccated state in particular contributes to the ability of dryland mosses to colonize and persist in unfavorable microclimates from which other less-tolerant species may be excluded (Kidron et al. 2000). Most biocrust species have perennial gametophytes, thus viability while desiccated enables shoots to persist throughout the entire year and bridge gaps in hydration occurring during the warmer months where growth cannot occur.
Temperature tolerance while hydrated in biocrust moss taxa, however, is similar to the range of many other bryophytes. Hydrated shoots are far less tolerant of temperature extremes, and tissue damage occurs at both the high and low ends of the temperature spectrum. When shoots are moist, tissue injury generally begins to occur when temperatures exceed 40 °C (Larcher 2003), with lethal high temperatures between 42 and 51 °C, depending on species and exposure time (Meyer and Santarius 1998; Proctor and Pence 2002). Less is known about responses to cold temperatures and although many dryland biocrust species survive and actively photosynthesize and grow throughout the winter, freezing (<−10 °C) of hydrated shoots may cause irreversible photoinhibition if repair mechanisms are not present (Lovelock et al. 1995). Production of isoprene (2-methyl-1, 3 butadiene) under conditions of temperature stress has been shown to have protective functions in some mosses that experience wide temperature fluctuations in nature (Hanson et al. 1999). Although thermoprotection via isoprene production has not yet been demonstrated in desert mosses, evidence for the evolution of its function in several early land plant lineages (Sharkey and Yeh 2001; Jobson and Qiu 2010) suggests it is worth considering its potential presence in this functional group.
Temperature exerts strong control on net carbon fixation in dryland species because of (a) biochemical limitations such as compromised membrane stability and enzyme denaturation, (b) increased respiration rates, and (c) reductions in environmental water availability due to increased rates of evaporation. Optimal temperatures for photosynthesis occur between 10 and 20 °C (Furness and Grime 1982; Alpert and Oechel 1987; K. K. Coe unpublished), and suppression of photosynthesis has been observed to occur at temperatures between 25 and 35 °C, depending on geographic locality and crust moss species (Grote et al. 2010; Coe et al. 2012b). At these temperatures, declines have been observed in net photosynthesis (Grote et al. 2010), carboxylation capacity, maximum rates of electron transport through photosynthetic membranes (Coe et al. 2012b), and efficiency of conversion of light energy to chemical energy (Hamerlynck et al. 2002; Coe et al. 2012b). Declines in photosynthetic performance are likely due to induced structural deficiencies such as enzyme denaturation, damage to chloroplast membranes and damage to the photosynthetic pigment apparatus (Larcher 2003), changes in membrane permeability associated with high heat while hydrated (Meyer and Santarius 1998; Liu et al. 2003), and/or offsets in the optimal temperature for photosynthesis as compared to respiration (Grote et al. 2010).
Because carbon fixation in dryland mosses is constrained at high temperatures, limited energy is available for physiological functioning, growth, stress tolerance, and reproduction. As a consequence, mosses growing in environments characterized by thermal stress are faced with a corresponding suite of energetic trade-offs. Male and female shoots in many biocrust moss taxa differ by an order of magnitude in energetic allocation to reproductive structures (Lackner 1939; Paolillo 1979; Bowker et al. 2000); based on dry biomass, antheridia cost approximately six times as much as archegonia to produce (Stark et al. 2000). Therefore, male shoots are constrained by the cost of their reproductive structures, and in stressful conditions, are forced to reduce proportional carbon allocation to growth or stress tolerance. Females, on the other hand, can allocate proportionally more carbon to growth and stress tolerance while continuing to produce reproductive structures. To illustrate, leaf growth and regeneration in female shoots occurred twice as fast and was more complete than in males exposed to incremental increases in temperature from 25 to 45 °C for 60 min (Stark and McLetchie 2006). These differences cause overall differences in stress tolerance abilities in male and female gametophytes, and have resulted in two common patterns in the field. First, dryland mosses exhibit sex ratios in nature that differ substantially from 1:1. Ratios are almost always female biased, with male shoots occurring <30 % of the time (or as low as 0 %) at the population level (Stark and McLetchie 2006). Such female-skewed ratios are commonly observed in Bryum (Stark et al. 2010), Didymodon (Ochyra and Zander 2002), Syntrichia (Mishler and Oliver 1991; Stark et al. 2005), and in an array of biocrust species from the Mojave Desert in North America (Brinda et al. 2007). Such patterns are directly related to the inability of males to grow and produce reproductive parts in environments characterized by thermal stress (Stark and McLetchie 2006). Second, dryland mosses exhibit spatial segregation of the sexes (SSS) based on gradients of environmental stress, where females are more common in thermally stressed environments such as plant interspaces, and often males are restricted to shrub understories (Bowker et al. 2000; Stark et al. 2005, 2010).
When female shoots do produce sporophytes (though it is very uncommon in dryland species due to SSS and low water availability for sperm transfer to archegonia), the cost of production and maintenance of these structures is much higher than production of archegonia alone. Abortion of sporophytes is an extremely common occurrence in dryland biocrust taxa for two reasons. First, the energetic cost of maintaining a sporophyte (due to investment of structural materials as well as maintenance of cellular machinery) is high, and places additional stress on the female gametophyte (Stark et al. 2000; Stark 2001). Second, sporophytes are less tolerant of thermal stress than gametophytes (illustrated in Microbryum heat shock experiments; McLetchie 2006). An understanding of the steps leading to sporophyte abortion is confounded by the fact that the sporophytes are connected to and dependent on gametophytes, making mechanistic inferences into the process and reasons for abortion difficult. Nonetheless, female shoots appear to be more stress tolerant and possess higher growth rates than males under conditions of thermal stress, unless they produce sporophytes.
In dryland systems, thermal stress, skewed sex ratios, and SSS result in rates of sexual reproduction that are low to absent (Bowker et al. 2000). Although high surface temperatures and large variation in temperatures impose significant amounts of stress on carbon acquisition and physiological functioning, correlates of temperature (such as water and nutrient availability or light levels) along environmental gradients can play a large role in biocrust moss physiological ecology and reproductive biology. For example, water availability is shown to correlate with temperature along a canopy – interspace gradient (Stark et al. 2010), and has been shown to influence sex expression in S. caninervis due to males’ inability to tolerate energy loss associated with repeated wet-dry cycles in plant interspace regions (Benassi et al. 2011).
V. Response to Variation in Light
Bryophytes as a group have high variation in light responses as well as seasonal and plastic variability (Proctor 2000). Dryland crust mosses may represent some of the extremes in plasticity of response as they typically occur in plant interspaces that experience high light conditions (PAR > 1,000 μmol m−2 s1), yet also can occupy habitats directly under low desert shrubs. When biocrust mosses are desiccated (and not physiologically active), they display higher tolerance of high light than wet shoots (Seel et al. 1992). Mosses that can persist in areas where they are exposed to extreme conditions (e.g. high irradiance plant interspaces) often, but not always, do so because they can survive in the desiccated state when the growth environment is extreme. Biocrust species are not always dry under conditions of high irradiance though, particularly following short rainstorms, thus still must be able to perform photosynthetically in and tolerate high light conditions while hydrated.
Most mosses fall into the category of shade plants based on their photosynthetic physiology (Valanne 1984). Their photosynthetic apparatuses saturate at low irradiances, and express low chlorophyll a:b ratios (Martin and Churchill 1982; Marschall 2004). Some biocrust taxa (e.g. Bryum) fall into this category and are often restricted to crusts under significant plant cover. Such mosses growing in lower light environments such as under shrubs exhibit higher photosynthetic rates at lower irradiances than those growing in exposed environments (Alpert and Oechel 1987). Further, even in biocrust taxa, photosynthetic activity typically occurs under <20 % full sun following rainfall events where cloud cover reduces PAR to 50–250 μmol m−2 s−1 (Marschall 2004). Some of the more common species (e.g. Syntrichia ruralis, S. caninervis, Rhacometrium spp.), however, saturate at higher irradiance levels. In open sun environments, S. ruralis exhibits 95 % saturation at PAR levels of 832–935 μmol m−2 s−1 and full saturation of S. ruralis (Proctor 2008) as well as S. caninervis (K. K. Coe unpublished) occur at PAR levels >1,000 μmol m−2 s−1.
Adaptations to protect tissues from excess light energy that are present in dryland biocrust mosses include photoprotection as well as energy dissipation mechanisms, though there is still some debate as to which mechanism is more important in these species (see Marschall 2004). Photoprotection by xanthophyll cycle carotenoids to scavenge free radicals appears to be important in both dry and wet tissues (Hamerlynck et al. 2002; Marschall 2004). Energy dissipation via non-photochemical quenching (NPQ) has been estimated using chlorophyll fluorescence techniques, and appears to also play an important role in response to high light. When exposed to irradiances >1,000 μmol m−2 s−1, S. ruralis displays NPQ levels that exceed that of more mesic species (Proctor 2000), and sun-exposed desiccation tolerant species tend to display NPQ values >2 times higher than more mesic mosses (Proctor and Smirnoff 2000; Hamerlynck et al. 2002; Proctor and Smirnoff 2011). However, NPQ levels diminish rapidly following several minutes in the dark and appear to be suppressed by violaxanthin inhibitors (Proctor and Smirnoff 2000). The current body of work (also see Chap. 7) suggests that photoprotection in biocrust mosses include strategies to dissipate excess energy as heat and regulation of xanthophyll-mediated protection appears to act as a control point that governs overall responses.
VI. Response to Elevated CO2
For all photosynthetic organisms, the concentration of CO2 at sites of carboxylation and, hence, responses to changes in CO2 concentration, depends upon the atmospheric CO2 concentration and diffusion rates to the point of carboxylation. Mosses differ from more commonly studied higher plants in that the diffusional pathway does not include stomata and is very sensitive to the saturation level of tissues in the diffusion path. However, biocrust mosses are similar to higher plants in that they are both responsive to both short-term and long-term elevated CO2 treatments, and responses have been studied in experiments that include laboratory fumigation treatments in chambers, open-top chambers in the field, and free air CO2 enrichment (FACE) rings.
Biocrust mosses display CO2 compensation points (the CO2 concentration at which net carbon assimilation becomes positive) that fall within the typical range for C3 plants (Dilks 1976), and short-term (<1 year) exposure to elevated CO2 can result in a stimulation of CO2 uptake by 30 % or more (Tuba et al. 1998) because of increased substrate for photosynthesis. Assimilation rates in S. ruralis were shown to increase by 30–35 % after initial exposure and remain significantly (~20 %) higher than ambient-grown mosses after growth for 5 months in a CO2 enriched environment (700 ppm; Tuba et al. 1998), yet starch and sugar content remain unchanged compared to ambient-grown mosses after this length of time (Csintalan et al. 1997). This suggests that photosynthesis is stimulated in the short term, that photosynthetic acclimation may not occur within the first year of fumigation, and that additional assimilated carbon is immediately used for energy rather than stored.
Following longer term (>1 year) exposure to elevated CO2, carbon content of shoots is altered in biocrust moss, and this change appears to influence physiological trade-offs among allocation to growth, sex expression, and stress tolerance. Research on S. caninervis collected from the Nevada FACE facility after 10 years exposure to CO2 enrichment has shown that increased photosynthetic efficiency results in increased percent carbon per unit mass of shoots (Brinda et al. 2011; Coe et al. 2012b), but it is also appears that this does not always translate into increased shoot growth, and shoot growth in elevated CO2 can even be significantly shorter compared to ambient-grown shoots if they express sex (Brinda et al. 2011). This may be because dryland biocrust species such as S. caninervis are slow growing stress tolerant species where growth is often sacrificed for other physiological processes, thus they may not respond to CO2 enrichment with a marked increase in structural biomass, and fixed carbon may be allocated to physiological functions or energetic requirements other than growth. Further, in arid systems, larger plants may even be at a disadvantage due to higher rates of moisture loss.
It is currently unclear if biocrust mosses will acclimate photosynthetically to long-term CO2 enrichment. Several studies have suggested that photosynthesis is downregulated after long-term exposure, and that reductions in Rubisco may occur (Long et al. 2004). However, Coe et al. (2012b) demonstrate that mosses after 10 years of CO2 enrichment display higher photosynthetic rates at field growth concentrations and no reductions in either supply of CO2 to carboxylation sites or rates of RuBP regeneration. The results from the Coe et al. study suggest that, because bryophytes possess neither the capacity to alter CO2 supply via stomatal conductance nor the limitations imposed by reduced sink strength in phloem transport, photosynthetic performance remains unaltered in the long term.
Carbon allocation patterns do appear to change after long-term elevated CO2 exposure, and may influence the tolerance of environmental stress and sex expression. Desiccation tolerance appears to be enhanced in biocrust moss grown in elevated CO2 and likely mechanisms include: (a) increased protection of cellular components with sugars and starch while in the desiccated state; and/or (b) increased regeneration potential of protonema following a desiccation event (Brinda et al. 2011) due to allocation of stored energy reserves to repair or to cellular processes that work to mitigate damage due to desiccation.
Growth in elevated CO2 appears to influence sex expression in biocrust species. Brinda et al. (2011) found that compared to shoots grown in ambient conditions, S. caninervis exposed to 10 years of CO2 enrichment displayed accelerated sexual maturation, and sex expression in shoots was twice as likely.
The thermotolerance of photosynthesis in biocrust mosses appears to be enhanced by long-term exposure to elevated CO2, and Syntrichia grown in elevated CO2 and exposed to high (35–40 °C) temperatures exhibited increased CO2 assimilation (Hearnshaw and Proctor 1982), increased conversion efficiency of light energy into chemical energy in the photosynthetic light reactions, increased electron transport rates during photosynthesis, and increased availability of CO2 at sites of carboxylation as compared to shoots grown at ambient CO2 (Coe et al. 2012b). Photosynthetic thermotolerance can be enhanced by a number of means, and two commonly cited mechanisms are enhanced membrane stability at high temperatures and increased Rubisco activase activity under stress (Sharkey et al. 2001; Sharkey and Schrader 2006). Either or both of these possibilities could account for elevated CO2-induced thermotolerance in desert mosses. Based on estimates of electron transport efficiency and diffusion of CO2 through photosynthetic membranes, Coe et al. (2012b) suggest enhanced membrane stability is the most parsimonious explanation for thermotolerance in S. caninervis exposed to elevated CO2 for 10 years. The role of Rubisco activase, however, in thermotolerance of biocrust mosses has received very little attention, yet could be equally important in protecting photosynthesis under high heat conditions. It is probable, based on work in other dryland plants (Sharkey et al. 2001) that increased amounts of Rubisco activase as a function of elevated CO2 exposure could lead to less diminished photosynthetic rates under high temperatures. In spite of multiple possible mechanisms, elevated CO2-induced photosynthetic thermotolerance is particularly important for biocrust mosses as shoots are exposed to a wide range of temperatures in dryland systems and are likely to experience increasingly heightened extremes in temperature in the future (Meehl et al. 2007).
In sum, stress tolerance is often favored at the expense of growth for dryland biocrust mosses, and elevated CO2 appears to accentuate this trade-off. Carbon supplementation influences allocation to growth, stress tolerance, and sex expression in many species, and excess carbon from a more efficient photosynthetic process is preferentially allocated towards processes such as thermotolerance of photosynthesis and desiccation tolerance of shoots, although productivity and biomass may not change (or even decline) under CO2 enrichment. This suggests that although growth rates may become even slower in these already slow-growing plants, biocrust mosses may be among the plants that exhibit sustained persistence in a CO2-enriched future atmosphere in dryland systems due to enhanced performance under environmental stress.
VII. Nutrient Relations
Second only to water, nitrogen often strongly limits primary production in arid regions (Vitousek and Howarth 1991; Smith et al. 1997). Mosses (including biocrust species) rely heavily on nutrient uptake from atmospheric deposition compared to uptake from soil. Therefore, they are often buffered from belowground nutrient limitation and increased nitrogen deposition from atmospheric sources is likely to influence productivity of dryland mosses and their relative abundance in crusts. Because biocrusts influence cycling of trace elements and are important in regional and global budgets (Zaady et al. 2000), such effects may extend to influence ecosystem biogeochemistry.
Nitrogen appears to be a limiting factor for certain aspects of reproductive biology in crust mosses. Sex expression in S. caninervis has been shown to be stimulated following 4 years fertilization with 10 kg N ha−1 year−1 and then suppressed under a higher treatment of 40 kg N ha−1 year−1 (Stark et al. 2011a, b) suggesting that reproduction is nitrogen limited, but excess levels can have detrimental effects. In this same study, productivity and regeneration vigor were unaffected by the high nitrogen treatment, suggesting some growth parameters were decoupled from environmental nitrogen levels. At larger spatial scales, increased nitrogen to mosses appears to alter certain ecological dynamics due to interactions with other members of dryland communities. In general, exposure to supplemental nitrogen has a negative fertilization effect on mosses due to tissue toxicity or competitive exclusion by vascular plants (van der Wal et al. 2005), and the latter effect has been partially implicated in facilitation of Cheatgrass (Bromus tectorum) invasions in western North America (Schwinning et al. 2005). Finally, there may be variation in levels of cyanosymbiosis in dryland biocrust species (with the cyanobacterial genera Microcoleus and/or Nostoc) under changes in nitrogen availability. Changes in levels of such associations are likely to influence nitrogen fixation rates and soil fertility on ecosystem scales.
It is important to point out that while nitrogen availability plays a role in certain ecophysiological responses of biocrust moss, water availability overwhelms these effects under most circumstances. Experiments that have altered water availability and nitrogen levels simultaneously (e.g. Stark et al. 2011a, b, and ongoing work in the Mojave Desert) show that productivity and sex expression are influenced to a greater degree by water than nitrogen. Additionally, compared to hydrated controls, intermittent hydration periods resulting in repeated wet-dry cycles reduces nitrogen uptake potential in crust moss (Bates 1997), suggesting that water relations and desiccation tolerance at the shoot level also influence response to environmental nitrogen levels.
Though nitrogen may be the most limiting nutrient for biocrust mosses, other elements such as phosphorus, potassium, and trace metals (Mg, Na, Ca, and Mo) appear to place constraints on growth and development (Belnap et al. 2001; Bowker et al. 2005). Atmospheric dust inputs significantly increase deposition of all of these bio-essential nutrients in Western North America (Belnap et al. 2001; Reynolds et al. 2001) and consequently enrich biocrusts and underlying soils (Belnap 2003). Mosses increase the surface roughness of biocrusts, and enhance their capacity to capture dust (Belnap 2003; Fig. 16.4). Augmented dust inputs due to moss presence thus may serve to (a) enrich moss through direct uptake of deposited nutrients onto shoots, and (b) enrich crusts and soil from throughfall and enhanced nutrient turnover.
VIII. Distributions and Ecological Roles of Biocrust Moss in a Future Climate
Biocrust mosses influence crust structure and function through their effects on surface texture and nutrient cycling. Climate change and physiology are likely to interact to affect these ecological roles of mosses in dryland systems. Mosses control carbon flux into crusts and soil through CO2 fixation, and photosynthetic rates are influenced primarily by water availability, but also by changes in CO2 concentration and temperature. Carbon cycling in crusts will probably be influenced on shorter (1–5 year) timescales by alterations in the magnitude and timing of precipitation, and on longer (>5 year) timescales by concomitant increases in average temperatures and atmospheric CO2. Nitrogen cycling in dryland crusts and soils is likely to be influenced by foliar uptake by moss shoots and the degree of cyanosymbiosis present, both of which will be influenced by future atmospheric deposition rates. Yet perhaps more importantly, the presence of moss influences microclimate biogeochemistry, and has been shown to control belowground nitrogen availability and cycling within crusts (Reed et al. 2012). Changes in biomass and moss percent cover due to alterations in intra-annual precipitation parameters may therefore exert more control on nitrogen cycling in aridlands compared to shoot-level physiological changes alone.
Distributions of dryland mosses are likely to be altered under future climate scenarios as well. Increases in average surface temperatures and in the frequency of extreme temperature events will place restrictions on suitable microhabitats of mosses within biocrusts and may exacerbate limited sexual reproduction due to spatial separation of the sexes. Rates of net carbon fixation and growth are likely to be suppressed under increased temperatures, limiting distributions in sun-exposed areas, yet elevated CO2 may offset these limitations by enhancing stress tolerance. Finally, sun-exposed microhabitats that intensify water stress and reduce effective rainfall event size due to evaporation are likely to show reduced moss growth and establishment of young shoots, and may show some degree of replacement over time by habitats under shrubs where water and nutrient availabilities are higher.
IX. Conclusions
Evolutionary pressure in desert ecosystems favors stress tolerance at the expense of growth for many organisms, and many of the adaptations present in biocrust moss and ecophysiological responses to environmental alterations reflect this trade-off. Owing to their growth environment, dryland biocrust mosses are generally small, clonally growing species with a high level of desiccation tolerance. This strategy ensures that tissues can be protected from temperature and light extremes during extended dry periods, and restricts periods of physiological function, photosynthesis, and growth to intermittent periods of hydration. Changes in intra-annual precipitation patterns will likely heavily influence performance of dryland mosses because of changes in carbon balance, which when compounded over long time scales has a large influence on survival and presence within crusts. Elevated CO2 is likely to interact with changes in temperature and rainfall to influence future performance in biocrust mosses by favoring stress tolerance and some aspects of sexual reproduction at the expense of growth.
As biocrusts are important in dryland regions for nutrient cycling and soil stability, and mosses play a key role in crust structure and function, the current body of knowledge contributing to our understanding of the physiological ecology of biocrust moss is essential in future ecosystem process modeling in dryland systems. We currently have little knowledge on cyanosymbiosis and its relation to nutrient status in dryland biocrust species, and only have limited information on the influences of CO2 on time scales longer than 1 year, thus future work should focus on these aspects of crust moss ecophysiology.
Abbreviations
- RWC:
-
relative water content;
- PAR:
-
photosynthetically active radiation;
- NPQ:
-
non-photochemical quenching;
- FACE:
-
Free-Air CO2 Enrichment;
- SSS:
-
Spatial segregation of the sexes
References
Alpert P, Oechel W (1987) Comparative patterns of net photosynthesis in an assemblage of mosses with contrasting microdistributions. Am J Bot 74:1787–1796
Barker D, Stark L, Zimpfer J, Mcletchie N, Smith S (2005) Evidence of drought-induced stress on biotic crust moss in the Mojave Desert. Plant Cell Environ 28(7):939–947
Bates J (1997) Effects of intermittent desiccation on nutrient economy and growth of two ecologically contrasted mosses. Ann Bot 79:299–309
Belnap J (1995) Surface disturbances: their role in accelerating desertification. Environ Monit Assess 37:39–57
Belnap J (2003) The world at your feet: desert biological soil crusts. Front Ecol Environ 1(4):181–189
Belnap J, Budel B, Lange OL (2001) Biological soil crusts: characteristics and distribution. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function and management, vol 150, Ecological studies. Springer, Berlin/Heidelberg, pp 3–30
Belnap J, Phillips S, Troxler T (2006) Soil lichen and moss cover and species richness can be highly dynamic: the effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Appl Soil Ecol 32(1):63–76
Benassi M, Stark L, Brinda J, Mcletchie D, Bonine M, Mishler B (2011) Plant size, sex expression and sexual reproduction along an elevation gradient in a desert moss. The Bryologist 114(2):277–288
Bewley JD (1979) Physiological aspects of desiccation tolerance. Annu Rev Plant Phys 30:195–238
Bowker M, Stark L, Mcletchie DN, Mishler B (2000) Sex expression, skewed sex ratios, and microhabitat distribution in the dioecious desert moss Syntrichia caninervis (Pottiaceae). Am J Bot 87(4):517–526
Bowker M, Belnap J, Davidson D, Phillips S (2005) Evidence for micronutrient limitation of biological soil crusts: importance to arid-lands restoration. Ecol Appl 15(6):1941–1951
Bowker M, Maestre F, Escolar C (2010) Biological crusts as a model system for examining the biodiversity-ecosystem function relationship in soils. Soil Biol Biochem 42(3):405–417
Brinda J, Stark L, Shevock J, Spence J (2007) An annotated checklist of the bryophytes of Nevada, with notes on collecting history in the state. The Bryologist 110(4):673–705
Brinda JC, Fernando C, Stark LR (2011) Ecology of bryophytes in Mojave Desert biological soil crusts: effects of elevated CO2 on sex expression, stress tolerance, and productivity in the moss Syntrichia caninervis Mitt. In: Tuba Z, Slack N, Stark LR (eds) Bryophyte ecology and climate change. Cambridge University Press, Cambridge, pp 169–191
Buitink J, Hoekstra FA, Leprince O (2002) Biochemistry and biophysics of tolerance systems. In: Black M, Pritchard HW (eds) Desiccation and survival in plants: drying without dying. CAB International, Wallingford, pp 293–318
Castro C, McKee T, Pielke R Sr (2010) The relationship of the North American monsoon to tropical and North Pacific sea surface temperatures as revealed by observational analyses. J Clim 14:4449–4473
Clair L, Johansen J (1993) Introduction to the symposium on soil crust communities. West N Am Nat 53(1):1–4
Coe KK, Belnap J, Sparks JP (2012a) Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology 93(7):1626–1636
Coe KK, Belnap J, Grote EE, Sparks JP (2012b) Physiological ecology of the desert moss Syntrichia caninervis after ten years exposure to elevated CO2: evidence for enhanced photosynthetic thermotolerance. Physiol Plant 144(4):346–356
Csintalan Z, Takacs Z, Tuba Z, Proctor MCF, Smirnof N, Grace J (1997) Desiccation tolerance of grassland cryptograms under elevated CO2: preliminary findings. Abstr Bot 21:309–315
Dilks TJK (1976) Measurement of the carbon dioxide compensation point and the rate of loss of 14 CO2 in the light and dark in some bryophytes. J Exp Bot 27:98–104
Dilks TJK, Proctor MCF (1974) The pattern of recovery of bryophytes after desiccation. J Bryol 8:97–115
Dilks TJK, Proctor MCF (1979) Photosynthesis, respiration and water content in bryophytes. New Phytol 82:97–114
Evans R, Belnap J (1999) Long-term consequences of disturbance on nitrogen dynamics in an arid ecosystem. Ecology 80(1):150–160
Furness S, Grime J (1982) Growth rate and temperature responses in bryophytes: II. A comparative study of species of contrasted ecology. J Ecol 70:525–536
Gaff DF (1997) Mechanisms of desiccation tolerance in resurrection vascular plants. In: Basra AS, Basra RK (eds) Mechanisms of environmental stress resistance in plants. Harwood Academic Publishers, London, pp 43–58
Grote EE, Belnap J, Housman D, Sparks JP (2010) Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Glob Change Biol 16(10):2763–2774
Hamerlynck E, Csintalan Z, Nagy Z, Tuba Z, Goodin D, Henebry G (2002) Ecophysiological consequences of contrasting microenvironments on the desiccation tolerant moss Tortula ruralis. Oecologia 131(4):498–505
Hanson D, Swanson S, Graham L, Sharkey T (1999) Evolutionary significance of isoprene emission from mosses. Am J Bot 86(5):634–639
Harper K, Pendleton R (1993) Cyanobacteria and cyanolichens: can they enhance availability of essential minerals for higher plants? West N Am Nat 53(1):59–72
Hearnshaw GF, Proctor MCF (1982) The effect of temperature on the survival of dry bryophytes. New Phytol 90:221–228
Hinshiri H, Proctor M (1971) The effect of desiccation on subsequent assimilation and respiration of the bryophytes Anomodon viticulosus and Porella platyphylla. New Phytol 70:527–538
Housman D, Powers H, Collins A, Belnap J (2006) Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J Arid Environ 66(4):620–634
Huxman T, Snyder K, Tissue D, Leffler A, Ogle K, Pockman W, Sandguist D, Potts D, Schwinning S (2004) Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141(2):1–15
Jobson RW, Qiu Y-L (2010) Amino acid compositional shifts during streptophyte transitions to terrestrial habitats. J Mol Evol 72(2):204–214
Kidron G, Barzilay E, Sachs E (2000) Microclimate control upon sand microbiotic crusts, western Negev Desert, Israel. Geomorphology 36(1–2):1–18
Lackner L (1939) Uber die Jahresperiodizitat in der Entwicklung der Laub-moose. Planta 29:534–616
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups, 4th edn. Springer, Berlin
Li Y, Wang Z, Xu T, Tu W, Liu C, Zhang Y, Yang C (2010) Reorganization of photosystem II is involved in the rapid photosynthetic recovery of desert moss Syntrichia caninervis upon rehydration. J Plant Physiol 167(16):1390–1397
Lioubimtseva E (2004) Climate change in arid environments: revisiting the past to understand the future. Prog Phys Geogr 28(4):502–530
Liu Y, Cao T, Glime J (2003) The changes of membrane permeability of mosses under high temperature stress. The Bryologist 106(1):53–60
Loik M, Breshears D, Lauenroth W, Belnap J (2004) A multi-scale perspective of water pulses in dryland ecosystems: climatology and ecohydrology of the western USA. Oecologia 141(2):269–281
Long S, Ainsworth E, Rogers A, Ort D (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55(1):591–628
Lovelock C, Jackson A, Melick D, Seppelt R (1995) Reversible photoinhibition in Antarctic moss during freezing and thawing. Plant Physiol 109(3):955–961
Marschall M (2004) Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann Bot 94(4):593–603
Martin CE, Churchill SP (1982) Chlorophyll concentrations and a/b ratios in mosses collected from exposed and shaded habitats in Kansas. J Bryol 12(2):297–304
Mcletchie D (2006) Sporophyte and gametophyte generations differ in their thermotolerance response in the moss microbryum. Ann Bot 97(4):505–511
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh AJ, Knutti R, Murphy JM, Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao Z-C (2007) Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge/New York
Meyer H, Santarius K (1998) Short-term thermal acclimation and heat tolerance of gametophytes of mosses. Oecologia 115(1):1–8
Mishler BD, Oliver MJ (1991) Gametophytic phenology of Tortula ruralis, a desiccation-tolerant moss, in the Organ Mountains of southern New Mexico. The Bryologist 94(2):143–153
Mishler BD, Oliver MJ (2009) Putting Physcomitrella patens on the tree of life: the evolution and ecology of mosses. Annu Plant Rev 36:1–15
Ochyra R, Zander R (2002) The genera Didymodon and Bryoerythrophyllum (Pottiaceae) in Antarctica. J Bryol 24(1):33–44
Oliver MJ, Bewley JD (1984) Plant desiccation and protein synthesis V. stability of Poly(A)− and Poly(B)+ RNA during desiccation and their synthesis upon rehydration in the desiccation tolerance moss Tortula ruralis and the intolerant moss Cratoneuron filicinum. Plant Physiol 74:917–922
Oliver MJ, Mishler BD, Quidenberry JE (1993) Comparative measures of desiccation-tolerance in the Tortula ruralis complex. I. Variation in damage control and repair. Am J Bot 80(2):127–136
Oliver MJ, Velten J, Wood AJ (2000a) Bryophytes as experimental models for the study of environmental stress tolerance: Tortula ruralis and desiccation tolerance in mosses. Plant Ecol 151:73–84
Oliver MJ, Tuba Z, Mishler BD (2000b) The evolution of vegetative desiccation tolerance in land plants. Plant Ecol 151:85–100
Oliver MJ, Velten J, Mishler BD (2005) Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integr Comp Biol 45:788–799
Paolillo DJ (1979) On the lipids of the sperm masses of three mosses. The Bryologist 82:93–96
Proctor MCF (1979) Structure and eco-physiological adaptation in bryophytes. In: Clarke GCS, Duckett JG (eds) Bryophyte systematics. Systematics Association special volume 14. Academic, London
Proctor MCF (2000) The bryophyte paradox: tolerance of desiccation, evasion of drought. Plant Ecol 151(1):41–49
Proctor MCF (2003) Experiments on the effect of different intensities of desiccation on bryophyte survival, using chlorophyll fluorescence as an index of recovery. J Bryol 25(3):201–210
Proctor MCF (2008) Physiological ecology. In: Shaw AJ, Goffinet B (eds) Bryophyte biology. Cambridge University Press, Cambridge
Proctor MCF, Pence VC (2002) Vegetative tissues: bryophytes, vascular resurrection plants and vegetative propagules. In: Black M, Pritchard HW (eds) Desiccation and survival in plants: drying without dying. CAB International, Wallingford, pp 207–239
Proctor MCF, Smirnoff N (2000) Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. J Exp Bot 51(351):1695–1704
Proctor MCF, Smirnoff N (2011) Ecophysiology of photosynthesis in bryophytes: major roles for oxygen photoreduction and non-photochemical quenching? Physiol Plant 141:130–140
Proctor MCF, Tuba Z (2002) Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytol 156:327–349
Proctor MCF, Nagy Z, Csintalan Z, Takács Z (1998) Water-content components in bryophytes: analysis of pressure-volume relationships. J Exp Bot 49(328):1845–1854
Proctor MCF, Oliver MJ, Wood A, Alpert P, Stark LR, Cleavitt N, Mishler B (2007) Desiccation-tolerance in bryophytes: a review. The Bryologist 110(4):595–621
Reed SC, Coe KK, Sparks JP, Housman DC, Zelikova TJ, Belnap J (2012) Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat Clim Change 2:752–755
Reynolds R, Belnap J, Reheis M, Lamothe P, Luiszer F (2001) Aeolian dust in Colorado Plateau soils: nutrient inputs and recent change in source. Proc Natl Acad Sci 98(13):7123–7127
Reynolds J, Kemp P, Ogle K, Fernandez R (2004) Modifying the pulse-reserve paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141(2):1–17
Rosentreter R, Bowker M, Belnap J (2007) A field guide to biological soil crusts of Western U.S. drylands. U.S. Government Printing Office, Denver
Sala O, Lauenroth W (1982) Small rainfall events: an ecological role in semiarid regions. Oecologia 53(3):301–304
Schonbeck MW, Bewley JD (1981a) Responses of the moss Tortula ruralis to desiccation treatments. I. Effects of minimum water content and rates of dehydration and rehydration. Can J Bot 59:2698–2706
Schonbeck MW, Bewley JD (1981b) Responses of the moss Tortula ruralis to desiccation treatments. II. Variations in desiccation tolerance. Can J Bot 59:2707–2712
Schwinning S, Starr B, Wojcik N, Miller M, Ehleringer J, Sanford R Jr (2005) Effects of nitrogen deposition on an arid grassland in the Colorado plateau cold desert. Rangel Ecol Manage 58(6):565–574
Seel WE, Hendry GAF, Lee JA (1992) The combined effects of desiccation and irradiance on mosses from xeric and hydric habitats. J Exp Bot 43(8):1023–1030
Sharkey TD, Schrader SM (2006) High temperature stress. In: Madhava Rao KV, Raghavendra AS, Reddy KJ (eds) Physiology and molecular biology of stress tolerance in plants. Springer, Dordrecht, pp 101–130
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52:407–436
Sharkey TD, Badger MR, von Caemmerer S, Andrews TJ (2001) Increased heat sensitivity of photosynthesis in tobacco plants with reduced Rubisco activase. Photosynth Res 67:147–156
Smirnoff N (1992) The carbohydrates of bryophytes in relation to desiccation tolerance. J Bryol 17:185–198
Smith SD, Monson RK, Anderson JE (1997) Physiological ecology of North American desert plants. Springer, Berlin/Heidelberg/New York
Stark LR (2001) Widespread sporophyte abortion following summer rains in Mojave Desert populations of Grimmia orbicularis. The Bryologist 104(1):115–125
Stark LR (2005) Phenology of patch hydration, patch temperature and sexual reproductive output over a four-year period in the desert moss Crossidium crassinerve. J Bryol 27(3):231–240
Stark LR, McLetchie D (2006) Gender-specific heat-shock tolerance of hydrated leaves in the desert moss Syntrichia caninervis. Physiol Plant 126(2):187–195
Stark LR, Mishler BD, Mcletchie DN (2000) The cost of realized sexual reproduction: assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. Am J Bot 87(11):1599–1608
Stark LR, McLetchie D, Mishler B (2005) Sex expression, plant size, and spatial segregation of the sexes across a stress gradient in the desert moss Syntrichia caninervis. The Bryologist 108(2):183–193
Stark LR, McLetchie D, Eppley S (2010) Sex ratios and the shy male hypothesis in the moss Bryum argenteum (Bryaceae). The Bryologist 113(4):788–797
Stark LR, Brinda JC, McLetchie DN (2011a) Effects of increased summer precipitation and N deposition on Mojave Desert populations of the biological crust moss Syntrichia caninervis. J Arid Environ 75:457–463
Stark LR, McLetchie DN, Smith SD, Oliver MJ (2011b) Responses of a biological soil crust moss to increased monsoon precipitation and nitrogen deposition in the Mojave Desert. In: Tuba Z, Slack N, Stark LR (eds) Bryophyte ecology and climate change. Cambridge University Press, Cambridge, pp 149–169
Thompson D, Walker L, Landau F, Stark L (2005) The influence of elevation, shrub species, and biological soil crust on fertile islands in the Mojave Desert, USA. J Arid Environ 61(4):609–629
Tuba Z, Lichtenthaler HK (2011) Ecophysiology of homoiochlorophyllous and poikilochlorophyllous desiccation-tolerant plants and vegetations. In: Luttge U, Beck E, Batrels D (eds) Plant desiccation tolerance, vol 215, Ecological studies. Springer, Berlin/Heidelberg, pp 157–185
Tuba Z, Lichtenthaler H, Csintalan Z, Nagy Z, Szente K (1994) Reconstitution of chlorophylls and photosynthetic CO2 assimilation upon rehydration of the desiccated poikilochlorophyllous plant Xerophyta scabrida (Pax) Th. Dur. et Schinz. Planta 192(3):414–420
Tuba Z, Csintalan Z, Proctor MCF (1996) Photosynthetic responses of a moss, Tortula ruralis, ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation, and their ecophysiological significance: a baseline study at present-day CO2 concentration. New Phytol 133:353–361
Tuba Z, Csintalan Z, Szente K, Nagy Z, Grace J (1998) Carbon gains by desiccation-tolerant plants at elevated CO2. Funct Ecol 12(1):39–44
Tucker E, Costerton J, Bewley J (1975) The ultrastructure of the moss Tortula ruralis on recovery from desiccation. Can J Bot 53(2):94–101
Valanne N (1984) Photosynthesis and photosynthetic products in mosses. In: Dyer AJ, Duckett JG (eds) The experimental biology of bryophytes. Academic, London, pp 257–273
Valko PG (2003) Monitoring biological soil crusts using hyperspectral remote sensing: determination of cyanobacteria, lichen, and moss contribution to spectral indices and observing community compositional changes due to global climate change. Senior thesis, George Washington University, St. Louis
Vitousek P, Howarth R (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13(2):87–115
Wal R, Pearce I, Brooker R (2005) Mosses and the struggle for light in a nitrogen-polluted world. Oecologia 142(2):159–168
Xu S, Jiang P, Wang Z, Wang Y (2009a) Crystal structures and chemical composition of leaf surface wax depositions on the desert moss Syntrichia caninervis. Biochem Syst Ecol 37:723–730
Xu S, Liu C, Jiang P, Cai W, Wang Y (2009b) The effects of drying following heat shock exposure of the desert moss Syntrichia caninervis. Sci Total Environ 407(7):2411–2419
Zaady E, Kuhn U, Wilske B, Sandoval-Soto L, Kesselmeier J (2000) Patterns of CO2 exchange in biological soil crusts of successional age. Soil Biol Biochem 32(7):959–966
Acknowledgements
We wish to thank Lloyd Stark for important discussions and insightful comments related to the information presented in this chapter, as well as use of photographs of Syntrichia caninervis. We also wish to acknowledge the members of the 2011–2012 Sparks Lab at Cornell University for helpful advice on previous versions of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Coe, K.K., Sparks, J.P., Belnap, J. (2014). Physiological Ecology of Dryland Biocrust Mosses. In: Hanson, D., Rice, S. (eds) Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration, vol 37. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6988-5_16
Download citation
DOI: https://doi.org/10.1007/978-94-007-6988-5_16
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6987-8
Online ISBN: 978-94-007-6988-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)